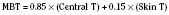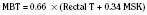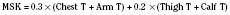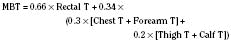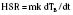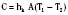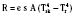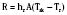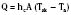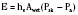CHAPTER 6 Thermoregulation: Physiology and Perioperative Disturbances
One of the many physiologic adaptations required for the survival of homeothermic species is the ability to maintain constant core body temperature within narrow limits. The significance of thermal regulation for neonates was first appreciated by two French obstetricians, Tarnier and later Budin, who in 1907 published the finding of significantly higher survival rates in normothermic versus hypothermic neonates (Budin, 1907). Several other investigators later confirmed the importance of thermal stability in the adaptive process and further elucidated the mechanisms by which neonates, infants, and children are able to behave as homeotherms (Silverman and Blanc, 1957; Cross et al., 1958; Silverman et al., 1958; Bruck, 1961). A homeothermic organism is characterized by its ability to maintain constant core (or central) body temperature despite changes in the ambient temperature. Not many physiologic parameters are as vigorously and effectively controlled as the core temperature. In humans, the central body temperature refers to the temperature of the vessel-rich group organs (i.e., brain, heart, lungs, liver, and kidneys) and is normally maintained within ±0.2° C of its set point of 37.0° C. This so-called interthreshold range defines the limits within which no thermoregulatory effector responses are triggered and the human organism behaves poikilothermically. The musculoskeletal system makes up the major part of the peripheral compartment, which is considered to be a dynamic buffer in the thermoregulatory system. The skin, representing the shell compartment, acts as a barrier to the environment.
Temperature control is subjected to circadian rhythms, some of which begin in the first days of life. The circadian rhythm of body temperature is generally age dependent (less pronounced at very young and very old ages) and generally closely associated with the sleep-wake cycle. In fertile women, a monthly rhythm in body temperature exists because of a higher set-point temperature in the luteal phase of the menstrual cycle (Hardy, 1961). Despite the fluctuations of body temperature within these rhythms, temperature control is tight and is accomplished by a sophisticated system that balances heat production and heat loss.
Accidental hypothermia occurs commonly in patients of any age who are undergoing anesthesia and surgery, and it has often been accepted as an unfortunate but unavoidable consequence of the surgical procedure. This high rate of hypothermia led Pickering to his famous statement, “The practical difficulty in cooling men is to break through the defenses of the body; the most effective means is to give an anesthetic…” (Pickering, 1958).
Temperature monitoring
Perioperative detection of changes in body temperature requires appropriate monitoring and monitoring sites. Most national anesthetic societies now have guidelines that require that one method for measuring body temperature during anesthesia be available (La Société Française d’Anesthésie et de Réanimation, 1994; American Society of Anesthesiologists, 2005; Australian and New Zealand College of Anaesthetists, 2006; Canadian Anesthesiologists’ Society, 2007).
Infrared thermometers (thermopiles) are quite popular in postanesthesia care units and on hospital wards; however, for continuous temperature monitoring during anesthesia they are not suitable. Despite a fast response time, their accuracy in clinical practice has not been confirmed (particularly when they are not used properly) (Craig et al., 2002; Heusch and McCarthy, 2005; Leon et al., 2005; Dodd et al., 2006).
Temperature-sensitive liquid crystals have been used to measure skin temperature. Although these devices are easy and convenient to handle, they generally do not meet the accuracy criteria required for clinical use. Their readouts can easily be affected by changes that are related not only to body temperature but also to skin blood flow (Leon et al., 1990; MacKenzie and Asbury, 1994). The suggestion of simply adding a constant correcting value (e.g., 2.2° C) to an arbitrary skin temperature to estimate central temperature has been shown to be unreliable (Burgess et al., 1978; Leon et al., 1990).
Body temperature varies widely within the body. Because of their high perfusion rates, core tissues tend to maintain a constant temperature, whereas peripheral tissues usually have significantly lower and less uniform temperatures that may differ by several degrees within a short distance from each other (Colin et al., 1971).
It has been suggested that hypothalamic temperature reflects core temperature, although there is no physiologic evidence that hypothalamic temperature precisely represents central temperature (Benzinger, 1969). Core-temperature measuring sites recommended for clinical use are the tympanic membrane, nasopharynx, distal esophagus, pulmonary artery, and, with some limitations, bladder and rectum. These sites usually provide equal readings in humans who are awake and in those who are anesthetized and undergoing noncardiac surgery (Cork et al., 1983). However, different temperatures may be measured at different monitoring sites and under certain clinical conditions, and the physiologic and clinical significance of these differences may vary.
The precision and accuracy of measurements at different body sites have been studied, and each site has its advantages and disadvantages (Cork et al., 1983; Bissonnette et al., 1989b). Ideally, the temperature-monitoring site reflects core temperature and is associated with only minimal or no morbidity.
Skin temperature measurements offer little as a reflection of core temperature (Lacoumenta and Hall, 1984; Bissonnette et al., 1989b). Because there is a wide variation in skin temperature, depending on the site of monitoring, several investigators have suggested monitoring between 4 and 15 sites, using different formulas to accurately describe the mean skin temperature (Shanks, 1975; Puhakka et al., 1994; Ram et al., 2002). For skin temperature to be of clinical value, it must closely reflect central temperature in the perioperative setting so that mild hypothermia and early signs of malignant hyperthermia (MH) can be detected. Beside the fact that increased body temperature is a late sign of MH, it is unlikely that skin temperature correlates well with central temperature during the early stages of MH, because circulating catecholamine concentrations may be up to 20 times higher than normal and result in significant changes of skin perfusion (Sessler, 1986; Sessler and Moayeri, 1990).
Tympanic-membrane temperature has been suggested as the most ideal temperature-monitoring site. Although it is not necessary for the temperature probe to be in direct contact with the tympanic membrane to accurately reflect tympanic temperature, the external auditory canal needs to be sealed by the probe to allow the air column trapped between the probe and the tympanic membrane to reach a steady-state temperature. During the initial postoperative period after infants and children have had cardiac reconstructive surgery, tympanic temperature does not correlate well with brain temperature and therefore does not provide a reliable estimate of central body temperature (Muma et al., 1991; Bissonnette et al., 2000). Because of difficulties associated with obtaining appropriate-sized thermistors and reports of tympanic membrane perforation, the clinical use of continuous intraoperative temperature measurement has been discouraged.
In contrast, oral temperature is generally considered less adequate and is therefore not recommended as an accurate site for intraoperative temperature-monitoring (Cork et al., 1983).
Esophageal temperature probes are often combined with an esophageal stethoscope, which makes this site particularly attractive for the pediatric population. In infants and children, and in patients who are cachetic, the thermal insulation between the tracheobronchial tree and the esophagus is minimal. Therefore, the respiratory gas flow may result in erroneous temperature readings, particularly when the fresh gas flow is high and its temperature differs significantly from body temperature (Bissonnette et al., 1989b). Furthermore, central temperature is measured only if the tip of the probe is placed in the distal third of the esophagus at the point where the heart sounds are the loudest (Bissonnette et al., 1989b; Stoen and Sessler, 1990). In patients with endotracheal tubes, monitoring of esophageal temperature is more reliable than rectal temperature and more practical than tympanic temperature.
Axillary temperature is not only the most commonly used method of measuring temperature, but it is also the most convenient site for temperature monitoring. It has been reported to be as accurate in measuring central temperature as tympanic membrane, esophageal, and rectal temperature sites. However, this accuracy is only achieved when the tip of the thermometer is carefully placed over the axillary artery and the arm is closely adducted (Bissonnette et al., 1989b). Unfortunately, malpositioning of the probe may result in unreliable estimates of core temperature. Infusion of cool solutions at high flow rates in small children on the ipsilateral side of the thermometer probe may result in falsely low temperature readings.
Rectal temperature monitoring can provide a central temperature reading; it is associated with minimal morbidity and its ease of insertion confers major advantages (Bissonnette et al., 1989b). Problems to be considered with its use pertain to the probe’s insulation by feces, its exposure to cooler blood returning from the legs, the influence of an open abdominal cavity during laparotomy, or irrigations of the bladder or the abdomen with either cold or warm solutions. Relative contraindications for rectal temperature probe insertion are inflammatory bowel disease, neutropenia and/or thrombocytopenia, and the need to irrigate the bowel or bladder.
Bladder-temperature monitoring is considered to be one of the most accurate methods of measuring core temperature. Its precision has been demonstrated to be identical to pulmonary-artery–temperature monitoring as long as urinary output is high; however, when urinary output is normal or less than normal, this site may become inaccurate in reflecting central temperature (Horrow and Rosenberg, 1988; Brauer et al., 2000).
Physiology of thermal regulation
Survival from body temperatures as low as 13.7° C has been reported, whereas death resulting from protein denaturation occurs within 7° C above normality at approximately 44° C (Gilbert et al., 2000). This illustrates a tolerance for cold that is more than three times higher than that for heat, which explains why the system for heat dissipation needs to be much more effective than the system for defense against cold.
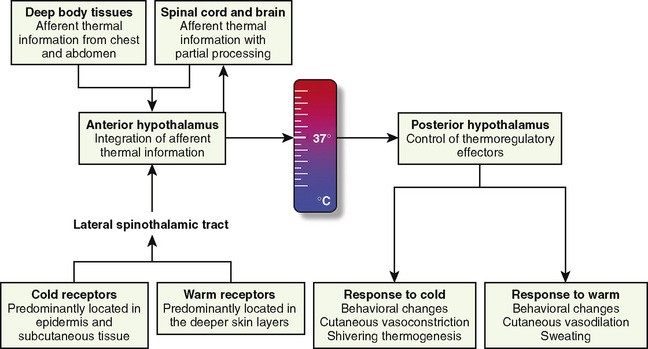
The thermoregulatory system is similar to other physiologic control systems in the sense that the brain uses negative feedback mechanisms to keep temperature variations from normal values minimal. The principal site of temperature regulation is the hypothalamus, which integrates afferent signals from temperature-sensitive cells found in most tissues, including other parts of the brain, spinal cord, central core tissues, respiratory tract, gastrointestinal tract, and the skin’s surface. The processing and regulation of thermoregulatory information occurs in three stages: afferent thermal sensing, central regulation, and efferent response (Fig. 6-1).
Afferent Thermal Sensing
Anatomically distinct warm and cold receptors in the body periphery sense the ambient temperature. The skin contains about 10 times more cold receptors than warm receptors, acknowledging the important function of the skin in the detection of cold (Poulos, 1981). Thermosensitive receptors are also located in close proximity to the great vessels, the viscera, and the abdominal wall, as well as in the brain and in the spinal cord. Each receptor type transmits its information through an afferent-nerve–conduction pathway. Although information originates from anatomically different nerve fibers, the speed of transmission is mainly influenced by the intensity of the stimulus rather than the type of nerve fiber. It is well established that the rate of change in skin temperature alters the apparent importance of the change. Rapid changes contribute up to five times as much to the central regulatory system as slower changes with comparable intensity (Wyss et al., 1975). However, other than in patients undergoing cardiopulmonary bypass surgery (where rapid temperature changes are common), the rate of change in core temperature does not appear to substantially influence the magnitude of the provoked regulatory responses.
Thermal information gathered from peripheral warm receptors, whose maximal discharge rate is between 45° and 50° C, is carried by unmyelinated C fibers. These C fibers also convey pain sensations, which explains why intense heat cannot be distinguished from severe pain (Pierau and Wurster, 1981; Poulos, 1981). Although most ascending thermal information travels along the spinothalamic tracts in the anterolateral spinal cord, no single spinal tract is solely responsible for conveying thermal information (Hellon, 1981).
Central Regulation
The preoptic area of the hypothalamus contains cold- and heat-sensitive neurons, with the latter predominating by a ratio of 4:1 (Boulant and Bignall, 1973). However, the vast majority of the neurons in this area are insensitive to temperature (Nakayama et al., 1963). This area also receives and processes nonthermic afferent information, which seems to be important in controlling the adaptive mechanisms and the behavior of the organism (Hori and Katafuchi, 1998).
Direct heat stimulation of this area results in increased discharge rates from the heat-sensitive neurons and activation of heat-loss mechanisms. Conversely, hypothalamic cold-sensitive neurons respond with increased discharge rates to direct cooling of the preoptic area of the hypothalamus (Boulant, 1974; Boulant and Demieville, 1977). Other centers involved in thermoregulation include the dorsomedial hypothalamus, periaqueductal gray matter, the nucleus raphe pallidus in the medulla oblongata, and the spinal cord, although their functions are not yet fully elucidated (Guieu and Hardy, 1970; Simon, 1974; Cabanac, 1975; Dickenson, 1977).
The contribution of the central thermoreceptors to thermal regulation under normal conditions is limited by the marked predominance of thermal input from peripheral receptors (Downey et al., 1964). These central receptors take over thermoregulation if the sensory input from peripheral sensors is disrupted (e.g., through central neuraxial anesthesia or spinal cord transection), but they are less efficient when compared with peripheral thermoreceptors (Downey et al., 1967).
The threshold temperature defines the central temperature at which a particular regulatory effector is activated (Box 6-1). When the integrated input from all sources is signaling that the interthreshold range is exceeded on either side, efferent responses are initiated from the hypothalamus to maintain normal body temperature.
Box 6-1 Definition of Temperature Regulation Terms
Efferent Response
where T denotes the temperature measured in °C. Other formulas do exist (Ramanathan, 1964; Colin et al., 1971; Shanks, 1975; Puhakka et al., 1994). See the following example:
where MSK reflects the mean skin temperature (in °C), which then equals:
Skin temperature is the most important parameter in triggering behavioral changes; however, in terms of impact on the thermoregulatory autonomic response, the thermal input from the skin contributes only about 20% (Cheung and Mekjavic, 1995; Lenhardt et al., 1999). The main part of this autonomic response depends on afferent information from the central core, which includes the brain (parts other than the hypothalamus), the spinal cord, and deep abdominal and thoracic tissues, with each of them contributing about 20% to the central thermoregulatory control (Jessen and Mayer, 1971; Simon, 1974; Mercer and Jessen, 1978; Jessen et al., 1984).
The most commonly described thermoregulatory model is a set-point system in which hypothalamic integration of thermal information indicates a body temperature above or below a predetermined threshold and then triggers warm or cold defenses. This set-point model, borrowed from engineering models, provides an easy way to explain how the thermoregulatory system functions and how temperature is regulated. In this model, the body compares its actual central temperature against a set reference temperature and then balances heat loss and heat-generating mechanisms to keep the temperature at this set reference point. However, while convenient, this model may not be accurate. More recent research suggests that peripheral and central thermoreceptors are connected through several other neurons to a thermoregulatory effector cell to form a thermoeffector loop (Kobayashi, 1989). In this set-up, once the temperature reaches the range for which the particular thermosensitive neuron has its highest sensitivity, its firing rate increases significantly and—independent of a central nervous system controller—triggers a response in the thermoregulatory effector. Central body temperature in this model is then the averaged result of all the thermoeffector loop actions combined, basically making a central controller (i.e., the hypothalamus) redundant (Kobayashi, 1989; Romanovsky, 2004). Although this more recent model has received a fair amount of attention, it has not yet been widely accepted in clinical practice.
Regardless of the actual model used, if an effective thermoregulatory system is in place, behavioral responses (e.g., heating the home, looking for shelter, or putting on a jacket) to environmental temperatures outside the thermoneutral range (approximately 28° C for an unclothed adult) remain the quantitatively most important thermoregulatory effectors in humans and are far more efficient than all of the autonomic responses combined. Cutaneous vasoconstriction is the first and most consistent thermoregulatory response to hypothermia. Total digital skin blood flow can be divided into nutritional (capillaries) and thermoregulatory (arteriovenous shunts) components. Cold-mediated decreases in cutaneous blood flow are most pronounced (down to 1% of the normal blood flow seen in a thermoneutral environment) in arteriovenous shunts of the hands, feet, ears, lips, and nose (Grant and Bland, 1931; Hillman et al., 1982). These shunts are typically 100 µm in diameter, which means that one can divert 10,000 times as much blood as a capillary with a 10 µm diameter under otherwise unchanged conditions (i.e., same length and pressure gradient) (Hales, 1985).
Flow changes not only in the arteriovenous shunts, but also in the far more numerous capillaries (Coffman and Cohen, 1971). The impressive decrease in cutaneous perfusion secondary to thermoregulatory vasoconstriction results in a heat-loss reduction of 50% from the hands and feet, but only of 17% from the trunk, resulting in an overall heat-loss reduction of only 25% (Sessler et al., 1991).
Thermal regulation in the newborn
Premature infants, infants who are small for gestational age, and even full-term neonates have an exceptionally large skin surface area compared with their body mass (assuming a normal ratio for a full-term neonate of 1, then the ratio for an adult is approximately 0.40). Heat loss is further increased because there is only a thin layer of subcutaneous fat and reduced keratin content of the infant’s skin, which results in increased thermal conductance and increased evaporative heat loss. (Therefore, when compared with adults, neonates lose proportionately more heat through their skin in similar environments). In contrast to adults, the capabilities and the functional range of the neonate’s thermoregulatory system are significantly limited and easily overwhelmed by environmental factors. The lower ambient temperature limit of thermal regulation in adults is 0° C, whereas that in newborns is 22° C. The combination of increased heat loss and a diminished efficacy of the thermoregulatory response with a reduced ability to generate heat puts these infants at high risk for hypothermia. The same anatomic properties that are responsible for the increased risk of hypothermia also allow for rewarming that is three to four times faster in infants and children compared with adults (Szmuk et al., 2001).
The neutral temperature (or the thermoneutral zone) is defined as the ambient temperature range, at which the oxygen demand (as a reflection of metabolic heat production) is minimal and temperature regulation is achieved through nonevaporative physical processes only (i.e., vasoconstriction or vasodilation). The upper limit of this range is called the upper critical temperature and marks the ambient temperature at which evaporative heat losses are triggered. Similarly, the lower critical temperature defines the ambient temperature below which metabolic heat generation is activated (nonshivering and/or shivering thermogenesis). Depending on the neonate’s weight, this neutral temperature zone is in the range of 32° to 35° C (unclothed in a draft-free environment with uniform temperature and moderate humidity), whereas for an unclothed adult it is approximately 28° C (Hey, 1975). In a thermoneutral environment, the cutaneous arteriovenous shunts are open and skin blood flow is maximal.
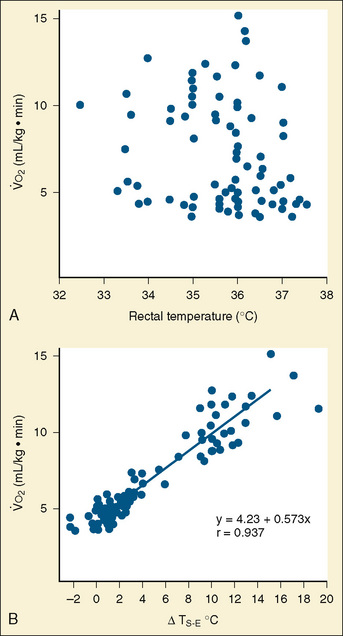
Maintaining core temperature in a cool environment results in increased oxygen consumption and potentially metabolic acidosis. It was demonstrated long ago that oxygen consumption does not correlate with rectal temperature in full-term neonates, but rather it increases directly with the skin surface-to-environment temperature gradient (Adamson et al., 1965). Oxygen consumption is minimal at gradients of 2° to 4° C. Thus, at environmental temperatures of 32° to 34° C and an abdominal skin temperature of 36° C, the resting newborn infant is in a state of minimal oxygen consumption (i.e., in a neutral thermal state). Normal rectal temperature, therefore, does not necessarily imply a state of minimal oxygen consumption in this age group, because the baby could activate all its physiologic defense mechanisms to maintain normal rectal temperature (Fig. 6-2).
Of particular concern in view of thermoregulation in the newborn is the head, which comprises up to 20% of the skin’s total surface area and shows the highest regional heat flux ability (Anttonen et al., 1995). In neonates and infants, the head may account for up to 85% of body-heat losses, which can be explained by the thin skull bones and the usually sparse scalp hair in combination with the close proximity to the highly perfused brain (core temperature) (Fleming et al., 1992). Facial cooling may increase oxygen requirements in the term and preterm infant by up to 23% and 36%, respectively, thereby further demonstrating the effectiveness of protecting the infant from heat loss by covering the head (Sinclair, 1972).
Thermoregulatory vasoconstriction and vasodilation are already present during the first day of life in both the premature and the full-term infant (Bruck, 1961; Lyons et al., 1996). With vasoconstriction, cutaneous blood flow decreases and the effect of tissue insulation increases, which results in an overall reduction in conductive and convective heat losses.
Heat loss mechanisms
The abilities to produce and dissipate heat are fundamental for a homeothermic organism. Controlled heat loss in homeotherms is accomplished in two stages, both governed by the physical laws of conduction, radiation, convection, and evaporation (Box 6-2) (Swyer, 1973). The second law of thermodynamics states that heat can be transferred from a warmer to a cooler object but never from a cooler to a warmer object. What this means is that the warmer object (in the operating room setting, this is almost exclusively the patient) is used to warm up the surrounding cooler objects (e.g., the operating room walls, tables, and instruments). Although most anesthesiologists consider heat loss to be a nuisance, without any heat loss to the environment (i.e., perfect insulation), the body of an awake adult at rest (assuming a metabolic rate of 75 W) would warm up by at least 1° C per hour. (Keep in mind that during exercise, metabolic heat generation can increase up to tenfold.) This can be calculated with the following formula:
Box 6-2 Mechanisms (by Percent) of Heat Loss for a Neonate in a Thermoneutral Environment
| Radiation | 39% |
| Convection | 34% |
| Evaporation | 24% |
| Conduction | 3% |
where HSR is the heat storage rate (W), m is the body mass (kg), k is the specific heat coefficient of the human body (3.5 • 103 J/°C), dTB is the change in body temperature (°C), and dt is the time interval (sec) (Burton, 1935).
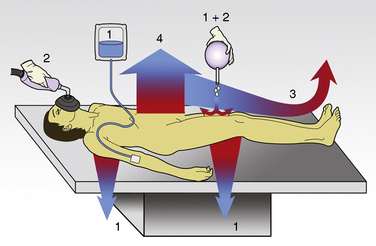
The first stage of heat loss during anesthesia occurs with the transfer of heat from the body core (central compartment) to the periphery and the skin’s surface, which is referred to as the concept of internal redistribution of heat. In the second stage, heat is dissipated from the skin’s surface to the environment (see “Anesthesia and Hypothermia,” p. 168). Physiologic manipulations of regional blood flow and changes in the thermal conductance properties of the insulation tissue can influence both gradients. Most studies of thermal regulation in infants and children have quantified the relative contributions of radiation, convection, evaporation, and conduction to heat loss. A study in newborns in a thermoneutral environment found radiation, convection, evaporation, and conduction to account for 39%, 34%, 24%, and 3%, respectively, of total heat loss (Hey, 1973). However, the conditions in the operating room rarely meet the criteria for thermoneutrality and the relative contributions of each of these four physical components to total heat loss can vary significantly. Figure 6-3 gives an overview of the heat loss mechanisms involved in the operating room setting.
Conduction
The coefficient hk is a property of the material or interface between the two objects that determines the rate of heat transfer per unit area per unit temperature difference (W/m2 • °C). During surgery, relatively little heat should be lost to the environment via conduction, because the patient is supposed to be well insulated from surrounding objects (Allen, 1987). However, conduction is also responsible for heat loss created by warming up cool intravenous fluids and irrigation solutions, which have the potential to significantly and quickly reduce body temperature. Attention should also be paid to ensure that the patient’s skin is not in contact with any metallic surfaces, because metals have a high thermal conductivity, thereby facilitating heat transfer. (In addition, contact with metallic surfaces during surgery must be avoided to prevent skin burns from electrocautery.) The physiologic factors controlling conductive heat loss are cutaneous blood flow and the thickness of the subcutaneous tissue (insulation).
Radiation
where hr denotes the radiation coefficient, an integration of emissivity and the Stefan Boltzmann constant. Heat transfer by radiation principally depends on the temperatures of the two surfaces concerned and is unaffected by air movement or the distance between the surfaces, and it can take place even across a vacuum (Allen, 1987).
As previously stated, newborns and infants have a large surface area-to-mass ratio, thus radiant heat loss is proportionally greater the smaller the infant. In both the infant who is awake and the infant who is anesthetized, radiation is the major factor for heat loss under normal conditions. The human body is an excellent emitter of energy at wavelengths relevant to heat transfer, and the probability of photon reflection in the standard operating room is almost zero. Radiant heat loss in the operating room is therefore a function of the temperature difference between the patient’s body and the room (i.e., the floor, walls, and ceiling) and all the objects in it. Warming up the operating room (and its contents) reduces not only the temperature gradient between the patient and the environment but also radiant heat losses. However, as long as a temperature gradient exists, the patient continues to warm up the surrounding environment. At a room temperature of 22° C, about 70% of the total heat loss is a result of radiation (Hardy et al., 1941). A simple single-layer covering of the body dramatically reduces the heat loss by convection and radiation; thus, a thin shirt (e.g., a silk blouse, although it provides only negligible insulation) already results in considerably increased thermal comfort.
Evaporation
Evaporative heat loss occurs through the skin and the respiratory system. Under conditions of thermal neutrality, evaporation accounts for 10% to 25% of heat loss. Physical factors governing evaporative heat loss include relative humidity of the ambient air, velocity of airflow, and lung minute ventilation. The driving force behind evaporation is the vapor pressure difference between the body surface and the environment. Evaporative losses include mainly three components: sweat (sensible water loss); insensible water loss from the skin, respiratory tract, and open surgical wounds; and evaporation of liquids applied to the skin, such as antibacterial solutions. The evaporation of water from a surface is dependent on energy that is absorbed from the surface during the transition from a liquid to a gaseous state. This energy is called the latent heat of vaporization, and in the case of sweat, it has a value of 2.5 × 106 J/kg. This figure emphasizes the extraordinary power of the human sweating mechanism as a means of dissipating heat, especially considering that an adult in excellent physical condition can produce up to 2 or 3 liters of sweat per hour (Armstrong et al., 1986; Godek et al., 2008). In an environment where the air temperature is equal to or higher than the skin temperature, sweating is the only mechanism available for dissipation of heat that originates from metabolic production. In this situation, anything that limits evaporation, such as high ambient humidity or impermeable clothing, may easily lead to heat storage and a potentially fatal rise in body temperature. Evaporative heat loss can be calculated as follows:
where V is the airflow velocity (m/sec). The important point to note is that evaporation is determined by the vapor pressure gradient between the exposed body surface and the ambient air and the rate of airflow across the surface (Allen, 1987).
Physiologic factors affecting evaporative losses relate to an infant’s ability to sweat and to increase the minute ventilation. Although the physical characteristics of the newborn predispose him or her to heat loss, it has been demonstrated that neonates are capable of sweating in a warm environment (Bruck, 1961). Full-term neonates begin to sweat when rectal temperature reaches 37.5° to 37.9° C and ambient temperature exceeds 35° C. Although the onset of sweat production in infants who are small for gestational age is slower than it is in full-term infants, the maximum rates of sweat production are comparable (Sulyok et al., 1973). However, premature infants with a gestational age below 30 weeks show no sweating response because the lumen of their sweat glands are not yet fully developed.
Only a small amount of heat is lost when dry, inspired respiratory gases are humidified by water evaporating from the tracheobronchial epithelium. In adults, respiratory losses account for only 5% to 10% of total heat loss during anesthesia and surgery, and total insensible losses account for approximately 25% of the total heat dissipated (Bickler and Sessler, 1990). Minute ventilation on a per-kilogram basis in infants and children is significantly higher than in adults; thus, respiratory heat loss represents about one third of the total heat loss. Obviously, respiratory heat loss increases if the patient breathes cool, dry air as opposed to warm, moisturized air (Bissonnette et al., 1989a, 1989b).
Evaporative heat loss from a large surgical incision may equal all other sources of intraoperative heat loss combined (Roe, 1971). Because of increased evaporative heat loss, hypothermia is also more likely to occur if the skin of the patient is wet or comes in contact with wet drapes.
Heat generation
The ability to produce heat by increasing the metabolic rate and oxygen consumption is the other prerequisite of thermal regulation for a homeothermic organism (Hull and Smales, 1978). Beside the fact that three of the physical mechanisms leading to heat loss (i.e., conduction, radiation, and convection) can theoretically also be used to passively warm up a patient, the body has the ability to actively produce heat. Heat generation can be achieved through four mechanisms:
The behavioral aspect of heat production (voluntary muscle activity) is usually not functional in the perioperative period and therefore its role in heat production will not be discussed further here. Of the three remaining mechanisms for heat production, nonshivering thermogenesis is the major component in the newborn, whereas shivering thermogenesis is the main mechanism for heat production in older children and adults. The contribution of nonshivering thermogenesis in adults is debatable (Jessen, 1980a).
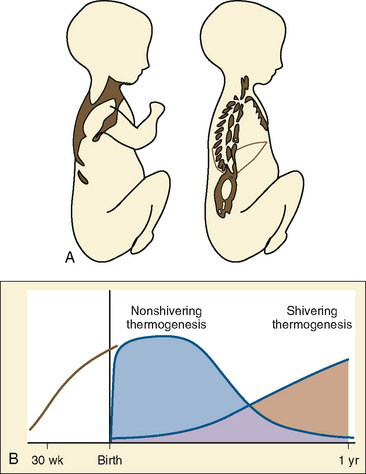
Although both the time course and the relationship between nonshivering and shivering thermogenesis in infants have been described, the exact time sequence and factors involved in the developmental aspects of switching on shivering thermogenesis and nonshivering thermogenesis off remain to be elucidated (Hull and Smales, 1978). The importance of nonshivering thermogenesis seems to decrease rapidly after the first year of life, while at the same time shivering thermogenesis is becoming more and more effective (Fig. 6-4B). Under normal conditions, dietary thermogenesis will not contribute significantly to thermogenesis during anesthesia. It only affects temperature if the patient is given food with a high protein or fructose content before or during anesthesia, which is normally not the case.
Nonshivering Thermogenesis
Brown fat differentiates in the human fetus between 20 and 30 weeks of gestational age (Lean et al., 1986a). It comprises only 2% to 6% of the infant’s total body weight and is found in six main locations: between the scapulae, in small masses around blood vessels of the neck, in large deposits in the axillae, in medium-size masses in the mediastinum, around the internal mammary vessels, and around the adrenal glands or kidneys (Fig. 6-4A).
Brown fat is a highly specialized tissue; the brown color is secondary to the abundant content of mitochondria in the cytoplasm of its multinucleated cells. These mitochondria are densely packed with cristae and have an increased content of respiratory-chain components (Himms-Hagen, 1976). They are unique in their ability to uncouple oxidative phosphorylation, resulting in heat production instead of generating adenosine triphosphate. This uncoupling process is mediated by the presence of the uncoupling protein 1 (UCP-1, or thermogenin) that is located on the inner mitochondrial membrane (Himms-Hagen, 1976; Ricquier and Kader, 1976).
Brown fat is highly vascularized and has a rich sympathetic innervation, which appears to be primarily β-sympathetic in origin and responsible for the uncoupling of oxidative phosphorylation (Karlberg et al., 1962, 1965). In respect to nonshivering thermogenesis, mature brown fat cells mainly rely on activation by β3-receptors. Cold stress increases sympathetic nervous system activity and norepinephrine release, which causes increased lipase activity in the brown fat tissue (Schiff et al., 1966). As a consequence, hydroxylation of triglycerides and release of free fatty acids occur. These free fatty acids act on UCP-1 and thereby increase the protein conductance across the mitochondrial membrane. In addition to norepinephrine, glucocorticoids and thyroxin have been implicated as factors that trigger nonshivering thermogenesis (Gale, 1973; Jessen, 1980b, 1980c). The heat produced by nonshivering thermogenesis is mainly a by-product of fatty acid metabolism, but to a minor degree it can also result from glucose metabolism. The activation of brown fat metabolism results in an increased proportion of the cardiac output being diverted through the brown fat. This proportion may reach as much as 25% of the cardiac output, which facilitates the direct warming of the blood.
Pharmacologic inhibition of nonshivering thermogenesis can be achieved with ganglionic and β-receptor blockade, inhalational anesthetics, and surgically by sympathectomy (Silverman et al., 1964; Stern et al., 1965; Ohlson et al., 1994). Inhibition of nonshivering thermogenesis by inhalational anesthetics starts as early as 5 minutes after turning on the vapor and starts to wean off within approximately 15 minutes after discontinuation of the inhalational anesthetic (Ohlson et al., 1994). Nonshivering thermogenesis is also inhibited in infants who have been anesthetized with fentanyl and propofol (Plattner et al., 1997).
In general, nonshivering thermogenesis seems to be quite variable in adults, but most often it does not appear to be functional or relevant (Ohlson et al., 1994; van Marken Lichtenbelt and Daanen, 2003). This assumption is supported by the fact that oxygen consumption does not increase significantly when patients exhibit thermoregulatory vasoconstriction (Mestyan et al., 1964; Dawkins and Scopes, 1965). However, it seems that adults have the potential to regenerate brown fat tissue under certain pathologic conditions, such as pheochromocytoma (secondary to high and sustained sympathetic stimulation), Chagas’ disease, hibernoma (a benign brown-fat tissue tumor), or marked cold acclimatization (Garruti and Ricquier, 1992; Lean et al., 1986b; Vybiral et al., 2000).
In contrast, premature and full-term neonates, as well as infants, are able to double their metabolic heat production during cold exposure (Mestyan et al., 1964; Dawkins and Scopes, 1965; Hey and Katz, 1969). Clinically significant nonshivering thermogenesis is possible within hours after birth and may persist up to the age of 2 years (Fig. 6-4, B) (Oya et al., 1997). Despite the fact that nonshivering thermogenesis is the main source of thermoregulatory heat production in infants, it should be kept in mind that its effect and sustainability are limited and do not compensate for the decreased ability of newborns and infants to effectively reduce heat loss through cutaneous vasoconstriction or the lack of heat production through shivering.
Core hypothermia or exposure to cold during general anesthesia with propofol and fentanyl do not trigger nonshivering thermogenesis in children; therefore, nonshivering thermogenesis seems to be nonfunctional (Plattner et al., 1997). Halothane anesthesia has been shown to block nonshivering thermogenesis in children (Ohlson et al., 1994; Dicker et al., 1995). It has been demonstrated in animal studies that pharmacologic inhibition of nonshivering thermogenesis by β-blockade also affects shivering thermogenesis (Bruck and Wunnenberg, 1965). In the animals studied, shivering did not fully compensate for the lack of heat produced by nonshivering thermogenesis. The magnitude of nonshivering thermogenesis in animals varies among species, but it appears that in newborn versus adult animals and in cold-adapted versus warm-adapted animals, the contribution of nonshivering thermogenesis to heat generation is significant (Himms-Hagen, 1976).
Shivering Thermogenesis
The precise mechanisms and factors that govern the onset of shivering and the decline of nonshivering thermogenesis are unclear. With increasing age, shivering thermogenesis takes over a more prominent role in thermoregulation. It has been shown that shivering is triggered only after all the other cold defense mechanisms, such as behavioral responses, nonshivering thermogenesis (both ineffective under anesthesia), and maximal thermoregulatory vasoconstriction, have failed to maintain body temperature within the interthreshold range (Hemingway, 1963; Hemingway and Price, 1968). Until recently, newborns and infants were considered to be unable to shiver, presumably because of the general immaturity of the musculoskeletal system on the one hand and the limited muscle mass on the other hand, which would render muscle activity ineffective in defense against cold. However, a few reports exist about shivering in neonates occurring at rectal temperatures of 35.0° to 35.3°C (Brück, 1992; Petrikovsky et al., 1997). It is debatable whether this shivering was indeed thermoregulatory in origin or whether drugs and other factors (all mothers received an intrapartum amnioinfusion) were to blame. For clinical purposes, it is probably reasonable to say that neonates do not shiver, and if they do, it is of no (or only minor) significance for thermoregulation.
For a short time and only in an otherwise healthy and young person, shivering can result in an up to a sixfold increase in metabolic heat production and oxygen consumption, but only a twofold increase is sustainable (Horvath et al., 1956; Benzinger, 1969; Ciofolo et al., 1989; Just et al., 1992; Giesbrecht et al., 1994). Oxygen consumption in elderly patients (usually the age group with the highest risk for adverse cardiac events) increases on average by approximately 130% during shivering (Bay et al., 1968).
Shivering is characterized by involuntary, irregular muscular activity usually beginning in the muscles of the upper body (commonly the masseter). Overt shivering is preceded by a generalized increase in muscle tone, and only once this muscle tone reaches a certain threshold will shivering be detectable (Guyton, 2000). The intensity of shivering is higher in central muscles than it is in peripheral muscles (Bell et al., 1992).
Shivering occurs in two different electromyographic patterns: a basal, continuous shivering with low intensity at a rate of 4 to 8 Hz and superimposed bursts of high intensity at a rate of 0.1 to 0.2 Hz. The former is associated with type 1 and the latter with type 2 muscle fibers, with the bursts creating the typical “waxing and waning” pattern in the electromyogram (Stuart et al., 1966; Haman et al., 2004).
In healthy patients, this rise in oxygen consumption is easily met by increased cardiac output without any signs of cardiopulmonary compromise. In patients with already limited hemodynamic, coronary, or pulmonary reserves, this increase in oxygen demand can lead to decreased mixed venous oxygen content and eventually to decreased arterial oxygen content and tissue hypoxia. An inverse correlation has been shown between intraoperative temperature and postoperative oxygen demand, as well as between different anesthetics (see “Thermoregulation and General Anesthesia,” p. 172) (Roe et al., 1966). Shivering is not only an unpleasant experience for the patient in the postoperative period; it has also been implicated in increased intracranial and intraocular pressure, wound dehiscence, and dental damage (Mahajan et al., 1987; Rosa et al., 1995; Alfonsi, 2001).
Although the incidence of postoperative shivering is inversely related to the core temperature, shivering was also found in patients who were kept strictly normothermic during isoflurane or desflurane anesthesia, indicating that a substantial fraction of shivering is nonthermoregulatory, with pain being one potential trigger (Horn et al., 1998, 1999). Inhibition of shivering with meperidine in awake, actively cooled volunteers resulted in a more than threefold higher and more than four-times prolonged core temperature afterdrop with a rewarming rate decreased by 37% when compared with the shivering control group (Giesbrecht et al., 1997).
Dietary Thermogenesis
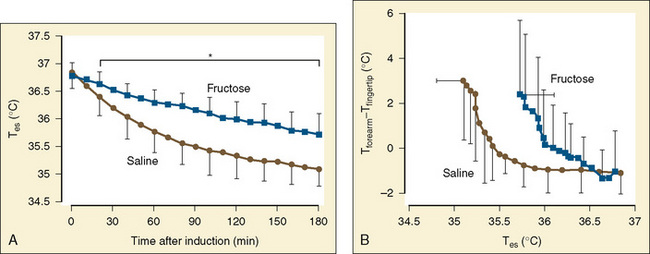
Stimulation of energy expenditure and thermogenesis by certain nutrients (i.e., proteins and amino acids) is a well-known phenomenon (Lindahl, 2006). Despite muscle paralysis and decreased metabolism during general anesthesia, the infusion of small amounts of amino acids resulted in up to a fivefold increase in heat generation during anesthesia compared with that in adults who were awake (Sellden et al., 1994). Using pre- and intraoperative amino-acid infusions, the same researchers were able to use this advantage clinically to achieve a core temperature of 36.5° ± 0.1° C at the end of surgery, whereas the temperature dropped to 35.7° ± 0.1° C in the control group (Sellden and Lindahl, 1999). Similar findings have been reported for preoperative amino-acid infusion in patients undergoing spinal anesthesia (Kasai et al., 2003). Although effective, the exact mechanism behind this form of thermogenesis has not yet been fully elucidated. It seems that stimulation of cellular amino-acid oxidation is crucial. Furthermore, protein synthesis and breakdown in extrasplanchnic tissues that require extra synthesis of ATP, could be a contributing factor as well. Approximately half of the heat generated in association with amino-acid infusions is splanchnic in origin. Blood flow in extrasplanchnic (but not splanchnic) tissues increases significantly, reflected by a raise in cardiac output of almost 20% (Brundin and Wahren, 1994). Except for a different time course, the average whole-body thermic effect of intravenous amino-acid administration is not different from the one seen with oral protein ingestion. Fructose administration has also been shown to increase the metabolic rate by 20% in anesthetized adult surgical patients. In a study of 20 patients, Mizobe et al. (2006) noted that intravenous fructose increased the intraoperative core temperature, oxygen consumption, and the vasoconstrictive threshold (Fig. 6-5). Dietary thermogenesis has the potential to cause hyperthermia not only during general anesthesia with attenuated thermoregulation, but also in the patient who is awake (Sellden, 2002).
Effect of anesthesia on thermoregulation
Anesthesia and Hypothermia
There is no generally accepted definition for hypothermia, but the distinction between mild (core temperature 34.0° to 35.9° C), moderate (32.0° to 33.9° C), and severe hypothermia (below 32.0° C) appears useful and reasonable for clinical purposes (Brux et al., 2005). General anesthesia reduces the threshold at which the body initiates a thermoregulatory response to cold stress (Boxes 6-3 and 6-4). Mild intraoperative hypothermia (1° to 3° C below normal) is common and results from a combination of the following events:
Box 6-4 Specific Effects of Anesthetics on Thermoregulation
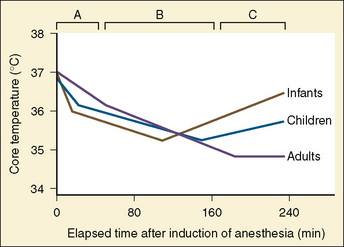
Hypothermia during general anesthesia has a typical profile and usually develops in three phases (Fig. 6-6):
Internal Redistribution
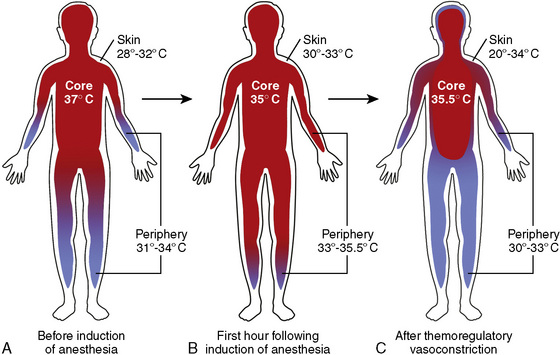
In order to understand the concept of internal redistribution, it is helpful to use the previously described division of the human body into three thermal compartments, the central, peripheral, and skin (or “shell”) compartments. The core temperature represents the central compartment temperature, which contains the vessel-rich group organs receiving approximately 75% of the cardiac output and representing about 10% of the body weight in adults and up to 22% in neonates. In an awake adult at rest, the entire central compartment accounts for approximately 66% of the body mass and extends to about 71% during general anesthesia (Deakin, 1998).
With induction of anesthesia, the central core temperature starts to decrease rapidly by approximately 0.5° to 1.5° C during the first 30 to 45 minutes of anesthesia (Fig. 6-7). Although this process results in reduced core temperature, the total body-heat content decreases only slightly. Heat—as the name implies—is mainly redistributed and not dissipated. This redistribution of heat accounts for 81% of the core temperature decrease in the first hour of anesthesia, whereas the remainder is the result of the anesthesia-induced reduction in metabolism and increased heat loss. For the subsequent 2 hours of anesthesia, the impact of redistribution on total heat loss decreases to approximately 43% (Matsukawa et al., 1995b). Accordingly, administration of a vasoconstrictor, such as phenylephrine, can limit the drop in core temperature caused by redistribution (Ikeda et al., 1999a).
Internal redistribution results in shrinkage of the peripheral compartment and enlargement of the central compartment, which explains not only the decreased core temperature, but also the increased temperature in the peripheral and skin compartments (Fig. 6-7). This is reflected by a more than fourfold increase in the perfusion of the forearms and particularly the legs after induction of anesthesia, and a forearm-fingertip or calf-toe temperature gradient that may exceed 8° C (Matsukawa et al., 1995b).
Thermal Imbalance
The second phase, which is the result of reduced heat production combined with increased heat loss to the environment, lasts about 2 to 3 hours (see Fig. 6-6). This heat loss leads to an approximately linear decrease in mean body temperature (typically 0.5° to 1.0° C/hr). Decreased heat production during anesthesia is caused by limited or absent muscle activity, work of breathing, and the reduced metabolic rate (Stoen and Sessler, 1990; Washington et al., 1992). Heat loss to the environment is a function of the temperature difference between body surface and ambient structures (concept of patient warming up the environment), and therefore decreases passively as the patient becomes more hypothermic. As mentioned earlier, radiation, convection, evaporation, and conduction all contribute to heat loss from the patient to the environment during anesthesia and surgery.
Thermal Steady State (Plateau or Rewarming Phase)
The third stage of the hypothermic response to anesthesia consists of a thermal steady state, in which metabolic heat production equals heat dissipation to the environment, and the core temperature therefore remains constant (see Fig. 6-6). This plateau normally occurs between a core temperature of 34.5° to 35.5° C. This is only possible if the patient increases the heat production, decreases the heat loss, or both to prevent further hypothermia.
A study of adults who were anesthetized with isoflurane showed that the effect of thermoregulatory vasoconstriction reduces heat loss by a maximum of 25%, which is relatively small compared with the fall in metabolic rate and the increase in evaporative heat loss from the surgical incision (Sessler et al., 1992). Heat loss to the environment is determined mainly by the capillary blood flow in large areas of the skin that cover the limbs and the trunk. These capillaries markedly outnumber the arteriovenous shunts, but they cannot constrict as effectively as the shunts. It is possible that vasoconstriction contributes to the thermal plateau by reestablishing the temperature gradient between the central and the peripheral compartments and thereby preventing metabolic heat from being transported to the periphery, from which it would dissipate (see Fig. 6-7). The metabolic heat produced in the body core is once again confined to a smaller central compartment, allowing the core temperature to remain constant.
To reinforce this theory of compartment size, it should be noted that the use of a limb tourniquet during surgical procedures can influence the thermoregulatory response in children and adults (Bloch et al., 1992; Estebe et al., 1996). The tourniquet may induce hyperthermia, which is most likely the result of decreased effective heat loss from distal skin areas, as well as from metabolic heat constraint to the central thermal compartment. Upon release of the tourniquets, the core temperature drops quickly to levels similar to those in patients who were part of a control group without a tourniquet (Estebe et al., 1996; Sanders et al., 1996; Akata et al., 1998). Despite having a constant core temperature, total body-heat content continues to decrease, because heat loss to the environment continues.
Unlike in adults, this third phase in infants and children is a rewarming rather than a plateau (see Fig. 6-6). As mentioned, general anesthesia decreases heat production by inhibiting muscular activity and nonshivering thermogenesis and by reducing the metabolic rate. Thus, the only possible explanation for this rewarming phase must be the occurrence of marked vasoconstriction within the peripheral and central compartments that results in shrinkage of the central compartment. The amount of metabolic heat produced is then distributed within a smaller central compartment volume and results in a higher core temperature. This is associated with a simultaneous increase in oxygen consumption, carbon dioxide production, and systemic norepinephrine levels, an effect that has been observed in infants who were anesthetized with isoflurane and paralyzed with vecuronium (Bissonnette, personal observation).
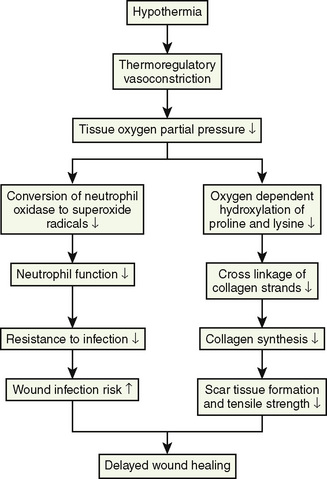
In contrast to adult responses, intraoperative thermoregulatory responses in infants are effective enough to significantly increase the core temperature despite a constant ambient temperature. A clinical study in anesthetized children found a twofold increase in oxygen consumption during mild hypothermia (Ryan, 1982). Both active and passive rewarming imposes significant physiologic stress on the infant. Passive surface rewarming (with the use of warm blankets, bundling, or other measures) turns off the skin’s cold receptors. If normal core temperature is not reached or maintained with passive surface rewarming, hypothermia may result in hypoventilation or even apnea, relative anesthetic overdose (reduced minimum alveolar concentration [MAC] at lower temperatures), and metabolic acidosis. The increased oxygen demand to maintain normal core temperature in the anesthetized infant may create or exacerbate a preexisting cardiopulmonary insufficiency. The release of norepinephrine to trigger vasoconstriction may contribute to the development of acidosis and hypoxia, thereby facilitating right-to-left pulmonary shunting. Sustained pulmonary artery hypertension and right-to-left pulmonary shunting may begin a vicious cycle of hypothermia (Fig. 6-8).
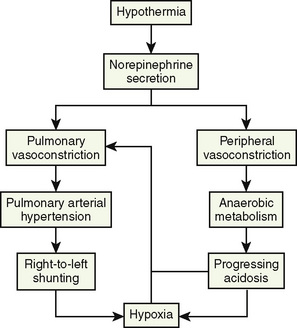
FIGURE 6-8 The vicious cycle of hypothermia in neonates and infants.
(Modified from Klaus M, Faranoff A: Care of the high-risk neonate, Philadelphia, 1986, WB Saunders.)
In infants, a correlation between intraoperative hypothermia and an early increase in postoperative oxygen consumption has been demonstrated (Roe et al., 1966).
Anesthesia and Hyperthermia
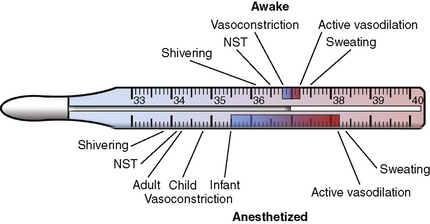
Similar to hypothermia, hyperthermia triggers important physiologic thermoregulatory responses by using thresholds and gains. Similar to hypothermia, the threshold temperature represents the central temperature for which a particular regulatory effector becomes active, whereas the gain quantifies the intensity of the response (Fig. 6-9). The effector mechanisms during hyperthermia are well preserved during anesthesia (Lopez et al., 1993). The efferent-response thresholds are shifted to higher temperatures with an expansion of the interthreshold range, which corresponds to the difference between the normal central temperature and the first efferent response triggered by the hypothalamus. An interesting observation with regard to the interthreshold range resides in the difference between the shift observed for hypothermia and the shift seen for hyperthermia.
The poikilothermic range to the hypothermic side in the anesthetized patient may be expanded by up to 3.5° C. In contrast, clinical studies in human volunteers have demonstrated that the threshold for active vasodilation and sweating is only 1.0° to 1.4° C higher in anesthetized patients than in those who are awake (Lopez et al., 1993). This observation suggests that the human physiology responds more aggressively to the threats from hyperthermia than from hypothermia. Thus, hyperthermia seems to be a far more dangerous threat to the body than a comparable degree of hypothermia (Lopez et al., 1993).
The efferent responses during hyperthermic stress are limited to two mechanisms: active thermoregulatory vasodilation and sweating. Vasodilation triggered in response to warm stress is not simply the absence of vasoconstriction, but rather an active process mediated by sympathetic cholinergic impulses and release of vasoactive substances (e.g., vasoactive intestinal peptide [VIP], histamine) that results in increased dissipation of heat (Detry et al., 1972; Rübsamen and Hales, 1984; Kellogg et al., 1998; Bennett et al., 2003; Wilkins et al., 2004). It has been demonstrated that the effect of hyperthermia on the peripheral vasculature results in a significant increase in blood flow (Tankersley et al., 1991; Matsukawa et al., 1995b). The observation of active cutaneous vasodilation in infants under anesthesia, although difficult to quantify (skin flushing), suggests that this thermoregulatory response to hyperthermia is preserved.
Sweating represents an increase in evaporative cutaneous heat loss during episodes of heat stress. The relatively high heat of vaporization of sweat (2.5 × 106 J/kg) makes sweating an extremely effective process. This allows for up to a fivefold increase in heat loss to the environment, making it proportionally more effective than all the cold-defense mechanisms combined (Fusi et al., 1989).
A study in adult volunteers showed that sweating remains functional during isoflurane anesthesia (Sessler, 1991b).
The benefits provided by induced hyperthermia (vasodilation) may be desirable during peripheral microvascular surgery, where an increase in regional blood flow is important. One of the clinical limitations of induced hyperthermia to increase cutaneous blood flow is the efficiency of the sweating mechanism. Despite active transfer of approximately 50 W of heat across the patient’s skin via convection and radiation, it was possible to demonstrate that the central temperature remains relatively constant or even decreases for exactly this reason (Sessler, 1993). Although shivering can easily double the heat production, sweating can result in the dissipation of more than 10 times the amount of normal basal heat production (Guyton, 2000).
Thermoregulation and General Anesthesia
Both intravenous and inhalational anesthetics can interfere with thermal regulation at peripheral and central receptor sites. In adults, general anesthesia has been shown to lower the thermoregulatory threshold temperature, triggering an average response to hypothermia by approximately 2.5° C, while the increases in the threshold temperature initiating a response to hyperthermia is less pronounced (approximately 1.3° C) (see Fig. 6-9) (Sessler et al., 1988a; 1988b). This anesthesia-induced expansion of the interthreshold range results in a wider temperature range over which active thermoregulatory responses are absent. Within this range, humans behave poikilothermically, with the body temperature changing passively in proportion to the difference between metabolic heat production and heat loss to the environment. Vasoconstriction and nonshivering thermogenesis are the only thermoregulatory responses available to anesthetized, paralyzed, hypothermic infants and children. Patients with mild hypothermia during surgery (e.g., a central body temperature of about 34.5° C) demonstrate profound peripheral vasoconstriction, which can easily be verified using the skin’s surface temperature gradients (e.g., forearm versus fingertip skin temperature), volume plethysmography, a laser Doppler flowmeter, or other techniques (Stuart et al., 1966; Sessler et al., 1988a, 1988b).
Thermoregulation and Inhalational Anesthetics
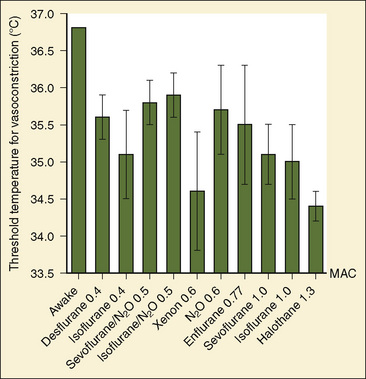
The maximum intensity of peripheral thermoregulatory vasoconstriction during anesthesia is similar to that in volunteers who are awake, indicating that the gain of the response is preserved, but that it is triggered at a markedly lower threshold temperature. The only exception seems to be desflurane, which lowers not only the threshold temperature for vasoconstriction but also its gain (Kurz et al., 1995b). The temperature at which vasoconstriction and nonshivering thermogenesis are triggered identifies the corresponding lower thermoregulatory threshold for the anesthetic agent at any given concentration or dose.
Halothane administration in a concentration of 1.0% in oxygen to healthy adults undergoing donor nephrectomy reduced the threshold temperature for thermoregulatory vasoconstriction to 34.4° ± 0.2° C (Sessler et al., 1988a). In infants and children who were anesthetized with 0.6% halothane combined with a caudal epidural block with bupivacaine, the threshold temperature for vasoconstriction was 35.7° C (Bissonnette and Sessler, 1992). Of note, in children with a body weight of over 30 kg, the central temperature continued to drop even after the vasoconstriction threshold temperature had been reached, whereas children and infants with a body weight below 30 kg were able to maintain or even slightly increase their central temperature. This shows that thermoregulatory defense in infants and younger children is more effective than in older children and adults.
The administration of subanesthetic concentrations of nitrous oxide (10% to 25% in a normoxic mixture) to healthy adult volunteers resulted in a significant but dose-independent reduction of shivering thermogenesis (Cheung and Mekjavic, 1995).
In a concentration of 0.6 (63%) MAC, administration of nitrous oxide resulted in a calculated reduction of the vasoconstriction threshold to 35.7° ± 0.6° C (Goto et al., 1999). Overall, nitrous oxide decreased the thermoregulatory vasoconstriction threshold less than equally potent concentrations of sevoflurane and isoflurane.
In a small study of adults anesthetized with isoflurane, the decrease in the threshold temperature for thermoregulatory vasoconstriction was found to be inversely correlated to the isoflurane concentration, with the threshold temperature decreasing by approximately 3° C per 1% increase in end-tidal isoflurane concentration (Stoen and Sessler, 1990). In a more recent study, the same group found that this dependence on dose was not linear, with isoflurane reducing the threshold temperature disproportionately at higher anesthetic concentrations (Xiong et al., 1996). In adults anesthetized with 0.7% isoflurane, the shivering-temperature threshold decreased, as did the maximum intensity of shivering. The gain of shivering increased significantly and was associated with a pattern of clonic muscular activity that was not a component of regular shivering (Ikeda et al., 1998a).
The thermoregulatory vasoconstriction-threshold temperature in infants and children anesthetized with isoflurane differs only slightly from that in adults (Bissonnette and Sessler, 1990). In pediatric patients anesthetized with 1 MAC halothane in 70% nitrous oxide, this same threshold temperature is higher (35.8° ± 0.5° C) than reported for adults (34.4° C) (Bissonnette and Sessler, 1990; Sessler et al., 1988a). In the adult study, the patients were anesthetized without nitrous oxide and the administered halothane concentration (1.3 MAC) was significantly higher than the one used in the pediatric study (1.0 MAC). In a separate study, a similar thermoregulatory threshold (35.8° ± 0.3° C) was found in pediatric patients who were anesthetized with 1 MAC of halothane in oxygen combined with a caudal epidural block with bupivacaine (Nebbia et al., 1996).
Thermoregulatory inhibition during general anesthesia with equally potent concentrations of halothane is therefore likely to affect adults and children similarly. The high surface area-to-mass ratio in infants, which allows for a rapid loss of heat to the environment, is largely offset by the high intrinsic metabolic rate. Heat loss is further reduced by the well-developed thermoregulatory vasoconstriction mechanism (Bissonnette and Sessler, 1990). Although a trend toward increased threshold temperatures was noted in smaller infants and children anesthetized with similar (age-corrected) concentrations of isoflurane, differences between groups were not statistically significant and spanned only about 0.3° C. This indicates that inhibition of thermoregulatory vasoconstriction is similar in anesthetized infants and children and relatively independent of body weight (Bissonnette and Sessler, 1990). This relatively constant degree of thermoregulatory inhibition in infants and children of different ages is in marked contrast to the age-related changes in the MAC of isoflurane. In infants 1 to 6 months of age, the MAC for isoflurane is approximately 1.5 times higher than the MAC for adults.
With regard to decreasing the threshold temperature for thermoregulatory vasoconstriction, sevoflurane was found to be similar to, although slower than, isoflurane, (Ozaki et al., 1997; Saito, 1997).
Desflurane increases the sweating threshold temperature in a concentration-dependent, linear way. The threshold temperatures for vasoconstriction and shivering at 0.8 MAC are comparable with the other volatile anesthetics, however, at 0.5 MAC, the drop in vasoconstriction threshold temperature was less pronounced. Thus, for desflurane there may be a nonlinear concentration-response relationship for cold-defense mechanisms (Annadata et al., 1995b). Desflurane also reduces the gain of thermoregulatory vasoconstriction (Kurz et al., 1995).
Enflurane is a peculiar inhalational agent with respect to its thermoregulatory effects. In healthy adult volunteers anesthetized with 1.3% enflurane (equivalent to approximately 0.77 MAC), the threshold temperature for thermoregulatory vasoconstriction was 35.1° ± 0.6° C without any patient stimulation and 35.5° ± 0.8° C during painful electrical stimulation. This result demonstrated a slight, although clinically insignificant, effect of nociception in offsetting the anesthesia-induced thermoregulatory inhibition (Washington et al., 1992). Combination with caudal or lumbar epidural blockade eliminates this effect during abdominal and peripheral surgical procedures. Thermoregulatory studies with enflurane in the pediatric population are hampered by the lack of enflurane MAC studies for this age group. In pediatric patients aged 1 to 12 years, 1.67% enflurane (equal to 1 MAC in adults, which is estimated to be equivalent to 0.75 to 1.0 MAC for the age group studied) combined with caudal bupivacaine caused a profound drop in the threshold temperature for thermoregulatory vasoconstriction (Nebbia et al., 1996). Most patients in this study failed to achieve thermoregulatory vasoconstriction despite reaching a mean core temperature of 33.9° ± 0.9° C. It was therefore concluded that the risk of hypothermia with enflurane is significantly higher when compared with the risk for isoflurane or halothane.
The effects of different inhalational anesthetics on the threshold temperature for thermoregulatory vasoconstriction are summarized in Figure 6-10. In addition, hypothermia can affect the physical characteristics of inhalational anesthetics, as well as the pharmacokinetics and pharmacodynamics of intravenous anesthetic drugs. Hypothermia reduces the MAC of inhalational agents (for isoflurane there is a linear MAC decrease of 5.1% per °C drop in core temperature) and increases their tissue solubility (Vitez et al., 1974; Eger and Johnson, 1987; Antognini, 1993; Antognini et al., 1994; Liu et al., 2001). Thus, for any inspired concentration of an inhalational anesthetic agent in a hypothermic patient, an increased amount of the agent is delivered to the tissues, when in fact the anesthetic requirements are decreased. Hypothermia also affects the pharmacokinetics of barbiturates and narcotics (Kadar et al., 1982; Koren et al., 1987).
Thermoregulation and Intravenous Agents
The effect of opioids on thermoregulation remained unclear until a few years ago. Alfentanil has been shown to significantly reduce the threshold temperature for thermoregulatory vasoconstriction. This reduction appears to be linear and in proportion to the plasma drug concentration (Kurz et al., 1995a). Meperidine and sufentanil linearly reduce the shivering threshold temperature (Alfonsi et al., 1998). Meperidine reduces the threshold temperature for shivering twice as much as it does the temperature for vasoconstriction, a side effect that is clinically used to treat postoperative shivering (Ikeda et al., 1997). Neither meperidine nor alfentanil reduce the gain and the maximum shivering intensity (Ikeda et al., 1998b). Tramadol slightly decreases the threshold temperature for sweating, whereas the threshold temperatures for vasoconstriction and shivering decrease linearly with the tramadol plasma concentration. In adult surgical patients, Mohta et al. (2009) have shown that tramadol is an effective prophylactic drug for reducing the incidence of postanesthetic shivering. Overall, with a doubling only of the interthreshold range, its effects on thermoregulation can be considered mild (De Witte et al., 1998).
A comparison between the temperature effects in children anesthetized with either ketamine or halothane showed that halothane decreases rectal temperature more than ketamine. Regardless of the agent used, children with the highest surface area-to-body weight ratio (i.e., the smallest children) had the greatest decrease in body temperature (Engelman and Lockhart, 1972). Core hypothermia in adults who are induced with ketamine is less pronounced when compared with the effect when they are induced with propofol. This finding was preserved during maintenance of anesthesia with sevoflurane in nitrous oxide/oxygen (Ikeda et al., 2001).
In the case of propofol, a small study of adult volunteers showed a significant and linear decrease in the threshold temperatures for vasoconstriction and shivering, whereas in another study the sweating threshold temperature increased only slightly (Leslie et al., 1994; Matsukawa et al., 1995c). Furthermore, induction of anesthesia with a single bolus dose of propofol (2.5 mg/kg) in adults and maintenance of anesthesia with sevoflurane in 60% nitrous oxide/oxygen resulted in lower core temperatures (35.5° ± 0.3° C) when compared with patients who only received sevoflurane in nitrous oxide/oxygen for induction and maintenance of anesthesia (36.2° ± 0.2° C) (Ikeda et al., 1999b). This led to the suggestion that brief propofol-induced vasodilation is sufficient enough to facilitate the core-to-peripheral redistribution of body heat, resulting in nonrecoverable heat loss to the environment.
Midazolam slightly reduces the threshold temperature for sweating, whereas the decrease in the threshold temperature for vasoconstriction is more profound, resulting in a threefold expansion of the interthreshold range, which is comparable with the results found for central neuraxial nerve blockade (Kurz et al., 1995c). These results contrast with the findings for inhalational anesthetics, propofol or opioids, which can expand the interthreshold range by a factor of 10 to 15 (Kurz et al., 1995c).
A bolus dose of clonidine followed by an infusion results in a dose-independent increase in the threshold temperature for sweating, but its gain remains unchanged (Delaunay et al., 1996). The use of clonidine for premedication neither affects redistribution hypothermia nor does it worsen hypothermia during general anesthesia (Bernard et al., 1998).
Dexmedetomidine given to healthy adult volunteers does not affect the threshold temperature for sweating, but reduces the threshold temperatures for vasoconstriction and shivering in a dose-dependent way (Talke et al., 1997). Easley et al. (2007) have reported that dexmedetomidine (0.5 mcg/kg) was effective in the treatment of shivering within 5 minutes.
Atropine blocks sympathetic cholinergic-mediated sweating and increases the threshold temperature for sweating and therefore may lead to hyperthermia (Fraser, 1978).
Thermoregulation and Regional Anesthesia
During regional anesthesia, central thermoregulation remains functional, as does metabolic heat generation, thereby providing some protection against hypothermia (Hynson et al., 1991). Regional anesthesia interferes with regional thermal sensation (afferent and efferent pathways) and inhibition of thermoregulatory vasoconstriction and shivering in the anesthetized area. Increased internal redistribution of body heat followed by increased heat loss to the environment may contribute to intraoperative hypothermia. In many aspects, the factors responsible for intraoperative hypothermia during neuraxial blockade are similar to those for patients under general anesthesia: redistribution of body heat from the core to the peripheral compartment accounts for 89% of the initial drop (i.e., in the first hour) in core temperature. In the following 2 hours, redistribution contributes to 62% of the core temperature decrease (Matsukawa et al., 1995a; 1995b). The extent of this redistribution, and thus the decrease in core temperature, depend on inhibition of peripheral vasoconstriction rather than on centrally mediated effects. Neuraxial anesthesia usually affects a major part of the body mass and the decrease in core temperature can be quite pronounced. However, heat production during regional anesthesia is only minimally decreased (Hynson et al., 1991).
In contrast to patients undergoing general anesthesia, patients under central neuraxial anesthesia may fail to reach a steady state in which heat loss and heat generation are equal, because peripheral vasoconstriction is completely abolished in the area affected by neuraxial blockade. In addition, extensive regional anesthesia may alter or even block the thermal input to the hypothalamus from the anesthetized body part, with the number of dermatomes blocked being directly proportional to the inhibition of central thermoregulation (Ozaki et al., 1994; Leslie and Sessler, 1996; Frank et al., 2000). Heat loss may therefore be an ongoing issue until sympathetic function and consequently the ability for vasoconstriction have been restored. Under these circumstances, hypothermia during regional anesthesia may become even more profound than during general anesthesia. In an attempt at compensation, peripheral vessels not affected by regional anesthesia are maximally vasoconstricted, although this is often not sufficient to prevent a further drop in core temperature, particularly when the body mass affected by regional anesthesia is approximately the same or even larger than the unblocked mass.
Shivering is initiated once the patient’s core temperature reaches the shivering threshold; however, neuraxial blockade reduces the gain of shivering by more than 60%, mainly because the upper body muscles fail to compensate for lower body paralysis (Kim et al., 1998). It seems unlikely that systemic absorption of local anesthetics contributes to the thermoregulatory changes seen under regional anesthesia, because a study generating equal plasma drug concentrations without regional anesthesia failed to reproduce these thermoregulatory disturbances (Glosten et al., 1991).
Compared with general anesthesia, the use of regional anesthesia reduces the risk of hypothermia, especially during surgery with small incisions where the patient can be kept well insulated. In contrast, regional anesthesia for large surgical incisions predisposes a patient to more profound hypothermia than with general anesthesia, and recovery to normal body temperature may be prolonged (Cattaneo et al., 2000).
In adults, the changes in threshold temperatures for sweating, vasoconstriction, and shivering during spinal anesthesia and epidural anesthesia seem to be comparable and result in a twofold expansion of the interthreshold range (Ozaki et al., 1994). In adults, the combination of general anesthesia with thoracic epidural anesthesia further reduced the threshold temperature for thermoregulatory vasoconstriction and thus significantly aggravated hypothermia compared with general anesthesia alone (Joris et al., 1994). Interestingly enough, diabetic patients with autonomic neuropathy showed lower core temperatures and delayed thermoregulatory vasoconstriction during general anesthesia than diabetic patients without autonomic dysfunction (Kitamura et al., 2000).
Unlike the thermoregulatory effects of regional anesthesia in adults, the institution of a caudal block in children anesthetized with halothane does not significantly alter the threshold temperature for thermoregulatory vasoconstriction in children (35.7° C without versus 35.9° C with caudal block) (Bissonnette and Sessler, 1992). Nevertheless, temperatures during regional anesthesia should be monitored in adult and pediatric patients, because significant hypothermia is common and remains otherwise undetected and therefore also untreated. However, a survey revealed that only a third of clinicians monitors body temperature during regional anesthesia in adults (Frank et al., 1999).
Adverse effects of hypothermia
Heat loss in children undergoing surgery can occur for a variety of reasons. Exposure of body cavities to low environmental temperatures and humidity, infusion of cold fluids, and ventilation with cold and dry gases, in combination with the infant’s physical characteristics of the large body surface area-to-mass ratio and the minimal insulating tissue layer, the potential for an infant or a child to become hypothermic during anesthesia is significantly increased. Nevertheless, hypothermia must not be viewed as an inevitable consequence of surgery. Although hypothermia may be protective for a small subgroup of patients with certain ischemic conditions, in the majority of patients the adverse effects outweigh the benefits, and inadvertent core hypothermia must be avoided (Illievich et al., 1994). In a review of temperature monitoring and perioperative thermoregulation, Sessler (2008) noted that hypothermia-related complications include increased morbidity, surgical wound infections, coagulopathies, increased allogenic transfusions, negative nit-rogen balance, delayed wound healing, delayed postoperative anesthetic recovery, prolonged hospitalization, shivering, and patient discomfort.
A study of 200 adult patients scheduled for colorectal surgery showed that patients who were allowed to become hypothermic (core temperature 34.7° ± 0.6° C) during the procedure suffered from a more than threefold higher rate of surgical wound infections than the group who was actively warmed and kept normothermic (36.6° ± 0.5° C) (Kurz et al., 1996). Furthermore, the times until suture removal and discharge from hospital in the hypothermia group were prolonged by 1 and 2.6 days, respectively. It has been suggested by these and other researchers that the vasoconstriction triggered by hypothermia may result in a decreased tissue partial pressure of oxygen, which leads to increased wound infection and finally delayed wound healing, even in the absence of an infection (Fig. 6-11) (Jonsson et al., 1991). A shorter hospital stay (2.7 fewer days) for normothermic versus hypothermic patients has also been reported by others (Sellden and Lindahl, 1999). In addition, hypothermia itself has been shown to reduce chemotaxis and phagocytosis of granulocytes, natural killer cell cytotoxicity, migration of macrophages, and synthesis of immunoglobulins, thereby directly affecting the immune response (Leijh et al., 1979; van Oss et al., 1980; Wenisch et al., 1996; Beilin et al., 1998).
Hypothermia significantly delays the reactions in the coagulation cascade (most likely because of a direct effect on the activity of the coagulation factors) with prolongation of prothrombin time and partial thromboplastin time (Rohrer and Natale, 1992). Inhibition of platelet function with prolonged bleeding time was found during hypothermia, secondary to inhibited up-regulation of platelet surface protein GMP-140 and down-regulation of the glycoprotein GP Ib-IX complex, reduced platelet aggregation, and thromboxane B2 generation (the stable metabolite of thromboxane A2) (Michelson et al., 1994). This platelet dysfunction is fully reversible with rewarming. Thromboelastography confirms these findings with a prolongation of the reaction and coagulation times, as well as a reduction in the clot formation rate (Douning et al., 1995). It is therefore not surprising that blood loss in patients undergoing hip arthroplasty was found to be significantly higher in the hypothermia group (core temperature, 35° ± 0.5° C) than in the normothermia group (core temperature, 36.6° ± 0.4° C) (Schmied et al., 1996). Similar results have been confirmed by other researchers (Bock et al., 1998; Rajagopalan et al., 2008).
Notably greater incidences of myocardial ischemia and PaO2 values below 80 mm Hg have been reported in hypothermic patients (defined by sublingual temperature measured on arrival to the recovery room) when compared with normothermic patients in the first 24 hours after lower extremity vascular surgery with either epidural or general anesthesia (Frank et al., 1993). Although these findings are most likely not relevant to most pediatric patients because of the lack of coronary heart disease, they nevertheless demonstrate the widespread and potential impact of hypothermia on the body in surgical patients.
Hypothermia results in diminished metabolism of drugs and prolongs their action. Although intraoperative hypothermia in adults resulted in delayed recovery from anesthesia when compared with normothermic patients, no such differences could be demonstrated in children (Bissonnette and Sessler, 1993; Lenhardt et al., 1997).
Studies on the effects of hypothermia and muscle relaxants have demonstrated that hypothermia decreases the requirements for nondepolarizing muscle relaxants because there is an increased sensitivity of the neuromuscular junction, as well as diminished biliary (indicating a reduced affinity for the drug substrate to microsomal enzymes) and renal elimination of the drug (Ham et al., 1978; Miller et al., 1978). Similar findings have been confirmed for other medications (McAllister and Tan, 1980).
Prevention of hypothermia
Operating Room Temperature
Keeping the operating room temperature at an optimal level is crucial in the prevention of hypothermia. The major source of heat loss in the anesthetized patient is caused by radiation. As mentioned previously, radiant heat loss is a function of the temperature difference between the patient and the environment. The effectiveness of controlling ambient operating room temperature in controlling the temperature of newborns during surgery has been confirmed (Bennett et al., 1977). In adults, 21° C is reported as the critical ambient temperature for maintaining normal nasopharyngeal or esophageal temperatures (36° to 37.5° C) (Morris and Wilkey, 1970; Morris, 1971a, 1971b). Operating room temperatures of 27° and 29° C are recommended for full-term and premature newborns, respectively. It is essential that every operating room be equipped with an individual thermistor control unit so that the temperature in each operating room can be controlled independently to meet the needs of each patient.
Reflecting Blankets
The use of reflective blankets in adults has produced conflicting results, and data on their use in infants and children are sparse. In adults, it has been reported that intraoperative normothermia can be maintained when the reflective blanket covers 60% or more of the patient’s body surface area (Bourke et al., 1984). One drawback of reflective blankets is the potential build-up of condensation on the inner side, which can result in wet clothes or skin, putting the patient at high risk for evaporative heat loss. With regard to heat conservation, it seems not that important what material the cover is made of; instead, it is important to cover as much of the body surface as possible (Sessler et al., 1991). The practice of covering the head with a plastic bag provides an easy, inexpensive way to significantly reduce radiant, convective, and evaporative heat losses (Fig. 6-12).
Skin-Surface–Warming Devices
The use of skin-surface–warming devices in adults before the induction of anesthesia reduces the magnitude of hypothermia that results from internal redistribution of heat (Glosten et al., 1993). Aggressive skin-surface warming induces peripheral vasodilation and favorably increases the temperature of the peripheral compartment to values approaching those of the central compartment. The net result is an increased mean body temperature, and the skin-surface warming reduces the amount of energy transferred from the central to the peripheral compartment after induction of anesthesia. A variety of passive and active skin-surface warmers are available, including circulating hot-water blankets, infrared radiant heaters, and convective forced-air heaters, which blow warm air through a disposable blanket to raise the effective ambient temperature immediately surrounding the patient (Stephen et al., 1960; Vale and Lunn, 1969; Morris, 1971b; Goldblat and Miller, 1972; Sessler and Moayeri, 1990; Steele et al., 1996). A newer system that simulates water immersion using a special garment and feedback algorithms analyzed by computer to achieve a preset body temperature is available, seems to perform well, and is also safe in children (Nesher et al., 2001a, 2001b; Nesher et al., 2002). Of all these devices, convective forced-air warmers are by far the most effective, not only from a thermoregulatory perspective, but also from a cost and convenience point of view. Not only can they maintain normal body temperature, but they can also rewarm a hypothermic patient (Sessler and Moayeri, 1990; Kurz et al., 1993; Ciufo et al., 1995; Karayan et al., 1996). In patients with decreased peripheral perfusion (e.g., because of high doses of vasoconstrictors, aortic cross clamping for coarctation or abdominal aortic aneurysm repair, or severe peripheral arterial occlusive disease) the device should be used with great caution only and not for extended lengths of time to avoid skin burns, particularly in infants and neonates (Azzam and Krock, 1995; Truell et al., 2000; Siddik-Sayyid et al., 2008).
Warming Mattresses
The use of warming mattresses reduces conductive heat loss. Warming mattresses set at 40° C and covered with two layers of cotton blankets have been demonstrated to effectively conserve heat (Goudsouzian et al., 1973). This measure is mainly efficient for infants with a body surface area of less than 0.5 m2. In older children and adults, only a small proportion of the skin’s surface area is in direct contact with the heating mattress, which makes these devices generally less effective to maintain normothermia.
Warming of Intravenous Fluids
Rapid infusion of chilled intravenous fluids (1° to 6° C) can be used effectively to induce hypothermia. The administration of 1 L of an ice-cold infusion in an adult is expected to decrease the core temperature by approximately 1.7° C, but values of up to 3° C are possible (Baumgardner et al., 1999; Rajek et al., 2000). Thus, intravenous fluids and blood products should be warmed before administration. It is particularly important to warm fluids in instances of rapid or massive fluid administration. In addition, attention should be paid to the length and the type of the infusion tubing, because significant heat loss of the infusion during transit from the warming device to the intravenous cannula may occur (Faries et al., 1991; Bissonnette and Paut, 2002). Even when warming the infusion to 37° C, a flow rate of more than 750 mL/hr is required to keep its temperature above 32° C at a distance of 25 cm away from the warmer when the infusion tubing is exposed to ambient air (20° C) (Faries et al., 1991). Because this flow rate is usually beyond the needs of regular pediatric cases, the length of the infusion tubing distal to the warmer should be kept as short as possible. Although a study demonstrated that conservative fluid management (1 mL/kg per hour of crystalloids warmed to 37° C) in patients aged 1 to 3 years resulted in less core hypothermia when compared with the control group who received a more aggressive fluid replacement (10 mL/kg per hour) (Ezri et al., 2003), this should not preclude pediatric patients from receiving appropriate intraoperative fluid resuscitation.
Humidified and Heated Gases
When breathing spontaneously (without an endotracheal tube or laryngeal mask airway in place), the tracheobronchial epithelium heats and moistens the inspired air, an energy-dependent process (evaporation and convection). Intubation significantly reduces the epithelial surface area that is available for warming and humidification of inspired gases. In order to minimize convective and evaporative heat losses from the respiratory tract and to minimize adverse effects on the tracheobronchial epithelium, inspiratory gases should be heated and humidified. Airway humidification in intubated patients prevents tracheal damage caused by dry inspired gases, increases tracheal mucus flow, and minimizes respiratory heat losses (Berry et al., 1973; Forbes 1974; Mercke 1975; Chalon et al., 1979b; Tollofsrud et al., 1984). Heat and humidity can be added to inspired gases either actively by evaporative or ultrasonic heated humidifiers or passively by heat and moisture exchanging filters (“artificial noses”) (Newton, 1975; Chalon et al., 1979a, 1984; Bissonnette et al., 1989a). Furthermore, there is considerable evidence that a relative humidity level of at least 50% is required to maintain normal ciliary function in the respiratory tract and to help prevent bronchospasm (Forbes, 1974; Mercke, 1975; Chalon et al., 1979a; Chan et al., 1980). More recent research, however, suggests an even higher humidity level of 75% to 100% to effectively maintain mucociliary function (Kilgour et al., 2004). Humidification to 50% (but not to 75% to 100%) is easily obtained with heat and moisture exchanging filters and should be standard for long procedures (Forbes, 1974; Mercke, 1975; Chalon et al., 1979a). In adults, heating and humidification of gases to 37° C and 100% relative humidity not only effectively helps maintain normothermia, but it also helps reverse hypothermia during general surgery (Pflug et al., 1978; Stone et al., 1981).
Because of the higher minute ventilation per kilogram of body weight in pediatric patients, airway humidification for infants is even more important and effective in helping to maintain normothermia than it is for adults (Bissonnette and Sessler, 1989a; Bissonnette et al., 1989). In newborns, heat loss during general anesthesia is significantly reduced when gases are heated and when humidified gases are used instead of dry anesthetic gases (Fonkalsrud et al., 1980). Although high- temperature humidification devices can decrease intraoperative evaporative heat loss, they may cause tracheal burns. Because there may be a large temperature gradient between the humid-ifier and the endotracheal tube, it is important to measure airway temperature as close to the patient as possible. This has the advantage of preventing heated gases from accidentally burning the trachea while providing the warmest possible inspiratory gas. In addition, intraoperative heat loss can be minimized. If these devices are used, it is recommended to heat inspiratory gases to normal body temperature only. Although heat and moisture exchanging filters are less effective than active humidifiers (especially in the first hour after induction of anesthesia), they seem to provide a reasonable, convenient, and cost-effective alternative (Bissonnette et al., 1989a).
Additional advantages of heat and moisture exchangers in small infants include the lack of risk for airway burns and overhumidification with the consequences of overhydration, and the reduced risk for breathing-circuit disconnection (Smith and Allen, 1986; Shroff and Skerman, 1988). Heat and mois-ture may falsely increase the esophageal temperature by about 0.35° C when compared with tympanic or mean body temperature (Bissonnette et al., 1989b).
All heat and moisture exchangers increase the dead space of the anesthesia tubing; however, the smallest ones have a dead space of less than 2 mL. At a fresh gas flow of 30 to 60 L/min, the increase in airway resistance is minimal, typically creating a pressure gradient of less than 2 cm H2O. Most heat and moisture exchangers now commonly have an in-built filter for bacteria, viruses, and latex particles (Barbara et al., 2001). Copious secretions from the tracheobronchial tree may result in partial or complete obstruction of the filter and thereby severely affect ventilation (Prasad and Chen, 1990; Barnes and Normoyle, 1996; Stacey et al., 1996). The in vitro specifications of the heat and moisture exchangers given by the manufacturers do not always match the actual in vivo performance (Lemmens and Brock-Utne, 2004).
Summary
For questions and answers on topics in this chapter, go to “Chapter Questions” at www.expertconsult.com.
Adamson K.J., Gandy G., James L. The influence of thermal factors upon oxygen consumption of the newborn human infant. J Pediatr. 1965;66:495.
Akata T., Kanna T., Izumi K., et al. Changes in body temperature following deflation of limb pneumatic tourniquet. J Clin Anesth. 1998;10:17.
Alfonsi P. Postanaesthetic shivering: epidemiology, pathophysiology, and approaches to prevention and management. Drugs. 2001;61:2193.
Alfonsi P., Sessler D.I., Du Manoir B., et al. The effects of meperidine and sufentanil on the shivering threshold in postoperative patients. Anesthesiology. 1998;89:43.
Allen J.A. The thermal environment and human heat exchange. In: Ernsting J., King P., editors. Aviation medicine. London: Butterworths; 1987:219.
American Society of Anesthesiologists. Standards for basic anesthetic monitoring. 2005.www.asahq.org/publicationsAndServices/standards/02.pdf.
Annadata R., Sessler D.I., Tayefeh F., et al. Desflurane slightly increases the sweating threshold but produces marked, nonlinear decreases in the vasoconstriction and shivering thresholds. Anesthesiology. 1995;83:1205.
Antognini J.F. Hypothermia eliminates isoflurane requirements at 20 degrees C. Anesthesiology. 1993;78:1152.
Antognini J.F., Lewis B.K., Reitan J.A. Hypothermia minimally decreases nitrous oxide anesthetic requirements. Anesth Analg. 1994;79:980.
Anttonen H., Puhakka K., Niskanen J., et al. Cutaneous heat loss in children during anaesthesia. Br J Anaesth. 1995;74:306.
Armstrong L.E., Hubbard R.W., Jones B.H., et al. Preparing Alberto Salazar for the heat of the 1984 olympic marathon. Phys Sportsmed. 1986;14:73.
Australian and New Zealand College of Anaesthetists. Recommendations on monitoring during anaesthesia. 2006.http://www.anzca.edu.au/resources/professional-documents/professional-standards/ps18.html.
Azzam F.J., Krock J.L. Thermal burns in two infants associated with a forced air warming system. Anesth Analg. 1995;81:661.
Barbara J., Chabane M.H., Leynadier F., et al. Retention of airborne latex particles by a bacterial and viral filter used in anaesthesia apparatus. Anaesthesia. 2001;56:231.
Barnes S.D., Normoyle D.A. Failure of ventilation in an infant due to increased resistance of a disposable heat and moisture exchanger. Anesth Analg. 1996;83:193.
Baumgardner J.E., Baranov D., Smith D.S., et al. The effectiveness of rapidly infused intravenous fluids for inducing moderate hypothermia in neurosurgical patients. Anesth Analg. 1999;89:163.
Bay J., Nunn J.F., Prys-Roberts C. Factors influencing arterial PO2 during recovery from anaesthesia. Br J Anaesth. 1968;40:398.
Beilin B., Shavit Y., Razumovsky J., et al. Effects of mild perioperative hypothermia on cellular immune responses. Anesthesiology. 1998;89:1133.
Bell D.G., Tikuisis P., Jacobs I. Relative intensity of muscular contraction during shivering. J Appl Physiol. 1992;72:2336.
Bennett E.J., Patel K.P., Grundy E.M. Neonatal temperature and surgery. Anesthesiology. 1977;46:303.
Bennett L.A., Johnson J.M., Stephens D.P., et al. Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol. 2003;552:223.
Benzinger T.H. Heat regulation: homeostasis of central temperature in man. Physiol Rev. 1969;49:671.
Bernard J.M., Fulgencio J.P., Delaunay L., et al. Clonidine does not impair redistribution hypothermia after the induction of anesthesia. Anesth Analg. 1998;87:168.
Berry F.A.Jr., Hughes-Davies D.I., DiFazio C.A. A system for minimizing respiratory heat loss in infants during operation. Anesth Analg. 1973;52:170.
Bickler P.E., Sessler D.I. Efficiency of airway heat and moisture exchangers in anesthetized humans. Anesth Analg. 1990;71:415.
Bissonnette B. Thermoregulation and paediatric anaesthesia. Curr Opin Anaesthesiol. 1993:6.
Bissonnette B., Holtby H.M., Davis A.J., et al. Cerebral hyperthermia in children after cardiopulmonary bypass. Anesthesiology. 2000;93:611.
Bissonnette B., Paut O. Active warming of saline or blood is ineffective when standard infusion tubing is used: an experimental study. Can J Anaesth. 2002;49:270.
Bissonnette B., Sessler D.I. Passive or active inspired gas humidification increases thermal steady-state temperatures in anesthetized infants. Anesth Analg. 1989;69:783.
Bissonnette B., Sessler D.I. The thermoregulatory threshold in infants and children anesthetized with isoflurane and caudal bupivacaine. Anesthesiology. 1990;73:1114.
Bissonnette B., Sessler D.I. Thermoregulatory thresholds for vasoconstriction in pediatric patients anesthetized with halothane or halothane and caudal bupivacaine. Anesthesiology. 1992;76:387.
Bissonnette B., Sessler D.I. Mild hypothermia does not impair postanesthetic recovery in infants and children. Anesth Analg. 1993;76:168.
Bissonnette B., Sessler D.I., LaFlamme P. Passive and active inspired gas humidification in infants and children. Anesthesiology. 1989;71:350.
Bissonnette B., Sessler D.I., LaFlamme P. Intraoperative temperature monitoring sites in infants and children and the effect of inspired gas warming on esophageal temperature. Anesth Analg. 1989;69:192.
Bloch E.C., Ginsberg B., Binner R.A.Jr, et al. Limb tourniquets and central temperature in anesthetized children. Anesth Analg. 1992;74:486.
Bock M., Muller J., Bach A., et al. Effects of preinduction and intraoperative warming during major laparotomy. Br J Anaesth. 1998;80:159.
Boulant J.A. The effect of firing rate on preoptic neuronal thermosensitivity. J Physiol. 1974;240:661.
Boulant J.A., Bignall K.E. Hypothalamic neuronal responses to peripheral and deep-body temperatures. Am J Physiol. 1973;225:1371.
Boulant J.A., Demieville H.N. Responses of thermosensitive preoptic and septal neurons to hippocampal and brain stem stimulation. J Neurophysiol. 1977;40:1356.
Bourke D.L., Wurm H., Rosenberg M., et al. Intraoperative heat conservation using a reflective blanket. Anesthesiology. 1984;60:151.
Brauer A., Martin J.D., Schuhmann M.U., et al. [Accuracy of intraoperative urinary bladder temperature monitoring during intra-abdominal operations]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2000;35:435.
Brismar B., Hedenstierna G., Lundh R., et al. Oxygen uptake, plasma catecholamines and cardiac output during neurolept-nitrous oxide and halothane anaesthesias. Acta Anaesthesiol Scand. 1982;26:541.
Bruck K. Temperature regulation in the newborn infant. Biol Neonate. 1961;3:65.
Bruck K., Wunnenberg B. The influence of ambient temperature in the process of replacement of non-shivering thermogenesis during postnatal development. Fed Proc. 1965;25:1332.
Brück K. Neonatal thermal regulation. In: Polin R.A., Fox W.W., editors. Fetal and neonatal physiology. Philadelphia: WB Saunders Co; 1992:493.
Brundin T., Wahren J. Effects of i.v. amino acids on human splanchnic and whole body oxygen consumption, blood flow, and blood temperatures. Am J Physiol. 1994;266:E396.
Brux A., Girbes A.R., Polderman K.H. [Controlled mild-to-moderate hypothermia in the intensive care unit]. Anaesthesist. 2005;54:225.
Budin P. The nursling. London: Caxton, 1907.
Burgess G.E.3rd, Cooper J.R., Marino R.J., et al. Continuous monitoring of skin temperature using a liquid-crystal thermometer during anesthesia. South Med J. 1978;71:516.
Burton A.C. Human calorimetry: the average temperature of the tissues of the body. J Nutr. 1935;9:261.
Cabanac M. Temperature regulation. Annu Rev Physiol. 1975;37:415.
Canadian Anesthesiologists’ Society. Guidelines to the practice of anesthesia. 2007.http://www.cas.ca/members/sign_in/guidelines/practice:of_anesthesia/default.asp?load=patient_monitoring.
Cattaneo C.G., Frank S.M., Hesel T.W., et al. The accuracy and precision of body temperature monitoring methods during regional and general anesthesia. Anesth Analg. 2000;90:938.
Chalon J., Ali M., Ramanathan S., et al. The humidification of anaesthetic gases: its importance and control. Can Anaesth Soc J. 1979;26:361.
Chalon J., Patel C., Ali M., et al. Humidity and the anesthetized patient. Anesthesiology. 1979;50:195.
Chalon J., Markham J.P., Ali M.M., et al. The pall ultipor breathing circuit filter: an efficient heat and moisture exchanger. Anesth Analg. 1984;63:566.
Chan P.K., Reddy C.S., Hayes A.W. Acute toxicity of penicillic acid and its interaction with pentobarbital and other compounds. Toxicol Appl Pharmacol. 1980;52:1.
Cheung S.S., Mekjavic I.B. Human temperature regulation during subanesthetic levels of nitrous oxide-induced narcosis. J Appl Physiol. 1995;78:2301.
Ciofolo M.J., Clergue F., Devilliers C., et al. Changes in ventilation, oxygen uptake, and carbon dioxide output during recovery from isoflurane anesthesia. Anesthesiology. 1989;70:737.
Ciufo D., Dice S., Coles C. Rewarming hypothermic postanesthesia patients: a comparison between a water coil warming blanket and a forced-air warming blanket. J Post Anesth Nurs. 1995;10:155.
Coffman J.D., Cohen A.S. Total and capillary fingertip blood flow in Raynaud’s phenomenon. N Engl J Med. 1971;285:259.
Colin J., Timbal J., Houdas Y., et al. Computation of mean body temperature from rectal and skin temperatures. J Appl Physiol. 1971;31:484.
Cork R.C., Vaughan R.W., Humphrey L.S. Precision and accuracy of intraoperative temperature monitoring. Anesth Analg. 1983;62:211.
Craig J.V., Lancaster G.A., Taylor S., et al. Infrared ear thermometry compared with rectal thermometry in children: a systematic review. Lancet. 2002;360:603.
Cross K., Tizard J., Trythall D. Gaseous metabolism of newborn infant breathing 15% oxygen. Acta Paediatr. 1958;47:217.
Dawkins M.J., Scopes J.W. Non-shivering thermogenesis and brown adipose tissue in the human new-born infant. Nature. 1965;206:201.
De Witte J., Rietman G.W., Vandenbroucke G., et al. Post-operative effects of tramadol administered at wound closure. Eur J Anaesthesiol. 1998;15:190.
Deakin C.D. Changes in core temperature compartment size on induction of general anaesthesia. Br J Anaesth. 1998;81:861.
Delaunay L., Herail T., Sessler D.I., et al. Clonidine increases the sweating threshold, but does not reduce the gain of sweating. Anesth Analg. 1996;83:844.
Detry J.M., Brengelmann G.L., Rowell L.B., et al. Skin and muscle components of forearm blood flow in directly heated resting man. J Appl Physiol. 1972;32:506.
Dickenson A.H. Specific responses of rat raphe neurones to skin temperature. J Physiol. 1977;273:277.
Dicker A., Ohlson K.B., Johnson L., et al. Halothane selectively inhibits nonshivering thermogenesis: possible implications for thermoregulation during anesthesia of infants. Anesthesiology. 1995;82:491.
Dodd S.R., Lancaster G.A., Craig J.V., et al. In a systematic review, infrared ear thermometry for fever diagnosis in children finds poor sensitivity. J Clin Epidemiol. 2006;59:354.
Douning L.K., Ramsay M.A., Swygert T.H., et al. Temperature corrected thrombelastography in hypothermic patients. Anesth Analg. 1995;81:608.
Downey J., Mottram R., Pickering G. The location by regional cooling of central temperature receptors in the conscious rabbit. J Physiol (London). 1964;170:415.
Downey J.A., Chiodi H.P., Darling R.C. Central temperature regulation in the spinal man. J Appl Physiol. 1967;22:91.
Easley R.B., Brady K.M., Tobias J.D. Dexmedetomidine for the treatmentof postanesthesia shivering in children. Paediatr Anaesth. 2007;17:341-346.
Eger E.I.2nd, Johnson B.H. MAC of I-653 in rats, including a test of the effect of body temperature and anesthetic duration. Anesth Analg. 1987;66:974.
Engelman D.R., Lockhart C.H. Comparisons between temperature effects of ketamine and halothane anesthesia in children. Anesth Analg. 1972;51:98.
Estebe J.P., Le Naoures A., Malledant Y., et al. Use of a pneumatic tourniquet induces changes in central temperature. Br J Anaesth. 1996;77:786.
Ezri T., Szmuk P., Weisenberg M., et al. The effects of hydration on core temperature in pediatric surgical patients. Anesthesiology. 2003;98:838.
Faries G., Johnston C., Pruitt K.M., et al. Temperature relationship to distance and flow rate of warmed i.v. fluids. Ann Emerg Med. 1991;20:1198.
Fleming P.J., Azaz Y., Wigfield R. Development of thermoregulation in infancy: possible implications for SIDS. J Clin Pathol. 1992;45:17.
Fonkalsrud E.W., Calmes S., Barcliff L.T., et al. Reduction of operative heat loss and pulmonary secretions in neonates by use of heated and humidified anesthetic gases. J Thorac Cardiovasc Surg. 1980;80:718.
Forbes A.R. Temperature, humidity and mucus flow in the intubated trachea. Br J Anaesth. 1974;46:29.
Frank S.M., Beattie C., Christopherson R., et al. Unintentional hypothermia is associated with postoperative myocardial ischemia. The Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology. 1993;78:468.
Frank S.M., El-Rahmany H.K., Cattaneo C.G., et al. Predictors of hypothermia during spinal anesthesia. Anesthesiology. 2000;92:1330.
Frank S.M., Nguyen J.M., Garcia C.M., et al. Temperature monitoring practices during regional anesthesia. Anesth Analg. 1999;88:373.
Fraser J.G. Iatrogenic benign hyperthermia in children. Anesthesiology. 1978;48:375.
Fusi L., Steer P.J., Maresh M.J., et al. Maternal pyrexia associated with the use of epidural analgesia in labour. Lancet. 1989;1:1250.
Gale C.C. Neuroendocrine aspects of thermoregulation. Annu Rev Physiol. 1973;35:391.
Garruti G., Ricquier D. Analysis of uncoupling protein and its mRNA in adipose tissue deposits of adult humans. Int J Obes Relat Metab Disord. 1992;16:383.
Giesbrecht G.G., Goheen M.S., Johnston C.E., et al. Inhibition of shivering increases core temperature afterdrop and attenuates rewarming in hypothermic humans. J Appl Physiol. 1997;83:1630.
Giesbrecht G.G., Sessler D.I., Mekjavic I.B., et al. Treatment of mild immersion hypothermia by direct body-to-body contact. J Appl Physiol. 1994;76:2373.
Gilbert M., Busund R., Skagseth A., et al. Resuscitation from accidental hypothermia of 13.7 degrees C with circulatory arrest. Lancet. 2000;355:375.
Glosten B., Hynson J., Sessler D.I., et al. Preanesthetic skin-surface warming reduces redistribution hypothermia caused by epidural block. Anesth Analg. 1993;77:488.
Glosten B., Sessler D.I., Ostman L.G., et al. Intravenous lidocaine does not cause shivering-like tremor or alter thermoregulation. Reg Anesth. 1991;16:218.
Godek S.F., Bartolozzi A.R., Burkholder R., et al. Sweat rates and fluid turnover in professional football players: a comparison of National Football League linemen and backs. J Athl Train. 2008;43:184.
Goldblat A., Miller R. Prevention of incidental hypothermia in neurosurgical patients. Anesth Analg. 1972;51:536.
Goto T., Matsukawa T., Sessler D.I., et al. Thermoregulatory thresholds for vasoconstriction in patients anesthetized with various 1-minimum alveolar concentration combinations of xenon, nitrous oxide, and isoflurane. Anesthesiology. 1999;91:626.
Goto T., Saito H., Nakata Y., et al. Effects of xenon on the performance of various respiratory flowmeters. Anesthesiology. 1999;90:555.
Goudsouzian N.G., Morris R.H., Ryan J.F. The effects of a warming blanket on the maintenance of body temperatures in anesthetized infants and children. Anesthesiology. 1973;39:351.
Grant R.J., Bland E. Observations on arteriovenous anastomoses in human skin and in bird’s foot with special reference to the reaction to cold. Heart. 1931;15:385.
Guieu J.D., Hardy J.D. Effects of heating and cooling of the spinal cord on preoptic unit activity. J Appl Physiol. 1970;29:675.
Gurtner C., Paut O., Bissonnette B. Temperature regulation: physiology and pharmacology. In: Bissonnette B., Dalens B., editors. Pediatric anesthesia: principles and practice. New York: McGraw-Hill; 2002:173.
Guyton A.C. Body temperature, temperature regulation and fever. In: Guyton A.C., Hall J.E., editors. Textbook of medical physiology. Philadelphia: WB Saunders; 2000:822.
Hales J.R.S. Skin arteriovenous anastomoses, their control and role in thermoregulation. In: Johansen K., Burggren W., editors. Cardiovascular shunts: phylogenetic, ontogenetic and clinical aspects. Munksgaard: Copenhagen; 1985:433.
Ham J., Miller R.D., Benet L.Z., et al. Pharmacokinetics and pharmacodynamics of d-tubocurarine during hypothermia in the cat. Anesthesiology. 1978;49:324.
Haman F., Legault S.R., Weber J.M. Fuel selection during intense shivering in humans: EMG pattern reflects carbohydrate oxidation. J Physiol. 2004;556:305.
Hardy J.D. Physiology of temperature regulation. Physiol Rev. 1961;41:521.
Hardy J.D., Milhorat A.T., Dubois E.F. Basal metabolism and heat loss of young women at temperatures from 22°C to 35°C. J Nutr. 1941;21:383.
Hellon R.F. Neurophysiology of temperature regulation: problems and perspectives. Fed Proc. 1981;40:2804.
Hemingway A. Shivering. Physiol Rev. 1963;43:397.
Hemingway A., Price W.M. The autonomic nervous system and regulation of body temperature. Anesthesiology. 1968;29:693.
Heusch A.I., McCarthy P.W. The patient: a novel source of error in clinical temperature measurement using infrared aural thermometry. J Altern Complement Med. 2005;11:473.
Hey E. Thermal neutrality. Br Med Bull. 1975;31:69.
Hey E.N. Physiological principles involved in the care of the pre-term human infant. In: Austin C.R., editor. The mammalian fetus in vitro. London: Chapman and Hall; 1973:251.
Hey E.N., Katz G. Temporary loss of a metabolic response to cold stress in infants of low birthweight. Arch Dis Child. 1969;44:323.
Hillman P.E., Scott N.R., van Tienhoven A. Vasomotion in chicken foot: dual innervation of arteriovenous anastomoses. Am J Physiol. 1982;242:R582.
Himms-Hagen J. Cellular thermogenesis. Annu Rev Physiol. 1976;38:315.
Hori T., Katafuchi T. Cell biology and the functions of thermosensitive neurons in the brain. Prog Brain Res. 1998;115:9.
Horn E.P., Sessler D.I., Standl T., et al. Non-thermoregulatory shivering in patients recovering from isoflurane or desflurane anesthesia. Anesthesiology. 1998;89:878.
Horn E.P., Schroeder F., Wilhelm S., et al. Postoperative pain facilitates nonthermoregulatory tremor. Anesthesiology. 1999;91:979.
Horrow J.C., Rosenberg H. Does urinary catheter temperature reflect core temperature during cardiac surgery? Anesthesiology. 1988;69:986.
Horvath S.M., Spurr G.B., Hutt B.K., et al. Metabolic cost of shivering. J Appl Physiol. 1956;8:595.
Hull D., Smales O. Heat production in the newborn. In: Sinclair J., editor. Temperature regulation and energy metabolism in the newborn. New York: Grune and Stratton; 1978:129.
Hynson J.M., Sessler D.I., Glosten B., et al. Thermal balance and tremor patterns during epidural anesthesia. Anesthesiology. 1991;74:680.
Ikeda T., Kazama T., Sessler D.I., et al. Induction of anesthesia with ketamine reduces the magnitude of redistribution hypothermia. Anesth Analg. 2001;93:934.
Ikeda T., Kim J.S., Sessler D.I., et al. Isoflurane alters shivering patterns and reduces maximum shivering intensity. Anesthesiology. 1998;88:866.
Ikeda T., Sessler D.I., Tayefeh F., et al. Meperidine and alfentanil do not reduce the gain or maximum intensity of shivering. Anesthesiology. 1998;88:858.
Ikeda T., Kurz A., Sessler D.I., et al. The effect of opioids on thermoregulatory responses in humans and the special antishivering action of meperidine. Ann N Y Acad Sci. 1997;813:792.
Ikeda T., Ozaki M., Sessler D.I., et al. Intraoperative phenylephrine infusion decreases the magnitude of redistribution hypothermia. Anesth Analg. 1999;89:462.
Ikeda T., Sessler D.I., Kikura M., et al. Less core hypothermia when anesthesia is induced with inhaled sevoflurane than with intravenous propofol. Anesth Analg. 1999;88:921.
Illievich U.M., Zornow M.H., Choi K.T., et al. Effects of hypothermia or anesthetics on hippocampal glutamate and glycine concentrations after repeated transient global cerebral ischemia. Anesthesiology. 1994;80:177.
Jessen C., Mayer E.T. Spinal cord and hypothalamus as core sensors of temperature in the conscious dog. I. Equivalence of responses. Pflugers Arch. 1971;324:189.
Jessen C., Pongratz H., Merker J., et al. Natural brain cooling and temperature regulation. Arch Exp Veterinarmed. 1984;38:336.
Jessen K. An assessment of human regulatory nonshivering thermogenesis. Acta Anaesthesiol Scand. 1980;24:138.
Jessen K. The cortisol fluctuations in plasma in relation to human regulatory nonshivering thermogenesis. Acta Anaesthesiol Scand. 1980;24:151.
Jessen K. The relation between thyroid function and human regulatory nonshivering thermogenesis. Acta Anaesthesiol Scand. 1980;24:144.
Jonsson K., Jensen J.A., Goodson W.H.3rd, et al. Tissue oxygenation, anemia, and perfusion in relation to wound healing in surgical patients. Ann Surg. 1991;214:605.
Joris J., Ozaki M., Sessler D.I., et al. Epidural anesthesia impairs both central and peripheral thermoregulatory control during general anesthesia. Anesthesiology. 1994;80:268.
Just B., Delva E., Camus Y., et al. Oxygen uptake during recovery following naloxone: relationship with intraoperative heat loss. Anesthesiology. 1992;76:60.
Kadar D., Tang B.K., Conn A.W. The fate of phenobarbitone in children in hypothermia and at normal body temperature. Can Anaesth Soc J. 1982;29:16.
Karayan J., Thomas D., Lacoste L., et al. Delayed forced air warming prevents hypothermia during abdominal aortic surgery. Br J Anaesth. 1996;76:459.
Karlberg P., Moore R., Oliver T.J. The thermogenic response of the newborn infant to noradrenaline. Acta Paediatr. 1962;51:284.
Karlberg P., Moore R., Oliver T.J. Thermogenic and cardiovascular response of the newborn baby to noradrenaline. Acta Paediatr. 1965;54:225.
Kasai T., Nakajima Y., Matsukawa T., et al. Effect of preoperative amino acid infusion on thermoregulatory response during spinal anaesthesia. Br J Anaesth. 2003;90:58.
Kellogg D.L.Jr, Crandall C.G., Liu Y., et al. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824.
Kilgour E., Rankin N., Ryan S., et al. Mucociliary function deteriorates in the clinical range of inspired air temperature and humidity. Intensive Care Med. 2004;30:1491.
Kim J.S., Ikeda T., Sessler D.I., et al. Epidural anesthesia reduces the gain and maximum intensity of shivering. Anesthesiology. 1998;88:851.
Kitamura A., Hoshino T., Kon T., et al. Patients with diabetic neuropathy are at risk of a greater intraoperative reduction in core temperature. Anesthesiology. 2000;92:1311.
Kobayashi S. Temperature-sensitive neurons in the hypothalamus: a new hypothesis that they act as thermostats, not as transducers. Prog Neurobiol. 1989;32:103.
Koren G., Barker C., Goresky G., et al. The influence of hypothermia on the disposition of fentanyl: human and animal studies. Eur J Clin Pharmacol. 1987;32:373.
Kurz A., Go J.C., Sessler D.I., et al. Alfentanil slightly increases the sweating threshold and markedly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1995;83:293.
Kurz A., Xiong J., Sessler D.I., et al. Desflurane reduces the gain of thermoregulatory arteriovenous shunt vasoconstriction in humans. Anesthesiology. 1995;83:1212.
Kurz A., Sessler D.I., Annadata R., et al. Midazolam minimally impairs thermoregulatory control. Anesth Analg. 1995;81:393.
Kurz A., Kurz M., Poeschl G., et al. Forced-air warming maintains intraoperative normothermia better than circulating-water mattresses. Anesth Analg. 1993;77:89.
Kurz A., Sessler D.I., Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209.
Lacoumenta S., Hall G.M. Liquid crystal thermometry during anaesthesia. Anaesthesia. 1984;39:54.
La Société Française d’Anesthésie et de Réanimation. [Recommandations concernant la surveillance des patients en cours d’anesthésie]. 1994.http://www.sfar.org/recomperop.html.
Lean M.E., James W.P., Jennings G., et al. Brown adipose tissue uncoupling protein content in human infants, children and adults. Clin Sci (Lond). 1986;71:291.
Lean M.E., James W.P., Jennings G., et al. Brown adipose tissue in patients with phaeochromocytoma. Int J Obes. 1986;10:219.
Leijh P.C., van den Barselaar M.T., van Zwet T.L., et al. Kinetics of phagocytosis of Staphylococcus aureus and Escherichia coli by human granulocytes. Immunology. 1979;37:453.
Lemmens H.J., Brock-Utne J.G. Heat and moisture exchange devices: are they doing what they are supposed to do? Anesth Analg. 2004;98:382.
Lenhardt R., Greif R., Sessler D.I., et al. Relative contribution of skin and core temperatures to vasoconstriction and shivering thresholds during isoflurane anesthesia. Anesthesiology. 1999;91:422.
Lenhardt R., Marker E., Goll V., et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997;87:1318.
Leon C., Rodriguez A., Fernandez A., et al. Infrared ear thermometry in the critically ill patient. J Crit Care. 2005;20:106.
Leon J.E., Bissonnette B., Lerman J. Liquid crystalline temperature monitoring: does it estimate core temperature in anaesthetized paediatric patients? Can J Anaesth. 1990;37:S98.
Leslie K., Sessler D.I. Reduction in the shivering threshold is proportional to spinal block height. Anesthesiology. 1996;84:1327.
Leslie K., Sessler D.I., Bjorksten A.R., et al. Propofol causes a dose-dependent decrease in the thermoregulatory threshold for vasoconstriction but has little effect on sweating. Anesthesiology. 1994;81:353.
Lindahl S. Setting the “furnace” during anesthesia (editorial). Anesthesiology. 2006;104:1119-1120.
Liu M., Hu X., Liu J. The effect of hypothermia on isoflurane MAC in children. Anesthesiology. 2001;94:429.
Lopez M., Ozaki M., Sessler D.I., et al. Physiologic responses to hyperthermia during epidural anesthesia and combined epidural/enflurane anesthesia in women. Anesthesiology. 1993;78:1046.
Lyons B., Taylor A., Power C., et al. Postanaesthetic shivering in children. Anaesthesia. 1996;51:442.
MacKenzie R., Asbury A.J. Clinical evaluation of liquid crystal skin thermometers. Br J Anaesth. 1994;72:246.
Mahajan R.P., Grover V.K., Sharma S.L., et al. Intraocular pressure changes during muscular hyperactivity after general anesthesia. Anesthesiology. 1987;66:419.
Matsukawa T., Sessler D.I., Christensen R., et al. Heat flow and distribution during epidural anesthesia. Anesthesiology. 1995;83:961.
Matsukawa T., Sessler D.I., Sessler A.M., et al. Heat flow and distribution during induction of general anesthesia. Anesthesiology. 1995;82:662.
Matsukawa T., Kurz A., Sessler D.I., et al. Propofol linearly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1995;82:1169.
McAllister R.G.Jr, Tan T.G. Effect of hypothermia on drug metabolism: in vitro studies with propranolol and verapamil. Pharmacology. 1980;20:95.
Mercer J.B., Jessen C. Central thermosensitivity in conscious goats: hypothalamus and spinal cord versus residual inner body. Pflugers Arch. 1978;374:179.
Mercke U. The influence of varying air humidity on mucociliary activity. Acta Otolaryngol. 1975;79:133.
Mestyan J., Bata G., Jarai I., et al. The significance of facial skin temperature in the chemical heat regulation of premature infants. Biol Neonat. 1964;7:243.
Michelson A.D., MacGregor H., Barnard M.R., et al. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Thromb Haemost. 1994;71:633.
Miller R.D., Agoston S., van der Pol F., et al. Hypothermia and the pharmacokinetics and pharmacodynamics of pancuronium in the cat. J Pharmacol Exp Ther. 1978;207:532.
Mizobe T., Nakajima Y., Ueno H., Sessler D.I. Fructose administration increases intraoperative core temperature by augmenting both metabolic rate and the vasoconstriction threshold. Anesthesiology. 2006;104:1124-1130.
Mohta M., Kumari N., Tyagi A., et al. Tramadol for prevention of postanaesthetic shivering: a randomized double-blind comparison with pethidine. Anaesthesia. 2009;64:141-146.
Morris R.H. Operating room temperature and the anesthetized, paralyzed patient. Arch Surg. 1971;102:95.
Morris R.H. Influence of ambient temperature on patient temperature during intraabdominal surgery. Ann Surg. 1971;173:230.
Morris R.H., Wilkey B.R. The effects of ambient temperature on patient temperature during surgery not involving body cavities. Anesthesiology. 1970;32:102.
Muma B.K., Treloar D.J., Wurmlinger K., et al. Comparison of rectal, axillary, and tympanic membrane temperatures in infants and young children. Ann Emerg Med. 1991;20:41.
Nakayama T., Hammel H.T., Hardy J.D., et al. Thermal stimulation of electrical activity of single units of the preoptic region. Am J Physiol. 1963;204:1122.
Nebbia S.P., Bissonnette B., Sessler D.I. Enflurane decreases the threshold for vasoconstriction more than isoflurane or halothane. Anesth Analg. 1996;83:595.
Nesher N., Insler S.R., Sheinberg N., et al. A new thermoregulation system for maintaining perioperative normothermia and attenuating myocardial injury in off-pump coronary artery bypass surgery. Heart Surg Forum. 2002;5:373.
Nesher N., Wolf T., Kushnir I., et al. Novel thermoregulation system for enhancing cardiac function and hemodynamics during coronary artery bypass graft surgery. Ann Thorac Surg. 2001;72:S1069.
Nesher N., Wolf T., Uretzky G., et al. A novel thermoregulatory system maintains perioperative normothermia in children undergoing elective surgery. Paediatr Anaesth. 2001;11:555.
Newton D.E. Proceedings: The effect of anaesthetic gas humidification on body temperature. Br J Anaesth. 1975;47:1026.
Ohlson K.B., Mohell N., Cannon B., et al. Thermogenesis in brown adipocytes is inhibited by volatile anesthetic agents: a factor contributing to hypothermia in infants? Anesthesiology. 1994;81:176.
Oya A., Asakura H., Koshino T., et al. Thermographic demonstration of nonshivering thermogenesis in human newborns after birth: its relation to umbilical gases. J Perinat Med. 1997;25:447.
Ozaki M., Sessler D.I., Suzuki H., et al. Nitrous oxide decreases the threshold for vasoconstriction less than sevoflurane or isoflurane. Anesth Analg. 1995;80:1212.
Ozaki M., Sessler D.I., Suzuki H., et al. The threshold for thermoregulatory vasoconstriction during nitrous oxide/sevoflurane anesthesia is lower in elderly than in young patients. Ann N Y Acad Sci. 1997;813:789.
Ozaki M., Kurz A., Sessler D.I., et al. Thermoregulatory thresholds during epidural and spinal anesthesia. Anesthesiology. 1994;81:282.
Petrikovsky B., Silverstein M., Schneider E.P. Neonatal shivering and hypothermia after intrapartum amnioinfusion. Lancet. 1997;350:1366.
Pflug A.E., Aasheim G.M., Foster C., et al. Prevention of post-anaesthesia shivering. Can Anaesth Soc J. 1978;25:43.
Pickering G. Regulation of body temperature in health and disease. Lancet. 1958;1:59.
Pierau F.K., Wurster R.D. Primary afferent input from cutaneous thermoreceptors. Fed Proc. 1981;40:2819.
Plattner O., Semsroth M., Sessler D.I., et al. Lack of nonshivering thermogenesis in infants anesthetized with fentanyl and propofol. Anesthesiology. 1997;86:772.
Poulos D.A. Central processing of cutaneous temperature information. Fed Proc. 1981;40:2825.
Prasad K.K., Chen L. Complications related to the use of a heat and moisture exchanger. Anesthesiology. 1990;72:958.
Puhakka K., Anttonen H., Niskanen J., et al. Calculation of mean skin temperature and changes in body heat content during paediatric anaesthesia. Br J Anaesth. 1994;72:548.
Rajagopalan S., Mascha E., Na J., et al. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108:71.
Rajek A., Greif R., Sessler D.I., et al. Core cooling by central venous infusion of ice-cold (4 degrees C and 20 degrees C) fluid: isolation of core and peripheral thermal compartments. Anesthesiology. 2000;93:629.
Ram D., Hermida L.B., Peretz B. A comparison of warmed and room-temperature anesthetic for local anesthesia in children. Pediatr Dent. 2002;24:333.
Ramanathan N.L. A new weighting system for mean surface temperature of the human body. J Appl Physiol. 1964;19:531.
Ricquier D., Kader J.C. Mitochondrial protein alteration in active brown fat: a sodium dodecyl sulfate-polyacrylamide gel electrophoretic study. Biochem Biophys Res Commun. 1976;73:577.
Roe C.F. Effect of bowel exposure on body temperature during surgical operations. Am J Surg. 1971;122:13.
Roe C.F., Santulli T.V., Blair C.S. Heat loss in infants during general anesthesia and operations. J Pediatr Surg. 1966;1:266.
Rohrer M.J., Natale A.M. Effect of hypothermia on the coagulation cascade. Crit Care Med. 1992;20:1402.
Romanovsky A.A. Do fever and anapyrexia exist? Analysis of set point-based definitions. Am J Physiol Regul Integr Comp Physiol. 2004;287:R992.
Rosa G., Pinto G., Orsi P., et al. Control of post anaesthetic shivering with nefopam hydrochloride in mildly hypothermic patients after neurosurgery. Acta Anaesthesiol Scand. 1995;39:90.
Rübsamen K., Hales J.R. Role of arteriovenous anastomoses in determining heat transfer across the hindleg skin of sheep. In: Hales J.R., editor. Thermal physiology. New York: Raven Press; 1984:259.
Ryan J.F. Altered temperature regulation section two: unintentional hypothermia. In: Cooperman L., Orkin F., editors. Complications in anesthesiology. Philadelphia: J.B. Lippincott Co; 1982:284.
Saito T. A comparison of the body temperature during sevoflurane anesthesia and isoflurane anesthesia. Ann N Y Acad Sci. 1997;813:786.
Sanders B.J., D’Alessio J.G., Jernigan J.R. Intraoperative hypothermia associated with lower extremity tourniquet deflation. J Clin Anesth. 1996;8:504.
Schiff D., Stern L., Leduc J. Chemical thermogenesis in newborn infants: catecholamine excretion and the plasma non-esterified fatty acid response to cold exposure. Pediatrics. 1966;37:577.
Schmied H., Kurz A., Sessler D.I., et al. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289.
Sellden E. Peri-operative amino acid administration and the metabolic response to surgery. Proc Nutr Soc. 2002;61:337.
Sellden E., Brundin T., Wahren J. Augmented thermic effect of amino acids under general anaesthesia: a mechanism useful for prevention of anaesthesia-induced hypothermia. Clin Sci (Lond). 1994;86:611.
Sellden E., Lindahl S.G. Amino acid-induced thermogenesis reduces hypothermia during anesthesia and shortens hospital stay. Anesth Analg. 1999;89:1551.
Sessler D.I. Malignant hyperthermia. J Pediatr. 1986;109:9.
Sessler D.I. Central thermoregulatory inhibition by general anesthesia. Anesthesiology. 1991;75:557.
Sessler D.I. Sweating threshold during isoflurane anesthesia in humans. Anesth Analg. 1991;73:300.
Sessler D.I. Perianesthetic thermoregulation and heat balance in humans. FASEB J. 1993;7:638.
Sessler D.I. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109:318-338.
Sessler D.I., McGuire J., Hynson J., et al. Thermoregulatory vasoconstriction during isoflurane anesthesia minimally decreases cutaneous heat loss. Anesthesiology. 1992;76:670.
Sessler D.I., McGuire J., Moayeri A., et al. Isoflurane-induced vasodilation minimally increases cutaneous heat loss. Anesthesiology. 1991;74:226.
Sessler D.I., McGuire J., Sessler A.M. Perioperative thermal insulation. Anesthesiology. 1991;74:875.
Sessler D.I., Moayeri A. Skin-surface warming: heat flux and central temperature. Anesthesiology. 1990;73:218.
Sessler D.I., Olofsson C.I., Rubinstein E.H., et al. The thermoregulatory threshold in humans during halothane anesthesia. Anesthesiology. 1988;68:836.
Sessler D.I., Olofsson C.I., Rubinstein E.H. The thermoregulatory threshold in humans during nitrous oxide-fentanyl anesthesia. Anesthesiology. 1988;69:357.
Sessler D.I., Ponte J. Disparity between thermal comfort and psychological thermoregulatory responses during epidural anesthesia. Anesthesiology. 1982;71:A682.
Shanks C.A. Mean skin temperature during anaesthesia: an assessment of formulae in the supine surgical patient. Br J Anaesth. 1975;47:871.
Shroff P.K., Skerman J.H. Humidifier malfunction: a cause of anesthesia circuit occlusion. Anesth Analg. 1988;67:710.
Siddik-Sayyid S.M., Abdallah F.W., Dahrouj G.B. Thermal burns in three neonates associated with intraoperative use of Bair Hugger warming devices. Paediatr Anaesth. 2008;18:337.
Silverman W., Blanc W. Effects of humidity on survival of newly born premature infants. Pediatrics. 1957;22:876.
Silverman W., Fertig J., Bergen A. Influence of thermal environment upon survival of newly born premature infants. Pediatrics. 1958;22:876.
Silverman W.A., Zamelis A., Sinclair J.C., et al. Warm nap of the newborn. Pediatrics. 1964;33:984.
Simon E. Temperature regulation: the spinal cord as a site of extrahypothalamic thermoregulatory functions. Rev Physiol Biochem Pharmacol. 1974:1.
Sinclair J.C. Thermal control in premature infants. Annu Rev Med. 1972;23:129.
Smith H.S., Allen R. Another hazard of heated water humidifiers. Anaesthesia. 1986;41:215.
Stacey M.R., Asai T., Wilkes A., et al. Obstruction of a breathing system filter. Can J Anaesth. 1996;43:1276.
Steele M.T., Nelson M.J., Sessler D.I., et al. Forced air speeds rewarming in accidental hypothermia. Ann Emerg Med. 1996;27:479.
Stephen C.R., Dent S., Hall K. Body temperature regulation during anesthesia in infants and children. JAMA. 1960;174:1579.
Stern L., Lees M.H., Leduc J. Environmental temperature, oxygen consumption, and catecholamine excretion in newborn infants. Pediatrics. 1965;36:367.
Stoen R., Sessler D.I. The thermoregulatory threshold is inversely proportional to isoflurane concentration. Anesthesiology. 1990;72:822.
Stone D.R., Downs J.B., Paul W.L., et al. Adult body temperature and heated humidification of anesthetic gases during general anesthesia. Anesth Analg. 1981;60:736.
Stuart D., Ott K., Ishikawa K., et al. The rhythm of shivering. 3. Central contributions. Am J Phys Med. 1966;45:91.
Sulyok E., Jequier E., Prod’hom L.S. Thermal balance of the newborn infant in a heat-gaining environment. Pediatr Res. 1973;7:888.
Swyer P. Heat loss after birth. In: Sinclair J.C., editor. Temperature regulation and energy metabolism in the newborn. New York: Grune and Stratton; 1973:91.
Szmuk P., Rabb M.F., Baumgartner J.E., et al. Body morphology and the speed of cutaneous rewarming. Anesthesiology. 2001;95:18.
Talke P., Tayefeh F., Sessler D.I., et al. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly decreases the vasoconstriction and shivering thresholds. Anesthesiology. 1997;87:835.
Tankersley C.G., Smolander J., Kenney W.L., et al. Sweating and skin blood flow during exercise: effects of age and maximal oxygen uptake. J Appl Physiol. 1991;71:236.
Tollofsrud S.G., Gundersen Y., Andersen R. Peroperative hypothermia. Acta Anaesthesiol Scand. 1984;28:511.
Truell K.D., Bakerman P.R., Teodori M.F., et al. Third-degree burns due to intraoperative use of a Bair Hugger warming device. Ann Thorac Surg. 2000;69:1933.
Vale R.J., Lunn H.F. Heat balance in anaesthetized surgical patients. Proc R Soc Med. 1969;62:1017.
van Marken Lichtenbelt W.D., Daanen H.A. Cold-induced metabolism. Curr Opin Clin Nutr Metab Care. 2003;6:469.
van Oss C.J., Absolom D.R., Moore L.L., et al. Effect of temperature on the chemotaxis, phagocytic engulfment, digestion and O2 consumption of human polymorphonuclear leukocytes. J Reticuloendothel Soc. 1980;27:561.
Vitez T.S., White P.F., Eger E.I.2nd. Effects of hypothermia on halothane MAC and isoflurane MAC in the rat. Anesthesiology. 1974;41:80.
Vybiral S., Lesna I., Jansky L., et al. Thermoregulation in winter swimmers and physiological significance of human catecholamine thermogenesis. Exp Physiol. 2000;85:321.
Washington D.E., Sessler D.I., McGuire J., et al. Painful stimulation minimally increases the thermoregulatory threshold for vasoconstriction during enflurane anesthesia in humans. Anesthesiology. 1992;77:286.
Wenisch C., Narzt E., Sessler D.I., et al. Mild intraoperative hypothermia reduces production of reactive oxygen intermediates by polymorphonuclear leukocytes. Anesth Analg. 1996;82:810.
Wilkins B.W., Chung L.H., Tublitz N.J., et al. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J Appl Physiol. 2004;97:1291.
Wyss C.R., Brengelmann G.L., Johnson J.M., et al. Altered control of skin blood flow at high skin and core temperatures. J Appl Physiol. 1975;38:839.
Xiong J., Kurz A., Sessler D.I., et al. Isoflurane produces marked and nonlinear decreases in the vasoconstriction and shivering thresholds. Anesthesiology. 1996;85:240.