The Ureter
Embryology
The ureter develops from a branch of the mesonephric duct called the ureteric bud. It arises during the fourth to fifth week of pregnancy and grows dorsally and upward until it contacts the nephrogenic cord. This contact of the ureteric bud with the metanephric blastema induces normal kidney development. The ureterovesical junction (UVJ) develops as the distal parts of the mesonephric duct are incorporated into the enlarging bladder originating from the urogenital sinus. As the mesonephric ducts are absorbed, the ureters come to open separately into the urinary bladder, with the orifice moving superolaterally and the distal ureteral segments entering obliquely through the base of the bladder. During this complex course of development, numerous variations concerning the position of the kidney, the course of the ureter, and the anatomy of the UVJ, as well as the ureteropelvic junction (UPJ), may arise.1–11
Imaging: On ultrasonography, the ureter can only be visualized when it is sufficiently filled with urine. The UPJ usually can be sonographically visualized in well-hydrated patients with a tapering of the pelvis toward the proximal ureter, using the kidney as an acoustic window from a dorsal or lateral approach. For visualizing the distal ureter, a sufficiently distended urinary bladder is mandatory, because it serves as a window to the retrovesical space. With sufficient bladder filling, the normal ureter can be detected and followed superiorly for several centimeters. Further visualization up to the iliac vessels or higher is only possible in cases with a significantly dilated ureter and sufficient sonographic access.1
The patency of the ureteral orifice can be sonographically assessed by observing the urine inflow jet into the urinary bladder. This ureteral jet sometimes can be seen on gray scale as a rush of pseudoechoes from the ureteral ostium into the bladder lumen, but it is more easily seen with color Doppler ultrasonography. Color Doppler imaging allows not only visualization of ureteral patency and osteal position but also evaluation of the frequency of the inflow jet, indicating ureteric peristaltic activity. Thus asymmetric jets, atypical direction of the ureteral jet, lateralized orifice position, and unusual ostial shape and impaired ureteral peristalsis may be used as indirect signs for ureteral pathology or dysfunction such as obstruction or vesicoureteral reflux (VUR).1,12–14
Modern multislice computed tomography (CT) is a powerful imaging tool that provides high spatial and anatomic resolution, but it may impose a significant radiation burden even using low-dose pediatric protocols. Thus in pediatric uroradiology, CT should be restricted to uncommon and complicated cases. Contrast-enhanced CT allows for excellent delineation of the entire ureter. By using three-dimensional reformatting and viewing techniques, an intravenous urography–like comprehensive overview over the entire ureter can be achieved. This technique usually is applied in rare or difficult cases, such as for assessment of a retrocaval ureter, ureteral tumors, and paraureteral pathology compressing or displacing the ureter.1,15–17
Magnetic resonance urography (MRU) is a powerful new tool for imaging the ureter and urinary tract. By applying heavily T2-weighted sequences, a sufficiently distended or dilated ureter can be visualized in its entire course without administration of a contrast agent. However, diuretic stimulation before the investigation is helpful and even mandatory in many cases. Diuretic contrast-enhanced MRU additionally allows for functional assessment by using fast T1-weighted sequences (usually three-dimensional gradient-echo or turbo-flash). The spatial resolution of MRU is less than on CT, and thus small folds or tiny stones can be overlooked or only inferred from indirect signs. In the lower parts of the ureter, where motion from breathing is less likely, high-resolution magnetic resonance (MR) sequences can improve spatial resolution for depiction of small structures, such as an unusual course or insertion site of an ectopic ureter.1,18–22
The current best method for assessment of ureteral function and drainage is dynamic nuclear scintigraphy. Serial image acquisitions are obtained after intravenous injection of technetium-99m–labeled mercaptoacetyltriglycine and diuretic stimulation. Ureteral drainage can be visualized and quantified in a standardized fashion. However, the normal ureter usually is difficult to assess by this method and, despite its excellent functional information, anatomic resolution is poor.1,23–25
Obstruction of the Ureter
Overview: Obstruction may be severe, threatening renal function and urine drainage, or it may be partial and minor without any clinical consequence. Most ureteral obstructions occur at the UPJ or UVJ. Obstructive lesions between these two points are uncommon and include retrocaval ureter, retroiliac ureter, ureteral obstruction caused by other vessels, ureteral valves, acquired ureteral strictures, ureteral urolithiasis, ureteral neoplasms, and extrinsic lesions affecting the ureter.1
Ureteral Valves and Striations
Overview: Ureteral valves are said to consist of a cusplike fold or an iris diaphragm composed of ureteral mucosa and smooth muscle fibers (Fig. 119-1). They are more commonly found in the lower third of the ureter. In neonates and infants, ureteral valves may be difficult to differentiate from physiologic ureteral folds, which are immature remnants that disappear during the first years of life. Ureteral striations are longitudinal mucosal folds that may be seen in normal ureters but often are a sign of inflammatory disease, VUR, or previous obstruction. They must be distinguished from submucosal hemorrhage and collateral circulation resulting from renal vein or inferior vena cava thrombosis.1,26–32
Vascular Obstruction
Overview: A retrocaval or circumcaval ureter (Fig. 119-2) is an uncommon anomaly in which the right ureter passes behind the inferior vena cava, emerges between the cava and the aorta, and then curves around and in front of the cava to return to its normal position in the pelvis. This anomaly results from abnormal persistence of the subcardinal vein in the definitive inferior vena cava. The ureter descends from the renal pelvis and crosses behind the inferior vena cava near its bifurcation. It then curves medially and upward, forming a reversed-J appearance (Fig. 119-3). Ureteral obstruction at this level is common. The retrocaval ureter is more common in males than in females and usually manifests in adult life, perhaps because the hydronephrosis is slow to develop. The anomaly may be demonstrated with contrast-enhanced multidetector CT, but MRU (with or without contrast) provides a less invasive and nonionizing imaging option.1,33–35 Ureteral obstructions caused by accessory renal arteries, iliac vessels, ovarian arteries, and hypogastric arteries (e-Fig. 119-4) are rare. Normal vascular impressions occasionally are seen on imaging (e-Fig. 119-5).36–37
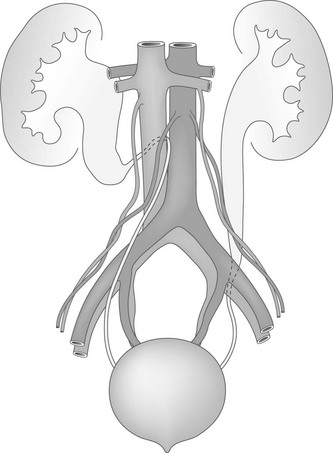
Figure 119-2 Abnormal ureteral course.
An anatomic diagram shows a right retrocaval ureter and a left retroiliac ureter.
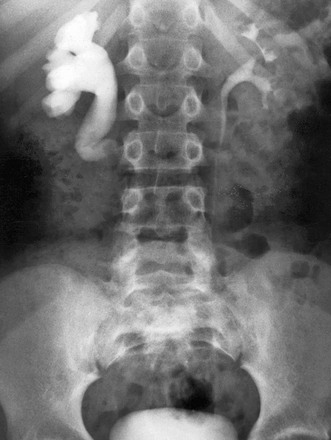
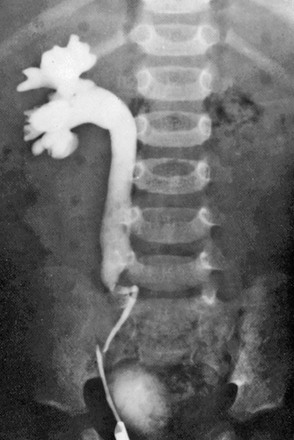
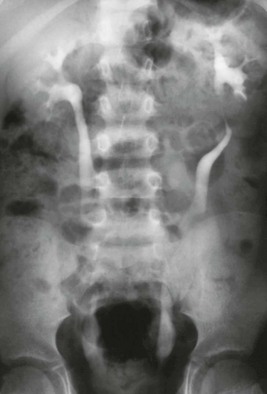
Figure 119-3 Retrocaval ureter.
Intravenous urography image shows right hydronephrosis. The dilated right ureter passes behind the inferior vena cava in front of the L3 pedicle.
Ureteral Ectopia: Intravesical Versus Extravesical
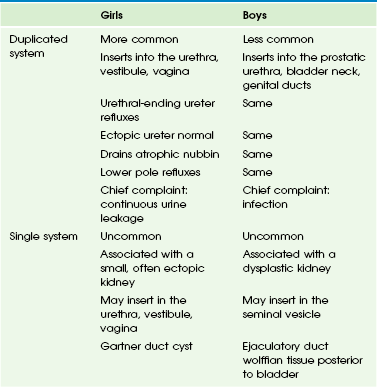
Overview: An ectopic ureter is one that drains in an abnormal location (outside the posterolateral angle of the trigone) either within the bladder or extravesically. Extravesical ureteral ectopia is more common and clinically more important than the intravesical type. It also is more common in girls than in boys, with some anatomic and functional differences between the two sexes (Table 119-1). It is more common in ureteral duplication anomalies (up to 80%).1,11,38–41
Intravesical Ureteral Ectopia: Two types of intravesical ureteral ectopia are recognized: lateral and caudal. In lateral ectopia, the more common of the two, one or both ureters (the lower pole ureter in a duplicated system) drain into the bladder more superior and laterally than normal. The intramural submucosal tunnel of the affected ureter tends to be short or otherwise defective, leading to VUR in many cases. In the second type, one or both ureters (the upper pole ureter in a duplicated system) drain inferior and medial to the usual site along a line extending from the normal lateral corner of the trigone to the bladder neck. These ureters are less prone to VUR than the lateral type.1,38,41
Extravesical Ureteral Ectopia: Extravesical ureteral ectopia in girls (Fig. 119-6) is associated with a duplicated system in at least 85% of cases and affects the upper pole ureter in practically all cases. The ectopic ureter may end in the urethra or in the vestibule or, less commonly, in the vagina (Fig. 119-7). A common presenting complaint is continuous leakage of urine in the context of an otherwise normal voiding pattern. Leakage of urine is observed even if the anomalous ureter ends in the proximal urethra, as a result of the relative weakness of the external urethral sphincter in girls. The renal parenchyma drained by the ectopic ureter often is dysplastic with decreased or absent function. The ipsilateral lower pole ureter may be normal or dilated and frequently is the site of reflux.1,42–46
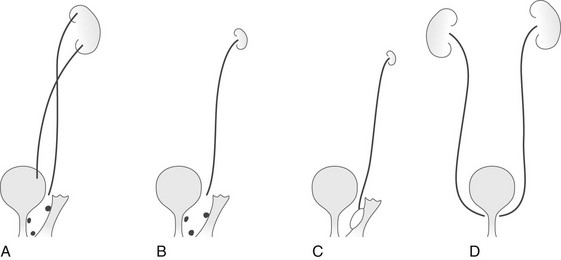
Figure 119-6 Types of ureteral ectopia in females.
A, In the most common variant, ureteral duplication is present, with the upper pole ureter draining into the urethra, into the perineum near the urethral meatus, or into the vagina. B, Ectopic ureteral drainage as in A, but from a single collecting system. The kidney may be small and dysplastic. C, Ectopic drainage from a single system into a Gartner duct cyst in the wall of the vagina. The ipsilateral kidney may not be identifiable. D, Bilateral single-system ectopic drainage into the bladder neck or proximal urethra. This uncommon form is found almost exclusively in females and usually is associated with a wide bladder neck, a defective internal sphincter, sometimes a malformed urethra, and urinary incontinence.
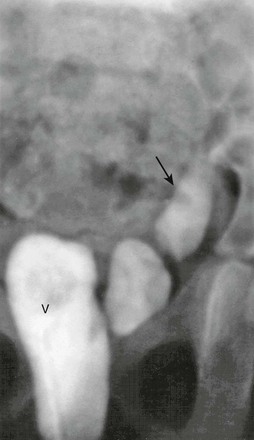
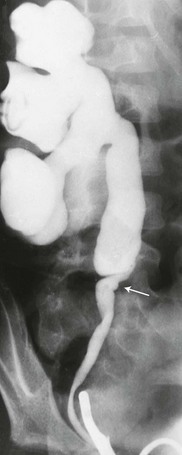
Figure 119-7 Ureteral ectopia.
Contrast medium injected into the vagina (V) is seen filling the distal left ureter (arrow).
Single (unduplicated) ureteral ectopia in girls is uncommon and usually unilateral. The corresponding kidney frequently is small and dysplastic and may be ectopic. Sometimes the ectopic ureter is connected with a multicystic dysplastic kidney or ends blindly superiorly without renal tissue (renal agenesis). The anomalous ureter may terminate in the urethra, vestibule, or vagina. Occasionally, a single ectopic ureter ends in a blind cystic structure in the lateral wall of the vagina (a Gartner duct cyst) (Fig. 119-8). Developmental anomalies of the vagina, uterus, and ipsilateral ovary may be encountered.1,42–46
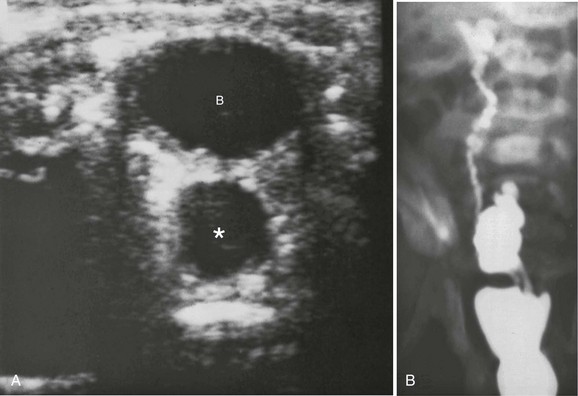
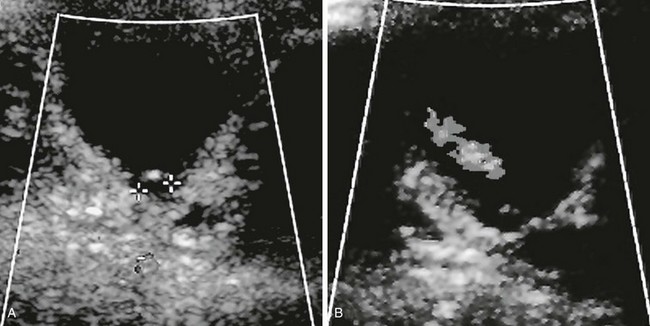
Figure 119-8 Ureteral ectopia into Gartner duct cyst.
A, A transverse sonogram shows a cystic structure (asterisk) posterior to the bladder (B). B, A voiding cystourethrogram image shows filling of the bladder, through the Gartner duct cyst, and through the ureter to the upper pole.
Extravesical ureteral ectopia in boys occurs much less commonly than in girls. The anomaly commonly involves the upper pole ureter in a duplex system but also may occur in a single, unduplicated ureter. The anomalous ureter may insert in the prostatic urethra, sometimes near the bladder neck, and much less often in the genital ducts (seminal vesicle, vas deferens, or ejaculatory duct) (Fig. 119-9). Ureteral ectopia to the posterior urethra usually ends slightly above or at the level of the verumontanum (Fig. 119-10). Ureteral ectopia to the genital ducts ends in a markedly dilated, cystic mass behind the trigone of the bladder. The renal parenchyma drained by the ectopic ureter is commonly small and dysplastic and often nonfunctioning. Urinary incontinence rarely is a problem in boys because the ectopic ureter drains above the strongly developed external urethral sphincter. Cryptorchidism or testicular hypoplasia on the side of the lesion is common.1,11,38,47–49
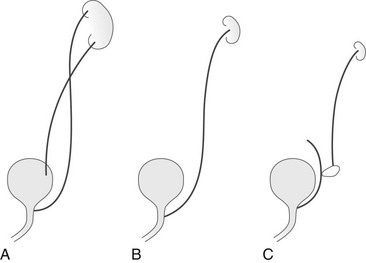
Figure 119-9 Types of ureteral ectopia in males.
A, In the most common variant, ureteral duplication is present with the upper pole ureter draining into the posterior urethra. B, Ectopic ureteral drainage as in A but from a single collecting system. The kidney may be small and dysplastic. C, Ectopic drainage from a single system into a seminal vesicle, vas deferens, or ejaculatory duct. The ipsilateral kidney may not be identifiable.
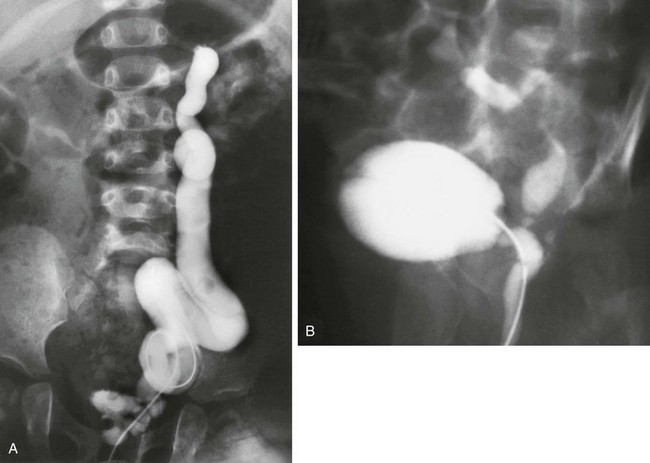
Figure 119-10 Ureteral ectopia into the urethra.
A, A voiding cystourethrogram image shows that the catheter has passed from the urethra directly into the ureter, which drains an atrophic upper pole. B, A voiding cystourethrogram image during voiding shows vesicoureteral reflux into the ureter, which drains into the posterior urethra.
Imaging: Ultrasound is the initial imaging method of choice and often is diagnostic, particularly when the anomalous ureter is dilated and sufficient renal parenchyma or even cysts of the corresponding renal moiety are present. The course of this ureter often may be followed down to and beyond the bladder. A vaginal ectopic ureter may be shown by ultrasound to be connected with a urine-filled vagina (e-Fig. 119-11). A nonfunctioning system is best visualized by MRU (Fig. 119-12). When the aberrant ureter empties in the urethra, VUR into the ectopic ureter occasionally is demonstrated by voiding cystourethrogram (VCUG) (Fig. 119-13 and e-Fig. 119-14). A vaginogram or genitogram may show reflux in the affected ureter if it terminates in the vagina (see Fig. 119-7). Note that many of these anomalies are accompanied by ipsilateral malformations of the genitalia. A thorough investigation of the vagina, the uterus, and the ovaries by ultrasound or magnetic resonance imaging (MRI) is recommended.1,11,18,20–22,38,39,50–52
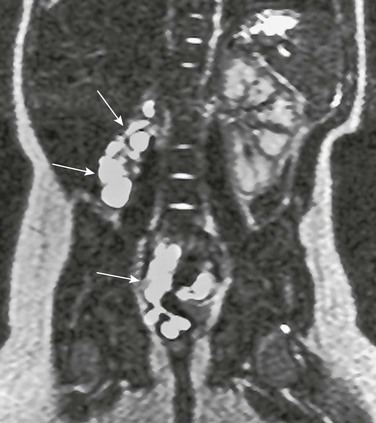
Figure 119-12 Ectopic ureteral insertion.
Magnetic resonance urography demonstrating a cystic remnant of an ectopic renal bud (top arrows) with only minimal residual function and a dilated and tortuous dysplastic ureter (bottom arrow) ectopically inserting into the vagina.
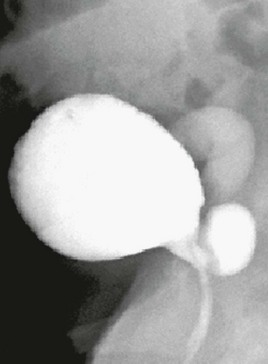
Figure 119-13 A voiding cystourethrogram image series in a girl with a refluxing ectopic ureterocele inserting into the urethra and filling of a diverticula-shaped ureterocele connected to the posterior urethra during voiding (see e-Fig. 119-14).
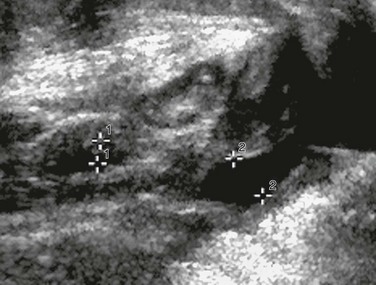
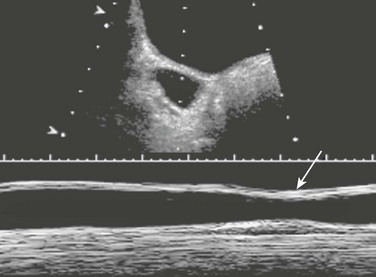
e-Figure 119-11 Ectopic ureteral insertion.
A longitudinal sonogram demonstrates a urine-filled vagina (cursors labeled “2”) with a nearly empty bladder (cursors labeled “1”) in a girl with ectopic vaginal ureteral insertion.
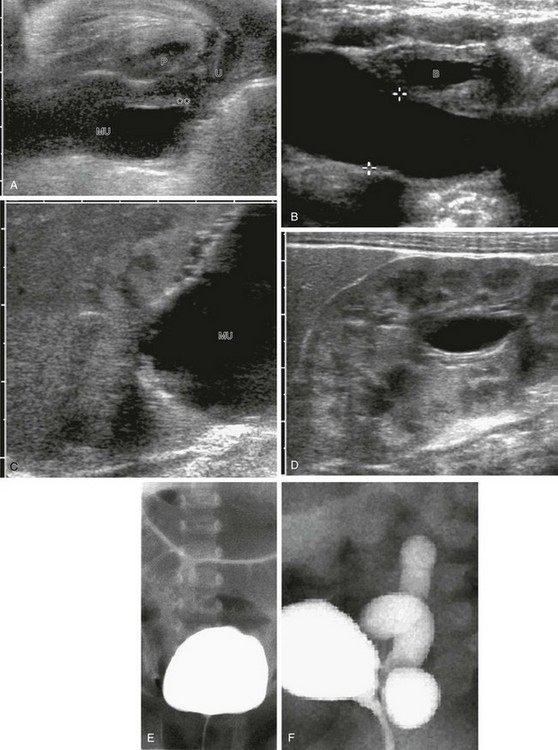
e-Figure 119-14 Ultrasound and voiding cystourethrogram (VCUG) in two patients with an ectopic ureter inserting into the urethra.
A, Perineal ultrasound of the urethra and the distal megaureter (MU) inserting ectopically (asterisks). U, Urethra; P, pubis. B, Megaureter (cursors) behind the urinary bladder (B). C, A longitudinal sonogram shows the upper dysplastic moiety of the kidney draining into the megaureter (MU). D, A longitudinal sonogram shows some dilatation of the lower moiety of the duplex kidney as a result of compression of the ureteropelvic junction by the upper pole megaureter. E and F, A VCUG image series in a girl with a refluxing ectopic ureterocele inserting into the urethra: no vesicoureteral reflux during filling (E) and with consecutive slow vesicoureteral reflux into the fluid-filled corresponding megaureter (F).
Ureterocele
Overview: A ureterocele is a cystlike expansion of the terminal segment of the ureter projecting into the lumen of the urinary bladder. The anomaly is relatively common and has two subtypes: (1) intravesical ureterocele, which is located entirely within the bladder, and (2) ectopic ureterocele, which usually is large and extends to the bladder neck area or proximal urethra. Both forms may be associated with a duplicated or an unduplicated system. More than 90% of the cases are discovered in children younger than 3 years of age, and most cases are diagnosed prenatally or in the newborn period. Urinary tract infection (UTI) is the most common presenting clinical manifestation. Failure to thrive, difficulty voiding, urinary retention, flank pain, and sometimes chronic renal failure are prominent manifestations of the anomaly in older infants and in children.1,53,54
The intravesical ureterocele (also called stenotic, orthotopic, simple, or adult-type ureterocele) is more common in adults than in children, suggesting that it may be acquired in many cases. In children it is more frequently associated with significant hydroureteronephrosis. The ureterocele is located at the orthotopic ureteric ostium position (i.e., at the lateral angle of the trigone) and is entirely within the bladder (Fig. 119-15). The ureteral orifice of the ureterocele is variably stenotic. Intravesical ureteroceles may be bilateral and, although commonly seen in single (unduplicated) ureters, may involve the upper and sometimes the lower pole ureter of a duplicated system.1,53,54
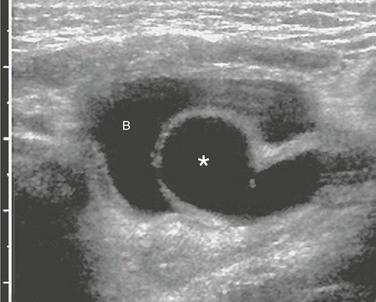
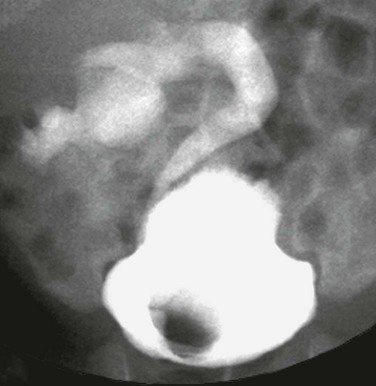
Figure 119-15 A ureterocele.
A typical sonogram of a large ureterocele (asterisk) protruding into the bladder (B) lumen.
The ectopic or infantile ureterocele is more common in children and is five to seven times more frequent in females than males. It is unilateral in 90% of cases and bilateral in 10% of cases. Ectopic ureteroceles most often occur in a duplicated system and almost always are connected with the upper pole ureter. The ureter connected with the ureterocele enters the bladder wall at the normal site, descends toward the bladder neck submucosally, passes through the internal urethral sphincter, and terminates ectopically in the proximal urethra. In contrast to a simple ureterocele, in which the “cyst” is formed by herniation of the distal end of the ureter, the ectopic ureterocele represents a dilatation and protrusion of the entire submucosal segment of the ureter into the lumen of the bladder and at the bladder neck. The ureterocele may obstruct the bladder neck or the opposite ureteral orifice, and in patients with ureteral duplication, it may deform the musculature or position and course of the adjacent ureter so that VUR into the lower pole ureter occurs (in 40% to 50% of cases). Occasionally the ureterocele herniates into the urethra, causing urethral obstruction.1,11,53–55
Imaging: Ultrasound imaging demonstrates an intravesical cystic lesion of variable size attached to the posterolateral wall of the bladder and protruding into the lumen of the bladder (“cyst within cyst”), which often is associated with a cystic structure in the upper pole moiety of the ipsilateral kidney (in duplicated systems) (Fig. 119-16 and e-Fig. 119-17). The dilated ureter connecting with the ureterocele may be demonstrated, as well as the corresponding renal unit. In duplex systems, both ureters on the side of the ureterocele may be dilated.1,10,11,55
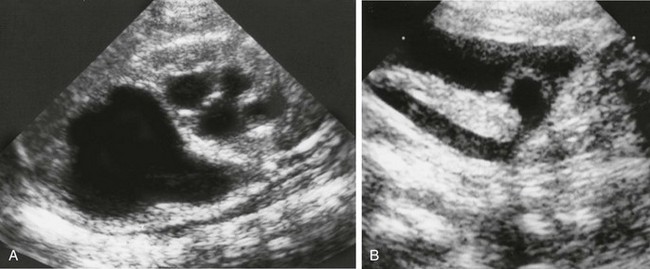
Figure 119-16 A ureterocele.
A, A longitudinal sonogram of the right kidney shows marked upper pole hydronephrosis and parenchymal thinning along with mild lower pole hydronephrosis. B, A longitudinal sonogram of the right pelvis shows a dilated right ureter ending in a ureterocele that protrudes into the bladder.
A radiolucent filling defect within the bladder is demonstrated on VCUG and is best imaged during the early filling. As bladder capacity is reached and luminal pressure rises, the ureterocele may be compressed and flattened against the bladder wall (Fig. 119-18 and e-Fig. 119-19) and can even evert (e-Fig. 119-20), simulating a paraureteral diverticulum. During voiding the VCUG may show prolapse of the ureterocele into the urethra (Fig. 119-21), where it may produce bladder outlet obstruction. VUR into an ureterocele is rare initially, but common after endoscopic incision (e-Fig. 119-22).
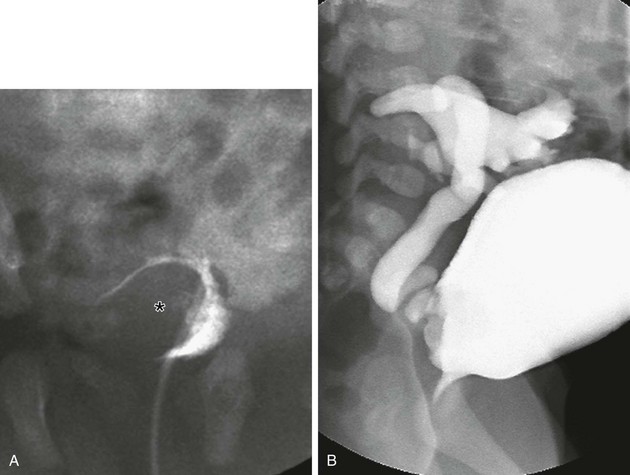
Figure 119-18 Ureterocele and vesicoureteral reflux.
A voiding cystourethrogram series demonstrating the defect caused by the ureterocele (asterisk) during early bladder filling (A) and effacement of the ureterocele during late filling (B).
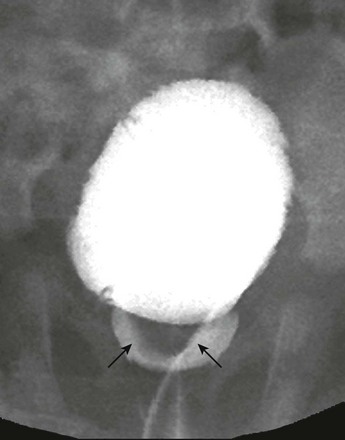
Figure 119-21 A ureterocele.
A voiding cystourethrogram demonstrates inferior herniation of a ureterocele (arrows) into the bladder neck.
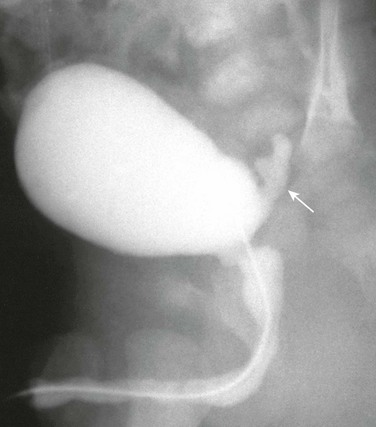
e-Figure 119-20 An everting ureterocele.
A voiding cystourethrogram image during voiding shows a diverticulum-like structure (arrow) from the posterior bladder.
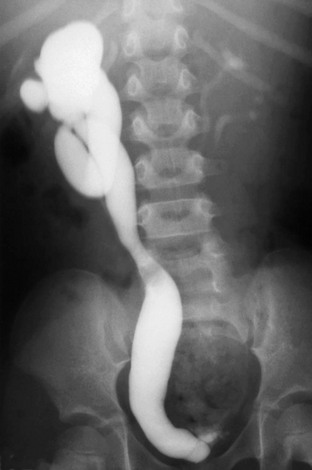
e-Figure 119-22 A ureterocele and vesicoureteral reflux.
A voiding cystourethrogram image shows reflux into the right-sided ureterocele and a distorted right upper pole collecting system. Grade II vesicoureteral reflux is present on the left as well.
Renal scintigraphy reveals decreased function at the superior pole of the kidney and often a photopenic area in the bladder corresponding to the ureterocele. Intravenous urography has been replaced by methods such as dynamic MRU as the modality of choice for preoperative imaging because it provides all the functional information along with superior anatomic detail and can reveal associated renal abnormalities and genital malformations (Fig. 119-23 and e-Fig. 119-24) (Box 119-1).1,10,11,18–25
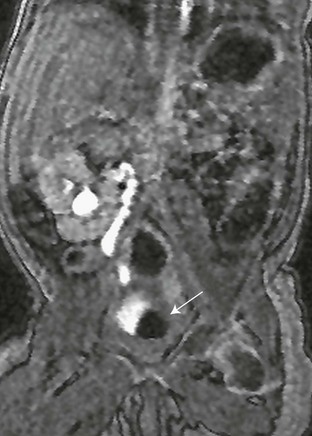
Figure 119-23 A typical magnetic resonance urography finding in ureteroceles.
A contrast-enhanced T1-weighted gradient-recalled echo sequence delineating the non–contrast filled ureterocele in the urinary bladder (arrow).
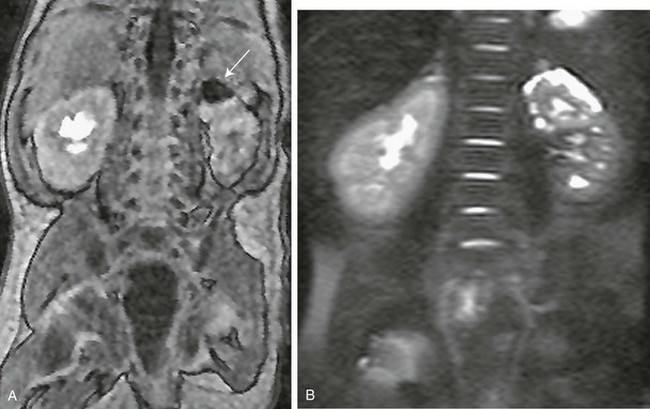
e-Figure 119-24 Additional typical magnetic resonance urography findings in ureteroceles.
A, A contrast-enhanced T1-weighted gradient-recalled echo sequence; note the nonenhanced collecting system of the upper moiety of the left kidney (arrow) that drains into the ureterocele. B, A T2-weighted, nonenhanced image demonstrates the cystic/dysplastic nature of the left upper pole parenchyma.
Acquired Ureteral Obstruction
Overview: Acquired strictures of the body of the ureter are uncommon. They may be due to a local surgical procedure, instrumentation, ureteral wall inflammation, or periureteral infection; they may be a complication of Henoch-Schönlein purpura, periarteritis nodosa, tuberculosis, or granulomatous disease of childhood (e.g., Crohn disease); they may be the result of trauma or submucosal hemorrhage in patients on anticoagulant medication; or they may follow local radiation. Other acquired intrinsic ureteral obstructions include sludge balls, blood clots, various calculi, and fungus balls. Note that acute obstructions may exhibit only minor collecting system dilatation, but patients will have severe pain and renal colic.
Imaging usually starts with ultrasound, with assessment of the distal ureter and the ureteral inflow jet through a sufficiently distended urinary bladder. The upper ureter and pelvocalyceal system then are evaluated before looking at the renal parenchyma and renal blood flow and Doppler changes. Ultrasound usually is supplemented with an abdominal radiograph. Although it is rarely used in Europe, CT (with or without contrast medium enhancement) often is used in North America. Advanced imaging such as MRU is rarely necessary.1,58–64
Primary Ureteral Neoplasms
Overview: Primary neoplasms of the renal pelvis and ureters, such as rhabdomyosarcoma or urothelial cell carcinoma, are extremely rare in children. Secondary involvement of the pelvocalyceal system by Wilms tumor, as well as Wilms tumor implants in the ureter, may occur. The ureter may be infiltrated or secondarily involved by other retroperitoneal tumors of infancy and childhood, such as neuroblastoma, rhabdomyosarcoma, peripheral neuroectodermal tumor, or malignant teratoma. Instances of benign and usually pedunculated fibrous polyps in the upper third of the ureter (occasionally bilateral) have been reported (Fig. 119-25 and e-Fig. 119-26). They may cause hematuria, or rarely obstruction, and have no malignant potential. Fibrous polyps are more common in adults. Ultrasound is only useful to detect large tumors with a significant extraureteral component or to visualize indirect signs caused by the obstruction. Otherwise, imaging relies on CT, MRI, or even ureterography.1,65–71
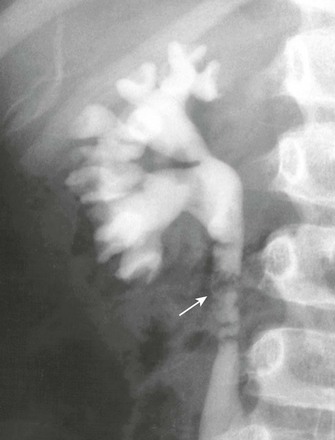
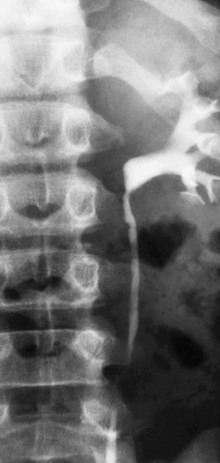
Figure 119-25 Ureteral obstruction from ureteral polyps.
An intravenous urography image shows mild hydronephrosis and proximal ureteral filling defects (arrow) surgically proven to be polyps.
Primary Megaureter
Overview: Two forms of megaureter are described: (1) a megaureter resulting from an organic lesion (Box 119-2) and (2) a primary megaureter, which is mostly functional in origin. A primary megaureter is an uncommon, nonhereditary lesion that is probably congenital in origin. It is caused by an adynamic distal ureteral segment, usually 0.5 to 4 cm in length, which prevents normal caudal propagation of ureteral peristalsis. The disorder is discovered in patients of any age and is more common in males than in females. Three fourths of cases are unilateral, with the left ureter being involved more often than the right ureter. Bilateral cases are more common in children younger than 1 year. The affected ureter is variously dilated but tapers down rather abruptly to a curved, short distal segment that appears normal in caliber or slightly narrowed on imaging. The dilatation often is limited to (or is most marked in) the lower half of the ureter. The renal parenchyma usually is of normal thickness, but in more severe cases it is variously thinned or even dysplastic and atrophic. The symptoms of a primary megaureter include recurrent UTI, abdominal pain (and distention if the megaureter is severe and bilateral), and hematuria. Ureteral stone formation has been reported. However, a primary megaureter often is discovered as an incidental finding in asymptomatic patients.
At gross pathology, the distal segment of the ureter appears normal, without evidence of intrinsic narrowing upon probing with ureteral catheters. Histologically, however, the ureter shows normal ganglion cells (which may be reduced in number), hypoplasia and atrophy of muscle fibers, and an increase in collagen tissue. The remaining muscle fibers are predominantly circular, and in severe cases little or no muscle tissue is present. The proximal dilated segment of the ureter shows muscular hypertrophy that results from hyperperistalsis. Cytoscopically the ureteral orifices are normal.1,72,73
Imaging: The findings on imaging reflect the pathologic changes in the ureter and kidney (Fig. 119-27 and e-Fig. 119-28). As seen fluoroscopically on ureterography, by ultrasound, and by dynamic MRU, the ureter shows normal or hyperactive peristalsis with waves starting in the proximal ureter and increasing in amplitude and fading distally into the dilated portion of the ureter. Antiperistaltic waves may be observed. A VCUG shows no organic or functional abnormalities of the bladder or urethra. VUR is not a typical feature of the disorder but does occur in some children. Renal agenesis and other contralateral abnormalities have been described, and ipsilateral megacalycosis and hydronephrosis may occur.1,74–77
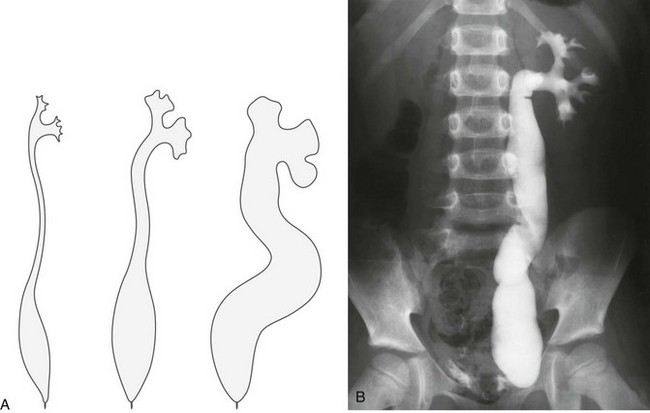
Figure 119-27 Megaureter.
A, A diagram of the ureter and collecting system in primary megaureters of varying severity. B, A postvoid voiding cystourethrogram image shows left vesicoureteral reflux and ureterovesical junction obstruction, suggesting a primary megaureter. The ureteral dilatation is most pronounced in the lower ureter; the renal pelvis and calyces are little affected.
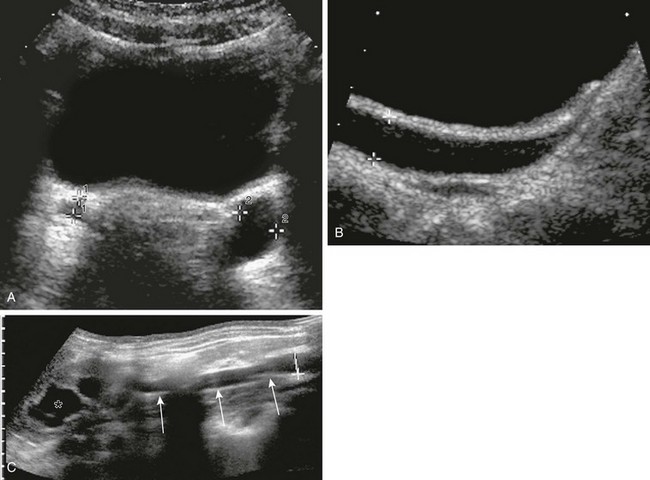
e-Figure 119-28 Megaureter.
A, A transverse sonogram through the bladder shows the typical appearance of a left megaureter (cursors labeled “2”). Note the visible but nondilated right ureter (cursor labeled “1”). B, A parasagittal oblique sonogram shows the dilated left (mega) ureter (cursors), in the same patient as in A, with some mild ureteral wall thickening; note the visible but nondilated right ureter in the transverse section. C, Using extended field of view ultrasound techniques, a panoramic overview of a dilated ureter (arrows) may become possible; the asterisk indicates the dilated pelvocalyceal system.
Hypotonia, Hypomotility, and Dyskinesia
Overview and Imaging: Hypotonia, hypomotility, and dyskinesia are disturbances of the ureter that primarily affect motility and function. An adynamic or dysplastic ureteral segment may cause functional impairment and cause segmental ureteral dilatation, with urine drainage relying on gravity. Furthermore, peristalsis and loss of muscular tone may be seen during and shortly after UTI, as well as after instrumentation, operations, or with in situ foreign bodies such as nephroureteral stents, causing reactive hypotonia with an adynamic or hypoperistaltic ureter. Finally, transient hypomotility of the ureter may be observed in postoperative settings and as a result of medications that affect the smooth muscles. Ureteral peristalsis may be assessed and documented using ultrasound cine-loop clips (e-Fig. 119-29); ureteral drainage and function is evaluated by mercaptoacetyltriglycine scintigraphy or dynamic MRU.1,23–25,78,79
Miscellaneous Conditions
Overview: Preoperative imaging needs to reveal all relevant information the surgeon requires for either planning or performing the operation. Because a comprehensive overview and display may be crucial, ultrasound alone often is considered insufficient, particularly because of its limited potential to visualize the entire ureter. Therefore VCUG (in refluxing units) and MRU usually are integrated in a standard preoperative imaging workup; more invasive procedures such as ureterography may be performed at the beginning of or during surgery in the course of the same anesthesia.1
Postoperative imaging is heavily based on ultrasound. However, ultrasound is poor in assessment and grading of urinary drainage; therefore a functional scintigraphic study, as well as MRU (or CT), sometimes may prove helpful. If postoperative complications occur (e.g., ureteral obstruction after antireflux procedures, ureteral obstruction due to blood clots, manifestation of UPJ obstruction after ureteral surgery, ureteral fibrosis, stenosis, and compression), imaging not only may detect them but also may offer treatment options. These image-guided interventions and treatment strategies consist of ureteral recanalization, balloon dilatation of fibrous stenoses and ureteral stenting for keeping a compressed or recanalized ureter patent, and percutaneous nephrostomy.1,80–82
Cerwinka, WH, Damien Grattan-Smith, J, Kirsch, AJ. Magnetic resonance urography in pediatric urology. J Pediatr Urol. 2008;4(1):74–82. quiz 82-83
Ehammer, T, Riccabona, M, Maier, E. High resolution MR for evaluation of lower urogenital tract malformations in infants and children: Feasibility and preliminary experiences. Eur J Radiol. 2011;78(3):388–393.
Gordon, I, Riccabona, M. Investigating the newborn kidney—update on imaging techniques. Semin Neonatol. 2003;8:269–278.
Jones, RA, Perez-Brayfield, MR, Kirsch, AJ, et al. Renal transit time with MR urography in children. Radiology. 2004;233(1):41–50.
Kim, S, Jacob, JS, Kim, DC, et al. Time-resolved dynamic contrast-enhanced MR urography for the evaluation of ureteral peristalsis: initial experience. J Magn Reson Imaging. 2008;28(5):1293–1298.
Riccabona, M, Fotter, R. Radiographic studies in children with kidney disorders: what to do and when. In: Hogg R, ed. Kidney disorders in children and adolescents. Birmingham: Taylor & Francis, 2006.
Riccabona, M, Fritz, G, Ring, E. Potential applications of three-dimensional ultrasound in the pediatric urinary tract: pictorial demonstration based on preliminary results. Eur Radiol. 2003;13:2680–2687.
Riccabona, M, Lindbichler, F, Sinzig, M. Conventional imaging in paediatric uroradiology. Eur J Radiol. 2002;43:100.
Riccabona, M, Simbrunner, J, Ring, E, et al. Feasibility of MR-urography in neonates and infants with anomalies of the upper urinary tract. Eur Radiol. 2002;12:1442.
Riccabona, M, Sorantin, E, Hausegger, K. Imaging guided interventional procedures in paediatric uroradiology—a case-based overview. Eur J Radiol. 2002;43:167.
Riccabona, M, Uggowitzer, M, Klein, E, et al. Contrast enhanced color Doppler sonography in children and adolescents. J Ultrasound Med. 2000;19:783.
Roy Choudhury, S, Chadha, R, Bagga, D, et al. Spectrum of ectopic ureters in children. Pediatr Surg Int. 2008;24(7):819–823.
References
1. Riccabona, M. The ureter and vesicoureteral reflux. In Slovis T, ed.: Caffey’s pediatric diagnostic imaging, 11th ed, Philadelphia: Mosby, 2008.
2. Shapiro, E. Clinical implications of genitourinary embryology. Curr Opin Urol. 2009;19(4):427–433.
3. Thomas, JC, DeMarco, RT, Pope, JC, 4th. Molecular biology of ureteral bud and trigonal development. Curr Urol Rep. 2005;6(2):146–151.
4. Mackie, GG. Abnormalities of the ureteral bud. Urol Clin North Am. 1978;5(1):161–174.
5. Weiss, JP. Embryogenesis of ureteral anomalies: a unifying theory. Aust N Z J Surg. 1988;58(8):631–638.
6. Viana, R, Batourina, E, Huang, H, et al. The development of the bladder trigone, the center of the anti-reflux mechanism. Development. 2007;134(20):3763–3769.
7. Mendelsohn, C. Using mouse models to understand normal and abnormal urogenital tract development. Organogenesis. 2009;5(1):306–314.
8. Marshall, FF. Embryology of the lower genitourinary tract. Urol Clin North Am. 1978;5(1):3–15.
9. Vaughan, ED, Jr., Middleton, GW. Pertinent genitourinary embryology: review for practicing urologist. Urology. 1975;6(2):139–149.
10. Herman, TE, McAlister, WH. Radiographic manifestations of congenital anomalies of the lower urinary tract. Radiol Clin North Am. 1991;29(2):365–382.
11. Berrocal, T, López-Pereira, P, Arjonilla, A, et al. Anomalies of the distal ureter, bladder, and urethra in children: embryologic, radiologic, and pathologic features. Radiographics. 2002;22(5):1139–1164.
12. de Bessa, J, Jr., Dénes, FT, Chammas, MC, et al. Diagnostic accuracy of color Doppler sonographic study of the ureteric jets in evaluation of hydronephrosis. J Pediatr Urol. 2008;4(2):113–117.
13. Riccabona, M. Potential of modern sonographic techniques in paediatric uroradiology. Eur J Radiol. 2002;43:110–121.
14. Strehlau, J, Winkler, P, de la Roche, J. The ureterovesical jet as a functional diagnostic tool in childhood hydronephrosis. Pediatr Nephrol. 1997;11:460.
15. Lin, WC, Wang, JH, Wei, CJ, et al. Assessment of CT urography in the diagnosis of urinary tract abnormalities. J Chin Med Assoc. 2004;67(2):73–78.
16. Maudgil, DD, McHugh, K. The role of CT in modern pediatric uroradiology. Eur J Radiol. 2002;43:129.
17. Korogi, Y, Takahashi, M, Fujimura, N, et al. Computed tomography demonstration of renal dysplasia with a vaginal ectopic ureter. J Comput Tomogr. 1986;10:273.
18. Cerwinka, WH, Damien Grattan-Smith, J, Kirsch, AJ. Magnetic resonance urography in pediatric urology. J Pediatr Urol. 2008;4(1):74–82. [quiz 82-83].
19. Avni, F, Bali, MA, Regnault, M, et al. MR urography in children. Eur J Radiol. 2002;43:154.
20. Avni, FE, Nicaise, N, Hall, M, et al. The role of MR imaging for the assessment of complicated duplex kidneys in children: preliminary report. Pediatr Radiol. 2001;31:215.
21. Kibar, Y, Avci, A, Akay, O, et al. Dribbling of urine due to ectopic vaginal insertion of an upper pole ureter diagnosed by magnetic resonance urography. Int Urol Nephrol. 2005;37(4):695–697.
22. Gylys-Morin, VM, Minevich, E, Tackett, LD, et al. Magnetic resonance imaging of the dysplastic renal moiety and ectopic ureter. J Urol. 2000;164:2034.
23. Kass, EJ, Fink-Bennett, D. Contemporary techniques for the radioisotopic evaluation of the dilated urinary tract. Urol Clin North Am. 1990;17:273.
24. Piepsz, A. Radionuclide studies in paediatric nephro-urology. Eur J Radiol. 2002;43(2):146–153.
25. Piepsz, A, Ham, HR. Pediatric applications of renal nuclear medicine. Semin Nucl Med. 2006;36(1):16–35.
26. Kannaiyan, L, Karl, S, Mathai, J, et al. Congenital ureteric stenosis: a study of 17 children. Pediatr Surg Int. 2009;25(6):513–517.
27. Rabinowitz, R, Kingston, TE, Wesselhoeft, C, et al. Ureteral valves in children. Urology. 1998;51(suppl 5A):7–11.
28. Maizels, M, Stephens, FD. Valves of the ureter as a cause of primary obstruction of the ureter: anatomic, embryologic and clinical aspects. J Urol. 1980;123(5):742–747.
29. Reinberg, Y, Alaibadi, H, Johnson, P, et al. Congenital ureteral valves in children: case report and review of the literature. J Pediatr Surg. 1987;22:379.
30. Docimo, SG, Lebowitz, RL, Retik, AB, et al. Congenital midureteral obstruction. Urol Radiol. 1989;11:156.
31. Brugnara, M, Cecchetto, M, Manfredi, R, et al. Prenatal diagnosis of a rare form of congenital mid-ureteral stricture: a case report and literature revisited. BMC Urol. 2007;7:8.
32. Hwang, AH, McAleer, IM, Shapiro, E, et al. Congenital mid ureteral strictures. J Urol. 2005;174(5):1999–2002.
33. Li, HZ, Ma, X, Qi, L, et al. Retroperitoneal laparoscopic ureteroureterostomy for retrocaval ureter: report of 10 cases and literature review. Urology. 2010;76(4):873–876.
34. Acharya, SK, Jindal, B, Yadav, DK, et al. Retrocaval ureter: a rare cause of hydronephrosis in children. J Pediatr Surg. 2009;44(4):846–848.
35. Lautin, EM, Haramati, N, Frager, D, et al. CT diagnosis of circumcaval ureter. AJR Am J Roentgenol. 1988;150:591.
36. Calder, AD, Hiorns, MP, Abhyankar, A, et al. Contrast-enhanced magnetic resonance angiography for the detection of crossing renal vessels in children with symptomatic ureteropelvic junction obstruction: comparison with operative findings. Pediatr Radiol. 2007;37(4):356–361.
37. Rigas, A, Karamanolakis, D, Bogdanos, I, et al. Pelvi-ureteric junction obstruction by crossing renal vessels: clinical and imaging features. BJU Int. 2003;92(1):101–103.
38. Roy Choudhury, S, Chadha, R, Bagga, D, et al. Spectrum of ectopic ureters in children. Pediatr Surg Int. 2008;24(7):819–823.
39. Churchill, BM, Abara, EO, McLorie, GA. Ureteral duplication, ectopy and ureteroceles. Pediatr Clin North Am. 1987;34:1273.
40. Bisset, GS, Strife, JL. The duplex collecting system in girls with urinary tract infection: prevalence and significance. AJR Am J Roentgenol. 1987;148:497.
41. Amis, ES, Cronan, JJ, Pfister, RC. Lower moiety hydronephrosis in duplicated kidneys. Urology. 1985;26:82.
42. Sheih, CP, Li, YW, Liao, YJ, et al. Diagnosing the combination of renal dysgenesis, Gartner’s duct cyst and ipsilateral Müllerian duct obstruction. J Urol. 1998;159(1):217–221.
43. Currarino, G. Single vaginal ectopic ureter and Gartner’s duct cyst with ipsilateral renal hypoplasia (or agenesis). J Urol. 1982;128:988.
44. Sen, S, Ahmed, S. Single system ureteroceles in childhood. Aust N Z J Surg. 1988;58:903.
45. Braverman, RM, Lebowitz, RL. Occult ectopic ureter in girls with urinary incontinence: diagnosis using CT. AJR Am J Roentgenol. 1991;156:365.
46. Kaefer, M, Barnewolt, C, Retik, AB, et al. The ultrasound diagnosis of infravesical obstruction in children: evaluation of bladder wall thickness indexed to bladder filling. J Urol. 1997;157:989.
47. Giglio, M, Medica, M, Germinale, F, et al. Renal dysplasia associated with ureteral ectopia and ipsilateral seminal vesicle cyst. Int J Urol. 2002;9(1):63–66.
48. MacDonald, GR. The ectopic ureter in men. J Urol. 1986;135:1269.
49. Zaontz, MR, Kass, EJ. Ectopic ureter opening into seminal vesicle cyst associated with ipsilateral renal agenesis. Urology. 1987;24:523.
50. Plaire, JC, Pope, JC, 4th., Kropp, BP, et al. Management of ectopic ureters: experience with the upper tract approach. J Urol. 1997;158(3 pt 2):1245–1247.
51. Nussbaum, AR, Dorst, JP, Jeffs, RD, et al. Ectopic ureter and ureterocele: their varied radiographic manifestations. Radiology. 1986;159:227.
52. Share, JC, Lebowitz, RL. Ectopic ureterocele without ureteral and calyceal dilatation (ureterocele disproportion): findings on urography and sonography. AJR Am J Roentgenol. 1989;152:567.
53. Merlini, E, Lelli Chiesa, P. Obstructive ureterocele-an ongoing challenge. World J Urol. 2004;22(2):107–114.
54. Shokeir, AA, Nijman, RJ. Ureterocele: an ongoing challenge in infancy and childhood. BJU Int. 2002;90(8):777–783.
55. Cremin, BJ. A review of ultrasonic appearances of posterior urethral valves and ureteroceles. Pediatr Radiol. 1986;16:357.
56. Keating, MA, Retik, AB. Management of dilated obstructed ureter. Urol Clin North Am. 1990;17:291.
57. Reitelman, C, Perlmutter, AD. Management of obstructing ectopic ureterocele. Urol Clin North Am. 1990;17:318.
58. Matsumoto, J. Acquired lesions involving the ureter in childhood. Semin Roentgenol. 1986;21:166.
59. Wasserman, NF. Inflammatory disease of the ureter. Radiol Clin North Am. 1996;34:1131.
60. Sorantin, E, Fotter, R, Aigner, R, et al. The sonographically thickened wall of the upper urinary tract: correlation with other imaging methods. Pediatr Radiol. 1997;27:667.
61. Pfister, C, Liard-Zmuda, A, Dacher, J, et al. Total bilateral ureteral replacement for stenosing ureteritis in Henoch-Schönlein purpura. Eur Urol. 2000;38(1):96–99.
62. Miller, OF, Smith, LJ, Ferrara, EX, et al. Presentation of idiopathic retroperitoneal fibrosis in the pediatric population. J Pediatr Surg. 2003;38(11):1685–1688.
63. Ritchey, ML, Gunderson, LL, Smithson, WA, et al. Pediatric urological complications with intraoperative radiation therapy. J Urol. 1990;143(1):89–91.
64. Kraus, SJ, Lebowitz, RL, Royal, SA. Renal calculi in children: imaging features that led to diagnoses: a pictorial essay. Pediatr Radiol. 1999;29:624.
65. Rutigliano, DN, Georges, A, Wolden, SL, et al. Ureteral reconstruction for retroperitoneal tumors in children. J Pediatr Surg. 2007;42(2):355–358.
66. Ritchey, M, Daley, S, Shamberger, RC, et al. Ureteral extension in Wilms’ tumor: a report from the National Wilms’ Tumor Study Group (NWTSG). J Pediatr Surg. 2008;43(9):1625–1629.
67. Droc, CL, Khanna, P, Paidas, CN, et al. Embryonal rhabdomyosarcoma arising in the ureter. Fetal Pediatr Pathol. 2008;27(1):1–11.
68. Townsend, MF, 3rd., Gal, AA, Thoms, WW, et al. Ureteral rhabdomyosarcoma. Urology. 1999;54(3):561.
69. Kojima, Y, Lambert, SM, Steixner, BL, et al. Multiple metachronous fibroepithelial polyps in children. J Urol. 2011;185(3):1053–1057.
70. Adey, GS, Vargas, SO, Retik, AB, et al. Fibroepithelial polyps causing ureteropelvic junction obstruction in children. J Urol. 2003;169(5):1834–1836.
71. Niu, ZB, Yang, Y, Hou, Y, et al. Ureteral polyps: an etiological factor of hydronephrosis in children that should not be ignored. Pediatr Surg Int. 2007;23(4):323–326.
72. Simoni, F, Vino, L, Pizzini, C, et al. Megaureter: classification, pathophysiology, and management. Pediatr Med Chir. 2000;22(1):15–24.
73. Vereecken, RL, Proesmans, W. A review of ninety-two obstructive megaureters in children. Eur Urol. 1999;36(4):342–347.
74. Merlini, E, Spina, P. Primary non-refluxing megaureters. J Pediatr Urol. 2005;1(6):409–417.
75. Peters, CA, Mandell, J, Lebowitz, RL, et al. Congenital obstructed megaureters in early infancy: diagnosis and treatment. J Urol. 1989;142:641.
76. Meyer, JS, Lebowitz, RL. Primary megaureter in infants and children: a review. Urol Radiol. 1992;14(4):296–305.
77. Pfister, RC, Hendren, WH. Primary megaureter in children and adults: clinical and pathophysiologic features of 150 ureters. Urology. 1978;12:160.
78. Kim, S, Jacob, JS, Kim, DC, et al. Time-resolved dynamic contrast-enhanced MR urography for the evaluation of ureteral peristalsis: initial experience. J Magn Reson Imaging. 2008;28(5):1293–1298.
79. Riccabona, M, Sorrantin, E, Fotter, R. Application of functional m-mode sonography in pediatric patients. Eur Radiol. 1998;8:1457.
80. Rypens, F, Avni, F, Bank, WO, et al. The uretero-vesical junction in children: ultrasound findings after surgical or endoscopic treatment. AJR Am J Roentgenol. 1992;158:837.
81. Horgan, JG, Rosenfield, NS, Weiss, RM, et al. Is renal ultrasound a reliable indicator of a nonobstructed duplication anomaly? Pediatr Radiol. 1984;14:388.
82. Leighton, DM, Mayne, V. Obstruction in the refluxing urinary tract: a common phenomenon. Clin Radiol. 1989;40:271.