Chapter 24 Thoracolumbar Spine Fractures
• The thoracolumbar junction is a flexible transition region in the spine, susceptible to injury due to transfer of kinetic energy.
• Clinicians should maintain a high suspicion of injury with thoracolumbar trauma because the incidence of a second vertebral fracture is 10% to 15%, and soft tissue injury may be as high as 50%.
• The most common mechanism of abdominal injuries is distraction or seat-belt injuries. Blunt abdominal aortic dissections are associated with distraction-rotational injuries of the thoracolumbar region.
• The three-column model of spine injury suggests that when all three columns are injured surgery may be necessary. Goals of surgery should be restoration of stability, balancing of opposing biomechanical forces, and decompression of the spinal canal with the aim to improve neurological outcome.
• Dorsal decompression via multilevel laminectomy alone after thoracic and thoracolumbar injuries has been shown to be ineffective and should not be performed as an isolated treatment strategy. Pedicle screw fixation provides for instrumentation of vertebrae with fractured or absent laminae, with purchase through all three columns. Increased rigidity by pedicle screw fixation permits fewer segments of fixation, leading to the preservation of more motion segments.
Approximately 160,000 patients a year in the United States suffer traumatic spinal column injuries, with 10% to 30% of them having a concurrent spinal cord injury.1–4 Although the majority of these injuries involves cervical (C1-C2) and lumbar (L3-L5) spine fractures, 15% to 20% of traumatic fractures occur at the thoracolumbar junction (T11-L2), whereas 9% to 16% occur in the thoracic spine (T1-T10).5,6 Paraplegia secondary to thoracic fractures have a first-year mortality rate of 7%,3,7 illustrating the devastating effects of thoracolumbar trauma.
Biomechanics
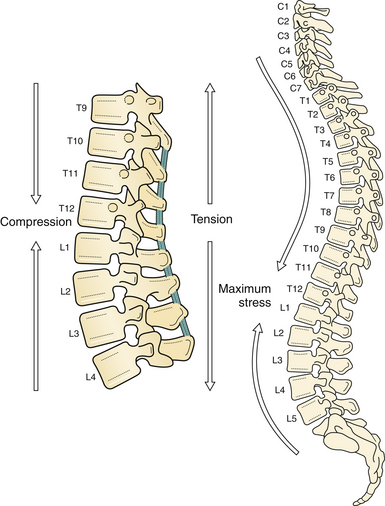
Forces along the long, rigid kyphotic thoracic spine catalyze an abrupt switch into the shorter, mobile lordotic lumbar spine at the thoracolumbar junction (Fig. 24.1). Biomechanically, this transition zone is susceptible to injury and is the most commonly injured portion of the spine. High-energy trauma (motor vehicle accidents) is the leading cause of injury over this region, followed by falls and sports-related injuries.8 Owing to the higher energy mechanisms of injury, additional organ systems are often injured in up to 50% of thoracolumbar trauma patients.8
The vertebral body is the primary load-bearing structure of the spine, with the intervertebral disk transferring all forces applied to the adjacent vertebral bodies.9,10 The annulus fibrosus of the intervertebral disk supports a significant portion of all applied axial and lateral loads and resists tension and shearing.11 The spinal ligamentous structures are essential in maintaining overall sagittal balance. The posterior longitudinal ligament (PLL) is a relatively weak ligament that provides some restriction to hyperflexion, along with the ligamentum flavum. The thick anterior longitudinal ligament (ALL) functions in resisting spinal hyperextension and distraction.12
The thoracic spine differs from the remainder of the spinal column because it is supported by and maintains articulations with the ribs. The intact rib cage increases the axial load-resisting capacity of the thoracic spine by a magnitude of four. The rib cage and facet articulations limit rotation, and therefore most thoracic spine fractures occur from a flexion or axial compression force vector.13 The majority of stability in flexion is provided by the costovertebral articulations.14,15 A significant factor in the degree and extent of fracture character is the rate of force impact loading.16
The thoracolumbar vertebrae are at an increased risk for developing compression fractures after trauma as a consequence of axial loads resulting from the natural kyphotic curvature of the thoracic spine.17 The kyphotic posture results in the placement of axial forces on the ventral portion of the vertebral body. If the strength of the ventral vertebral body is exceeded, a fracture of the vertebral body occurs, resulting in a vertebral compression fracture (VCF). The traumatic forces may also exceed the strength of the dorsal vertebral body and ligamentous elements, resulting in disruption of the dorsal tension band.
The osseous structures, ligaments, rib cage, and inherent anatomy impart great integrity on the thoracic and lumbar spine. The great kinetic energy needed for a fracture to the spine here is dissipated on impact through the soft tissue and viscous elements contained within and around the thoracic cavity, resulting in a high incidence of concurrent injuries. The incidence of concurrent injuries is reported to be greater than 80%, and these injuries involve the thorax, appendicular skeleton, and abdominal region.18–20 These high-energy impacts also affect remote areas from the trauma, such as the cranial vault. Petitjean and associates18 reported a 65% incidence of head injuries after high-velocity impacts, which resulted in incomplete thoracic spinal cord injury with 12% of these injuries classified as severe (Glasgow Coma Scale [GCS] score less than 8).18
Tearing or rupture of the aorta, with associated hemodynamic compromise, has been associated with thoracic vertebral fractures.18,21–23 Hemothorax in up to one third is another comorbidity.24,25 Pulmonary injuries have been reported in 85% of patients and typically consist of pulmonary contusions.26 Infrequently, perforation of the esophagus and tracheal injuries have also been associated with thoracic fractures.27–29
The thoracolumbar region is more vulnerable to concurrent injuries than the thoracic region because it is not provided the protection of the thoracic rib cage. Typically these injuries consist of hollow viscous injuries, such as intestinal perforations, mesenteric avulsions, or solid organ injuries.18,26,30
The most common mechanism of abdominal injuries is distraction or seat-belt injuries.18,26,31 Blunt abdominal aortic dissections are associated with distraction-rotational injuries of the thoracolumbar region.2,31 Multiple-level thoracic and lumbar fractures are also associated with a high incidence of abdominal injuries.2
Axial load injuries, particularly in patients who have jumped or fallen and landed on their feet, may manifest as both thoracolumbar fractures and calcaneal fractures. Miller and colleagues reported a 48% incidence of concurrent abdominal injuries associated with transverse process fractures.32 Therefore, a physician treating vertebral column injuries must be aware of not only the presence of spinal fractures but also the possibility of concurrent, nonspinal, soft tissue and bony injuries.
Radiographic Evaluation
In trauma patients, fractures are commonly missed in the early resuscitative period. Reportedly, between 5% and 15% of multisystem trauma patients have occult fractures missed on initial evaluation.33–35 Composing a minor proportion of traumatic fractures, thoracic spine fractures are extremely difficult to visualize compared to other vertebral or appendicular fractures. Approximately 20% to 50% of superior thoracic spinal fractures are not diagnosed by admission plain radiographs.5,20,36 Therefore, all suspected spinal trauma admissions should be immobilized until a thorough and detailed spinal evaluation can be performed. If appropriate stabilization precautions are not taken in this patient population, unforeseen neurological compromise may result.6
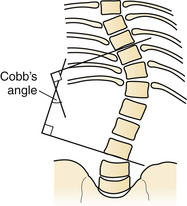
Initial radiographic assessment includes anteroposterior (AP) and lateral spine films assessing for loss of vertical body height, fracture of the pedicles, increased interpedicular distance, transverse process or rib fractures, and malalignment of vertebral bodies. One should examine the lateral radiograph for loss of body height, disruption of rostral or caudal end plate, dorsal cortical wall fracture with retropulsed bone, fracture of spinous processes, widening of interspinous distance, and subluxation or angulation of vertebral bodies.37 Malalignment in the AP plane is suggestive of fracture dislocation.38–40 The Cobb angle may also be calculated, for assessment of deformity.41
The Cobb angle is a measurement commonly used for evaluation of coronal spinal curves in scoliosis on an AP radiographic projection. When measuring the Cobb angle, first one must identify the apical vertebra of the curve in question. This is the vertebra that is the most displaced and rotated from the midline alignment. The end or transitional vertebrae are also identified from the curve above and below the apical vertebra. The end vertebrae are defined as the most superior and inferior vertebrae that are least displaced and least rotated but still present in the deformity’s curve. Two lines are drawn, one along the superior end plate of the superior end vertebra and a second line drawn along the inferior end plate of the inferior end vertebra. Because these lines typically will not meet in the space provided, unless they are severe curves, perpendicular lines are drawn. The angle between the intersecting perpendicular lines is the same as the original lines and is referred to as the Cobb angle (Fig. 24.2).
In the presence of a vertebral body injury, the entire spine should be imaged in an orthogonal manner because of the high incidence (5% to 20%) of noncontiguous spinal fractures.19,42–44 Radiographically, a typical superior end plate thoracic fracture has loss of vertebral height, with or without malalignment, a widened paraspinal line, and possibly a widened mediastinum.20 Difficulties in imaging the upper thoracic region (T1-T4) have led to decreased reliance on diagnosis with plain films. Computed tomography (CT) is more sensitive in detecting fractures than plain radiographs.45 CT is particularly adept at showing the integrity of the middle column, the degree of canal compromise, as well as subluxations or fractures of facets and lamina.
Sagittal reconstructions are helpful in visualizing flexion-distraction injuries and fracture dislocations. CT image reconstruction is also invaluable at the cervicothoracic junction because of the overlay of the scapula, shoulders, and surrounding tissues. In the obtunded patient this technique has been reported to identify more than 10% of fractures not visualized on plain radiographs.46 CT, however, has a limited capacity to visualize disk herniations, epidural or subdural hematomas, ligamentous disruption, or spinal cord parenchymal changes.47
Magnetic resonance imaging (MRI) has further improved the ability to visualize and comprehend the pathological anatomy of the soft tissue, ligamentous, intervertebral disk, and neural element disruption that occurs after spinal injury. MRI has supplanted CT myelography as the imaging tool of choice of the neuraxis, because it is faster, is noninvasive, and allows improved visualization of the spinal cord parenchyma.48,49
MRI evaluation is especially useful at the thoracolumbar junction because of the variable location of the cauda equina and conus medullaris in the adult population at this level.50 A neurological examination can be difficult to interpret at the conus/cauda equina transition level, as a result of the presence of lumbar spinal nerve sparing, the presence of concurrent injuries, sedation, in-dwelling catheters, and delayed reflex recovery. Accurate neural visualization may help in clarifying the pathological anatomy in this clinical situation.
Classification of Thoracolumbar Fractures
Injuries to the thoracic and lumbar spine account for more than 50% of all spinal fractures and a large portion of acute spinal cord injuries.51 Given this frequency and the significant impact of these injuries, significant advancements have been made in the surgical treatment of thoracolumbar trauma. Nonetheless, although there has been progress in the invention and continued evolution of spinal instrumentation and surgical techniques, medical decision making in spine trauma remains controversial.
A number of classification systems have been developed in an attempt to better define thoracolumbar trauma and aid treatment decision making. These systems are typically based on either anatomical structures (the Denis three-column system) or on proposed mechanisms of injury (Ferguson and Allen).52,53
One of the earliest classifications of spinal fractures was by Watson-Jones in 1931, which was based primarily on diagnosis and treatment of flexion injuries.54 This was followed by Nicholl55 who developed the first detailed thoracic and thoracolumbar spinal fracture classification scheme and attempted to define unstable versus stable fractures after trauma in a series of flexion and flexion-rotation injuries.55 Later, Holdsworth56 (Fig. 24.3) further studied the importance of the spinal ligamentous complexes after thoracolumbar junction injuries and classified fractures according to their mechanism of injury into four main types: flexion, flexion and rotation, extension, and compression. Holdsworth56 further classified these fractures as unstable if the posterior ligamentous complex, consisting of the intervertebral disk, spinous ligaments, facet capsules, and the ligamentum flavum, was disrupted.57
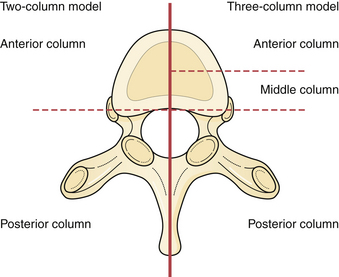
Kelly and Whiteside58 reported that without dislocation of the dorsal elements of the spinal column neurological injuries rarely occur. They classified fractures based on structural criteria and considered the spine to consist of not one, but rather two separate supportive columns. The primary ventral column is composed of the vertebral bodies, and a second structural column consists of the posterior neural arches and ligaments. Later, Louis59 further modified this structural classification scheme by proposing a third column. Louis’ three-column concept consisted of one ventral column and two dorsal columns involving each facet articulation.
Denis used the enhanced CT imaging techniques, along with in vitro biomechanical data, to further modify the spinal column theories into a different three-column classification scheme (see Fig. 24.3). In this classification the ventral column consists of the ALL, the anterior annulus fibrosis, and the anterior half of the vertebral bodies. The middle column consists of the PLL, the dorsal annulus fibrosis, and the dorsal half of the vertebral bodies. Lastly, the posterior column, analogous to what Holdsworth defined as the dorsal ligamentous complex, consists of the bony neural arch, posterior spinous ligaments, and ligamentum flavum, as well as the facet joints. According to the Denis classification scheme, rupture of the dorsal ligamentous complex creates instability only if and when there is concurrent disruption of at least the PLL and dorsal annulus.53
Denis defined failure of the anterior column alone under compression (compression fracture) with an intact posterior column as a stable fracture. Burst fractures were defined as being generated through an axial compressive load, and involved failure of the anterior and middle columns. Severe tensile injuries resulted in seat-belt fracture or flexion-distraction injuries, which involve a disruption of the posterior and middle columns with an intact anterior column that serves as a fulcrum or hinge. The last category in Denis’ scheme is fracture dislocations, which are defined as a mechanical failure of all three columns, which makes them extremely unstable injuries (see Fig. 24.3).
McCormack and co-workers60 created a classification system based on a load-sharing principle that uses a graded point system based on the integrity of the vertebral bodies or anterior and middle column. Points are based on the amount of vertebral body comminution, spread of fragments at the fracture site, and the amount of corrected traumatic kyphosis.61,62 This classification scheme assists the surgeon in deciding if ventral spinal support is necessary after dorsal instrumentation, based on the premise that inadequate anterior column support will result in excessive loads being transferred to the dorsal elements (and instrumentation), thus increasing the risk for failure.
Vaccaro and associates63 proposed the thoracolumbar injury severity score (TLISS) in 2005, designed to simplify the classification of thoracolumbar injury and increase consistency of treatment among surgeons. This system helped address many of its predecessor’s limitations.64–67 The TLISS system defines injuries according to injury morphology, and now for the first time combined with both the PLL status and the neurological status of the patient.
The TLISS was designed to aid in medical decision making by providing both diagnostic and prognostic information with a weighted injury severity score. Stable injury patterns (TLISS < 4) may be treated nonoperatively with brace immobilization and active patient mobilization. Unstable injury patterns (TLISS > 4) may be treated operatively with the guiding principles of deformity correction, neurological decompression if necessary, and spinal stabilization followed by active patient mobilization.64,67
So far, the TLISS system has shown good to excellent intra- and interobserver reliability in a number of countries, among spine surgeons, and throughout a spectrum of spine treatment providers with varying levels of experience.64 A study of 71 cases of thoracolumbar traumatic fracture was given to five spine surgeons, along with details of the TLISS and instructions for scoring, finding a 96.4% agreement among surgeons.63 Furthermore, use of the TLISS has yielded more than a 90% agreement in the management of thoracolumbar trauma across a number of providers.68
Although the TLISS system has demonstrated success, there are inherent limitations. To date, many of the investigations into the TLISS system have been performed by individuals involved with its development.69 Additionally, a prospective application of the TLISS system and severity score to the treatment of spinal injuries is needed to define any improvements in care and patient outcomes compared with conventional systems.
Fracture Management
No definitive treatment algorithm has been universally accepted for this spinal disorder, despite the numerous classification systems that exist. Stability of the vertebral column over the thoracic and thoracolumbar region, like the remainder of the spine, is dependent on the integrity of the osseous and ligamentous components. One difficulty in treating these fractures is that the definition of instability of the spine is difficult to assess, based on clinical and radiographic findings.
Treatment of two-column injuries, such as burst fractures, depends to a significant extent on the neurological status. In neurologically intact patients, nonoperative treatment is generally recommended consisting of bedrest, early mobilization in a thoracolumbosacral orthotic (TLSO) brace, and continued close monitoring for increased kyphosis and neurological changes.70 Defino and Canto demonstrated an excellent reduction of sagittal deformity on 2-year follow-up of 20 patients.71
Burst fractures, as defined by Denis, are vertebral body fractures involving the anterior and middle columns, such that the ALL and vertebral body, including the dorsal vertebral body cortex, are disrupted. As stated before, a burst fracture in a neurologically intact patient without posterior ligamentous or dorsal element fractures is usually considered a stable injury. Burst fractures are inherently more stable because of the presence of the costovertebral ligamentous complex, along with the support of the rib cage.72
Brown and colleagues73 advocated nonoperative treatment of burst fractures with less than 50% of vertebral body collapse, less than 30 degrees of kyphotic deformity, and no more than 3 cm of offset from the standard sagittal vertical angle on lateral scoliosis films. These recommendations are supported by the findings of Cantor and associates.42 Brown’s management protocol recommended immediate casting of the hemodynamically stable patient in a hyperextension body cast, followed by serial radiographs. Early mobilization was associated with reduced hospital stays and costs.
Nonoperative management therefore may be used in the neurologically intact patient, even with a large degree of spinal canal stenosis from retropulsed bone fragments, as long as there is no significant kyphosis representing significant disruption of the dorsal osteoligamentous complex.74 However, if there is a decline in the patient’s neurological status during nonoperative treatment, operative intervention is indicated in the presence of documented instability or neural compression.75
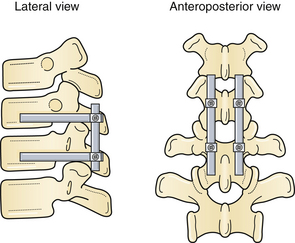
Siebenga and co-workers76 demonstrated the benefits of surgery in 34 patients with AO type A fractures without neurological deficits (compression fractures) who were randomized to short-segment posterior stabilization (Fig. 24.4) versus orthosis predominantly at the thoracolumbar junction. Superior correction of the kyphotic deformity in the surgical arm and functional outcome scores (Visual Analog pain score) were found. A higher percentage of patients in the surgical arm had returned to work at follow-up, in accordance with the findings of several nonrandomized trials.76 This differed from the earlier works of Wood and associates77 finding no difference in outcome when comparing operative and nonoperative treatment of neurologically intact burst fractures.
Harrington rods were the first spinal implants widely used for the treatment of vertebral fractures.77 Unfortunately, this artificially applied distraction force can result in the loss of the normal spinal curvature, now working at a biomechanical disadvantage.78,79 Newer segmental instrumentation systems were initially developed for scoliosis. Maximization of fixation was achieved by instrumenting across all three vertebral columns, which resulted in a relatively low incidence of fixation failure. Compression, distraction, and translation are all possible within the same construct.
Pedicle screw fixation allowed for instrumentation of vertebrae with fractured or absent laminae, with purchase through all three columns, an improvement on previous segmental fixation devices. Increased rigidity necessitates fewer segments of fixation, leading to the preservation of more motion segments. Preservation of motion is most important in the cervical and lumbar segments.72 Surgeon preference often plays a role, as does fracture morphology. Timing of surgery becomes an important issue in the treatment of thoracic spine fractures. Progressive neurological deficit in the presence of canal compromise is an accepted indication for immediate decompression and stabilization. Some studies suggest that patients with thoracic spine fractures treated within 72 hours, irrespective of concomitant injuries, do much better physiologically postoperatively than those in whom stabilization is delayed. Boerger and colleagues80 showed in a meta-analysis that patients with an incomplete neurological deficit who underwent an operative decompression and stabilization procedure in less than 72 hours had enhanced neurological recovery compared to nonoperatively treated patients.80,81
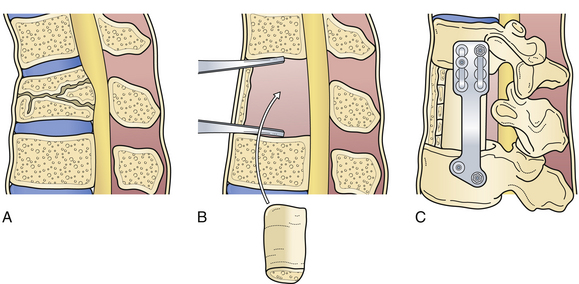
Flexion-distraction injuries result in disruption of the posterior and middle columns in tension.82 Very often, the anterior column remains intact, acting as a hinge. Surgical intervention for these fractures typically involves a posterior approach. Anterior approaches (Fig. 24.5) are not routinely used in these injuries, to preserve the intact anterior column.83
Fracture-dislocation injuries have a significant distraction component and differ from seat-belt-type injuries in that there is a large rotatory or torque component that causes a disruption of all three spinal columns.52 As a result, they carry a high incidence of complete spinal cord injury. Therefore, the main objective of surgical intervention is solely to provide posterior stabilization, facilitating early mobilization and rehabilitation. Anterior decompression and stabilization (see Fig. 24.5) are performed following posterior surgical realignment of the fracture in rare instances in which partial neurological deficit exists in the presence of significant anterior neural compression.84,85 Chapman and co-workers reported in their series that a “lapbelt-sign” had a positive predictive value of 0.69 and a negative predictive value of 0.91 for intra-abdominal injury, illustrating the high morbidity rate in flexion-distraction injuries.86
When partial neurological deficit is present, seen often with burst fractures, improving residual canal compromise is also the goal of surgery. Laminectomy with transpedicular decompression also can improve the canal clearance achieved through a posterior approach. The ventral approach is particularly useful for decompressing midline ventral lesions and correcting severe kyphotic deformities.80,87–89
Dorsal decompression via multilevel laminectomy alone after thoracic and thoracolumbar injuries has been shown to be ineffective and should not be performed as an isolated treatment strategy.89,90 Loss of the dorsal tension band and instability, along with the potential progression of a kyphotic deformity, may ensue. The immediate result of removing the dorsal osseous components is dorsal migration of the spinal cord if the spine has a lordotic alignment.
The osseous structures are fused concomitantly with posterior instrumentation. Some surgeons fuse only the injured vertebral segments with subsequent staged removal of hardware. Other surgeons fuse the entire length of the instrumentation. This results in loss of motion at additional segments. As mentioned, this is of less importance in the thoracic spine. With modern segmental fixation, fewer segments need to be instrumented to provide stability, and generally, the entire instrumented region is fused.91
Minimally invasive surgery (MIS) is seeing increased use in North America in tandem with the development of easier to implement systems. In a review of over 138 papers in the literature it has been cited that the average blood loss is 1 L and the anterior and posterior approach infection rate is 0.7% and 3.1%, respectively.92
Less commonly, anterior MIS procedures are implemented endoscopically. Posterior percutaneous pedicle screw instrumentation accounts for the majority of MIS procedures, along with balloon-assisted techniques. In higher energy burst fractures, the risk of epidural extrusion of cement and dorsal displacement of posterior bony fragments is relevant.92,93 Lastly, there is a paucity of level 1 or 2 evidence comparing MIS with open techniques.
Summary
The care of patients with thoracolumbar spine trauma with or without neurological deficits has evolved dramatically over the past 30 years. The development of more effective instrumentation techniques coupled with the establishment of spinal injury care centers where immediate treatment and rehabilitation can be administered successively has definitely improved the care of these patients. Despite these advances, the majority of patients with thoracolumbar injuries are still treated nonoperatively with cast or brace immobilization and early ambulation. More aggressive treatment is guided by the use of classification systems that detail the mechanism of injury, the degree of compromise of spinal structures, and the potential for late mechanical instability or neural injury. Guidelines such as the TLISS system are now in place to help the clinician make better educated decisions as to which patients to treat operatively and which to manage nonoperatively. As we have seen here, there is no clear consensus as to the absolute indications for surgical intervention in patients with many types of thoracolumbar fractures.
Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8(8):817-831.
Haber T.R., Filmy W.T., O’Brien M. Thoracic and lumbar fractures: diagnosis and management. In: Bridwell K.H., DeWald P.R., editors. The Textbook of Spinal Surgery. Philadelphia: Lippincott; 1991:857-910.
Patel A.A., Dailey A., Brodke D.S., et al. Spine trauma study group. Thoracolumbar spine trauma classification: the thoracolumbar injury classification and severity score system and case examples. J Neurosurg Spine. 2009;10(3):201-206.
White A.A., Panjabi M.M. Clinical Biomechanics of the Spine. Philadelphia: JB Lippincott; 1978.
Wood K., Buttermann G., Mehbod A., et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg Am. 2003;85A(5):773-781.
Please go to expertconsult.com to view the complete list of references.
1. Evans L. Risk of fatality from physical trauma versus sex and age. J Trauma. 1988;28:368-378.
2. Inaba K., Kirkpatrick A.W., Finkelstein J., et al. Blunt abdominal aortic trauma in association with thoracolumbar spine fractures. Injury. 2001;32(3):201-207.
3. National SCI Statistical Center (U.S.). Spinal Cord Injury Facts and Figures at a Glance 2008. Birmingham, AL: The National SCI Statistical Center; 2008. Available at http://www.spinalcord.uab.edu/show.asp?durki=116979
4. Price C., Makintubee S., Herndon W., Istre G.R. Epidemiology of traumatic spinal cord injury and acute hospitalization and rehabilitation charges for spinal cord injuries in Oklahoma. Am J Epidemiol. 1994;139:37-47.
5. El-Khoury G.Y., Whitten C.G. Trauma to the upper thoracic spine: anatomy, biomechanics and unique imaging features. AJR. 1993;160:95-102.
6. Gertzbein S.D. Fractures of the Thoracic and Lumbar Spine. Baltimore: Williams & Wilkins; 1992.
7. Chapman J.R., Anderson P.A. Thoracolumbar spine fractures and neurologic deficits (review). Orthop Clin North Am. 1994;25:595-612.
8. Hu R., Mustard C.A., Burns C. Epidemiology of incident spinal fracture in a complete population. Spine. 1996;21(4):492-499.
9. Gertzbein S.D. Neurologic deterioration in patients with thoracic and lumbar fractures after admission to the hospital. Spine. 1994;19:1723-1725.
10. Panjabi M.M., White A.A., Johnson R.M. Cervical spine mechanics as a function of transection of components. J Biomech. 1975;8:327-336.
11. Maiman D.J., Pintar F.A. Anatomy and clinical biomechanics of the thoracic spine. Clin Neurosurg. 1992;38:296-324.
12. Myklebust J.B., Pintar F., Yoganandan N. Tensile strength of spinal ligaments. Spine. 1971;13:526-531.
13. Hanley E.N., Eskay M.L. Thoracic spine fractures. Orthopedics. 1989;12:689-696.
14. Haber T.R., Filmy W.T., O’Brien M. Thoracic and lumbar fractures: diagnosis and management. In: Bridwell K.H., DeWald P.R., editors. The Textbook of Spinal Surgery. Philadelphia: Lippincott; 1991:857-910.
15. Ebraheim N.A., Xu R., Ahmad M., Yeasting R.A. Projection of the thoracic pedicle and its morphometric analysis. Spine. 1997;22:233-238.
16. Tran N.T., Watson N.A., Tencer A.F., et al. Mechanism of the burst fracture in the thoracolumbar spine. Spine. 1995;20:1984-1988.
17. Haher T.R., Tozzi J.M., Lospinuso M.F., et al. The contribution of the three columns of the spine to spinal stability: a biomechanical model. Paraplegia. 1989;27(6):432-439.
18. Petitjean M.E., Mousselard H., Pointillart V., et al. Thoracic spinal trauma and associated injuries: should early spinal decompression be considered? J Trauma. 1995;39:368-372.
19. Saboe L.A., Reid D.C., Davis L.A., et al. Spine trauma and associated injuries. J Trauma. 1991;31:43-48.
20. van Beek E.J., Been H.D., Ponsen K.K., Maas M. Upper thoracic spinal fractures in trauma patients—a diagnostic pitfall. Injury. 2000;31(4):219-223.
21. Dalvie S.S., Burwell M., Noordeen M.H.H. Haemothorax and thoracic spinal fracture; a case for early stabilization. Injury. 2000;31:269-270.
22. Sturm J.T., Hines J.T., Perry J.F. Thoracic spinal fractures and aortic rupture: a significant and fatal association. Ann Thorac Surg. 1990;50:931-933.
23. van Raaij T.M., Slis H.W., Hooglanb P.H., et al. Massive haemothorax following vertebral fracture. Injury. 2000;31:202-203.
24. Argenson C., Bouleau P., de Peretti F., Lovett J. Les fractures du rachis thoracique (T1-10). A propos de 105 cas. Rev Chir Orthop. 1989;75(6):370-386.
25. Freysz M., Adarnon O., Wilkening M., Sautreaux J.L. Hemothorax et fractures de la colonne dorsale. Semin Hop. 1983;59(32):2229-2231.
26. Beaunoyer M., St-Vil D., Lallier M., Blanchard H. Abdominal injuries associated with thoraco-lumbar fractures after motor vehicle collision. J Pediatr Surg. 2001;36(5):760-762.
27. Chiliimindris C.P. Rupture of the thoracic esophagus from blunt trauma. J Trauma. 1977;17:968-971.
28. Monzon J.R., Ryan B. Thoracic esophageal perforation secondary to blunt trauma. J Trauma Injury Infect Crit Care. 2000;49(6):1129-1131.
29. Stothert J.C.Jr., Buttorff J., Kaminski D.L. Thoracic esophageal and tracheal injury following blunt trauma. J Trauma. 1980;20:992-995.
30. Rabinovici R., Ovadia P., Mathiak G., Abdullah F. Abdominal injuries associated with lumbar spine fractures in blunt trauma. Injury. 1999;30(7):471-474.
31. Gumley G., Taylor T.K., Ryan M.D. Distraction fractures of the lumbar spine. J Bone Joint Surg. 1982;64B:520-525.
32. Miller C.D., Blyth P., Civil I.D. Lumbar transverse process fractures—a sentinel marker of abdominal organ injuries. Injury. 2000;31(10):773-776.
33. Chan R.N.W., Ainscow D., Sikorski J.M. Diagnostic failures in the multiple injured. J Trauma. 1980;20:684-687.
34. Enderson B.L., Reath D.B., Meadows J. The tertiary trauma survey: a prospective study of missed injury. J Trauma. 1990;30:666-669.
35. Laasonen E.M., Kivioja A. Delayed diagnosis of extremity injuries in patients with multiple injuries. J Trauma. 1991;31:257-260.
36. Stanislas M.J.C., Latham J.M., Porter K.M., et al. A high-risk group for thoracolumbar fractures. Injury. 1998;29:15-18.
37. Arigtuaco E.J., Binet E.F. Radiology of thoracic and lumbar fractures. Clin Orthop Relat Res. 1984;189:43-57.
38. Ballock R.T., Mackersie R., Abitbol J.J., et al. Can burst fractures be predicted from plain radiographs? J Bone Joint Surg. 1992;74B:147-150.
39. Boyle J.J., Singer K.P., Milne N. Morphological survey of the cervicothoracic junctional region. Spine. 1996;21(5):544-548.
40. Bradford D.S., Akbamia B.A., Winter R.B. Surgical stabilization of fractures and fracture dislocations of the thoracic spine. Spine. 1977;2:185-196.
41. Kuklo T.R., Polly D.W., Owens B.D., et al. Measurement of thoracic and lumbar fracture kyphosis: evaluation of intraobserver, interobserver, and technique variability. Spine. 2001;26(1):61-65.
42. Cantor J.B., Lebwohl N.H., Garvey T., Eismont F.J. Non-operative management of stable thoracolumbar burst fractures with early ambulation and bracing. Spine. 1993;19:1731-1740.
43. Daffner R.H., Deeb Z.L., Rothfus W.E. Thoracic fractures and dislocations in motorcyclists. Skeletal Radiol. 1987;16:280-284.
44. Posner I., White A.A.III, Edwards W.T. A biomechanical analysis of the clinical stability of the lumbar and lumbosacral spine. Spine. 1982;7:374-389.
45. Denis F., Burkus J.K. Shear fracture dislocations of the thoracic and lumbar spine associated with forceful hyperextension (lumberjack paraplegia). Spine. 1992;17:152.
46. Jelly L.M., Evans D.R., Easty M.J., et al. Radiography versus spiral CT in the evaluation of cervicothoracic junction injuries in polytrauma patients who have undergone intubation. Radiographics. 2000;20(Spec No):S251-S259.
47. Flanders A.E. Thoracolumbar trauma imaging overview. Instructional Course Lectures. 1999;48:429-431.
48. Karnaze M.G., Gado M.H., Sartor K.J., Hodges F.J. Comparison of MR and CT myelography in imaging the cervical and thoracic spine. AJR. 1988;150(2):397-403.
49. Schaefer D.M., Flanders A., Northrup B.E., et al. Magnetic resonance imaging of acute cervical spine trauma: correlation with severity of neurological injury. Spine. 1989;14:1090-1095.
50. Brightman R.P., Miller C.A., Rea G.L., et al. Magnetic resonance imaging of trauma to the thoracic and lumbar spine. Spine. 1992;17:541-550.
51. Singer K.P., Jones T.J., Breidahl P.D. A comparison of radiolographic and computer-assisted measurements of thoracic and thoracolumbar sagittal curvature. Skeletal Radiol. 1990;19:21-26.
52. Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8(8):817-831.
53. Ferguson R.L., Allen B.L.Jr. A mechanistic classification of thoracolumbar spine fractures. Clin Orthop. 1983;189:817-831.
54. Watson-Jones R. Manipulative reduction of crush fractures of the spine. Br Med J. 1931;1(3659):300-302.
55. Nicholl E.A. Fractures of the dorso-lumbar spine. J Bone Joint Surg. 1949;31B:376-394.
56. Holdsworth F.W. Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg. 1963;45B:6-20.
57. Chance C.Q. Note on a type of flexion fracture of the spine. Br J Radiol. 1948;21:452.
58. Kelly R.P., Whiteside T.E.Jr. Treatment of lumbo-dorsal fracture, dislocations. Ann Surg. 1968;167:705-717.
59. Louis R. Les theories de l’instabilise. Rev Chir Orthop. 1977;63:423-435.
60. McCormack T., Karaikovic E., Gaines R.W. The load sharing classification of spine fractures. Spine. 1994;19(15):1741-1744.
61. McLain R.F., Sparling E., Benson D.R. Early failure of short-segment pedicle instrumentation for thoracolumbar fractures. J Bone Joint Surg. 1993;75A:162-167.
62. Zindrick M.R., Wiltse L.L., Doornic A., et al. Analysis of the morphometric characteristics of the thoracic and lumbar pedicles. Spine. 1987;12:160-166.
63. Vaccaro A.R., Baron E.M., Sanfilippo J., et al. Reliability of a novel classification system for thoracolumbar injuries: the thoracolumbar injury severity score. Spine. 2006;31(11):S62-S69.
64. Patel A.A., Dailey A., Brodke D.S., et al. Spine trauma study group. Thoracolumbar spine trauma classification: the thoracolumbar injury classification and severity score system and case examples. J Neurosurg Spine. 2009;10(3):201-206.
65. Vaccaro A.R., Lehman R.A.Jr., Hulbert R.J., et al. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine. 2005;30(20):2325-2333.
66. Whang P.G., Vaccaro A.R., Poelstra K.A., et al. The influence of fracture mechanism and morphology on the reliability and validity of two novel thoracolumbar injury classification systems. Spine. 2007;32:791-795.
67. Vaccaro A.R., Zeiller S.C., Hulbert R.J., et al. The thoracolumbar injury severity score: a proposed treatment algorithm. J Spinal Disord Tech. 2005;18:209-215.
68. Patel A.A., Vaccaro A.R., Albert T.J., et al. The adoption of a new classification system: time-dependent variation in interobserver reliability of the thoracolumbar injury severity score classification system. Spine. 2007;32:E105-E110.
69. Rampersaud Y., Fisher C., Wilsey J., et al. Agreement between orthopedic surgeons and neurosurgeons regarding a new algorithm for the treatment of thoracolumbar injuries: a multicenter reliability study. J Spinal Disord Tech. 2006;19:477-482.
70. White A.A., Panjabi M.M. Clinical Biomechanics of the Spine. Philadelphia: JB Lippincott; 1978.
71. Defino H.L., Canto F.R. Low thoracic and lumbar burst fractures: radiographic and functional outcomes. Eur Spine J. 2007;16:1934-1943.
72. Bransford R., Bellabarba C., Thompson J.H., et al. The safety of fluoroscopically-assisted thoracic pedicle screw instrumentation for spine trauma. J Trauma. 2006;60(5):1047-1052.
73. Brown C.W., Gorup J.M., Chow G.H. Nonsurgical treatment of thoracic burst fractures in controversies. In: Zdeblick T.A., Benzel E.C., editors. Spine Surgery. St. Louis: Quality Medical Publishing; 1999:86-96.
74. Hashimoto T., Kaneda K., Abumi K. Relationship between traumatic spinal canal stenosis and neurologic deficits in thoracolumbar burst fractures. Spine. 1988;13:1268-1272.
75. Cotler J.M., Vernace J.V., Michalski J.A. The use of Harrington rods in thoracolumbar fractures. Orthop Clin North Am. 1986;17:87-103.
76. Siebenga J., Leferink V.J., Segers M.J., et al. Treatment of traumatic thoracolumbar spine fractures: a multicenter prospective randomized study of operative versus nonsurgical treatment. Spine. 2006;31:2881-2890.
77. Wood K., Buttermann G., Mehbod A., et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg Am. 2003;85A(5):773-781.
78. Gray H. Anatomy, descriptive, and surgical. In: Pick T.P., Howden R., editors. Gray’s Anatomy, Revised American Edition from the Fifteenth English Edition. New York: Bounty Books, 1977.
79. Jacobs R.R., Casey M.P. Surgical management of thoracolumbar spine injuries. Clin Orthop. 1984;189:22-35.
80. Boerger T.O., Limb D., Dickson R.A. Does “canal clearance” affect neurological outcome after thoracolumbar burst fractures? J Bone Joint Surg. 2000;82B:629-635.
81. Kaneda K., Abumi K., Jujiya M. Burst fractures with neurologic deficits of the thoracolumbar-lumbar spine. Results of anterior decompression and stabilization with anterior instrumentation. Spine. 1984;9:788-795.
82. Gertzbein S.D., Court-Brown C.M. Flexion-distraction injuries of the lumbar spine: mechanism of injury and classification. Clin Orthop. 1988;227:52-60.
83. Tezer M., Erturer R.E., Ozturk C. Conservative treatment of fractures of the thoracolumbar spine. Int Orthop. 2005;29:78-82.
84. Convery F.R., Minteer M.A., Smith R.W., Emerson S.M. Fracture-dislocation of the dorsal-lumbar spine: acute operative stabilization by Harrington instrumentation. Spine. 1978;3:160.
85. Denis F., Armstrong G.W., Searls K., Matta L. Acute thoracolumbar burst fractures in the absence of neurologic deficit. Clin Orthop Relat Res. 1984;189:142-149.
86. Chapman J.R., Agel J., Jurkovich G.J., et al. Thoracolumbar flexion-distraction injuries: associated morbidity and neurological outcomes. Spine. 2008;33(6):648-657.
87. Berry J.L., Moran J.M., Berg W.S., Steffee A.D. A morphological study of human lumbar and selected thoracic vertebrae. Spine. 1987;12:362-366.
88. Bohlman H.H. Traumatic fractures of the upper thoracic spine with paralysis. J Bone Joint Surg Am. 1974;56:1299.
89. Bohlman H.H., Freehafer A., Dejak J. The results of treatment of acute injuries of the upper thoracic spine with paralysis. J Bone Joint Surg Am. 1985;67:360-369.
90. Morgan T.H., Wharton G.W., Austin G.N. The results of laminectomy in incomplete spinal cord injury. J Bone Joint Surg Am. 1970;52:1115-1130.
91. McCullen G., Vaccaro A.R., Garfin S.R. Thoracic and lumbar trauma: rationale for selecting the appropriate fusion technnique. Orthop Clin North Am. 1998(4):813-828.
92. Rampersaud Y.R., Annand N., Dekutoski M.B. Use of minimally invasive techniques in the management of thoracolumbar trauma. Spine. 2006;33(11):S96-S102.
93. Cosar M., Sasani M., Oktenoglu T., et al. The major complications of transpedicular vertebroplasty. J Neurosurg Spine. 2009;11:607-613.