Chapter 61 Thoracoscopic Corpectomy and Reconstruction
Spinal instability caused by trauma or destructive disease has historically been treated through a dorsal approach. Purely dorsal techniques, however, often fail adequately to address ventrally located pathology. Dorsal decompression with thoracic laminectomy of ventral epidural masses has been associated with increased risk of injury to the spinal cord. Ventral spinal canal decompression through dorsal and even posterolateral approaches can be challenging and ineffective.1–3 Furthermore, dorsal instrumentation may not sufficiently stabilize a significantly disrupted ventral load-bearing spinal column.4,5
To more effectively and directly decompress and stabilize the ventral spine, ventral thoracotomy and thoracoabdominal techniques were developed.6,7 Although these approaches demonstrated improved outcomes and are an acceptable treatment modality for ventral thoracolumbar disease, the high access morbidity of these open procedures often results in post-thoracotomy pain syndromes, postoperative pneumothorax or pleural effusion, shoulder dysfunction, abdominal wall relaxation, and significant scarring of the chest wall.8
Spine surgeons have more recently adapted the minimally invasive thoracoscopic techniques that have been applied by thoracic surgeons for many years. Thoracoscopic spine surgery was first used for the treatment of thoracic disc herniations and traumatic fractures. With advances in thoracoscopic video technology, instrumentation, and instrument systems, thoracoscopic spine surgery has improved significantly, and its use has been expanded to include the treatment of most ventral thoracolumbar disorders, including trauma, tumor, and degenerative disease, as well as deformity correction in select cases.4,9–15
Specialized tools for endoscopic spine surgery are used to access the thoracic cavity through small chest incisions, and the surgery is performed under two-dimensional video guidance. Minimizing chest wall dissection and retraction through the use of small thoracoscopic incisions has significantly improved outcomes and reduced postoperative morbidity without compromising long-term successful fusion rates.9–11,16–18 The minimally invasive thoracoscopic approach can now be safely and effectively performed to treat disease that had previously required an open thoracotomy.
Advantages and Disadvantages
Several advantages are offered by the minimally invasive ventrolateral thoracoscopic approach over an open thoracotomy. Multiple vertebral levels and the ventral spinal canal can be visualized and treated without increasing surgical exposure when access ports are properly placed. The surgical field can be imaged with excellent resolution using modern high-definition endoscopic technology. The small intercostal incisions negate the need for rib resection and retraction, unlike open thoracotomy approaches, which necessitate large incisions, extensive dissection of intercostal muscles, rib resection, and retraction of the chest wall. The thoracoscopic approach is associated with reduced blood loss, need for blood transfusion,19 days of mechanical ventilation, perioperative wound pain, incidence of pulmonary and shoulder dysfunction, length of hospital stay, and days to rehabilitation.16–18
For most spine surgeons, the major disadvantage of the thoracoscopic approach is unfamiliarity with the technique and high technical demand. The operation is performed distant from the surgical site in two dimensions based solely on thoracoscopic image guidance, which requires most spine surgeons to acquire a new set of skills. Before operating on a patient, the surgeon must gain familiarity with the new technique in practical and didactic training sessions. The surgeon and operating room staff must overcome a steep learning curve while gaining familiarity with the approach, and this can initially increase operative times by several hours. Anesthesia monitoring and double-lumen ventilation may also increase operative times. Conversion to an open thoracotomy may be required with difficult cases or when intraoperative complications cannot be resolved with the thoracoscopic technique. Finally, extensive intrathoracic disease, whether pulmonary or spinal, may be difficult to address with the thoracoscopic approach.
Indications and Contraindications
The thoracoscopic approach is best suited for patients with thoracolumbar disease limited to one vertebral body and the ventral spinal canal between T3 and L3, although multiple levels may be treated. The most common indication for thoracoscopic spinal surgery is in the setting of trauma. Among patients with traumatic spinal injury, ventral spinal reconstruction for biomechanical instability is the most common surgical indication. Traumatic spinal instability may be secondary to fracture, injury to the intervertebral discs, or significant ligamentous disruption. The mainstay of treatment for thoracolumbar fractures is rigid fixation with transpedicular screw and rod constructs. The decision to add ventral column reconstruction is based on the load-bearing capacity of the injured spinal segment. The load-sharing classification system developed by McCormack et al.5 established a correlation between failure of dorsal short segment fixation and the characteristics of the most significantly injured vertebrae. Fractures with a high degree of vertebral body comminution, fragment apposition, and postoperative deformity correction were found to be at high risk for dorsal instrumentation failure. Thoracoscopic surgery for reconstruction of the ventral load-bearing elements is indicated in these patients. Although patients with neurologic deficits from fracture intrusion into the ventral spinal canal comprise a minor subgroup of patients with traumatic spine injury, they are also indicated for spinal canal decompression and ventral stabilization.
In cases of spinal tumor, thoracolumbar surgery is indicated for treatment of spinal instability, radiation treatment failures, most cases of spinal stenosis secondary to epidural tumor causing neural compression, and pain intractable to conservative measures or to obtain a histologic diagnosis.3,20–23 The thoracoscopic approach is indicated for resection of vertebral body tumors with or without ventral spinal canal involvement and for ventral column reconstruction with interbody placement and ventrolateral instrumentation.
Preoperative Assessment and Planning
Planning of Resection and Reconstruction
Detailed preoperative examination of the patient’s bony anatomy is important for planning the reconstruction. The appropriate lengths of the vertebral body screws are determined by measuring the widths of the vertebrae on the preoperative images. The height of the interbody is approximated by measuring the distance between the inferior end plate of the cranial level and the superior end plate of the caudal level. The plate dimensions can also be approximated preoperatively by measuring the distance between the lower third of the cranial vertebral body and the upper third of the caudal vertebral body. The extent of pathologic canal intrusion is also measured to determine the amount of bony resection needed for sufficient ventral spinal canal decompression.
Operative Technique
Thoracoscopic Instruments and Instrumentation Systems
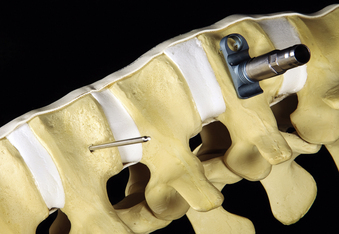
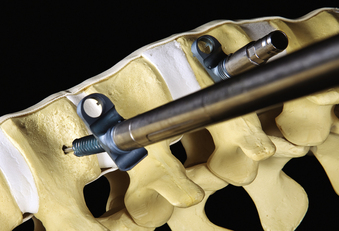
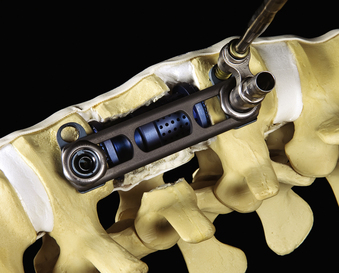
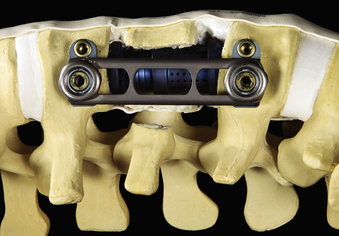
In addition to specialized instruments, the MACS-TL ventrolateral thoracolumbar spinal implant (Aesculap, Tuttlingen, Germany) was specifically designed for thoracoscopic use (Fig. 61-1). It consists of a rigid plate that is secured to the ventrolateral vertebral body with two pairs of triangulated fixation screws for increased strength: two dorsal polyaxial screws and two ventral stabilizing screws. The screws are implanted into the normal vertebrae adjacent to the diseased vertebra(e). The fixation plate is then rigidly secured to the screws. The biomechanical properties of the MACS-TL plating system have been characterized in monosegmental and bisegmental partial and full corpectomy models both with and without dorsal ligamentous injury. Case series have also demonstrated its clinical efficacy.10,13,15,24–26
Localization
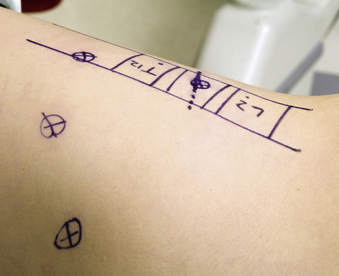
The relation of the spine and identified sites of the access portals is determined with intraoperative fluoroscopy. After optimal patient positioning, a lateral image centered over the pathologic area is obtained and projected orthograde onto the chest wall. The level of interest is often evident because of changes in spinal alignment and bony architecture. The skin is marked with a diagram outlining the vertebral bodies of the pathologic and adjacent levels by drawing the ventral and dorsal spinal lines and intervertebral discs. The four portal access sites are then marked (Fig. 61-2). The positioning of these portals determines working distances and is essential for proper retraction and intraoperative thoracoscopic image guidance.
Dissection and Exposure
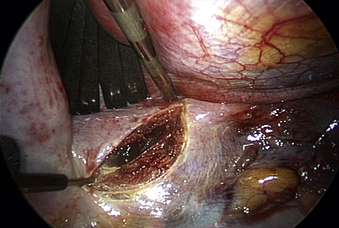
The spine is oriented horizontally on the video monitor by rotating the endoscope. The aorta is situated ventral to the spine, and its dorsal margin demarcates the ventral border of the vertebral bodies. The dome of the diaphragm is then retracted to expose the diaphragmatic insertion, which is typically located between T12 and L1. The spine is exposed by incising the diaphragm when operating at or below the insertion (Fig. 61-3). The diaphragm is incised with a harmonic scalpel where it is naturally thin, which is parallel to and 1 to 2 cm away from the insertion site. Closure of the diaphragm at the end of the case is also facilitated by this margin of tissue. The layers of the diaphragm are incised in a semicircular line along the spine and ribs. A 2- to 3-cm incision is generally sufficient for lesions at L1. For exposure of L2, the length is increased caudally to approximately 5 cm. For lesions at L3, which may be difficult to approach with the thoracoscopic technique, a more extensive incision is made in the diaphragm, and a thoracoscopic-assisted, mini-open retroperitoneal exposure is used. Once the diaphragm is incised, the retractor is inserted through the opening. The ventral spine is exposed by mobilizing the peritoneal sac and retroperitoneal fat from the psoas muscle fascia with blunt dissection and retracting the tissue ventrally and caudally.
Vertebral Body Screw Instrumentation
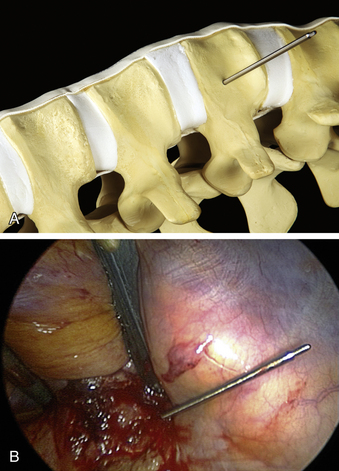
The ventrolateral polyaxial screws for the MACS-TL plating system are inserted into the uninvolved vertebral bodies adjacent to the pathologic level. The first polyaxial screw is placed into the caudal level, which prevents the surgeon having to “look over” a cranially placed screw and keeps the visual field unobstructed. The screw entry point is positioned approximately 10 mm ventral to the spinal canal and 10 mm away from the end plate in the upper third of the vertebra (Fig. 61-4A), away from the midportion of the vertebral body where the segmental blood vessels lie. A short Kirschner wire (K-wire) is inserted under fluoroscopic guidance perpendicular to the cortical surface with a radiolucent targeting device. When a perpendicular position is attained, the concentric rings on the targeting device will align and the K-wire will appear on-end. A mallet is used to strike the device attached to the K-wire until a stable depth of approximately 25 mm is reached (Fig. 61-4B). The K-wire is then detached from the targeting instrument. The cortical surface is perforated with a cannulated decorticator, which is inserted over the K-wire, in preparation for screw instrumentation.
The screws for the MACS-TL system are inserted into a specialized polyaxial clamp before placement. The screw-clamp assembly is attached to a centralizer tube and inserted over the K-wire. The clamp is oriented with the ventral stabilizing screw hole located ventrally. The K-wire is removed after the screw is advanced past the cortical surface. Removal of the K-wire prevents its advancement during screw insertion and the possibility of vascular perforation or soft tissue injury. The screw is then safely inserted to its full depth (Fig. 61-5). Screw placement is then performed at the cranial level using a similar technique (Fig. 61-6). The insertion site is approximately 10 mm ventral to the spinal canal and in the inferior third of the vertebral body, approximately 10 mm cranial to the inferior end plate.
Vertebral Body Resection and Spinal Canal Decompression
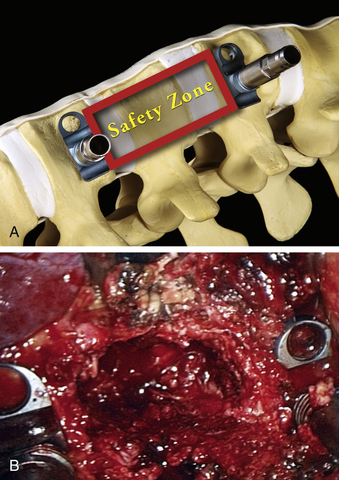
The corpectomy boundaries are defined cranially and caudally by the intervertebral discs. The polyaxial clamps are oriented parallel to the end plates. The ventral and dorsal edges of the clamps define the ventromedial and posterolateral margins of the corpectomy. These boundaries define an area that includes both the corpectomy region itself and a safety zone that protects critical structures (Fig. 61-7A). The instruments are kept within these safe boundaries during the vertebral body resection.
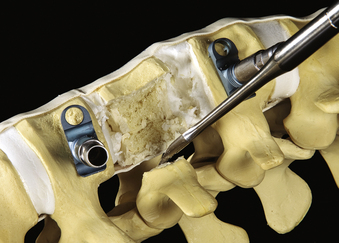
Thoracoscopic discectomies at the disc spaces above and below the pathologic level are performed using the same technique as in open procedures. A thoracoscopic scalpel is used to perform an anulectomy and thoracoscopic rongeurs are used for disc resection. Cartilage and soft tissue are removed for end-plate preparation. A central corpectomy is performed by removing the vertebral body bone between the resected disc spaces. Osteotomes are used to create a rectangular trough in the vertebrae. Curettes, rongeurs, or a high-speed drill may be used to extend the corpectomy cavity (Fig. 61-7B). The cortical bone of the ventral, dorsal, and contralateral margins is kept intact. The depth of the corpectomy across the midline is confirmed with intraoperative fluoroscopy. In cases of tumor, bone and tissue samples are collected for frozen and permanent pathology sectioning.
Spinal canal decompression is performed for lesions significantly encroaching on the spinal canal beyond the dorsal wall of the vertebral body. The ventral spinal canal is accessed by identifying and removing the ipsilateral rib head and pedicle (Fig. 61-8). The rib head is followed to its attachment to the ventrolateral spine and resected with a high-speed drill. Removal of the proximal 2 cm exposes the underlying pedicle, which may be palpated and demarcated with a blunt hook. The pedicle is resected with thoracoscopic rongeurs and a high-speed drill to simultaneously decompress the neuroforamen and provide ventral spinal canal access. The epidural space is carefully cleared of bone and other pathologic tissues, such as blood and tumor, by gentle manipulation into the corpectomy cavity and removal. During this process, direct visualization of the thecal sac prevents injury to the underlying ventral spinal cord. Vertebral body resection is completed after the ventral spinal canal is adequately decompressed and the corpectomy cavity has been enlarged to accommodate the interbody device for reconstruction and stabilization.
Vertebral Body Reconstruction and Completion of Instrumentation
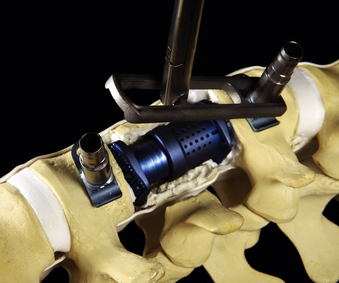
After the vertebral body is stabilized, the ventrolateral MACS-TL plate is fit and secured over the screw-clamp assembly. A suitable plate length is chosen by measuring the distance between the screw heads and adding 30 mm to the measurement. The plate is first mounted over the centralizer and caudal screw-clamp assembly and then positioned onto the cranial assembly (Fig. 61-9). Leaving the screws slightly loose when fitting the plate allows angulation of the polyaxial heads and facilitates plate positioning. Fixation nuts are screwed onto the polyaxial head over the plate and secured with a torque handle and then the centralizers are removed from the polyaxial clamps.
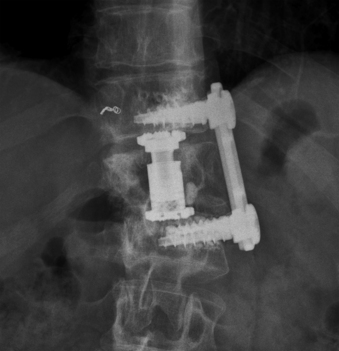
The reconstruction is completed by inserting the ventral stabilizing screws and tightening and locking the polyaxial screws. Final tightening of the polyaxial screws down to the vertebral bodies eliminates the rotational freedom of the screw-clamp assembly and plate. The guide sleeve for the ventral stabilizing screw is attached to the polyaxial clamp. The decorticator is inserted through the guide sleeve and ventral hole in the clamp. A driver is used to insert the ventral stabilizing screw into the vertebral body (Fig. 61-10), and the guide sleeve is removed. The ventral stabilizing screws for the MACS-TL system are triangulated and should be 5 mm shorter than the posterolateral screw to prevent contact of the two screws. Finally, the gold locking screw is inserted into the polyaxial head and locked with a torque wrench, converting the polyaxial screw-clamp system into a rigid and completed construct (Figs. 61-11 and 61-12). Anteroposterior and lateral fluoroscopic imaging verifies the final construct position.
Chest Tube Placement and Wound Closure
The patient may be extubated immediately after surgery. Lung inflation without significant pneumothorax is also verified in the immediate postoperative period with a chest radiograph. Initially, the chest tube is connected to intermittent wall suction. If proper lung inflation is demonstrated on the chest radiograph, the chest tube is advanced to water seal on postoperative day 1. Patient mobilization, incentive spirometry training, and postoperative plain radiographs and CT scans centered on the construct are also obtained on postoperative day 1. Once chest radiographs continue to demonstrate lung inflation without pneumothorax and the chest tube output decreases below 100 mL/day, the chest tube is removed, typically on postoperative day 2. A final chest radiograph is used to verify stable lung inflation after chest tube removal. After discharge, the patient returns to the clinic for follow-up examination and plain radiographs at 1-, 3-, 6-, 12-, and 24-month intervals. Work with light duty is typically allowed after 4 weeks and full activity after 3 months.
Technical Tips
Patients with poor bone quality may require construct reinforcement to achieve solid instrumented fixation. Cementable MACS-TL screws with increased thread diameter and pitch to prevent pullout are available for this purpose (Figs. 61-13 and 61-14); these have been demonstrated to increase fixation strength and biomechanical stability.27,28 In challenging cases, these screws can also be used for rescue. Injectable cement is delivered into the hollow screw core and through three longitudinal slits into the surrounding cancellous bone. The posterolateral MACS-TL screws are replaced by the cementable screws in the polyaxial clamps, and screw implantation is performed with the same technique. The construct is tightened flush against the bone into its final position before 1.5 mL of cement is injected because adjusting the position of the construct is extremely difficult once the cement has set. Securing the locking nuts completes the construct.
Outcomes
Results
Minimally invasive thoracoscopic spine surgery has demonstrated safety and efficacy, with improved outcomes over open surgery. The limited exposure and approach are the primary factors in decreasing overall morbidity. Thoracoscopic procedures have complication rates between 0% and 5.4%,10,13,16–18 whereas open thoracotomy morbidity rates are between 14% and 29.5%.8,16–18,29 The complication rate of postoperative pneumothorax, pleural effusion, and intercostal muscle neuralgia for the thoracoscopic approach is reported to be 5.4%, compared with 14% for the open approach.18 In cases of tumor, the reported mortality rate for open thoracotomy is 8.2%,8 whereas thoracoscopic surgery has had no reported mortality.10,16 Both thoracotomy and thoracoscopic surgery are associated with low infection rates of around 0.5%.16–18
Postoperative pain after thoracoscopic surgery is significantly reduced.10 Postoperative analgesic therapy dosages and durations are reported to decrease by 42% and 31%, respectively.16–18 Although reported rates of chronic pain with open thoracotomy range from 7% to 55%,18 favorable rates between 4% and 35% are associated thoracoscopic surgery. The median length of stay in the hospital was 7 days (range, 4–10 days) in a series of patients who underwent thoracoscopic tumor surgery,10 compared with a median of 9 days (range, 4 to 57 days) for patients who underwent open thoracotomy for tumor resection.8 Fusion rates at 1-year radiographic follow-up have been reported to be up to 90% using the MACS-TL ventrolateral plating system.17
While the surgeon and surgical staff learn the new thoracoscopic technique, operative times increase initially to an average of 6 hours or more.9,10,13,16–18 Once the procedure has been mastered, total surgical time for resection and reconstruction is decreased to 2.5 to 3 hours in cases of trauma18 and to 4 hours for cases of tumor.10 The average estimated blood loss for thoracoscopic spine surgery in trauma patients ranges from 250 to 450 mL,18 which is less than with many dorsal fusion procedures. In cases of tumor, blood loss of 600 mL is reported for thoracoscopic cases,10 whereas studies of open thoracotomy cases for tumor have reported 1 L of blood loss.8 Major intraoperative complications such as injury to the viscera or vasculature, uncontrollable hemorrhage, and cerebrospinal fluid or chyle leak can be avoided in most instances with meticulous adherence to surgical technique.10 In skilled hands, the rate of conversion to an open thoracotomy is 1% or less.17
Complications
The incidence of significant intraoperative complications with thoracoscopic surgery is low.10,11,13,15 Injury to the aorta or vena cava constitutes the most hazardous complication. Direct injury may occur from excessive use of sharp instruments; indirect extension of injury to the segmental vessels may also damage the great vessels. Injury to the segmental vessels themselves can result in considerable blood loss and is typically the result of insufficient exposure and coagulation. The vasculature may also be injured by a breach through the cortex opposite the corpectomy site, where the segmental vessels may be in close proximity to the vertebral body. To prevent a breach through the cortical bone, the integrity of the opposite cortex is verified with direct thoracoscopic visualization and intraoperative fluoroscopy. Overaggressive retraction, suction, or instrument handling can result in local injury to the lung parenchyma, which is usually sufficiently addressed with stapling or suturing as needed. Aggressive diaphragmatic incisions or retraction can cause injury to the kidneys, spleen, and ureters.
Pulmonary complications are most common during the early postoperative period. Patients may experience atelectasis, persistent pneumothorax or hemothorax, pleural effusions, or pneumonia, and with time may develop intrathoracic adhesions.17,18 Aggressive pulmonary toilet, early ambulation, avoidance of narcotic overuse, and judicious use of the chest tube can prevent these complications in most cases. Although their incidence is lower than with open thoracotomy, intercostal neuralgia and shoulder pain and dysfunction are still possible. The risk of both superficial and deep wound infections and wound dehiscence exists with all surgeries, and may be decreased with a careful multilevel closure.
Potential late complications include instrumentation and fusion failures. Although minimally invasive technology has undergone major advances and improvements, the potential complications of hardware fracture, pullout, and loosening cannot altogether be prevented. The incidence of hardware complications may be decreased by carefully choosing a suitable construct and by improving surgical technique. Furthermore, patients with significant spinal column disruption, as may be the case in trauma, or with advanced osteoporosis may benefit from dorsal instrumentation for supplementation of ventral thoracoscopic reconstruction and to increase overall spinal stability.30
Conclusions
The recent advances in thoracoscopic surgical techniques, instruments, and instrumentation have made this treatment modality one of the standard procedures for thoracolumbar vertebral body resection and ventral spinal canal decompression. The technology continues to be improved and refined. The development of instrumentation specific to thoracoscopic technique has made ventral spinal stabilization and reconstruction achievable after decompression. With current methods, the thoracoscopic technique is safe and effective, and patient outcomes are improved after thoracoscopic surgery compared with open thoracotomy. Given familiarity with the operative technique, an understanding of local and patient-specific anatomy, and appropriate patient selection, a diversity of ventral thoracolumbar disease can be successfully treated with minimally invasive thoracoscopic surgery.
Beisse R. Endoscopic anterior repair in spinal trauma. In: Regan J.J., Lieberman I.H., editors. Atlas of minimal access spine surgery. 2nd ed. St. Louis: Quality Medical Publishing; 2004:285-320.
Beisse R. Thoracoscopic decompression and fixation (MACS-TL). In: Kim D., Fessler R.G., Regan J.J., editors. Endoscopic spine surgery and instrumentation. New York: Thieme; 2004:180-198.
Beisse R. Thoracoscopically assisted anterior approach to thoracolumbar fractures. In: Mayer H.M., editor. Minimally invasive spine surgery: a surgical manual. New York: Springer; 2005:203-214.
Beisse R., Muckley T., Schmidt M.H., et al. Surgical technique and results of endoscopic anterior spinal canal decompression. J Neurosurg Spine. 2005;2:128-136.
Kan P., Schmidt M.H. Minimally invasive thoracoscopic approach for anterior decompression and stabilization of metastatic spine disease. Neurosurg Focus. 2008;25(2):E8.
Khoo L.T., Beisse R., Potulski M. Thoracoscopic-assisted treatment of thoracic and lumbar fractures: a series of 371 consecutive cases. Neurosurgery. 2002;51(Suppl 5):S104-S117.
1. Grant R., Papadopoulos S.M., Greenberg H.S. Metastatic epidural spinal cord compression. Neurol Clin. 1991;9:825-841.
2. Grant R., Papadopoulos S.M., Sandler H.M., et al. Metastatic epidural spinal cord compression: current concepts and treatment. J Neurooncol. 1994;19:79-92.
3. Klimo P.Jr., Kestle J.R., Schmidt M.H. Treatment of metastatic spinal epidural disease: a review of the literature. Neurosurg Focus. 2003;15(5):E1.
4. Beisse R. Video-assisted techniques in the management of thoracolumbar fractures. Orthop Clin North Am. 2007;38:419-429. abstract vii
5. McCormack T., Karaikovic E., Gaines R.W. The load sharing classification of spine fractures. Spine (Phila Pa 1976). 1994;19:1741-1744.
6. Gokaslan Z.L., York J.E., Walsh G.L., et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg. 1998;89:599-609.
7. Schwarzenbach O., Boos N., Aebi M. Spinal metastases and metastasis-induced pathological fractures of the spine [in German]. Unfallchirurg. 1990;93:457-466.
8. Walsh G.L., Gokaslan Z.L., McCutcheon I.E., et al. Anterior approaches to the thoracic spine in patients with cancer: indications and results. Ann Thorac Surg. 1997;64:1611-1618.
9. Amini A., Beisse R., Schmidt M.H. Thoracoscopic spine surgery for decompression and stabilization of the anterolateral thoracolumbar spine. Neurosurg Focus. 2005;19(6):E4.
10. Kan P., Schmidt M.H. Minimally invasive thoracoscopic approach for anterior decompression and stabilization of metastatic spine disease. Neurosurg Focus. 2008;25(2):E8.
11. Beisse R., Muckley T., Schmidt M.H., et al. Surgical technique and results of endoscopic anterior spinal canal decompression. J Neurosurg Spine. 2005;2:128-136.
12. Caputy A., Starr J., Riedel C. Video-assisted endoscopic spinal surgery: thoracoscopic discectomy. Acta Neurochir (Wien). 1995;134:196-199.
13. Khoo L.T., Beisse R., Potulski M. Thoracoscopic-assisted treatment of thoracic and lumbar fractures: a series of 371 consecutive cases. Neurosurgery. 2002;51(Suppl 5):S104-S117.
14. McAfee P.C., Regan J.R., Fedder I.L., et al. Anterior thoracic corpectomy for spinal cord decompression performed endoscopically. Surg Laparosc Endosc. 1995;5:339-348.
15. Schultheiss M., Kinzl L., Claes L., et al. Minimally invasive ventral spondylodesis for thoracolumbar fracture treatment: surgical technique and first clinical outcome. Eur Spine J. 2003;12:618-624.
16. Beisse R. Thoracoscopically assisted anterior approach to thoracolumbar fractures. In: Mayer H.M., editor. Minimally invasive spine surgery: a surgical manual. New York: Springer; 2005:203-214.
17. Beisse R. Thoracoscopic decompression and fixation (MACS-TL). In: Kim D., Fessler R.G., Regan J.J., editors. Endoscopic spine surgery and instrumentation. New York: Thieme; 2004:180-198.
18. Beisse R. Endoscopic anterior repair in spinal trauma. In: Regan J.J., Lieberman I.H., editors. Atlas of minimal access spine surgery. ed 2. St. Louis: Quality Medical Publishing; 2004:285-320.
19. Ragel B.T., Kan P., Schmidt M.H. Blood transfusions after thoracoscopic anterior thoracolumbar vertebrectomy. Acta Neurochir (Wien). 2010;152:597-603.
20. Heary R.F., Bono C.M. Metastatic spinal tumors. Neurosurg Focus. 2001;11(6):E1.
21. Hosono N., Yonenobu K., Fuji T., et al. Orthopaedic management of spinal metastases. Clin Orthop Relat Res. 1995;312:148-159.
22. Klimo P.Jr., Thompson C.J., Kestle J.R., Schmidt M.H. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro-oncol. 2005;7:64-76.
23. Witham T.F., Khavkin Y.A., Gallia G.L., et al. Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nat Clin Pract Neurol. 2006;2:87-94. quiz 116
24. Schultheiss M., Hartwig E., Kinzl L., et al. Thoracolumbar fracture stabilization: comparative biomechanical evaluation of a new video-assisted implantable system. Eur Spine J. 2004;13:93-100.
25. Schultheiss M., Hartwig E., Sarkar M., et al. Biomechanical in vitro comparison of different mono- and bisegmental anterior procedures with regard to the strategy for fracture stabilisation using minimally invasive techniques. Eur Spine J. 2006;15:82-89.
26. Schreiber U., Bence T., Grupp T., et al. Is a single anterolateral screw-plate fixation sufficient for the treatment of spinal fractures in the thoracolumbar junction? A biomechanical in vitro investigation. Eur Spine J. 2005;14:197-204.
27. Schultheiss M., Claes L., Wilke H.J., et al. Enhanced primary stability through additional cementable cannulated rescue screw for anterior thoracolumbar plate application. J Neurosurg. 2003;98(Suppl 1):50-55.
28. Schultheiss M., Hartwig E., Claes L., et al. Influence of screw-cement enhancement on the stability of anterior thoracolumbar fracture stabilization with circumferential instability. Eur Spine J. 2004;13:598-604.
29. Oskouian R.J.Jr., Johnson J.P. Vascular complications in anterior thoracolumbar spinal reconstruction. J Neurosurg. 2002;96(1 Suppl):1-5.
30. Bishop F., Samuelson M., Finn M., et al. The biomechanical contribution of varying posterior constructs after anterior thoracolumbar corpectomy and reconstruction. J Neurosurg Spine. 2010;13:234-239.