Chapter 77 Thoracic Discectomy
It has been estimated that up to 20% of the population has a thoracic disc herniation, as evidenced by MRI.1,2 However, the need for discectomy is relatively rare. Surgery for removal of thoracic disc herniations is thought to constitute less than 4% of all disc operations.3,4 Historically, these operations have been associated with suboptimal outcomes for various reasons, in part because of diagnostic delays. These delays are a result of the rarity of symptomatic thoracic discs and the lack of a characteristic presentation pattern. It is hoped that a growing awareness of this disorder will lead to better outcomes as a result of earlier treatment. Additionally, there is uncertainty about the natural history of this disease. There is no consensus regarding the indications for disc removal. Most surgeons generally avoid prophylactic surgery for disc herniation; however, this practice has not been based on prospective studies. Generally, surgery is reserved for patients with severe, intractable radicular pain or for those with myelopathy, especially when it is progressive or severe. Finally, there are numerous operations for the removal of these lesions. Regrettably, no universally accepted selection criteria exist to help determine the best operation for each individual situation. Recently, surgical selection guidelines were proposed based on a large series of ventrolateral and lateral operations, the well-documented success of groups using the transpedicular approach, and preliminary experience with newer procedures, including transthoracic thoracoscopy, retropleural thoracotomy, and transfacet pedicle-sparing approaches (Table 77-1).5–9
TABLE 77-1 Surgical Approaches for Herniated Thoracic Discs
| Surgical Approach | General Indications |
|---|---|
| Ventral | |
| Transsternal | Upper thoracic spine Densely calcified centrolateral |
| Ventrolateral | |
| Transthoracic/ thoracoscopic | Densely calcified centrolateral Selected mildly calcified centrolateral |
| Retropleural | Selected high-medical-risk static severe myelopathy |
| Lateral | |
| A. Extracavitary | |
| B. Costotransversectomy | A and B, selected densely calcified centrolateral Mildly calcified centrolateral |
| C. Parascapular | C, upper thoracic spine calcified centrolateral |
| Dorsolateral | |
| Transfacet pedicle-sparing/transpedicular | All soft herniated discs Calcified lateral Mildly calcified centrolateral All high-medical-risk except densely calcified centrolateral |
Until the 1950s, laminectomy, with or without disc removal, was the treatment of choice for the surgical management of this disorder. Logue’s historical review in 1952 revealed the poor results achieved using laminectomy.10 Consequently, other methods of performing discectomy were developed. These operations were intended to improve the exposure to the ventral spinal canal throughout the thoracic spine. Although these approaches were successful at improving access to the disc space, they were technically formidable and associated with considerable morbidity. Each of these approaches has a unique set of potential complications; it is important to be aware of them so that they can be avoided. This chapter emphasizes these other methods and focuses on the advantages and disadvantages of each procedure. Fourteen contemporary thoracic disc series are summarized, with special attention given to the reported complications. In closing, a new management paradigm for the treatment of symptomatic thoracic discs is presented.
Surgical Approaches for Thoracic Discectomy
Dorsal Approaches
Laminectomy
The initial approach used for thoracic disc herniation was laminectomy, either with or without disc removal. In 1952, Logue10 reviewed the thoracic discectomy literature and found that the results were poor. A significant percentage of patients were left paraplegic. Although the precise reasons for these results were not known, it was postulated that laminectomy alone did not significantly reduce the ventral forces created by a thoracic disc herniation acting on the spinal cord.4 Additionally, when discectomy was performed, spinal cord manipulation was generally poorly tolerated. The limited space available for the spinal cord, as well as the comparatively tenuous blood supply, was thought to increase the susceptibility of the thoracic spinal cord to injury during disc removal (Fig. 77-1).
Advantages and Disadvantages
Although laminectomy is technically the simplest decompressive spinal operation, the only indication for its use in thoracic disc disease may be in the treatment of thoracic spondylosis.11
Recent Experience
Black described a modified laminotomy/medial facet approach using a unilateral interlaminar laminotomy involving the superior and inferior laminar arches, as well as the medial 2 to 3 mm of the facet joint. The dural edge is exposed to establish boundaries for the cord, and an Epstein stomping curet is used to push the disc downward and away from the cord. This technique was used to remove 11 discs in seven patients. Effective treatment was reported; only one patient reported transient increased weakness compared with preoperative weakness.12
Dorsolateral Approaches
Transpedicular Approach
Patterson and Arbit13 first reported the transpedicular approach in 1978. It was initially performed on three patients with thoracic disc herniations. Two of the patients had complete resolution of their symptoms, and the third markedly improved. Subsequently, several other series have also reported excellent results (Fig. 77-2).
Technique
The patient is placed in the prone position and taped to the table to facilitate rotation away from the surgeon during the disc removal. The spinous process, lamina, and facet joints are exposed using a linear midine incision. Most of the facet joint is removed, as is the pedicle caudal to the disc space. The pedicle is drilled out flush with the vertebral body. A small cavity measuring 1.5 to 2.0 cm in depth is created in the vertebral body to enable depressing the overlying disc away from the ventral dura mater. Additionally, whenever necessary, hemilaminotomies are performed to visualize the dorsolateral dura mater.11,14–19
Advantages
This approach is considerably less invasive than most other operations for thoracic disc removal, particularly the transthoracic (TT) and the lateral extracavitary (LECA) approaches. Using this less invasive approach is thought to lessen perioperative pain, shorten hospital stays, and enable earlier return to premorbid activity.4,8,16,20,21 The surgery avoids problems associated with thoracotomy, rib resection, and extensive muscle dissection. Operating time and blood loss also appear to be less than with other surgeries.
Disadvantages
Critics of this procedure point out the limited ability to visualize across the spinal canal, making decompression of the central and contralateral portions of the disc a relatively blind procedure. Sometimes, difficulty is encountered in managing calcified and intradural disc fragments. We8,13,16 believe that safety is enhanced by using specially designed thoracic microdiscectomy instrumentation (Fig. 77-3) and endoscopic techniques.4,8,16 Although some report being able to remove intradural fragments and central, densely calcified discs using the dorsolateral techniques,5,13,16,19,22 we4 have not had success with these entities. Dense calcifications involving the dorsal margin of the disc space tend to adhere strongly to the ventral dura mater.4,17 The possibility of significant dural adherence may lead one to use one of the ventrolateral or lateral procedures in those situations.4,17
The final disadvantage of this procedure relates to the removal of the facet-pedicle complex, without the ability to place an interbody graft. It has been reported that patients operated on using the transpedicular approach have somewhat disappointing results from the standpoint of localized back pain.8,13,16,19,23 The desire to improve these results led to the development of the transfacet pedicle-sparing approach.4,8,18 It was postulated that the avoidance of pedicle removal should minimize postoperative back pain. The preliminary experience has suggested that this, in fact, may be the case.
Recent Experience
Bilsky reviewed 20 cases performed between 1982 and 1992 at New York Hospital in which the transpedicular approach was used for thoracic discs (lateral or centrolateral calcified or soft discs). No postoperative instrumentation was used for stabilization; however, no patient suffered from postoperative spinal instability-related pain or delayed kyphosis.15 Levi et al. have published a contemporary series of 35 patients operated upon via a unilateral transpedicular approach. Good results were obtained in 15 patients, fair results in 11 patients, and no improvement in 8 patients, and 1 patient was paraplegic after surgery. They also did not find evidence of clinical or radiographic instability.24 Chi et al. describe a mini-open transpedicular thoracic discectomy in which tubular retractors and microscope visualization are used in performing a transpedicular thoracic discectomy. Eleven patients were operated on using the mini-open disectomy approach versus four patients operated on using an open dorsolateral approach. Patients operated on using the mini-open approach had less blood loss and a greater improvement in their modified Prolo score compared with the open procedure. At long-term follow-up, the modified Prolo score was similar for both groups of patients.25 Jho published a series of endoscopic transpedicular thoracic discectomies in 25 patients. A 1.5-cm trocar is placed at the junction of the lamina and facet via a paramedian incision. The medial portion of the facet, the very lateral portion of the lamina, and the rostral one third of the pedicle are removed using a high-speed drill. A limited amount of spinal cord dura and nerve root are exposed, and then a 70-degree lens endoscope is introduced, allowing for a discectomy to be performed under direct visualization. This surgery was able to be performed on an outpatient or overnight stay basis; no surgery-related complications were reported.26
Transfacet Pedicle-Sparing Approach
The transfacet pedicle-sparing approach was developed as a simpler alternative to the formidable ventrolateral and lateral operations for the treatment of thoracic disc disease.4,8 Initially, morphometric studies were carried out and improved the authors’ orientation to the disc space and ventral spinal canal, thereby aiding discectomy. Cadaveric studies demonstrated that a keyhole facetectomy alone, without associated pedicle or lamina removal, did not sacrifice the exposure achieved with the transpedicular approach.4,8 Although the safety and overall effectiveness of the transpedicular approach have been well documented,13,16 it was hoped that avoidance of pedicle removal and limiting the amount of facet resected would improve localized back pain results.8
Technique
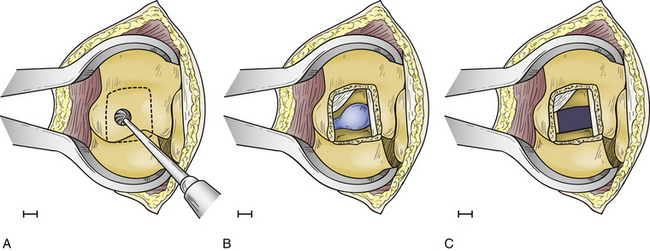
The operation is performed with the patient in the prone position on a radiolucent frame and spinal table. The arms are placed at the sides, and the patient is taped to the table. Anteroposterior (AP) fluoroscopic imaging is used to identify the appropriate disc space. A 4-cm linear skin incision is centered over the disc space. The paraspinal muscles are subperiosteally reflected laterally, exposing the ipsilateral spinous process, lamina, facet joint, and transverse processes above and below the disc space. A small, self-retaining retractor is placed, and the fluoroscope is introduced to verify the correct level and the precise location of the underlying disc relative to the facet joint. Once this relationship is ascertained, a high-speed drill is used to facilitate a partial facetectomy (Fig. 77-4A). Care is taken to preserve the lateral margin of the inferior and superior articular processes of the facet joint and the entire pedicle directly caudal to the disc. When the partial facetectomy is completed, the underlying neuroforaminal fat is coagulated with bipolar cautery. The nerve root, which exits the spinal canal under the more rostral pedicle, is rarely encountered, except in the upper thoracic spine (Fig. 77-4B). The underlying anulus is coagulated and incised. The disc herniation is removed using conventional microdiscectomy techniques (Fig. 77-4C). As with the transpedicular approach, no fusion is required.8
Advantages
Advantages of the transfacet pedicle-sparing approach may include diminished operating time, decreased blood loss, and limited bone and soft tissue removal. Like the transpedicular approach, perioperative pain, hospital stay, and return to premorbid activity appear to compare favorably with the more formidable ventrolateral and lateral approaches.4,17,19 When necessary, multiple disc herniations may be treated.14 The exposure is identical to that provided by the transpedicular approach. Preservation of the pedicle may improve long-term localized back pain results.4,17,19
Disadvantages
The disadvantages are the same as for the transpedicular approach.13,16 Specially designed thoracic microdiscectomy instrumentation8 and, on occasion, open endoscopic visualization of the disc and ventral dural mater have been helpful in enhancing safety during the disc removal.8,16
Recent Experience
Zhang et al. have recently published a series of 18 patients treated with the transfacet pedicle-sparing approach with dorsal instrumentation and fusion. Although the transfacet approach was published with the goal of avoiding dorsal instrumentation, the patients in this series all had additional segmental instrumentation. All patients had good exposure of the disc space and good decompression of the spinal cord. These investigators experienced a complication rate of 33%, with six of the patients requiring additional surgery; five had wound infections or seromas requiring washout and one required revision of a misplaced screw.27
Minimally Invasive Approaches
A number of minimally invasive microendoscopic discectomy techniques have been advocated, all using a dorsolateral approach and an endoscope for visualization. Although the series are not large, they have all demonstrated adequate access and visualization with removal of the thoracic discs with decreased morbidity compared with open approaches.22
Technique
The basic underlying instrumentation used in all approaches involves a dorsolateral approach, endoscope, and tubular dilators. The patient is usually placed in a prone position; however, Jho has placed the patient in a lateral position for an endoscopic transpedicular thoracic discectomy.26 The approach used by Fessler et al. involves a prone position on a radiolucent Wilson frame.28 A localizing radiograph is obtained to determine the level of the thoracic disc. A paramedian skin incision is made at the level of the disc, approximately 3 to 4 cm lateral to the midline. A series of tubular muscle dilators are then placed, and the tubular retractor is placed to form a working port for the endoscope. The endoscope is passed down the center of the tubular retractor. Under endoscopic visualization, the muscle and soft tissue is cleaned off at the level of the transverse process and lateral aspect of the facet. A drill then excises the junction of the transverse process and lateral facet, allowing access to the disc. The pedicle is identified, and drilling of the superior aspect of the caudal pedicle is performed to allow access to the thoracic disc. The disc anulus is identified and cut, and the endoscope is tilted for a 30-degree visualization similar to a transforaminal-type approach. The disc is removed, and a down-going curet or a Woodson elevator is used to push down the disc fragment away from the spinal cord. After the discectomy is performed, the tubular retractor is removed and the wound closed.
Disadvantages
The main disadvantage of a minimally invasive endoscopic approach is the learning curve and familiarity with the anatomy. This approach should be performed by individuals with facility using the endoscope and previous experience with the open dorsolateral approach. Minimally invasive approaches can turn out to be maximally invasive approaches when the learning curve is steep. Visualization is good at the point of entry and is limited to familiarity with the anatomy seen on the endoscope.
Recent Experience
Two main series using this approach have been published.22,26 Perez-Cruet et al. have a series of seven patients operated on using a minimally invasive thoracic microendoscopic discectomy (TMED).22 Results demonstrated no morbidity with this approach, good access, and quick return to work. No case required conversion to an open procedure. Jho reported a series of endoscopic transpedicular approaches also with excellent results. His series is based on a more extensive pedicle takedown, coming in more medially than Perez-Cruet. Results were also excellent for visualization, disc removal, and quick return to work.26
Lateral Approaches
Lateral Extracavitary Approach
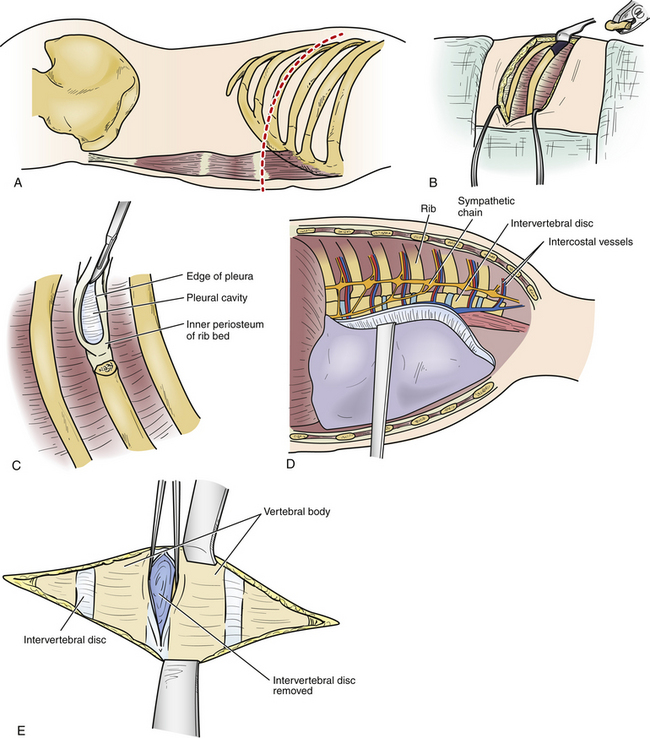
LECA was developed and refined by Larson.29 It was first performed for the management of Pott disease. It provides the best exposure to the ventral spinal canal of all lateral operations. Large thoracic discectomy series have documented its safety and efficacy17,30 (Fig. 77-5).
Technique
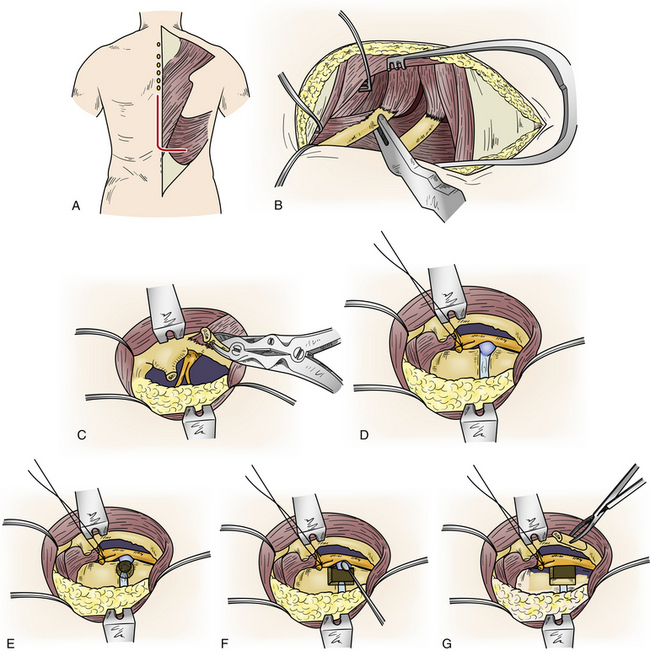
The procedure is performed with the patient prone and taped to the table with arms at the side. The skin incision consists of a hockey-stick incision, with the vertical portion centered over the area of pathology11,18,29,30 (see Fig. 77-5A). Caudally, the incision is gently curved off the midline for 8 to 12 cm, enabling the skin, subcutaneous tissue, and fascial flap to be rotated far laterally. Alternatively, a paramedian lunar-shaped incision may be used. The erector spinae muscles are subperiosteally dissected off the dorsal ribs and transverse processes and flapped medially.31 The erector spinae muscle complex may be wrapped in a moistened laparotomy pad and gently retracted medially. Intraoperative imaging helps ensure removal of the rib that articulates with the correct disc space (see Fig. 77-5B). Once the proximal 8 to 12 cm of rib is resected, the underlying intercostal nerve is identified and traced into the neural foramen (see Fig. 77-5C). The pedicle caudal to the disc is identified and removed, exposing the lateral aspect of the dura mater (see Fig. 77-5D). At this point, the dorsal third of the disc space is removed. Care is taken to leave intact the dorsal-most margin of disc and the posterior longitudinal ligament (see Fig. 77-5E). The dorsal-caudal quarter of the rostral vertebra is drilled out, as is the dorsal-rostral quarter of the caudal vertebra. This creates a cavity so that the remaining dorsal disc and posterior longitudinal ligament can be gently depressed away from the spinal cord (see Fig. 77-5F). The ventral dura mater can then be inspected either directly, with small dental mirrors, or with an endoscope. The rib, which was resected to facilitate exposure of the disc space, is then fashioned into strut grafts and carefully impacted into the cavity (see Fig. 77-5G). The ventral dura mater and spinal canal are then inspected again to ensure that there is no encroachment by the bone grafts.
Advantages
One major advantage of this procedure is the enhanced safety during the disc removal because of direct visualization of the dura mater before and during the decompression, which is facilitated by the removal of the pedicle.11,18,29,30 Additionally, because of the extensive amount of rib removed compared with the other lateral procedures, the exposure to the ventral spinal canal is improved. Surgeon orientation is almost truly lateral. By remaining extrapleural, the procedure avoids the complications observed with intrathoracic surgery.17 These include the need to place a thoracostomy tube and various pulmonary complications (cerebrospinal fluid [CSF]–pleural) fistula, pneumonia, and complications related to the need to take down the diaphragm at the thoracolumbar junction). A ventral strut graft can be placed with relative ease. Finally, in the rare instance of multiple symptomatic discs,4,32 multiple levels can be exposed.17,30
Disadvantages
The major disadvantage of LECA is that it is a formidable operation with a potential for significant perioperative pain.30 Additionally, there is a potential for a prolonged operating time and considerable blood loss, especially early on in a surgeon’s experience. In experienced hands, however, these parameters appear to be comparable to the ventrolateral approaches.29,30 Densely calcified central discs, intradural fragments, ventral dural tears, or discs that are completely enveloped by the dura mater may be difficult to remove without the need to elevate the dura mater.4 These conditions may be better treated using a ventrolateral approach. Finally, as is the case with ventrolateral surgeries, we do not generally recommend LECA in the medically high-risk patient.4
Lateral Parascapular Extrapleural Approach
Fessler et al.28 developed a modification of LECA called the lateral parascapular extrapleural approach (LPEA). This operation simplifies removal of a thoracic disc herniation in the upper thoracic spine.
Technique
Patients are placed prone and taped to the table so they can be rotated 15 to 20 degrees away from the surgeon during the decompression. The arms are kept at the sides. A midline incision extends two spinous processes above and below the disc to be removed. Caudally, the incision is gently curved laterally to the scapular line on the side of pathology. To minimize postoperative seroma formation, the incision is carried down to the deep fascia, with only minimal subcutaneous undermining. The rhomboid and trapezius muscles are then dissected off the spinous processes. A myocutaneous flap is rotated toward the medial scapular border. The caudal fibers of the trapezius muscle are transected to reflect the flap. It is important to protect the rostral latissimus dorsi fibers while cutting the inferior portion of the trapezius muscle. The musculocutaneous flap is limited by the skin incision and the medial scapula. The scapula rotates laterally as the trapezius and rhomboid muscles are mobilized. This increases the exposure of the ribs laterally for better orientation to the ventral spinal cord and central disc space.28,31
Advantages
The major advantage of this technique is that it simplifies removal of upper thoracic disc herniations by providing a far lateral orientation to the disc space at this level. This far lateral exposure enhances safety during decompression by improving visualization across the ventral spinal canal. Superior mediastinal structures, which may be traumatized with other approaches to the upper thoracic spine, are avoided. Recurrent laryngeal nerve palsies are also avoided.28
Costotransversectomy
The costotransversectomy approach was initially reported in 1894 by Menard for the treatment of Pott disease.33 The orientation to the disc space is more dorsal than with either LECA or LPEA. This is the result of a more limited rib resection.
Technique
Different costotransversectomy techniques have been proposed. Patient positioning options vary from prone to modified lateral decubitus.33 Skin incisions likewise vary and include the paramedian incision along the lateral border of the erector spinae muscles and a semilunar incision. A skin, subcutaneous tissue, and fascial flap may be rotated medially toward the spinous process. The trapezius muscle can be incised in line with the skin incision and retracted medially. The erector spinae muscles are then dissected from their attachments and can be reflected medially. Some surgeons prefer to cut this muscle complex and reapproximate it at the time of closure. From this point, surgical decompression is the same as for LECA.
Recent Experience
LECA has been augmented by the use of a neuronavigational system for frameless stereotaxy by Kim et al., who used the Stealth surgical navigation system (Stealth Surgical, Gordonsville, VA) to localize the anatomy and provide feedback regarding the dura relative to the disc. Five patients were operated on in this manner with good complete removal of the herniated disc relative to the spinal cord. These investigators cite the advantage of accurate knowledge of the surgical instrumentation relative to the surrounding structures not directly visible in the operating field.34
Ventrolateral Approaches
Transthoracic Thoracotomy
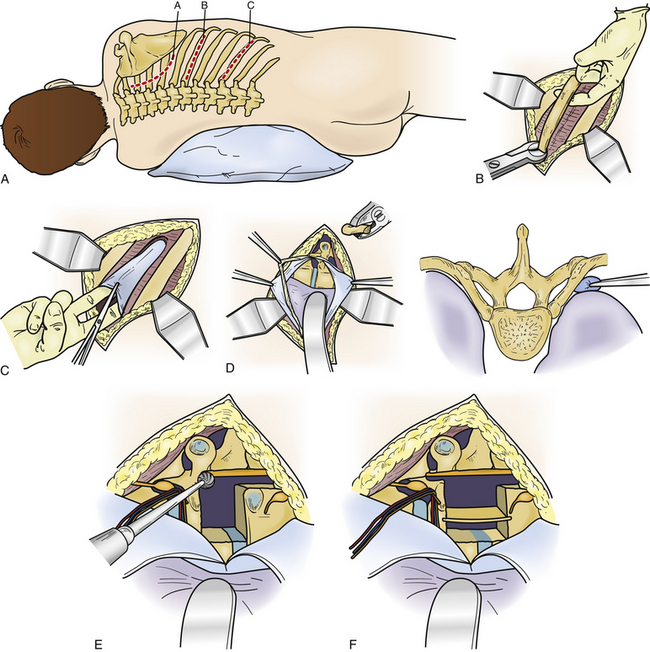
The transthoracic thoracotomy approach was first reported independently in 1969 by Perot and Munro23 and Ransohoff et al.35 These two groups reported on a combined number of five patients. Despite the limited number of patients initially undergoing this approach, it has developed into one of the surgical cornerstones for the management of symptomatic thoracic disc herniations.4,19 Two large series of thoracic discectomy patients were compared in 1992.17 One group primarily used the transthoracic thoracotomy approach, and the other group exclusively used the lateral extracavitary approach. It was determined that these two formidable procedures provided very good results with minimal associated morbidity. Transthoracic thoracotomy provides excellent exposure to the anterior column between T3 and LI (Fig. 77-6).
Technique
The preferred side of approach in the upper thoracic spine is through a right thoracotomy. This avoids the heart as well as the carotid and subclavian arteries. When the disc herniation involves the middle and lower portions of the thoracic spine, most spine surgeons perform a left thoracotomy. Approaching the disc from this side avoids manipulation of the delicate inferior vena cava, which may prove difficult to repair if injured. Additionally, at the thoracolumbar junction the liver is avoided. A double-lumen endotracheal tube may be used to facilitate single-lung ventilation (particularly in the upper thoracic spine). Care is taken to place the patient in a true lateral decubitus position. A bean bag and tape are used to maintain this position so that the patient may be rolled during the decompression. When possible, the area of pathology is centered over the break in the table. All pressure points are padded. A tangential incision is made over the rib to be resected, enabling the removal of additional ribs when necessary. The skin and subcutaneous tissue are incised from the lateral border of the paraspinal muscles to the sternocostal junction (see Fig. 77-6A). The thoracic muscular layers are then incised, and a rib retractor is introduced. It is helpful to verify that the proper rib is being resected by intraoperative AP fluoroscopy. The periosteum of the rib is then incised and exposed using subperiosteal dissection. A Doyen elevator is used to strip the periosteum from the ventral surface of the rib without violating the underlying endothoracic fascia and pleura. Small defects in these layers should be closed primarily; the rib is then resected. Bone edges of the rib are waxed, and the resected portion of rib is saved so that it can be used to form an interbody bone graft on completion of the discectomy. The parietal pleura is then incised along the line of the rib bed, and the wound is held open by introducing a rib spreader (see Fig. 77-6C). The lung is covered with a moistened laparotomy pad and can be gently retracted medially and ventrally. It may be selectively collapsed to facilitate additional exposure of the spinal column. The parietal pleura, which covers the vertebral bodies, is incised. A Cobb elevator can be used to prepare the vertebral bodies. This dissection must avoid injury to the segmental vessels, as well as the sympathetic chain that crosses the middle portion of each vertebral body (see Fig. 77-6D). It may be necessary to ligate or clip these vessels. To expose the dorsolateral disc space, ventral spinal canal, and entire neuroforamen, it is necessary to incise the radiate ligament and to drill off the head of the overlying rib. The overlying transverse process, neurovascular foramen, and rostral and caudal boundaries of the pedicle that is directly caudal to the appropriate disc space are palpated with a small nerve hook. The pedicle can be removed, exposing the lateral dura mater. When necessary, additional exposure of the neuroforamen can be facilitated by sectioning the intercostal nerve proximal to the dorsal root ganglia. At this point, the surgeon should have precise orientation regarding the location of the ventral floor of the spinal canal. The dorsal portion of the anulus is incised, and the discectomy is carried across the vertebral body approaching the other side. Care is taken to leave intact the most dorsal margin of disc and the posterior longitudinal ligament. A high-speed drill is used to create a small cavity in the dorsal-caudal quadrant of the rostral vertebral body and the dorsal-rostral portion of the caudal vertebral body. Once this cavity has been created, the overlying dorsal disc margin and posterior longitudinal ligament can be incised and gently depressed into the cavity, away from the ventral dura mater. A reverse-angled curet may aid the decompression without manipulating the spinal cord (see Fig. 77-6E). The ventral spinal canal and dura mater should be directly inspected subsequent to placement of bone grafts to ensure that there is no encroachment of the spinal canal. The lung is then expanded under direct visualization. The parietal pleura is closed over a thoracostomy tube.
Advantages
The main advantage of the transthoracic thoracotomy relates to the ventrolateral exposure of the disc space and the ventral spinal canal. This orientation enables improved exposure for the removal of midline densely calcified discs and intradural fragments. Repair of ventral dural tears is also simplified as is interbody bone graft placement. Finally, it is possible to remove more than one disc in the rare situation of multiple symptomatic herniations.32
Disadvantages
The main disadvantage is similar to that for the more extensive lateral exposures: this is a formidable operation with significant potential for considerable postoperative pain.4,17 Because of the visceral exposure, several unique risks are possible. These include CSF–pleural fistulas and pulmonary complications secondary to lung collapse. Additionally, closed chest drainage is required in the postoperative period. Disc herniations that occur at the thoracolumbar junction may require at least partial takedown of the diaphragm. Finally, the operation is technically demanding and may require collaboration with a thoracic surgeon. Patients may have an epidural placed preoperatively prior to surgery for postoperative pain. The chest tube is usually removed 3 to 6 days after surgery, prolonging the time of discharge.
Transthoracic Thoracoscopy
The use of thoracoscopy for the treatment of thoracic disc herniations was reported as early as 1994.36,37 Subsequently, growing enthusiasm for this procedure has developed, with several surgeons demonstrating proficiency.38–41 The technique, as described by Rosenthal et al.,37 uses a right-sided approach. The patient is placed in a lateral decubitus position with the side of access turned upward. Four small incisions are made along the midaxillary line so that four trocars may be inserted in a triangular fashion along this line converging on the disc space. An endoscope with a 30-degree angled lens is introduced through one of the trocars, leaving the other three as working ports. Specially designed long instruments are introduced through the working ports. The rib head and pedicle are removed to expose the dura. At this point, the discectomy proceeds similarly to an open thoracotomy.
Advantages
Proponents of this approach believe that compared with transthoracic thoracotomy, transthoracic thoracoscopy allows for complete disc removal with a reduction in surgical trauma. Thus it is thought to be associated with significantly less pain and morbidity and enables a faster recovery time.37,38
Disadvantages
In its present form, this technique has several limitations, including the need for pleural entry with all the attendant risks of intrathoracic surgery (e.g., the need for a postoperative thoracostomy tube). A steep learning curve accompanies this method because of the need to develop techniques requiring new hand-eye coordination skills, limited tactile feedback during the surgery, and difficulties with the three-dimensional visualization during disc removal. Additionally, in an era when growing cost constraints affect surgical methodology, the cost-effectiveness of this procedure must be evaluated from the standpoint of duration of hospitalization, length of surgery, and cost of the instrumentation required. Finally, despite being touted by some as a minimally invasive technique for thoracic disc removal, it is important to keep in mind that this operation currently requires multiple stab incisions for the insertion of the camera, two working ports, and, on occasion, a retractor for the diaphragm or an incompletely collapsed lung. In its present form, this procedure is perhaps more invasive than other techniques.4 The exposure this technique provides is identical to thoracotomy. If this procedure is proven clinically safe and effective over long-term follow-up, it would appear to be best suited as an alternative to open thoracotomy in distinction to being a substitute for the dorsolateral, lateral, and retropleural.operations.4
Recent Experience
Johnson et al. reported experience with endoscopic thoracic discectomy in 36 patients, which they compared with 8 patients undergoing standard open thoracotomy for thoracic discs. These investigators concluded that patients who underwent thoracoscopic discectomy had shorter operative times, less blood loss, a shorter period of chest tube drainage, less narcotic usage, and shorter lengths of stay. The incidence of complications (minor and major) was felt to be 31% in the thoracoscopic group and greater than 100% (more than one complication per patient) in the thoracotomy/discectomy group. These investigators emphasized that the thoracoscopic discectomy, although safer in experienced hands, had a steep learning curve, which may warrant open surgeries for surgeons not familiar with endoscopic techniques.42 Rosenthal and Dickman reported on a series of 55 patients who underwent thoracic endoscopic disc removal. There were no neurologic complications from the procedure. They reported a 16% rate of intercostal neuralgia in the endoscopic thoracic discectomy group, versus a 50% rate in the open thoracotomy group. The thorascopic group did not have an increased incidence of a retained disc fragment compared with an open thoracotomy. Surgery time was comparable between the thorascopic group and the open surgery technique. The operative time and hospital discharge were less than for conventional surgery.6 These investigators also examined the incidence of retained discs and found that the open thoracotomy group had no retained fragments, the thorascopic group had a 4% retention rate, and the open costotransversectomy group had a 13% retention rate.6 In a series of patients undergoing reoperation for herniated thoracic discs, Dickman et al. found that calcified, large broad-based, centrally located discs were the hardest to remove easily and had higher recurrence rates.43 Anand and Regan have recently published a report on a series of 100 patients operated on with video-assisted thorascopic surgery with a 2-year follow-up. There were no permanent postoperative neurologic deficits. They found that patients with the most severe presentation (i.e., myelopathy) had the greatest improvement in the Oswestry Disability score. At the end of 2 years, 68 patients responded to the final questionnaire. There was an overall 84% satisfaction rate, with objective long-term clinical success achieved in 70% of the patients after 2 years.44
Retropleural Thoracotomy Approach
Retropleural thoracotomy was refined and popularized by McCormick.5 This surgical option in the management of ventral spinal pathology provides an orientation similar to the other ventrolateral approaches and avoids key complications (Fig. 77-7).
Technique
Like the transthoracic procedures, the side of the approach is determined by the location of the pathology. The patient is placed in the lateral decubitus position on a bean bag with a small roll under the dependent axilla. Lesions that occur at the thoracolumbar junction should be centered over the break in the table. Between T5 and T10, a 12-cm skin incision is made, extending from the dorsal axillary line to a point 4 cm lateral to the dorsal midline over the rib at the level of pathology. For upper thoracic spine lesions, a hockey-stick incision is made that parallels the medial and caudal scapular border (see Fig. 77-7A). The incision is then carried through the scapular muscles (trapezius and rhomboids) to the ribs. The scapula is rotated rostrally to expose the appropriate rib. After the appropriate level is identified, a subperiosteal detachment of intercostal muscles is performed over 8 to 10 cm of rib. The rib is then resected and removed (see Fig. 77-7B). The most proximal 4 cm of rib that has attachments to the vertebral body and transverse process remains intact. Once the rib resection is completed, a well-defined layer of tissue is identified in the rib bed. This layer is the endothoracic fascia. It is analogous to the transversalis fascia in the abdomen.
The endothoracic fascia lines the entire chest cavity. The underlying parietal pleura maintains an attachment to the inner chest wall through this layer. There is a potential space between the endothoracic fascia and parietal pleura that may contain loose areolar tissue. The endothoracic fascia is continuous with the inner periosteum of the rib and the thoracic vertebral body. It is important to remember that the thoracic sympathetic chain, the intercostal neurovascular elements, the thoracic duct, and the azygos veins are contained against the thoracic wall and the vertebral bodies within this fascial layer. Once identified, the fascia is incised in line with the rib bed (see Fig. 77-7C). The underlying parietal pleura is bluntly dissected from the undersurface of the endothoracic fascia, in much the same way that the peritoneum is freed from the transversalis fascia. Proximally, the pleura is dissected from the vertebral column. At this point, a table-mounted malleable retractor maintains retraction of a laparotomy pad-covered lung (see Fig. 77-7D). The opening can be further widened by placement of a rib retractor.
The endothoracic fascia should be opened over the remaining 4 cm of proximal rib. The costotransverse ligaments are divided, and the rib head is removed. The fascia and periosteum over the vertebral body are elevated away from the disc space. The intercostal vessels that cross the midvertebral body transversely may be preserved. Once the rib head is resected, the boundaries of the neuroforamen and pedicle are identified and the rostral portion of the pedicle directly caudal to the disc space may be resected. From this point on, the decompression is identical to the other ventrolateral approaches (see Figs. 77-7E and 77-7F). At the thoracolumbar junction, because of the diaphragm and the greater angulation of the ribs, the procedure is modified. A 12- to 14-cm skin incision is usually made over the 10th rib extending from the dorsal axillary line to 4 cm off the midline (see Fig. 77-7A). A 10-cm portion of rib is then exposed and resected and the pleural surface of the diaphragm is identified. It should be noted that the endothoracic fascia is reflected over the diaphragm and tightly applied to its surface.
The initial exposure may be somewhat cramped because of the diaphragm’s attachment to the ribs. If the endothoracic fascia is present in the rib bed, it is opened. Caudally, the pleural surface of the diaphragm is depressed and detached from the ventral surface of the T11 and T12 ribs with Cobb elevators. Once this is completed, there is a communication between the retropleural and retroperitoneal space. Medially, the detachment is continued so that the arcuate ligaments are elevated off the quadratus lumborum and psoas muscles. Division of the crus of the diaphragm completes the mobilization of the diaphragm. At this point, table-mounted malleable retractors are inserted as rib spreaders and the proximal 4 cm of rib is then removed. The decompression at this point continues as described earlier.30
Advantages
This operation provides exposure to the ventral spinal canal and dura mater that is identical to the intrathoracic operations discussed earlier. However, it avoids pleural entry; thus, many of the significant complications that may arise from these procedures are avoided. Also important is that the retropleural thoracotomy approach provides the shortest direct surgical route to the ventral thoracic and thoracolumbar spine. This may enhance safety during the decompression, as well as enable the surgery to be performed using smaller incisions with less soft-tissue dissection, which should lessen perioperative pain and diminish the length of hospitalization. In contrast to the ventrolateral approaches, the diaphragm can be mobilized quite easily at the thoracolumbar junction without its incision. Additionally, the exposure rendered is obscured by the aorta and vena cava. In contrast to the lateral approaches, exposure to the lateral spinal canal can be achieved without dissection or sacrifice of the intercostal nerve or potential occlusion of the intraforaminal radicular medullary artery. Overall, this procedure appears to offer advantages over the other ventrolateral surgeries (both closed and open) and lateral approaches.36
Disadvantages
When compared with the dorsolateral operations (transfacet and transpedicular), the orientation to the disc space and ventral spinal canal is more direct. This more direct orientation must be weighed against the more extensive muscle and bone manipulation required by this surgery. If safety and efficacy are demonstrated clinically, the retropleural thoracotomy approach may be ideally suited for disc herniations that cannot be reliably treated using one of the less invasive dorsolateral techniques.
Ventral Approaches
Transsternal Approach
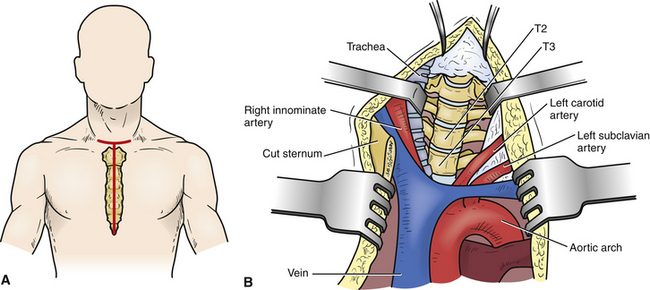
The transsternal approach may be an option for the management of midline, densely calcified herniations between T2 and T5 (Fig. 77-8).11,17 When the surgery requires access to the T4-5 levels, preoperative imaging must define the relationship between the aortic arch and the ventral spinal column.
Technique
This operation is generally performed through a T-shaped skin incision that has its vertical limb extending from the lower cervical skin crease to a few centimeters below the xiphoid process (see Fig. 77-8A). The strap muscles are then divided, and the precervical fascia and pretracheal fascia incised at the level of the sternal notch. The sternum is then split and retracted laterally. After the exposure of the pericardium and thymus, the thymus is retracted to the right, exposing the left innominate vein, which can be sacrificed. The working space is between the left common carotid artery and the innominate artery, trachea, esophagus, and thyroid. Gentle retraction of these structures exposes the ventral region of the spinal column (see Fig. 77-8B). Once the ventral spine is exposed, the disc is removed using standard microdiscectomy techniques that are used during ventral cervical microdiscectomy.11,17
Recently, Teng et al. proposed an easy MRI method to measure and categorize individual anatomic variations within the cervicothoracic junction (CTJ). They divided a group of normal patients into three groups based on their lesion relative to the suprasternal notch (STN). In the majority of patients, the T3 vertebra is above the STN, and lesions may be approached via a suprasternal approach. In patients with long necks, this exposure could be carried down one or two vertebral levels lower than those in the short-necked group. In approximately one third of the cases, the lesion is below the STN, and a combined approach involving a manubriotomy and sternotomy is required.9
Advantages and Disadvantages
The primary advantage of this approach is the management of thoracic disc herniations that are midline and densely calcified in the upper thoracic spine.4 The other advantages are similar to those for the ventrolateral approaches. This is clearly a challenging operation with the potential for significant physiologic cost to the patient. Fortunately, disc herniations in the upper thoracic spine are quite rare, constituting just a fraction of symptomatic disc herniations.4 Most of these herniations can be treated successfully using one of the other, less invasive approaches. In addition to all the disadvantages encountered with the ventrolateral approaches, the transsternal approach has the unique risk of injury to the left recurrent laryngeal nerve and the thoracic duct.17
Complications
Thoracic Disc Series
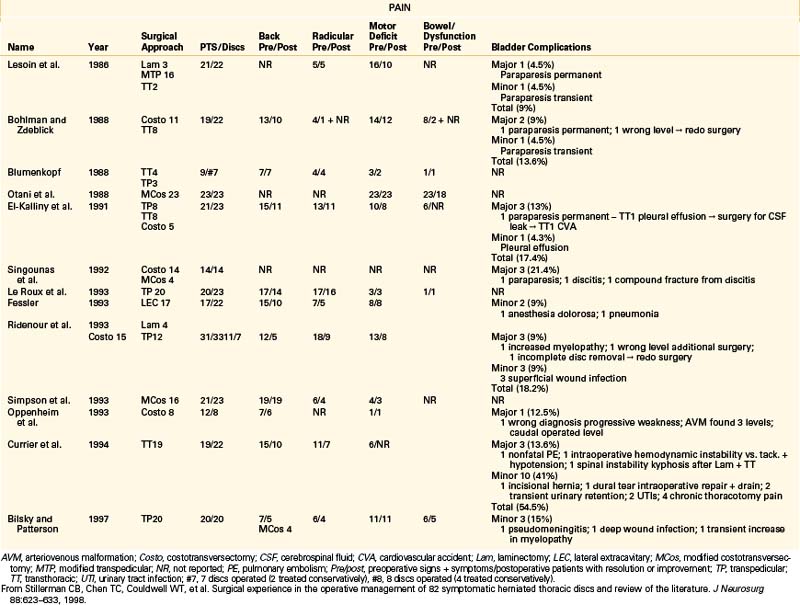
Several independently reported reviews of thoracic disc herniations have appeared in the literature.1,2,44–47 Many of these reports provide excellent historic perspectives that include surgeries performed before the 1930s. For a better understanding of the overall effectiveness of modern thoracic disc herniation management, a focused review of several large series is presented in Table 77-2.4 Chen has summarized the results of outcomes of various thoracic disc procedures. Outcomes were measured as improvement in pain control or myelopathy (if present). Duration of surgery, length of hospital stay, and patient satisfaction were reported for the various approaches. Moreover, complications of thoracic discectomy, including paraparesis, instability, dural tear, and pseudomeningocele, were reported.48 Recently, Stillerman et a1.4 reported their experience with the operative management of 82 symptomatic herniated thoracic discs. In this series the overall complication rate was 12/82 (14.6%). Three major complications (3.6%) occurred, consisting of one perioperative death from cardiopulmonary complications in a patient at high medical risk, spinal instability requiring further surgery, and an increase in the severity of a preoperative paraparesis. There were 9/82 (11%) minor complications, which consisted of one transient paraparesis that completely resolved after 48 hours, one compression fracture treated with 3 months of bracing, three superficial wound infections, three pneumonias, and one postoperative seizure. All of the minor complications were treated medically with no sequelae (Table77-3).4 Results of this series compared with 13 other contemporary series demonstrated a 6.1% major complication rate (16/263) compared with a 3.6% rate (3/82) in the series. This difference was not found to be statistically significant (chi-square test). Although several deaths were reported in earlier studies,13,14,21,45,49 none occurred in the 13 contemporary review series. The minor complications reported in the review group constituted 8.7% (23/263), compared with 11% (9/82) in the series. The total complication rate reported was 14.8% (39/263) in the review group and 14.6% (12/82) in the series. Like the case with major complications, there was no significant difference in either the total complication rate or minor complication rate.
TABLE 77-3 Surgical Complications in 82 Thoracic Microdiscectomies
| Complication Type | No. of Complications |
|---|---|
| Death | 1 (1.2%) |
| Loss of spinal integrity | |
| Stable compression | |
| Fx: braced | 1 (1.2%) |
| Fx with kyphosis: surgery | 1 (1.2%) |
| Increased weakness | |
| Transient (resolved 48 hr) | 1 (1.2%) |
| Permanent mild residual deficit | 1 (1.2%) |
| Superficial wound infection | 3 (3.7%) |
| Pneumonia | 3 (3.7%) |
| Seizure | 1 (1.2%) |
| Total | 12 (14.6%) |
Fx, fracture.
From Stillerman CB, Chen TC, Couldwell WT, et al: Surgical experience in the operative management of 82 symptomatic herniated thoracic discs and review of the literature. J Neurosurg 88:623–633, 1998.
Based on experience gained with the ventrolateral and lateral surgeries during the course of the series of 82 thoracic discectomies, the well-documented success by others using the dorsolateral transpedicular approach, and the preliminary experience reported with the transthoracic thoracoscopy, retropleural thoracotomy, and transfacet pedicle-sparing approaches, guidelines for selection of a surgical approach have been developed4 (see Table 77-1).
New Paradigm for Thoracic Disc Herniations
Based on an evaluation of the aforementioned contemporary series, a management paradigm was developed for treating this disorder4 (Fig. 77-9). The natural history of the disease, the patient’s initial presentation, correlation with MRI findings, and current neurologic examination are crucial to the decision process for surgical versus conservative management. Patients with symptoms from thoracic disc herniations are placed into one of three groups: localized/axial pain, radicular pain, or myelopathy.
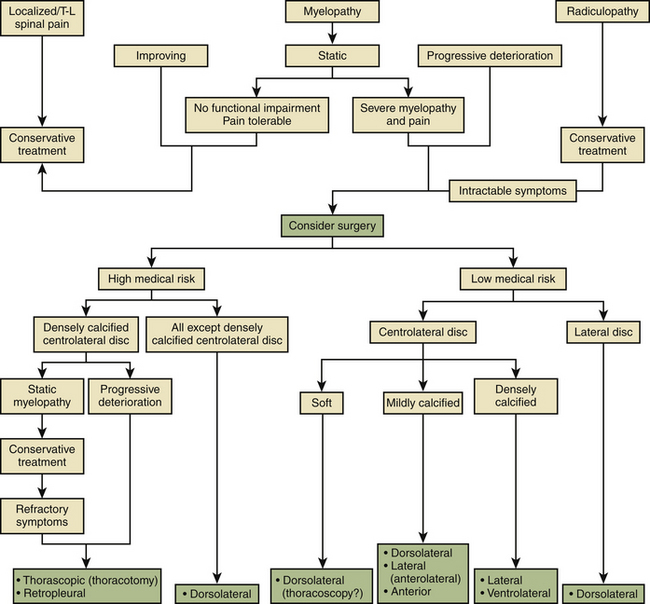
FIGURE 77-9 Management scheme for treating symptomatic thoracic disc herniations.
(From Stillerman CB, Chen TC, Couldwell WT, et al: Surgical experience in the operative management of 82 symptomatic herniated thoracic discs and review of the literature. J Neurosurg 88:623–633, 1998.)
Localized/Axial Pain
Patients with back pain alone who do not have myelopathy or severe unrelenting radicular pain constitute a difficult management problem because of the nonspecific nature of their pain syndrome. Surgery has a high failure rate in these patients and must be individualized.
Myelopathy
Patients with myelopathy who are shown to have a disc herniation are treated based on the status of their neurologic deficit. The following are intended to serve as suggested considerations; the ultimate choice of procedure must be influenced by the surgeon’s familiarity and skill with the different approaches. Patients who have a nonsevere static deficit without functional impairment and whose pain is tolerable are treated nonoperatively. Patients who are improving from the standpoint of their myelopathy are likewise treated conservatively. When the myelopathy is either severe and nonprogressive or progressing, the patients are entered into either a high-medical-risk or a low-medical-risk group. In the low-medical-risk group, the parameters influencing the selection of a particular approach include the position of the disc in the spinal canal (centrolateral vs. lateral herniation) and the consistency of the disc (soft vs. mildly calcified vs. densely calcified). Additionally, the location of the calcium in the disc (i.e., ventral vs. dorsal) is important in ascertaining the likelihood of significant dural adherence by the disc material. High-medical-risk patients with severe (static or progressive) myelopathy should undergo dorsolateral decompression unless the disc is midline or central-lateral,1 large, and densely calcified. Patients with these types of discs are operated on, using either a transthoracic thoracoscopy or retropleural approach, or, on occasion, a transthoracic thoracotomy. The high-medical-risk patients with densely calcified central-lateral discs and static myelopathies are initially treated conservatively. However, if their symptoms prove to be refractory, they are operated on with the same surgical approaches used for patients found to have progressive deterioration in their myelopathy.
Future Trends
Future trends for thoracic discectomy emphasize enhanced imaging, visualization, and smaller incisions. These approaches may be performed dorsolaterally as demonstrated by the minimally invasive endoscopic approaches advocated by Jho26 and Fessler.50 Thorascopic endoscopic approaches via a ventrolateral approach are also popular because of the increased visualization provided by the endoscope. Lastly, intraoperative imaging using neuronavigational systems has been advocated to enhance visualization with open procedures.
Brown C.W., Deffer P.A., Akmakjian J., et al. The natural history of thoracic disc herniation. Spine (Phila Pa 1976). 1992;17:S97-S102.
Maiman D.J., Larson S.J., Luck E., El-Ghatit A. Lateral extracavitary approach to the spine for thoracic disc herniation: report of 23 cases. Neurosurgery. 1984;14:178-182.
McCormick P.C. Retropleural approach to the thoracic and thoracolumbar spine. Neurosurgery. 1995;37:1-7.
Patterson R.H.Jr., Arbit E. A surgical approach through the pedicle to protruded thoracic discs. J Neurosurg. 1978;48:768-772.
Perez-Cruet M.J., Kim B.S., Sandhu F., et al. Thoracic microendoscopic discectomy. J Neurosurg Spine. 2004;1:58-63.
Perot P.L., Munro D.D. Transthoracic removal of midline thoracic disc protrusions causing spinal cord compression. J Neurosurg. 1969;31:452-458.
Stillerman C.B., Chen T.C., Couldwell W.T., et al. Surgical experience in the operative management of 82 symptomatic herniated thoracic discs and review of the literature. J Neurosurg. 1998;88:623-633.
Stillerman C.B., Chen T.C., Day J.D., et al. The transfacet pedicle-sparing approach for thoracic disc removal: cadaveric morphometric analysis and preliminary clinical experience. J Neurosurg. 1995;83:971-976.
1. Arseni C., Nash F. Thoracic intervertebral disc protrusion: a clinical study. Neurosurgery. 1960;17:418-430.
2. Awad E.E., Martin D.S., Smith K.R.Jr., et al. Asymptomatic versus symptomatic herniated thoracic discs: their frequency and characteristics as detected by computed tomography after myelography. Neurosurgery. 1991;28:180-186.
3. Russell T. Thoracic intervertebral disc protrusion: experience of 67 cases and review of the literature. Br J Neurosurg. 1989;3:153-160.
4. Stillerman C.B., Chen T.C., Couldwell W.T., et al. Surgical experience in the operative management of 82 symptomatic herniated thoracic discs and review of the literature. J Neurosurg. 1998;88:623-633.
5. McCormick P.C. Retropleural approach to the thoracic and thoracolumbar spine. Neurosurgery. 1995;37:1-7.
6. Rosenthal D., Dickman C.A. Thorascopic microsurgical excision of herniated thoracic discs. J Neurosurg. 1998;89:224-235.
7. Sheikh H., Samartzis D., Perez-Cruet M.J. Techniques for the operative management of thoracic disc herniation: minimally invasive thoracic microdiscectomy. Orthop Clin North Am. 2007;3:351-361.
8. Stillerman C.B., Chen T.C., Day J.D., et al. The transfacet pedicle-sparing approach for thoracic disc removal: cadaveric morphometric analysis and preliminary clinical experience. J Neurosurg. 1995;83:971-976.
9. Teng H., Hsiang J., Wu C., et al. Surgery in the cervicothoracic junction with an anterior low suprasternal approach alone or combined with manubriotomy and sternotomy: an approach selection method based on the cervicothoracic angle. J Neurosurg Spine. 2009;10(6):532-544.
10. Logue V. Thoracic intervertebral disc prolapse with spinal cord compression. J Neurol Neurosurg Psychiatry. 1952;15:227-241.
11. Stillerman C.B., Weiss M.H. Principles of surgical approaches to the thoracic spine. In: Tarlov E.C., editor. Neurosurgical topics: neurosurgical treatment of disorders of the thoracic spine. Park Ridge, IL: AANS; 1991:1-19.
12. Black P. Laminotomy/medial facet approach in the excision of thoracic disc herniation. Neurosurg Focus. 2000;9(4):E6.
13. Patterson R.H.Jr., Arbit E. A surgical approach through the pedicle to protruded thoracic discs. J Neurosurg. 1978;48:768-772.
14. Bilsky M.H., Patterson R.H. The transpedicular approach for thoracic disc herniations. In: Benzel E.C., Stillerman C.B., editors. The thoracic spine. St Louis: Quality Medical, 1998.
15. Bilsky M.H. Transpedicular approach for thoracic disc herniations. Neurosurg Focus. 2000;9(4):E3.
16. Le Roux P.D., Haglund M.M., Harris A.B. Thoracic disc disease: experience with the transpedicular approach in twenty consecutive patients. Neurosurgery. 1993;33:58-66.
17. Stillerman C.B., Weiss M.M. Management of thoracic disc disease. Olin Neurosurg. 1992;38:325-352.
18. Stillerman C.B., Couldwell W.T., Chen T.C., et al. Thoracic disc (invited response) neurosurgical forum. Neurosurgery. 1996;85:187-190.
19. Stillerman C.B., Weiss M.M. Surgical management of thoracic disc herniation and spondylosis. In: Menezes A.H., Sonntag V.K.H., Benzel E.C., Cahill D.W., editors. Principles of spinal surgery. New York: McGraw-Hill; 1996:581-601.
20. Patterson R.H. Thoracic disc herniation: operative approaches and results. Neurosurgery. 1983;12:305.
21. Patterson R.H. Thoracic disc disease: experience with the transpedicular approach in twenty consecutive patients. Neurosurgery. 1993;33:66.
22. Perez-Cruet M.J., Kim B.S., Sandhu F., et al. Thoracic microendoscopic discectomy. J Neurosurg Spine. 1, 2004. 58–63, 2004
23. Perot P.L., Munro D.D. Transthoracic removal of midline thoracic disc protrusions causing spinal cord compression. J Neurosurg. 1969;31:452-458.
24. Levi N., Gjerris F., Dons K. Thoracic disc herniation. Unilateral transpedicular approach in 35 consecutive patients. J Neurosurg Sci. 1999;43(1):37-42.
25. Chi J.H., Dhell S., Kanter A.S., Mummaneni P.V. The mini-open transpedicular thoracic discectomy: surgical technique and assessment. Neurosurg Focus. 2008;25(2):E5.
26. Jho H.D. Endoscopic transpedicular thoracic discectomy. Neurosurg Focus. 2000;9(4):E4.
27. Zhang B.R., Konodi B.C., Chapman J.R. Early experience treating thoracic disc herniations using a modified transfacet pedicle-sparing decompression and fusion. J Neurosurg Spine. 2010;12(2):221-331.
28. Fessler R.C., Dietze D.D.Jr., MacMillan M., et al. Lateral parascapular extrapleural approach to the upper thoracic spine. J Neurosurg. 1991;75:349-355.
29. Larson S.J., Holst R.A., Hemmy D.C., Sances A.Jr. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45:628-637.
30. Maiman D.J., Larson S.J., Luck E., El-Ghatit A. Lateral extracavitary approach to the spine for thoracic disc herniation: report of 23 cases. Neurosurgery. 1984;14:178-182.
31. Dietze D.D.Jr., Fessler R.C. Thoracic disc herniations. Neurosurg Clin North Am. 1993;4:75-90.
32. Chin L.S., Black K.L., Hoff J.T. Multiple thoracic disc herniations: case report. J Neurosurg. 1987;66:290-292.
33. Borges L.F. Thoracic disc disease and spondylosis. In: Tindall C.T., Cooper P.R., Barrow D.L., editors. The practice of neurosurgery. Baltimore: Williams & Wilkins; 1996:2461-2471.
34. Kim K.D., Barbitz J.D., Mimbs J. Imaging-guided costotransversectomy for thoracic disc herniation. Neurosurg Focus. 2000;9(4):E7.
35. Ransohoff J., Spencer F., Siew F., et al. Transthoracic removal of thoracic disc: report of three cases. J Neurosurg. 1969;31:459-461.
36. Horwitz M.B., Moossy J.J., Julian T., et al. Thoracic discectomy using video assisted thoracoscopy. Spine (Phila Pa 1976). 1994;9:1082-1086.
37. Rosenthal D., Rosenthal R., De Simonc A. Removal of a protruded thoracic disc using microsurgical endoscopy: a new technique. Spine. 1994;19:1087-1091.
38. Dickman C.A., Rosenthal D., Karahalios D.G., et al. Thoracic vertebrectomy and reconstruction using a microsurgical thoracoscopic approach. Neurosurgery. 1996;38:201-219.
39. Krasna M.J., Mack M.J. Atlas of thoracoscopic surgery. St Louis, 1994, Quality Medical. 1994:206-211.
40. McAfee P.C., Regan J.R., Zdeblick T., et al. The incidence of complications in endoscopic anterior thoracolumbar spinal reconstructive surgery. Spine (Phila Pa 1976). 1995;20:1624-1632.
41. Regan J.J., Mack M.J., Picetti C.D. A technical report on video-assisted thoracoscopy in thoracic spinal surgery. Spine (Phila Pa 1976). 1995;20:831-837.
42. Johnson J.P., Filler A.G., McBride D.Q. Endoscopic thoracic discectomy. Neurosurg Focus. 2000;9(4):E11.
43. Dickman C.A., Rosenthal D., Regan J.J. Reoperation for herniated thoracic discs. J Neurosurg. 1999;91(Suppl 2):157-162.
44. Anand N., Regan J.J. Video-assisted thorascopic surgery for thoracic disc disease: classification and outcome study of 100 consecutive cases with a 2-year minimum follow-up period. Spine (Phila Pa 1976). 2002;27(8):871-879.
45. Arce C.A., Dohrmann C.J. Thoracic disc herniation: improved diagnosis with computed tomographic scanning and a review of the literature. Surg Neurol. 1985;23:356-361.
46. Arce C.A., Dohrmann G.J. Herniated thoracic discs: symposium on neurosurgery. Neurol Olin. 1985;3:383-392.
47. Brown C.W., Deffer P.A., Akmakjian J., et al. The natural history of thoracic disc herniation. Spine (Phila Pa 1976). 1992;17:S97-S102.
48. Chen T.C. Surgical outcome for thoracic disc surgery in the postlaminectomy era. Neurosurg Focus. 2000;9(4):e12.
49. Benjamin V. Diagnosis and management of thoracic disc disease. Olin Neurosurg. 1983;30:577-605.
50. Eichholz K.M., O’Toole J.E., Fessler R.G., et al. Thoracic microenscopic discectomy. Neurosurg Clin N Am. 2006;17:441-446.