CHAPTER 92 Thoracic Aortic Aneurysms
Thoracic aortic aneurysms can result from a variety of causes. The underlying cause of a thoracic aortic aneurysm can typically be predicted by its location and morphologic features and by the age of the patient.1 Whereas the overarching goal of therapy remains similar (i.e., to prevent complications, notably aortic rupture), the nature, timing, and associated operative interventions can differ significantly according to the location and cause of the aneurysm. An example is that of the ascending aortic aneurysm, which by its location is associated with the additional considerations of whether to replace the aortic valve, to reimplant the coronary arteries, and to repair the arch vessels. In addition, assessment of global cardiovascular function is paramount in directing appropriate treatment strategy.
Thoracic aortic aneurysms are less common than their abdominal counterpart.2 Imaging plays a critical role in diagnosis, treatment planning (i.e., assessment of the need for intervention, urgency of intervention, and type of intervention), and postsurgical surveillance. Moreover, some complications, such as spinal cord ischemia, are germane to surgical repair of thoracic aortic aneurysms, and imaging can provide a road map that may allow prospective modification of surgical technique to reduce the chances of such complications.
THORACIC AORTIC ANEURYSM
Definition
The traditional definition of an aneurysm is dilation of a blood vessel wall so that the resulting caliber is 50% greater.3 This size-based definition does not account for morphologic characteristics such as focal saccular dilation of the aorta due to trauma, penetrating atherosclerotic ulcer, and infection. These scenarios require an “aneurysm mentality” because saccular aortic dilations are at particular risk for rupture and are thus also classified as aneurysms.
In true aneurysms, the dilation involves all layers of the blood vessel wall. False aneurysms (also known as pseudoaneurysms or saccular aneurysms) occur from disruption of one or more layers of the aortic wall.4
Prevalence and Epidemiology
Thoracic aortic aneurysms are less common than abdominal aneurysms, and the prevalence depends on the etiology (Fig. 92-1). Of note, aneurysms due to systemic arterial disease have less male preponderance and a more advanced age at presentation than abdominal aneurysms do, resulting in greater comorbid disease.2
The population incidence of detected descending thoracic and thoracoabdominal aortic aneurysms is estimated to be 5.9 new aneurysms per 100,000 person-years. The lifetime probability of rupture in these aneurysms is 75% to 80%.5
Etiology and Pathophysiology
Etiology and Pathogenesis
Atherosclerosis
Atherosclerosis affects the aortic wall in many ways, such as through erosion of the internal elastic lamina and subsequent exposure of the medial layer of the aortic wall to the pulse pressure or through ischemia of the media from reduced blood supply due to disease in the vasa vasorum. Another mechanism of aneurysm formation is through progression of atherosclerotic plaque ulceration to a penetrating atherosclerotic ulcer with breakage of the intimal layer. Penetrating atherosclerotic ulcer may result in a saccular aneurysm (Fig. 92-2). Atherosclerotic aneurysms are associated with hypertension, coronary artery disease, and abdominal aortic aneurysms.4
Cystic Medial Necrosis
As the name implies, cystic medial necrosis affects the medial layer of the arterial wall; degeneration of the smooth muscle creates “cystic spaces,” resulting in a fusiform aneurysm.4 This is the most common cause of aneurysms of the ascending aorta. The pathophysiologic process involves the aortic root, resulting in dilation of the annulus of the aortic valve. Associated aortic regurgitation may require concomitant replacement of the aortic valve.6 Cystic medial necrosis is the hallmark of the pathologic changes in Marfan syndrome (Figs. 92-3 to 92-6). Other connective tissue disorders, such as Ehlers-Danlos and Loeys-Dietz (Fig. 92-7) syndromes, also affect the medial layer of the aorta.4 These entities are both familial and have an identifiable gene leading to the abnormal biochemistry.
Marfan syndrome is the most common of the connective tissue processes, with an incidence of 1:10,000.7 Marfan syndrome is an autosomal dominant condition that results in a mutation in the gene encoding fibrillin 1,8 an essential protein for elastic properties, causing cardiovascular and musculoskeletal abnormalities. The elastin-depleted aorta is stiffer and more prone to dilation as it incurs higher pulse pressure than the normally distensible aorta does. The dilation starts in the root and extends to the mid-ascending aorta.9 Aortic rupture and dissection are the leading causes of death in patients with Marfan syndrome.7 Repair in patients with Marfan syndrome is recommended in the asymptomatic patient when the aortic root or ascending aorta exceeds 5 cm in diameter because of the high risk of aortic rupture and aortic dissection.10 Associated cardiovascular abnormalities include aortic insufficiency and mitral valve prolapse, which frequently necessitate valve repair, and pulmonary artery aneurysms.9
Loeys-Dietz syndrome has only recently been characterized as a distinct phenotype that is caused by mutations in genes encoding type 1 or type 2 transforming growth factor β.11 Aneurysms form at an earlier age than in other connective tissue disorders and tend to rupture at a smaller size, with a greater propensity for dissection. The arteriopathy tends to be more systemic than in Marfan syndrome, and the postoperative surveillance must factor both the repaired artery and the remote arteries including the intracranial circulation.
Vascular Ehlers-Danlos syndrome is an autosomal dominant disorder caused by heterozygous mutations of the COL3A1 gene. The syndrome is characterized by fragile arterial tissue that not only is prone to aneurysm, dissection, and rupture but can also make surgical repair difficult. Noninvasive imaging, such as computed tomographic angiography (CTA) and magnetic resonance angiography (MRA), is preferred because of the risk of dissection and rupture with arterial access. Unlike Marfan and Loeys-Dietz syndromes, which have a predilection for the aortic root, Ehlers-Danlos syndrome more often affects the visceral arteries.12
Dissection
Aortic dissection is different enough in pathogenesis and management to be considered separately from thoracic aortic aneurysms. The term dissecting aneurysm is discouraged. Nonetheless, a dissected aorta may become aneurysmal (Fig. 92-8), and an aneurysm may dissect; both scenarios alter the management and approach. Of note, any cause of an acute aortic syndrome can result in aneurysm formation.
Trauma
Blunt thoracic trauma with a sudden deceleration mechanism injures the aorta at its points of relative fixation (due to shear), which includes the aortic isthmus (in the region of the ligamentum arteriosum), the hiatus, and the aortic root, in descending frequency. Injury may cause transection or intimal disruption and, if unrecognized, may be manifested as a saccular aneurysm that may involve disruption of the intima and media (pseudoaneurysm or false aneurysm).13
Mild bulging of the proximal descending aorta at the attachment of the ligamentum arteriosum (ductus bump) is a common normal finding and should not be confused with aneurysms in that region, particularly those due to trauma (Fig. 92-9).
Inflammation and Infection
Infection may primarily involve the artery and secondarily lead to aneurysm, or an aneurysm may become infected. The most common site of infection is the ascending aorta near the sinus of Valsalva (Fig. 92-10).14 Infected aortic aneurysms, despite the pseudonym mycotic aneurysms, are rarely due to fungal agents and mostly caused by bacteria in an acute or chronic setting, classically due to syphilis.
Infected aortitis leading to aneurysms may occur by direct extension from endocarditis or seeding of the vasa vasorum with infected complexes from remote septic foci. These remote foci may not be clinically evident, and Salmonella and Staphylococcus are the most common etiologic agents in this situation. Predisposing factors include intravenous drug abuse and immunocompromised state.15
Vasculitides such as Takayasu arteritis and giant cell arteritis may lead to aneurysmal dilation, but mural thickening is the predominant finding in such inflammatory conditions. The mural thickening may lead to reduction in luminal caliber even if the adventitia-adventitia measurement indicates arterial enlargement.16
Post-stenotic Dilation
Aortic stenosis can cause dilation of the ascending aorta, usually the right lateral and posterior wall, from the post-stenotic jet. This is commonly seen in those with a bicuspid aortic valve (Fig. 92-11), although the dilation may be partly due to a systemic arteriopathy because this condition is also associated with coarctation of the aorta.17 Coarctation can also lead to post-stenotic dilation.
Location
Thoracoabdominal aortic aneurysms are further divided by the Crawford classification (Fig. 92-12), which is used to determine the operative approach and to counsel the patient about postoperative complications. Crawford I and II start distal to the origin of the left subclavian artery, with Crawford II extending below the renal artery origin. Crawford III starts more distal than Crawford I in the descending thoracic aorta. Crawford IV is essentially an aneurysm of the abdominal aorta that extends to the diaphragmatic hiatus.18
True and False Aneurysms
All causes of arteriopathy can cause true aneurysms. The causes of false aneurysms, on the other hand, are more typically trauma, infection (i.e., mycotic aneurysm), and penetrating atherosclerotic ulcer. False aneurysms have a saccular morphology, that is, an eccentric bulge, and can be considered a contained rupture (Fig. 92-13). They occur from the disruption of layers of the blood vessel wall and are often contained only by the adventitia and fibrous tissue. This makes them more likely than true aneurysms to rupture for any given size.1
Manifestations of Disease
Clinical Presentation
Aneurysms may be detected incidentally, be manifested through local mass effect or systemic symptoms, or cause symptoms from acute rupture.1,19
Rupture
The strongest predictor of rupture is the initial size of the aneurysm. Also, the etiology of the aneurysm must be taken into consideration as certain connective tissue disorders are associated with rupture at a smaller aneurysm size. For thoracoabdominal aortic aneurysms due to atherosclerosis, the rupture rate is 18% at 2 years for aneurysms greater than 5 cm. Another risk factor for rupture is aneurysm growth of more than 5 mm in 6 months. Aneurysm growth correlates with smoking, forced expiratory volume in 1 second (FEV1) of less than 1.5 L/ min, female sex, and advancing age.20 The diameter at which elective surgery on the ascending aorta is recommended is considered to be 5.5 cm.10 Ascending aortic aneurysms grow faster in association with a bicuspid aortic valve (0.19 cm/yr) than with a non–bicuspid valve (0.13 cm/yr).21
Imaging Indications and Algorithm
Aneurysm and Its Extent
The questions that need to be answered are
The following information concerning the aneurysm and vascular tree needs to be determined:
The state of the axillary and iliofemoral arteries is important to note for many reasons. The iliofemoral arteries provide stent graft access and must be of a certain minimal diameter to allow the sheath to enter without complication. Bypass may use the axillary arteries. In addition, an axillary-femoral bypass may achieve distal aortic perfusion that is critical to reducing visceral ischemia during the cross-clamp period.1
Associated Abnormalities
Cardiac
The major cause of perioperative death and morbidity in patients undergoing vascular surgery for atherosclerotic arterial disease is ischemic heart disease; only 8 % of such patients have normal coronary arteries.22 It is important to identify patients with reduced contractile and perfusion reserve. Concomitant or prophylactic revascularization has not been shown to reduce postoperative outcomes from the cardiac viewpoint, but medical therapy does reduce adverse events in high-risk patients and may influence the surgical approach to reduce operating time.
In the patient with an ascending aortic aneurysm, the state of the aortic root and aortic valve requires evaluation. The degree of aortic regurgitation may be mild and might not merit a concomitant aortic valve replacement during ascending aortic aneurysm repair, but it affects the decision with regard to cross-clamping.1
Respiratory
Some of the risk factors for atherosclerotic aneurysms are also those for obstructive lung disease, the diagnosis of which can direct early attention to respiratory support in the perioperative period or favor the use of an endovascular stent graft rather than the open surgical approach. In addition, reduced FEV1 is an independent determinant of growth of an aneurysm.20
Imaging Technique and Findings
Radiography
Chest radiography can suggest the presence of an aneurysm and its complications. Aneurysms often present incidentally on chest radiography. Findings on chest radiography include dilation of the aortic contour (Fig. 92-15) and calcification. Associated cardiac abnormalities will lead to an abnormal cardiac size and configuration.23
Aneurysms of the sinus of Valsalva may mimic a mass on chest radiography. Sinus of Valsalva aneurysms are symmetric or asymmetric. Connective tissue disease typically causes symmetric dilation of the sinuses. The location of the contour abnormality or “mass” on chest radiography can predict the involved sinus. Aneurysms of the right sinus project anteriorly (best seen on the lateral view) and can erode the sternum. Aneurysms from the anterior portion of the noncoronary sinus project over the right atrium, and those from the posterior portion of this sinus project over the left atrium.24
Rupture of an aneurysm and the presence of a mediastinal hematoma are suggested by the following1: displacement of the esophagus, obscuration of the aortic contour, pleural effusion (left), and apical cap (left).
Ultrasonography
Two-dimensional echocardiography (either transthoracic or transesophageal) is not primarily used for the characterization of the aneurysm or the arterial tree. However, the transesophageal approach can provide useful information about the presence of plaque and a dissection flap. The one area not usually visualized by the more invasive but more sensitive transesophageal approach is that part of the ascending aorta overlapped by the trachea because of loss of the acoustic window.25
Echocardiography is performed for assessment of valvular function, particularly that of the aortic valve. As mentioned, aortic regurgitation may influence the decision to operate on the aortic valve concurrently. Also, in the presence of aortic regurgitation, cross-clamping should be done with caution, possibly with bypass of the left side of the heart.1 The intra-aortic balloon pump, a frequent intensive care unit support device, is contraindicated in patients with significant aortic regurgitation.
Computed Tomography
With the advent of multidetector CTA and novel contrast injection mechanisms and protocols, CTA has become the most accessible, used, and important modality for the assessment of patients with thoracic aortic aneurysms. Although the risk of radiation exposure and the nephrotoxicity of iodinated contrast medium cannot be discounted, CTA gives excellent spatial and vascular information enabling improved computer postprocessing, such as multiplanar reformation, maximum intensity projection, and volume rendering. Postprocessed viewing of data provides a surgeon or interventionalist with a valuable three-dimensional perspective of the aneurysm, its location, and its position relative to its branch vessels and adjacent structures. The combination of fast acquisition and spatial resolution enables evaluation of the arch to the femoral arteries (and even the toes, if necessary) with the administration of one contrast media bolus (“run”) and with only one breath-hold requirement. In addition, ECG-gated cardiac CT can provide information on cardiac function and the coronary arteries. CT of the aortic valve, however, does not provide direct measurement of blood flow, as can be achieved with MRI. Nonetheless, planimetry of CT data can detect and to some extent quantify aortic stenosis (e.g., aortic valve area) or aortic regurgitation (e.g., comparison of right and left ventricular volumes). CTA will assess nonvascular organs, notably the lungs, and may direct the need for pulmonary function tests.26
Images should be inspected before the administration of contrast material for signs of aortic rupture and for intramural hematoma (Fig. 92-16). Signs of aortic rupture (Fig. 92-17) include irregular aortic wall with broken intimal calcifications, periaortic and mediastinal hematoma, hemothorax (left sided for rupture of aneurysms of the descending aorta), hemopericardium (rupture of ascending aortic aneurysms), and soft tissue “stranding” in the mediastinal fat. Intramural hematoma is indicated by a crescentic high attenuation (50 to 80 HU) along the aortic wall. High attenuation within the mural thrombus is important to identify because this sign is associated with instability and subsequent rupture of aneurysms. Adjacent atelectatic lung should be distinguished from periaortic hematoma.
A distinction must be made between high attenuation within a mural thrombus and an intramural hematoma. Intramural hematoma is due to bleeding within the medial layer of the aorta and has a smooth margin and a partially circumferential and somewhat helical configuration. Intimal calcifications, if present, are displaced by the intramural hematoma toward the lumen, that is, the hematoma is peripheral to the calcification. Mural thrombus is usually chronic and adherent to the arterial wall. This has an irregular border. The intima is at the periphery of the mural thrombus, that is, the thrombus is internal to the wall of the aorta. Thrombus may calcify, giving the appearance of a displaced intima. However, the distinction can be made by the irregular edge of the thrombus.27
Infected aneurysms present as a saccular structure eccentrically from the aortic wall with rapid enlargement, periaortic gas, and erosion of the adjacent osseous structures such as the vertebral body or sternum. Associated reactive adenopathy is frequent.15
Magnetic Resonance
MRI can provide much of the same information that CT can, with no radiation and without the complications of iodinated contrast media.28 However, the use of gadolinium-based contrast agents in patients with severe renal impairment may be a problem secondary to their increased risk for nephrogenic systemic fibrosis.29 MRI is not as readily available, takes longer to perform than CTA, and may be demanding for the unstable patient. Importantly, MRI does not assess the lungs and cannot detect calcium reliably.
Angiography
The use of angiography is declining with the availability of CTA and MRA. Certain indications remain, notably the evaluation of the coronary arteries. Aortography will show the dilated aorta, although it is only a luminogram and does not provide information about the aortic wall, thus underestimating aneurysm size.30
Differential Diagnosis
From Clinical Presentation
The acute presentation of thoracic aortic aneurysm is typically ominous as it suggests aortic rupture. Patients may have chest pain that can be confused with any number of also dire thoracic conditions, such as myocardial infarction, pulmonary embolism, and aortic dissection.1 This clinical spectrum is that of acute aortic syndrome and is further discussed in Chapter 93. In most cases, however, the presentation of aortic aneurysms is often asymptomatic, detected incidentally on routine chest radiography or on echocardiography.
From Imaging Findings
The assessment of aneurysms is fairly unequivocal on cross-sectional imaging. On contrast-enhanced CT, paravertebral processes such as lymphoma and extramedullary hematopoiesis can simulate aortic rupture. Evaluation of the images obtained before the administration of contrast material should make the distinction between an inherently high-attenuation process (hematoma) and an enhancing process (malignant neoplasm).29
Enlargement of the aorta on plain radiography simulates mediastinal, pulmonary, and paracardiac masses requiring broader differential diagnostic considerations.23,24 Saccular aneurysms in particular may simulate a mediastinal mass.
Synopsis of Treatment Options
Medical
Medical treatment depends on the cause. Vasculitides such as Takayasu arteritis are treated with steroids. Appropriate antibiotics are used for infected aortic aneurysms and syphilitic aneurysms.19
Surgical/Interventional
Stent Graft
Stent grafts can be used for aneurysms in the descending thoracic aorta in both the emergent and elective scenario.31 Not all aneurysms are suitable for treatment by stents. The proximal and distal neck of the aneurysm must be long enough to provide a suitable cuff for proper deployment of a stent. The neck should not exceed 36 mm in diameter, be relatively free of plaque or thrombus, and not be excessively angulated or calcified. The proximal landing zone should be at least 15 mm and may be defined from the origin of either the left subclavian artery or left common carotid artery; if the latter is chosen, the left subclavian artery is necessarily occluded. In that situation, an ipsilateral carotid-subclavian bypass graft is performed to avoid the steal phenomenon and vertebrobasilar insufficiency. Saccular aneurysms due to trauma (including iatrogenic trauma) or penetrating ulcer, because of their focality and relative absence of disease in the proximal and distal landing zones, have the best response rate to stent graft treatment.
Stent migration is more likely to occur in those with atherosclerotic aneurysms whose aorta is more tortuous. Strategies to reduce the chances for or complications of stent migration include a longer proximal landing zone (20 mm) and the use of overlapping stents.31
An endoleak is diagnosed by the identification of contrast agent opacification of the excluded aneurysms (Fig. 92-18). Endoleaks due to failure of proximal and distal attachment (type 1) may occur immediately in the period after intervention or after a delay. Delayed type 1 endoleaks suggest graft migration. A proximal landing zone that is short, wide, angulated, ulcerated, or of reverse tapering or trapezoidal morphology predisposes to type 1 endoleak. Retrograde flow through a collateral artery such as the intercostal artery is a type 2 endoleak, which is less common after stent placement in the thoracic aorta than in the abdominal aorta. Type 3 and type 4 endoleaks (due to graft disruption or separation of components in a modular stent graft or porosity, respectively) are less common than type 1 and type 2. In the absence of a visualized endoleak, the continued expansion of the sac is classified as a type 5 endoleak or endotension.
Prosthetic Graft
A description of open surgery for thoracic aortic aneurysms is beyond the scope of this chapter. A brief description is made of the surgical principles. Imagers must be familiar with the surgery to detect complications and to avoid the misinterpretation of expected surgical findings for pathologic changes (Fig. 92-19).
The placement of a prosthetic graft may be without disruption of vascular continuity (interposition graft) or with disruption of vascular continuity (extra-anatomic bypass graft). Interposition grafts may be placed within the native artery (inclusion repair), that is, the aneurysm sac is not repaired and is peripheral to the graft. Alternatively, the aneurysm sac is resected (total repair). Repair of the ascending aorta is accompanied by root repair if the root exceeds 4.5 cm. Surgery of the aortic root may be accompanied by valve repair or coronary artery reimplantation. Surgery may involve the arch in a partial or complete manner, with or without reimplantation of the great arteries. In those with aneurysms of both the ascending and descending aorta, one strategy is a prosthetic graft repair of the ascending aorta and a stent graft repair of the descending aorta.32
Complications of Prosthetic Grafts
The most devastating postoperative complication is paraplegia. Despite progress in perioperative care, the incidence of paraplegia has not been reduced to acceptable levels. Risk factors include cross-clamp time of more than 30 minutes, hypotension, and mesenteric ischemia. Operative maneuvers such as sequential reperfusion of the intercostal arteries could be of benefit (Fig. 92-20). Preoperative localization of the artery of Adamkiewicz may be of value, and both CTA and MRA have been used for this purpose (Fig. 92-21). This artery arises from the anterior branch of the radiculomedullary artery, which is a branch of the left posterior intercostal artery (usually 9th to 12th). The diameter of the artery is 0.8 to 1.3 mm, and it has a characteristic hairpin course. Owing to its small diameter and tortuous course, high spatial resolution acquisition with the ability to render three-dimensional reformations is critical.33
Reporting: Information for the Referring Physician
Nonvascular Findings
Nonvascular findings are described, such as the lungs and solid abdominal viscera. For lung nodule follow-up, the guidelines of the Fleischner Society are used.34
KEY POINTS
 Aneurysms of the ascending aorta are usually caused by connective tissue disease such as Marfan syndrome and involve the aortic root.
Aneurysms of the ascending aorta are usually caused by connective tissue disease such as Marfan syndrome and involve the aortic root. Aortic root dilation is accompanied by aortic regurgitation, which may require concomitant valve repair.
Aortic root dilation is accompanied by aortic regurgitation, which may require concomitant valve repair. Aneurysms of the descending thoracic aorta are amenable to endovascular stent graft placement, particularly those caused by penetrating atherosclerotic ulcer or trauma.
Aneurysms of the descending thoracic aorta are amenable to endovascular stent graft placement, particularly those caused by penetrating atherosclerotic ulcer or trauma. The baseline imaging modality (CTA or MRA) should be similar to the modality used for postoperative surveillance.
The baseline imaging modality (CTA or MRA) should be similar to the modality used for postoperative surveillance. Description of the artery of Adamkiewicz is of value in patients undergoing open repair of a descending thoracic aortic aneurysm.
Description of the artery of Adamkiewicz is of value in patients undergoing open repair of a descending thoracic aortic aneurysm.Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816-828.
Johnson PT, Chen J, Loeys B, et al. Loeys-Dietz syndrome: MDCT angiography findings. Am J Radiol. 2007;189:W29-W35.
Macedo TA, Stanson AW, Oderich GS, et al. Infected aortic aneurysms: imaging findings. Radiology. 2004;231:250-257.
Patel HJ, Deeb GM. Ascending and arch aorta: pathology, natural history, and treatment. Circulation. 2008;118:188-195.
Pyeritz RE. The Marfan syndrome. Annu Rev Med. 2000;51:481-510.
Zilocchi M, Macedo TA, Oderich GS, et al. Vascular Ehlers-Danlos syndrome: imaging findings. AJR Am J Roentgenol. 2007;189:712-719.
1 Curci JA. Arterial aneurysms—etiologic considerations. In: Rutherford RB, editor. Vascular Surgery. 6th ed. Philadelphia: WB Saunders; 2005:475-492.
2 Bickerstaff LK, Pairolero PC, Hollier LH, et al. Thoracic aortic aneurysms: a population-based study. Surgery. 1982;92:1103-1108.
3 Aronberg DJ, Glazer HS, Madsen K, Sagel SS. Normal thoracic aortic diameters by computed tomography. J Comput Assist Tomogr. 1984;8:247-250.
4 Schoen FJ. Blood vessels. In: Robbins SL, Cotran RS, editors. Pathologic Basis of Disease. 7th ed. Philadelphia: WB Saunders; 2004:511-555.
5 Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17-27.
6 Brinster DR, Rizzo RJ, Bolman RMIII. Ascending aortic aneurysms. In: Kohn LH, editor. Cardiac Surgery in the Adult. 3rd ed. New York: McGraw-Hill; 2007:1223-1250.
7 Pyeritz RE, Dietz HC. The Marfan syndrome and other microfibrillar disorders. In: Royce PM, Steinman B, editors. Connective Tissue and Its Heritable Disorders. 2nd ed. New York: Wiley-Liss; 2002:585-626.
8 Dietz HC, Pyeritz RE, Hall BD, et al. The Marfan syndrome locus: confirmation of assignment to chromosome 15 and identification of tightly linked markers at 15q15-q21.3. Genomics. 1991;9:355.
9 Marsalese DL, Moodie DS, Vacante M, et al. Marfan’s syndrome: natural history and long-term follow-up of cardiovascular involvement. J Am Coll Cardiol. 1989;14:422.
10 Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877-S1880.
11 Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-ß receptor. N Engl J Med. 2006;355:788-798.
12 Germain DP. Clinical and genetic features of vascular Ehlers-Danlos syndrome. Ann Vasc Surg. 2002;16:391-397.
13 Mirvis SE, Shanmuganathan K, Miller BH, et al. Traumatic aortic injury: diagnosis with contrast-enhanced thoracic CT—five-year experience at a major trauma center. Radiology. 1996;200:413-422.
14 Feigl D, Feigl A, Edwards JE. Mycotic aneurysms of the aortic root: a pathologic study of 20 cases. Chest. 1986;90:553.
15 Hsu RB, Lin FY. Infected aneurysm of the thoracic aorta. J Vasc Surg. 2008;47:270-276.
16 Procter CD, Hollier LH. Takayasu’s arteritis and temporal arteritis. Ann Vasc Surg. 1992;6:195-198.
17 Bonderman D, Gharehbaghi-Schnell E, Wollenek G, et al. Mechanisms underlying aortic dilatation in congenital aortic valve malformation. Circulation. 1999;99:2138.
18 Crawford ES, Crawford JL, Safi HJ, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg. 1986;3:389-404.
19 Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816-828.
20 Cambria RA, Gloviczki P, Stanson AW, Cherry KJJr. Outcome and expansion rate of 57 thoracoabdominal aortic aneurysms managed nonoperatively. Am J Surg. 1995;170:213-217.
21 Davies RR, Kaple RK, Mandapati D, et al. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg. 2007;83:1338-1344.
22 Hertzer NR, Beven EG, Young JR, et al. Coronary artery disease in peripheral vascular patients: a classification of 1000 coronary angiograms and results of surgical management. Ann Surg. 1984;199:223-233.
23 Miller WT. Thoracic aortic aneurysms: plain film findings. Semin Roentgenol. 2001;36:288-294.
24 Ominsky SH, Kricun ME. Roentgenology of sinus of Valsalva aneurysms. AJR Am J Roentgenol. 1975;125:571-581.
25 Scott CH, Keane MG, Ferrari VA. Echocardiographic evaluation of the thoracic aorta. Semin Roentgenol. 2001;36:325-333.
26 Nguyen BT. Computed tomography diagnosis of thoracic aortic aneurysms. Semin Roentgenol. 2001;36:309-324.
27 Macura KJ, Corl FM, Fishman EK, Bluemke DA. Pathogenesis in acute aortic syndromes: aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. AJR Am J Roentgenol. 2003;181:309-316.
28 Roberts DA. Magnetic resonance imaging of thoracic aortic aneurysm and dissection. Semin Roentgenol. 2001;36:295-308.
29 http://www.fda.gov/cder/drug/infopage/gcca/default.htm.
30 Angiography of the aorta and peripheral arteries. In: Baim DS, editor. Grossman’s Cardiac Catheterization, Angiography, and Intervention. Philadelphia: Lippincott Williams & Wilkins; 2005:254-281.
31 Garzón G, Fernández-Velilla M, Martí M, et al. Endovascular stent-graft treatment of thoracic aortic disease. Radiographics. 2005;25:S229-S244.
32 Sundaram B, Quint LE, Patel HJ, Deeb GM. CT findings following thoracic aortic surgery. Radiographics. 2007;27:1583-1594.
33 Yoshioka K, Niinuma H, Ohira A, et al. MR angiography and CT angiography of the artery of Adamkiewicz: noninvasive preoperative assessment of thoracoabdominal aortic aneurysm. Radiographics. 2003;23:1215-1225.
34 MacMahon H, Austin JH, Gamsu G, et al. Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237:395-400.

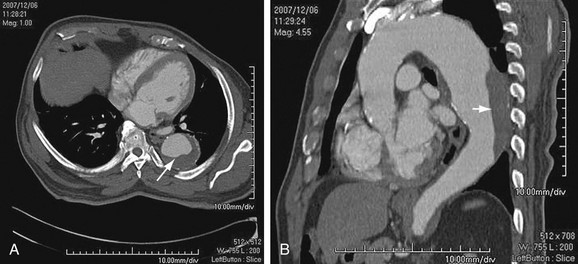
 FIGURE 92-1
FIGURE 92-1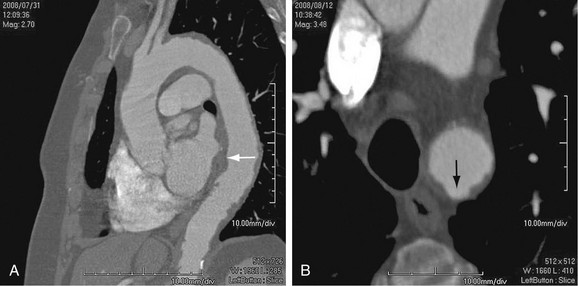
 FIGURE 92-2
FIGURE 92-2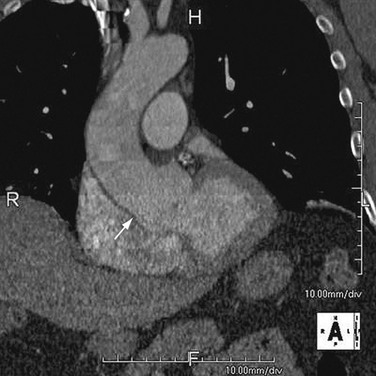
 FIGURE 92-3
FIGURE 92-3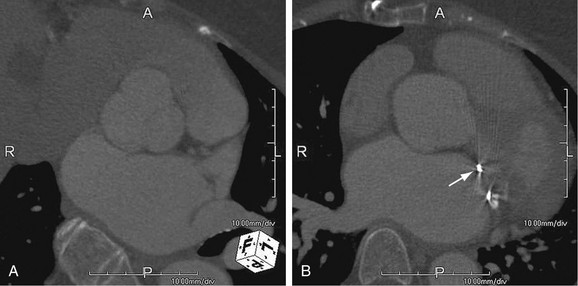
 FIGURE 92-4
FIGURE 92-4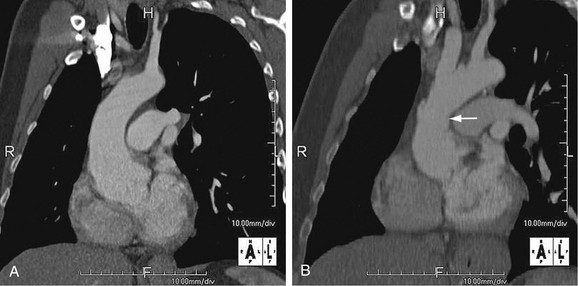
 FIGURE 92-5
FIGURE 92-5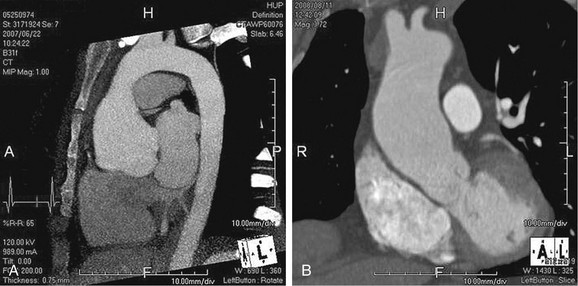
 FIGURE 92-6
FIGURE 92-6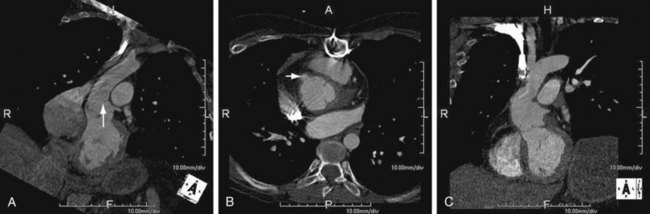
 FIGURE 92-7
FIGURE 92-7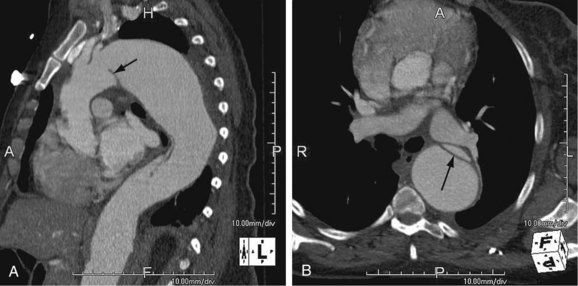
 FIGURE 92-8
FIGURE 92-8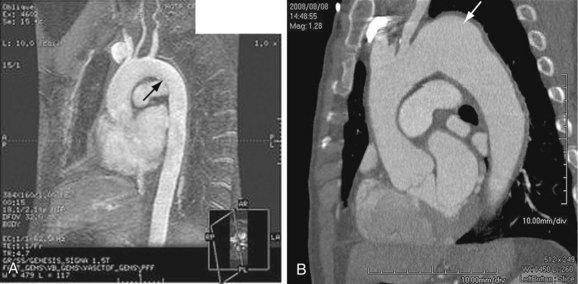
 FIGURE 92-9
FIGURE 92-9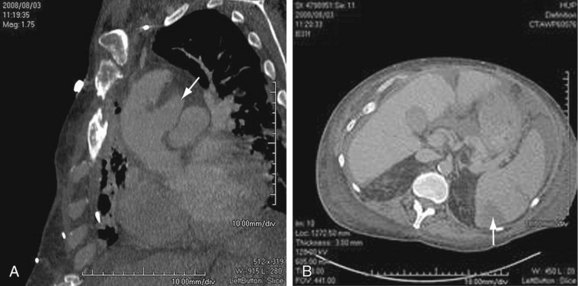
 FIGURE 92-10
FIGURE 92-10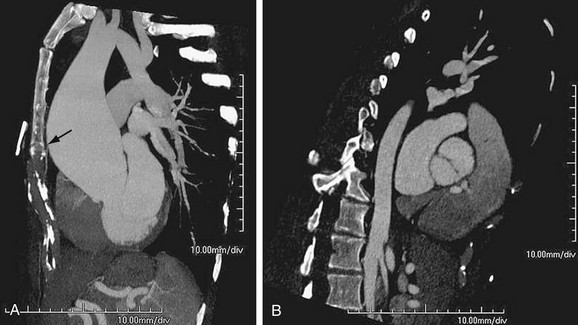
 FIGURE 92-11
FIGURE 92-11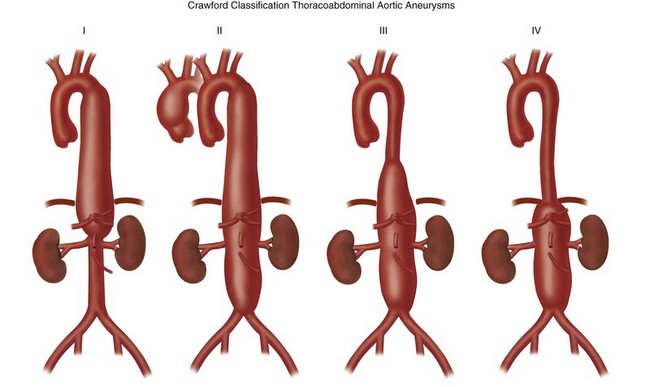
 FIGURE 92-12
FIGURE 92-12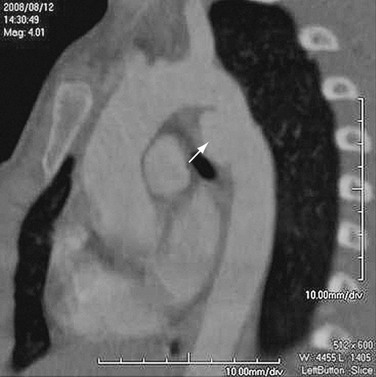
 FIGURE 92-13
FIGURE 92-13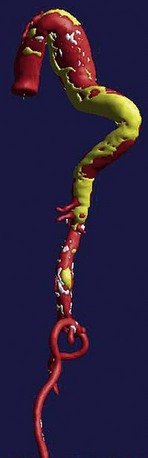
 FIGURE 92-14
FIGURE 92-14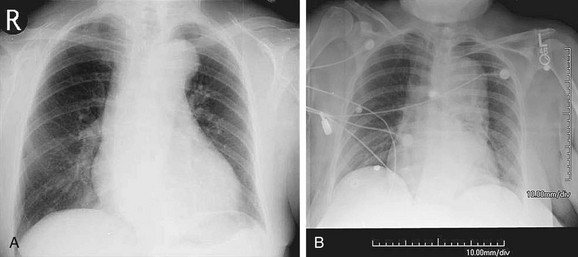
 FIGURE 92-15
FIGURE 92-15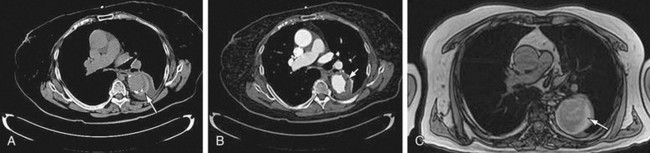
 FIGURE 92-16
FIGURE 92-16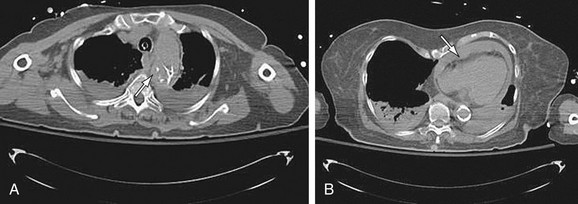
 FIGURE 92-17
FIGURE 92-17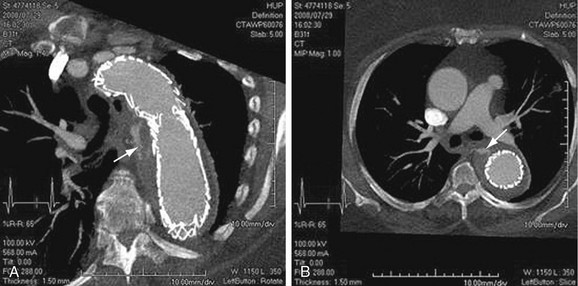
 FIGURE 92-18
FIGURE 92-18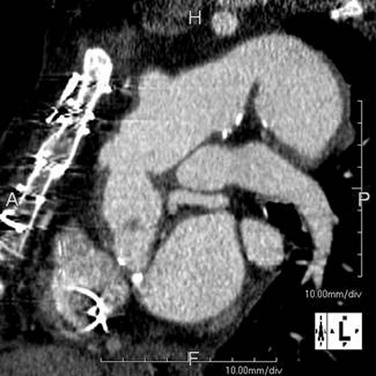
 FIGURE 92-19
FIGURE 92-19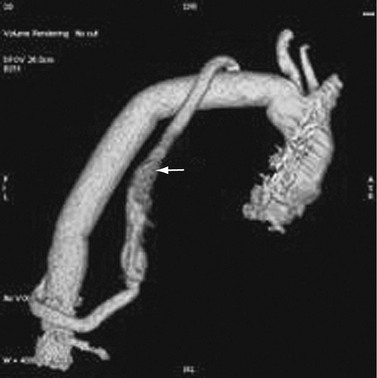
 FIGURE 92-20
FIGURE 92-20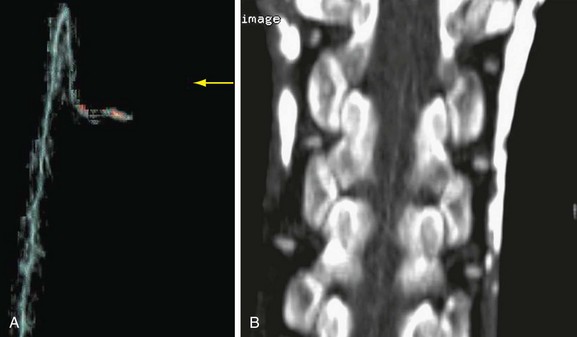
 FIGURE 92-21
FIGURE 92-21




