Thermal Burns
Perspective
Burns are relatively common injuries caused by direct or indirect contact with heat, electrical current, radiation, or chemical agents. The common denominator in all burns is protein denaturation and cell death. With thermal burns, the degree of injury is dependent on the temperature, the duration of the exposure, and the intrinsic structure of the burned tissue that determines its heat conductivity.1–3 Temperatures below 44° C are generally tolerated for prolonged periods without causing burns. As the temperature rises, there is an exponential decrease in the time to produce injury, because the proportion of unfolded protein molecules increases dramatically at supraphysiologic temperatures.4 At temperatures above 60° C, most tissue proteins are denatured. The proteins most predisposed to thermal denaturation are the lipid bilayer and membrane-bound adenosine triphosphatases. Thermal alteration of the plasma membrane is likely to be the most significant cause of the tissue necrosis.4 Cell death occurs by one of two mechanisms.5 Necrosis (or oncosis) is a passive process characterized by cellular swelling, depletion of intracellular energy stores, and massive release of inflammatory mediators. In contrast, apoptosis is a highly programmed, active process characterized by shrinkage and condensation of cell contents, DNA fragmentation, and budding without cellular swelling and a minimal inflammatory response. Both necrosis and apoptosis play a role in burn injury progression, although their relative role may vary.6,7 The underlying mechanisms leading to cellular necrosis and apoptosis are discussed further in the section on pathophysiology.
Epidemiology
According to the American Burn Association (ABA), 450,000 burn injuries are treated in medical facilities each year.8 This includes 3500 deaths, which occur mostly in residential fires. Of the 45,000 burn admissions each year, more than 55% are admissions to specialized burn centers. The majority of burns occur from fire or flame (44%), scalds (33%), contact with hot objects (9%), electricity (4%), or chemical agents (3%). More than one third of admissions (38%) exceed 10% total body surface area (TBSA), and 10% exceed 30% TBSA. Most admissions include severe burns of such vital body areas as the face, hands, and feet. The overall survival rate from burns in the years 1995 to 2005 was 94.4%.9
The National Center for Health Statistics indicates a decrease in the number of burn visits from 1996 to 2000, possibly because of improved fire prevention strategies, with no further changes in trends from 2000 to 2005.9 Half of burn patients in the emergency department (ED) are age 19 to 44 years. The most commonly affected body areas are the upper extremities (41%), lower extremities (26%), and head and neck (17%). Most of the burns are partial-thickness injuries, and less than 5% are full thickness.
Principles of Disease
Unlike mechanical injuries, in which the amount of tissue damage is determined at the time of injury, burns are dynamic injuries that often progress in depth and size over the first few days after injury. Traditionally the burn injury has been divided into three concentric zones.10 At the center of the burn is a zone of irreversible coagulative necrosis that is formed immediately. Surrounding this central core is a zone of ischemia in which there is a reduction in the dermal microcirculation, putting this area at risk for subsequent necrosis if the perfusion is not improved. The zone of ischemia is the focus of intense research, directed toward reversing or preventing the conversion from ischemia to necrosis. The third and outermost zone of injury is the zone of hyperemia, characterized by an immediate and transient increase in perfusion. The ability of the skin to regenerate itself without scar formation is highly dependent on the level of injury.11 Regeneration of the damaged epidermis occurs from two primary sources. The basal layer of cells from the uninjured adjacent epidermis can give rise to reepithelialization of the burn; however, this is limited to 1 cm from the wound edges. With large burns the major source of regenerative epidermal cells comes from the dermal skin appendages: hair follicles and sebaceous glands. More damage to the epidermal appendages increases the likelihood of scar formation.
Although the exact mechanisms that drive burn injury progression are unknown, several processes are thought to play a role.12,13 The inflammatory response results in the release of a large number of toxic cytokines, mediators, and free oxygen and nitrogen radicals that contribute to further injury.14–16 Occlusion of the dermal microcirculation with a combination of red blood cells, neutrophils, and microthrombi results in reduced perfusion and tissue ischemia that is exacerbated by a large amount of tissue edema.17 Cytokines, such as tumor necrosis factor alpha, result in cellular apoptosis and further inflammation; free oxygen and nitrogen radicals damage vital proteins, lipids, and DNA. Cellular membranes are especially vulnerable to oxidative stress caused by lipid peroxidation.18 The importance of these mechanisms is demonstrated by the ability of several inhibitors of inflammation and oxidative stress to reduce burn injury progression both in animals and humans.19–22 Unfortunately, none of these agents have demonstrated clinical utility.
A predisposition to infection, metabolic derangements, and other factors also predispose partial-thickness burns to progress to deeper burns.13 Lack of adequate fluid resuscitation and hypoperfusion contribute to the deepening of the burn wound. Impaired ability to combat infection is common in patients with large burns and appears to be cytokine mediated. In particular, interleukin-12 (IL-12) has been shown to depress immune function.23 Abnormal complement activity also predisposes to infection in large burns.
Pathophysiology of Inhalation Injury
Exposure to heat can cause significant injury and rapidly developing edema to the upper airways in up to one third of burn patients with inhalation injury.24 However, owing to effective heat dissipation, direct thermal injury is generally not seen below the vocal cords.25 Other components of smoke, such as products of incomplete combustion (e.g., carbon monoxide [CO], cyanide, the aldehydes and oxides of sulfur and nitrogen), and particulate matter often result in significant ventilation-perfusion (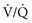 ) mismatch and pulmonary edema.26–28 (See Chapter 159.)
) mismatch and pulmonary edema.26–28 (See Chapter 159.)
The upper and lower airway pathology is secondary to airway edema and de-epithelialization of the injured tracheobronchial mucosa, with progressive shedding of the necrotic lining of the airway and the formation of pseudomembranous casts that partially or completely obstruct the airway. These casts consist of necrotic epithelium, mucus, cellular debris, fibrinous exudate, polymorphonuclear leukocytes, and clumps of bacteria. Damage to the mucociliary function of the endobronchial mucosa reduces the ability to eliminate excess mucus and secretions. In addition, the pulmonary parenchyma becomes infiltrated with leukocytes that release inflammatory mediators and reactive oxygen species that further contribute to bronchospasm, tissue inflammation, and destruction. In particular, the local formation of nitric oxide (NO) results in vasodilatation and reversal of hypoxic pulmonary vasoconstriction, further aggravating 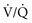 mismatch.28 NO combines with superoxide (O2−), which is released by neutrophils to form peroxynitrite (ONOO−). This reactive molecule then damages DNA and activates poly(adenosine diphosphate [ADP] ribose) polymerase, which is an enzyme that repairs damaged DNA while depleting the cell of large amounts of adenosine triphosphate (ATP), leading to necrosis.27–29
mismatch.28 NO combines with superoxide (O2−), which is released by neutrophils to form peroxynitrite (ONOO−). This reactive molecule then damages DNA and activates poly(adenosine diphosphate [ADP] ribose) polymerase, which is an enzyme that repairs damaged DNA while depleting the cell of large amounts of adenosine triphosphate (ATP), leading to necrosis.27–29
The increase in extravascular water content and pulmonary lymph flow causes a marked decrease in the compliance of the lungs. Inhalation injury also results in deactivation of pulmonary surfactant, leading to areas of microatelectasis causing further ventilation perfusion mismatch and pulmonary shunting, and results in progressive hypoxemia and ultimately the clinical syndrome of acute respiratory distress syndrome.29,30 Ventilator-induced barotrauma further contributes to tissue injury and the release of toxic mediators.
Clinical Features
Classification and Diagnosis of Burns
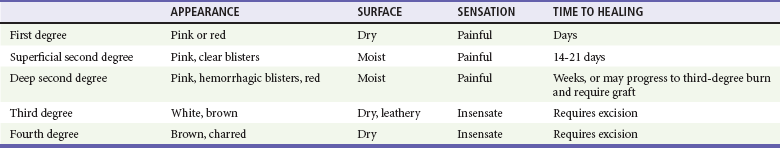
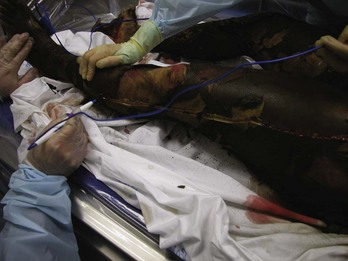
Burns are classified by mechanism of injury, depth, extent, and associated injuries and comorbidities. Determination of the depth of injury is crucial, as healing potential and appropriate treatments are contingent on the depth of injury (Table 63-1). The current burn classification system was first described by the French barber surgeon Ambroise Paré several hundred years ago. First-degree burns are limited to the epidermis and characterized by erythema and pain. They generally heal within several days to a week. Second-degree, or partial-thickness, burns extend through the epidermis and into the dermis. They may be divided into superficial and deep partial-thickness injuries. Superficial second-degree burns are limited to the superficial (papillary) dermis. The skin is erythematous and forms blisters, and the surface can be moist (Fig. 63-1). The wound blanches on pressure and is painful. Deep second-degree burns extend through the epidermis into the deep (reticular) dermis. They appear white with some erythematous areas with less blanching and moisture than the superficial second-degree burns (Fig. 63-2). The distinction between superficial and deep second-degree burns is important because deep second-degree burns do not heal within 2 to 3 weeks and often result in severe scarring and contractures, especially in children. Deep second-degree burns that do not heal within 21 days require excision and skin grafting to minimize scarring. Deep second-degree burns may also progress to third-degree burns over the course of several days after injury. Third-degree burns extend through the entire dermis and are referred to as “full-thickness” burns. Their appearance is stiff and white or tan. They are dry or may have a charred appearance (Fig. 63-3). They do not blanch and, because of destruction of the nerves, are not painful. Finally, fourth-degree burns extend through the skin and subcutaneous fat into the underlying muscle and bone. Fourth-degree burns are stiff and charred and have visibly thrombosed vessels. Most burns are not uniform in depth and have areas of varying depth of injury.
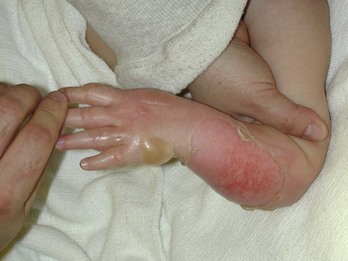
Figure 63-1 Superficial second-degree burn.
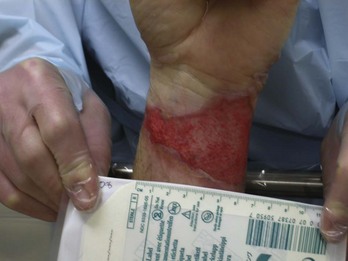
Figure 63-2 Deep second-degree burn.
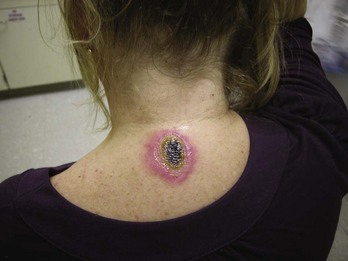
Figure 63-3 Third-degree burn.
Injection of radioactive isotopes and vital dyes, thermography, photometry, nuclear imaging, and ultrasound have been used to help determine burn depth and predict the potential of burns to heal.31 Currently the most promising tool is laser Doppler imaging; however, even this tool has little predictive value in the early stages in the ED. As a result, clinical evaluation in the ED is required to estimate burn depth. The accuracy of non–burn specialists in predicting burn depth is approximately 50%,32 and even among burn specialists the accuracy is only 75%.33 This emphasizes the need for close follow-up and serial assessments to distinguish between deep and superficial burns, as the injury is dynamic and often continues to evolve after initial presentation.
The extent or surface area of the injury must be evaluated. Estimation of the percentage of TBSA burned is often inaccurate on initial inspection, and clinical rules and charts are used for more precise estimation of the percentage of TBSA.34–36 Careful estimation is critical because the volume of fluid administered is based on this evaluation and an overestimation of the extent of injury can lead to overzealous fluid administration.37,38 In addition, the criteria for referral to a burn unit are dependent on the surface area of the burn. Only second-degree and deeper burns should be used to calculate the percentage of TBSA affected. A useful clinical rule for smaller burns or scattered areas of burn is that the area of a patient’s palm and fingers is approximately 1% TBSA.39 For larger burns, the “rule of nines” is useful. This rule states that the allocation of percentage of TBSA in an adult patient can be estimated as follows: 18% for the front and 18% for the back of the trunk, 18% for each lower extremity, 9% for the head and 9% for each upper extremity, and 1% for the perineal area. A Lund-Browder chart is useful for estimating the extent of burns in children (Fig. 63-4).40 The rule of nines should not be used for children as their head is larger and, in proportion, their extremities are smaller than those of adults.
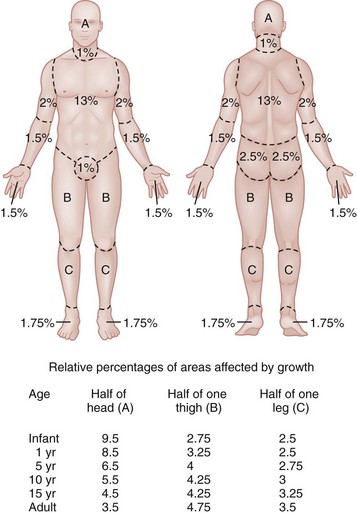
Figure 63-4 Lund-Browder chart.
Finally, burns are classified by severity into minor, moderate, and severe based on the TBSA burned, the percentage of full-thickness injury, and the involvement of specific areas such as the face, hands, feet, or perineum (Table 63-2). In addition, the presence of inhalation injury, high-voltage electrical burns, associated major trauma such as fractures, extremes of age, and comorbid conditions is considered.
Management
Burns should be covered with a clean dressing. Cooling with tap water or a commercially available cooling blanket helps reduce pain.41,42 Care should be taken to minimize hypothermia, especially when ambient temperature is low. Topical antimicrobial agents and greasy ointment should not be used during prehospital care because they offer few benefits during transport, must be removed when the patient arrives at the hospital, and may obscure accurate determination of burn depth.
Emergency Department Management of Burns
Airway Management
Management of the upper airway in burn patients may be extremely challenging. After exposure to superheated steam and toxic fumes, rapid and progressive upper airway edema may develop within minutes. If there is doubt regarding upper airway compromise, fiberoptic laryngoscopy should be performed. Endotracheal intubation guided by fiberoptic laryngoscopy or bronchoscopy is a useful technique, and if attempts at intubation are unsuccessful, surgical cricothyrotomy or needle cricothyrotomy may be necessary. After placement of an endotracheal tube, several devices are available to help secure it.43 With circumferential neck burns, the stiff burn eschar may compress the airway as well as the major neck vessels and require laterally placed escharotomies on either side of the neck, with extreme care taken to avoid damage to blood vessels and other underlying vital structures.
Recognizing Inhalation Injury
Smoke inhalation injury affects 5 to 35% of hospitalized burn patients. With improvements in fluid resuscitation, inhalation injury has become one of the two major causes of mortality in burn patients, and the presence of inhalation injury more than doubles the mortality rate in adults.44,45
Traditionally, inhalation injury was diagnosed based on clinical findings such as facial burns, singed nasal vibrissae, carbonaceous sputum, and a history of injury within a closed space. However, these findings are neither highly sensitive nor highly specific.46 Similarly, wheezing, crepitations, hypoxemia, and abnormalities on the initial chest radiograph may or may not be present in the ED, except in the most severely injured.
The diagnosis of inhalation injury in the ED is best made by direct visualization of the airways with fiberoptic laryngoscopy before intubation and bronchoscopy after intubation.47 Findings of inhalation injury include the presence of soot, charring, and mucosal inflammation, edema, or necrosis. Bronchoscopy can identify injury to the airway, but diagnosis of injury to the parenchyma of the lung is best made with xenon ventilation scanning, which demonstrates areas of decreased alveolar gas washout secondary to small airway obstruction. Xenon scanning is rarely used in the ED.
CO levels should be measured in serum via CO-oximetry. Noninvasive devices that measure CO levels are now available in some ambulances and hospitals; these devices expedite recognition of CO poisoning. Cyanide, a frequent combustion product of plastics, should be suspected in patients injured in a closed-space fire who have elevated CO and lactate levels. (See Chapter 159.)
Management of Inhalation Injury
Deciding when mechanical ventilation is required is critical. In addition to securement of the upper airway, there are several other indications for endotracheal intubation and mechanical ventilation (Table 63-3). Alveolar lavage may be required to help remove thick secretions and improve pulmonary toilette. This has been simplified by smaller and more flexible bronchoscopes.46
Table 63-3
Indications for Endotracheal Intubation and Mechanical Ventilation in Burn Patients
There are many modes of mechanical ventilation, each having its own advantages and disadvantages. In the ED most patients who are intubated will require heavy sedation with or without paralysis, and therefore the assist control mode, in which the ventilator cycles automatically at a preselected rate, is most appropriate. Rapid sequence induction is the preferred method unless airway obstruction is suspected, in which case awake intubation with sedation may be necessary. The goals of mechanical ventilation should include achieving an acceptable oxygen saturation (>92%) and limiting the plateau airway pressures to 35 cm H2O or less to reduce the incidence of barotrauma.48 This may require allowing the partial pressure of carbon dioxide (PCO2) levels to rise to 35 to 55 mm Hg (permissive hypercapnia) as long as the pH remains greater than 7.25. A recent randomized trial found that low tidal volumes (6 mL/kg of predicted weight) reduced mortality.49 Starting tidal volumes should therefore be set at 6 to 8 mL/kg of predicted body weight. Larger tidal volumes may be required if oxygenation is not adequate. Positive end-expiratory pressure may also help oxygenation. For hyperoxic lung injury to be minimized, oxygen concentrations should be titrated to the lowest concentrations that maintain adequate oxygenation. A sample of initial ventilator settings is presented in Table 63-4. High-frequency percussive ventilation that administers high-frequency, time-cycled, pressure-limited subtidal volumes may reduce the incidence of barotrauma.50 Continuous positive airway pressure (CPAP) and BL-PAP may be considered in alert and cooperative patients who are difficult to oxygenate.51 However, endotracheal intubation should not be delayed in patients who fail to rapidly improve with noninvasive ventilation. Airway pressure release ventilation that uses CPAP applied at a high level with intermittent releases of airway pressure has also been used to help promote alveolar recruitment while reducing the risk of barotrauma.52
Table 63-4
Recommended Initial Ventilator Settings
| Tidal volume | 6-8 mL/kg |
| Respiratory rate | 8-12 in adults |
| 12-45 in children | |
| Plateau pressures | <35 cm H2O |
| I/E ratio | 1 : 1 to 1 : 3 |
| Flow rates | 40-100 L/min |
| PEEP | 8 cm H2O |
I/E, inspiratory-expiratory; PEEP, positive end-expiratory pressure.
Bronchodilators should be used in patients with wheezing, and they may also improve mucociliary function. Frequent airway suctioning and chest physiotherapy are also helpful at removing secretions. Aerosolized N-acetylcysteine with or without aerosolized heparin also helps break down thick mucous secretions.53,54 An example of a treatment protocol for patients with inhalation injury may include 5000 to 10,000 units of heparin and 3 mL of normal saline nebulized every 4 hours, alternating with 3 to 5 mL of aerosolized 20% N-acetylcysteine.46
Circulation and Fluid Resuscitation
Before World War II, many burn patients died of hypovolemic shock and renal failure. The first formal fluid resuscitation protocol was developed during treatment of burn victims after the Coconut Grove nightclub fire in Boston in 1942.55 Since then, many fluid resuscitation regimens have been described; however, the Parkland formula described by Baxter remains the most popular.56
Burn injury results in the activation of the complement system and the release of a large number of inflammatory mediators, such as histamine, prostaglandins, and leukotrienes. These mediators increase the permeability of the local and systemic vasculature, resulting in the extravasation of intravascular fluids and proteins into the interstitial space, contributing to vascular fluid depletion and soft tissue edema.57 The leakage of plasma proteins during the first 3 to 5 hours reduces the intravascular oncotic pressure and increases the interstitial oncotic pressure, which leads to an increase in edema formation.58 With large burns, half of the fluid requirements are a result of extravasation into unburned areas.59
Patients with small burns (less than 20% TBSA in adults and less than 10-15% TBSA in children) can be successfully treated with oral fluids only.60 With larger burns, intravenous fluid resuscitation is required to restore intravascular volume and prevent the development of hypovolemic shock. One of the oldest controversies in the management of burn patients is the role of colloids for the resuscitation of burn patients. Because capillary leakage of proteins lasts at least 12 to 24 hours after injury, the consensus is that colloids should not be used during the initial fluid resuscitation unless the burn is very deep.61 A recent systematic review of 23 trials that included 7754 patients concluded that there is no evidence that resuscitation with colloids reduces the risk of death compared with crystalloids in patients with trauma or burns or after surgery.62 Recent ABA practice guidelines for burn shock resuscitation consider the addition of colloid-containing fluid after the first 12 to 24 hours to reduce overall fluid requirements.60
In 1968, Baxter reported that resuscitation of dogs with 50% TBSA burns with a volume of LR solution equal to 24 to 32% of their body weight corrected physiologic and metabolic derangements within 24 hours.63 The results were optimal when half of the volume was administered within the first 8 hours after injury. This method of fluid resuscitation was then evaluated in 11 patients with burns covering 30 to 85% TBSA, demonstrating that the required fluid volume of LR was 3.5 to 4.5 mL/kg/%TBSA (excluding first-degree burns). These findings led to the Parkland formula, which called for a volume of 4 mL/kg/%TBSA of LR to be given over the first 24 hours, with half of the volume given over the first 8 hours.64 Later studies demonstrated that most adults and children were successfully resuscitated with 24-hour fluid volumes of 3.7 to 4.3 mL/kg/%TBSA.65 Other fluid resuscitation formulas have also been described (Table 63-5). Based on studies at the U.S. Army Institute of Surgical Research, the “rule of ten” has recently been suggested for estimating the initial fluid requirements, with hourly adjustments based on clinical response.66 With this rule the estimated burn size (percent of TBSA) is multiplied by 10 to derive the initial fluid rate in milliliters per hour. For every 10 kg above 80 kg, 100 mL is added to this rate. This rule tends to overestimate fluid requirements in those weighing less than 40 kg and to underestimate fluid requirements in patients weighing more than 140 kg. The burn resuscitation index (BRI) is another simple method for calculating fluid resuscitation in burn victims.67 The burn size score (BSS) is first determined based on the rule of nines, in which each nine is replaced with the number 1. The head and arms are each assigned 1 point, and the legs, anterior torso, and posterior torso are assigned 2 points, for a total of 11 points for the entire body. If an area is burned more than 50%, it is assigned the full point value. An area burned less than 50% is assigned half the point value. All points are added, and then the result is rounded up to determine the BSS. Once the BSS has been obtained, the initial fluid resuscitation rate is found by cross-referencing the BSS with the patient’s weight (Table 63-6). Use of this index significantly increases the accuracy of calculated fluid requirements of burn victims, especially by emergency medicine residents.67
Table 63-5

Cl, chloride; LR, Ringer’s lactate; TBSA, total body surface area; NA, sodium; NS, normal saline.
Table 63-6
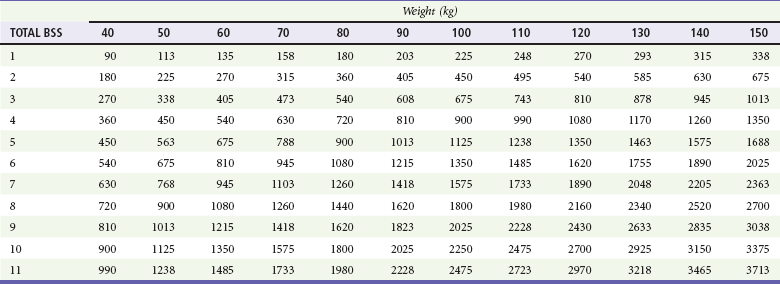
Adapted from Kahn SA, Schoemann M, Lentz CW: Burn resuscitation index: a simple method for calculating fluid resuscitation in the burn patient. J Burn Care Res 31:616, 2010.
All fluid resuscitation formulas should be used only as general guides, and adjustments must be to maintain adequate tissue and organ perfusion. Burn patients should be resuscitated with only as much fluid as is necessary to maintain organ perfusion. Organ perfusion can be estimated by heart rate, blood pressure, level of consciousness, capillary refill, and a urine output of 0.5 to 1.0 mL/kg/hr in adults or 1.0 to 1.5 mL/kg/hr in children.60 Certain patient groups may require additional fluid, including patients with inhalation injury, those with electrical burns, and those in whom resuscitation is delayed. Patients with inhalation injury require 35 to 65% more fluid than patients with similar-sized burns without inhalation injury.68
A study of transferred burn patients found that smaller burns tended to be overestimated with over-resuscitation, whereas larger burns tended to be underestimated with under-resuscitation.69 This further emphasizes the need for careful monitoring of the volume of urine output and clinical signs of tissue turgor and adequate resuscitation.
There have been rising concerns that overly aggressive fluid resuscitation, often termed “fluid creep,” may have adverse outcomes.61 In a study of 72 patients, increased fluid volumes were associated with pneumonia, sepsis, adult respiratory distress syndrome, multiorgan failure, and death.70 Patients who receive more fluid than needed are at risk for the abdominal compartment syndrome, which may lead to acute renal failure. Factors associated with fluid creep include a lack of careful, frequent observations by clinicians, increasing use of opioids (termed “opioid creep,” in which high doses of opioids are used, leading to a drop in blood pressure and the need for further fluid boluses), and the emergence of goal-directed resuscitation. Although clinicians are often eager to increase fluid administration in patients with poor urine output and unstable vital signs, they are less likely to reduce fluids in patients with adequate or increased urine output.71 Goal-directed therapy is aimed at achieving supranormal levels of cardiac output and oxygen delivery and consumption and normal acid-base status. In contrast, restoration of normal physiologic and metabolic conditions in severely burned patients generally requires at least 24 to 48 hours to occur, and “pushing” these parameters prematurely may be detrimental.61 Excessive initial resuscitation should be avoided, and fluid infusions should be frequently adjusted downward to avoid overhydration and subsequent cardiac failure, especially in the elderly.
Local Burn Wound Care
Cooling of the Burn
The benefits of cooling the burn surface have been recognized for many years. There is controversy regarding the best cooling agent and the optimal timing, duration, and temperature of cooling. Classic studies suggest that the optimal cooling temperature is in the range of 10 to 25° C,72 but cooling of large burns with ice water can result in hypothermia and increased mortality. Venter and colleagues found that ice water (1-8° C) resulted in more necrosis than no cooling at all, and cooling with tap water at 12 to 18° C resulted in the least necrosis and fastest healing.42 The beneficial effects of cooling are still present when treatment is initiated up to 30 minutes after injury. External cooling with tap water is associated with a reduction in pain and may reduce the progression of tissue necrosis and limit the requirement for skin grafting.73,74 If the patient is made comfortable by the cooling, it should be continued until the pain is reduced. Ice and ice water should be avoided because they may lead to increased tissue injury and hypothermia.42
Management of Burn Blisters
The management of burn blisters is a controversial topic. Some suggest that the fluid confined by the necrotic skin can lead to a closed-space infection, whereas others have argued that the intact blister creates a moist wound environment that is beneficial to wound healing. In vitro studies show both positive and negative effects of the burn blister fluid. Small clinical studies show that intact blisters heal faster and are less likely to become infected.75,76 However, in these studies the débrided burns were not treated with any of the advanced dressings currently available, limiting the conclusions. With blisters that have already ruptured, any necrotic epidermis should be gently removed while adherent epidermis is left intact. With large or tense blisters, the unruptured blister may be aspirated with a sterile needle.
Burn Dressings
The purposes of a burn dressing are to protect the wound, to reduce pain, to absorb wound exudate, and to reduce evaporative heat loss.77 The routine use of systemic antibiotics is not supported in the literature.78 Local care for first-degree burns is not required other than optional topical anesthetics, aloe vera, or topical nonsteroidal anti-inflammatory drugs (NSAIDs).79,80 There are two general methods of treating second-degree burns: (1) the open method, which consists of topical antimicrobials with or without standard dressings, and (2) the closed method, which uses synthetic or biologic occlusive dressings.
The open method is most appropriate for contaminated, large burns with large amounts of exudate. Topical antimicrobials should be used until the burn is completely reepithelialized to prevent bacterial colonization of the wound. The burn is reepithelialized when there is a pearly opalescent coating and it is dry to the touch. Antimicrobials used for burns include bacitracin, neomycin, mupirocin, silver sulfadiazine, iodophors, and mafenide.81 Choice of the specific topical antimicrobial should be based on physician and patient preferences. After the antimicrobial is applied, a nonadherent dressing can be used to cover the wound. With this method the dressings should be removed daily and the burns cleansed with water and soap followed by reapplication of the antimicrobial agent and dressing. Traditionally, silver sulfadiazine and mafenide were the mainstays of treatment. However, they may be associated with rare adverse reactions such as reversible bone marrow suppression and metabolic acidosis, respectively. Silver sulfadiazine should not be used in patients with sulfa allergy. The use of silver sulfadiazine is no longer recommended because there is evidence that it delays healing of partial thickness burns and in vitro is toxic to keratinocytes and fibroblasts.82,83
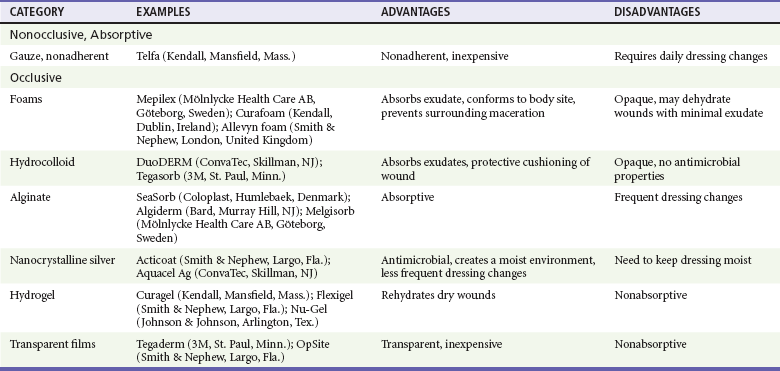
The second method of managing partial-thickness burns is with occlusive dressings. Occlusive dressings support a moist wound-healing environment that is optimal for healing.84 Because these dressings do not need to be changed daily, they also reduce costs and the pain associated with frequent dressing changes that are required when topical antimicrobial agents are used.85 Occlusive dressings are most appropriate for superficial second-degree burns with little to moderate exudate. Several major types of materials are used for occlusive dressings, including foams, transparent films, hydrocolloids, alginates, hydrogels, and composites (Table 63-7). The development of products containing nanocrystalline silver has renewed interest in silver-based products because silver has potent antimicrobial properties against both gram-negative and gram-positive organisms as well as fungi such as Candida. Nanocrystalline silver is less rapidly deactivated than the ionic form of silver and thus does not require frequent dressing changes and reapplication. Many of these dressing can be left in place for at least 1 week or until they become saturated or malodorous. Nanocrystalline silver has been incorporated into many new sustained delivery dressings, such as Aquacel Ag (ConvaTec, Skillman, NJ) and Acticoat (Smith & Nephew, Hull, United Kingdom). Compared with silver sulfadiazine, these dressings have been shown to reduce pain, speed healing, and reduce costs because few dressing changes are required.86–88 A silicone-coated polyurethane foam that releases silver (Mepilex Ag, Mölnlycke, Goteborg, Sweden) has recently been introduced to the market and is especially attractive as a burn dressing because the silicone base adheres to the wound bed while the foam absorbs exudate and prevents tissue maceration. The silicone base also allows easy, nontraumatic removal of the dressing after approximately 1 week. In addition, unlike some of the other silver-based dressings, this dressing does not have to be kept wet. In a recent randomized controlled trial, partial-thickness burns treated with this dressing healed faster and with fewer dressing changes than with silver sulfadiazine.89
Absorptive hydrocolloids (such as DuoDERM [ConvaTec, Skillman, NJ]) have also been shown to be effective and less painful than traditional silver sulfadiazine.90 Biosynthetic composite dressings such as Biobrane (UDL Laboratories, Sugar Land, Tex.) and Apligraf (Organogenesis, Canton, Mass.) are generally limited to use by burn specialists. A recent systematic review by the Cochrane group concluded that evidence from small trials, many with methodological limitations, suggests that superficial and partial-thickness burns may be managed with hydrocolloids, silicon nylon, topical antimicrobial agents (containing silver), polyurethane film, and biosynthetic dressings. There was no evidence to support the use of silver sulfadiazine.91
Escharotomy
Releasing the constriction of a burn eschar with a scalpel or cautery at the bedside is called escharotomy. Although infrequently needed, escharotomy can be a limb-saving or lifesaving procedure.92 The dead skin hardens and forms an eschar that appears as a hard covering. Patients with a large eschar require careful monitoring, especially those with circumferential burns or extensive burns to the chest or neck, because there is danger of the development of edema beneath the eschar, which leads to constriction and eventual interruption of arterial inflow. In the chest, the increased pressure impairs respiration. Pain, loss of sensation, and delayed capillary refill may be early signs of reduced perfusion. Traditionally, decreased Doppler signals in the extremity have been used as an indication for escharotomy, but one study suggests that a pulse oximetry value of less than 90% with or without Doppler signals in the involved extremity is an indication for escharotomy of the involved extremity.93 An increase in peak airway pressure or difficulty with ventilation may be a sign that escharotomy of the chest is required. Circumferential burns of the neck that compromise the airway or the large vessels require escharotomy of the neck with care to avoid the airway and vessels themselves.
The procedure is performed by making a longitudinal incision down to the fat in the constricting eschar (Fig. 63-5). Care should be taken to avoid underlying vessels and nerves. One study of patients transferred to burn centers found that escharotomy incisions were frequently not made deep enough to relieve the obstruction of flow.93 It is important to check that an adequate incision has been made by reassessing Doppler signals and pulse oximetry in the extremities and ensuring that the peak airway pressures are decreased after the procedure. Escharotomies may need to be extended into healthy tissue on either side of the eschar to allow adequate decompression. Use of cautery to perform escharotomy is preferred by some to minimize bleeding.
Pain Management
Burns are among the most painful injuries94; however, many patients do not receive analgesia while in the ED.95 In addition to being inhumane, inadequate pain management may contribute to an exaggerated inflammatory and stress response, increased pain from a “wind-up” phenomenon (in which the pain receptors are hypersensitized), long-term chronic pain and dysesthesias, and post-traumatic stress disorder.96–98 The release of second messenger proteins such as protein kinase C-epsilon and neurotrophins such as nerve growth factor may mediate acute-burn hyperalgesia and priming, resulting in a predilection toward the development of chronic hyperalgesia.99 Inadequate analgesia may be a result of the distraction of the often dramatic burn wound or other associated injuries, or concern that potent analgesics may impair ventilation. Pain management should be of primary concern for all burn patients.
There are three phases of burn recovery, each with a characteristic and different type of pain, and each requiring specific assessment tools and management strategies. These three phases include the emergent or resuscitative phase, the healing phase, and the rehabilitative phase.100 This chapter focuses on the emergent phase of pain management.
During the emergent phase of management, patients may experience three major types of pain. Background pain describes the underlying pain that results directly from the injury. The amount of background pain is highly variable and also depends on the patient’s anxiety. This pain is usually described as a continuous sensation of burning or throbbing that exists even when the patient is immobile. Background pain may be substantially reduced simply by covering the burn with a moist cool dressing.101 Breakthrough pain occurs when the patient experiences a sudden increase in the pain intensity, usually as the analgesic level decreases with time. Shortening the analgesic dosage interval usually addresses this type of pain. Procedural pain refers to the pain experienced when the burn wound is manipulated or débrided. This pain is generally very severe but transient in nature. Addition of an anxiolytic medication to parenteral opioids and use of music are helpful during this phase of management.102
Pharmacologic Therapies
Morphine sulfate and acetaminophen are the most commonly used analgesics in burn patients.103 Minor pain from burns may be managed with oral acetaminophen (1 g in adults or 15 mg/kg in children) every 4 to 6 hours or an NSAID such as ibuprofen (400-800 mg in adults or 10 mg/kg in children) every 6 to 8 hours. The pain of minor burns may also be treated with a topical NSAID or aloe vera.79,80
Moderate to severe burn pain is managed with parenteral opioids (such as morphine sulfate 0.05-0.1 mg/kg) titrated to effect. The intravenous route is recommended owing to its rapid and reliable effects and consistent absorption. Because of its short half-life, fentanyl 0.5 to 1.0 µg/kg may be used for managing breakthrough and procedural pain. Intranasal fentanyl at a dose of 1.4 µg/kg may be as effective as oral morphine in both children and adults.104 Intravenous infusion of lidocaine (1 mg/kg followed by 40 µg/kg/min infusion) has also been shown to reduce the pain in burn patients.105
Benzodiazepines have been useful adjuncts to pain therapy in burns. Care must be exercised in administering opioids and benzodiazepines together, as they may act synergistically to induce hypoventilation and hypotension. Lorazepam (1 mg IV or orally) and midazolam (1 mg IV or intranasally) are both widely used in burn patients.106,107
Other drugs (including gabapentin, stimulants, beta-blockers, low-dose ketamine, and antidepressants) are useful in managing chronic pain and burn pain in the burn unit; however, there is no evidence to support their use in the ED.108–110
Burn Prevention
The ED visit offers a unique “teachable moment” for physicians to educate patients and their families about preventing burns in the future. Prevention programs and safety legislation have made substantial contributions to decrease the incidence and severity of burn injury, especially for parents and school-age children. Areas in which burn prevention programs have been implemented have had a significant decrease in admissions for burn injury.111
In addition, several initiatives are targeting vulnerable segments of the population for prevention efforts.112,113 Mothers with less than a high school education who are younger than 20 years and have more than two children are at a much higher risk for a fatal fire event.114 Every visit to the ED provides an opportunity for education. Although prevention initiatives are reaching increasing numbers of people, there is still the need for further education of the public and in particular those subsegments of the population at high risk for burn injury. Specific preventive recommendations are listed in Table 63-8.
Disposition
Most patients will have small and superficial burns and can be discharged with close follow-up with a burn specialist or a primary care physician who has experience managing burns (see Table 63-2). When available, telemedicine and telephone consultation have also been shown to be helpful in decision-making and follow-up of burn patients, especially in questionable cases.116 Patients with large burns (>20% TBSA), deep burns, burns over sensitive areas (such as the face, perineum, or hands), or burns that require escharotomy should be admitted, preferably to a burn unit. Patients with inhalation injury or other significant injuries or illnesses should also be admitted. Patients with inadequate social support and in whom abuse or neglect is suspected should also be considered for admission.
References
1. Bláha, J. Physiology and pathology of skin after burns and derangement of gene expression. Acta Chir Plast. 2006;48:127–132.
2. Moritz, AR, Henriques, FC, Jr. Studies of thermal injury. II. The relative importance of time and surface temperatures in the causation of cutaneous burns. Am J Pathol. 1947;23:695.
3. Singer, AJ, Berruti, L, Thode, HC, Jr., McClain, SA. Standardized burn model using a multiparametric histologic analysis of burn depth. Acad Emerg Med. 2000;7:1–6.
4. Despa, F, Orgill, DP, Neuwalder, J, Lee, RC. The relative thermal stability of tissue macromolecules and cellular structure in burn injury. Burns. 2006;31:568.
5. Hotchkiss, RS, Strasser, A, McDunn, JE, Swanson, PE. Cell death. N Engl J Med. 2009;361:1570–1583.
6. Gravante, G, et al. Apoptotic death in deep partial thickness burns vs. normal skin of burned patients. J Surg Res. 2007;141:141–145.
7. Singer, AJ, McClain, SA, Taira, BR, Guerriero, JL, Zong, W. Apoptosis and necrosis in the ischemic zone adjacent to third degree burns. Acad Emerg Med. 2008;15:549–554.
8. American Burn Association. Burn Incidence and Treatment in the United States: 2011 Fact Sheet. www.ameriburn.org/resources_factsheet.php?PHPSESSID=fbf3c262c153c58e4c9123ef12b39682.
9. Singer, AJ, Lee, CC, Thode, HC, Jr. Epidemiology of burns in the ED, 1996-2004. Ann Emerg Med. 2007;14:S65.
10. Jackson, D. The diagnosis of the depth of burning. Br J Surg. 1953;40:588.
11. Dunkin, CS, et al. Scarring occurs at a critical depth of skin injury: Precise measurement in a graduated dermal scratch in human volunteers. Plast Reconstr Surg. 2007;119:1722.
12. Shupp, JW, et al. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res. 2010;31:839–873.
13. Singh, V, Devgan, L, Bhat, S, Milner, SM. The pathogenesis of burn wound conversion. Ann Plast Surg. 2007;59:109.
14. Singer, AJ, Clark, RA. Cutaneous wound healing. N Engl J Med. 1999;341:738.
15. Parihar, A, Parihar, MS, Milner, S, Bhat, S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns. 2008;34:6.
16. Jaskille, AD, Jeng, JC, Sokolich, JC, Lunsford, P, Jordan, MH. Repetitive ischemia-reperfusion injury: A plausible mechanism for documented clinical burn-depth progression after thermal injury. J Burn Care Res. 2007;28:13.
17. Demling, RH. The burn edema process: Current concepts. J Burn Care Rehabil. 2005;26:207.
18. Horton, JW. Free radicals and lipid peroxidation mediated injury in burn trauma: The role of antioxidant therapy. Toxicology. 2003;189:75.
19. Singer, AJ, McClain, SA, Hacht, G, Batchkina, G, Simon, M. Semapimod reduces the depth of injury resulting in enhanced re-epithelialization of partial-thickness burns in swine. J Burn Care Res. 2006;27:40.
20. Taira, BR, et al. Rosiglitazone, a PPAR-gamma ligand, reduces burn progression in rats. J Burn Care Res. 2009;30:499.
21. Giles, N, Rea, S, Beer, T, Wood, FM, Fear, MW. A peptide inhibitor of c-Jun promotes wound healing in a mouse full-thickness burn model. Wound Repair Regen. 2008;16:58.
22. Mileski, WJ, et al. Clinical effects of inhibiting leukocyte adhesion with monoclonal antibody to intercellular adhesion molecule-1 (enlimomab) in treatment of partial-thickness burn injury. Trauma. 2003;54:950.
23. O’Sullivan, ST, et al. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222:482.
24. Haponik, EF, et al. Acute upper airway injury in burn patients: Serial changes of flow-volume curves and nasopharyngoscopy. Am Rev Respir Dis. 1987;135:360.
25. Moritz, AR, Henriques, FC, Jr., McLean, R. The effects of inhaled heat on the air passages of lungs: An experimental investigation. Am J Pathol. 1945;21:311.
26. Prien, T, Traber, DL. Toxic compounds and inhalation injury—a review. Burns Incl Therm Inj. 1988;14:451.
27. Maybauer, MO, Rehberg, S, Traber, DL, Herndon, DN, Maybauer, DM. [Pathophysiology of acute ling injury in severe burn and inhalation injury]. Anaesthesist. 2009;58:805.
28. Enkhbaatar, P, Traber, DL. Pathophysiology of acute lung injury in combined burn and smoke inhalation injury. Clin Sci. 2004;107:137.
29. Murakami, K, Traber, DL. Pathophysiological basis of smoke inhalation injury. News Physiol Sci. 2003;18:125.
30. Nieman, GF, Clark, WR, Jr., Wax, SD, Webb, SR. The effect of smoke inhalation on pulmonary surfactant. Ann Surg. 1980;191:171.
31. Jaskille, AD, Shupp, JW, Jordan, MH, Jeng, JC. Critical review of burn depth assessment techniques: Part I. Historical review. J Burn Care Res. 2010;30:937.
32. Jaskille, AD, Ramella-Roman, JC, Shupp, JW, Jordan, MH, Jeng, JC. Critical review of burn depth assessment techniques: Part II. Review of laser Doppler technology. J Burn Care Res. 2010;31:151.
33. Hlava, P, Moserova, J, Konigova, R. Validity of clinical assessment of the depth of a thermal injury. Acta Chir Plast. 1983;25:202.
34. Hammond, JS, Ward, CG. Transfers from emergency room to burn center: Errors in burn size estimate. J Trauma. 1987;27:1161.
35. Berkebile, BL, Goldfarb, IW, Slater, H. Comparison of burn size estimates between prehospital reports and burn center evaluations. J Burn Care Rehabil. 1986;7:411.
36. Perry, RJ, Moore, CA, Morgan, BD, Plummer, DL. Determining the approximate area of a burn: An inconsistency investigated and re-evaluated. BMJ. 1996;312:1338.
37. Gomez, R, Cancio, LC. Management of burn wounds in the emergency department. Emerg Med Clin North Am. 2007;25:135.
38. Collis, N, Smith, G, Fenton, OM. Accuracy of burn size estimation and subsequent fluid resuscitation prior to arrival at the Yorkshire Regional Burns Unit: A three-year retrospective study. Burns. 1999;25:345.
39. Sheridan, RL, et al. Planimetry study of the percent of body surface represented by the hand and palm: Sizing irregular burns is more accurately done with the palm. J Burn Care Rehabil. 1995;16:605.
40. Lund, C, Browder, N. The estimate of areas of burn. Surg Gynecol Obstet. 1944;79:352.
41. Singer, AJ, Freidman, B, Modi, P, Soroff, HH. The effect of a commercially available burn-cooling blanket on core body temperatures in volunteers. Acad Emerg Med. 2006;13:686.
42. Venter, TH, Karpelowsky, JS, Rode, H. Cooling of the burn wound: The ideal temperature of the coolant. Burns. 2007;33:917.
43. Helvig, B, Mlcak, R, Nichols, RJ, Jr. Anchoring endotracheal tubes on patients with facial burns: Review from Shriner’s Burns Institute, Galveston, Texas. J Burn Care Rehabil. 1987;8:236.
44. Barrow, RE, Spies, M, Barrow, LN, Herndon, DN. Influence of demographics and inhalation injury on burn mortality in children. Burns. 2004;30:72.
45. Colohan, SM. Predicting prognosis in thermal burns with associated inhalation injury: A systematic review of prognostic factors in adult burn victims. J Burn Care Res. 2010;31:529.
46. Mlcak, RP, Suman, OE, Herndon, DN. Respiratory management of inhalation injury. Burns. 2007;33:2.
47. Carr, JA, Phillips, BD, Bowling, WM. The utility of bronchoscopy after inhalation injury complicated by pneumonia in burn patients: Results from the National Burn Repository. J Burn Care Res. 2009;30:967.
48. Slutsky, AS. Mechanical ventilation: American College of Chest Physicians’ Consensus Conference. Chest. 1993;104:1833.
49. Oba, Y, Salzman, GA. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury. N Engl J Med. 2000;343:813.
50. Allan, PF, Osborn, EC, Chung, KK, Wanek, SM. High-frequency percussive ventilation revisited. J Burn Care Res. 2010;31:510.
51. Endorf, FW, Dries, DJ. Noninvasive ventilation in the burned patient. J Burn Care Res. 2010;31:217.
52. Dries, DJ, Marini, JJ. Airway pressure release ventilation. J Burn Care Res. 2009;30:929.
53. Toon, MH, Maybauer, MO, Greenwood, JE, Maybauer, DM, Fraser, JF. Management of acute smoke inhalation injury. Crit Care Resusc. 2010;12:53.
54. Miller, AC, Rivero, A, Ziad, S, Smith, DJ, Elamin, EM. Influence of nebulized unfractionated heparin and N-acetylcysteine in acute lung injury after smoke inhalation injury. J Burn Care Res. 2009;30:249.
55. Saffle, JR. The 1942 fire at Boston’s Cocoanut Grove nightclub. Am J Surg. 1993;166:581.
56. Baxter, CR. Fluid volume and electrolyte changes of the early postburn period. Clin Plast Surg. 1974;1:693.
57. Arturson, G. Forty years in burns research: The postburn inflammatory response. Burns. 2000;26:599.
58. Pitt, RM, Parker, JC, Jurkovich, GJ, Taylor, AE, Curreri, PW. Analysis of altered capillary pressure and permeability after thermal injury. J Surg Res. 1987;42:693.
59. Demling, RH, Mazess, RB, Witt, RM, Wolberg, WH. The study of burn wound edema using dichromatic absorptiometry. J Trauma. 1978;18:124.
60. Pham, TN, Cancio, LC, Gibran, NS. American Burn Association: American Burn Association Practice Guidelines burn shock resuscitation. J Burn Care Res. 2008;29:257.
61. Saffle, JI. The phenomenon of “fluid creep” in acute burn resuscitation. J Burn Care Res. 2007;28:382.
62. Perel, P, Roberts, I. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 4, 2007.
63. Baxter, CR, Shires, T. Physiological response to crystalloid resuscitation of severe burns. Ann N Y Acad Sci. 1968;150:874.
64. Baxter, C. Fluid resuscitation, burn percentage, and physiologic age. J Trauma. 1979;19(11 Suppl):864.
65. Baxter, CR. Problems and complications of burn shock resuscitation. Surg Clin North Am. 1978;58:1313.
66. Chung, KK, et al. Simple derivation of the initial fluid rate for the resuscitation of severely burned adult combat casualties: In silico validation of the rule of 10. J Trauma. 2010;69(Suppl 1):S49.
67. Kahn, SA, Schoemann, M, Lentz, CW. Burn resuscitation index: A simple method for calculating fluid resuscitation in the burn patient. J Burn Care Res. 2010;31:616.
68. Dai, NT, et al. The comparison of early fluid therapy in extensive flame burns between inhalation and noninhalation injuries. Burns. 1998;24:671.
69. Freiburg, C, Igneri, P, Sartorelli, K, Rogers, F. Effects of differences in percent total body surface area estimation on fluid resuscitation of transferred burn patients. J Burn Care Res. 2007;28:42.
70. Klein, MB, et al. The association between fluid administration and outcome following major burn: A multicenter study. Ann Surg. 2007;245:622.
71. Cancio, LC, et al. Predicting increased fluid requirements during the resuscitation of thermally injured patients. J Trauma. 2004;56:404.
72. Ofeigsson, OJ. First-aid treatment of scalds and burns by water cooling. Postgrad Med. 1961;30:330.
73. Ofeigsson, OJ. Water cooling: First-aid treatment for scalds and burns. Surgery. 1965;57:391.
74. Jandera, V, Hudson, DA, de Wet, PM, Innes, PM, Rode, H. Cooling the burn wound: Evaluation of different modalities. Burns. 2000;26:265.
75. Swain, AH, Azadian, BS, Wakeley, CJ, Shakespeare, PG. Management of blisters in minor burns. BMJ. 1987;295:181.
76. Gimbel, NS, Kapetansky, DI, Weissman, F, Pinkus, HK. A study of epithelization in blistered burns. AMA Arch Surg. 1957;74:800.
77. Fan, K, Tang, J, Escandon, J, Kirsner, RS. State of the art topical wound-healing products. Plast Reconstr Surg. 2011;127(Suppl):44S.
78. Ugburo, AO, Atoyebi, OA, Oyeneyin, JO, Sowemimo, GO. An evaluation of the role of systemic antibiotic prophylaxis in the control of burn wound infection at the Lagos University Teaching Hospital. Burns. 2004;30:43.
79. Magnette, JL, et al. Diclofenac systemic exposure is not increased when topical diclofenac is applied to ultraviolet-induced erythema. Eur J Clin Pharmacol. 2004;60:591.
80. Maenthaisong, R, Chaiyakunapruk, N, Niruntraporn, S, Kongkaew, C. The efficacy of aloe vera used for burn wound healing: A systematic review. Burns. 2007;33:713.
81. Palmieri, TL, Greenhalgh, DG. Topical treatment of pediatric patients with burns: A practical guide. Am J Clin Dermatol. 2002;3:529.
82. Hussain, S, Ferguson, C. Best evidence topic report: Silver sulphadiazine cream in burns. Emerg Med J. 2006;23:929.
83. Atiyeh, BS, Costagliola, M, Hayek, SN, Dibo, SA. Effect of silver on burn wound infection control and healing: Review of the literature. Burns. 2007;33:139.
84. Singer, AJ, Dagum, AB. Current management of acute cutaneous wounds. N Engl J Med. 2008;359:1037.
85. Eaglstein, WH. Experiences with biosynthetic dressings. J Am Acad Dermatol. 1985;12(2 Pt 2):434.
86. Cuttle, L, et al. A retrospective cohort study of Acticoat versus Silvazine in a paediatric population. Burns. 2007;33:701.
87. Caruso, DM, et al. Randomized clinical study of Hydrofiber dressing with silver or silver sulfadiazine in the management of partial-thickness burns. J Burn Care Res. 2006;27:298.
88. Paddock, HN, et al. A silver impregnated antimicrobial dressing reduces hospital length of stay for pediatric patients with burns. J Burn Care Res. 2007;28:409.
89. Silverstein, P, et al. An open, parallel, randomized, comparative, multicenter study to evaluate the cost-effectiveness, performance, tolerance, and safety of a silver-containing soft silicone foam dressing (intervention) vs silver sulfadiazine cream. J Burn Care Res. 2011;32:617–626.
90. Martin, FT, O’Sullivan, JB, Regan, PJ, McCann, J, Kelly, JL. Hydrocolloid dressing in pediatric burns may decrease operative intervention rates. J Pediatr Surg. 2010;45:600.
91. Wasiak, J, Cleland, H, Campbell, F. Dressings for superficial and partial thickness burns. Cochrane Database Syst Rev. 4, 2008.
92. Piccolo, NS, et al. Escharotomies, fasciotomies and carpal tunnel release in burn patients—review of the literature and presentation of an algorithm for surgical decision making. Handchir Mikrochir Plast Chir. 2007;39:161.
93. Brown, RL, Greenhalgh, DG, Kagan, RJ, Warden, GD. The adequacy of limb escharotomies-fasciotomies after referral to a major burn center. J Trauma. 1994;37:916.
94. Summer, GJ, Puntillo, KA, Miaskowski, C, Green, PG, Levine, JD. Burn injury pain: The continuing challenge. J Pain. 2007;8:533.
95. Singer, AJ, Thode, HC, Jr. National analgesia prescribing patterns in emergency department patients with burns. J Burn Care Rehabil. 2002;23:361.
96. Kawasaki, Y, et al. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310.
97. Ikeda, H, et al. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659.
98. Van Loey, NE, Maas, CJ, Faber, AW, Taal, LA. Predictors of chronic posttraumatic stress symptoms following burn injury: Results of a longitudinal study. J Trauma Stress. 2003;16:361.
99. Summer, GJ, et al. TrkA and PKC-epsilon in thermal burn-induced mechanical hyperalgesia in the rat. J Pain. 2006;7:884.
100. Ratcliff, SL, et al. The effectiveness of a pain and anxiety protocol to treat the acute pediatric burn patient. Burns. 2006;32:554.
101. Nguyen, NL, Gun, RT, Sparnon, AL, Ryan, P. The importance of immediate cooling: A case series of childhood burns in Vietnam. Burns. 2002;28:173.
102. Tan, X, Yowler, CJ, Super, DM, Fratianne, RB. The efficacy of music therapy for decreasing pain, anxiety, and muscle tension levels during burn dressing changes: A prospective randomized crossover trial. J Burn Care Res. 2010;31:590.
103. Martin-Herz, SP, et al. Pediatric pain control practices of North American Burn Centers. J Burn Care Rehabil. 2003;24:26.
104. Finn, J, et al. A randomised crossover trial of patient controlled intranasal fentanyl and oral morphine for procedural wound care in adult patients with burns. Burns. 2004;30:262.
105. Jonsson, A, Cassuto, J, Hanson, B. Inhibition of burn pain by intravenous lignocaine infusion. Lancet. 1991;338:151.
106. Cassuto, J, Tarnow, P. Potent inhibition of burn pain without use of opiates. Burns. 2003;29:163.
107. Patterson, DR, Ptacek, JT, Carrougher, GJ, Sharar, SR. Lorazepam as an adjunct to opioid analgesics in the treatment of burn pain. Pain. 1997;72:367.
108. Rice, TL, Kyff, JV. Intranasal administration of midazolam to a severely burned child. Burns. 1990;16:307.
109. Cuignet, O, Pirson, J, Soudon, O, Zizi, M. Effects of gabapentin on morphine consumption and pain in severely burned patients. Burns. 2007;33:81.
110. Pereira, CT, Jeschke, MG, Herndon, DN. Beta-blockade in burns. Novartis Found Symp. 2007;280:238.
111. Richardson, P, Mustard, L. The management of pain in the burns unit. Burns. 2009;35:921.
112. Peleg, K, Goldman, S, Sikron, F. Burn prevention programs for children: Do they reduce burn-related hospitalizations? Burns. 2005;31:347.
113. Ytterstad, B, Smith, GS, Coggan, CA. Harstad injury prevention study: Prevention of burns in young children by community-based intervention. Inj Prev. 1998;4:176.
114. Taira, BR, et al. Predictors of sustaining burn injury: does the use of common prevention strategies matter? J Burn Care Res. 2011;32:20–25.
115. Scholer, SJ, Hickson, GB, Mitchel, EF, Jr., Ray, WA. Predictors of mortality from fires in young children. Pediatrics. 1998;101:E12.
116. Turk, E, et al. Use of telemedicine and telephone consultation in decision making and follow-up of burn patients: Initial experience from two burn units. Burns. 2011;37:415.