CHAPTER 42 The Use and Misuse of Antibiotics in Neurosurgery
The Importance of Antibiotics in Neurosurgery
Few neurosurgeons would be willing to practice modern neurosurgery without the ready availability of antibiotics. They have made it possible to treat infections of the brain, meninges, and surgical site effectively and to salvage excellent results from what would otherwise be devastating complications of neurosurgical operations. Although some still argue based on the extraordinary results of Harvey Cushing, who reported one infection in 149 patients (0.7%), that careful technique overcomes almost all sources of infection, a very special understanding of the available evidence is required to deliberately omit perioperative antibiotic prophylaxis in modern neurosurgical practice.1 Antibiotics are an integral part of the daily life of the neurosurgeon.
Risks Associated with Antibiotic Administration
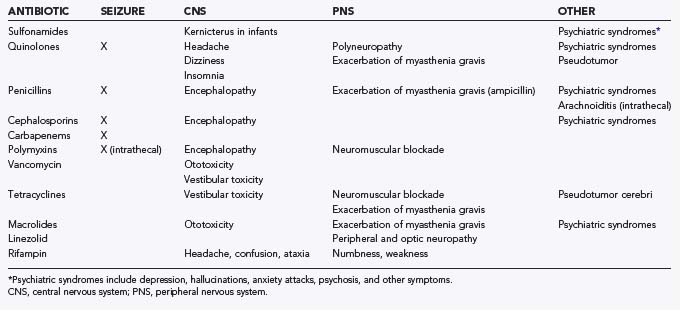
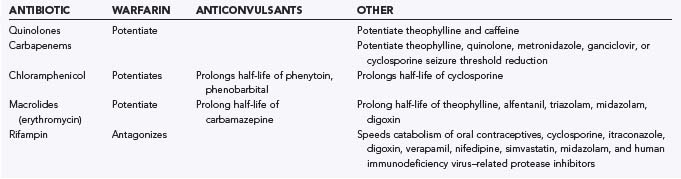
Antibiotic therapy in neurosurgical patients is implemented in various situations, including prophylaxis for procedures, empirical treatment of a presumed infection, or treatment of a specific infection. Administration of antibiotics is not without consequence, however. Adverse drug reactions that may result include central nervous system (CNS) toxicities, systemic toxicities, allergic reactions, side effects, and drug-drug interactions. Moreover, there is the potential for antibiotic resistance with careless administration. The emergence of antibiotic resistance is a growing and potentially catastrophic problem that should not be taken lightly. Drug toxicity is a consequence of either excessive dosing or impaired drug metabolism, the latter possibly being due to hepatic or renal insufficiency. An allergic drug reaction is a hypersensitivity reaction to a medication that may be immunologically mediated and can result in urticaria, bronchospasm, anaphylactic shock, or angioedema. Side effects are other adverse drug reactions that are neither due to drug toxicity or to a hypersensitivity reaction. In this section, commonly used antibiotics in neurosurgery are addressed, along with their local toxicity, systemic toxicity, side effects, drug-drug interactions, and potential for resistance. Hypersensitivity reactions are discussed briefly and only in regard to specific antibiotics. Table 42-1 summarizes the most important neurotoxicities encountered with current antibiotic use. Table 42-2 summarizes selected drug interactions of importance to the neurosurgical patient.
Sulfonamides
Sulfonamide neurotoxicity can occur in the premature and newborn period because sulfonamides have the potential to displace bilirubin from albumin, with the resultant free bilirubin being deposited in the basal ganglia and subthalamic nuclei and resulting in kernicterus.2 Moreover, there are reports in the literature of other CNS toxicities, including ataxia, depression, and psychosis with associated visual and auditory hallucinations.3,4
Quinolones
The incidence of neurotoxicity from quinolones ranges from 1% to 2%, and symptoms may include headache, dizziness, and insomnia.5–8 Additionally, instances of delirium, acute psychosis, and seizures have been reported.5,6,9 Moreover, there have been reports of the development of demyelinating polyneuropathy,10 exacerbation of myasthenia gravis,11–15 and peripheral sensory disturbances16 with fluoroquinolone use. Signs of pseudotumor cerebri may develop in infants and young children with high doses of nalidixic acid, the first quinolone to be introduced.17 These CNS effects should generally resolve with the discontinuation of therapy.
Penicillins
Neurotoxicity after parenteral administration of penicillin G is most likely to occur in patients with renal insufficiency, intracranial lesions, or alteration of the blood-brain barrier (BBB).18 This toxicity can occur when the concentration of penicillin G in cerebrospinal fluid (CSF) exceeds 10 µg/mL and may be manifested as lethargy, confusion, twitching, multifocal myoclonus, or seizures.18 Arachnoiditis and encephalopathy may follow the intrathecal injection of penicillin G, and therefore intraventricular and intrathecal administration should be avoided.18 The development of symptoms that may resemble panic attacks or acute psychosis with seizures or hallucinations is known as Hoigne’s syndrome and can follow the intravascular injection of penicillin.19,20 Furthermore, ampicillin may exacerbate weakness in patients with myasthenia gravis.21
Cephalosporins
Many cephalosporins have been associated with neurotoxicity, and ceftazidime, in particular, has been reported to cause hallucinations,22 confusion, encephalopathy,23 and status epilepticus.22,24 There have also been published reports of cephalosporin-associated recurrent aseptic meningitis.25
Carbapenems
The most common adverse events encountered with carbapenem administration are nausea and vomiting.26 Other frequent adverse events include diarrhea, rash, fever, and laboratory abnormalities, such as elevated liver enzyme levels, eosinophilia, thrombocytopenia, and increased prothrombin time.26 Patients at risk for seizures are those with preexisting renal insufficiency or an intracranial mass lesion who are given high doses of imipenem.27 Furthermore, there are reports that concomitant administration of imipenem with theophylline, quinolones, metronidazole, ganciclovir, or cyclosporine may reduce the seizure threshold.28–30 In contrast to imipenem, meropenem is less likely to induce seizures.31–34
Aminoglycosides
The most important and serious side effects of aminoglycosides are nephrotoxicity and ototoxicity. The other dose-related adverse effect of clinical importance is neuromuscular blockade, which occurs rarely and is usually related to an underlying condition.35 The first signs of ototoxicity may be seen histologically, with the outer hair cells of the cochlea affected first, followed by the inner hair cells.36 Damage to the hair cells results in high-frequency hearing loss, followed by progressive loss of hearing at lower frequencies.35 After degeneration of the hair cells, there can also be damage to nerve fibers.37 The first clinical symptom of cochlear damage is often high-pitched and continuous tinnitus.38 In regard to the vestibular apparatus, hair cell damage occurs along with deterioration of the otoconial membrane and otolith structures.39,40 Clinical signs and symptoms of vestibular damage include disequilibrium, ataxia, transient positional vertigo, and oscillopsia.35,38 In rare instances, aminoglycosides have the potential to cause neuromuscular blockade and paralysis.35 Patients with preexisting myasthenia gravis are at a higher risk for the development of neuromuscular blockade.41 Risk factors for ototoxicity include renal insufficiency, preexisting impaired hearing, old age, sepsis, dehydration, fever, previous aminoglycoside exposure, and concomitant use of vancomycin, cisplatin, or carboplatin.35 Anosmia after aminoglycoside therapy has also been described in the literature, with the sense of smell returning after time.42,43 Intrathecal or intraventricular administration is used rarely because it may cause local inflammation and can result in aseptic meningits.44
Polymyxins
The major adverse drug effects of polymyxins are nephrotoxicity, neurotoxicity, and neuromuscular blockade. Renal insufficiency and giddiness may result when polymyxins are used parenterally, and pain, numbness, paresthesias, confusion, coma, and convulsions may result when they are used intrathecally.45–47
Neurotoxicity from parenteral polymyxin B and colistin is seen most frequently in patients with compromised renal function, with an overall incidence of approximately 7.3%.48 With increasing doses of polymyxin, patients may experience circumoral paresthesias, convulsions, apnea, distal paresthesias, ptosis, diplopia, dysphagia, dysphonia, areflexia, ataxia, and dizziness.49–51 Intrathecal dosages greater than 50,000 U/day have been reported to cause chemical meningitis.18,52
Vancomycin
Neurotoxicity with the administration of vancomycin can include both vestibular damage and cochlear damage, which can result in tinnitus and sensory hearing loss.53,54 Tinnitus may be an early symptom and can indicate the development of deafness.55 Ototoxicity in the early stages, characterized by tinnitus and dizziness, appears to be reversible.56 However, by the time that the patient experiences a noticeable hearing deficit, the toxicity is often irreversible.57 The mechanism by which vancomycin causes ototoxicity is thought to be direct damage to the auditory nerve, which leads to irreversible loss of the sensory hairs in the cochlea that initially affects high-frequency sensory hearing.57 Subsequently, lower frequencies are affected, and eventually, total hearing loss may result.58
Tetracyclines
Neurotoxicity is a well-recognized side effect of tetracycline therapy, and there are several reports in the literature documenting increased intracranial pressure with medium- to long-term use.59–62 In infants, a bulging fontanelle may be apparent but resolves on discontinuation of the therapy.63 Vestibular symptoms are also reported with tetracycline administration and may include dizziness and ataxia.64–66 Additionally, tetracyclines are thought to block the neuromuscular junction by both prejunctional and postjunctional effects that depress the sensitivity of muscle to acetylcholine.3,67 This neuromuscular blockade has been reported to exacerbate myasthenia gravis.18
Chloramphenicol
Neurotoxic effects of chloramphenicol administration are rare. Optic neuropathy and peripheral neuropathy have been cited in the literature in children with cystic fibrosis.68–71 Symptoms of visual toxicity may include blurred vision followed by loss of visual acuity and impaired red-green color discrimination, whereas peripheral nerve toxicities result in burning, tingling, or numbness of extremities.3 The aforementioned symptoms generally resolve after discontinuation of the therapy.3 Additionally, there have been reports of chloramphenicol-induced encephalopathy that can progress to delirium.72
The most feared toxicities of chloramphenicol administration include bone marrow suppression, aplastic anemia, and gray baby syndrome.73
Macrolides
Neurotoxicity is mostly associated with erythromycin and may result in neuropsychiatric symptoms or ototoxicity.74 The neuropsychiatric symptoms that have been reported in the literature include confusion, hysteria, anxiety, and nightmares, all of which disappeared after therapy was terminated.75,76 The ototoxicity that is induced by erythromycin is a result of high doses, or it can occur in patients with preexisting hepatic or renal insufficiency.74,77 In contrast to aminoglycosides, the hearing loss with macrolides is reversible with cessation of the drug.78 There are also reports cited in the literature of the potential for erythromycin to exacerbate weakness in patients with myasthenia gravis.79,80
Linezolid
Neurotoxicity can result in peripheral neuropathy with prolonged use of linezolid.81–83 Most cases of peripheral neuropathy have occurred when linezolid is used beyond the maximal recommended duration of therapy of 28 days.83 Additionally, there have been several reports documenting linezolid-associated optic neuropathy.84–86
Rifampin
The neurotoxic effects of rifampin may include ataxia, confusion, dizziness, numbness, muscular weakness, inability to concentrate, and headache.18,87
The adverse reactions most commonly reported include rash, fever, headache, general malaise, gastrointestinal symptoms, nausea, and vomiting.88,89
General Principles of Antibiotic Use
The Blood-Brain and Blood–Cerebrospinal Fluid Barriers
The BBB is formed by endothelial cells of the cerebral vasculature, supported by astroglia and pericytes, with tight junctions between the endothelial cells and minimal fenestrations or bulk transport across the cells. For the BCSFB, this barrier is located at the epithelial layer of the choroid plexus, not at the endothelium, but is similar in character, with tight junctions between the epithelial cells. For both barriers, active influx and efflux transporters located on the endothelial/epithelial cell surface may drastically alter the distribution of an antibiotic into the desired compartment.90,91 Many factors can contribute to the permeability of any substance across the BBB:
Each of these factors affects a substance’s ability to cross the brain barriers and reach its intended site of action. Increasing molecular weight, ionization, plasma protein binding, and metabolism at the barrier and the presence of efflux transporters decrease the permeability of substances across the brain barriers.91,92 Increased lipophilicity, influx transporters, and inflammation can increase the permeability of the barriers to antibiotics.93 The inflammation associated with infections may decrease over the course of and in response to treatment. Antibiotics may cross into the CSF or brain parenchyma more readily at the initiation of treatment, but as the inflammatory response to the infection abates, either from the antibiotic treatment or from the administration of other medications such as dexamethasone, the ability of an antibiotic to cross the brain barriers diminishes.94,95 However, this effect may not alter outcome.96–100
The overall goal of antibiotic treatment is to deliver an adequate concentration of the drug to the proper compartment. Antibiotics that are bactericidal are generally preferred for the treatment of CNS infections as well because of the low concentration of immunologic proteins such as complement and immunoglobulins and the relatively low numbers of phagocytic cells, although this contention may be changing.101–103 This goal may be accomplished in several ways. First, the dose of the drug may be increased. This method is helpful for drugs with low systemic toxicity and relatively low permeability across the brain barriers, such as β-lactam antibiotics. Second, the choice of antibiotic may be changed to a drug that has greater penetration into the CNS, such as chloramphenicol or quinolones. Third, antibiotics may be delivered directly across the brain barriers (usually by indwelling ventricular or lumbar catheters). This method is especially helpful when using antibiotics that have higher systemic toxicity and poor permeability across the brain barriers, which may limit the systemic dose that can be administered; such drugs include vancomycin and aminoglycosides. For example, administration of chloramphenicol via an intraventricular route does not provide the same advantage that intraventricular administration of vancomycin does because chloramphenicol’s penetration of the brain barriers already exceeds that needed to provide a therapeutic concentration. In addition, bypassing the brain barriers does not provide a significant advantage because chloramphenicol also needs to be hydrolyzed to be effective, which is usually done in the liver. Although hydrolysis has been shown to occur in the CSF, intraventricular administration is probably not necessary.104 Alternatively, using higher doses of β-lactam antibiotics to reach bactericidal concentrations is easily accomplished and generally associated with minimal systemic toxicity. Intraventricular administration of β-lactams is probably more toxic than systemic administration, although whether the source of the toxicity is the antibiotic or the underlying infection is not clear.104
Pharmacokinetics of Antibiotic Delivery
To achieve effective antibiotic dosing, the pharmacokinetics of antibiotic administration must be understood. For infections occurring outside the barriers of the CNS, the principles of systemically administering antibiotics are less complicated. Peak concentrations depend on bioavailability, the amount delivered, the volume of distribution, and elimination via metabolism and excretion.103 However, the pharmacokinetics of antibiotic administration to the CNS depends on both systemic pharmacokinetics and the behavior of the antibiotic in its access to and elimination from the CNS. The sum of these factors can be ascertained by experimentally determining the proportion of antibiotic that reaches the CNS. Most data relating to antibiotic pharmacokinetics in the CNS come from studies on CSF and meningitis. Much less data exist on these parameters for the brain parenchyma itself.105,106
In determining this proportion, careful interpretation of experimental data is required. Many studies looking at the proportion of antibiotic reaching the CNS use simple plasma-CSF ratios at a single time point. These ratios can vary widely during a dosing cycle and can be quite misleading.106,107 The most useful data are derived from using plasma-CSF AUC (AUC is the area under the drug concentration–versus-time curve) ratios in intermittent dosing or steady-state concentrations during continuous infusion. For β-lactam antibiotics, the AUC ratio generally ranges from 0.01 to 0.1. Less hydrophilic antibiotics, such as rifampicin, trimethoprim-sulfamethoxazole, and the fluoroquinolones, have ratios that range from 0.1 to 0.9. Vancomycin and the aminoglycosides also exhibit low penetration into CSF, with ratios of less than 0.1.106,108 However, data suggest that these ratios may be different in the treatment of infections of the brain parenchyma (i.e., brain abscess). One study showed equivalent levels of antibiotic in abscess fluid and plasma 6 hours after administration; however, these are also point ratios, not AUC ratios.109 Whether the antibiotics are as effective in abscess fluid is a separate consideration. Concentrations of antibiotics throughout the CSF are not constant. Ventricular CSF will have a lower concentration of protein and antibiotic than will lumbar CSF because the CSF produced in the ventricles has not yet mixed with exuded extracellular fluid from the brain parenchyma. Therefore, CSF concentrations of antibiotics rely on the permeability of both the BBB and the BCSFB. Penetration of antibiotics through the blood-lesion barrier (specifically, the blood-abscess barrier) will vary with the stage of formation of the abscess, the relative vascularity of the lesion, and even the cause of the lesion. However, it is impossible to differentiate the individual contributions of the blood-lesion barrier and the surrounding BBB to antibiotic concentrations by measuring antibiotic levels within abscesses.105,109–111
The half-life of the antibiotic in CSF is also an important consideration. Most antibiotics are not metabolized in CSF. Elimination is achieved either by diffusion back through the BBB and BCSFB or from turnover of the CSF. Generally, the CSF half-life of antibiotics is significantly longer than the plasma half-life. The CSF half-life of antibiotics may also be increased in CNS infections because of decreased turnover of CSF. Conversely, in patients with CSF shunts or external CSF drains, the CSF half-life may be quite variable because the circulation of CSF is altered by the presence of the shunt or drain.112,113
Central Nervous System Toxicity of Antibiotic Therapy
Intrathecal antibiotics may also have significant neurological toxicities, although the most commonly used intrathecal antibiotics, vancomycin and gentamicin, appear to have relatively low toxicity when administered intrathecally. Additionally, discerning these effects may be difficult in patients with a coexisting serious CNS infection. Intraventricular vancomycin appears to be relatively free of toxicity, even at high CSF levels.114 Intraventricular gentamicin may lead to CNS toxicity and cause ototoxicity or epilepsy; however, these effects are not clearly related to CSF levels of gentamicin.115,116 Other antibiotics less commonly used intraventricularly, such as the β-lactam antibiotics, may cause similar effects, especially seizures, as when they are administered systemically.117,118
Antibiotic Prophylaxis
Systemic Antibiotic Prophylaxis
Antibiotic prophylaxis should be considered in terms of the inherent risk for infection associated with the procedure being contemplated. The standard approach to estimating risk for infection at the surgical site is the classification endorsed by the Centers for Disease Control and Prevention (Table 42-3).119
| WOUND CLASS | DESCRIPTION | EXAMPLES |
|---|---|---|
| Clean | Uninflamed, uncontaminated, no trauma or infection, primarily closed with no break in sterile technique |
Expected infection rates range from less than 1% in clean wounds with antibiotic prophylaxis to 6% to 10% in dirty wounds, even with antibiotic treatment.120
Clean wounds in neurosurgery are generally subdivided into those with and without implantation of a substantial foreign body. The prototypical neurosurgical foreign body is the shunt. Clean shunt implantations with antibiotic prophylaxis have approximately an 8% to 10% infection rate, a rate much higher than that for non–foreign body operations.121
Clean Neurosurgical Procedures
The value of systemic antibiotic prophylaxis in clean neurosurgical operations is supported by level I evidence from multiple randomized clinical trials and high-quality meta-analysis.122 The same is true of systemic antibiotic prophylaxis for shunt operations.123,124 Additional meta-analyses have supported its value in preventing meningitis after craniotomy125 and in spine neurosurgery.126
The value of systemic antibiotic prophylaxis in clean-contaminated operations has not been adequately studied to allow a confident conclusion to be reached. The current accepted practice in transnasal surgery varies from limited perioperative use127 to use as though the procedure were contaminated (i.e., therapeutic doses for a therapeutic duration).128
It is not feasible to study the differential effectiveness of different antibiotics. If one antibiotic reduces the expected infection rate to 1% and another is twice as good (infection rate of 0.5%), a study consisting of more than 5000 patients would be required to have a reasonable chance (power of 0.8) of finding that result to be statistically significant (P ≤ .05). Although the duration of prophylactic antibiotic administration in neurosurgery has not been specifically studied in a randomized trial, the general principles of systemic prophylactic antibiotic administration are well established in many disciplines119:
External Ventricular Drains
There is insufficient evidence to support a firm conclusion about the value of systemic antibiotic prophylaxis in reducing infections associated with external ventricular drains.130 One underpowered trial compared systemic antibiotic prophylaxis with placebo for ventriculostomy and found no difference in the infection rate.131 A single trial compared short-term and long-term antibiotic administration and suggested that long-term use reduced infection rates but selected for resistant organisms.132 The infection rates in this study were high (extracranial infection rates of 40% with short-term use and 20% with long-term use). This is an area in which further study could be useful.
Topical Antibiotic Prophylaxis
The topical use of antibiotics during neurosurgical procedures to prevent postoperative infection has a long history but has not been studied with sufficient rigor to produce a definitive conclusion about its effectiveness. Two reviews have been published.134,135
When antibiotics first became available, they were sprinkled into the wound in powdered form. Pennybacker and coauthors reported a reduction in infection rates to modern levels (0.9%),136 but a large review of practice at Massachusetts General Hospital did not confirm this benefit.137 A report by Malis in 1979, however, renewed enthusiasm for the practice (now with antibiotic solutions rather than powder).138 Subsequent case series have supported the concept, although a randomized comparison of systemic plus topical antibiotic prophylaxis against systemic antibiotics alone has not been done.135,139,140
The issue of toxicity requires special consideration for topical administration. The tendency of penicillin to produce seizures when applied to the cerebral cortex is well known, therefore topical penicillins should be avoided.141 Limited study of the effect of topical application on the cerebral cortex suggests that bacitracin and metronidazole have the lowest likelihood of producing epileptiform activity.142 pH is likely to be an important parameter, and topical solutions applied directly to the nervous system should be adjusted to physiologic pH when possible.
Cerebrospinal Fluid Shunts
Topical antibiotic prophylaxis is commonly used during CSF shunt procedures in two ways: wound irrigation plus filling the shunt with antibiotic solution and the use of antibiotic-impregnated shunt catheters. The topical use of solutions has the same quality of evidence as it does for other neurosurgical procedures (see earlier). A recent Cochrane Systematic Review of the prevention of CSF shunt infection examined the evidence regarding antibiotic-impregnated shunt catheters and concluded that the evidence did support their effectiveness in reducing shunt infection rates.123
Antibiotic Treatment
Soft Tissue Infections
Initial treatment in most cases should be directed against staphylococci and streptococci. Oral antibiotic therapy with a semisynthetic penicillin, a first-generation cephalosporin, clindamycin, or erythromycin should be considered.143 If methicillin-resistant Staphylococcus aureus is suspected, vancomycin should be used until cultures prove otherwise. The duration of antimicrobial therapy is dictated by how the infection responds but should typically continue for 7 to 10 days.
Necrotizing Soft Tissue Infections
Because of the polymicrobial nature of necrotizing soft tissue infections, empirical treatment with broad-spectrum antibiotics is often initiated. A number of different regimens may be used to achieve broad-spectrum coverage, the most common of which include the following144:
The most common antibiotic prescribing error is inadequate coverage of Enterococcus, which optimally requires ampicillin or vancomycin plus an aminoglycoside.144
Meningitis
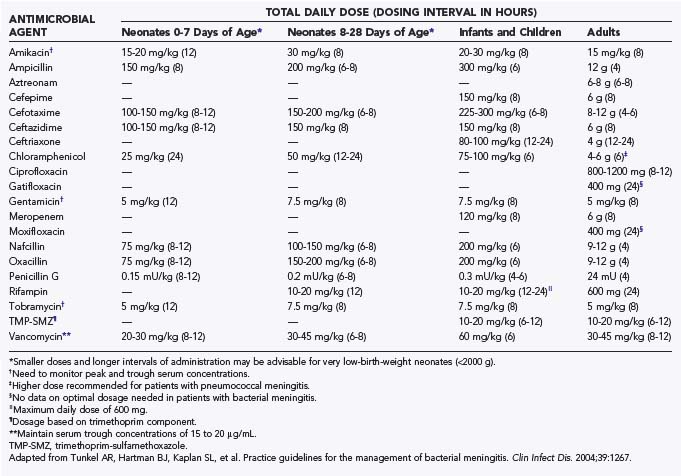
Antibiotic therapy for neurosurgical patients with postoperative bacterial meningitis is initially empirical. Practice guidelines for the initial empirical antimicrobial treatment of bacterial meningitis have been published by the Infectious Disease Society of America along with recommended dosages (Table 42-4). First-line empirical therapy includes a third-generation cephalosporin such as cefotaxime or ceftriaxone and vancomycin.145 In suspected cases of Pseudomonas aeruginosa, ceftazidime should be used. Unfortunately, there have been reports of the emergence of resistant gram-negative bacilli (i.e., resistant Enterobacter146–149 and Acinetobacter150–152 species) with plasmid-encoded or inducible chromosomal β-lactamases that hydrolyze extended-spectrum cephalosporins.153 In these cases, amikacin can be added, although this does not always prevent the development of resistance, as Chow and associates reported in their study.154 Therefore, for β-lactamase–producing Enterobacteriaceae or Acinetobacter species, an extended-spectrum carbapenem such as meropenem may be used because these organisms tend to be resistant to multiple antibiotics. Moreover, Parodi and coauthors reported good clinical and microbiologic responses with carbapenems for Enterobacter meningitis.155 Judicious use of this antibiotic needs to be ensured because carbapenem-resistant Enterobacter strains have been reported.156 For resistant Acinetobacter meningitis, Nguyen and colleagues demonstrated that intravenous imipenem and amikacin with or without intrathecal amikacin could be used successfully with the caveat that all patients have their ventriculostomy catheters removed as part of the treatment.151 Although their study consisted of a small number of cases, Rodriguez Guardado and coworkers demonstrated that intravenous and intrathecal colistin is an option and is as safe and effective as carbapenems for the treatment of nosocomial meningitis caused by Acinetobacter.157 Furthermore, a review by Falagas and associates documented that therapy with intraventricular and intrathecal polymyxins alone or in combination with systemic antimicrobial agents is effective against gram-negative meningitis.52
TABLE 42-4 Recommendations for Empirical Antimicrobial Therapy for Purulent Meningitis Based on Patient Age and Specific Predisposing Condition (A-III)
| PREDISPOSING FACTOR | COMMON BACTERIAL PATHOGENS | ANTIMICROBIAL THERAPY |
|---|---|---|
| Age | ||
| <1 mo | Streptococcus agalactiae, Escherichia coli, Listeria monocytogenes, Klebsiella species | Ampicillin plus cefotaxime or ampicillin plus an aminoglycoside |
| 1-23 mo | Streptococcus pneumoniae, Neisseria meningitidis, S. agalactiae, Haemophilus influenzae, E. coli | Vancomycin plus a third-generation cephalosporin*† |
| 2-50 yr | N. meningitidis, S. pneumoniae | Vancomycin plus a third-generation cephalosporin*† |
| >50 yr | S. pneumoniae, N. meningitidis, L. monocytogenes, aerobic gram-negative bacilli | Vancomycin plus ampicillin and a third-generation cephalosporin*† |
| Head trauma | ||
| Basilar skull fracture | S. pneumoniae, H. influenzae, group A β-hemolytic streptococci | Vancomycin plus a third-generation cephalosporin* |
| Penetrating trauma | Staphylococcus aureus, coagulase-negative staphylococci (especially Staphylococcus epidermidis), aerobic gram-negative bacilli (including Pseudomonas aeruginosa) | Vancomycin plus cefepime, vancomycin plus ceftazidime, or vancomycin plus meropenem |
| Neurosurgery | Aerobic gram-negative bacilli (including P. aeruginosa), S. aureus, coagulase-negative staphylococci (especially S. epidermidis) | Vancomycin plus cefepime, vancomycin plus ceftazidime, or vancomycin plus meropenem |
| Cerebrospinal fluid shunt | Coagulase-negative staphylococci (especially S. epidermidis), S. aureus, aerobic gram-negative bacilli (including P. aeruginosa), Propionibacterium acnes | Vancomycin plus cefepime,‡ vancomycin plus ceftazidime,‡ or vancomycin plus meropenem‡ |
† Some experts would add rifampin if dexamethasone is also given.
‡ In infants and children, vancomycin alone is reasonable unless Gram stains reveal the presence of gram-negative bacilli.
Adapted from Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267.
As mentioned previously, gram-negative bacilli have the potential to become resistant to β-lactam antibiotics, therefore CSF needs to be sampled at regular intervals to ensure that it is being sterilized. When the response to systemic antibiotic treatment is poor during the treatment of gram-negative meningitis, the use of intraventricular antibiotics in combination with intravenous antibiotics should be considered early. The most commonly used intraventricular agents include gentamicin, amikacin, and polymyxin E (colistin) (Table 42-5).104,153
TABLE 42-5 Recommended Dosages of Antimicrobial Agents Administered by the Intraventricular Route (A-III)
| ANTIMICROBIAL AGENT | DAILY INTRAVENTRICULAR DOSE (mg) |
|---|---|
| Vancomycin | 5-20* |
| Gentamicin | 1-8† |
| Tobramycin | 5-20 |
| Amikacin | 5-50‡ |
| Polymyxin B | 5§ |
| Colistin | 10 |
| Quinupristin/dalfopristin | 2-5 |
| Teicoplanin | 5-40 |
NOTE: There are no specific data that define the exact dose of an antimicrobial agent that should be administered by the intraventricular route. Virtually all intrathecal use of antibiotics is considered “off label.”
* Most studies have used a 10- or 20-mg dose.
† The usual daily dose is 1 to 2 mg for infants and children and 4 to 8 mg for adults.
‡ The usual daily intraventricular dose is 30 mg.
§ The dosage in children is 2 mg daily.
 Dosage of 5 to 10 mg every 48 to 72 hours.
Dosage of 5 to 10 mg every 48 to 72 hours.
Adapted from Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267.
As results from cultures are confirmed for susceptibility, the antibiotic treatment should be adjusted accordingly. Practice guidelines for pathogen-specific antimicrobial treatment of bacterial meningitis have been published by the Infectious Disease Society of America along with recommended dosages (Tables 42-6 and 42-7). The length of treatment when treating gram-negative bacilli is most often 2 weeks after culture results have been negative.158,159 Other authors have recommended a treatment duration between 2 and 4 weeks.153,160,161 When treating S. aureus, 2 weeks of treatment is reasonable. However, the duration of therapy should always be individualized based on the patient’s clinical response to treatment.
TABLE 42-6 Recommendations for Specific Antimicrobial Therapy for Bacterial Meningitis Based on Isolated Pathogen and Susceptibility Testing
| MICROORGANISM, SUSCEPTIBILITY | STANDARD THERAPY | ALTERNATIVE THERAPIES |
|---|---|---|
| Streptococcus pneumoniae | ||
| Penicillin MIC | ||
| <0.1 µg/mL | Penicillin G or ampicillin | Third-generation cephalosporin,* chloramphenicol |
| 0.1-1.0 µg/mL† | Third-generation cephalosporin* | Cefepime (B-II), meropenem (B-II) |
| ≥2.0 µg/mL | Vancomycin plus a third-generation cephalosporin*‡ | Fluoroquinolone§ (B-II) |
| Cefotaxime or ceftriaxone MIC ≥1.0 µg/mL | Vancomycin plus a third-generation cephalosporin*‡ | Fluoroquinolone§ (B-II) |
| Neisseria meningitidis | ||
| Penicillin MIC | ||
| <0.1 µg/mL | Penicillin G or ampicillin | Third-generation cephalosporin,* chloramphenicol |
| 0.1-1.0 µg/mL | Third-generation cephalosporin* | Chloramphenicol, fluoroquinolone, meropenem |
| Listeria monocytogenes | Ampicillin or penicillin G |
Trimethoprim-sulfamethoxazole, meropenem (B-III) |
| Streptococcus agalactiae | Ampicillin or penicillin G |
Third-generation cephalosporin* (B-III) |
| Escherichia coli and other Enterobacteriaceae¶ | Third-generation cephalosporin (A-II) | Aztreonam, fluoroquinolone, meropenem, trimethoprim-sulfamethoxazole, ampicillin |
| Pseudomonas aeruginosa¶ | Cefepime or ceftazidime or ceftazidime (A-II) (A-II) |
Aztreonam, ciprofloxacin, ciprofloxacin, meropenem meropenem |
| Haemophilus influenzae | ||
| β-Lactamase negative | Ampicillin | Third-generation cephalosporin,* cefepime, chloramphenicol, fluoroquinolone |
| β-Lactamase positive | Third-generation cephalosporin (A-I) | Cefepime (A-I), chloramphenicol, fluoroquinolone |
| Staphylococcus aureus | ||
| Methicillin susceptible | Nafcillin or oxacillin | Vancomycin, meropenem (B-III) |
| Methicillin resistant | Vancomycin** | Trimethoprim-sulfamethoxazole, linezolid (B-III) |
| Staphylococcus epidermidis | Vancomycin** | Linezolid (B-III) |
| Enterococcus species | ||
| Ampicillin susceptible | Ampicillin plus gentamicin | — |
| Ampicillin resistant | Vancomycin plus gentamicin | — |
| Ampicillin and vancomycin resistant | Linezolid (B-III) | — |
NOTE: All recommendations are A-III unless otherwise indicated.
MIC, minimal inhibitory concentration.
† Ceftriaxone/cefotaxime-susceptible isolates.
‡ Consider addition of rifampin if the MIC of ceftriaxone is greater than 2 µg/mL.
§ Gatifloxacin or moxifloxacin.
 Addition of an aminoglycoside should be considered.
Addition of an aminoglycoside should be considered.
¶ Choice of a specific antimicrobial agent must be guided by in vitro susceptibility test results.
** Consider addition of rifampin.
Adapted from Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267.
Empyema
Antibiotic treatment is initially broad spectrum and is directed against streptococci, staphylococci, and anaerobes. Empirical therapy includes a third-generation cephalosporin, vancomycin, or penicillin and metronidazole, with the latter providing anaerobic coverage. The suggested duration of treatment includes 2 weeks of intravenous therapy with an additional 6 weeks of oral therapy.162,163 Nathoo and colleagues use a regimen consisting of a penicillin, chloramphenicol, and metronidazole administered intravenously for 2 weeks followed by a 4-week oral course.164 The antibiotic regimen will require appropriate modification to either narrow or broaden the coverage based on bacterial culture and susceptibility results.
Brain Abscess
Initial coverage should begin with vancomycin, a third-generation cephalosporin, and metronidazole for anaerobic coverage.165 As cultures and sensitivities are reported, the antibiotic regimen should be tailored accordingly. Proteus, Escherichia coli, and Serratia species, which are common causes of cerebral abscesses in neonates, should be covered with a combination of cefotaxime and gentamicin or amikacin. If the infection is refractory to the aforementioned management, craniotomy with eradication of the primary foci should be undertaken.
The duration of therapy is still a point of debate. In most cases, parenteral treatment for 6 to 8 weeks is recommended.166,167 Some authors advocate an additional course of oral antibiotics for 2 to 3 months to eliminate any residual foci.167
Ventriculitis
The initial antibiotic regimen should include broad-spectrum coverage for possible resistant gram-positive and gram-negative organisms. Appropriate antibiotic therapy may include vancomycin plus a cephalosporin with antipseudomonal coverage such as cefepime or ceftazidime. Alternatively, vancomycin can be combined with meropenem to achieve similar coverage. Intraventricular vancomycin has also been reported to be successful in the treatment of ventriculitis caused by Staphylococcus and Enterococcus species.168,169
Intravenous polymyxin E (colistin) may be an option for gram-negative organisms, such as Acinetobacter and Pseudomonas, that are resistant to first-line antibacterial treatments.170 There have also been case reports documenting the success of intrathecal colistin in the treatment of resistant Acinetobacter ventriculitis.171–173
Shunt Infections
Initial antibiotic selection should be broad spectrum and include coverage for methicillin-resistant S. aureus and Staphylococcus epidermidis, along with coverage for resistant gram-negative organisms such as Pseudomonas species, which have a propensity to adhere to foreign material.174 Recommended empirical antibiotic therapy should consist of vancomycin to cover gram-positive organisms along with a cephalosporin that has antipseudomonal activity, such as cefepime or ceftazidime.175–177 Rifampin can also be added for gram-positive shunt infections that fail to clear with vancomycin monotherapy.174 Intrathecal administration of vancomycin or aminoglycosides may need to be initiated for shunt infections that are difficult to eradicate and fail to clear with systemic therapy.174 For the treatment of Propionibacterium acnes, small studies have documented success with intravenous penicillin combined with shunt externalization and replacement.178
An alternative treatment of gram-positive shunt infections is intravenous linezolid, which has been demonstrated to be successful in case reports.179,180 Linezolid possesses many attractive features that may increase its use, such as excellent CSF penetration and broad-spectrum activity. Additionally, Cruciani and coworkers demonstrated that intraventricular administration of teicoplanin was effective in seven patients with staphylococcal neurosurgical shunt infections.181
The introduction of antibiotic-impregnated shunt catheters may prove beneficial. A randomized controlled study performed by Eymann and associates demonstrated that antibiotic-impregnated shunt catheters reduce the infection rate significantly in both pediatric and adult populations.182
Infection with Spinal Instrumentation
Empirical antibiotic therapy should be directed against staphylococcal species along with gram-negative organisms. Initial treatment with vancomycin and a third-generation cephalosporin until culture and susceptibility results return is appropriate. In most cases, removal of hardware is not necessary and can potentially have devastating complications because of spine instability and lack of bony fusion. Retrospective reviews of clinical practice have suggested that titanium instrumentation does not interfere with the treatment of spinal infection to the extent that stainless steel or other foreign bodies do.183 Therefore, instrumentation should be left in place.184
The duration of antibiotic therapy for postoperative infection after spinal instrumentation is still debated. Lonstein proposes intravenous antibiotics for 10 to 14 days followed by oral antibiotics for 3 to 6 months.185 Perry and coauthors recommend antibiotic treatment until the fever and leukocytosis resolve or for a minimum of 10 days.186
Vertebral Osteomyelitis
Specific antibiotic treatment should be based on culture and susceptibility results. For methicillin-resistant S. aureus, combination therapy with rifampin and intravenous vancomycin is the preferred regimen,187,188 whereas linezolid can serve as an alternative. Previous studies have recommended that antibiotics be administered for a minimum of 6 to 8 weeks.189–191 Gasbarrini and colleagues recommend that a minimum of 6 weeks of intravenous antibiotics be administered followed by 6 weeks of oral antibiotics.192 For tuberculous vertebral osteomyelitis, the current standard of practice is to initiate isoniazid and rifampin for a 6- to 9-month period.193 Nussbaum and coworkers have recommended that the course of treatment be at least 12 months in duration and consist of at least two antituberculous drugs.194
Osteomyelitis of the Skull
Initial antibiotic therapy should be directed against staphylococci with vancomycin plus a third-generation cephalosporin to cover gram-negative bacilli. If there is concern about anaerobes, metronidazole should be initiated. Once culture and susceptibility results return, the antibiotic therapy can be modified. Intravenous antibiotic treatment should be continued for at least 4 weeks and possibly followed by an additional oral course of antibiotics.195
Diskitis
For antibiotic treatment of diskitis, Cushing recommends empirical treatment directed against methicillin-resistant S. aureus for 5 to 7 days, followed by oral therapy for 1 to 2 weeks.196 An appropriate empirical regimen may include a combination of vancomycin and a third-generation cephalosporin. Jansen and associates recommend intravenous antibiotics for 4 to 6 weeks followed by oral antibiotics until the symptoms resolve.197
Conclusion
Barker FG. Efficacy of prophylactic antibiotics against meningitis after craniotomy: a meta-analysis. Neurosurgery. 2007;60:887.
Barker FG. Efficacy of prophylactic antibiotic therapy in spinal surgery: a meta-analysis. Neurosurgery. 2002;51:391.
Barker FG. Efficacy of prophylactic antibiotics for craniotomy: a meta-analysis. Neurosurgery. 1994;35:484.
Chow KM, Hui AC, Szeto CC. Neurotoxicity induced by beta-lactam antibiotics: from bench to bedside. Eur J Clin Microbiol Infect Dis. 2005;24:649.
Eymann R, Chehab S, Strowitzki M, et al. Clinical and economic consequences of antibiotic-impregnated cerebrospinal fluid shunt catheters. J Neurosurg Pediatr. 2008;1:444.
Falagas ME, Bliziotis IA, Tam VH. Intraventricular or intrathecal use of polymyxins in patients with gram-negative meningitis: a systematic review of the available evidence. Int J Antimicrob Agents. 2007;29:9.
Haines SJ, Walters BC. Antibiotic prophylaxis for cerebrospinal fluid shunts: a metaanalysis. Neurosurgery. 1994;34:87.
Kearney BP, Aweeka FT. The penetration of anti-infectives into the central nervous system. Neurol Clin. 1999;17:883.
Lee MC, Wang MY, Fessler RG, et al. Instrumentation in patients with spinal infection. Neurosurg Focus. 2004;17:E7(6).
Mangram AJ, Horan TC, Pearson ML, et al. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97.
Nagarajan L, Lam GC. Tetracycline-induced benign intracranial hypertension. J Paediatr Child Health. 2000;36:82.
Nau R, Prange HW, Muth P, et al. Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob Agents Chemother. 1993;37:1518.
Nau R, Sorgel F, Prange HW. Pharmacokinetic optimisation of the treatment of bacterial central nervous system infections. Clin Pharmacokinet. 1998;35:223.
Neuwelt EA. Mechanisms of disease: the blood-brain barrier. Neurosurgery. 2004;54:131.
Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts: a systematic review. J Neurosurg Pediatr. 2008;1:48.
Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for preventing meningitis in patients with basilar skull fractures. Cochrane Database Syst Rev. 2006;1:CD004884.
Snavely SR, Hodges GR. The neurotoxicity of antibacterial agents. Ann Intern Med. 1984;101:92.
Thomas RJ. Neurotoxicity of antibacterial therapy. South Med J. 1994;87:869.
Wagner C, Sauermann R, Joukhadar C. Principles of antibiotic penetration into abscess fluid. Pharmacology. 2006;78:1.
Wen DY, Bottini AG, Hall WA, et al. Infections in neurologic surgery. The intraventricular use of antibiotics. Neurosurg Clin N Am. 1992;3:343.
1 Cushing HC. Concerning the results of operations for brain tumor. JAMA. 1915;64:189.
2 Stern L, Brodersen R. Kernicterus research and the basic sciences: a prospect for future development. Pediatrics. 1987;79:154.
3 Snavely SR, Hodges GR. The neurotoxicity of antibacterial agents. Ann Intern Med. 1984;101:92.
4 Lehr D. Clinical toxicity of sulfonamides. Ann N Y Acad Sci. 1957;69:417.
5 Lipsky BA, Baker CA. Fluoroquinolone toxicity profiles: a review focusing on newer agents. Clin Infect Dis. 1999;28:352.
6 Fish DN. Fluoroquinolone adverse effects and drug interactions. Pharmacotherapy. 2001;21:253S.
7 Janknegt R. Fluoroquinolones. Adverse reactions during clinical trials and postmarketing surveillance. Pharmaceutisch Weekblad—Scientific Edition. 1989;11:124.
8 Ball P. Adverse reactions and interactions of fluoroquinolones. Clin Invest Med. 1989;12:28.
9 Kushner JM, Peckman HJ, Snyder CR. Seizures associated with fluoroquinolones. Ann Pharmacother. 2001;35:1194.
10 Murray CK, Wortmann GW. Trovafloxacin-induced weakness due to a demyelinating polyneuropathy. South Med J. 2000;93:514.
11 Moore B, Safani M, Keesey J. Possible exacerbation of myasthenia gravis by ciprofloxacin. Lancet. 1988;1:882.
12 Mumford CJ, Ginsberg L. Ciprofloxacin and myasthenia gravis. BMJ. 1990;301:818.
13 Roquer J, Cano A, Seoane JL, et al. Myasthenia gravis and ciprofloxacin. Acta Neurol Scand. 1996;94:419.
14 Rauser EH, Ariano RE, Anderson BA. Exacerbation of myasthenia gravis by norfloxacin. DICP. 1990;24:207.
15 Azevedo E, Ribeiro JA, Polonia J, et al. Probable exacerbation of myasthenia gravis by ofloxacin. J Neurol. 1993;240:508.
16 Hedenmalm K, Spigset O. Peripheral sensory disturbances related to treatment with fluoroquinolones. J Antimicrob Chemother. 1996;37:831.
17 Riyaz A, Aboobacker CM, Sreelatha PR. Nalidixic acid induced pseudotumour cerebri in children. J Indian Med Assoc. 1998;96:308.
18 Thomas RJ. Neurotoxicity of antibacterial therapy. South Med J. 1994;87:869.
19 Silber TJ, D’Angelo L. Psychosis and seizures following the injection of penicillin G procaine. Hoigne’s syndrome. Am J Dis Child. 1985;139:335.
20 Araszkiewicz A, Rybakowski JK. Hoigne’s syndrome, kindling, and panic disorder. Depress Anxiety. 1996;4:139.
21 Howard JFJr. Adverse drug effects on neuromuscular transmission. Semin Neurol. 1990;10:89.
22 al-Zahawi MF, Sprott MS, Hendrick DJ. Hallucinations in association with ceftazidime. BMJ. 1988;297:858.
23 Douglas MA, Quandt CM, Stanley DA. Ceftazidime-induced encephalopathy in a patient with renal impairment. Arch Neurol. 1988;45:936.
24 Jackson GD, Berkovic SF. Ceftazidime encephalopathy: absence status and toxic hallucinations. J Neurol Neurosurg Psychiatry. 1992;55:333.
25 Creel GB, Hurtt M. Cephalosporin-induced recurrent aseptic meningitis. Ann Neurol. 1995;37:815.
26 Calandra GB, Brown KR, Grad LC, et al. Review of adverse experiences and tolerability in the first 2,516 patients treated with imipenem/cilastatin. Am J Med. 1985;78:73.
27 Calandra G, Lydick E, Carrigan J, et al. Factors predisposing to seizures in seriously ill infected patients receiving antibiotics: experience with imipenem/cilastatin. Am J Med. 1988;84:911.
28 Semel JD, Allen N. Seizures in patients simultaneously receiving theophylline and imipenem or ciprofloxacin or metronidazole. South Med J. 1991;84:465.
29 Alvan G, Nord CE. Adverse effects of monobactams and carbapenems. Drug Saf. 1995;12:305.
30 Balfour JA, Bryson HM, Brogden RN. Imipenem/cilastatin: an update of its antibacterial activity, pharmacokinetics and therapeutic efficacy in the treatment of serious infections. Drugs. 1996;51:99.
31 Schmutzhard E, Williams KJ, Vukmirovits G, et al. A randomised comparison of meropenem with cefotaxime or ceftriaxone for the treatment of bacterial meningitis in adults. Meropenem Meningitis Study Group. J Antimicrob Chemother. 1995;36:85.
32 Klugman KP, Dagan R. Randomized comparison of meropenem with cefotaxime for treatment of bacterial meningitis. Meropenem Meningitis Study Group. Antimicrob Agents Chemother. 1995;39:1140.
33 Patel JB, Giles RE. Meropenem: evidence of lack of proconvulsive tendency in mice. J Antimicrob Chemother. 1989;24:307.
34 Linden P. Safety profile of meropenem: an updated review of over 6,000 patients treated with meropenem. Drug Saf. 2007;30:657.
35 Barclay ML, Begg EJ. Aminoglycoside toxicity and relation to dose regimen. Adverse Drug React Toxicol Rev. 1994;13:207.
36 Huizing EH, de Groot JC. Human cochlear pathology in aminoglycoside ototoxicity—a review. Acta Otolaryngol Suppl. 1987;436:117.
37 Matz GJ. Aminoglycoside cochlear ototoxicity. Otolaryngol Clin North Am. 1993;26:705.
38 Black FO, Pesznecker SC. Vestibular ototoxicity. Clinical considerations. Otolaryngol Clin North Am. 1993;26:713.
39 Canizares FJ, Baeyens JM, Gonzalez MR, et al. Ototoxicity caused by gentamicin in the otolytic membrane of the saccule. Adv Otorhinolaryngol. 1990;45:94.
40 Harada Y, Sugimoto Y. Metabolic disorder of otoconia after streptomycin intoxication. Acta Otolaryngol. 1977;84:65.
41 Sanders DB, Kim YI, Howard JFJr, et al. Intercostal muscle biopsy studies in myasthenia gravis: clinical correlations and the direct effects of drugs and myasthenic serum. Ann N Y Acad Sci. 1981;377:544.
42 Welge-Luessen A, Wolfensberger M. Reversible anosmia after amikacin therapy. Arch Otolaryngol Head Neck Surg. 2003;129:1331.
43 Henkin RI. Drug-induced taste and smell disorders. Incidence, mechanisms and management related primarily to treatment of sensory receptor dysfunction. Drug Saf. 1994;11:318.
44 Haase KK, Lapointe M, Haines SJ. Aseptic meningitis after intraventricular administration of gentamicin. Pharmacotherapy. 2001;21:103.
45 Everett ED, Strausbaugh LJ. Antimicrobial agents and the central nervous system. Neurosurgery. 1980;6:691.
46 Clark KL, Freiman DG, Hamburger M, et al. The toxicity of intracisternally injected polymyxin B in the rhesus monkey. Antibiot Annu. 1955;3:428.
47 Michalopoulos A, Falagas ME. Colistin and polymyxin B in critical care. Crit Care Clin. 2008;24:377.
48 Koch-Weser J, Sidel VW, Federman EB, et al. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann Intern Med. 1970;72:857.
49 Wolinsky E, Hines JD. Neurotoxic and nephrotoxic effects of colistin in patients with renal disease. N Engl J Med. 1962;266:759.
50 Lindesmith LA, Baines RDJr, Bigelow DB, et al. Reversible respiratory paralysis associated with polymyxin therapy. Ann Intern Med. 1968;68:318.
51 Bosso JA, Liptak CA, Seilheimer DK, et al. Toxicity of colistin in cystic fibrosis patients. DICP. 1991;25:1168.
52 Falagas ME, Bliziotis IA, Tam VH. Intraventricular or intrathecal use of polymyxins in patients with gram-negative meningitis: a systematic review of the available evidence. Int J Antimicrob Agents. 2007;29:9.
53 Farber BF, Moellering RCJr. Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother. 1983;23:138.
54 Traber PG, Levine DP. Vancomycin ototoxicity in patient with normal renal function. Ann Intern Med. 1981;95:458.
55 Fekety R. Vancomycin. Med Clin North Am. 1982;66:175.
56 Mellor JA, Kingdom J, Cafferkey M, et al. Vancomycin toxicity: a prospective study. J Antimicrob Chemother. 1985;15:773.
57 Duffull SB, Begg EJ. Vancomycin toxicity. What is the evidence for dose dependency? Adverse Drug React Toxicol Rev. 1994;13:103.
58 Bailie GR, Neal D. Vancomycin ototoxicity and nephrotoxicity. A review. Med Toxicol Adverse Drug Experience. 1988;3:376.
59 Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol. 1996;134:693.
60 Lander CM. Minocycline-induced benign intracranial hypertension. Clin Exp Neurol. 1989;26:161.
61 Monaco F, Agnetti V, Mutani R. Benign intracranial hypertension after minocycline therapy. Eur Neurol. 1978;17:48.
62 Nagarajan L, Lam GC. Tetracycline-induced benign intracranial hypertension. J Paediatr Child Health. 2000;36:82.
63 Fields JP. Bulging fontanel: a complication of tetracycline therapy in infants. J Pediatr. 1961;58:74.
64 Williams DN, Laughlin LW, Lee YH. Minocycline: possible vestibular side-effects. Lancet. 1974;2:744.
65 Jacobson JA, Daniel B. Vestibular reactions associated with minocycline. Antimicrob Agents Chemother. 1975;8:453.
66 Fanning WL, Gump DW, Sofferman RA. Side effects of minocycline: a double-blind study. Antimicrob Agents Chemother. 1977;11:712.
67 Singh YN, Marshall IG, Harvey AL. Pre- and postjunctional blocking effects of aminoglycoside, polymyxin, tetracycline and lincosamide antibiotics. Br J Anaesth. 1982;54:1295.
68 Ramilo O, Kinane BT, McCracken GHJr. Chloramphenicol neurotoxicity. Pediatr Infect Dis J. 1988;7:358.
69 Godel V, Nemet P, Lazar M. Chloramphenicol optic neuropathy. Arch Ophthalmol. 1980;98:1417.
70 Harley RD, Huang NN, Macri CH, et al. Optic neuritis and optic atrophy following chloramphenicol in cystic fibrosis patients. Trans Am Acad Ophthalmol Otolaryngol. 1970;74:1011.
71 Cocke JGJr, Brown RE, Geppert LJ. Optic neuritis with prolonged use of chloramphenicol. Case report and relationship to fundus changes in cystic fibrosis. J Pediatr. 1966;68:27.
72 Levine PH, Regelson W, Holland JF. Chloramphenicol-associated encephalopathy. Clin Pharmacol Ther. 1970;11:194.
73 Holt D, Harvey D, Hurley R. Chloramphenicol toxicity. Adverse Drug React Toxicol Rev. 1993;12:83.
74 Periti P, Mazzei T, Mini E, et al. Adverse effects of macrolide antibacterials. Drug Saf. 1993;9:346.
75 Cohen IJ, Weitz R. Psychiatric complications with erythromycin. Drug Intell Clin Pharm. 1981;15:388.
76 Black RJ, Dawson TA. Erythromycin and nightmares. BMJ. 1988;296:1070.
77 Umstead GS, Neumann KH. Erythromycin ototoxicity and acute psychotic reaction in cancer patients with hepatic dysfunction. Arch Intern Med. 1986;146:897.
78 Eichenwald HF. Adverse reactions to erythromycin. Pediatr Infect Dis. 1986;5:147.
79 Absher JR, Bale JFJr. Aggravation of myasthenia gravis by erythromycin. J Pediatr. 1991;119:155.
80 May EF, Calvert PC. Aggravation of myasthenia gravis by erythromycin. Ann Neurol. 1990;28:577.
81 Ferry T, Ponceau B, Simon M, et al. Possibly linezolid-induced peripheral and central neurotoxicity: report of four cases. Infection. 2005;33:151.
82 Bressler AM, Zimmer SM, Gilmore JL, et al. Peripheral neuropathy associated with prolonged use of linezolid. Lancet Infect Dis. 2004;4:528.
83 Rho JP, Sia IG, Crum BA, et al. Linezolid-associated peripheral neuropathy. Mayo Clin Proc. 2004;79:927.
84 Saijo T, Hayashi K, Yamada H, et al. Linezolid-induced optic neuropathy. Am J Ophthalmol. 2005;139:1114.
85 Rucker JC, Hamilton SR, Bardenstein D, et al. Linezolid-associated toxic optic neuropathy. Neurology. 2006;66:595.
86 Narita M, Tsuji BT, Yu VL. Linezolid-associated peripheral and optic neuropathy, lactic acidosis, and serotonin syndrome. Pharmacotherapy. 2007;27:1189.
87 Radner DB. Toxicologic and pharmacologic aspects of rifampin. Chest. 1973;64:213.
88 Grosset J, Leventis S. Adverse effects of rifampin. Rev Infect Dis. 1983;5:S440.
89 Stout JE, Engemann JJ, Cheng AC, et al. Safety of 2 months of rifampin and pyrazinamide for treatment of latent tuberculosis. Am J Respir Crit Care Med. 2003;167:824.
90 Neuwelt EA. Mechanisms of disease: the blood-brain barrier. Neurosurgery. 2004;54:131.
91 Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6:591.
92 Nau R, Sorgel F, Prange HW. Lipophilicity at pH 7.4 and molecular size govern the entry of the free serum fraction of drugs into the cerebrospinal fluid in humans with uninflamed meninges. J Neurol Sci. 1994;122:61.
93 Spector R. Ceftriaxone pharmacokinetics in the central nervous system. J Pharmacol Exp Ther. 1986;236:380.
94 Dacey RG, Sande MA. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974;6:437.
95 Quagliarello VJ, Long WJ, Scheld WM. Morphologic alterations of the blood-brain barrier with experimental meningitis in the rat. Temporal sequence and role of encapsulation. J Clin Invest. 1986;77:1084.
96 Friedland IR, Klugman KP. Cerebrospinal fluid bactericidal activity against cephalosporin-resistant Streptococcus pneumoniae in children with meningitis treated with high-dosage cefotaxime. Antimicrob Agents Chemother. 1997;41:1888.
97 Doit C, Barre J, Cohen R, et al. Bactericidal activity against intermediately cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with bacterial meningitis treated with high doses of cefotaxime and vancomycin. Antimicrob Agents Chemother. 1997;41:2050.
98 Quagliarello VJ, Scheld WM. Treatment of bacterial meningitis. N Engl J Med. 1997;336:708.
99 Quagliarello V, Scheld WM. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327:864.
100 Girgis NI, Farid Z, Mikhail IA, et al. Dexamethasone treatment for bacterial meningitis in children and adults. Pediatr Infect Dis J. 1989;8:848.
101 Rahal JJ, Simberkoff MS. Host defense and antimicrobial therapy in adult gram-negative bacillary meningitis. Ann Intern Med. 1982;96:468.
102 Simberkoff MS, Moldover NH, Rahal J. Absence of detectable bactericidal and opsonic activities in normal and infected human cerebrospinal fluids. A regional host defense deficiency. J Lab Clin Med. 1980;95:362.
103 Quintiliani RSr, Quintiliani RJr. Pharmacokinetics/pharmacodynamics for critical care clinicians. Crit Care Clin. 2008;24:335.
104 Wen DY, Bottini AG, Hall WA, et al. Infections in neurologic surgery. The intraventricular use of antibiotics. Neurosurg Clin N Am. 1992;3:343.
105 Wagner C, Sauermann R, Joukhadar C. Principles of antibiotic penetration into abscess fluid. Pharmacology. 2006;78:1.
106 Kearney BP, Aweeka FT. The penetration of anti-infectives into the central nervous system. Neurol Clin. 1999;17:883.
107 Nau R, Sorgel F, Prange HW. Pharmacokinetic optimisation of the treatment of bacterial central nervous system infections. Clin Pharmacokinet. 1998;35:223.
108 Lutsar I, Friedland IR. Pharmacokinetics and pharmacodynamics of cephalosporins in cerebrospinal fluid. Clin Pharmacokinet. 2000;39:335.
109 Sjolin J, Eriksson N, Arneborn P, et al. Penetration of cefotaxime and desacetylcefotaxime into brain abscesses in humans. Antimicrob Agents Chemother. 1991;35:2606.
110 Wood JH, Doppman JL, Lightfoote WE, et al. Role of vascular proliferation on angiographic appearance and encapsulation of experimental traumatic and metastatic brain abscesses. J Neurosurg. 1978;48:264.
111 Yamamoto M, Jimbo M, Ide M, et al. Penetration of intravenous antibiotics into brain abscesses. Neurosurgery. 1993;33:44.
112 Nau R, Prange HW, Muth P, et al. Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob Agents Chemother. 1993;37:1518.
113 Nau R, Prange HW, Kinzig M, et al. Cerebrospinal fluid ceftazidime kinetics in patients with external ventriculostomies. Antimicrob Agents Chemother. 1996;40:763.
114 Golledge CL, McKenzie T. Monitoring vancomycin concentrations in CSF after intraventricular administration. J Antimicrob Chemother. 1988;21:262.
115 Welch K. Residual shunt infection in a program aimed at its prevention. Z Kinderchir Grenzgeb. 1979;28:374.
116 Mangi RJ, Holstein LL, Andriole VT. Treatment of gram-negative bacillary meningitis with intrathecal gentamicin. Yale J Biol Med. 1977;50:31.
117 Chow KM, Hui AC, Szeto CC. Neurotoxicity induced by beta-lactam antibiotics: from bench to bedside. Eur J Clin Microbiol Infect Dis. 2005;24:649.
118 De Sarro A, De Sarro GB, Ascioti C, et al. Epileptogenic activity of some beta-lactam derivatives: structure-activity relationship. Neuropharmacology. 1989;28:359.
119 Mangram AJ, Horan TC, Pearson ML, et al. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97.
120 Narotam PK, van Dellen JR, du Trevou MD, et al. Operative sepsis in neurosurgery: a method of classifying surgical cases. Neurosurgery. 1994;34:409.
121 Drake JM. The surgical management of pediatric hydrocephalus. Neurosurgery. 2008;62(Suppl 2):633.
122 Barker FG. Efficacy of prophylactic antibiotics for craniotomy: a meta-analysis. Neurosurgery. 1994;35:484.
123 Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts. Cochrane Database Syst Rev. 2006;3:CD005365.
124 Haines SJ, Walters BC. Antibiotic prophylaxis for cerebrospinal fluid shunts: a metanalysis. Neurosurgery. 1994;34:87.
125 Barker FG. Efficacy of prophylactic antibiotics against meningitis after craniotomy: a meta-analysis. Neurosurgery. 2007;60:887.
126 Barker FG. Efficacy of prophylactic antibiotic therapy in spinal surgery: a meta-analysis. Neurosurgery. 2002;51:391.
127 Brown SM, Anand VK, Tabaee A, et al. Role of perioperative antibiotics in endoscopic skull base surgery. Laryngoscope. 2007;117:1528.
128 Kraus DH, Gonen M, Mener D, et al. A standardized regimen of antibiotics prevents infectious complications in skull base surgery. Laryngoscope. 2005;115:1347.
129 Hammond CJ, Gill J, Peto TE, et al. Investigation of prevalence of MRSA in referrals to neurosurgery: implications for antibiotic prophylaxis. Br J Neurosurg. 2002;16:550.
130 Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts: a systematic review. J Neurosurg Pediatr. 2008;1:48.
131 Blomstedt GC. Results of trimethoprim-sulfamethoxazole prophylaxis in ventriculostomy and shunting procedures. A double-blind randomized trial. J Neurosurg. 1985;62:694.
132 Poon WS, Ng S, Wai S. CSF antibiotic prophylaxis for neurosurgical patients with ventriculostomy: a randomised study. Acta Neurochir Suppl. 1998;71:146.
133 Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for preventing meningitis in patients with basilar skull fractures. Cochrane Database Syst Rev. 2006;1:CD004884.
134 Haines SJ. Topical antibiotic prophylaxis in neurosurgery. Neurosurgery. 1982;11:250.
135 Maurice-Williams RS, Pollock J. Topical antibiotics in neurosurgery: a re-evaluation of the Malis technique. Br J Neurosurg. 1999;13:312.
136 Pennybacker JB, Taylor M, Cairns H. Penicillin in the prevention of infections during operations on the brain and spinal cord. Lancet. 1947;4:160.
137 Wright RL. A survey of possible etiologic agents in postoperative craniotomy infections. J Neurosurg. 1966;25:125.
138 Malis LI. Prevention of neurosurgical infection by intraoperative antibiotics. Neurosurgery. 1979;5:339.
139 Haines SJ, Goodman ML. Antibiotic prophylaxis of postoperative neurosurgical wound infection. J Neurosurg. 1982;56:103.
140 Yamamoto M, Jimbo M, Ide M, et al. Perioperative antimicrobial prophylaxis in neurosurgery: clinical trial of systemic flomoxef administration and saline containing gentamicin for irrigation. Neurol Med Chir (Tokyo). 1996;36:370.
141 Grondahl TO, Langmoen IA. Epileptogenic effect of antibiotic drugs. J Neurosurg. 1993;78:938.
142 Reid KH, Shields CB, Raff MJ, et al. Effect of the topical application of some newer antibiotics on the cerebral cortex. Neurosurgery. 1987;20:868.
143 Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373.
144 Elliott D, Kufera JA, Myers RA. The microbiology of necrotizing soft tissue infections. Am J Surg. 2000;179:361.
145 Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267.
146 Cherubin CE, Eng RH. Experience with the use of cefotaxime in the treatment of bacterial meningitis. Am J Med. 1986;80:398.
147 Ralph ED, Behme RJ. Enterobacter meningitis—treatment complicated by emergence of mutants resistant to cefotaxime. Scand J Infect Dis. 1987;19:577.
148 Wolff MA, Young CL, Ramphal R. Antibiotic therapy for Enterobacter meningitis: a retrospective review of 13 episodes and review of the literature. Clin Infect Dis. 1993;16:772.
149 Lu CH, Chang WN, Chuang YC. Resistance to third-generation cephalosporins in adult gram-negative bacillary meningitis. Infection. 1999;27:208.
150 Livermore DM. β-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557.
151 Nguyen MH, Harris SP, Muder RR, et al. Antibiotic-resistant Acinetobacter meningitis in neurosurgical patients. Neurosurgery. 1994;35:851.
152 Siegman-Igra Y, Bar-Yosef S, Gorea A, et al. Nosocomial Acinetobacter meningitis secondary to invasive procedures: report of 25 cases and review. Clin Infect Dis. 1993;17:843.
153 O’Neill E, Humphreys H, Phillips J, et al. Third-generation cephalosporin resistance among gram-negative bacilli causing meningitis in neurosurgical patients: significant challenges in ensuring effective antibiotic therapy. J Antimicrob Chemother. 2006;57:356.
154 Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585.
155 Parodi S, Lechner A, Osih R, et al. Nosocomial Enterobacter meningitis: risk factors, management, and treatment outcomes. Clin Infect Dis. 2003;37:159.
156 De Gheldre Y, Maes N, Rost F, et al. Molecular epidemiology of an outbreak of multidrug-resistant Enterobacter aerogenes infections and in vivo emergence of imipenem resistance. J Clin Microbiol. 1997;35:152.
157 Rodriguez Guardado A, Blanco A, Asensi V, et al. Multidrug-resistant Acinetobacter meningitis in neurosurgical patients with intraventricular catheters: assessment of different treatments. J Antimicrob Chemother. 2008;61:908.
158 Briggs S, Ellis-Pegler R, Raymond N, et al. Gram-negative bacillary meningitis after cranial surgery or trauma in adults. Scand J Infect Dis. 2004;36:165.
159 Luby JP. Infections of the central nervous system. Am J Med Sci. 1992;304:379.
160 Landesman SH, Cherubin CE, Corrado ML. Gram-negative bacillary meningitis. New therapy and changing concepts. Arch Intern Med. 1982;142:939.
161 The management of neurosurgical patients with postoperative bacterial or aseptic meningitis or external ventricular drain-associated ventriculitis. Infection in Neurosurgery Working Party of the British Society for Antimicrobial Chemotherapy. Br J Neurosurg. 2000;14:7.
162 Dill SR, Cobbs CG, McDonald CK. Subdural empyema: analysis of 32 cases and review. Clin Infect Dis. 1995;20:372.
163 Bok AP, Peter JC. Subdural empyema: burr holes or craniotomy? A retrospective computerized tomography–era analysis of treatment in 90 cases. J Neurosurg. 1993;78:574.
164 Nathoo N, Nadvi SS, van Dellen JR, et al. Intracranial subdural empyemas in the era of computed tomography: a review of 699 cases. Neurosurgery. 1999;44:529.
165 Calfee DP, Wispelwey B. Brain abscess. Semin Neurol. 2000;20:353.
166 Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763.
167 Bamberger DM. Outcome of medical treatment of bacterial abscesses without therapeutic drainage: review of cases reported in the literature. Clin Infect Dis. 1996;23:592.
168 Luer MS, Hatton J. Vancomycin administration into the cerebrospinal fluid: a review. Ann Pharmacother. 1993;27:912.
169 Pfausler B, Spiss H, Beer R, et al. Treatment of staphylococcal ventriculitis associated with external cerebrospinal fluid drains: a prospective randomized trial of intravenous compared with intraventricular vancomycin therapy. J Neurosurg. 2003;98:1040.
170 Levin AS, Barone AA, Penco J, et al. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28:1008.
171 Ho YH, Wang LS, Chao HJ, et al. Successful treatment of meningitis caused by multidrug-resistant Acinetobacter baumannii with intravenous and intrathecal colistin. J Microbiol Immunol Infect. 2007;40:537.
172 Al Shirawi N, Memish ZA, Cherfan A, et al. Post-neurosurgical meningitis due to multidrug-resistant Acinetobacter baumannii treated with intrathecal colistin: case report and review of the literature. J Chemother. 2006;18:554.
173 Vasen W, Desmery P, Ilutovich S, et al. Intrathecal use of colistin. J Clin Microbiol. 2000;38:3523.
174 Shah SS, Smith MJ, Zaoutis TE. Device-related infections in children. Pediatr Clin North Am. 2005;52:1189.
175 Chapman PH, Borges LF. Shunt infections: prevention and treatment. Clin Neurosurg. 1985;32:652.
176 Swanberg L, Tuazon CU. Rifampin in the treatment of serious staphylococcal infections. Am J Med Sci. 1984;287:49.
177 Ziai WC, Lewin JJ3rd. Update in the diagnosis and management of central nervous system infections. Neurol Clin. 2008;26:427.
178 Collignon PJ, Munro R, Sorrell TC. Propionibacterium acnes infection in neurosurgical patients. Experience with high-dose penicillin therapy. Med J Aust. 1986;145:408.
179 Gill CJ, Murphy MA, Hamer DH. Treatment of Staphylococcus epidermidis ventriculo-peritoneal shunt infection with linezolid. J Infect. 2002;45:129.
180 Cook AM, Ramsey CN, Martin CA, et al. Linezolid for the treatment of a heteroresistant Staphylococcus aureus shunt infection. Pediatr Neurosurg. 2005;41:102.
181 Cruciani M, Navarra A, Di Perri G, et al. Evaluation of intraventricular teicoplanin for the treatment of neurosurgical shunt infections. Clin Infect Dis. 1992;15:285.
182 Eymann R, Chehab S, Strowitzki M, et al. Clinical and economic consequences of antibiotic-impregnated cerebrospinal fluid shunt catheters. J Neurosurg Pediatr. 2008;1:444.
183 Lee MC, Wang MY, Fessler RG, et al. Instrumentation in patients with spinal infection. Neurosurg Focus. 2004;17:E7(6).
184 Levi AD, Dickman CA, Sonntag VK. Management of postoperative infections after spinal instrumentation. J Neurosurg. 1997;86:975.
185 Lonstein JE. Diagnosis and treatment of postoperative spinal infections. Surg Rounds Orthop. 1989;3:25.
186 Perry JW, Montgomerie JZ, Swank S, et al. Wound infections following spinal fusion with posterior segmental spinal instrumentation. Clin Infect Dis. 1997;24:558.
187 Henry NK, Rouse MS, Whitesell AL, et al. Treatment of methicillin-resistant Staphylococcus aureus experimental osteomyelitis with ciprofloxacin or vancomycin alone or in combination with rifampin. Am J Med. 1987;82:73.
188 Dworkin R, Modin G, Kunz S, et al. Comparative efficacies of ciprofloxacin, pefloxacin, and vancomycin in combination with rifampin in a rat model of methicillin-resistant Staphylococcus aureus chronic osteomyelitis. Antimicrob Agents Chemother. 1990;34:1014.
189 Muhle I, Rau J, Ruskin J. Vertebral osteomyelitis due to Actinobacillus actinomycetemcomitans. JAMA. 1979;241:1824.
190 Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med. 1970;282:198.
191 Wedge JH, Oryschak AF, Robertson DE, et al. Atypical manifestations of spinal infections. Clin Orthop Relat Res. 1977;123:155.
192 Gasbarrini AL, Bertoldi E, Mazzetti M, et al. Clinical features, diagnostic and therapeutic approaches to haematogenous vertebral osteomyelitis. Eur Rev Med Pharmacol Sci. 2005;9:53.
193 Schlesinger N, Lardizabal A, Rao J, et al. Tuberculosis of the spine: experience in an inner city hospital. J Clin Rheumatol. 2005;11:17.
194 Nussbaum ES, Rockswold GL, Bergman TA, et al. Spinal tuberculosis: a diagnostic and management challenge. J Neurosurg. 1995;83:243.
195 Bullitt E, Lehman RA. Osteomyelitis of the skull. Surg Neurol. 1979;11:163.
196 Cushing AH. Diskitis in children. Clin Infect Dis. 1993;17:1.
197 Jansen BR, Hart W, Schreuder O. Discitis in childhood. 12-35-year follow-up of 35 patients. Acta Orthop Scand. 1993;64:33.