CHAPTER 237 Thoracic Outlet Syndrome
Vascular TOS is relatively uncommon and accounts for less than 1% of cases of TOS, but it is usually easy to detect on clinical examination or vascular imaging modalities.1 Neither vascular type of TOS (arterial or venous) directly affects the brachial plexus but may cause neurological symptoms related to ischemia.
History of Thoracic Outlet Syndrome
TOS has had a long and tumultuous history. Galen first described the presence of a cervical rib in 150 AD, and Vesalius further characterized this anomaly in the 16th century.2 In 1742, Hunauld introduced the cervical rib and its associated symptomatology, and in 1860, Wilshire noted the relationship between a cervical rib and upper extremity paresthesias.2 One year later, Coote described surgical removal of an exostosis from the C7 transverse process for the treatment of an ischemic hand.3 In 1910, Murphy published his experience with first rib resection and its clinical efficacy,4 and in 1927, Adson advocated scalenotomy without rib resection.5 Until the 1930s, resection of the first rib was the mainstay of treatment. In 1929, Naffziger and Grant described neurovascular compression at the thoracic outlet secondary to scalene muscle anomalies, and in the 1930s, they performed scalenectomies for relief of the symptoms. In 1935, Ochsner and colleagues termed this condition scalenus anticus syndrome.6 It was not until 1956 that the term thoracic outlet syndrome was coined; in this paper, Peet and coauthors reported a series of 55 patients with TOS, 71% of whom improved with conservative treatment.7 In the 1960s there was a resurgence of first rib resection procedures for the treatment of TOS after Clagett’s description of the posterior approach and Roos’s description of the transaxillary approach.8,9 Dr. Kline has advocated the posterior subscapular approach to brachial plexus decompression in selected cases,10,11 a procedure similar to the one described by Clagett. More recently, the choice of approach has usually depended on the surgeon’s preference.
Anatomy
The thoracic outlet refers to the communication of the thoracic cavity with the root of the neck. Although considered to be a misnomer by anatomists, it is designated an outlet because important vessels and nerves emerge to enter the neck and upper extremities.12
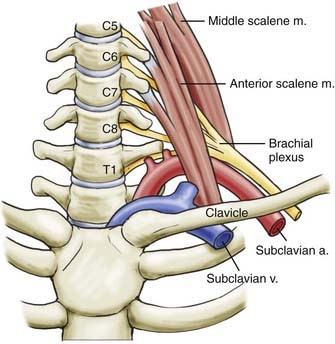
Three sites within the thoracic outlet where neurovascular compression may occur have been well described: the interscalene triangle, the costoclavicular space, and the subpectoral tunnel.13 The most important passageway clinically is the interscalene triangle, which is bordered by the anterior scalene muscle anteriorly, the middle scalene muscle posteriorly, and the medial surface of the first rib inferiorly. This triangle contains the trunks of the brachial plexus and the subclavian artery. It is important to note that the subclavian vein runs anterior to the anterior scalene muscle. Immediately distal to the interscalene triangle, the neurovascular bundle enters the costoclavicular triangle, which is bordered anteriorly by the middle third of the clavicle, posteromedially by the first rib, and posterolaterally by the upper border of the scapula (Fig. 237-1). The neurovascular bundle then enters the subcoracoid space, also referred to as the retropectoralis minor space, beneath the coracoid process deep to the pectoralis minor tendon.
Compression or irritation of the brachial plexus, or both, have been described within each of these spaces. Demondion and coworkers used magnetic resonance imaging (MRI) to demonstrate narrowing of these anatomic spaces with certain upper limb movements. Hyperabduction and external rotation of the arm produced compression of the neural elements within the costoclavicular space; arm elevation compressed these elements within the subcoracoid space.14 Using computed tomographic (CT) angiography, Remy-Jardin and associates have also demonstrated vascular compression within these spaces with similar arm movements.15
For practical purposes, the vast majority of neurogenic TOS cases involve compression within the interscalene triangle. Anomalous structures such as cervical ribs, hypertrophied musculature, and fibrous bands may further constrict this space.16,17 Anomalous bands are more common than cervical ribs and may originate from a cervical or thoracic rib, the transverse process of C7, the suprapleural membrane (Sibson’s fascia), or the scalene muscles.16–18
Clinical Findings
In true or classic neurogenic TOS, the typical patient is a young, thin female with a long neck and drooping shoulders,19,20 but this condition is also seen in athletes with overdeveloped scalene musculature.21 Dull aching in the lateral aspect of the neck, shoulder, axilla, parascapular region, and inner portion of the arm is often described. Discomfort may be provoked by repetitive use of the extremity, particularly with overhead activities. Sensory disturbances can include numbness or paresthesias, or both, in a dermatomal pattern over the ulnar aspect of the forearm and hand. Vasomotor disturbances such as changes in skin color and temperature may be seen in advanced cases, presumably related to compression of sympathetic fibers in the lower trunk, C8, or the T1 spinal nerve, versus the circulatory alterations seen in vascular TOS. Muscle wasting and atrophy in the hand can be seen in advanced cases of neurogenic TOS. A classic finding is the so-called Gilliatt-Sumner hand,22 in which a dramatic degree of atrophy occurs in the abductor pollicis brevis and lesser atrophy in the interosseous and hypothenar muscles. It is important to note that the motor findings in patients with true neurogenic TOS include muscles in both the median and ulnar nerve distributions whereas the sensory findings are confined to the ulnar nerve distribution. In most cases the upper and middle trunk elements are not involved, and thus the median nerve sensory distribution is spared. These clinical findings correspond to the typical findings on electrodiagnostic studies.
No provocative tests for neurogenic TOS are very reliable, but the 90-degree shoulder abduction and external rotation test and a Tinel sign over the supraclavicular brachial plexus seem to have the best predictive value. Classic provocative maneuvers include the Roos test (elevated arm stress test to induce reproduction of the neurological symptoms), the Adson test (full neck extension and head rotation toward the side being examined, during deep inhalation, to detect a reduction in radial pulse amplitude), and the Wright test (progressive shoulder abduction to reproduce the symptoms).23,24 Overall, these tests have a sensitivity and specificity of 72% and 53%, respectively.23 False-positive results may occur in as many as 45% of patients with the Adson test, 77% with the Roos test, and 61% with the supraclavicular pressure test, particularly in patients with other entrapment neuropathies.25
Unfortunately, the more common finding in patients with neurogenic TOS is a chronic pain syndrome with features suggestive of brachial plexus irritation or intermittent compression. These patients usually fall into the disputed neurogenic TOS category.26 Physical examination is difficult in these patients because of the tendency to guard the extremity, provide an unreliable sensory examination, and demonstrate a give-way type of weakness.
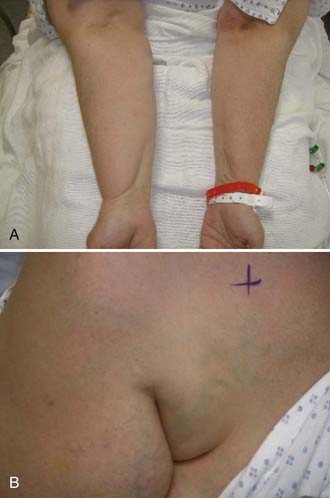
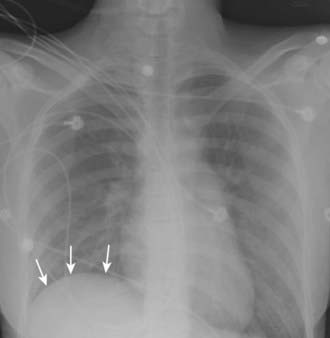
Vascular-type TOS usually occurs in young adults with a history of vigorous arm activity. Patients with arterial TOS often have complaints of a cold, diffusely painful arm that is easily fatigued with activity. Clinical signs can include coolness, pallor, and cyanosis of the affected hand with diminished or absent distal pulses. In later stages of this disease, gangrene of the digits may occur. A supraclavicular mass or bruit may also be present. Thrombosis of the subclavian vein, also known as Paget-von Schrotter syndrome, is manifested as upper extremity edema and cyanosis with distended superficial veins of the shoulder and chest, often without complaints of pain1 (Fig. 237-2).
Diagnostic Evaluation
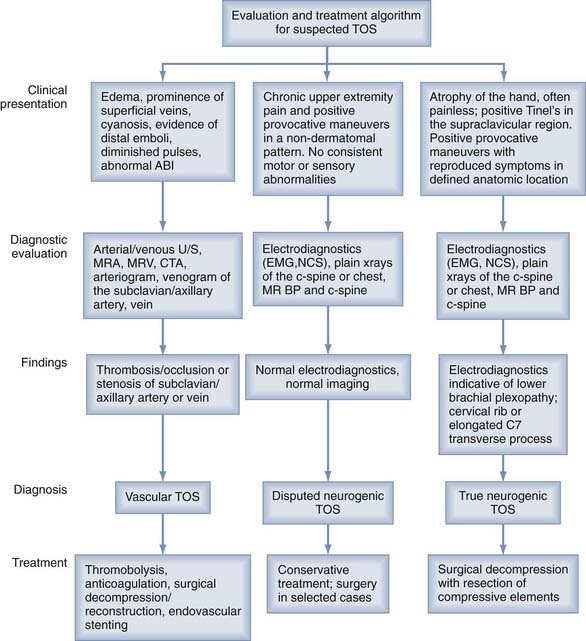
Neurophysiologic testing, including electromyography (EMG) and nerve conduction studies (NCSs), can be helpful in the evaluation of patients with suspected neurogenic TOS (Table 237-1). In true neurogenic TOS, EMG and NCSs are most useful. EMG of the affected hand muscles reveals a reduced number of motor units under voluntary control. There may also be an increased incidence of large, long-duration polyphasic potentials, but abnormal spontaneous activity is unusual. Needle examination of the cervical paraspinal muscles should produce normal results. Maximal motor conduction velocity may be slowed in the median nerve but normal in the ulnar nerve, and distal motor latencies for both nerves are normal. Compound motor action potentials recorded over the thenar muscles are reduced in situations of marked axonal loss, whereas those recorded over the hypothenar muscles are generally normal. Sensory nerve action potentials recorded at the median nerve in the wrist have normal amplitude and latency, but they are often small or absent when recorded from the ulnar nerve after stimulation of the fifth finger. Ulnar somatosensory evoked potentials may or may not be abnormal. In disputed neurogenic TOS, electrophysiologic studies are usually normal.27 Nerve conduction velocities for the medial antebrachial cutaneous nerve have been reported to be abnormal in patients with neurogenic TOS in the absence of other electrophysiologic findings28,29 (Fig. 237-3).
TABLE 237-1 Differential Diagnoses for Neurogenic Thoracic Outlet Syndrome
| Spinal | Cervical disk disease or foraminal stenosis |
| Cervical spinal cord tumor | |
| Cervical syrinx | |
| Peripheral nerve | Brachial plexitis |
| Median nerve entrapment neuropathy | |
| Ulnar nerve entrapment neuropathy | |
| Nerve sheath tumor | |
| Orthopedic | Shoulder abnormalities (rotator cuff injury) |
| Other | Complex regional pain syndrome |
| Fibromyalgia | |
| Apical lung lesion (Pancoast’s tumor) |
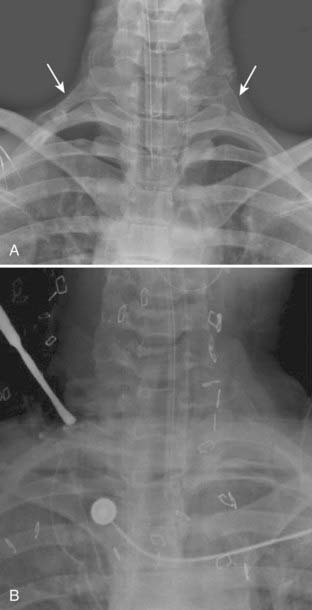
Radiographic studies are an important component in the evaluation of TOS. Imaging studies of the cervical spine, such as plain radiography, CT myelography, and MRI, are recommended to identify spondylotic disease and to rule out spinal tumor or syrinx. Plain radiographs of the cervical spine or chest, or both, should be obtained to rule out a cervical rib or an enlarged C7 transverse process (Fig. 237-4). The finding of an elongated, tapering, downward-pointing (“beaked”) C7 transverse process or a partial cervical rib strongly suggests the presence of a fibrous band extending to the first thoracic rib. Sites of thoracic outlet compression may be visualized on MRI, and studies have correlated decreased size of the costoclavicular space with TOS symptomatology, particularly with provocative maneuvers.14,30,31 MRI of the brachial plexus is useful to identify mass lesions such as nerve sheath tumors or a Pancoast tumor. If a vascular cause is suspected, a vascular study (MR angiography or venography, CT angiography or venography, ultrasonography, or catheter angiography) of the subclavian vessels should be performed. MR neurography, a technique based on enhancing signal differences between nerves and surrounding tissues, has increasingly been used for the diagnosis of neurogenic TOS.32
Conservative Treatment
Most patients with either true or disputed neurogenic TOS deserve a trial of conservative therapy. First, lifestyle modification should be established immediately with avoidance of activities that exacerbate or provoke symptoms, particularly overhead activities, hyperabduction of the arm, carrying of heavy bags over the shoulder, and sleeping in positions with the arms overhead. Physical therapy should be directed at the shoulder girdle and scalene musculature, as well as focused toward correcting poor posture.33–35 Pain management physicians can offer important adjunctive diagnostic information on and treatment of disputed neurogenic TOS. Jordan and coauthors reported that patients who respond to scalene muscle blocks are more likely to respond to surgery. In addition, the authors noted that patients with a diagnosis of fibromyalgia, complex regional pain syndrome, or depression (or any combination of these diagnoses) were more likely to be resistant to treatment.36,37 Scalene muscle denervation through targeted injection of botulinum toxin has been reported to result in improved pain and decreased analgesic use in 80% to 90% of patients at 6 months.38,39 Conservative management of TOS may be successful in 50% to 90% of cases.26,34,40–42 Proponents of conservative treatment of neurogenic TOS emphasize the correlation between favorable outcome and patient compliance with a home exercise program and modification of behavior both at home and at work.35
The state of Washington funded a large retrospective analysis of TOS in their workers’ compensation population; more specifically, outcomes after nonoperative and operative management were compared. In the majority of these patients, disputed neurogenic TOS was diagnosed. Patients who received operative intervention had 50% higher medical costs and were three to four times more likely to be work-disabled.43
Certain financial factors may play a role in the evaluation and treatment of neurogenic TOS. Cherington and colleagues reviewed reimbursement patterns and patient profiles for patients who underwent TOS surgery in Colorado in 1989. This study showed that TOS was rarely diagnosed and treated surgically in patients who did not have either private health insurance or workers’ compensation.44
Surgical Approaches
Anterior Supraclavicular Approach
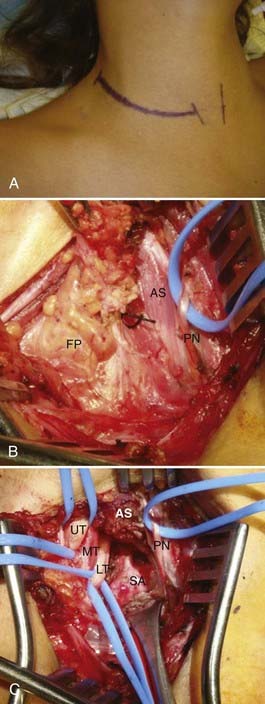
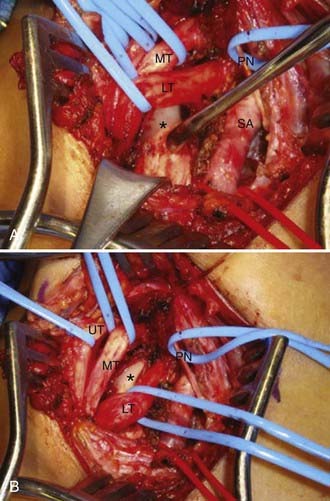
The anterior supraclavicular approach is favored by most neurosurgeons, who frequently use this exposure to treat traumatic or neoplastic lesions of the brachial plexus. This approach allows wide exposure of the supraclavicular plexus and the middle two thirds of the first rib, where most potential anomalous fibrous bands are attached.21,45 The incision is either transverse within a skin crease (our preference for cosmesis) or L shaped and centered on the posterior cervical triangle (Fig. 237-5). During exposure, important anatomic landmarks to identify are the posterior border of the sternocleidomastoid muscle, the omohyoid muscle, the supraclavicular fat pad, the transverse cervical artery and vein, the phrenic nerve, and the anterior scalene muscle. Our preferred technique is to make a 6- to 8-cm transverse incision approximately one to two fingerbreadths above the clavicle, preferably along a preexisting skin crease. The medial extent of the incision is the midpoint of the sternocleidomastoid. Sharp dissection down to the platysma muscle is performed. We attempt to preserve sizable cutaneous nerves to avoid a painful neuroma. The platysma muscle is opened parallel to the incision, with the intent of reapproximating its edges on closure. Next, the omohyoid is identified running transversely across the exposure and is retracted laterally (it may be divided with impunity, but this is not usually necessary; it may serve as a guide to the suprascapular nerve more distally). The supraclavicular fat pad is then identified and reflected carefully in an inferomedial-to-superolateral direction. Frequently, sizable lymphatic channels are encountered within the fat pad, and they must either be preserved or, more likely, dissected with bipolar electrocautery. The transverse cervical vessels are deep to or within the fat pad, and they are usually ligated and divided. The phrenic nerve has a unique course; it runs superolaterally to inferomedially on the anterior surface of the anterior scalene muscle, beneath its investing fascia. The identity of the phrenic nerve is confirmed by stimulating it and feeling contraction of the ipsilateral hemidiaphragm. The nerve is then gently mobilized and a vessel loop is placed. The medial and lateral margins of the anterior scalene muscle are identified and bluntly dissected. Once the anterior scalene is isolated, the muscle is transected. Typically, we perform the transection in piecemeal fashion with bipolar coagulation and scissors while carefully protecting the overlying phrenic nerve. The upper, middle, and lower trunks of the brachial plexus are running laterally and inferiorly deep to the lateral edge of the anterior scalene. An identifying loop is placed around each trunk. The subclavian artery is found by palpation and visual inspection running inferiorly in the plane of the brachial plexus and is controlled with a vessel loop. Frequently, glistening white fascial bands are seen within the anterior and middle scalene muscles and, in many cases, are the presumed culprits in compression/irritation of the plexus elements. The neural elements are inspected in circumferential fashion, and any compressive bands or anomalous structures are resected (Fig. 237-6). Occasionally, the suprapleural membrane (Sibson’s fascia) is prominent and may need to be divided. The lower trunk in particular is dissected proximally until the C8 and T1 spinal nerves are identified. The first rib can be identified and resected as well, although we generally find that the soft tissue elements are much more likely to contact the plexus. Significant traction must be applied to the trunks to safely resect the first rib, and thus we rarely do this. Intraoperative EMG is used to confirm the identities of the neural elements, and nerve action potentials may also be recorded to assess damaged nerve segments. Before closure, the wound cavity is filled with saline and a Valsalva maneuver is performed to check for a pleural leak. A chest radiograph is always obtained postoperatively to check for pneumothorax, hemothorax, or hemidiaphragm elevation.
This procedure can be performed with minimal morbidity by surgeons experienced in this approach. Numbness over the supraclavicular region, lasting approximately 6 weeks, may occur as a result of manipulation of or injury to the supraclavicular nerve during the approach; in certain circumstances, painful neuromas or neuropathic pain, or both, may form at the site of the nerve injury.46 Major complications from this approach include pneumothorax (1% to 2%), phrenic nerve injury (3% to 6%) (Fig. 237-7), and chylothorax (1% to 2%).47,48 Vascular injury occurs in approximately 1% to 2% of patients in whom the first rib is removed via the supraclavicular approach.48 Transient paresthesias or weakness in the arm or hand is seen occasionally and generally resolves within days to a few weeks.
Posterior Subscapular Approach
The posterior subscapular approach as described by Kline and associates provides excellent exposure of the C8 and T1 spinal nerves and the lower trunk of the brachial plexus.10,11 This approach is particularly useful in patients who have previously undergone anterior approaches or received radiation therapy to the area.
Higher rates of injury to the long thoracic, dorsal scapular, and spinal accessory nerves are seen in this procedure, along with a 5% incidence of scapular winging.10,11
Transaxillary Approach
The transaxillary approach with resection of the first rib was popularized by Roos in 1966 and is still commonly used by many thoracic and vascular surgeons.9,49–51 The patient is placed in the posterolateral position with the arm elevated above the head. An incision is made over the first palpable rib (usually the third rib) in the axillary fossa. The axillary fat, lymph nodes, and vessels are dissected away, and the anterior and middle scalene muscles are divided. The first rib is identified and resected. The advantage with this approach is that it allows easy and almost complete access to the first rib, unhindered by adjacent neurovascular structures. The posterior third of the first rib may, in large patients, be difficult to excise with this approach. The major shortcoming of this approach is limited exposure of the neurovascular elements, behind which congenital bands or a cervical rib may be located. Endoscopically assisted transaxillary techniques have recently been developed and are reported to be safe and effective.52,53 Major complications with this approach include brachial plexus injury (1% to 3%), venous injury (2%), and pneumothorax (9%).48,54,55 Other reported complications have included Horner’s syndrome and damage to the thoracic duct.1 Patients may also experience paresthesias or hypersensitivity in the distribution of the intercostobrachial nerve because this nerve is vulnerable to injury with this approach.46
Clinical Outcomes After Surgery
Overall, surgery results in resolution of pain or paresthesias, or both, in 50% to 60% of patients and a partial response in another 20% to 30%.56–58 For true neurogenic TOS with radiographic abnormalities, Nannapaneni and Marks reported complete resolution of pain in 59% (33/56) and marked improvement of pain in another 21% (12/56) of patients treated via the supraclavicular approach with excision of the compressive elements. A majority of patients reported improved sensory function (75%), improved hand function (72%), and improved muscle strength (65%).58
Murovic and coworkers recently reported on the surgical experience with disputed neurogenic TOS at Louisiana State University Health Sciences Center (LSUHSC). Of 99 operative cases involving the disputed type, 52 (52%) were performed through the posterior subscapular approach and 47 (47%) were performed via an anterior supraclavicular approach. Cervical ribs or elongated C7 transverse processes were found in 38% of patients. Postoperatively, 65% had relief of pain or paresthesias, or both, and 33% had partial improvement in pain. Of the 35 patients with preoperative motor deficits, 24 (69%) improved and 11 (31%) were unchanged.57 Over a similar period at LSUHSC, 33 patients with true neurogenic TOS (1 patient with bilateral neurogenic TOS) and a Gilliatt-Sumner hand were treated surgically. Of the 22 patients with pain, 21 (95%) had improvement postoperatively. Moreover, severe motor deficits were improved in 70% (14/20), and mild motor deficits were improved in 86% (12/14). Bony abnormalities, such as a cervical rib or elongated C7 transverse process, were found in 41% (14/34) of these patients.59
In 1998, Urschel and Razzuk published a review of more than 15,000 patients seen over a 50-year period (1947 to 1996) who were evaluated for TOS at the University of Texas Southwestern Medical School. In this period, 3914 patients underwent primary transaxillary decompression, first rib resection, and stellate ganglion resection. The authors reported that 95% of patients had relief of their symptoms with a 0.0008% incidence of significant neurological injury.60 Although these results seem outstanding, it is unclear how the authors assessed the outcome of these patients.
Using the posterior subscapular approach for the surgical treatment of true neurogenic TOS, Dr. Kline reported improvement of pain in 54% and improvement of motor function in 67% of patients at 1 year.61
Unfortunately, 15% to 20% of patients will experience recurrence of symptoms after either transaxillary rib resection or scalenectomy. Sanders and coauthors reported that either approach can be successful in the treatment of recurrent TOS; the combined success rate (transaxillary or supraclavicular) for the operative treatment of recurrent TOS in their series of 134 operations was 84% at 3 months but decreased to 50% at 3 to 5 years.62 Atasoy reported that the recurrence rate is lowered to 5% to 10% when a combination of transaxillary rib resection and supraclavicular scalenectomy is used as the primary surgery.63
Comparisons of Surgical Approach
In 1989, Sanders and Pearce published a retrospective analysis of outcomes from their 18-year experience with both the supraclavicular and transaxillary approaches. This study included 668 primary operations on 491 patients. With use of the life-table analysis method based on subjective patient experience, success after surgery was found to be equivalent between the two approaches at up to 15 years after surgery. Their conclusions were that the supraclavicular approach (with or without rib resection) was as successful as the transaxillary approach for traumatic neurogenic TOS and had fewer complications.48
In 2005, Sheth and Campbell published a randomized trial comparing supraclavicular neuroplasty with transaxillary first rib resection in a relatively small group of patients with disputed neurogenic TOS. Their results suggested that the transaxillary approach provides better pain relief than does the supraclavicular approach.64
Adson AW. Surgical treatment for symptoms produced by cervical ribs and the scalenus anticus muscle. Surg Gynecol Obstet. 1947;85:687.
Atasoy E. Recurrent thoracic outlet syndrome. Hand Clin. 2004;20:99.
Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20:15.
Filler AG, Howe FA, Hayes CE, et al. Magnetic resonance neurography. Lancet. 1993;341:659.
Huang JH, Zager EL. Thoracic outlet syndrome. Neurosurgery. 2004;55:897.
Jordan SE, Ahn SS, Gelabert HA. Differentiation of thoracic outlet syndrome from treatment-resistant cervical brachial pain syndromes: development and utilization of a questionnaire, clinical examination and ultrasound evaluation. Pain Physician. 2007;10:441.
Kline DG. Thoracic outlet syndrome. In: Kim DH, Midha R, Murovic JA, et al, editors. Kline & Hudson’s Nerve Injuries. 2nd ed. Philadelphia: WB Saunders; 2008:139.
Lindgren KA. Conservative treatment of thoracic outlet syndrome: a 2-year follow-up. Arch Phys Med Rehabil. 1997;78:373.
Martinez BD, Wiegand CS, Evans P, et al. Computer-assisted instrumentation during endoscopic transaxillary first rib resection for thoracic outlet syndrome: a safe alternate approach. Vascular. 2005;13:327.
Maxey TS, Reece TB, Ellman PI, et al. Safety and efficacy of the supraclavicular approach to thoracic outlet decompression. Ann Thorac Surg. 2003;76:396.
Murovic J, Kim DH, Kim SH, et al. Thoracic outlet syndrome: part two. Neurosurg Q. 2007;17:13.
Murovic J, Kim DH, Kim SH, et al. Thoracic outlet syndrome: part one. Neurosurg Q. 2007;17:1.
Nannapaneni R, Marks SM. Neurogenic thoracic outlet syndrome. Br J Neurosurg. 2003;17:144.
Roos DB. The thoracic outlet syndrome is underrated. Arch Neurol. 1990;47:327.
Tender GC, Thomas AJ, Thomas N, et al. Gilliatt-Sumner hand revisited: a 25-year experience. Neurosurgery. 2004;55:883.
Vanti C, Natalini L, Romeo A, et al. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eur Medicophysica. 2007;43:55.
Weigel G, Schmidt M, Gradl B, et al. TOS-surgery via a single supraclavicular incision. Acta Neurochir Suppl. 2007;100:141.
1 Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46:601.
2 Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20:15.
3 Coote H. Exostosis of the left transverse process of the seventh cervical vertebra, surrounded by blood vessels and nerves: successful removal. Lancet. 1861;1:360.
4 Murphy T. Brachial neuritis caused by pressure of the first rib. Aust Med J. 1910;15:582.
5 Adson AW. Surgical treatment for symptoms produced by cervical ribs and the scalenus anticus muscle. Surg Gynecol Obstet. 1927;85:687.
6 Oschner A, Gage M, Debakey M. Scalenus anticus (Naffziger) syndrome. Am J Surg. 1935;28:669.
7 Peet RM, Henriksen JD, Anderson TD, et al. Thoracic outlet syndrome: evaluation of a therapeutic exercise program. Proc Mayo Clin. 1956;131:281.
8 Clagett OT. Research and prosearch. Thorac Cardiovasc Surg. 1962;44:153.
9 Roos DB. Transaxillary approach for first rib resection to relieve thoracic outlet syndrome. Ann Surg. 1966;163:354.
10 Dubuisson AS, Kline DG, Weinshel SS. Posterior subscapular approach to the brachial plexus. Report of 102 patients. J Neurosurg. 1993;79:319.
11 Tender GC, Kline DG. Posterior subscapular approach to the brachial plexus. Neurosurgery. 2005;57:377.
12 Snell RS. Clinical Anatomy, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
13 Ranney D. Thoracic outlet: an anatomical redefinition that makes clinical sense. Clin Anat. 1996;9:50.
14 Demondion X, Bacqueville E, Paul C, et al. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227:461.
15 Remy-Jardin M, Remy J, Masson P, et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol. 2000;174:1667.
16 Murovic J, Kim DH, Kim SH, et al. Thoracic outlet syndrome: part one. Neurosurg Q. 2007;17:1.
17 Roos DB. Congenital anomalies associated with thoracic outlet syndrome. Anatomy, symptoms, diagnosis, and treatment. Am J Surg. 1976;132:771.
18 Pang D, Wessel HB. Thoracic outlet syndrome. Neurosurgery. 1988;22:105.
19 Clein LJ. The droopy shoulder syndrome. Can Med Assoc J. 1976;114:343.
20 Swift TR, Nichols FT. The droopy shoulder syndrome. Neurology. 1984;34:212.
21 Huang JH, Zager EL. Thoracic outlet syndrome. Neurosurgery. 2004;55:897.
22 Gilliatt RW, Le Quesne PM, Logue V, et al. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry. 1970;33:615.
23 Gillard J, Perez-Cousin M, Hachulla E, et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68:416.
24 Rayan GM, Jensen C. Thoracic outlet syndrome: provocative examination maneuvers in a typical population. J Shoulder Elbow Surg. 1995;4:113.
25 Nord KM, Kapoor P, Fisher J, et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48:67.
26 Wilbourn AJ. The thoracic outlet syndrome is overdiagnosed. Arch Neurol. 1990;47:328.
27 Aminoff MJ. Electromyography in Clinical Practice, 3rd ed. New York: Churchill Livingstone; 1998.
28 Machanic BI, Sanders RJ. Medial antebrachial cutaneous nerve measurements to diagnose neurogenic thoracic outlet syndrome. Ann Vasc Surg. 2008;22:248.
29 Seror P. Medial antebrachial cutaneous nerve conduction study, a new tool to demonstrate mild lower brachial plexus lesions. A report of 16 cases. Clin Neurophysiol. 2004;115:2316.
30 Demondion X, Boutry N, Drizenko A, et al. Thoracic outlet: anatomic correlation with MR imaging. AJR Am J Roentgenol. 2000;175:417.
31 Demondion X, Herbinet P, Van Sint Jan S, et al. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26:1735.
32 Filler AG, Howe FA, Hayes CE, et al. Magnetic resonance neurography. Lancet. 1993;341:659.
33 Lindgren KA. Conservative treatment of thoracic outlet syndrome: a 2-year follow-up. Arch Phys Med Rehabil. 1997;78:373.
34 Novak CB, Collins ED, Mackinnon SE. Outcome following conservative management of thoracic outlet syndrome. J Hand Surg Am. 1995;20:542.
35 Vanti C, Natalini L, Romeo A, et al. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eur Medicophysica. 2007;43:55.
36 Jordan SE, Ahn SS, Gelabert HA. Differentiation of thoracic outlet syndrome from treatment-resistant cervical brachial pain syndromes: development and utilization of a questionnaire, clinical examination and ultrasound evaluation. Pain Physician. 2007;10:441.
37 Jordan SE, Machleder HI. Diagnosis of thoracic outlet syndrome using electrophysiologically guided anterior scalene blocks. Ann Vasc Surg. 1998;12:260.
38 Jordan SE, Ahn SS, Freischlag JA, et al. Selective botulinum chemodenervation of the scalene muscles for treatment of neurogenic thoracic outlet syndrome. Ann Vasc Surg. 2000;14:365.
39 Jordan SE, Ahn SS, Gelabert HA. Combining ultrasonography and electromyography for botulinum chemodenervation treatment of thoracic outlet syndrome: comparison with fluoroscopy and electromyography guidance. Pain Physician. 2007;10:541.
40 Aligne C, Barral X. Rehabilitation of patients with thoracic outlet syndrome. Ann Vasc Surg. 1992;6:381.
41 Kenny RA, Traynor GB, Withington D, et al. Thoracic outlet syndrome: a useful exercise treatment option. Am J Surg. 1993;165:282.
42 Nakatsuchi Y, Saitoh S, Hosaka M, et al. Conservative treatment of thoracic outlet syndrome using an orthosis. J Hand Surgery Br. 1995;20:34.
43 Franklin GM, Fulton-Kehoe D, Bradley C, et al. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54:1252.
44 Cherington M, Cherington C. Thoracic outlet syndrome: reimbursement patterns and patient profiles. Neurology. 1992;42:943.
45 Weigel G, Schmidt M, Gradl B, et al. TOS-surgery via a single supraclavicular incision. Acta Neurochirur Suppl. 2007;100:141.
46 Mackinnon SE, Novak CB. Thoracic outlet syndrome. Curr Probl Surg. 2002;39:1070.
47 Janjua RM, Tender GC, Tiel RL, et al. Thoracic outlet syndrome. In: Slutsky DJ, Hentz VR, editors. Peripheral Nerve Surgery: Practical Applications in the Upper Extremity. Philadelphia: Churchill Livingstone, 2008.
48 Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10:626.
49 Roos DB. The thoracic outlet syndrome is underrated. Arch Neurol. 1990;47:327.
50 Urschel HCJr. Management of the thoracic-outlet syndrome. N Engl J Med. 1972;286:1140.
51 Urschel HCJr, Razzuk MA. Neurovascular compression in the thoracic outlet: changing management over 50 years. Ann Surg. 1998;228:609.
52 Abdellaoui A, Atwan M, Reid F, et al. Endoscopic assisted transaxillary first rib resection. Interact Cardiovasc Thorac Surg. 2007;6:644.
53 Martinez BD, Wiegand CS, Evans P, et al. Computer-assisted instrumentation during endoscopic transaxillary first rib resection for thoracic outlet syndrome: a safe alternate approach. Vascular. 2005;13:327.
54 Chang DC, Lidor AO, Matsen SL, et al. Reported in-hospital complications following rib resections for neurogenic thoracic outlet syndrome. Ann Vasc Surg. 2007;21:564.
55 Dale WA. Thoracic outlet compression syndrome. Critique in 1982. Arch Surg. 1982;117:1437.
56 Maxey TS, Reece TB, Ellman PI, et al. Safety and efficacy of the supraclavicular approach to thoracic outlet decompression. Ann Thorac Surg. 2003;76:396.
57 Murovic J, Kim DH, Kim SH, et al. Thoracic outlet syndrome: part two. Neurosurg Q. 2007;17:13.
58 Nannapaneni R, Marks SM. Neurogenic thoracic outlet syndrome. Br J Neurosurg. 2003;17:144.
59 Tender GC, Thomas AJ, Thomas N, et al. Gilliatt-Sumner hand revisited: a 25-year experience. Neurosurgery. 2004;55:883.
60 Urschel HCJr, Razzuk MA. Neurovascular compression in the thoracic outlet: changing management over 50 years. Ann Surg. 1998;228:609.
61 Kline DG. Thoracic outlet syndrome. In: Kim DH, Midha R, Murovic JA, et al, editors. Kline & Hudson’s Nerve Injuries. 2nd ed. Philadelphia: WB Saunders; 2008:139.
62 Sanders RJ, Haug CE, Pearce WH. Recurrent thoracic outlet syndrome. J Vasc Surg. 1990;12:390.
63 Atasoy E. Recurrent thoracic outlet syndrome. Hand Clin. 2004;20:99.
64 Sheth RN, Campbell JN. Surgical treatment of thoracic outlet syndrome: a randomized trial comparing two operations. J Neurosurg Spine. 2005;3:355.