W7 Arthroscopic Thermal Shrinkage for Scapholunate Ligament Injuries
The use of arthroscopic thermal shrinkage with radiofrequency (RF) for the treatment of scapholunate ligament injuries is a more recent technique, and the real effectiveness is undetermined.1–3 The ability of RF probes to débride and shrink tissues makes them an attractive alternative to the use of a mechanized resector for débridement of scapholunate ligament tears, and provides a means for stabilizing the scapholunate joint.
What Is Shrinkage?
Shrinkage is a physical phenomenon that occurs with heat modification of type I collagen in ligamentous tissue. When the collagen is heated to a critical temperature, the heat-labile intramolecular hydrogen bonds break.4 The protein undergoes a phase transition from a highly ordered crystalline structure to a random-coil state, which is similar to melting, and the tissue tensile properties change.5 Typically, this thermal denaturation of collagen type I occurs at approximately 60°C to 65°C. The heat in ligamentous tissues is generated by a RF pulse, which results in the oscillation of molecules as their polarity changes (i.e., ohmic resistance). The RF probe imparts a high-frequency (350 kHz to 1 MHz) alternating current from an electrical generator to the tissue. This current creates an ionic agitation in the tissue as the ions attempt to follow the changes in direction of the alternating current. This ionic agitation results in frictional heating within the tissue. The current passes either between the probe tip and a grounding pad (monopolar) or between two points on the probe tip (bipolar).
Molecular Effects of Thermal Shrinkage
Transmission electron microscopy shows significant alterations in the collagen architecture. These changes are characterized by the loss of the classic 67-nm periodicity of the type I collagen fibril that is evidenced by the loss of the periodical cross-striations in the collagen fibril. There also is an increase in the cross-sectional area of the collagen fibril. The margins of the fibrils begin to lose their distinct edge, while maintaining their circular shape. These ultrastructural effects are caused by unwinding of the collagen triple helix as a result of the temperature increase in the tissue.6,7
Biological Response to Thermal Shrinkage
At time 0, after thermal shrinkage, under light microscopy there is evidence of diffuse hyalinization and fusion of the collagen fiber. By day 7, there is fibroblast proliferation around and within the hyalinized regions. By day 30, large fibroblasts have migrated into the region and produced new matrix. These newly arrived fibroblasts use the acellular “hyalinized” collagen as a scaffold for migration and matrix synthesis. At 3 months, active reparative changes are evident with an increase in vascularity. The fibroblasts have now regained a more normal appearance under transmission electron microscopy. At 7 months, the cell morphology and vascularity have returned to normal without evidence of any permanent tissue injury or severe inflammation.8,9
Biomechanical Effects of Thermal Shrinkage
The aim of thermal shrinkage is to improve joint stability when the ligaments or capsular tissue are lax or incompetent. Data are conflicting, however, with regard to the biomechanical properties of thermally treated soft tissue. Some of these inconsistencies may be accounted for by differences in experimental protocols, which do not allow for direct comparison between studies. Only a few, but important, basic concepts may be extrapolated from these studies as pertains to shrinkage of the scapholunate ligament. Experimental studies have shown that (1) ligaments and joint capsular tissue can be modified significantly (shortened) by thermal energy at the temperature range of 70°C to 80°C; (2) thermal energy causes immediate deleterious effects, such as loss of the mechanical properties, collagen denaturation, and cell necrosis; (3) thermally treated tissue is repaired actively by a residual population of fibroblasts and vascular cells, with concomitant improvement of mechanical properties; (4) the shrunken tissue stretches with time if the tissue is subjected to physiological loading immediately after surgery; and (5) leaving viable tissue between treated regions significantly improves the healing process.10
Rationale for Shrinkage of Scapholunate Ligament Injuries
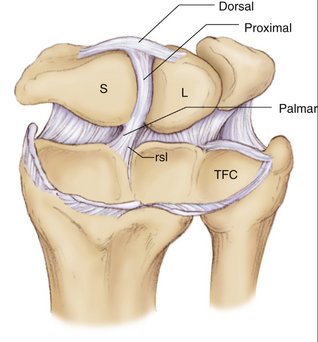
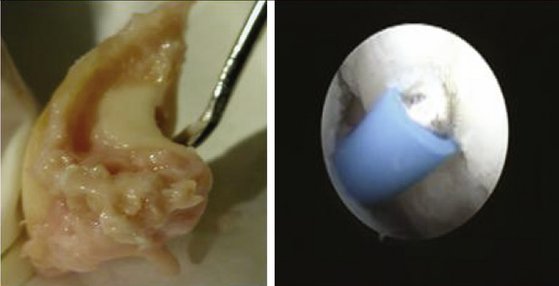
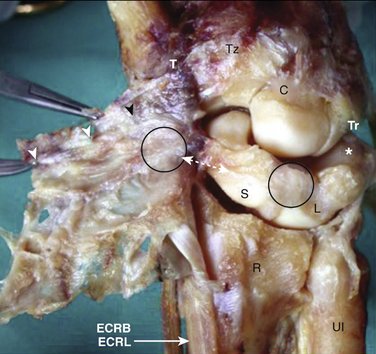
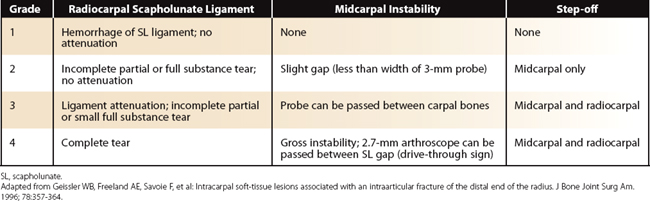
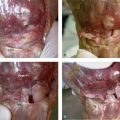
Our concept for the use of thermal shrinkage for the treatment of instability of the carpus with scapholunate ligament injuries arose from previous published work on the use of thermal shrinkage on other articulations and the favorable results that were achieved after mechanical débridement of partial scapholunate ligament tears.11,12 We also were influenced by the biomechanical importance of the scapholunate ligament for stability of the carpus and the paucity of treatment methods for carpal instability, and the relative ease of performing an arthroscopic shrinkage of the scapholunate ligament. The scapholunate ligament (SL) is not a homogeneous structure. It is divided into three parts: dorsal, proximal, and palmar (Fig. W7-1). The dorsal part is the strongest subregion of the scapholunate ligament. It meets all the criteria for the definition of an articular ligament in that it is composed of collagen fascicles surrounded by connective tissue with intertwined neurovascular bundles.13–15 It has a thickness of 2 to 3 mm and a length of 4 to 5 mm (Fig. W7-2) and merges with the dorsal capsule (Fig. W7-3).
The proximal portion is grossly anisotropic. It is composed mainly of fibrocartilaginous tissue, which is weak owing to its avascularity. The transition zone between the proximal and palmar portions is marked by the radioscapholunate ligament, which inserts on the palmar aspect of the scapholunate ligament. The palmar portion is composed of thin collagen fascicles (1 mm thick) with a length of 4 to 5 mm. This portion is invisible through the standard dorsal arthroscopic portals in the face of an intact radioscapholunate ligament. The three parts do not have the same tensile strength. The dorsal part is most resistant to shear forces with an ultimate yield strength of 300N. The palmar part fails at a load of 150N, whereas the proximal portion can withstand only 25N to 50N of stress. The triquetrolunate ligament, which also is divided into three parts, has the exact reverse characteristics on loading to failure as those of the scapholunate ligament. Biomechanical studies also have shown that the dorsal subregion of the scapholunate ligament is responsible for controlling scaphoid flexion and the extension motion, whereas the palmar subregion controls rotational motion.16–19
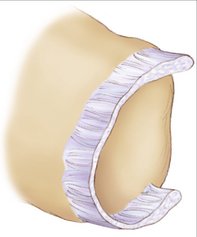
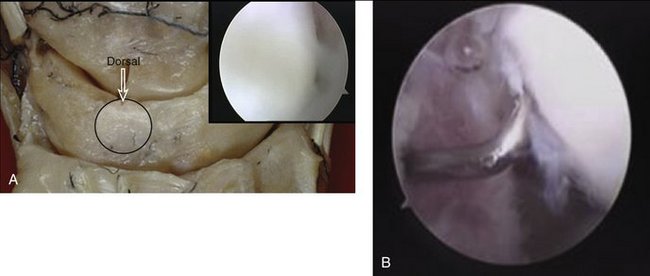
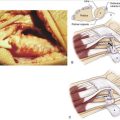
Based on this evidence, it was apparent to us that the use of thermal shrinkage of the scapholunate ligament was feasible and most appropriate for the dorsal part of the ligament. When considering the kinematics and the instability of the carpus in scapholunate ligament injuries, it is important to remember the role of the dorsal radiocarpal ligaments (Fig. W7-4) and the dorsal capsule (Fig. W7-5). They are intimately connected with the scapholunate ligament and must be included in the thermal shrinkage (Fig. W7-6).
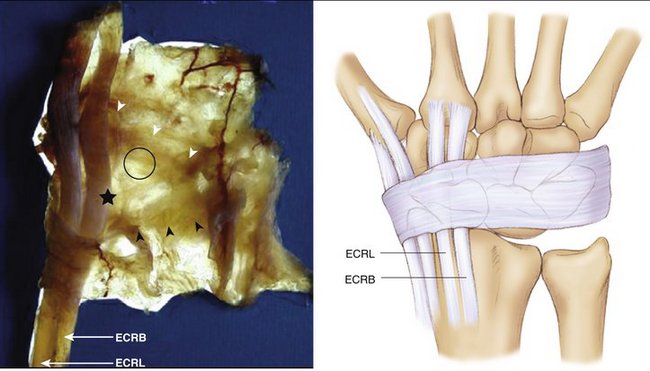
Figure W7-5 The dorsal capsule. ECRB, extensor carpi radialis brevis; ECRL, extensor carpi radialis longus.
The aim of thermal shrinkage of the scapholunate ligaments along with the dorsal ligaments and the dorsal capsule is to maintain the ligament and capsular shortening that is achieved during shrinkage, while awaiting the secondary fibroplasia and resultant thickening of the joint capsule and ligament. Another theoretical goal is the interruption of any painful afferent sensory pathways through the destruction of sensory receptors.20–22
Technique
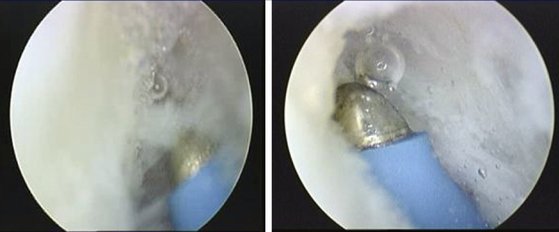
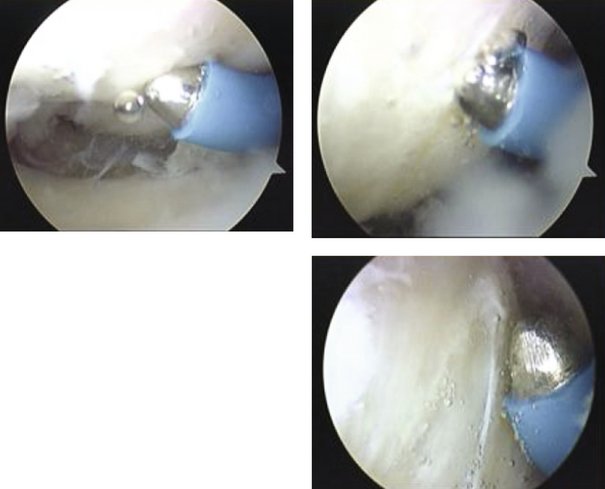
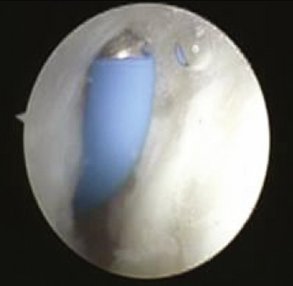
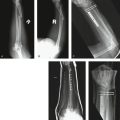
Wrist arthroscopy is performed using a standard technique. The arthroscopy is done by placing the affected extremity in a distraction tower with 3 to 5 kg of distraction. The correct amount and direction of the distraction force are monitored fluoroscopically, to avoid iatrogenic injury to the carpal ligaments, and to control the palmar flexion of the scaphoid. The joint is insufflated with 5 to 7 mL of normal saline followed by the establishment of the 3,4 and 4,5 portals as a viewing and working portal and a midcarpal portal. The wrist is examined from radial to ulnar, and the stability of the scapholunate interval stability is assessed by probing the transition zone between the dorsal portion of the scapholunate ligament, which is thick and taut, and the weaker proximal portion, which is identified by palpation. All patients undergo stress testing of the scapholunate ligament under direct visualization. Any ligamentous injury is classified according to the arthroscopic classification scheme described by Geissler.23
The shrinkage of the scapholunate ligament is performed with a 2.3-mm monopolar RF probe that is dedicated for shrinkage (Micro-Tacs with an angled tip and a controlled temperature system). The shrinkage is performed on the entire dorsal section of the ligament (Fig. W7-7) extending up to its confluence with the dorsal capsule (Fig. W7-8). The palmar subregion of the scapholunate ligament is not included in the shrinkage. The scapholunate ligament and capsular tissue are treated with multiple single linear passes (grid pattern) to leave more viable tissue adjacent to the treated areas, which may result in faster cellular invasion and matrix formation. There is no objective way to measure the effect of RF probes, so the surgeon relies on the visual assessment of the morphological ligament tissue changes and capsular volume reduction to quantify the degree of tissue shrinkage.
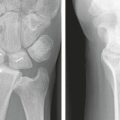
Using the Geissler classification of scapholunate ligament injuries (Table W7-1), symptomatic grade 1 lesions are treated with the standard technique for shrinkage as described. Grade 2 lesions that show dynamic instability (i.e., an increased scapholunate gap with loading) are treated with shrinkage as described in addition to Kirschner wire (K-wire) fixation of the scaphoid and lunate.24,25 In grade 3 lesions, in which there is a complete scapholunate ligament perforation, the shrinkage is mainly performed on the dorsal capsule and radiocarpal ligaments with only marginal shrinkage of the torn dorsal ligament, combined with K-wire fixation of the scaphoid and lunate. Acute and subacute grade 3 lesions less than 4 months old that show a static scapholunate dissociation and rotational instability require an arthroscopic reduction and K-wire fixation.
Scientific Study
Results
No complications were noted from the use of RF probes (Table W7-1). The scores for overall clinical outcome showed that patients in group A had better outcomes than patients in group B. Patients in group C also had better outcomes than patients in group D. No statistically significant changes were noted between the preoperative and postoperative x-rays for groups A and B. There was a statistically significant reduction, however, in the scapholunate interval seen on x-rays during loading for group C.
1. Hirsh L, Sodha S, Bozentka D, et al. Arthroscopic electrothermal collagen shrinkage for symptomatic laxity of the scapholunate interosseous ligament. J Hand Surg.. 2005;30(6):643-647.
2. Darlis NA, Weiser RW, Sotereanos DG. Partial scapholunate ligament injuries treated with arthroscopic debridement and thermal shrinkage. J Hand Surg.. 2005;30(5):908-914.
3. Shih JT, Lee HM. Monopolar radiofrequency electrothermal shrinkage of the scapholunate ligament. Arthroscopy.. 2006;22(5):553-557.
4. Arnoczky SP, Aksan A. Thermal modification of connective tissues: basic science considerations and clinical implications. J Am Acad Orthop Surg.. 2000;8(5):305-313.
5. Owens BD, Stickles BJ, Busconi BD. Radiofrequency energy: applications and basic science. Am J Orthop. 2003;32:117-120.
6. Wallace AL, Hollinshead RM, Frank CB. The scientific basis of thermal capsular shrinkage. J Shoulder Elbow Surg.. 2000;9:354-360.
7. Medvecky MJ, Ong BC, Rokito AS, Sherman OH. Thermal capsular shrinkage: basic science and clinical applications. Arthroscopy. 2001;17:624-635.
8. Vangsness CT, Mitchell W III, Nimni M, et al. Collagen shortening: an experimental approach with heat. Clin Orthop.. 1997;337:267-271.
9. Berger RA, Garcia-Elias M. General anatomy of the wrist. In: An KN, Berger RA, Cooney WP III, editors. Biomechanics of the Wrist Joint. New York: Springer-Verlag; 1991:1-22.
10. DeWal H, Ahn A, Raskin KB. Thermal energy in arthroscopic surgery of the wrist. Clin Sports Med.. 2002;21:727-735.
11. Lopez MJ, Hayashi K, Fanton GS, et al. The effect of radiofrequency energy on the ultrastructure of joint capsular collagen. Arthroscopy.. 1998;14:495-501.
12. Lu Y, Hayashi K, Edwards RB, et al. The effect of monopolar radiofrequency treatment pattern on joint capsular healing: in vitro and in vivo studies using an ovine model. Am J Sports Med.. 2000;28:711-719.
13. Wall MS, Deng XH, Torzilli PA, et al. Thermal modification of collagen. J Shoulder Elbow Surg.. 1998;8:339-344.
14. Fanton GS. Arthroscopic electrothermal surgery of the shoulder. Oper Tech Sports Med.. 1998;6:139-146.
15. Obrzut LS, Hecht P, Hayashi K, et al. The effect of radiofrequency energy on the length and temperature properties of the glenohumeral joint capsule. Arthroscopy.. 1998;14:395-400.
16. Ruch DS, Arthroscopic Poehling GG:. management of partial scapholunate and lunotriquetral injuries of the wrist. J Hand Surg [Am]. 1996;21:412-417.
17. Hayashi K, Markel MD. Thermal capsulorrhaphy treatment of shoulder instability: basic science. Clin Orthop.. 2001;390:59-72.
18. Hecht P, Hayashi K, Cooley AJ, et al. The thermal effect of monopolar radiofrequency energy on the properties of joint capsule: an in vivo histologic study using a sheep model. Am J Sports Med.. 1998;26:808-814.
19. Berger RA. The gross and histologic anatomy of the scapholunate interosseous ligament. J Hand Surg [Am]. 1996;21:170-178.
20. Sokolow C, Saffar P. Anatomy and histology of the scapholunate ligament. Hand Clin.. 2001;17:77-81.
21. Geissler WB, Haley T. Arthroscopic management of scapholunate instability. Atlas Hand Clin.. 2001;6:253-274.
22. Goldberg SH, Strauch RE, Rosenwasser MP. Scapholunate and lunotriquetral instability in the athlete: diagnosis and management. Oper Tech Sports Med.. 2006;14:108-121.
23. Geissler WB, Freeland AE, Savoie F, et al. Intracarpal soft-tissue lesions associated with an intra-articular fracture of the distal end of the radius. J Bone Joint Surg Am. 1996;78:357-364.
24. Weiss APC, Sachar K, Glowacki KA. Arthroscopic debridement alone for intercarpal ligament tears. J Hand Surg [Am].. 1997;22:344-349.
25. Whipple TL, Schengel D, Caffrey WD. Arthroscopic reduction and internal fixation of scapholunate dissociation. Arthroscopy.. 1992;8(3):410.