Chapter 12 Thoracic Surgery
In this chapter the care of patients undergoing noncardiac thoracic surgery is discussed. The emphasis is on postoperative care, but preoperative evaluation and intraoperative management are also reviewed. Thoracic surgery patients, particularly those undergoing pulmonary resection or lung volume reduction surgery (LVRS), are commonly elderly, current or former heavy smokers, and highly likely to have other smoking-related diseases. Careful preoperative selection and optimization are essential for good postoperative outcomes.
This chapter is divided into two sections: (1) pulmonary resection; (2) other thoracic surgery. Pulmonary resection is essentially a discussion of the diagnosis, selection, treatment, and postoperative care of patients undergoing surgery for lung cancer. Other topics covered include LVRS, mediastinal tumors, and esophageal resection. Thoracic trauma, massive hemoptysis, and empyema are covered in Chapters 25, 26, and 35.
PULMONARY RESECTION
Surgery for Lung Cancer
Diagnosis
Patients usually have one or more symptoms related to their tumors, such as cough, chest pain, dyspnea, wheeze, or weight loss. Central tumors can obstruct a large airway, causing atelectasis or pneumonia. Other symptoms and signs are suggestive of tumor extension beyond the lung. Pleuritic chest pain may indicate direct tumor invasion of the chest wall. Dysphagia may indicate esophageal involvement. Hoarseness, Horner syndrome, and arm pain are indicative of recurrent laryngeal nerve, sympathetic chain, and brachial plexus involvement, respectively. Lung cancers commonly metastasize to the brain, skeleton, liver, and adrenals. Extrapulmonary or metastatic spread generally precludes surgery. A number of paraneoplastic syndromes are associated with lung cancer (Table 12-1).
Table 12-1 Paraneoplastic Syndromes Associated with Lung Cancer
| Syndrome (hormone secretion) | Clinical Manifestation | Cancer |
|---|---|---|
| Hypercalcemia (parathyroid) | Hypercalcemia, polyuria, hypovolemia, confusion | NSCLC |
| SIADH (ADH) | Hypernatremia | SCLC/NSCLC |
| Cushing syndrome (ACTH, CRH) | Hypertension, fluid retention, weakness, hypokalemia, hyperglycemia | SCLC/Carcinoid |
| Acromegaly (GH, GHRH) | Bony overgrowth | SCLC/Carcinoid |
| Gynecomastia (HCG) | Breast enlargement | SCLC/NSCLC |
| Myositis/myopathy | Proximal weakness, myalgia | SCLC/NSCLC |
| Myasethenic syndrome | Weakness, fatigability | SCLC |
| Brain/spinal cord/peripheral neuropathy | Multiple | SCLC |
ACTH, adrenocorticotropic hormone; ADH, antidiuretic hormone; CRH, corticotropin releasing hormone; GH, growth hormone; GHRH, growth hormone releasing hormone; HCG, human chorionic gonadotropin; NSCLC, non-small cell lung cancer; SCLC, small cell cancer; SIADH, syndrome of inappropriate antidiuretic hormone secretion.
Occasionally, lung cancer is asymptomatic and is an incidental finding on a routine chest radiograph. The presence of a large noncalcified mass (<3 cm in diameter) with spiculated margins is highly suggestive of malignancy. The chest radiograph may show consolidation distal to the mass, mediastinal lymphadenopathy, or pleural effusion. Recently, there has been interest in screening high-risk, asymptomatic patients by means of computed tomography (CT) scanning.1
Surgical Suitability for Resection
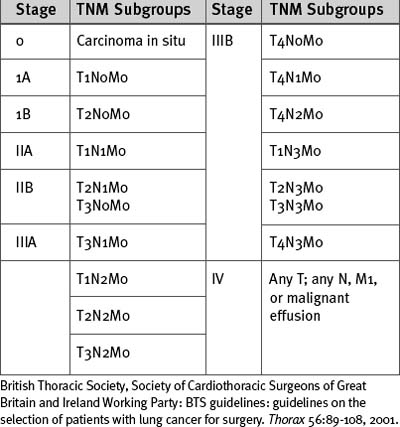
Lung cancer is staged according to the TNM (tumor, node, metastases) classification (Tables 12-2 and 12-3). All patients being considered for surgery should have a CT scan of the chest, liver, and adrenal glands. Percutaneous needle biopsy may be considered for peripheral lesions but is not mandatory. Patients with mediastinal lymph nodes greater than 1 cm diameter on CT scan should undergo a staging biopsy or mediastinoscopy prior to lung resection. The presence of a malignant effusion is a contraindication to surgery, but an effusion due to consolidation distal to an obstructing lesion is not; if there is doubt, a pleural aspirate should be obtained.
| Primary Tumor (T) | |
| Tx | Primary tumor cannot be assessed or proven |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor <3 cm in greatest dimension, surrounded by lung or visceral pleura, without evidence of invasion more proximal than lobar bronchus |
| T2 | Tumor with any of the following features: >3 cm in greatest dimension involves main bronchus >2 cm distal to the carina invades the visceral pleura associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not involve the entire lung |
| T3 | Tumor of any size that directly invades the following: chest wall, diaphragm, mediastinal pleura, parietal pericardium; or tumor in the main bronchus <2 cm distal to the carina but without carinal involvement; or associated atelectasis or obstructive pneumonitis of the whole lung |
| T4 | Tumor of any size that involves any of the following: mediastinum, heart, great vessels, trachea, esophagus, vertebral body, carina; or tumor with a malignant pleural or pericardial effusion or with satellite tumor nodule within the ipsilateral primary tumor lobe of the lung |
| Regional Lymph Nodes (N) | |
| Nx | Regional nodes cannot be assessed |
| N0 | No regional lymph node metastases |
| N1 | Metastases to ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes involved by direct extension of primary tumor |
| N2 | Metastases to ipsilateral mediastinal and/or subcarinal nodes |
| N3 | Metastases to contralateral mediastinal, contralateral hilar, ipsilateral, or contralateral scalene or supraclavicular lymph nodes |
| Distant Metastases (M) | |
| Mx | Presence of distant metastases cannot be assessed |
| M0 | No distant metastases |
| M1 | Distant metastases present (includes those in a different lobe of the same lung) |
British Thoracic Society, Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party: BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 56:89-108, 2001.
Fitness for Pulmonary Resection
Curative lung resection involves the removal of a considerable amount of tissue, which results in permanent loss of pulmonary function. Quantitative assessment of pulmonary function is therefore important for stratifying a patient’s surgical risk. However, given that the outcome of nonsurgical treatment of lung cancer is very poor, it may still be appropriate to proceed with pulmonary resection in a patient deemed to be at high risk based on the results of lung function tests.2–5
Routine Assessment
Many patients undergoing pulmonary resection are elderly. Advanced age (<80 years) does not itself preclude pulmonary resection. However, pneumonectomy is associated with an increased risk for mortality in elderly patients, and age should be a factor in deciding suitability for surgery in this patient group.2 The patient’s general clinical state should be evaluated, including changes in exercise capacity or the occurrence of weight loss or anemia. Preoperative weight loss of more than 10%, a body mass index less than 18.5, and a low serum albumin are markers of advanced disease and indicate increased perioperative risk.
All patients should undergo simple spirometry and oximetry measurements prior to pulmonary resection. Some centers also measure the pulmonary diffusing capacity of carbon monoxide (DLCO) in all patients. A forced expiratory volume in the first second (FEV1) that is greater than 1.5 l is considered adequate for lobectomy; an FEV1 greater than 2 l is considered adequate for pneumonectomy. Values less than those are indications for further pulmonary testing. A resting arterial carbon dioxide tension greater than 6 kPa (45 mmHg) also indicates increased perioperative risk.
Pulmonary Assessment of High-Risk Patients
Predicted Postoperative FEV1.
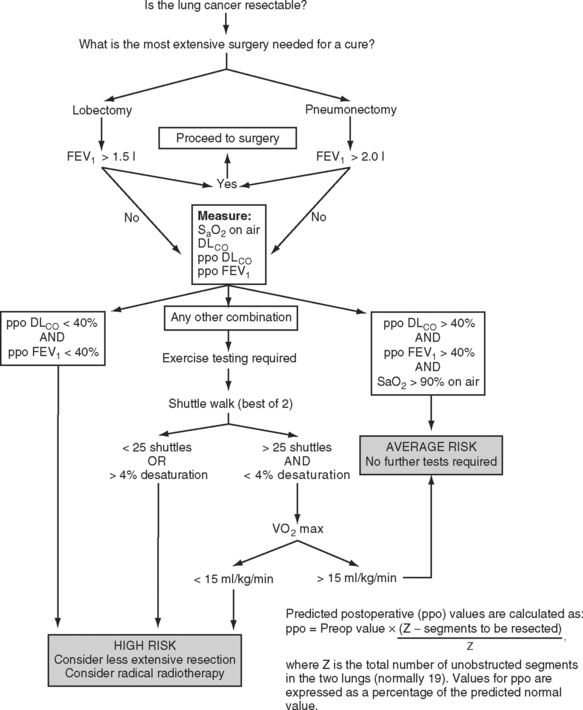
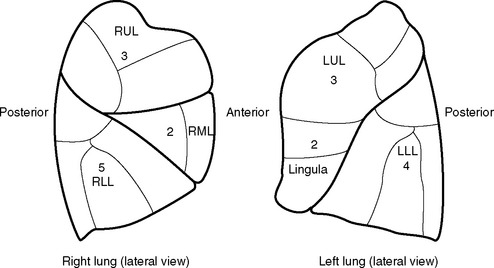
The predicted postoperative FEV1 (ppoFEV1) is calculated from the preoperative FEV1 (preopFEV1) and the proportion of functional lung that is to be removed (Fig. 12-1). The most accurate way to measure the functional contribution of the lung tissue to be resected is by means of split perfusion scanning using technetium (Tc99) macroaggregates or quantitative CT scanning.6 A simple alternative is to estimate the proportion on the basis of the number of anatomic pulmonary segments that are to be removed (Fig. 12-2). To allow safe resection, the predicted postoperative FEV1 should be greater than 40% of normal.
Diffusing Capacity.
The DLCO provides a measure of the severity of parenchymal lung disease—although the measurement probably reflects ventilation-perfusion inequalities rather than impaired function of the alveolar capillary membrane. Reduced DLCO is strongly associated with adverse postoperative outcome. Historically, patients undergoing pulmonary resection with a preoperative DLCO of less than 60% of the predicted value experienced a mortality rate of 25% and a pulmonary morbidity rate of 45%.7 More recent data suggest that if the preoperative DLCO is greater than 45% of the predicted value, acceptable surgical results may be obtained.8
Exercise Testing.
A Vo2 max of less than 15 ml/kg/min indicates a high risk for developing a postoperative cardiorespiratory complication following lung resection.3 A Vo2 max less than 10 ml/kg/min (<40% predicted) indicates that the patient is unsuitable for any form of pulmonary resection, whereas a value greater than 20 ml/kg/min (<75% of predicted) indicates resection up to pneumonectomy is reasonable.3 As with FEV1 and DLCO, a predicted postoperative Vo2 max can also be calculated; a value less than 10 ml/kg/min (<40% predicted) indicates unsuitability for pulmonary resection.9
Integrated Approach to Pulmonary Testing
A number of algorithms have been proposed for pulmonary testing to identify high-risk patients and assess their suitability for surgery. One algorithm, proposed by the British Thoracic Society, is shown in Figure 12-1.2
Preoperative Cardiovascular Evaluation
Clinical predictors of increased perioperative cardiac risk are summarized in Table 12-4. Patients at major risk should have a formal cardiologic assessment, and those with severe coronary artery or valvular lesions should be considered for a revascularization procedure or valve surgery prior to pulmonary resection. Patients at intermediate risk who have good functional capacity do not require further cardiac investigation but, in the presence of poor functional capacity, referral to a cardiologist and cardiac stress testing are warranted. Patients at increased risk for cerebrovascular disease (e.g., a carotid bruit or a history of stroke or transient ischemic attack) should undergo a preoperative carotid Doppler ultrasound study.
Table 12-4 Predictors of Increased Cardiovascular Risk Following Major Noncardiac Surgery
| Major Predictors |
| Unstable coronary syndromes: recent myocardial infarction with evidence of important ischemic risk based on symptoms or noninvasive study; unstable or severe angina |
| Decompensated congestive cardiac failure |
| Significant cardiac arrhythmias: high-grade atrioventricular block; symptomatic ventricular arrhythmias in the presence of underlying heart disease; supraventricular arrhythmias with uncontrolled ventricular rate |
| Severe valvular heart disease |
| Intermediate Predictors |
| Mild angina |
| Previous myocardial infarction based on history of pathologic Q waves |
| Compensated or prior congestive cardiac failure |
| Diabetes |
| Minor Predictors |
| Advanced age |
| Abnormal ECG findings (e.g., left ventricular hypertrophy, left bundle branch block, ST or T wave abnormalities) |
| Rhythm other than sinus rhythm (e.g., atrial fibrillation) |
| History of stroke |
| Poorly controlled hypertension |
Adapted from Eagle KA, Berger PB, Calkins H, et al: ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol 39:542-553, 2002; and from British Thoracic Society, Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party: BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 56:89-108, 2001.
Intraoperative Management
Pulmonary resection for cancer is usually carried out via a lateral thoracotomy incision and involves a lobectomy, bilobectomy, or pneumonectomy. Selective lung ventilation is usually achieved using a double-lumen endotracheal tube. Pneumonectomy is required for a centrally located carcinoma, particularly when the tumor is adherent to hilar structures. Pneumonectomy or bilobectomy is required for tumors that have crossed a lung fissure. Resection of part of the pericardium (intracardiac pneumonectomy) or chest wall may be required for tumor clearance. For tumors involving a bronchus, a “sleeve lobectomy” may be performed; in this surgery a segment of bronchus along with a lobe are excised. The remaining lobes are then reattached to the residual bronchus. Sleeve lobectomy is performed as an alternative to pneumonectomy with the aim of preserving lung function. Bronchotracheal resection with bronchial reattachment is required in cases in which tumors involve the carina. Limited wedge or segmental resection is appropriate for benign tumors, for metastases, and for primary lung cancer in a patient who would not tolerate full lobectomy. However, in patients with primary lung cancer, sublobar resection is associated with increased local recurrence rates compared to lobectomy. Concurrent with pulmonary resection, mediastinal lymph node sampling is usually performed for the purpose of staging the tumor.
Routine Postoperative Care Following Pulmonary Resection
Ideally, patients are extubated following pulmonary resection to minimize air leak and to reduce the tension on bronchial or pulmonary staple lines. However, a period of elective postoperative ventilation is justified for patients with severely impaired gas exchange, hypothermia, acidosis, or ongoing bleeding. Hypothermia significantly increases the incidence of myocardial ischemia; therefore, patients who are hypothermic (<35.5°C) should be warmed to 36.5°C using a forced-air warming blanket.10 Immediately following surgery, a chest radiograph should be obtained to assess lung expansion and the position of drains and to rule out hemothorax. The absence of significant mediastinal shift should be confirmed after a pneumonectomy.
Routine Chest Drain Management
Management of the chest drains differs according to procedure. After lobectomy (and most other thoracic procedures), apical and basal drains are inserted and connected to an underwater seal. Traditionally, these drains have also been connected to low-pressure suction at a level of about −20 cm H2O (approximately 15 mmHg or 2 kPa). More recently, there has been a move away from using suction, with drains just connected to an underwater seal. This strategy can be used in patients with and without air leaks and may lead to a shorter requirement for chest drainage and reduced hospital stay, particularly in patients without air leaks.11 Chest drains are kept in place until drainage is minimal, the residual lung has reexpanded, and any air leak has stopped.
Analgesia
Epidural Analgesia.
Thoracic epidural analgesia is a widely used and effective strategy for postthoracotomy pain, and it reduces the risk for postoperative respiratory complications in patients undergoing pneumonectomy.12 The epidural catheter should be placed at the dermatomal level of the surgical incision (usually between T5 and T8) by the anesthesiologist prior to the induction of anesthesia. Opioids, local anesthetics, or a combination of the two may be used as a continuous infusion, with top-ups if required (Table 12-5). One widely used regime is the combination of bupivacaine 0.125% and fentanyl 2 μg/ml administered as a continuous epidural infusion at 0.1 ml/kg/hr.13,14
| Top-up or Intermittent Bolus Dose | Continuous Infusion | |
|---|---|---|
| Opioid only | ||
| Morphine | 2-4 mg | 0.2-0.3 mg/hr |
| Meperidine | 25-50 mg | 10-20 mg/hr |
| Local anesthetic only | ||
| Bupivacaine 0.125-0.25% (maximum 24-hour dose, 400 mg) | 5-10 ml | 5-12 ml/hr |
| Ropivacaine 0.2-0.4% | 5-10 ml | 5-12 ml/hr |
| Combination | ||
| Bupivacaine 0.125% + fentanyl 2-4 μg/ml | 5-10 ml | 5-12 ml/hr |
| Ropivacaine 0.2% + fentanyl 2-4/ml | 5-10 ml | 5-12 ml/hr |
Blockade of the thoracic and cervical sympathetic nerves can cause hypotension and bradycardia, particularly in the presence of hypovolemia. Catheter migration into the subarachnoid space can result in a high, dense block and in marked hypotension, which may progress to a “total spinal” with profound hypotension, apnea, and loss of consciousness. Urinary retention is common with epidural analgesia so a urinary catheter should be inserted. Weakness can limit mobilization; a patient with a thoracic epidural should not be allowed to walk unaccompanied.
Despite an apparently adequately functioning epidural, patients may complain of ipsilateral shoulder-tip pain. The mechanism of this is not clear but may involve irritation of the diaphragm by the intercostal tube. Shoulder-tip pain does not respond well to intravenous opioids or to increase in the epidural infusion. It does not appear to be caused by shoulder distraction because it is not abolished by suprascapular nerve block.15
Patient-Controlled Analgesia.
Patient-controlled analgesia may be the primary analgesic modality, or it may be used in combination with a regional technique. Dosing regimes are provided in Table 4-4.
Multimodal Analgesia.
For most patients, a multimodal approach to analgesia is used. It may include regular acetaminophen and, if not contraindicated, a nonsteroidal antiinflammatory drug (see Chapter 4). To this basic regime should be added a regional technique and patientcontrolled analgesia. Nonsteroidal antiinflammatory drugs may decrease the effectiveness of a pleurodesis and should be avoided in patients undergoing this procedure.16
Assessment of Analgesia and Regional Blocks.
Pain can be subjectively graded by the patient on a scale from 0 to 10, where 0 indicates no pain and 10 indicates the worst pain imaginable; the goal is to achieve pain scores of less than 3/10 at rest and less than 5/10 with coughing (see Chapter 4).
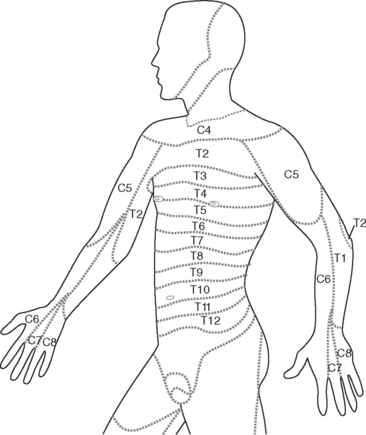
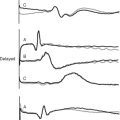
For regional nerve blocks (epidural, paravertebral, extrapleural) with local anesthetic drugs, the extent of the block should be assessed with reference to the dermatome chart (Fig. 12-3). This should be done every 2 to 4 hours or more frequently if the block is high or after a top-up. Pain signals are carried by the same nerve fibers that carry temperature sensation. Thus, the ability to perceive cold (response to an ice cube) can be used to evaluate the distribution of the block. Block height should be measured in conjunction with pain scores and vital signs.
If the block height is above the T2 dermatome there is the potential for severe bradycardia (cervical sympathetic blockade); respiratory insufficiency (blockade of phrenic nerve); and loss of consciousness. Tingling in the fourth and fifth digits (the C8 dermatome; see Fig. 12-3) is an indication that the block is too high. In this situation, the infusion should be stopped and the patient reviewed every 15 minutes until the block height regresses.
Early Complications Following Pulmonary Resection
The perioperative mortality rates for lobectomy and pneumonectomy should be less than 4% and 8%, respectively.2 Respiratory and cardiac and complications are summarized in Tables 12-6 and 12-7.
Table 12-6 Causes of Acute Respiratory Failure Following Pulmonary Resection
| Early (typically < 72 hours) |
| Residual effects of anesthesia (sedatives, neuromuscular blockade, opioids) |
| Inadequate analgesia |
| Atelectasis and retained secretions |
| Pneumothorax |
| Hemothorax |
| Bronchospasm |
| Phrenic nerve injury |
| Acute respiratory distress syndrome |
| Late (typically > 48 hours) |
| Pneumonia |
| Bronchopleural fistula |
| Pulmonary embolism |
Table 12-7 Causes of Acute Hemodynamic Instability Following Pulmonary Resection
| Mismanagement of chest drains: |
| Suction applied to a pneumonectomy drain, resulting in mediastinal shift toward operative side* |
| Kinking of drain applied to lobectomy patient, resulting in tension pneumothorax with mediastinal shift away from operative side |
| High regional block |
| Concealed hemorrhage with massive hemothorax |
| Arrhythmias* |
| Right ventricular failure* |
| Pericardial tamponade† |
| Myocardial ischemia or infarction |
| Cardiac herniation† |
| Pulmonary embolus |
* Particularly following pneumonectomy
† Particularly following intrapericardial pneumonectomy
Respiratory Insufficiency in the Early Postoperative Period
Hypoxemia, hypercarbia, and increased work of breathing can occur during the early postoperative period due to the combined effects of atelectasis, retained secretions, and inadequate analgesia. Clotted blood within a large airway may cause lobar collapse. There may be preexisting chronic lung disease or pneumonia. Following lobectomy, residual lung tissue may not have fully expanded. Persisting effects of anesthetic agents may contribute to postoperative respiratory insufficiency: opioids cause respiratory depression; inhaled anesthetic agents (isoflurane, sevoflurane) impair hypoxic pulmonary vasoconstriction; residual neuromuscular blockade causes weakness. Myasthenic syndrome (see Table 12-1) is typically associated with small cell cancer (which is rarely amenable to surgery) and is therefore uncommon following pulmonary resection. The treatment of acute respiratory failure is outlined in Chapter 27.
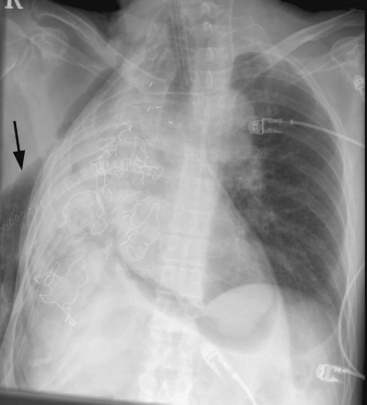
Mediastinal and Subcutaneous Emphysema
Air from disrupted alveoli or small bronchi can pass through a breach in the pleura into the extrathoracic subcutaneous tissues or can track along pulmonary vessels and the bronchial tree into the mediastinum (Fig. 12-4). The possibility of air leakage from the esophagus, which may have been injured during surgery, also must be considered.
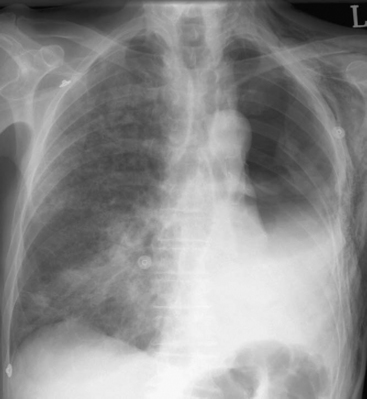
Acute Respiratory Distress Syndrome
Pulmonary edema due to acute respiratory distress syndrome (ARDS; postpneumonectomy syndrome) is a well recognized complication of pulmonary resection (Fig. 12-5); its incidence is 3% to 7% and its mortality rate is 20% to 80%.17,18 Despite the term postpneumonectomy syndrome, the condition also occurs following lobectomy. The condition is more common following pneumonectomy than lobectomy, and more common on the right side than the left.19 It has been linked to preoperative alcohol abuse, excessive perioperative fluid administration, and peak ventilatory pressures above 25 cm H2O intraoperatively.19
Patients typically present within 3 days of surgery with respiratory distress and hypoxemia. The chest radiograph demonstrates diffuse alveolar infiltrates which, in the case of lobectomy, may be bilateral. Patients are often extremely unwell. Preventive measures include (1) fluid restriction, aiming to replace only surgical and physiologic fluid losses; (2) the use of vasopressors instead of volume to treat nonhemorrhagic hypotension; and (3) if ventilated, the use of a lung-protective strategy (see Chapter 24).20 Noninvasive ventilation may reduce the mortality rate resulting from ARDS after pulmonary resection.21
Other Early Respiratory Complications
Hypotension and Low Cardiac Output
There are many possible reasons for postoperative hemodynamic instability, but certain causes must be specifically considered in patients who have undergone pulmonary resection (see Table 12-7). Patients should be carefully examined (including their chest drains). Investigations include a 12-lead electrocardiogram, a chest radiograph, and an echocardiogram.
Arrhythmias.
Supraventricular arrhythmias, particularly atrial fibrillation, occur in more than 20% of patients following pulmonary resection.22 Advanced age, pneumonectomy, upper lobectomy, and a history of chronic obstructive pulmonary disease (COPD) are risk factors.22,23 The use of amiodarone in the prevention of supraventricular arrhythmias following pulmonary resection has been implicated in the development of ARDS.24 However, this concern has not been borne out by more recent studies.25,26
Late Complications Following Pulmonary Resection
Pneumonia
Pneumonia occurs in up to 20% of patients following pneumonectomy and is associated with increased mortality rates.27 Pneumonia typically develops later than 48 hours after surgery but may present earlier if pulmonary infection was present preoperatively. The diagnosis and treatment of pneumonia is discussed in Chapter 35.
Persistent Air Leak Following Lobectomy
Persistent bubbling of the chest drain and incomplete expansion of the lung can be a troublesome problem. Various strategies have been tried to prevent this complication, including the application of fibrin glue to suture lines28 and the early cessation of chest drain suction.29,30 Treatment is usually expectant and in most circumstances the leak gradually settles. A new large air leak, with incomplete expansion of the lung, should raise suspicion of a bronchopleural fistula.
Bronchopleural Fistula
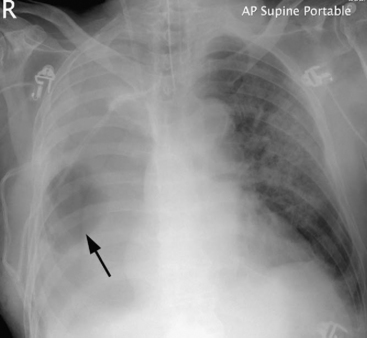
A bronchopleural fistula occurs when there is breakdown of the bronchial stump or tracheobronchial anastomosis. The incidence is 4.5% to 20% after pneumonectomy and 0.5% after lobectomy.31 Risk factors include preoperative radiotherapy, preoperative pulmonary infection, diabetes, right pneumonectomy, a long bronchial stump, residual cancer at the stump, and the need for postoperative ventilation.31
A bronchopleural fistula typically presents with fever, cough, and purulent or hemorrhagic expectoration. With pneumonectomy, the chest radiograph typically demonstrates an increased air content and reduced fluid level within the pleural space. There may be soiling of the nonoperative lung, which is identified by pulmonary infiltrates on the chest radiograph (Fig. 12-6). Less commonly, patients develop systemic sepsis and acute respiratory failure. The diagnosis may be confirmed by thoracic CT or bronchoscopy. Alternatively, dye (e.g., methylene blue) can be instilled into the pleural space and recovered from the sputum.
Initial treatment involves placement of an intercostal drain and nursing patients with the operative side down to minimize soiling of the nonoperative lung. Sputum and chest drain fluid should be sent for microbiologic analysis, and antibiotics should be given to cover the most likely causative organisms: Streptococcal species, Staphylococcus aureus, enteric gram-negative bacilli, and anerobes (see Chapter 35).
Deep Venous Thrombosis and Pulmonary Embolus
Thoracic surgery patients typically have at least two major risk factors for deep venous thrombosis: malignancy and major surgery. In one study, 26% of patients experienced thromboembolic events during their hospitalizations for thoracotomy.32 Thus, all patients undergoing pulmonary resection should receive prophylactic treatment by means of graduated compression stockings and low molecular weight heparin. Low molecular weight heparin should commence the evening before surgery so that the time interval between heparin administration and the insertion of an epidural is at least 12 hours. Similarly, prior to removal of an epidural, the evening dose should be withheld and the epidural removed the following morning.
OTHER THORACIC SURGERY
Lung Volume Reduction Surgery (LVRS)
The rationale for LVRS is that the removal of grossly emphysematous lung tissue will improve residual pulmonary and chest wall mechanics.33,34 In particular, it is thought that the remaining lung tissue will be repositioned on a more favorable part of the pulmonary compliance curve and that the length/tension relationship of the respiratory muscles will be optimized, resulting in reduced work of breathing.
The recently completed National Emphysema Treatment Trial demonstrated that LVRS does not confer a survival advantage but does improve exercise capacity more than medical therapy does.35 However, in the subgroup of patients with predominantly upper lobe emphysema (heterogeneous disease) and low baseline exercise capacity, a survival advantage was shown in patients undergoing LVRS. Patients with non-upper lobe disease and high baseline exercise capacities experienced increased mortality rates and negligible functional gains.
Patients should be extubated at the end of the procedure, possibly reducing the risk for air leak. Intercostal tubes should be left on free drainage regardless of the size of any air leak. Provision of high-quality analgesia, preferably via an epidural, is absolutely essential for adequate postoperative respiratory function. Residual air leak and persisting pneumothorax are associated with considerable morbidity and mortality rates. Enteral feeding should be commenced within 1 to 2 days of surgery. The 90-day mortality rate for LVRS is about 8%.35
Mediastinal Tumors
If a mediastinal mass is suspected, the diagnosis may be confirmed by CT scan (Fig. 12-7). Histologic diagnosis, which is essential for treatment, may be obtained by bronchoscopy, esophagoscopy, needle biopsy, or surgical biopsy (mediastinoscopy, mediastinotomy). Some tumors are treated primarily medically (e.g., lymphoma, germ cell tumor), with surgical intervention being limited to diagnostic biopsy and postchemotherapy resection of residual mass. For other tumors, notably thymoma, surgical excision is indicated.
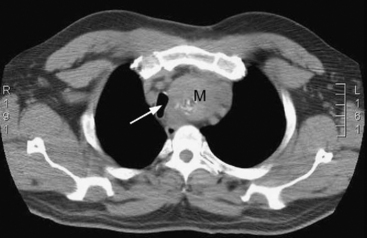
Figure 12.7 CT scan showing a superior mediastinal mass (M). The trachea (arrow) is displaced to the right by the mass.
Intraoperative Management
Surgical resection is performed via a median sternotomy or thoracotomy. Depending on the position of the tumor, resection of part of the pericardium, chest wall, or lung may be required, along with excision and reconstruction of the superior vena cava and innominate vein.36 Operative complications include bleeding and section of phrenic and recurrent laryngeal nerves.36
Postoperative Issues
Postoperatively, there is a risk for respiratory insufficiency due to the effects of major thoracic surgery, pneumothorax, tracheomalacia, possible section of the phrenic and recurrent laryngeal nerves and, in some patients, concomitant myasthenia gravis. Hemorrhage into the mediastinum can cause tamponade and hemothorax. In one series, the operative mortality rate was 6%.36
Patients with myasthenia gravis are at particular risk for postoperative respiratory failure. It is essential to ensure that neuromuscular blocking drugs have completely worn off or been fully reversed prior to extubation (see Chapter 4). Patients should be given their regular doses of pyridostigmine prior to surgery and as soon as practical following surgery. The drug is only available orally so postoperative administration may have to be via a nasogastric tube. Patients with severe myasthenia gravis may benefit from preoperative corticosteroids or, occasionally, plasmapheresis so as to prevent postoperative respiratory failure.
Esophageal Surgery
Treatment of Esophageal Cancer
There are a number of surgical approaches to the esophagus. For lesions of the lower esophagus, an upper abdominal or left thoracotomy may be used. For more proximal lesions, a combined upper abdominal and right thoracotomy (the Ivor Lewis procedure) is used. There is also a three-staged operation (McKeown) that involves a laparotomy, right thoracotomy, and neck incision. Some surgeons perform endoscopic intrathoracic mobilization of the esophagus and intraabdominal mobilization of the stomach.37 The transhiatal approach (Orringer) avoids a thoracotomy altogether. The stomach and esophagus are freed via neck and upper abdominal incisions using blunt finger dissection. The stomach is elevated into the neck through the esophageal hiatus and anastomosed to the esophagus within the neck. The advantages of a transhiatal approach include the avoidance of a thoracotomy and the creation of the esophageal anastomosis within the neck. The main advantage of an intrathoracic approach is the ability to achieve a more complete lymph node dissection. However, pulmonary complications are more common and anastomotic leaks, although less likely, are more difficult to manage.
Postoperative Care
Routine Postoperative Care.
Patients may arrive in the intensive care unit extubated or intubated. A period of elective mechanical ventilation is common following prolonged or difficult intrathoracic resection. A nasogastric drainage tube and, usually, a nasojejunal feeding tube are in situ. Following an intrathoracic resection, one or more intercostal tubes are also present and are connected to underwater seal drains. The nasogastric and nasojejunal tubes should initially be placed on free drainage. These tubes traverse the esophageal anastomosis; thus, if they are inadvertently removed, they should be reinserted only under fluoroscopic control and under the direction of the surgeon. At the time of a patient’s arrival in the intensive care unit, a chest radiograph should be obtained and inspected for the presence of pneumothorax and to confirm the correct position of all lines and tubes.
Significant third-space fluid losses are to be expected over the first 24 to 72 hours. Therefore, maintenance fluids of 100 to 200 ml/hr of a crystalloid solution are appropriate. Because of the risk for pulmonary edema (see subsequent material), close attention to intravascular volume status is required, along with invasive monitoring of arterial blood pressure, central venous pressure, and hourly urine output. A positive daily fluid balance and weight gain of a few kilograms can be expected during the first few postoperative days. Patients undergoing esophagectomy are often malnourished, and commencement of nutrition within 24 to 48 hours of surgery is indicated. If enteral feeding via a jejunal feeding tube is not possible, parenteral nutrition is appropriate. Provision of adequate pain relief, preferably by means of an epidural, is essential to avoid postoperative cardiorespiratory complications.38,39
Complications Following Esophagectomy.
A number of complications may occur following esophagectomy (Table 12-8). Respiratory failure is common; as much as 20% of patients require prolonged mechanical ventilation.40 Pneumonia is a common occurrence and has many causes, including atelectasis and retained secretions, prolonged ventilation, poor nutritional state, and aspiration. Aspiration may occur preoperatively as the result of esophageal obstruction; it may occur postoperatively due to the effects of a nasogastric tube, due to swallowing dysfunction caused by dissection of the cervical esophagus, loss of the normal gastroesophageal junction, or recurrent laryngeal nerve palsy. Patients are at particular risk for acute lung injury and ARDS following esophagectomy. There may be preexisting radiation pneumonitis or bleomycin pulmonary toxicity. Pulmonary lymphatics are partially destroyed at the time of surgery, and chylothorax is commonly noted after radical lymph node dissection. Lung injury may occur because of prolonged one-lung ventilation or as a consequence of the systemic inflammatory response syndrome. Postoperative sepsis also may precipitate ARDS. The risk factors for postoperative respiratory failure are advanced age, preoperative chemoradiation, and an FEV1 less than 65% of the predicted rate.40
| Respiratory failure |
| Anastomotic leak |
| Sepsis and the systemic inflammatory response syndrome |
| Airway necrosis and tracheoesophageal fistula |
| Recurrent laryngeal nerve palsy |
| Chylothorax |
| Deep vein thrombosis |
| Dumping syndrome |
| Alcohol withdrawal |
If an anastomotic leak is suspected, confirmation of the diagnosis may be made by performing a radiographic contrast swallow. However, there should be a low threshold for a thoracic CT scan with oral contrast. Particular note should be made that the drains are well positioned and that they take up the leaked contrast. The decision has to be made whether to manage the patient conservatively (with strict nil per mouth and reliance on existing or additional chest drains) or whether the thorax should be reopened (with pleural lavage and possible complete esophagectomy and formation of a cervical esophagostomy and tube gastrostomy). The mortality rate seen in intrathoracic esophageal anastomotic leak is about 35%.41
Tracheal Surgery
Tracheal resection is most commonly carried out in cases of cancers involving the trachea and in tracheal stenosis secondary to trauma or following tracheostomy. Patients present with signs of intrathoracic or extrathoracic airway obstruction (see Chapter 27), particularly when lying flat.
1 Kawahara M. Screening for lung cancer. Curr Opin Oncol. 2004;16:141-145.
2 British Thoracic Society, Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001;56:89-108.
3 Bolliger CT. Evaluation of operability before lung resection. Curr Opin Pulm Med. 2003;9:321-326.
4 Burke JR, Duarte IG, Thourani VH, et al. Preoperative risk assessment for marginal patients requiring pulmonary resection. Ann Thorac Surg. 2003;76:1767-1773.
5 Beckles MA, Spiro SG, Colice GL, et al. The physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest. 2003;123:105S-114S.
6 Bolliger CT, Guckel C, Engel H, et al. Prediction of functional reserves after lung resection: comparison between quantitative computed tomography, scintigraphy, and anatomy. Respiration. 2002;69:482-489.
7 Ferguson MK, Little L, Rizzo L, et al. Diffusing capacity predicts morbidity and mortality after pulmonary resection. J Thorac Cardiovasc Surg. 1998;96:894-900.
8 Win T, Jackson A, Sharples L, et al. Relationship between pulmonary function and lung cancer surgical outcome. Eur Resp J. 2005;25:594-599.
9 Bolliger CT, Wyser C, Roser H, et al. Lung scanning and exercise testing for the prediction of postoperative performance in lung resection candidates at increased risk for complications. Chest. 1995;108:341-348.
10 Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events: a randomized clinical trial. JAMA. 1997;277:1127-1134.
11 Antanavicius G, Lamb J, Papasavas P, et al. Initial chest tube management after pulmonary resection. Am Surg. 2005;71:416-419.
12 Licker M, Spiliopoulos A, Frey JG, et al. Risk factors for early mortality and major complications following pneumonectomy for non-small cell carcinoma of the lung. Chest. 2002;121:1890-1897.
13 Pennefather SH, Russell GN. Postthoracotomy analgesia: recent advances and future directions. In: Slinger PD, editor. Progress in Thoracic Anesthesia. Baltimore: Lippincott Williams & Wilkins; 2004:163-185.
14 Minzter BH, Johnson RF, Grimm BJ. The practice of thoracic epidural analgesia: a survey of academic medical centers in the United States. Anesth Analges. 2002;95:472-475.
15 Tan N, Agnew NM, Scawn ND, et al. Suprascapular nerve block for ipsilateral shoulder pain after thoracotomy with thoracic epidural analgesia: a double-blind comparison of 0.5% bupivacaine and 0.9% saline. Anesth Analges. 2002;94:199-202.
16 Lardinois D, Vogt P, Yang L, et al. Non-steroidal anti-inflammatory drugs decrease the quality of pleurodesis after mechanical pleural abrasion. Eur J Cardiothorac Surg. 2004;25:865-871.
17 Hayes JP, Williams EA, Goldstraw P, et al. Lung injury in patients following thoracotomy. Thorax. 1995;50:990-991.
18 Kutlu CA, Williams EA, Evans TW, et al. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Ann Thorac Surg. 2000;69:376-380.
19 Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97:1558-1565.
20 Bigatello LM, Allain R, Gaissert HA. Acute lung injury after pulmonary resection. Minerva Anestesiol. 2004;70:159-166.
21 Auriant I, Jallot A, Herve P, et al. Noninvasive ventilation reduces mortality in acute respiratory failure following lung resection. Am J Respir Crit Care Med. 2001;164:1231-1235.
22 Rena O, Papalia E, Oliaro A, et al. Supraventricular arrhythmias after resection surgery of the lung. Eur J Cardiothorac Surg. 2001;20:688-693.
23 Sekine Y, Kesler KA, Behnia M, et al. COPD may increase the incidence of refractory supraventricular arrhythmias following pulmonary resection for non-small cell lung cancer. Chest. 2001;120:1783-1790.
24 van Mieghem W, Coolen L, Malysse I, et al. Amiodarone and the development of ARDS after lung surgery. Chest. 1994;105:1642-1645.
25 Lanza LA, Visbal AI, DeValeria PA, et al. Low-dose oral amiodarone prophylaxis reduces atrial fibrillation after pulmonary resection. Ann Thorac Surg. 2003;75:223-230.
26 Ciriaco P, Mazzone P, Canneto B, et al. Supraventricular arrhythmia following lung resection for non-small cell lung cancer and its treatment with amiodarone. Eur J Cardiothorac Surg. 2000;18:12-16.
27 Ploeg AJ, Kappetein AP, van Tongeren RB, et al. Factors associated with perioperative complications and long-term results after pulmonary resection for primary carcinoma of the lung. Eur J Cardiothorac Surg. 2003;23:26-29.
28 Fabian T, Federico JA, Ponn RB. Fibrin glue in pulmonary resection: a prospective, randomized, blinded study. Ann Thorac Surg. 2003;75:1587-1592.
29 Rice TW, Okereke IC, Blackstone EH. Persistent air-leak following pulmonary resection. Chest Surg Clin North Am. 2002;12:529-539.
30 Marshall MB, Deeb ME, Bleier JI, et al. Suction vs water seal after pulmonary resection: a randomized prospective study. Chest. 2002;121:831-835.
31 Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Sem Thorac Cardiovasc Surg. 2001;13:3-7.
32 Ziomek S, Read RC, Tobler HG, et al. Thromboembolism in patients undergoing thoracotomy. Ann Thorac Surg. 1993;56:223-226.
33 McKenna RJJr, Gelb A, Brenner M. Lung volume reduction surgery for chronic obstructive pulmonary disease: where do we stand ? World J Surg. 2001;25:231-237.
34 Cordova FC, Criner GJ. Surgery for chronic obstructive pulmonary disease: the place for lung volume reduction and transplantation. Curr Opin Pulm Med. 2001;7:93-104.
35 Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. New Engl J Med. 2003;348:2059-2073.
36 Bacha EA, Chapelier AR, Macchiarini P, et al. Surgery for invasive primary mediastinal tumors. Ann Thorac Surg. 1998;66:234-239.
37 Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg. 2003;238:486-494.
38 Tsui SL, Law S, Fok M, et al. Postoperative analgesia reduces mortality and morbidity after esophagectomy. Am J Surg. 1997;173:472-478.
39 Flisberg P, Tornebrandt K, Walther B, et al. Pain relief after esophagectomy: thoracic epidural analgesia is better than parenteral opioids. J Cardiothorac Vasc Anesth. 2001;15:282-287.
40 Avendano CE, Flume PA, Silvestri GA, et al. Pulmonary complications after esophagectomy. Ann Thorac Surg. 2002;73:922-926.
41 Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg. 2004;10:71-75.
42 Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol. 2002;39:542-553.