Chapter 134
Thoracic and Thoracoabdominal Aortic Aneurysms
Evaluation and Decision Making
Gilbert R. Upchurch Jr.,
Aortic diseases, including aortic aneurysms, are the 12th leading cause of death in the United States.1 Although abdominal aortic aneurysms (AAAs) and ascending aortic aneurysms are more common, descending thoracic aortic aneurysms (TAAs) and thoracoabdominal aortic aneurysms (TAAAs) are not rare, with an estimated incidence of 5.9 cases per 100,000 person-years.2 A study by Clouse and associates suggested that this incidence is increasing and is closer to 10.4 cases per 100,000.3 TAA repair is associated with a high morbidity and mortality. Even though TAAs, including those of the ascending aorta, aortic arch, and descending aorta, course anatomically from the ascending aorta to the diaphragm, the focus of this chapter is on descending TAAs and TAAAs. TAAAs, which can occur from the left subclavian artery to the aortic bifurcation, are defined by anatomic criteria.
Thoracic aortic disease is complex and induced by a myriad of histopathologic processes. Most TAAs and TAAAs are secondary to either medial degeneration or aortic dissection. Medial degeneration is characterized by disruption and loss of elastic fibers and increased deposition of proteoglycans. Many other thoracic aortic diseases, including acute aortic syndromes (aortic dissection, intramural hematoma, and penetrating aortic ulcers) and various forms of vasculitis (giant cell arteritis, Takayasu’s arteritis, and Behçet’s disease), can induce TAAAs. Whereas isolated AAAs were once called atherosclerotic, TAAs associated with frank atherosclerosis are much rarer. Other histopathologic processes associated with thoracic aortic disease include coarctations, various brachiocephalic anomalies (i.e., aberrant right subclavian arteries), and tumors. Disastrous complications of thoracic aortic disease also include pulmonary and enteric fistulae.
This chapter is intended to provide an overview of thoracic aortic disease to help the caregiver of patients with these pathologic conditions in decision making. Guidelines for the diagnosis and management of patients with thoracic aortic disease have recently been published4; contemporary reviews on the management of thoracic and thoracoabdominal aortic aneurysms are also available.5–7
Thoracic Aorta: Anatomy and Epidemiology of Thoracic and Thoracoabdominal Aortic Aneurysms
Whereas the techniques of open and endovascular TAAA repair are discussed in more detail in Chapters 135 and 136, it is important to provide a description of the normal anatomy as well as of the many and varied pathologic changes that are associated with TAAs (Fig. 134-1).
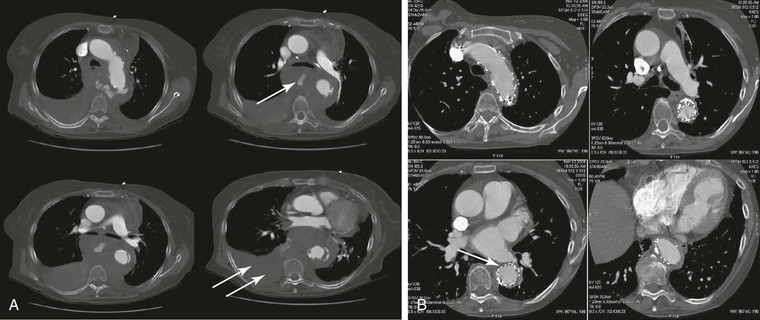
Figure 134-1 A, Multiple CT scan levels documenting a ruptured thoracoabdominal aortic aneurysm (TAAA). The single arrow denotes dissection associated with the rupture. The double arrow points to a right-sided pleural effusion. B, A repeated CT scan 8 months after placement of a thoracic endograft shows resolution of the effusion and exclusion of the TAAA. The arrow denotes a stent-graft.
Definition
TAAAs are localized dilatations in the thoracic and abdominal aorta secondary to weakening and subsequent expansion of the aortic wall. A TAAA by definition is a dilatation at least 1.5 times its normal value.8 When all aneurysms of the thoracic aorta are considered, those of the ascending aorta are the most common (40%). Aneurysms of the descending thoracic aorta account for 35% of TAAs, whereas aortic arch aneurysms (15%) and TAAAs (10%) account for a smaller percentage (Table 134-1).2,9 Defining these anatomic aortic sizes is critical to help identify pathologic aortic growth because TAAA diameter is the strongest predictor of rupture, with a reported mean aortic diameter of ruptured TAAAs of 6.1 cm.10
Normal and Pathologic Aortic Size
The normal thoracic aorta is divided into four parts: the aortic root, the ascending aorta, the aortic arch, and the descending thoracic aorta.4 The normal thoracic aortic wall is composed of three layers, similar to all blood vessels, which include the intima, media, and adventitia. The aorta normally enlarges as it progresses from the aortic root to the terminal aorta.9 In addition, gender, age, and body surface area influence aortic diameter (Table 134-2). Even after adjustment for age and body surface area, mean aortic size is significantly smaller, usually 2 to 3 mm, in women than in men.11–14 Body surface area is reported to be a better predictor of aortic diameter than height or weight.14 Normal and pathologic growth rates are also important. A mean increase in TAAA cross-sectional diameter of 0.4 cm/y has been reported.10 Importantly, the growth rates of TAAAs are not predictable or linear. However, there is consensus that TAAAs, similar to AAAs, have growth rates that accelerate as they enlarge.15 For example, TAAAs larger than 5 cm expand at a growth rate of 0.79 cm/y, whereas TAAAs less than 5 cm experience growth rates of 0.17 cm/y.10 Dapunt and associates documented that patients with ruptured TAAAs experienced growth rates of 0.7 cm/y.10
Table 134-2
Normal Adult Thoracic Aortic Diameters by Gender4
| Thoracic Aorta | Range of Reported Mean (cm) | Assessment |
| Mid-descending, female | 2.45-2.64 | CT |
| Mid-descending, male | 2.39-2.98 | CT |
| Diaphragmatic, female | 2.40-2.44 | CT |
| Diaphragmatic, male | 2.43-2.68 | CT |
CT, Computed tomography.
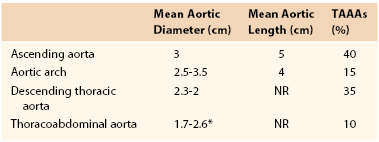
The terms saccular and fusiform should also be defined for TAAAs because the morphology of an aneurysm will often dictate treatment options as well as indicate specific causes (i.e., mycotic aneurysms are often saccular and at high risk of rupture).16 Most TAAAs are fusiform, a chronic uniform dilatation involving the whole circumference of the aorta. In contrast, saccular aneurysms often represent an eccentric dilatation of the aorta (Fig. 134-2). The size at which eccentric saccular aneurysms are repaired has not been well studied; however, most would concur that a lower threshold is an acceptable indication for repair.
Population Affected
TAAAs are primarily a disease of the elderly. The average age of patients with TAAAs is 65 years, with a male-to-female ratio of 1.7 : 1.2 In contrast, in patients with AAAs whose mean age is 75 years, the male-to-female ratio is 6 : 1.17 TAAAs clearly have a genetic component in that more than 20% of patients will have a first-degree relative affected by aneurysm disease.18–20
Risk Factors for Disease and Rupture
Many risk factors common in patients with AAAs, including hypertension, smoking, and atherosclerosis in other arteries, are also common in patients with TAAAs.2,21–23 Although TAAAs are most often described as degenerative, in up to 20% of patients they are the sequelae of chronic aortic dissection,24 wherein aneurysm dilatation of the outer wall of the false lumen necessitates late aortic repair in up to 40% of patients irrespective of initial medical or surgical treatment of the dissection. Systemic hypertension (in particular, elevated diastolic blood pressure higher than 100 mm Hg) has been associated with aortic growth and rupture.10,25
Multiple genetic syndromes, in addition to the standard connective tissue syndromes such as Marfan’s syndrome, have been associated with TAAs and dissections. Familial aggregation studies have shown that between 11% and 19% of patients referred for TAA or dissection repair have a first-degree relative with a TAA or dissection.21,26,27 Patients with a strong family history of TAA tend to present at a younger age than those without a positive history and yet at an older age than those with connective tissue disease. This disease appears to be inherited in an autosomal dominant fashion with variable expression.28 Mapping studies have shown that there is significant genetic heterogeneity in these patients with familial TAAs and dissection (TAAD). Causative genes have been identified for TAAD2 (due to TGFBR2 mutation), 16p (due to MYH11 mutation), and TAAD4 (due to ACTA2 mutation), suggesting that mutations to the basic aortic wall building blocks of actin and myosin are responsible for these syndromes.29–31 Further studies have suggested that rare copy number variants disrupt genes responsible for smooth muscle cell adhesion and contraction in patients with both sporadic and familial TAA disease.32 The genetics of TAAs and dissections is the subject of many contemporary reviews.33,34
Because most patients with TAAAs are asymptomatic, treatment is aimed at preventing rupture. Natural history studies of TAAAs are rarer than those of isolated infrarenal AAAs, probably related to their much less frequent occurrence. In addition, studies on TAAAs often include patients with both acute and chronic aortic dissection, which serves to complicate natural history data. Initial studies from the 1970s by Pressler and McNamara documented that approximately 40% of patients who did not undergo surgical repair died of TAAA rupture, whereas 32% died of other cardiovascular diseases. Mean survival was less than 3 years. During the extended period of observation, more than 90% of patients sustained aortic rupture, with 68% of ruptures occurring more than 1 month after the diagnosis.35,36 The 5-year survival rate for patients with 6.0-cm TAAAs is 54%, with a risk of rupture of 3.7%/y and a risk of death of 12%/y. Median survival in patients with untreated TAAAs is poor at only 3.3 years.37 In a natural history study of patients who were not candidates for surgery by Crawford et al, the survival rate was just 24% at 2 years, with more than half the deaths related to aneurysm rupture. Chronic obstructive pulmonary disease (COPD) was noted in 80% of the subgroup with rupture.38 Similar studies in patients with small infrarenal AAAs have confirmed COPD as a significant risk factor for rupture.39 Cambria and colleagues followed a series of 57 patients with TAAAs who were not considered operative candidates. In addition to COPD, the authors found an association (P = .06) between rupture and chronic renal failure.40 In a study by Griepp and colleagues, 165 patients with TAAAs were monitored after being assigned nonoperative status. Twenty percent of patients eventually suffered rupture. Important risk factors for rupture included older age, COPD, uncharacteristic continued pain, and aortic diameter. Patients with aortic dissection ruptured at smaller aortic diameters than did those with nondissected degenerative aneurysms.41
Aneurysm diameter is the most important risk factor for rupture. Dapunt and coworkers documented that TAAAs larger than 8 cm have an 80% risk of rupture within 1 year of diagnosis.10 However, the size at which TAAAs rupture is unpredictable. Similar to AAAs, it appears that aneurysm growth rates play a role. The average expansion rate of a TAAA is approximately 0.10 to 0.42 cm/y.37,42–44 Coady and associates suggested that expansion by more than 1 cm/y signals impending rupture.42 Juvonen and colleagues examined 114 patients with TAAAs in detail.45 Multivariate analysis suggested that increasing age, pain (even atypical), COPD, descending thoracic aortic diameter, and abdominal aortic diameter were predictive of rupture (Table 134-3).
Table 134-3
Risk Factors for Thoracoabdominal Aortic Aneurysm Rupture45
| Risk Factor | Relative Rate | P Value |
| Chronic obstructive pulmonary disease | 3.6* | .004 |
| Age | 2.6† | .02 |
| Pain | 2.3 | .04 |
| Descending aortic diameter | 1.9 | .003 |
| Abdominal aortic diameter | 1.50‡ | .05 |
* The relative rate increases by a factor of 1.9 for each centimeter of descending aortic diameter.
† The relative rate increases by a factor of 2.6 for each decade of age.
‡ The relative rate increases by a factor of 1.5 for each centimeter of abdominal aortic diameter.
Incidence and Prevalence
The incidence and prevalence of TAAAs have been increasing during the last several decades.2 Clouse and coauthors documented that the incidence of TAAAs in the United States is 10.4 cases per 100,000.3 Olsson examined the prevalence of TAAAs in a large contemporary population. All subjects with thoracic aortic dissections or aneurysms from 1987 to 2002 were identified in the Swedish National Healthcare Registry. Of 14,229 individuals with thoracic aortic disease, the diagnosis was made in 11,039 (78%) before death. The incidence of thoracic aortic disease rose by 52% in men and 28% in women to reach 16.3 and 9.1 per 100,000 per year, respectively. The authors concluded that the prevalence and incidence of thoracic aortic disease were higher than previously reported and increasing (Fig. 134-3).46 The increasing prevalence of TAAAs has been attributed to a number of factors, including improved imaging techniques, an aging population, and increased patient and physician awareness.47
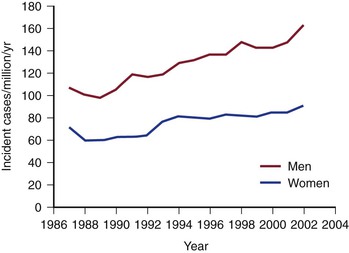
Figure 134-3 Increasing incidence of thoracoabdominal aortic aneurysms and dissection in the Swedish population (men and women), 1987 to 2002 (cases per million). (From Olsson C, et al: Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 114:2611-2618, 2006.)
Pathogenesis
The development of a TAAA is a multifactorial event that involves a complex interaction of genetic factors, cellular imbalance, and altered hemodynamic factors.48 The increased incidence of TAAAs in patients with Marfan’s syndrome and other connective tissue disorders inherited in a mendelian manner as well as the familial inheritance of nonsyndromic TAADs typically inherited in an autosomal dominant fashion with marked variability in the age at onset and decreased penetrance suggests a varying genetic role along different segments of the aorta.28–31 More recent studies have suggested that genetic variation in extracellular matrix (ECM) actin and myosin may contribute to the development of TAAAs.49,50 Wang and coauthors examined genetic signatures in the peripheral blood of patients with TAAAs (n = 58) and control patients (n = 36). This study documented a number of gene families that were different between the two groups, including those involved in the cell cycle, DNA metabolism, glycolysis, interferon-γ signaling, and transcription. The authors concluded that analysis of peripheral blood to determine expression signatures may help identify patients with TAAAs with high accuracy.51
Formation of TAAAs, similar to that of other aneurysms, is therefore a complicated, dynamic process involving both extracellular and cellular processes (see Chapter 129). Once the process is initiated through a combination of the aforementioned factors, inflammation and pathologic remodeling of the ECM occur. A large body of evidence suggests that ECM degradation by matrix metalloproteinases (MMPs) exceeds matrix production and repair during aneurysm formation. Although the biomechanical properties and composition of the aorta, including the role of elastin and collagen in the arterial matrix, are discussed in Chapter 129, it is important to emphasize that there are significant differences in the composition of the aortic wall as one progresses from the ascending aorta to the iliac bifurcation. Andreotti and colleagues documented that the ascending aorta has a greater concentration of elastin and is therefore more compliant than the descending aorta. This alteration in elastin concentration leads to a progressive decrease in the elastin-collagen ratio as the aorta progresses from the ascending aorta to the abdominal aorta.52 The media also becomes thinner as one progresses from proximal to distal along the aorta.53
A number of studies have documented the overexpression and increased activity of various ECM proteinases, specifically the MMPs, in human TAAAs.54 These proteolytic enzymes, which have been more extensively studied in AAAs, are clearly critical as well during the formation of TAAAs. Sinha and coworkers documented asymmetrical production of MMP9 in the expanding human TAAA wall, which correlated with increased numbers of macrophages.55 In contrast, MMP2 was documented to be increased in the wall of the TAAA that was preserved, in particular, at the point where smooth muscle cells were more abundant and the wall was preserved. Ikonomidis and associates studied the role of MMPs in a murine model of TAAAs.54 These authors documented that MMP9 gene deletion attenuated TAA formation despite increases in MMP2 activity. They concluded that interactions between MMP9 and MMP2 are necessary to facilitate TAAA progression. Studies by Ikonomidis and others have documented altered membrane type 1 MMP and tissue inhibitor of MMP2 (TIMP2, an inhibitor of MMPs) in the murine TAA model.56
Aortic Wall Histology
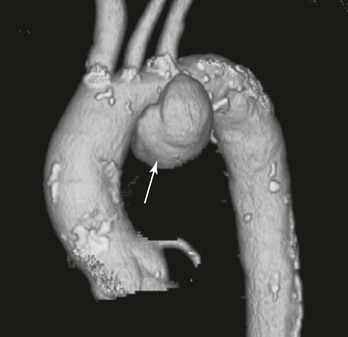
The histology of TAAAs is most closely associated with medial degeneration, formerly known as cystic medial necrosis (Fig. 134-4). Medial degeneration is characterized by fragmentation and loss of elastic fibers; loss of smooth muscle cells; and collections of interstitial collagenous tissue, basophilic ground substance, and proteoglycans.57,58 Although medial degeneration is considered part of the normal aging process, it is accelerated by certain clinical conditions, such as hypertension and atherosclerosis.59 Given the relatively widespread process along the thoracic aorta, medial degeneration is most closely associated with the development of fusiform aneurysms. Genetic abnormalities, such as Marfan’s syndrome, also accelerate aortic medial degeneration.60 Although TAAAs were originally described as noninflammatory, studies have shown that infiltration of leukocytes contributes to the formation and development of TAAAs. The significant difference in the epidemiology and histology of TAAAs and AAAs suggests distinct causes.
Etiology
Eighty percent of TAAAs are secondary to medial degeneration; approximately 15% to 20% are caused by aortic dissection (Box 134-1) (see Chapter 138). TAAAs secondary to aortic dissection typically occur in younger patients and involve more extensive aortic segments than do degenerative aneurysms.61 The aorta in patients with Marfan’s syndrome is particularly prone to aortic dissection and subsequent TAAA formation.62 Both systemic autoimmune disorders, such as Takayasu’s arteritis, and chronic nonspecific aortitis can destroy the aortic media with progressive aneurysm formation. Aneurysms associated with arteritis are seen in women more commonly than degenerative aneurysms are. Aneurysms of the upper thoracic aorta can also be secondary to congenital aortic coarctations, either in unrepaired coarctations or after repair.63–65
TAAAs may also form secondary to infection. Although saccular aneurysms have been described as mycotic, these aneurysms are most often bacterial and occur because of hematogenous spread of emboli laden with bacteria. Infected TAAAs usually arise as a result of seeding of atherosclerotic plaque in the aorta, the development of a focal inflammatory process in the aortic wall, and ultimately the formation of a false aneurysm. Infected TAAAs present many challenging dilemmas. The goals of therapy are to debulk the infection and to restore arterial continuity. For years this has involved in situ repair with its attendant lifetime risk of reinfection. Recent reports of treatment of infected TAAAs with endovascular prostheses are beginning to accumulate.66,67
Anatomic Classification
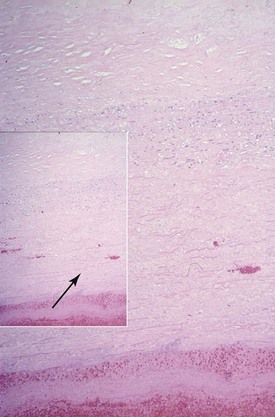
Evaluation of a patient with a TAAA as well as the technical performance of either open or endovascular repair is closely aligned with the Crawford classification (Fig. 134-5).68–70 Classification of TAAAs also has important therapeutic implications for the operation to be performed as well as for the risk of specific complications. Type I TAAAs account for approximately 25% of all TAAAs; they involve the entire descending thoracic aorta and extend only to the upper abdominal aorta. Type II TAAAs (approximately 30% of TAAAs) involve the entire descending thoracic aorta and most or all of the abdominal aorta (Fig. 134-6A). Type III TAAAs (less than 25%) involve variable lengths of the descending thoracic aorta and extend into the abdominal aorta (Fig. 134-6B). Type IV TAAAs (<25%) are limited to most or all of the abdominal aorta, including the visceral and renal arteries.
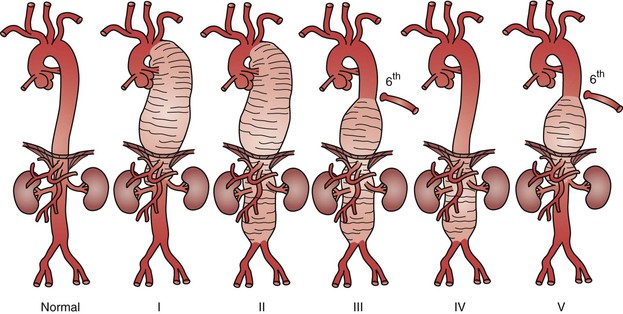
Figure 134-5 Crawford classification of thoracoabdominal aortic aneurysms. Type I, distal to the left subclavian artery to above the renal arteries. Type II, distal to the left subclavian artery to below the renal arteries. Type III, from the sixth intercostal space to the renal arteries. Type IV, from the 13th intercostal space to the iliac bifurcation (entire abdominal aorta). Type V, below the sixth intercostal space to just above the renal arteries.
Clinical Findings
History and Physical Examination
Most patients with TAAAs have no symptoms attributable to their disease at the time of diagnosis.23,36,71 The diagnosis is therefore often made when the patient is being evaluated for unrelated conditions. Although most patients with TAAAs are often asymptomatic, most aneurysms will become symptomatic before they rupture.71 Panneton et al71 documented that 57% of patients with degenerative TAAAs will have symptoms before rupture. The recent American Heart Association guideline for thoracic aortic disease has issued recommendations regarding the history and physical examination for the general approach to the patient with suspected thoracic aortic disease4 (Box 134-2). The authors have suggested on the basis of class I, level C evidence that “the clinician should perform a focused physical examination, including a careful and complete search for arterial perfusion differentials in both upper and lower extremities, evidence of visceral ischemia, focal neurologic deficits, a murmur of aortic regurgitation, bruits, and findings compatible with possible cardiac tamponade.”72–74
The most common initial symptom in patients with TAAAs is vague pain, which can occur in the chest, back, flank, or abdomen. The differential diagnosis in a patient with a symptomatic TAAA therefore includes angina, aortic dissection, and degenerative disease of the spine. The chronic pain associated with TAAAs may easily be dismissed in patients with TAAAs before the diagnosis of a TAAA has been made. Typical of most aneurysms when they are enlarging, pain may increase in severity dramatically. Juvonen and coworkers suggested that the back pain associated with a TAAA can indeed be chronic and have a pattern and implication different from those noted with AAAs, in which the back pain is more often acute and suggests pending rupture.45 Symptoms may also occur in patients with TAAAs from compression of the thoracic aorta by structures in the thoracic cavity. Hoarseness can develop in patients with TAAAs as a result of stretching or compression of the left recurrent laryngeal nerve. Tracheal deviation, persistent cough, or other respiratory symptoms may also be present.35,75 Dysphagia is an uncommon, nonspecific complaint reported by patients with TAAAs that is due to compression of the aneurysm by the esophagus.76 Sudden and catastrophic hemoptysis or hematemesis may occur as a result of erosion of the TAAA into the bronchial and pulmonary space or the esophagus, respectively. Patients with TAAAs may rarely have neurologic deficits, including paraplegia. This is much more common in those with aortic dissection. Embolization to the visceral, renal, and lower extremity arteries has been reported.77 Patients with an abdominal component of their aneurysm may have gastrointestinal hemorrhage, aortoenteric fistulae, or a functional small bowel obstruction secondary to compression of the duodenum. Most patients with symptoms have TAAAs that have attained a diameter greater than 5 cm.
Patients with TAAAs generally have no obvious physical findings in the chest area unless tracheal deviation is present.78 Patients with an abdominal component of their TAAA may have a pulsatile abdominal mass similar to pure AAAs. No controlled or blinded study has been performed that reproducibly stratifies patients with TAAAs into groups predictive of poor outcomes.4 The critical issue is to identify those patients who are acutely ill from their thoracic aortic disease and to triage them accordingly.
Synchronous Aneurysm Risk
Studies examining the natural history of patients with TAAAs indicate that between 20% and 30% will also have an AAA.35,79 In a series of 1500 patients, Crawford et al documented that 13% harbored aneurysms in other segments.80 This appears to be true of patients who undergo TAAA repair as well, of whom as many as a third will have undergone previous aortic repair.81 Most commonly, patients with TAAAs have undergone previous infrarenal AAA repair. Patients with TAAAs are also at risk of discontinuous aneurysms or aneurysms proximal to their TAAA. Synchronous proximal ascending and arch aneurysms are noted in 6% to 13% of patients with TAAAs.71 Even after repair, at least 10% of patients with TAAAs will require subsequent aneurysm repair in noncontiguous aortic segments.82 Patients with Marfan’s syndrome who have suffered a type A dissection appear to be at high risk for this type of manifestation. As discussed in subsequent chapters, there is clearly an increasing trend in treating complex arch aneurysms and TAAAs with sequential open and endovascular therapies.83–85
Diagnostic Evaluation
Imaging
Accurate and precise determination of the anatomy of a patient with a TAAA is mandatory in determining both the need for and the details of operative repair as well as the prescribed follow-up in patients with TAAAs who have not reached a diameter threshold for repair. Recommendations for aortic imaging techniques to determine the presence of progression of thoracic aortic disease have been modified and are summarized in Box 134-3.
Patients with TAAAs frequently have significant coexisting medical conditions, including hypertension, coronary artery disease, COPD, congestive heart failure, cerebrovascular occlusive disease, and peripheral vascular occlusive disease. Cross-sectional imaging, mainly by computed tomography (CT), is the primary method to visualize the thoracic and abdominal aorta for determining aneurysm diameter and extent. Because most TAAAs are small when they are discovered, serial imaging is required; however, level A and B evidence regarding the timing of surveillance is lacking. Serial imaging is typically performed at 6- to 12-month intervals but varies by the initial diameter and extent of the aneurysm.
Chest Radiography
A chest radiograph (Fig. 134-7) or plain films of the abdomen in patients with TAAA may suggest an enlarged thoracic aorta secondary to a calcified aortic wall. Indirect findings suggestive of TAAA on chest radiography include a widened mediastinum, enlargement of the aortic knob, and tracheal deviation. Whereas chest films are part of the routine preoperative workup of every patient going for TAAA repair, they are inadequate to definitely exclude the presence or absence of thoracic aortic disease. A study of data pooled from 10 studies suggested that the predictive sensitivity of a widened mediastinum or an abnormal aortic contour associated with thoracic aortic disease is 64% and 71%, respectively.86 In contrast, a prospective study of more than 200 patients with thoracic aortic disease documented a specificity of 86% for chest radiography.87
Computed Tomography
Spiral CT scanning with three-dimensional reconstructions is now the “gold standard” for evaluating the aorta in patients with TAAAs as it helps diagnose the pathologic process as well as measure the aortic diameter. New-generation multidetector CT scans have been shown to have sensitivities and specificities of well above 95%.88–91 Spiral CT using 360-degree rotation of the x-ray beam source can document the extent of the aortic aneurysm and provide accurate serial measurement of aneurysm diameter. Computer programs can generate sagittal, coronal, and oblique images as well as three-dimensional renderings that can be used to determine whether a patient is a candidate for a stent-graft.92 A general assessment of other organs in the chest, abdomen, and pelvis is also obtained with CT, which allows the detection of other pathologic processes, including cancer, that might affect patient management and surgical planning.93,94 In patients with renal insufficiency, CT without iodinated contrast agents can be useful to determine TAAA size or extent but will not provide accurate data about the atherosclerotic burden of branch vessels or the presence of a dissection. It is my general impression, supported by studies,95 that given the standard use of low-osmolar, smaller boluses of contrast material in performing computed tomographic angiography (CTA), contrast-related acute renal failure after CTA is in fact rare and, when it does occur, mild.
CTA also importantly allows other essential information to be obtained, including assessment of occlusive disease in branch vessels. In addition, CTA documents the patency of important intercostals that might be reimplanted into the aortic graft if extensive open TAAA repair is being considered. CTA further documents the presence of thrombus, inflammatory changes, dissection, and retroperitoneal blood suggestive of a ruptured aneurysm. The relative advantages of CT scanning over magnetic resonance imaging (MRI) include its being less expensive, quicker to perform and thus less claustrophobia inducing, useful in patients with previous implanted ferromagnetic devices, and widely available.
A word of caution is needed regarding CTA and the timing of TAAA repair. Because of the nephrotoxicity of iodinated contrast agents, in the elective surgery and endovascular therapy setting, repair is best delayed for at least 24 hours after CT, especially in patients with chronic renal insufficiency. The administration of N-acetylcysteine and intravenous hydration are current strategies used to limit contrast-induced nephropathy.96,97 If contrast-induced nephropathy does occur, elective repairs should be delayed until renal function returns to baseline.
Magnetic Resonance Imaging and Magnetic Resonance Angiography
MRI and magnetic resonance angiography (MRA) can be considered in the evaluation of patients with TAAAs, especially those with renal insufficiency, because it avoids the use of iodinated contrast material (Fig. 134-8). MRA has also been used to map the spinal cord circulation before TAAA repair.98 Although MRI is believed to provide better contrast resolution, its spatial resolution is poorer than that of CT scanning. Initially, there was enthusiastic support for use of MRI and MRA to identify the patency of visceral and renal vessels in patients with TAAAs.99 However, recent concerns about the use of gadolinium in patients with renal insufficiency has made MRA less attractive in those with aortic aneurysms.100 MRI and MRA are also technically limited in that thrombus and calcium are not prominently displayed. In addition, the increased time required to acquire the images, the associated claustrophobia, the increased cost, and the interference from metallic implants make MRA of limited use in the treatment of TAAAs.
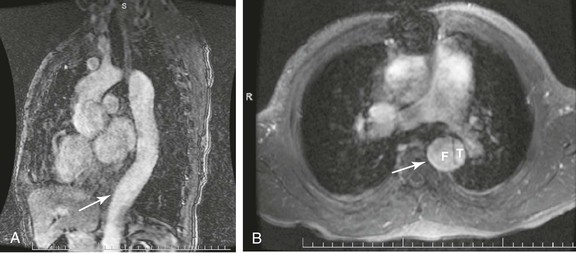
Figure 134-8 MRA of thoracoabdominal aortic aneurysm (TAAA). A, Sagittal MRA view with gadolinium enhancement in a patient with a type B aortic dissection and subsequent TAAA formation. The arrow denotes an area of maximal aneurysmal dilatation. B, Axial cut at same level (arrow) documenting the true (T) and false (F) lumen of dissection.
Arteriography
Although aortography was routinely performed in the past as part of the preoperative evaluation of patients with TAAAs to define the extent and location of the aneurysm, angiography is presently considered obsolete except for special situations, such as attempting to map the spinal cord circulation. Williams and coworkers used angiography to identify the spinal artery of Adamkiewicz, or the great radicular artery, to help prevent paraplegia by knowing in advance of TAAA repair which intercostal artery is providing this important branch to enable prompt and focused revascularization.101 Indications for angiography also include evaluation of branch anatomy in a patient with a TAAA and occlusive disease in the cerebrovascular, visceral, renal, or iliac beds when one wants to limit the use of iodinated contrast material in those with chronic renal failure. Selective angiography with dilute contrast material or carbon dioxide might also be used to treat patients with occlusive disease before TAAA repair, even though few specific studies documenting this approach have been performed.
Laboratory Testing
At present, there are no available biomarkers to suggest the presence of a TAAA. Initial research in patients with acute aortic dissections has focused on a number of proteins, including lipoprotein (a),102 S-100B,103 and calponin.104 Touat and associates have suggested that platelet activation and thrombin generation occur in patients with large dilated ascending aortic aneurysms, particularly in areas of mucoid degeneration.105 A recent review by Trimarchi has suggested that “our current knowledge is expanding at a rapid rate and the future [for biomarkers] is very promising.”106 Laboratory testing, including a complete blood count with platelets, coagulation studies, and determination of blood urea nitrogen and creatinine levels, is standard before TAAA repair. Reports have suggested that the rare patient with a TAAA may have low-grade disseminated intravascular coagulation based on consumptive coagulopathy from the aneurysm.107
Medical Therapy
There is no level A or B evidence comparing modern medical therapy (aspirin, beta blocker, statin, angiotensin-converting enzyme inhibitor, smoking cessation medications) with open or endovascular TAAA repair. In addition, most of the literature on which we base our medical management of patients with TAAAs is extrapolated from literature on patients with ascending aortic aneurysms or aortic dissections.108–111 In general, nonoperative management consists of strict blood pressure control with beta blockade, cessation of smoking, and periodic imaging to monitor the size of the TAAA. There are guidelines to help us in the management and preoperative preparation of patients with AAAs, but not specifically for patients with TAAAs. In addition, reports focused on medical therapies to limit or to slow the growth of TAAAs are few. The American Heart Association guidelines have suggested that “stringent control of hypertension, lipid profile optimization, smoking cessation, and other atherosclerosis risk–reduction measures should be instituted for patients with small aneurysms not requiring surgery, as well as for patients who are not considered to be surgical or stent graft candidates.”4
Antihypertensive Medication
Recent guidelines have suggested that antihypertensive therapy should be administered to keep patients with TAAAs at a goal blood pressure of 140/90 mm Hg in patients without diabetes and 130/80 mm Hg in patients with diabetes or chronic renal failure.4 It was recommended that patients with Marfan’s syndrome and aortic aneurysms be administered beta blockers to reduce the rate of aortic dilatation unless contraindicated. A class IIa recommendation was made to reduce blood pressure with beta blockers, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers in patients with TAAs to the lowest point at which patients can tolerate it without adverse effects.
Beta Blockers
Wheat et al are credited with documenting that decreasing the force of myocardial contraction (dP/dt) slows aortic growth and may prevent TAAA rupture secondary to aortic dissection, but not degenerative TAAAs.112 This observation is the basis for prescribing β-adrenergic blocking agents as first-line antihypertensive therapy for patients with TAAAs. Shores and colleagues used propranolol during a 10-year period and showed a significant reduction in growth of the aortic root, aortic events, and mortality in patients with Marfan’s syndrome.113 Results in animal models of AAA have not translated into beneficial effects of slowing AAA growth in humans.114,115 It is therefore not clear that beta blockers retard aortic growth in patients with degenerative TAAAs. Regardless, the safety and efficacy of beta blockers in preventing death secondary to myocardial infarction are well documented, and therefore they should be prescribed, especially in the preoperative setting.
Angiotensin-Converting Enzyme Inhibitors or Receptor Blockers
There is increasing evidence that oxidative stress plays an important role in the development of degenerative TAAs. Ejiri and associates documented a role for the renin-angiotensin system in the pathogenesis of TAAAs. Human thoracic aneurysmal (n = 40) and nonaneurysmal (control, n = 39) aortic sections were examined, and the results documented markedly increased in situ production of reactive oxygen species throughout the TAAA wall. Multiple regression analysis revealed that medical treatment with angiotensin II type 1 receptor blockers suppressed expression of reactive oxygen species in TAAA.116 A study by Moltzer and colleagues specifically focusing on TAAs also suggests that angiotensin II type 1 receptor blockers, and not angiotensin-converting enzyme or renin inhibitors, should be preferred treatment of patients with TAAs.117
Statins
A well-studied class of drugs with important cholesterol-lowering properties is the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors. HMG-CoA reductase inhibitors, also known as statins, in addition to lowering cholesterol also have pleiotropic effects that inhibit inflammation.118,119 Specific to TAAAs, studies in human TAAAs have shown a role for p22phox-based reduced nicotinamide adenine dinucleotide/reduced nicotinamide adenine dinucleotide phosphate (NADH/NADPH) oxidase in the pathogenesis of TAAAs. This study suggested that statins might have inhibitory effects on the formation of TAAAs through the suppression of NADH/NADPH oxidase.116 Although data examining TAAA growth in humans are lacking, Schouten and associates documented that statins inhibit the growth of AAAs.120 One study suggested that statins are effective in lowering mortality after endovascular AAA repair but not after TAAA repair.121 These authors concluded that this variable response to statins indirectly supports the concept of a distinct pathogenesis of AAAs and TAAAs. Nonetheless, most patients with TAAAs have other indications for statin therapy.
Smoking Cessation
Patients who smoke or who have COPD are at increased risk for the development of TAAAs. TAAAs also grow faster and rupture more often in smokers.10,45 Cannon et al suggested that this is secondary to increased elastolytic activity.122 On the basis of the AAA literature, few would argue that patients who smoke are at risk for the development of TAAAs.123,124 These studies suggest that active pursuit of cessation of cigarette smoking is an important adjunct in those under observation with a TAAA. Yet, similar to statin use, no randomized or prospective trials have ever shown that smoking cessation slows thoracic aortic growth.
Selection of Treatment
The decision when to operate on a patient with a TAAA involves assessment of the likelihood of aortic rupture versus the operative risk of the individual patient.125 It is unclear at present what the impact of thoracic endovascular aortic repair (TEVAR) will be, with its attendant lower short-term mortality and morbidity, on decision making about what aortic diameter should serve as a threshold for TAAA repair. Two major factors, the patient’s physiologic reserve and vascular anatomy, play a significant role in determining whether a patient is best suited for open repair or an endovascular approach. 126 Recent guidelines specifically tailored for when and how to repair descending thoracic and thoracoabdominal aortic aneurysms have been issued4 (Box 134-4).
Size Criteria
Size criteria for TAAA repair are not as clearly defined as for infrarenal AAAs because there are no level A or B scientific data about the timing of operative intervention.127,128 This issue is further complicated by the observation that degenerative TAAAs are often not uniform in size and involve aortic segments of varying diameters and morphology. In addition, it is important to recognize the impact of body size on aortic size. Adjustment for body surface area or height needs to be incorporated into decision making about the threshold for repair and risk for rupture.129 For the average older man (5′6″ to 5′10″), the normal proximal descending aorta is 2.8 cm, the mid-descending aorta is 2.7 cm, and the distal descending aorta is 2.6 cm. It has been suggested that the accepted size indications for TAAA repair, independent of etiology, might be a diameter of 5.2 to 5.6 cm or twice the diameter of the normal contiguous aorta, depending on the aortic segment being repaired. Algorithms suggest that 0.6 cm can be added to or subtracted from these figures for individuals taller than 6 feet or shorter than 5 feet.68,130 Others have suggested that repair of TAAAs should be considered in patients with aortas twice the size of a normal continuous segment or approximately 6 cm in diameter.78
On the basis of natural history studies that have documented an extremely high risk of rupture and death if TAAAs are left untreated, all patients with TAAAs should be considered for repair.35,41,131 The natural history of ruptured TAAAs was first studied extensively by Crawford and colleagues.132 In a series of more than 100 ruptured TAAAs, the authors noted that 80% of ruptures occurred in patients with aneurysms that were less than 10 cm in diameter. The presence of an aortic dissection portended rupture at smaller diameters, with a reported 13% of ruptures occurring in aneurysms smaller than 6 cm. Rupture occurred in the thoracic and abdominal cavity with approximately equal frequency. They concluded by suggesting that because elective surgery is associated with a 92% survival rate, TAAA repair should be considered before rupture when aneurysms are 5 cm or larger in good-risk patients, in patients with symptomatic aneurysms, and in most patients with larger aneurysms. In a large Scandinavian autopsy series, Juvonen and colleagues suggested that 6 cm should be the threshold for open TAAA repair.45 More recently, Elefteriades suggested that the size criterion for surgical TAAA intervention should be larger than 6.5 cm.133 The author suggested that this threshold should be lowered for patients with connective tissue disorders, in whom repair should be offered when the aneurysm attains a diameter of 6.0 cm.133 A positive family history for aortic rupture or dissection might also serve to lower the threshold for repair. Coady and coauthors suggested that in asymptomatic TAAA patients who are monitored longitudinally, there are “hinge points” that demarcate highly dangerous aortic thresholds.42 For the descending thoracic aorta, the hinge point is 7 cm, where a 43% risk for rupture is encountered. This suggests a “conservative” criterion of 6.5 cm for surgical intervention in TAAAs. Because multiple studies have suggested increased mortality and morbidity for types I and II TAAAs, perhaps a higher threshold for repair (6 cm) should be used in these patients. In contrast, patients with less extensive aneurysms, such as type IV TAAAs, might be better served by repair at a diameter of 5.5 cm. Although patients with symptoms should undergo TAAA repair even if their aneurysm has not attained a certain diameter, the threshold for repair should be tailored to the individual surgeon and institutional results.
Preoperative Evaluation
The physiologic stress on a patient undergoing open TAAA repair is unparalleled, and it is to be emphasized that the preoperative evaluation detailed herein will probably differ substantially as a function of the mode of operative repair. The initial evaluation of a patient with a TAAA begins with a thorough history and physical examination focusing on the patient’s cardiac, pulmonary, and renal function. Imaging studies are reviewed to determine the operative plan. Standard preoperative laboratory testing, electrocardiography, and chest radiography should be performed. Guidelines for perioperative evaluation before open surgical and endovascular thoracic aortic repairs have been created 4 (see Box 134-2).
Cardiac
The presence of coronary artery disease varies by the aortic disease being treated. Because the typical patient undergoing TAAA repair is elderly, compared with a patient with a connective tissue disorder with a dissection, impaired myocardial function and the presence of atherosclerotic disease of the coronary arteries are common in patients undergoing aneurysm repair.134,135 In landmark studies of patients before AAA repair, Hertzer and colleagues documented that 42% had significant coronary artery disease in the setting of ischemic cardiac symptoms. Importantly, 19% of the patients who were symptom free also had significant coronary artery disease. Given the high prevalence of coronary disease combined with the stress of this operation, cardiac disease is the leading cause of mortality after open TAAA repair.69,71 Cardiac disease has been reported to be responsible for 49% of early deaths as well as for a third of late deaths after TAAA repair. Therefore, before TAAA repair, all patients should undergo extensive evaluation of their coronary arteries and heart valves. The introduction of endovascular TAAA repair may have an impact on the extensive and invasive nature of this evaluation, but to date data are lacking.
The preoperative electrocardiogram in a hypertensive patient with a degenerative TAAA may suggest left ventricular hypertrophy. There may also be evidence of ischemic heart disease. Although noninvasive stress testing can often predict areas of reversible ischemia and may be useful in an asymptomatic patient, the use of coronary angiography in patients with suspected atherosclerotic coronary disease (e.g., angina, low ejection fraction, previous coronary artery bypass grafting) may be indicated (see Chapter 39).
In the elective setting, coronary artery revascularization, either by coronary angioplasty and stenting or by coronary artery bypass grafting, may be indicated. Details about the specifics of coronary revascularization are important. For example, if the patient is to undergo coronary stenting, clopidogrel is used for a minimum of 6 weeks, which will delay TAAA repair. Avoidance of drug-eluting coronary stents, with their attendant lifelong indication for clopidogrel, is also not indicated. In addition, if the patient is to undergo coronary artery bypass grafting, use of the left internal mammary artery is discouraged, primarily because it serves as an important collateral to the spinal cord as well as to the chest wall.
Echocardiography, most often transesophageal echocardiography (TEE), is helpful in demonstrating left ventricular function and concomitant valvular abnormalities. TEE is also useful in evaluating the ascending and descending thoracic aorta. There are many advantages to TEE over other modalities, including the ability to perform it in the emergency department or operating room. It can also be safely used in patients with renal insufficiency to assess ascending and descending thoracic aortic size as well as the presence of dissection. However, TEE may not be widely available in the emergency setting and is operator dependent.
Pulmonary
Pulmonary complications after open TAAA repair are common, with the incidence of COPD estimated to be between 30% and 40%. COPD is also associated with increased perioperative mortality after TAAA repair.69,136 Pulmonary function tests and arterial blood gas analysis are performed routinely. Preoperative maneuvers to improve pulmonary function before TAAA repair include immediate cessation of smoking, use of appropriate bronchodilators, and rarely the preoperative administration of steroids when an acute exacerbation of reactive airway disease occurs. Patients can also be prescribed an exercise program to improve lung capacity and to lose weight if obesity accompanies their lung disease. In a study of patients with COPD undergoing elective open AAA repair, fewer prescribed inhalers, lower hematocrit, renal insufficiency, and coronary artery disease were associated with unfavorable outcomes.137 Preservation of the left recurrent laryngeal and phrenic nerves is an important adjunct to decrease the incidence of pulmonary complications in these patients.
Renal
Much has been written about the impact of renal failure on the outcome of patients undergoing TAAA repair. A large series of patients undergoing open TAAA repair suggested that the rate of renal failure postoperatively ranges from 5% to 40%, with an attendant mortality rate as high as 70%.138–141 Acute renal failure also predicts increased nonrenal complications postoperatively, including respiratory failure. Second only to aortic rupture, chronic renal insufficiency is the strongest predictor of perioperative acute renal failure and mortality after TAAA repair (Fig. 134-9).142,143 Therefore, evaluation of renal function before TAAA repair, whether open or endovascular, is imperative.142 A full 15% of patients with TAAAs will have some degree of chronic renal insufficiency, as defined by a creatinine level of 1.8 mg/dL (159 µmol/L) or greater.21 Preoperative renal insufficiency is such a strong predictor of poor outcome after type II to type V TAAA repair that it has been suggested to be a relative contraindication to proceeding with repair. Renal function is routinely assessed with standard laboratory tests, including blood urea nitrogen and creatinine concentrations. Huynh and coworkers documented that calculation of the glomerular filtration rate is superior to calculation of creatinine as a predictor of outcome after TAAA repair.144 Cross-sectional imaging will aid the surgeon in evaluating kidney size and the often associated renovascular atherosclerotic disease. If physiologic testing is required, duplex ultrasonography will provide information about the severity of ostial atherosclerotic disease, based on velocities, as well as parenchymal function, based on resistive indices. When TAAA repair is performed in renal failure patients, it is imperative that severe renal artery occlusive disease be treated intraoperatively by endarterectomy, stenting, or bypass grafting with the expectation that renal function will not worsen.145,146 Stenting before TAAA repair to theoretically improve renal function has been reported.147
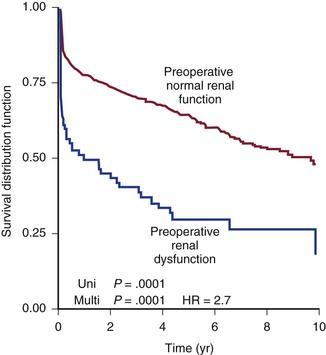
Figure 134-9 Ten-year actuarial survival for patients with and without preoperative renal failure. HR, Hazard ratio.
Preoperative intravenous hydration as well as avoidance of nephrotoxic agents, such as iodinated contrast material, is indicated. Although national trends have moved away from admitting patients preoperatively to the hospital, this group of patients, especially if their visceral arteries are to be bypassed, should probably still be admitted to the hospital and undergo both bowel preparation and intravenous hydration. The rationale has to do with attempting to decrease the risk of bacterial translocation, especially in the setting of visceral ischemia during placement of a synthetic graft at the time of TAAA repair.148
Functional Status
All surgeons know that the preoperative functional status of patients undergoing TAAA repair is critical, even though few objective studies in patients with TAAA have examined this subject in detail. Hua and coworkers, using the National Surgical Quality Improvement Program database in patients undergoing AAA repair, suggested that “poor functional status” predicted mortality.149 Although TAAA repair has been shown to improve survival, a report by Zierer and coauthors examined the late functional status and quality of life of asymptomatic patients undergoing elective repair.150 During a 5-year period, 110 asymptomatic patients underwent elective thoracic aortic replacement for ascending, descending, or thoracoabdominal aneurysms. Functional status, physical and psychological quality of life (Medical Outcomes Study 36-Item Short Form Health Survey, in which 50 represents the normalized age-matched U.S. population), and survival (Kaplan-Meier) were assessed. The results documented that return to normal activity levels was independent of age and procedure. At a mean of 35 months, psychological quality of life was similar between the various surgical groups, but physical quality of life was lower after thoracoabdominal than after ascending or descending aneurysm repair (P < .02). Importantly, age did not affect physical quality of life, and interestingly, older patients had improved psychological quality of life. The overall survival rate was 70% at 4 years, but once again it was lower for thoracoabdominal than for ascending or descending aneurysms (P < .002). The authors suggested that advanced age does not impair return to normal functional status and that patients with asymptomatic thoracic aneurysms thus should not be denied elective replacement on the basis of age alone because functional recovery is not significantly impaired.150
Addressing quality of life issues between open aortic repair and TEVAR, Dick and associates performed a post hoc analysis of a prospectively collected consecutive series of 136 patients with surgical diseases of the descending aorta between January 2001 and December 2005. Endpoints included perioperative and late mortality rates as well as long-term quality of life as assessed by the Short Form Health Survey and Hospital Anxiety and Depression Scale questionnaires. The study documented no differences in perioperative mortality rates (9% for open repair vs. 8% for TEVAR; P = .254). Cumulative long-term mortality rates were similar in both cohorts. Overall quality of life scores were 93 (63 to 110) for open repair and 83 (60 to 112) for TEVAR and were not significantly different. Anxiety and depression scores were not increased after open surgery. The authors suggested that both TEVAR and open aortic repair have excellent long-term results in the treatment of thoracic aortic disease and thus provide little aid in helping us council patients on which type of repair they should seek.151
Although most of these studies have focused on perioperative functional status, Rigberg and colleagues documented that late (1-year) survival was poor after TAAA repair and decreased with patient age.152 Another study by Crawford and coworkers also investigated late (5-year) functional status after TAA repair. These authors concluded that permanent loss of functional capacity occurs only rarely in survivors of TAA repair but noted the difficulty in obtaining appropriate controls for studies such as this.153
Open Versus Endovascular Repair for Thoracoabdominal Aortic Aneurysms
Until recently, elective surgical therapy for TAAAs involved major surgery with a significant risk of perioperative mortality and morbidity. Centers of excellence in this procedure report elective mortality and paraplegia rates of 4.8% and 4.6%, respectively.132 Mortality after surgical treatment of ruptured TAAA is extremely high, even though rates of 26% have been reported.154 In contrast, national mortality rates before the introduction of endovascular technology were 22%.155 Surviving patients experience many postoperative complications and have lengthy hospital stays. Given the continued high mortality and morbidity in contemporary surgical practice, it is not surprising that new techniques for repair were developed. Data suggest that TEVAR of isolated descending TAAs is a safe alternative to open surgery and is associated with lower mortality and morbidity.156,157 However, long-term (>10 years) results are not yet available. In addition, no specific risk scoring system has been developed to predict mortality in patients undergoing TEVAR.158
The decision about which therapy is appropriate for a particular patient is an evolving aspect of care of these complicated patients. There are few guidelines on indications for TEVAR versus open TAAA repair as determined in a prospective, randomized comparison. A Cochrane review comparing thoracic stent-grafting with surgery for TAAs concluded that although stent-grafting of the thoracic aorta is technically feasible and nonrandomized studies suggest reduction of early outcomes, such as paraplegia, mortality, and hospital stay, high-quality randomized controlled trials assessing clinically relevant outcomes including open conversion, aneurysm exclusion, endoleaks, and late mortality are needed.6 However, three industry-sponsored comparative trials are now available. As of this writing, stent-graft devices are approved to treat only degenerative aneurysm disease as well as aortic transections. In addition, there are no randomized, controlled prospective trials comparing open and endovascular TAA repair, even though industry-sponsored trials suggest clinical equipoise.156,159–161
Industry-Sponsored Endovascular Graft Trials
Although all of the device-specific trials are prospective, these trials are not randomized156,159–161 (see Chapter 136). They also suffer from primary use of historical control patients undergoing open TAA repair. In addition, they were not designed to help us determine which patients are best served by stent-grafting versus open repair and do not include patients with visceral or renal artery involvement. Clearly, this is an evolving field, with branched and fenestrated endografts perhaps being available in the near future. The treatment of this section will continue to be controversial as some believe that the hybrid procedure to treat TAAAs is preferable to open repair for Crawford types I to III TAAAs.162
Long-Term Results
Long-term follow-up data from the preclinical Gore TAG trial were recently published. Makaroun and coauthors reported 5-year follow-up with the Gore TAG device in treating degenerative TAAAs and documented no difference in all-cause mortality between endovascular and open TAAA repair at 5 years (67% vs. 68%).163 Major adverse events at 5 years were significantly reduced in the TEVAR group (57.9% vs. 78.7%; P = .001). Endoleaks in the TAG group decreased from 8.1% at 1 month to 4.3% at 5 years. Five TAG patients have undergone major aneurysm-related re-interventions at 5 years (3.6%), including one arch aneurysm repair for a type 1 endoleak and migration, one open conversion, and five endovascular procedures for endoleaks in three patients. For the TEVAR patients, sac size at 60 months decreased in 50% and increased in 19% with respect to the 1-month baseline. At 5 years, there have been no ruptures, one migration, no collapses, and 20 instances of stent fracture in 19 patients, all before revision of the TAG graft. Although the authors acknowledged that the rates of secondary intervention were much higher in the stent-graft group, they concluded that stent-graft repair of TAAA is superior to open repair at 5 years. Even though this study is prospective, it was not randomized. In addition, it was not designed to help us determine which patients are best served by stenting versus open repair.
A meta-analysis reviewing open TAAA repair and stent-grafting by Walsh and colleagues included 17 eligible studies totaling 1109 patients and demonstrated that stenting was associated with a significant reduction in mortality (pooled odds ratio, 0.36; P < .0001) and major neurologic injury (pooled odds ratio, 0.39; P < .0001), with no difference in the major re-intervention rate after elective TAAA repair. Importantly, there was no effect on mortality in patients with thoracic aortic trauma or rupture. The authors concluded by suggesting that endovascular TAAA repair reduces perioperative mortality and neurologic complications in patients undergoing elective TAAA repair, although they did suggest that there may be less benefit in other thoracic aortic conditions.164
Decision Making
Current Status
A report from the Society of Thoracic Surgeons Endovascular Surgery Task Force is useful in trying to determine which patients should undergo open TAAA repair versus endovascular stent-grafting.165 The panel acknowledged that diseases of the thoracic aorta are increasingly being treated by stent-grafts. However, no prospective randomized trials comparing the two types of repair for the same type of disease have been performed. At present in the United States, there are three endografts approved by the Food and Drug Administration for the treatment of degenerative TAAAs. First-generation stent-grafts suffered a number of graft-related complications (stent fractures, graft collapse) as well as complications from the introduction of new technology (increased stroke risk, ascending aortic dissections). Thoracic stent-grafting is relatively new with little long-term follow-up. The long-term consequences of repeated radiation exposure as a result of serial CT scanning are also unknown. In general, although TAAA is a serious disease, it is also relatively indolent. The authors concluded by emphatically stating that identification of small TAAAs does not justify the use of endovascular stent-grafting because the rates of rupture, dissection, and death with TAAAs smaller than 5 cm are relatively low.
Contemporary results of open surgical repair of TAAAs are primarily generated at institutions with great expertise in this area. There is little doubt that TAAA repair, although the disease is relatively rare, is one of the few operations for which surgeon and hospital volume influence mortality.155,166 Based on 1898 cases, a mortality rate of 4.8% can be attained after open TAAA repair in centers of excellence,165 as opposed to a national mortality rate of more than 20% from administrative data.155 In addition, the risk of paralysis and stroke is 3.4% and 2.7%, respectively, after open TAAA repair in centers of excellence. Five- and 10-year survival rates are 60% and 38%, respectively.165
With the technology presently available, few would argue for treatment of all thoracic aortic disease by stent-grafting alone or some combination of stent-grafting and extra-anatomic bypasses to the exclusion of open TAAA repair. Indications for endovascular intervention are not clearly defined at present and instead are based on reports of open TAAA repair from institutions with great expertise. A recent call from the French National Authority has suggested that a prospective registry of all thoracic aortic procedures be initiated. The goals of such a registry would be (1) to select patients who could be treated by TEVAR, (2) to assess the feasibility of a randomized controlled study comparing TEVAR with open repair, (3) to assess the medium-term outcome of different devices, and (4) to obtain a better understanding of the economics of stent-grafting compared with open repair.167
Long-term results after endografting are not yet generally available, with the first 5-year follow-up report of the only Food and Drug Administration–approved endograft recently published.163 Mortality rates after endovascular TAAA repair vary widely between 2% and 26%, depending largely on the nature of the indication for the operation (elective versus urgent versus emergency), the comorbid conditions of the patient, and the experience of the endovascular specialist.165 Midterm results suggested that moderate-term survival rates (3 to 8 years) also varied greatly between 25% and 90%. Although there should clearly be enthusiasm for the use of endovascular therapies to treat many thoracic aortic diseases, late complications, such as endoleaks, stent fractures, and stent migration, are more common after endovascular TAAA repair than after open repair. The issue of increased cost (initial cost of the stent-grafts, multiple CT scans in follow-up, secondary procedures) associated with endografting will also need to be figured into the equation in considering which therapy is correct for a particular patient. Importantly, it is also not clear at present whether a more aggressive endovascular approach will affect long-term survival or freedom from aortic complications compared with open surgical therapy.
Subpopulations Benefiting from Thoracic Endovascular Aortic Repair
There are clear subpopulations of patients best served by TEVAR. It is reasonable to conclude that patients older than 75 years, if anatomically suitable, should be directed toward stent-grafting.155 The subset of patients with significant COPD is also likely to benefit from stent-grafting when possible to avoid the attendant risks incurred from a thoracotomy. Patients with increasing pain or with ruptured TAAAs should also be considered for TEVAR, which can often be performed more expeditiously than open repair if the anatomy is appropriate for an endovascular approach. A recent meta-analysis of open repair versus TEVAR for ruptured TAAs suggested that TEVAR is associated with significantly lower 30-day mortality rates compared with open surgical repair. However, TEVAR was associated with an increased number of aneurysm-related deaths in follow-up.168 Until recently, most would suggest that all patients with TAAs would benefit from treatment at a high-volume center. However, a study examining TEVAR versus open TAA repair suggested that TEVAR is associated with shorter hospital stay, similar mortalities, and fewer complications but increased hospital charges. This suggests that a more widespread application of TEVAR to even community and smaller regional hospital centers that are considered low-volume providers is occurring.169
Current Indications for Thoracic Endovascular Aortic Repair
At present, outside of off-label use of thoracic and abdominal stent-grafts or investigational device exemptions, indications for TEVAR are only for degenerative TAAAs with adequate proximal and distal aortic landing zones. Fenestrated and multibranched endografts, which are widely available in Europe and Australia, may in the future ultimately make the hybrid procedure that is performed in the United States today mostly obsolete. As with the evolution of many new technologies, the indication for stent-grafting is based on a lower short-term mortality rate in comparison to open TAAA repair or medical therapy, not necessarily on long-term outcomes. New less invasive therapies such as TEVAR often need to document only clinical equipoise to gain market share. Regardless, the physician caring for a patient with a TAAA should consider a constellation of issues, including age, comorbid diseases, symptoms, life expectancy, quality of life, aortic diameter, aneurysm morphology and extent, suitability of a landing zone for a stent-graft, cost of therapies, and operator experience, in deciding to commit a patient to any intervention.165
Thoracic Endovascular Aortic Repair Versus Observation
The issue of whether to offer patients with TAAAs endovascular repair when they are not candidates for open repair because of excessive comorbid conditions has not been addressed in a prospective randomized fashion. In patients with infrarenal AAAs, the EVAR-2 trial suggested that such patients did not benefit in terms of survival when randomized to endovascular repair versus observation, although this was related to the rather high (9%) operative mortality associated with stent-graft repair in this study.170 Patel and colleagues examined this issue in 46 asymptomatic patients with descending thoracic aortic disease who were considered high risk for open surgery for reasons of age of 80 years or older (47.8%) and comorbid conditions (84.8%). Twenty-one patients underwent TEVAR, whereas another 25 either were excluded from TEVAR on the basis of unfavorable anatomy or refused intervention. All-cause mortality in the entire cohort was 50%. Yet the median actual time to mortality was different between the two groups (control, 9.2 months; TEVAR, 24.9 months; P = .01). Life-table analysis demonstrated improved survival with TEVAR at 24 months (P = .05) (Fig. 134-10).171
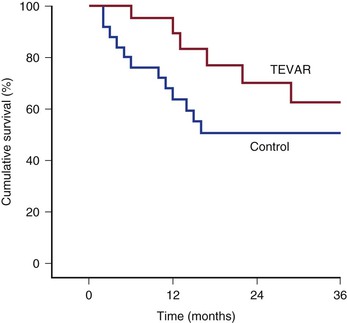
Figure 134-10 Life-table analysis demonstrating that elective thoracic endovascular aortic repair (TEVAR) results in an early-term to intermediate-term survival advantage for high-risk asymptomatic patients with thoracic aortic disease. Note that the 1- and 2-year life-table survival rates for TEVAR are 95% and 70%, respectively; in contrast, the 1- and 2-year life-table survival rates for the control arm are 68% (P = .03) and 51% (P = .05), respectively. (From Patel HJ, et al: Survival benefit of endovascular descending thoracic aortic repair for the high-risk patient. Ann Thorac Surg 83:1628-1633; discussion 1633-1634, 2007.)
In a study by Roselli and associates, patients with thoracoabdominal aneurysms considered “high risk” for conventional surgery were enrolled in a prospective trial to evaluate a novel endovascular grafting system. Devices were custom designed for each patient with the use of high-resolution CT. Although there was no control group, the authors treated 73 patients by endovascular repair for type I, II, or III (n = 28) or type IV (n = 45) TAAAs. Technical success was achieved in 93% of patients (68 of 73), and the 30-day mortality rate was 5.4% (4 of 73). Major perioperative complications occurred in 11 patients (15%), including paraplegia (2.7%, 2 of 73), new onset of dialysis (1.4%, 1 of 73), prolonged ventilator support (6.8%, 5 of 73), myocardial infarction (5.4%, 4 of 73), and minor hemorrhagic stroke (1.4%, 1 of 72). The authors concluded that endovascular repair of aortic aneurysms involving the visceral segment in nonsurgical high-risk candidates is feasible with acceptable morbidity and mortality rates. They also suggested that assessment of durability will require longer follow-up.172
Other Pathologic Processes Associated with The Thoracic Aorta
Aberrant Right Subclavian Artery
Multiple and various brachiocephalic congenital abnormalities have been described, but the most common variant is an aberrant right subclavian artery. In this anomaly, the right subclavian artery takes a course posterior to the esophagus. As many as 80% of individuals with this anomaly will develop dysphagia (dysphagia lusoria) as the artery enlarges when the patient grows.4 The artery itself as it enlarges develops a Kommerell diverticulum. This artery will often become aneurysmal and is associated with thoracic aortic dissections and aneurysms. Whereas initial treatment of this condition was surgical resection of the proximal right subclavian and replacement of it and the abnormal section of the descending thoracic aorta with a graft, reports describe a hybrid approach in managing the aberrant right subclavian in which a right common carotid artery to distal right subclavian artery bypass is performed with ligation of the right subclavian artery proximal to the vertebral artery. This is followed by stent-grafting of the abnormal descending thoracic aorta.173 Before the origin of the right subclavian artery is covered, it is imperative to perform coil embolization of side branches off the aberrant subclavian artery.
Coarctation of the Aorta
Coarctation of the aorta, a relatively common birth abnormality, occurs in approximately 40 to 50 per 100,000 live births.4 On physical examination, patients may have disparate pulses in the upper extremity and lower extremity. Although this finding is most often managed soon after birth or in childhood by nonvascular surgeons, some patients may present later with resultant heart failure, refractory hypertension, or diminished pulses. It is important to diagnose and to treat this abnormality because if it is left untreated, it is uniformly fatal. Surgical options depend on the location of the coarctation, but those discovered in the thoracoabdominal aorta are typically managed by patch aortoplasty or thoracoabdominal bypass.174,175 Congenital narrowings of the renal and mesenteric arteries are also commonly seen in these patients and can be managed concurrently in the same surgical setting.176–178
Vascular surgeons may be called on to help manage previously repaired coarctations in the descending aorta in patients who present later in life with patch pseudoaneurysms. The use of both uncovered and covered stents has been described and appears to be effective (Fig. 134-11). A recent review by the Cochrane group concluded that there is insufficient evidence with regard to the best treatment of coarctation of the thoracic aorta. The authors suggested that a randomized, controlled trial is needed.179
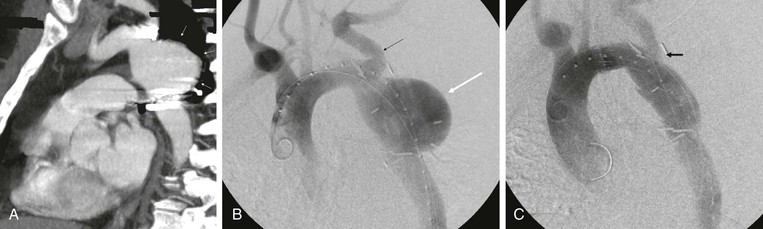
Figure 134-11 CTA of a patient who presented with chest pain as an adult after having a previous coarctation repair as a child. The patient was found to have a large pseudoaneurysm by CTA (A, arrows) and angiography (B, white arrow; black arrow, left subclavian artery), which was managed with thoracic aortic stent-grafting and left subclavian fenestration with stent grafting (C, arrow).
Thoracic Aortic Atheroma
Patients with severe aortic atheroma are at significant risk for the development of sequelae of distal embolization. Patients with plaques characterized as larger than 4 mm or greater in thickness proximal to the left subclavian in the aortic arch in particular are at increased risk for development of a stroke4 (Fig. 134-12). The presence of thoracic aortic atheroma appears to be especially relevant in the patient with an unexplained stroke.180 Patients with aortic arch plaques, even receiving appropriate antiplatelet therapy, have an ischemic stroke rate of 11% per year. Risk factors for the development of aortic atheroma include those that are typical for atherosclerosis in general. The likelihood of embolization is increased in the setting of a complex aortic plaque.181 Diagnosis of this syndrome is made by the standard methods of imaging of the thoracic aorta (i.e., echocardiography, CTA, MRA, angiography). There are no specific recommendations about how this high-risk group of patients should be treated as no randomized trial has or likely will be performed. The data regarding the choice of anticoagulation with warfarin compared with antiplatelet therapy are confusing but in balance slightly support the use of warfarin if it is not contraindicated.4 In general, the use of statins because of their plaque regression capabilities also seems prudent. The use of stent-grafting in the thoracic aorta to treat this condition has been described.182
Tumors of the Thoracic Aorta
Tumors of the thoracic aorta are extremely rare, with only a total of 53 thoracic and 10 thoracoabdominal tumors in the literature.183 Most neoplasms of the thoracic aorta are related to contiguous spread of adjacent malignant neoplasms or subsequent metastases.184–187 Patients will typically present with constitutional symptoms, but patients have presented with distal embolization as well. Sarcoma and malignant fibrous histiocytoma are the two primary neoplasms of the thoracic aorta.183 Whereas attempted resection and reconstruction have been described, overall the prognosis is exceedingly poor.188
Selected Key References
Abraha I, Romagnoli C, Montedori A, Cirocchi R. Thoracic stent graft versus surgery for thoracic aneurysm. Cochrane Database Syst Rev. 2009;(1).
Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res. 2007;139:292–307.
Cowan JA Jr, Dimick JB, Henke PK, Huber TS, Stanley JC, Upchurch GR Jr. Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume-related outcomes. J Vasc Surg. 2003;37:1169–1174.
Hiratzka LF, Barkis GL, Beckman JA, Bersin RM, Carr VF, Caset DE, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchhoukos NT, Lytle BW, Milewicz DM, Reich DL, Shinn JA, Svensson LG, Williams DM. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. [Writing Group Members] Circulation. 2010;121:e266–e369.
McNamara JJ, Pressler VM. Natural history of arteriosclerotic thoracic aortic aneurysms. Ann Thorac Surg. 1978;26:468–473.
One of the earliest descriptions of the natural history of thoracic aortic aneurysms..
Roselli EE, Greenberg RK, Pfaff K, Francis C, Svensson LG, Lytle BW. Endovascular treatment of thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2007;133:1474–1482.
Largest experience in the United States with fenestrated and multibranched endografts for TAAAs..
Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA, Eggebrecht H, Elefteriades JA, Erbel R, Gleason TG, Lytle BW, Mitchell RS, Nienaber CA, Roselli EE, Safi HJ, Shemin RJ, Sicard GA, Sundt GA, Sundt TM 3rd, Szeto WY, Wheatley GH 3rd. Society of Thoracic Surgeons Endovascular Surgery Task Force: Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008;85(Suppl):S1–S41.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. National Center for Health Statistics, National Vital Statistics System, WISQARS Query. 20 leading causes of death, United States, 1999-2004, all races, both sexes. [Available at] http://webappa.cdc.gov/sasweb/ncipc/leadcaus10.html; 2007 [Accessed July 25] .
2. Bickerstaff LK, et al. Thoracic aortic aneurysms: a population-based study. Surgery. 1982;92:1103–1108.
3. Clouse WD, et al. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA. 1998;280:1926–1929.
4. Hiratzka LF, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. [Writing Group Members] Circulation. 2010;121:e266–e369.
5. Conrad MF, et al. Contemporary management of descending thoracic and thoracoabdominal aortic aneurysms: endovascular versus open. Circulation. 2008;117:841–852.
6. Abraha I, et al. Thoracic stent graft versus surgery for thoracic aneurysm. Cochrane Database Syst Rev. 2009;(1).
7. Knepper J, et al. A review of clinical trials and registries in descending thoracic aortic aneurysm. Semin Vasc Surg. 2010;23:170–175.
8. Santilli JD, et al. Diagnosis and treatment of abdominal aortic aneurysms. Am Fam Physician. 1997;56:1081–1090.
9. Vasan RS, et al. Echocardiographic reference values for aortic root size: the Framingham Heart Study. J Am Soc Echocardiogr. 1995;8:793–800.
10. Dapunt OE, et al. The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 1994;107:1323–1332.
11. Agmon Y, et al. Is aortic dilatation an atherosclerosis-related process? Clinical, laboratory, and transesophageal echocardiographic correlates of thoracic aortic dimensions in the population with implications for thoracic aortic aneurysm formation. J Am Coll Cardiol. 2003;42:1076–1083.
12. Garcier JM, et al. Normal diameter of the thoracic aorta in adults: a magnetic resonance imaging study. Surg Radiol Anat. 2003;25:322–329.
13. Hager A, et al. Diameters of the thoracic aorta throughout life as measured with helical computed tomography. J Thorac Cardiovasc Surg. 2002;123:1060–1066.
14. Liddington MI, et al. The relationship between aortic diameter and body habitus. Eur J Vasc Surg. 1992;6:89–92.
15. Brady AR, et al. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21.
16. Roman MJ, et al. Prognostic significance of the pattern of aortic root dilation in the Marfan syndrome. J Am Coll Cardiol. 1993;22:1470–1476.
17. Pleumeekers HJ, et al. Aneurysms of the abdominal aorta in older adults. The Rotterdam Study. Am J Epidemiol. 1995;142:1291–1299.
18. Biddinger A, et al. Familial thoracic aortic dilatations and dissections: a case control study. J Vasc Surg. 1997;25:506–511.
19. Coady MA, et al. Familial patterns of thoracic aortic aneurysms. Arch Surg. 1999;134:361–367.
20. Hasham SN, et al. Mapping a locus for familial thoracic aortic aneurysms and dissections (TAAD2) to 3p24-25. Circulation. 2003;107:3184–3190.
21. Guo DC, et al. Pathogenesis of thoracic and abdominal aortic aneurysms. Ann N Y Acad Sci. 2006;1085:339–352.
22. Coady MA, et al. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin. 1999;17:615–635.
23. Moreno-Cabral CE, et al. Degenerative and atherosclerotic aneurysms of the thoracic aorta. Determinants of early and late surgical outcome. J Thorac Cardiovasc Surg. 1984;88:1020–1032.
24. Cambria RP, et al. Thoracoabdominal aneurysm repair: perspectives over a decade with the clamp-and-sew technique. Ann Surg. 1997;226:294–303.
25. Juvonen T, et al. Risk factors for rupture of chronic type B dissections. J Thorac Cardiovasc Surg. 1999;117:776–786.
26. Coady MA, et al. Familial patterns of thoracic aortic aneurysms. Arch Surg. 1999;134:361–367.
27. Biddinger A, et al. Familial thoracic aortic dilatations and dissections: a case control study. J Vasc Surg. 1997;25:506–511.
28. Milewicz DM, et al. Reduced penetrance and variable expressivity of familial thoracic aortic aneurysms/dissections. Am J Cardiol. 1998;82:474–479.
29. Guo D, et al. Familial thoracic aortic aneurysms and dissections: genetic heterogeneity with a major locus mapping to 5q13-14. Circulation. 2001;103:2461–2468.
30. Hasham SN, et al. Mapping a locus for familial thoracic aortic aneurysms and dissections (TAAD2) to 3p24-25. Circulation. 2003;107:3184–3190.
31. Vaughan CJ, et al. Identification of a chromosome 11q23.2-q24 locus for familial aortic aneurysm disease, a genetically heterogeneous disorder. Circulation. 2001;103:2469–2475.
32. Prakash SK, et al. Rare copy number variants disrupt genes regulating vascular smooth muscle cell adhesion and contractility in sporadic thoracic aortic aneurysms and dissections. Am J Hum Genet. 2010;87:743–756.
33. Jondeau G, et al. Genetics of thoracic aortic aneurysms. Curr Atheroscler Rep. 2012;14:219–226.
34. Milewicz DM, et al. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Ann Rev Genomics Hum Genet. 2008;9:283–302.
35. McNamara JJ, et al. Natural history of arteriosclerotic thoracic aortic aneurysms. Ann Thorac Surg. 1978;26:468–473.
36. Pressler V, et al. Aneurysm of the thoracic aorta. Review of 260 cases. J Thorac Cardiovasc Surg. 1985;89:50–54.
37. Davies RR, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–27.
38. Crawford ES, et al. Thoracoabdominal aortic aneurysm: observations regarding the natural course of the disease. J Vasc Surg. 1986;3:578–582.
39. Cronenwett JL, et al. Actuarial analysis of variables associated with rupture of small abdominal aortic aneurysms. Surgery. 1985;98:472–483.
40. Cambria RA, et al. Outcome and expansion rate of 57 thoracoabdominal aortic aneurysms managed nonoperatively. Am J Surg. 1995;170:213–217.
41. Griepp RB, et al. Natural history of descending thoracic and thoracoabdominal aneurysms. Ann Thorac Surg. 1999;67:1927–1930.
42. Coady MA, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg. 1997;113:476–491.
43. Masuda Y, et al. Expansion rate of thoracic aortic aneurysms and influencing factors. Chest. 1992;102:461–466.
44. Rizzo JA, et al. Procedures for estimating growth rates in thoracic aortic aneurysms. J Clin Epidemiol. 1998;51:747–754.
45. Juvonen T, et al. Prospective study of the natural history of thoracic aortic aneurysms. Ann Thorac Surg. 1997;63:1533–1545.
46. Olsson C, et al. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–2618.
47. LaRoy LL, et al. Imaging of abdominal aortic aneurysms. AJR Am J Roentgenol. 1989;152:785–792.
48. Barbour JR, et al. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res. 2007;139:292–307.
49. Guo DC, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–1493.
51. Wang Y, et al. Gene expression signature in peripheral blood detects thoracic aortic aneurysm. PLoS One. 2007;2:e1050.
52. Andreotti L, et al. Aortic connective tissue in ageing—a biochemical study. Angiology. 1985;36:872–879.
53. Wolinsky H, et al. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20:99–111.
54. Ikonomidis JS, et al. Effects of deletion of the matrix metalloproteinase 9 gene on development of murine thoracic aortic aneurysms. Circulation. 2005;112(Suppl):I242–I248.
55. Sinha I, et al. A biologic basis for asymmetric growth in descending thoracic aortic aneurysms: a role for matrix metalloproteinase 9 and 2. J Vasc Surg. 2006;43:342–348.
56. Jones JA, et al. Alterations in membrane type-1 matrix metalloproteinase abundance after the induction of thoracic aortic aneurysm in a murine model. Am J Physiol Heart Circ Physiol. 2010;299:H114–H124.
57. Erdheim J. Medionecrosis aortae idiopathica cystica. Virchows Arch. 1930;276:187.
58. Klima T, et al. The morphology of ascending aortic aneurysms. Hum Pathol. 1983;14:810–817.
59. Schlatmann TJ, et al. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977;39:13–20.
60. Carlson RG, et al. Cystic medial necrosis of the ascending aorta in relation to age and hypertension. Am J Cardiol. 1970;25:411–415.
61. Cambria RP. Thoracoabdominal aortic aneurysms. Rutherford R. Vascular surgery. ed 5. WB Saunders: Philadelphia; 2000.
62. LeMaire SA, et al. Spectrum of aortic operations in 300 patients with confirmed or suspected Marfan syndrome. Ann Thorac Surg. 2006;81:2063–2078.
63. Kang N, et al. Circulatory arrest for repair of postcoarctation site aneurysm. Ann Thorac Surg. 2004;77:2029–2033.
64. Heikkinen L, et al. Long-term results of direct aortoplasty for repair of aortic coarctation in adults. Ann Thorac Surg. 1990;49:948–950.
65. Ala-Kulju K, et al. Aneurysms after patch graft aortoplasty for coarctation of the aorta: long-term results of surgical management. Ann Thorac Surg. 1989;47:853–856.
66. Kan CD, et al. Outcome after endovascular stent graft treatment for mycotic aortic aneurysm: a systematic review. J Vasc Surg. 2007;46:906–912.
67. Kpodonu J, et al. Endovascular management of a descending thoracic mycotic aneurysm: mid-term follow-up. Eur J Cardiothorac Surg. 2007;32:178–179.
68. Crawford ES, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg. 1986;3:389–404.
69. Svensson LG, et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17:357–368.
70. Safi HJ, et al. Distal aortic perfusion and cerebrospinal fluid drainage for thoracoabdominal and descending thoracic aortic repair: ten years of organ protection. Ann Surg. 2003;238:372–380.
71. Panneton JM, et al. Nondissecting thoracoabdominal aortic aneurysms: part I. Ann Vasc Surg. 1995;9:503–514.
72. Isselbacher E. Cecil medicine. ed 23. Elsevier Health Sciences: Philadelphia; 2008.
73. Libby P, et al. Braunwald’s heart disease: a textbook of cardiovascular medicine. ed 8. WB Saunders: Philadelphia; 2007.
74. Townsend CM, et al. Sabiston textbook of surgery. ed 18. Elsevier Health Sciences: Philadelphia; 2008.
75. Cooke JC, et al. Simultaneous tracheobronchial and esophageal obstruction caused by a descending thoracic aneurysm. J Vasc Surg. 1993;18:90–94.
76. Furukawa H, et al. Saccular descending thoracic aortic aneurysm with dysphagia. Jpn J Thorac Cardiovasc Surg. 1999;47:277–280.
77. Arnold SE, et al. Spontaneous cerebral embolism from descending thoracic aortic aneurysm—a case report. Angiology. 1991;42:69–72.
78. Fann JI. Descending thoracic and thoracoabdominal aortic aneurysms. Coron Artery Dis. 2002;13:93–102.
79. Svensjo S, et al. Thoracic and thoracoabdominal aortic aneurysm and dissection: an investigation based on autopsy. Br J Surg. 1996;83:68–71.
80. Crawford ES, et al. Aortic aneurysm: a multifocal disease. Presidential address. Arch Surg. 1982;117:1393–1400.
81. Coselli JS, et al. Impact of previous thoracic aneurysm repair on thoracoabdominal aortic aneurysm management. Ann Thorac Surg. 1997;64:639–650.
82. Clouse WD, et al. Late aortic and graft-related events after thoracoabdominal aneurysm repair. J Vasc Surg. 2003;37:254–261.
83. Schoenhoff FS, et al. The frozen elephant trunk: an interesting hybrid endovascular-surgical technique to treat complex pathologies of the thoracic aorta. J Vasc Surg. 2007;45:597–599.
84. Abou-Zamzam AM Jr, et al. Endovascular repair of a ruptured descending thoracic aortic aneurysm in a patient with an ascending aortic aneurysm: hybrid open arch reconstruction with simultaneous thoracic stent-graft deployment within elephant trunk. Ann Vasc Surg. 2008;22:168–172.
85. Resch TA, et al. Combined staged procedures for the treatment of thoracoabdominal aneurysms. J Endovasc Ther. 2006;13:481–489.
86. Klompas M. Does this patient have an acute thoracic aortic dissection? JAMA. 2002;287:2262–2272.
87. von Kodolitsch Y, et al. Chest radiography for the diagnosis of acute aortic syndrome. Am J Med. 2004;116:73–77.
88. Yoshida S, et al. Thoracic involvement of type A aortic dissection and intramural hematoma: diagnostic accuracy: comparison of emergency helical CT and surgical findings. Radiology. 2003;228:430–435.
89. Sommer T, et al. Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging. Radiology. 1996;199:347–352.
90. Zeman RK, et al. Diagnosis of aortic dissection: value of helical CT with multiplanar reformation and three-dimensional rendering. AJR Am J Roentgenol. 1995;164:1375–1380.
91. Shiga T, et al. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med. 2006;166:1350–1356.
92. Hemminger BM, et al. Assessment of real-time 3D visualization for cardiothoracic diagnostic evaluation and surgery planning. J Digit Imaging. 2005;18:145–153.
93. Loutsidis A, et al. Incidental finding of lung cancer in a patient with thoracic aortic aneurysm—simultaneous management. A case report. Eur J Cancer Care (Engl). 2007;16:387–389.
94. Morimoto Y, et al. Surgical strategy for advanced gastric cancer with a concomitant thoracoabdominal aortic aneurysm requiring arterial reconstruction of the visceral branches. Surg Today. 2007;37:817–821.
95. El-Hajjar M, et al. Incidence of contrast-induced nephropathy in patients with chronic renal insufficiency undergoing multidetector computed tomographic angiography treated with preventive measures. Am J Cardiol. 2008;102:353–356.
96. Schweiger MJ, et al. Prevention of contrast induced nephropathy: recommendations for the high risk patient undergoing cardiovascular procedures. Catheter Cardiovasc Interv. 2007;69:135–140.
97. Meschi M, et al. Facts and fallacies concerning the prevention of contrast medium–induced nephropathy. Crit Care Med. 2006;34:2060–2068.
98. Nijenhuis RJ, et al. Comparison of magnetic resonance with computed tomography angiography for preoperative localization of the Adamkiewicz artery in thoracoabdominal aortic aneurysm patients. J Vasc Surg. 2007;45:677–685.
99. Kaufman JA, et al. MR imaging (including MR angiography) of abdominal aortic aneurysms: comparison with conventional angiography. AJR Am J Roentgenol. 1994;163:203–210.
100. Clorius S, et al. Nephrogenic systemic fibrosis following exposure to gadolinium-containing contrast agent. Clin Nephrol. 2007;68:249–252.
102. Schillinger M, et al. Lipoprotein(a) in patients with aortic aneurysmal disease. J Vasc Surg. 2002;36:25–30.
103. Lases EC, et al. Clinical prospective study of biochemical markers and evoked potentials for identifying adverse neurological outcome after thoracic and thoracoabdominal aortic aneurysm surgery. Br J Anaesth. 2005;95:651–661.
104. Suzuki T, et al. Preliminary experience with the smooth muscle troponin-like protein, calponin, as a novel biomarker for diagnosing acute aortic dissection. Eur Heart J. 2008;29:1439–1445.
105. Touat Z, et al. Dilation-dependent activation of platelets and prothrombin in human thoracic ascending aortic aneurysm. Arterioscler Thromb Vasc Biol. 2008;28:940–946.
106. Trimarchi S, et al. In search of blood tests for thoracic aortic diseases. Ann Thorac Surg. 2010;90:1735–1742.
107. Mamiya S, et al. Disseminated intravascular coagulation accompanying thoracic and abdominal aortic aneurysm; report of three cases. Jpn J Med. 1988;27:91–95.
108. Genoni M, et al. Chronic beta-blocker therapy improves outcome and reduces treatment costs in chronic type B aortic dissection. Eur J Cardiothorac Surg. 2001;19:606–610.
109. Ladouceur M, et al. Effect of beta blockade on ascending aortic dilatation in children with the Marfan syndrome. Am J Cardiol. 2007;99:406–409.
110. Ahimastos AA, et al. Effect of perindopril on large artery stiffness and aortic root diameter in patients with Marfan syndrome: a randomized controlled trial. JAMA. 2007;298:1539–1547.
111. Mochizuki S, et al. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study. Lancet. 2007;369:1431–1439.
112. Wheat MW Jr, et al. Dissecting aneurysms of the aorta: present status of drug versus surgical therapy. Prog Cardiovasc Dis. 1968;11:198–210.
113. Shores J, et al. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. N Engl J Med. 1994;330:1335–1341.
114. Moursi MM, et al. Inhibition of aortic aneurysm development in blotchy mice by beta adrenergic blockade independent of altered lysyl oxidase activity. J Vasc Surg. 1995;21:792–799.
115. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg. 2002;35:72–79.
116. Ejiri J, et al. Oxidative stress in the pathogenesis of thoracic aortic aneurysm: protective role of statin and angiotensin II type 1 receptor blocker. Cardiovasc Res. 2003;59:988–996.
117. Moltzer R, et al. The role of the renin-angiotensin system in thoracic aortic aneurysms: clinical implications. Pharmacol Ther. 2011;131:50–60.
118. Steinmetz EF, et al. Treatment with simvastatin suppresses the development of experimental abdominal aortic aneurysms in normal and hypercholesterolemic mice. Ann Surg. 2005;241:92–101.
119. Nagashima H, et al. A 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, cerivastatin, suppresses production of matrix metalloproteinase-9 in human abdominal aortic aneurysm wall. J Vasc Surg. 2002;36:158–163.
120. Schouten O, et al. Statins are associated with a reduced infrarenal abdominal aortic aneurysm growth. Eur J Vasc Endovasc Surg. 2006;32:21–26.
121. Diehm N, et al. Statins are associated with decreased mortality in abdominal, but not in thoracic aortic aneurysm patients undergoing endovascular repair: propensity score–adjusted analysis. Vasa. 2008;37:241–249.
122. Cannon DJ, et al. Blood elastolytic activity in patients with aortic aneurysm. Ann Thorac Surg. 1982;34:10–15.
123. Rasmussen TE, et al. Human leukocyte antigen class II immune response genes, female gender, and cigarette smoking as risk and modulating factors in abdominal aortic aneurysms. J Vasc Surg. 2002;35:988–993.
124. Wilmink TB, et al. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999;30:1099–1105.
125. Coady MA, et al. Developing surgical intervention criteria for thoracic aortic aneurysms. Cardiol Clin. 1999;17:827–839.
126. Greenberg RK, et al. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg. 2003;38:990–996.
127. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants. Lancet. 1998;352:1649–1655.
128. Lederle FA, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437–1444.
129. Davies RR, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg. 2006;81:169–177.
130. Svensson LG. Device discordancy: lost cords, quick-fix seekers, quality, and ethics. J Thorac Cardiovasc Surg. 2006;131:261–263.
131. Coselli JS, et al. Mortality and paraplegia after thoracoabdominal aortic aneurysm repair: a risk factor analysis. Ann Thorac Surg. 2000;69:409–414.
132. Crawford ES, et al. Ruptured aneurysm of the descending thoracic and thoracoabdominal aorta. Analysis according to size and treatment. Ann Surg. 1991;213:417–425.
133. Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877–S1880.
134. Hertzer NR, et al. Routine coronary angiography prior to elective aortic reconstruction: results of selective myocardial revascularization in patients with peripheral vascular disease. Arch Surg. 1979;114:1336–1344.
135. Young JR, et al. Coronary artery disease in patients with aortic aneurysm: a classification of 302 coronary angiograms and results of surgical management. Ann Vasc Surg. 1986;1:36–42.
136. Estrera AL, et al. Descending thoracic aortic aneurysm: surgical approach and treatment using the adjuncts cerebrospinal fluid drainage and distal aortic perfusion. Ann Thorac Surg. 2001;72:481–486.
137. Upchurch GR Jr, et al. Predictors of severe morbidity and death after elective abdominal aortic aneurysmectomy in patients with chronic obstructive pulmonary disease. J Vasc Surg. 2003;37:594–599.
138. Cambria RP, et al. Thoracoabdominal aneurysm repair: results with 337 operations performed over a 15-year interval. Ann Surg. 2002;236:471–479.
139. Coselli JS, et al. Thoracoabdominal aortic aneurysm repair: review and update of current strategies. Ann Thorac Surg. 2002;74:S1881–S1884.
140. Kashyap VS, et al. Renal failure after thoracoabdominal aortic surgery. J Vasc Surg. 1997;26:949–955.
141. Kouchoukos NT, et al. Safety and efficacy of hypothermic cardiopulmonary bypass and circulatory arrest for operations on the descending thoracic and thoracoabdominal aorta. Ann Thorac Surg. 2001;72:699–707.
142. Safi HJ. Thoracoabdominal aortic aneurysm. Rutherford R. Vascular surgery. ed 6. Elsevier Saunders: Philadelphia; 2005.
143. Svensson LG, et al. Appraisal of adjuncts to prevent acute renal failure after surgery on the thoracic or thoracoabdominal aorta. J Vasc Surg. 1989;10:230–239.
144. Huynh TT, et al. Glomerular filtration rate is superior to serum creatinine for prediction of mortality after thoracoabdominal aortic surgery. J Vasc Surg. 2005;42:206–212.
145. Svensson LG, et al. Thoracoabdominal aortic aneurysms associated with celiac, superior mesenteric, and renal artery occlusive disease: methods and analysis of results in 271 patients. J Vasc Surg. 1992;16:378–389.
146. LeMaire SA, et al. Deployment of balloon expandable stents during open repair of thoracoabdominal aortic aneurysms: a new strategy for managing renal and mesenteric artery lesions. Eur J Cardiothorac Surg. 2004;26:599–607.
148. Woodcock NP, et al. Bacterial translocation in patients undergoing abdominal aortic aneurysm repair. Br J Surg. 2000;87:439–442.
149. Hua HT, et al. Early outcomes of endovascular versus open abdominal aortic aneurysm repair in the National Surgical Quality Improvement Program–Private Sector (NSQIP-PS). J Vasc Surg. 2005;41:382–389.
150. Zierer A, et al. Elective surgery for thoracic aortic aneurysms: late functional status and quality of life. Ann Thorac Surg. 2006;82:573–578.
151. Dick F, et al. Outcome and quality of life after surgical and endovascular treatment of descending aortic lesions. Ann Thorac Surg. 2008;85:1605–1612.
152. Rigberg DA, et al. Age stratified, perioperative, and one-year mortality after abdominal aortic aneurysm repair: a statewide experience. J Vasc Surg. 2006;43:224–229.
153. Crawford RS, et al. Functional outcome after thoracoabdominal aneurysm repair. J Vasc Surg. 2008;48:828–835.
154. Barbato JE, et al. Contemporary results of open repair of ruptured descending thoracic and thoracoabdominal aortic aneurysms. J Vasc Surg. 2007;45:667–676.
155. Cowan JA Jr, et al. Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume–related outcomes. J Vasc Surg. 2003;37:1169–1174.
156. Makaroun MS, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg. 2005;41:1–9.
157. Heijmen RH, et al. Endovascular stent-grafting for descending thoracic aortic aneurysms. Eur J Cardiothorac Surg. 2002;21:5–9.
158. Day CP, et al. Endovascular repair of the thoracic aorta: predictors of 30-day mortality in patients on the New Zealand Thoracic Aortic Stent Database (NZ TAS). Eur J Vasc Endovas Surg. 2009;37:160–165.
159. Bavaria JE, et al. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg. 2007;133:369–377.
160. Matsumura JS, et al. International controlled clinical trial of thoracic endovascular aneurysm repair with the Zenith TX2 endovascular graft: 1-year results. J Vasc Surg. 2008;47:247–257.
161. Fairman RM, et al. Pivotal results of the Medtronic Vascular Talent Thoracic Stent Graft System: the VALOR Trial. J Vasc Surg. 2008;48:546–554.
162. Black SA, et al. Complex thoracoabdominal aortic aneurysms: endovascular exclusion with visceral revascularization. J Vasc Surg. 2006;43:1081–1089.
163. Makaroun MS, et al. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J Vasc Surg. 2008;47:912–918.
164. Walsh SR, et al. Endovascular stenting versus open surgery for thoracic aortic disease: systematic review and meta-analysis of perioperative results. J Vasc Surg. 2008;47:1094–1098.
165. Svensson LG, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008;85(Suppl):S1–S41.
166. Cowan JA Jr, et al. Ruptured thoracoabdominal aortic aneurysm treatment in the United States: 1988 to 1998. J Vasc Surg. 2003;38:319–322.
167. Corbillon R, et al. The French National Authority for Health reports on thoracic aortic stent grafts. J Vasc Surg. 2008;47:1099–1107.
168. Jonker FHW, et al. Meta-analysis of open versus endovascular repair for ruptured descending thoracic aortic aneurysm. J Vasc Surg. 2010;51:1026–1032.
169. Gopaldas RR, et al. Superior nationwide outcomes of endovascular versus open repair for isolated descending thoracic aortic aneurysm in 11,669 patients. J Thorac Cardiovasc Surg. 2010;140:1001–1010.
170. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 2005;365:2187–2192.
171. Patel HJ, et al. Survival benefit of endovascular descending thoracic aortic repair for the high-risk patient. Ann Thorac Surg. 2007;83:1628–1633.
172. Roselli EE, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2007;133:1474–1482.
173. Yang C, et al. Aberrant subclavian artery pathologies and Kommerell’s diverticulum: a review and analysis of published endovascular/hybrid treatment options. J Endovasc Ther. 2012;19:373–382.
174. Coleman DM, et al. Long-segment thoracoabdominal aortic occlusions in childhood. J Vasc Surg. 2012;56:482–485.
175. Stanley JC, et al. Abdominal aortic coarctation: surgical treatment of 53 patients with a thoracoabdominal bypass, patch aortoplasty, or interposition aortoaortic graft. J Vasc Surg. 2008;48:1073–1082.
176. Upchurch GR Jr, et al. Pediatric splanchnic arterial occlusive disease: clinical relevance and operative treatment. J Vasc Surg. 2002;35:860–867.
177. Stanley JC, et al. Pediatric renovascular hypertension: 132 primary and 30 secondary operations in 97 children. J Vasc Surg. 2006;44:1219–1228.
178. Grigoryants V, et al. Iliorenal bypass: indications and outcomes following 41 reconstructions. Ann Vasc Surg. 2007;21:1–9.
179. Padua LMS, et al. Stent placement versus surgery for coarctation of the thoracic aorta. Cochrane Database Syst Rev. 2012;(5).
180. Khatibzadeh M, et al. Aortic atherosclerotic plaques as a source of systemic embolism. J Am Coll Cardiol. 1996;27:664–669.
181. The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. Ann Intern Med. 1998;128:639–647.
182. Wolf PS, et al. Endovascular treatment of massive thoracic aortic thrombus and associated ruptured atheroma. Ann Vasc Surg. 2010;24:416e9–416e12.
183. Oldenburg WA. Primary tumors of major blood vessels. ed 6. Elsevier Saunders: Philadelphia; 2005.
184. Deslauriers J, et al. Clinical and surgical staging of non–small cell lung cancer. Chest. 2000;117:96S–103S.
185. Kameda K, et al. Detection of T-factor in lung cancer using magnetic resonance imaging and computed tomography. J Thorac Imaging. 1988;3:73–80.
186. Ohtsuka T, et al. Cine computed tomography for evaluation of tumors invasive to the thoracic aorta: seven clinical experiences. J Thorac Cardiovasc Surg. 1996;112:190–192.
187. Schroder C, et al. Transesophageal echographic determination of aortic invasion by lung cancer. Chest. 2005;127:438–442.
188. Kouchoukos NT, et al. Primary sarcoma of the thoracic aorta. Ann Thorac Surg. 2007;83:e1.