Chapter 90 Therapeutic plasma exchange and intravenous immunoglobulin immunomodulatory therapy
Bloodletting to remove ‘evil humours’ has a long history of over 2000 years. Although not based on logic in the past, the removal of ‘noxious’ agents from the blood remains the rationale, but now there is scientific understanding of the pathophysiology of the diseases treated by plasma exchange.1 Exchange transfusions revolutionised the management of haemolytic disease in the newborn, and paved the way for therapeutic plasmapheresis and plasma exchange – the removal of plasma, with replacement by albumin-electrolyte solutions or fresh frozen plasma.
RATIONALE FOR PLASMA EXCHANGE
Theoretically, plasma exchange should be effective to treat any disorder in which there is a pathogenic circulating factor responsible for the disease. However, this premise is probably too simplistic, and other mechanisms may contribute to its beneficial effects (Table 90.1). It is a non-specific procedure, which brings about numerous, potentially undesirable, alterations in the plasma’s milieu interieur.
| Removal of circulating toxic factor antibodies |
| Monoclonal antibodies |
| Autoantibodies |
| Alloantibodies, immune complexes, chemicals, drugs |
| Depletion of the mediators of inflammation |
| Replacement of deficient plasma factor(s) |
| Potentiation of drug action |
| Enhanced reticuloendothelial function |
| Altered immunoregulation |
| Potentiating effects of plasma exchange on other modes of therapy |
PATHOPHYSIOLOGY OF AUTOIMMUNE DISEASE
In general, autoimmune disease can be an acute, self-limiting (‘one hit’) disorder, intermittent or a chronic perpetuating disorder. Acute autoimmune disease may have an identifiable trigger, such as an infection, followed by a 10-day to 3-week gap until the pathogenic humoral or cellular factors appear in the circulating blood. At this point, end-organ damage commences, and clinical features of the disease appear.
The course of the disease will be determined by several factors:
The extent of damage is a product of the:
Cells are broadly divided into three kinetic characteristics:
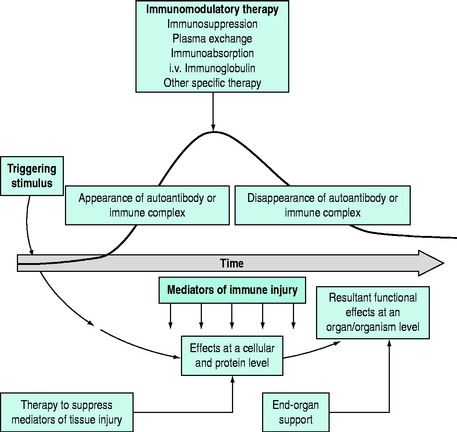
As most disorders are multifactorial, it is unlikely that a single form of therapy will be successful. A multipronged approach to therapy needs to be planned following an analysis of the basic underlying pathophysiology. The stage of the disease is also important (Figure 90.1). Clearly, plasma exchange will have a different response when autoantibody production is rising rapidly, compared with a stage when autoantibody has ceased production. Also, immunoregulation is a complex process, and therapies may interfere at different points in the immune mechanisms.
In some circumstances, specific and directed therapy may attack the most relevant link in the pathophysiological chain, but overall multiple approaches to therapy may be required. In general, plasma exchange is a temporising procedure and concomitant immunosuppressive therapy is required to maintain control. Plasma exchange for autoimmune disease should generally be regarded as a first step in immunomodulatory therapy, and restricted to acute or fulminant situations in which autoantibodies or immune complexes are responsible for life-threatening or end-organ damaging complications. There are some situations in which the humoral factor may be only transient (‘one antigen hit’ disorders), and no follow-up immunosuppression is required (e.g. acute postinfectious polyneuritis). Increasing monoclonal antibody therapy with rituximab (an anti-CD20 antibody) is finding a role in the longer term management of autoimmune diseases.2,3
The availability and recognition of the immunomodulatory effects of intravenous immunoglobulin has resulted in an exponential increase in its use in immune and inflammatory disorders, with its availability being one of the main drivers of the supply of human-derived plasma products. In most of the immune and inflammatory disorders in which plasma exchange has been used, intravenous immunoglobulin has been used. The mechanisms of action of intravenous immunoglobulin remain controversial. Intravenous immunoglobulin, fractionated from normal human plasma, was introduced as a replacement therapy for humoral immunodeficiency disorders, but is now widely used for a range of autoimmune and inflammatory diseases. Progress is being made in understanding the complex mechanisms by which IVIG has immunomodulatory actions. The mechanisms of action of IVIG involve modulation of expression and function of immunoglobulin Fc receptors, interference with activation of the complement system and the cytokine and immunoglobulin idiotype networks, and regulation of cell growth. There is also evidence for effects on activation, differentiation and effector functions of dendritic cells and T and B lymphocytes.4
TECHNICAL CONSIDERATIONS
Cell separators available for plasma exchange therapy are broadly divided into two groups:5
Either of these techniques for plasma exchange can be combined with immunoabsorption techniques, in which immunoglobulins are specifically or non-specifically removed.6
INDICATIONS (Table 90.2)
Table 90.2 Acute diseases in which plasma exchange may be beneficial*
| Immunoproliferative diseases with monoclonal immunoglobulins |
* This is an incomplete list and only includes disorders that are relatively common or in which plasma exchange has a definitive role to play.
MONOCLONAL ANTIBODIES ASSOCIATED WITH IMMUNOPROLIFERATIVE DISEASES
HYPERVISCOSITY SYNDROME
Characteristic clinical features of hyperviscosity in association with monoclonal proteins include visual disturbance, neurological dysfunction and hypervolaemia, all of which can be rapidly relieved by plasma exchange.7
IMMUNOLOGICAL DISEASES
DISEASES MEDIATED BY SPECIFIC AUTOANTIBODIES
Goodpasture’s syndrome
Circulating antiglomerular basement membrane antibodies can be demonstrated in most cases of Goodpasture’s syndrome. The disease classically has a fulminant presentation with rapidly progressive renal failure and life-threatening pulmonary haemorrhage. Early diagnosis and intensive plasma exchange may be necessary to preserve renal function and control pulmonary haemorrhage. Patients who are already in anuric renal failure rarely show improvement in renal function.8
Myasthenia gravis
Removal of the acetylcholine receptor autoantibody is associated with clinical improvement in the majority of patients with this disorder. The beneficial effects of plasma exchange are usually transient, and the procedure should normally be used in conjunction with other forms of therapy (see Chapter 49). The major role for plasma exchange is usually in myasthenic crisis, in patients whose condition is resistant to other forms of therapy, and prior to surgery. Therapy can be monitored by acetylcholine receptor autoantibody assays and respiratory function tests. Patients undergoing plasma exchange may transiently deteriorate during the procedure, due to a combination of the physical exertion and removal of medication from the circulation, and adequate ventilatory support should be available. IVIG may also be use in myasthenia gravis.9–11
Stiff-man syndrome
Stiff-man syndrome (STS) is a rare neurological disorder characterised by involuntary axial and proximal limb rigidity and continuous motor unit activity on electromyography. Autoantibodies to glutamic acid decarboxylase are usually demonstrable. Although plasma exchange is successful in STS, those who are autoantibody negative are less likely to respond.12,13
Autoimmune haematological disorders
Haemostatic failure due to autoantibodies directed against coagulation factors may present a major management problem. Antibodies directed against factor VIII are the commonest, occurring spontaneously or in association with replacement therapy in haemophiliacs. First-line therapy in patients with coagulation factor inhibitors usually requires activated factor VII therapy with recombinant FVIIa or appropriate prothrombin concentrates.14
IMMUNE COMPLEX DISEASE
Rapidly progressive glomerulonephritis
Immune complex-induced rapidly progressive glomerulonephritis may occur by itself or be associated with several systemic disorders (e.g. systemic lupus erythematosus, polyarteritis nodosa and Wagener’s granulomatosis).15–17 Plasma exchange may result in improvement in renal function even in patients who present with anuria. The decomplementing and defibrinating effects of plasma exchange may be partly responsible for clinical improvement. The therapeutic role of plasma exchange in rapidly progressive glomerulonephritis is difficult to assess, but most experienced physicians feel that the procedure leads to a more rapid and complete recovery of renal function in fulminant, rapidly deteriorating cases. However, the ultimate prognosis of the disease depends on adequate immunosuppression to inhibit immune complex formation, or spontaneous disappearance of the inciting antigen.
Systemic lupus erythematosus (SLE)
Plasma exchange has a role in acute life-threatening or organ-damaging relapses of SLE. Rapid deterioration in renal function, cerebritis and acute fulminant lupus pneumonitis are clinical situations in which plasma exchange should be considered.18
OTHER IMMUNE-MEDIATED DISEASES
Renal transplant rejection
Humoral mechanisms appear to play a part in hyperacute renal allograft rejection. Plasma exchange ± IVIG may be useful in tiding patients over episodes of acute graft rejection, but results of clinical trials have been conflicting.19,20 General opinion is that plasma exchange helps in a limited number of patients who cannot be currently preselected by any clinical or laboratory criteria.
Thrombotic thrombocytopenic purpura
TTP used to be a fatal disease in 90% of patients, but dramatic improvement in its outcome has occurred over the past two decades with the development of effective therapy. Plasma exchange has become the cornerstone of the treatment with fresh frozen plasma replacement.21,22 Cryoprecipitate-poor plasma (depleted in vWF) may offer advantages over whole fresh frozen plasma. It is now possible to achieve remissions in the majority of patients and cures are now common, though unfortunately relapse may occur. The clinical course at relapse is usually milder than the disease at presentation and less aggressive therapy may be needed.
Haemolytic uraemic syndrome (HUS)
This syndrome has many similarities to TTP, but renal involvement in contrast to cerebral in TTP is the hallmark in association with microangiopathy and thrombocytopenia.23 The prognosis and approach to management of HUS is similar to TTP. It usually occurs in children (related to bacterial or viral infections), but may rarely be seen in adults, in whom the disease may take a more chronic and sinister course. In adult cases medications are commonly implicated.
Inflammatory demyelinating neuropathies and the Guillain–Barré syndrome (GBS)
This acute self-limiting disease in which an acute demyelinating neuropathy occurs (usually following a viral infection) commonly results in admission to the intensive care unit (ICU) (see Chapter 49). There is demyelination due to postinfectious autoimmunity, with both cellular and humoral arms of the immune system attacking myelin. There is now wide experience in the use of plasma exchange and IVIG therapy in GBS, with controlled trials substantiating its benefits of shortening of the illness and complications. Therapy should be instituted early.11,24 As GBS also responds to high-dose IVIG there is debate as to which should be the first line of therapy. Decisions are usually made on the basis of local logistics and economics as the therapies are comparable in relationship to efficacy and safety.25 In some cases the onset of recovery after therapy may be delayed, probably due to time for remyelination to occur. Some patients show rapid improvement after plasma exchange, suggesting the presence of neuronal blocking factors. Chronic inflammatory demyelinating peripheral neuropathy (CIPD) is related to GBS, and plasma exchange and/or IVIG have important roles in treatment, in many cases requiring long-term therapy.
COMPLICATIONS
REACTIONS TO REPLACEMENT FLUIDS
The rapid infusion of any blood component may be associated with allergic or vasomotor reactions. Plasma exchange is a rather unique situation in which blood or blood products and plasma substitutes are being infused at resuscitation rates into normovolaemic, normotensive patients.
1 Isbister JP. Plasma exchange: a selective form of blood-letting. Med J Aust. 1979;2:167-173.
2 Scheinberg M, Hamerschlak N, Kutner JM, et al. Rituximab in refractory autoimmune diseases: Brazilian experience with 29 patients (2002–2004). Clin Exp Rheumatol. 2006;24:65-69.
3 Virgolini L, Marzocchi V. Rituximab in autoimmune diseases. Biomed Pharmacother. 2004;58:299-309.
4 Negi VS, Elluru S, Siberil S, et al. Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007;27:233-245.
5 Stegmayr BG. A survey of blood purification techniques. Transfus Apher Sci. 2005;32:209-220.
6 Ullrich H, Kuehnl P. New trends in specific immunoadsorption. Transfus Apher Sci. 2004;30:223-231.
7 Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost. 2003;29:467-471.
8 Shah MK, Hugghins SY. Characteristics and outcomes of patients with Goodpasture’s syndrome. Southern Med J. 2002;95:1411-1418.
9 Zhu KY, Feferman T, Maiti PK, et al. Intravenous immunoglobulin suppresses experimental myasthenia gravis: immunological mechanisms. J Immunol. 2006;176:187-197.
10 Gajdos P, Chevret S, Toyka K. Intravenous immunoglobulin for myasthenia gravis. Cochrane Database Syst Rev. 2006;2:CD002277.
11 Green DM. Weakness in the ICU: Guillain–Barré syndrome, myasthenia gravis, and critical illness polyneuropathy/myopathy. Neurologist. 2005;11:338-347.
12 Shariatmadar S, Noto TA. Plasma exchange in stiff-man syndrome. Ther Apher. 2001;5:64-67.
13 Cantiniaux S, Azulay JP, Boucraut J, et al. [Stiff man syndrome: clinical forms, treatment and clinical course]. Rev Neurol (Paris). 2006;162:832-839.
14 Abshire T, Kenet G. Recombinant factor VIIa: review of efficacy, dosing regimens and safety in patients with congenital and acquired factor VIII or IX inhibitors. J Thromb Haemost. 2004;2:899-909.
15 Stegmayr BG, Almroth G, Berlin G, et al. Plasma exchange or immunoadsorption in patients with rapidly progressive crescentic glomerulonephritis. A Swedish multi-center study. Int J Artif Organs. 1999;22:81-87.
16 Rahman T, Harper L. Plasmapheresis in nephrology: an update. Curr Opin Nephrol Hypertens. 2006;15:603-609.
17 Jayne DR, Gaskin G, Rasmussen N, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180-2188.
18 Pagnoux C, Korach JM, Guillevin L. Indications for plasma exchange in systemic lupus erythematosus in 2005. Lupus. 2005;14:871-877.
19 Lehrich RW, Rocha PN, Reinsmoen N, et al. Intravenous immunoglobulin and plasmapheresis in acute humoral rejection: experience in renal allograft transplantation. Hum Immunol. 2005;66:350-358.
20 Rocha PN, Butterly DW, Greenberg A, et al. Beneficial effect of plasmapheresis and intravenous immunoglobulin on renal allograft survival of patients with acute humoral rejection. Transplantation. 2003;75:1490-1495.
21 Brunskill SJ, Tusold A, Benjamin S, et al. A systematic review of randomized controlled trials for plasma exchange in the treatment of thrombotic thrombocytopenic purpura. Transfus Med. 2007;17:17-35.
22 Ozkalemkas F, Ali R, Ozkocaman V, et al. Therapeutic plasma exchange plus corticosteroid for the treatment of the thrombotic thrombocytopenic purpura: a single institutional experience in the southern Marmara region of Turkey. Transfus Apher Sci. 2007;36:109-115.
23 Amirlak I, Amirlak B. Haemolytic uraemic syndrome: an overview. Nephrology. 2006;11:213-218.
24 Raphael JC, Chevret S, Hughes RA, et al. Plasma exchange for Guillain–Barré syndrome. Cochrane Database Syst Rev. 2002;2:CD001798.
25 Tsai CP, Wang KC, Liu CY, et al. Pharmacoeconomics of therapy for Guillain–Barré syndrome: plasma exchange and intravenous immunoglobulin. J Clin Neurosci. 2007;14:625-629.