Chapter 83 Thoracic and Lumbar Spondylosis
Anatomy, Pathophysiology, and Biomechanics
The intervertebral disc is bound ventrally by the anterior longitudinal ligament, dorsally by the posterior longitudinal ligament, and rostrally and caudally by cartilaginous end plates that abut the vertebral bodies. The anulus fibrosus forms the outer ring of the disc and provides most of the structural integrity. A softer, notochord-derived nucleus pulposus forms the center portion of the disc and, although not as strong as the anulus, provides cushioning and some resistance, mainly to axial loads. With aging, the disc progressively desiccates and becomes less elastic, a process that has been termed disc degeneration. In the case of spondylosis, disc degeneration contributes to the overall pathology in several different ways. As the disc desiccates, a process that is demonstrable on MRI by loss of T2 signal (so-called dark disc disease) (Fig. 83-1), it has the potential, by virtue of its innervation by the recurrent sinuvertebral nerve, ventral rami, and rami communicantes, to contribute to back pain.1 With the loss of hydration, the disc may become incapable of resisting physiologic biomechanical forces, resulting in failure by herniation through a defect in the anulus. This herniation may result in neurologic deficit and/or pain. Even in the absence of true herniation, a broad-based bulge may compress the neural elements, causing symptoms. Apart from symptomatology, the desiccated disc is no longer able to perform a portion of its biomechanical function in the normal movement of the spine. Other elements of the spine must therefore bear the resultant biomechanical stresses, potentially accelerating their degeneration.

FIGURE 83-1 Sagittal T2-weighted MRI of the lumbar spine illustrating advanced disc degeneration at L5-S1.
Facet joints in the thoracic spine and the lumbar spine are alternatively referred to as zygapophyseal or apophyseal joints. Because they oppose the neural elements, degeneration and hypertrophy may cause compression of spinal roots, the spinal cord, and the thecal sac of the lumbar spine. Each joint is composed of the superior articular process of the caudal vertebra and the inferior articular process of the rostral vertebra. The opposing surfaces are covered with synovium, while the outer surface is covered by fibrous capsule. The joints of the thoracic spine from the C7-T1 joint to the T9-10 joint are typically oriented in the coronal plane and assume a configuration not unlike that of shingles on a roof. The T10-11 joint is often a transition area where the joint orientation becomes slightly tangential to the coronal plane. The portion of the joint that is visible dorsally in the lumbar spine is mostly composed of the inferior articular process of the rostral vertebra; this is readily demonstrated with removal of the joint capsule. The superior articular process of the inferior vertebra forms the ventral and lateral portion of the joint. Because of its location directly adjacent to the exit point of the nerve root laterally and the thecal sac medially, the superior facet process often comprises the point of maximal compression when joint hypertrophy leads to neural compromise. Because innervation of the facets themselves is via medial branches of the dorsal primary rami, degeneration of the facets may produce back pain by this mechanism.2–4
Signs and Symptoms
Back Pain
As previously stated, lumbar and thoracic spondylosis may cause back pain. This multifactorial complaint is very common and may occur in the absence of defined spinal pathology. In degenerative spondylosis, however, the generation of pain may be due to facet hypertrophy, disc degeneration, spinal instability, and/or referred pain from neural compression. These causes are generally difficult to separate from each other; even in the face of overt instability, the so-called pain generator may be protean and difficult to treat.
Nevertheless, the mechanisms by which possible sources of pain in spondylosis affect patients bear discussion. As was mentioned earlier, the intervertebral discs and facet joints are innervated and may cause pain with degeneration.1–4 Furthermore, as the spine ages, the degenerating facets and increasingly desiccated intervertebral discs lose some of their ability to maintain normal motion and support of the vertebral column. As a result, paraspinal muscles may be recruited to maintain posture, and this may contribute to painful paraspinal muscle spasm.
Radiculopathy
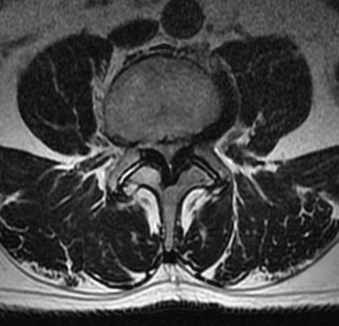
The underlying pathophysiology of radiculopathy is most likely multifactorial. Direct mechanical compression of a nerve root certainly plays some role in the generation of radiculopathy, particularly as removal of the offending lesion frequently results in marked improvement in symptoms. In the lumbar spine, this compression most commonly occurs in the lateral recess and affects the nerve root exiting the next most caudal neural foramen (Fig. 83-2). Thus, a paracentral herniation at L4-5 most commonly affects the L5 nerve root exiting the L5-S1 neural foramen. However, while it is intuitive that a herniated thoracic or lumbar disc causes pain by direct mechanical compression or stretch on the nerve root, many patients have disc pathology that abuts or compresses the neural elements without associated radicular symptoms. Disc material is both immunogenic and inflammatory.5,6 Animal models have demonstrated that nucleus pulposus material causes an inflammatory reaction and a demonstrable increase in reactive cytokines. Tumor necrosis factor alpha has been proposed as a possible underlying inflammatory factor.7–10 Both processes have been implicated in symptom generation.
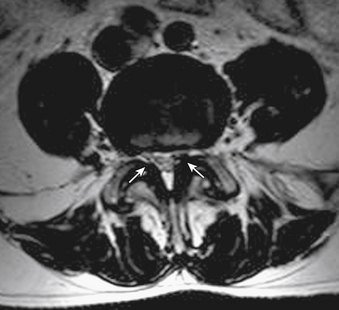
Radiculopathy is not always the result of disc pathology. As the thoracolumbar facet joints hypertrophy as the degenerative process progresses, compression of the ventrally lying nerve roots may occur. This compression generally occurs in the aforementioned region directly adjacent to the exit point of the nerve root laterally and the thecal sac medially, termed the lateral recess (Fig. 83-3). The ligamentum flavum may also hypertrophy, contributing to this compression.
Myelopathy
When spondylotic processes result in compression of the spinal cord, myelopathy may occur. Spondylotic processes in the thoracic spine, including disc pathology, facet hypertrophy, and ligamentum flavum hypertrophy, may cause direct compression of the spinal cord. In the lumbar spine, the situation is somewhat more complex, as the spinal cord in most adults ends in the region of L1-2. Thus, compression from spondylotic pathology at lumbar levels causes symptoms related to compression of the spinal cord, conus medullaris, or nerve roots. Symptoms from pathology at the thoracolumbar junction therefore vary according to the neural structures that are affected, although rarely true thoracic disc herniation can mimic lumbar radiculopathy.11,12
Claudication
When spondylotic processes result in compression of the thecal sac below the conus medullaris, neurogenic claudication may result. Most commonly, this occurs as the result of spondylotic spinal stenosis caused by hypertrophy of the ligamentum flavum, by facet arthropathy and subsequent overgrowth, by broad-based intervertebral disc bulges, or by a combination of any of the three. These symptoms are to be distinguished from vascular claudication.13 Pain often extends down the back of the legs into the calves in neurogenic claudication, while the pain of vascular claudication is often described as being in a “stocking” distribution. With vascular claudication, relief often comes quickly after rest; simply resting does not often help neurogenic claudication. Patients frequently must sit or assume a flexed or stooped posture (the so-called shopping cart sign) to relieve the pain of neurogenic claudication. Vascular problems that cause claudication usually result in diminished or absent peripheral pulses and cool extremities, whereas in neurogenic claudication, the lower-extremity examination may be entirely normal.
Correlative Diagnostics
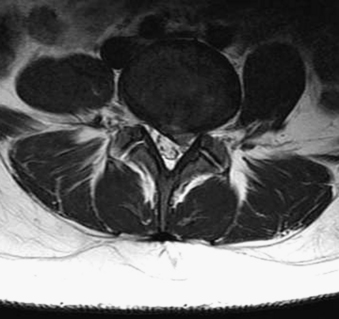
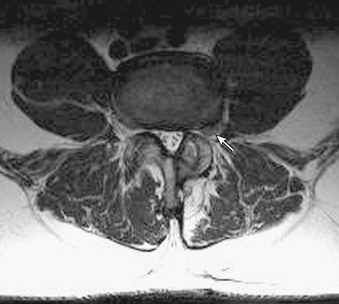
MRI findings in lumbar spondylosis generally differ from those in thoracic spondylosis. Degeneration, as was mentioned earlier in the chapter, may be associated with disc herniation, ligamentum flavum hypertrophy, and facet joint hypertrophy. The summation of these pathologic tissue responses may cause stenosis of the spinal canal in the midline or lateral recesses of the canal (Figs. 83-4 and 83-5). Facet hypertrophy, ligamentum flavum hypertrophy, or broad-based disc bulges (Fig. 83-6) may also contribute to stenosis of the central canal, lateral recesses, or foramina. Extraforaminal disc herniations may cause compression of the exiting nerve root lateral to the neural foramen (Fig. 83-7). As spondylosis progresses and joint arthropathy worsens, increased fluid within the facet capsule itself or the presence of synovial cysts associated with the joints may contribute to compression of the thecal sac and/or nerve roots (Fig. 83-8). In the absence of frank instability, these findings may also portend the development of instability following surgical decompression without stabilization.
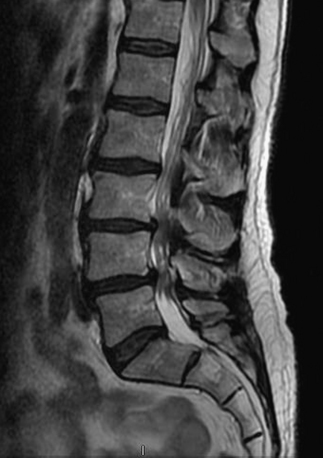
FIGURE 83-4 Sagittal T2-weighted MRI of the lumbar spine illustrating central stenosis at L2-3, L3-4, and L4-5.
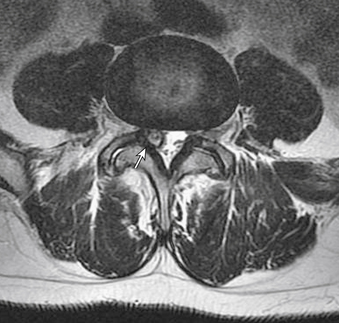
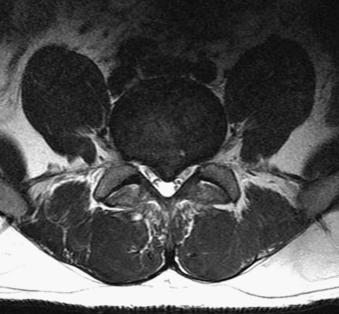
FIGURE 83-8 Axial T2-weighted MRI of the lumbar spine demonstrating a large synovial cyst at L4-5 on the right (arrow).
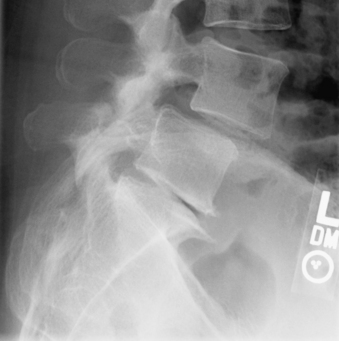
Typically, patients with lumbar spondylosis will undergo plain radiographic imaging of the affected area prior to or as a part of their initial evaluation. Most often, anteroposterior (AP), lateral, flexion, extension, and oblique images are obtained. Findings on AP and lateral radiographs include loss of disc space height, osteophyte formation, and possibly spondylolisthesis (Fig. 83-9). Flexion-extension films are used to assess the movement of the spine in sagittal plane rotation and AP translation, typically to evaluate for excessive translation. In the presence of a defect in the pars interarticularis, oblique radiographs demonstrate discontinuity of the pars (Fig. 83-10). In the presence of lumbar or thoracic spondylosis, attention should also be paid to the alignment of the spinal column as a whole, specifically regarding the presence of scoliosis, kyphosis, or any associated aberration of coronal or sagittal balance.
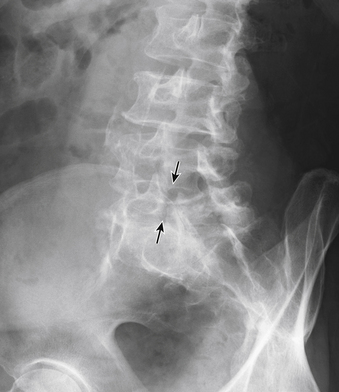
FIGURE 83-10 Oblique radiograph of the lumbar spine demonstrating a fracture of the pars interarticularis of L5 (arrows).
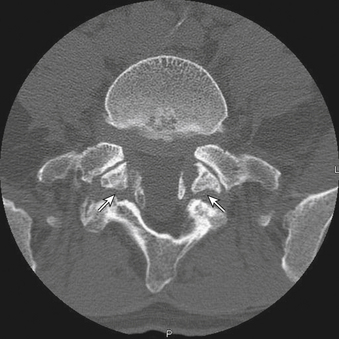
CT may also be a valuable adjunctive imaging modality in the presence of spondylosis. In patients with questionable bony anatomy, CT may help to define difficult or otherwise obscured anatomic relationships (Fig. 83-11). CT combined with myelography may be particularly helpful when metallic implants from prior surgical procedures obscure the relevant anatomy on MRI or when implanted medical devices prevent the safe acquisition of MRI. CT may also provide invaluable information about pedicle diameters and angles when the placement of spine instrumentation is under consideration. This is particularly important in the thoracic spine, where midthoracic and upper thoracic pedicles may be so small as to prohibit the safe placement of transpedicular instrumentation. It is likewise important in patients with known spondylolysis and spondylolisthesis, in whom CT may demonstrate very small pedicles at the affected level, especially if the listhesis is high grade and long-standing.
Treatment
The management of lumbar spondylosis must be individualized, as many patients have some combination of disc disease, facet hypertrophy, and ligamentum flavum hypertrophy, with or without spondylolisthesis. In many instances, however, treatment may be based on the predominant pathology.
In patients with radiculopathy and minimal weakness from a lumbar disc herniation, a course of medical management including physical therapy, muscle relaxants, nonsteroidal anti-inflammatory medications, and neuropathic pain medication can provide relief. Some patients may obtain relief from epidural steroid injections as well, although data are conflicting and this therapy does not work as well for back pain.14–16 Patients with acute intractable pain, cauda equina syndrome, or progressive neurologic deficit should be treated with surgery. Surgical treatment of disc herniation with radiculopathy has been the subject of much debate. The well-publicized Spine Patient Outcomes Research Trial was hailed as a definitive study demonstrating similar outcomes for surgical and nonsurgical treatment of lumbar disc herniation causing radiculopathy. However, statistical analysis did not account for crossover and other factors that decreased its validity and the weight of its findings.17–19 Therefore, while the statistically robust intent to treat analysis showed no advantage of surgical treatment over nonsurgical treatment, the as-treated analysis showed a substantial advantage of surgical treatment over nonsurgical treatment.
In the case of lumbar spinal stenosis, oral agents have not been found to be effective in treating the typically present claudication symptoms, and insufficient evidence exists regarding physical therapy.20 Epidural steroids may be of benefit for temporary relief.20 Although the data are limited, patients who are treated nonsurgically with these measures may require surgery 20% to 40% of the time. Of those who do not require surgery, 50% to 70% can expect to have improvement in their pain symptoms with time.20 Further study that was performed as a part of the aforementioned Spine Patient Outcomes Research Trial demonstrated an improvement in outcome for surgical treatment of symptomatic lumbar stenosis when compared to standard nonsurgical treatment.21
Regarding the management of lumbar spondylolisthesis with resultant stenosis, surgical treatment is indicated in patients with instability on flexion and extension radiographs or symptoms of stenosis that are recalcitrant to the previously mentioned nonsurgical treatments. Decompression with fusion with or without instrumentation is currently recommended.22 The need to reduce the listhesis to normal alignment is not supported in the literature, provided that adequate decompression has been accomplished.22
The indications for fusion in patients with lumbar spondylosis in the absence of spondylolisthesis are less well defined. There is some class I evidence to support lumbar fusion in carefully selected patients who have failed conservative therapy, albeit with a moderate amount of benefit over conservative therapy alone.23,24 There are also data to support the addition of transpedicular instrumentation to aid in fusion.25 In general, the addition of fusion to simple discectomy should be considered for patients with instability or other structural anomaly or in recurrent herniations with chronic intractable back pain.26 There are no convincing data to suggest that fusion in addition to decompression for lumbar stenosis conveys any benefit, except in cases of preoperative instability or spondylolisthesis.27,28
Consideration must also be given to the possibility of iatrogenic postlaminectomy instability that may be predicted prior to decompression. Postlaminectomy instability is a theoretical risk in all patients, but this risk is increased in patients with marked preoperative instability or deformity.29,30 If the preoperative imaging suggests that wide (i.e., more than one third of the joint) facetectomy or concomitant laminectomy and discectomy are required for adequate decompression, consideration should be given to adding fusion supplemented with transpedicular instrumentation.27
Technique
For laminectomy, the exposure must demonstrate the medial facet joints, lamina, and pars interarticularis at each level. Generally, the procedure begins by removal of the spinous processes at each affected level and often the inferior aspect of the next most rostral spinous process, to facilitate exposure. It is typically easiest and safest to approach the target area from the caudal aspect, owing to the angle of the lamina. Ligamentous attachments are dissected free by using curettes to clear the epidural plane. Bony resection is carried out by using rongeurs until the laminae are removed in a caudal-to-rostral fashion. The lateral border of resection is defined by the facet joints and pars interarticularis. As in the discectomy approach described earlier in the chapter, the medial one third of the facet can be removed safely without effect on the biomechanics of the spine. Ligament and bone are removed until the thecal sac is relaxed and the lateral recesses are decompressed. Anatomically, lateral recess decompression includes resection of the medial superior facet process of the caudal vertebra at any given segment until the pedicle and lower exiting nerve root can be visualized. Each foramen should be probed to assess the degree of decompression, and further foraminal decompression should be done as indicated. This must be done with care, as the nerve root is susceptible to injury at this stage. It must also be remembered that complete facetectomy will result in at least the potential for resultant instability.
Expected Outcomes
Following lumbar discectomy, varying degrees of success have been reported. Resolution of both back pain and radicular pain was found in 62% of patients in one large study.31 Overall satisfaction with the procedure ranges from 69% to 96%, the higher percentage being observed in a 5-year study.31,32 Up to 3% to 5% of patients may have worsening of motor symptoms, and up to 12% to 15% may have worsening of sensory findings after discectomy.33 Comparison of technical variations has also yielded conflicting data. While some authors have advocated the so-called sequestrectomy (removal of only the herniated fragment without curettage of the disc space), an analysis of the published literature from 1980 to 2007 showed varying results.34–37 While patients who had undergone aggressive discectomy demonstrated a lower incidence of recurrent disc herniation than did those with less aggressive removal (3.5% vs. 7%, respectively), they also demonstrated an increase in recurrent back and leg pain (27.8% vs. 11.6%, respectively) when compared with patients who had undergone limited discectomy.35 Some researchers have suggested that microdiscectomy through minimally invasive tubular systems may be less effective, but results in this study were measured at 1 year postoperatively.32
In patients for whom conservative therapy has failed, surgical decompression for lumbar stenosis can improve pain and claudication in up to 80% of cases.20 Patients who are treated with decompression and fusion for lumbar stenosis with spondylolisthesis may experience relief in back and leg symptoms in up to 86% of cases.38 Patients with solid fusion have also been shown to do better than those with pseudarthrosis, although this might not achieve true statistical significance.39
McCormick P.C. The Spine Patient Outcomes Research Trial results for lumbar disc herniation: a critical review. J Neurosurg Spine. 2007;6(6):513-520.
McGirt M.J., Ambrossi G.L., Datoo G., et al. Recurrent disc herniation and long-term back pain after primary lumbar discectomy: review of outcomes reported for limited versus aggressive disc removal. Neurosurgery. 2009;64(2):338-344. discussion 344–345
Resnick D.K., Choudhri T.F., Dailey A.T., et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: fusion in patients with stenosis and spondylolisthesis. J Neurosurg Spine. 2005;2(6):679-685.
Watters W.C.3rd, Baisden J., Gilbert T.J., et al. Degenerative lumbar spinal stenosis: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J. 2008;8(2):305-310.
Weinstein J.N., Lurie J.D., Tosteson T.D., et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006;296(20):2451-2459.
Weinstein J.N., Tosteson T.D., Lurie J.D., et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296(20):2441-2450.
Weinstein J.N., Tosteson T.D., Lurie J.D., et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358(8):794-810.
1. Bogduk N., Tynan W., Wilson A.S. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132(Pt 1):39-56.
2. Bogduk N., Long D.M. The anatomy of the so-called “articular nerves” and their relationship to facet denervation in the treatment of low-back pain. J Neurosurg. 1979;51(2):172-177.
3. Chua W.H., Bogduk N. The surgical anatomy of thoracic facet denervation. Acta Neurochir (Wien). 1995;136(3-4):140-144.
4. Giles L.G., Taylor J.R. Innervation of lumbar zygapophyseal joint synovial folds. Acta Orthop Scand. 1987;58(1):43-46.
5. Rutkowski M.D., Winkelstein B.A., Hickey W.F., et al. Lumbar nerve root injury induces central nervous system neuroimmune activation and neuroinflammation in the rat: relationship to painful radiculopathy. Spine (Phila Pa 1976). 2002;27(15):1604-1613.
6. Virri J., Gronblad M., Seitsalo S., et al. Comparison of the prevalence of inflammatory cells in subtypes of disc herniations and associations with straight leg raising. Spine (Phila Pa 1976). 2001;26(21):2311-2315.
7. Olmarker K., Rydevik B., Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine (Phila Pa 1976). 1993;18(11):1425-1432.
8. Mulleman D., Mammou S., Griffoul I., et al. Pathophysiology of disk-related sciatica. I.–Evidence supporting a chemical component. Joint Bone Spine. 2006;73(2):151-158.
9. Mulleman D., Mammou S., Griffoul I., et al. Pathophysiology of disk-related low back pain and sciatica. II. Evidence supporting treatment with TNF-alpha antagonists. Joint Bone Spine. 2006;73(3):270-277.
10. Shamji M.F., Allen K.D., So S., et al. Gait abnormalities and inflammatory cytokines in an autologous nucleus pulposus model of radiculopathy. Spine (Phila Pa 1976). 2009;34(7):648-654.
11. Tokuhashi Y., Matsuzaki H., Uematsu Y., Oda H. Symptoms of thoracolumbar junction disc herniation. Spine (Phila Pa 1976). 2001;26(22):E512-E518.
12. Lyu R.K., Chang H.S., Tang L.M., Chen S.T. Thoracic disc herniation mimicking acute lumbar disc disease. Spine (Phila Pa 1976). 1999;24(4):416-418.
13. Hawkes C.H., Roberts G.M. Neurogenic and vascular claudication. J Neurol Sci. 1978;38(3):337-345.
14. Spaccarelli K.C. Lumbar and caudal epidural corticosteroid injections. Mayo Clin Proc. 1996;71(2):169-178.
15. Vad V.B., Bhat A.L., Lutz G.E., Cammisa F. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine (Phila Pa 1976). 2002;27(1):11-16.
16. Resnick D.K., Choudhri T.F., Dailey A.T., et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 13: injection therapies, low-back pain, and lumbar fusion. J Neurosurg Spine. 2005;2(6):707-715.
17. Weinstein J.N., Tosteson T.D., Lurie J.D., et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296(20):2441-2450.
18. Weinstein J.N., Lurie J.D., Tosteson T.D., et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006;296(20):2451-2459.
19. McCormick P.C. The Spine Patient Outcomes Research Trial results for lumbar disc herniation: a critical review. J Neurosurg Spine. 2007;6(6):513-520.
20. Watters W.C.3rd, Baisden J., Gilbert T.J., et al. Degenerative lumbar spinal stenosis: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J. 2008;8(2):305-310.
21. Weinstein J.N., Tosteson T.D., Lurie J.D., et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358(8):794-810.
22. Watters W.C.3rd, Bono C.M., Gilbert T.J., et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J. 2009;9(7):609-614.
23. Chou R., Baisden J., Carragee E.J., et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine (Phila Pa 1976). 2009;34(10):1094-1109.
24. Resnick D.K., Choudhri T.F., Dailey A.T., et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 7: intractable low-back pain without stenosis or spondylolisthesis. J Neurosurg Spine. 2005;2(6):670-672.
25. Resnick D.K., Choudhri T.F., Dailey A.T., et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 12: pedicle screw fixation as an adjunct to posterolateral fusion for low-back pain. J Neurosurg Spine. 2005;2(6):700-706.
26. Resnick D.K., Choudhri T.F., Dailey A.T., et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 8: lumbar fusion for disc herniation and radiculopathy. J Neurosurg Spine. 2005;2(6):673-678.
27. Resnick D.K., Choudhri T.F., Dailey A.T., et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 10: fusion following decompression in patients with stenosis without spondylolisthesis. J Neurosurg Spine. 2005;2(6):686-691.
28. Resnick D.K., Choudhri T.F., Dailey A.T., et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: fusion in patients with stenosis and spondylolisthesis. J Neurosurg Spine. 2005;2(6):679-685.
29. Fox M.W., Onofrio B.M., Hanssen A.D. Clinical outcomes and radiological instability following decompressive lumbar laminectomy for degenerative spinal stenosis: a comparison of patients undergoing concomitant arthrodesis versus decompression alone. J Neurosurg. 1996;85(5):793-802.
30. Fox M.W., Onofrio B.M. Indications for fusion following decompression for lumbar spinal stenosis. Neurosurg Focus. 1997;3(2):e2. discussion e5
31. Lewis P.J., Weir B.K., Broad R.W., Grace M.G. Long-term prospective study of lumbosacral discectomy. J Neurosurg. 1987;67(1):49-53.
32. Arts M.P., Brand R., van den Akker M.E., et al. Tubular diskectomy vs conventional microdiskectomy for sciatica: a randomized controlled trial. JAMA. 2009;302(2):149-158.
33. Blaauw G. Braakman R, Gelpke GJ, Singh R: Changes in radicular function following low-back surgery. J Neurosurg. 1988;69(5):649-652.
34. Barth M., Weiss C., Thome C. Two-year outcome after lumbar microdiscectomy versus microscopic sequestrectomy: part 1: evaluation of clinical outcome. Spine (Phila Pa 1976). 2008;33(3):265-272.
35. McGirt M.J., Ambrossi G.L., Datoo G., et al. Recurrent disc herniation and long-term back pain after primary lumbar discectomy: review of outcomes reported for limited versus aggressive disc removal. Neurosurgery. 2009;64(2):338-344. discussion 344–345
36. Thome C., Barth M., Scharf J., et al. Outcome after lumbar sequestrectomy compared with microdiscectomy: a prospective randomized study. J Neurosurg Spine. 2005;2(3):271-278.
37. Barth M., Diepers M., Weiss C., et al. Two-year outcome after lumbar microdiscectomy versus microscopic sequestrectomy: part 2: radiographic evaluation and correlation with clinical outcome. Spine (Phila Pa 1976). 2008;33(3):273-279.
38. Booth K.C., Bridwell K.H., Eisenberg B.A., et al. Minimum 5-year results of degenerative spondylolisthesis treated with decompression and instrumented posterior fusion. Spine (Phila Pa 1976). 1999;24(16):1721-1727.
39. Kornblum M.B., Fischgrund J.S., Herkowitz H.N., et al. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine (Phila Pa 1976). 2004;29(7):726-733. discussion 733–734