The Transplant Patient
Background
Transplantation of tissues and organs has been of interest to physicians and surgeons since the 18th century. Advances in our understanding of the immune system, development of immunosuppressive medications, and techniques such as cardiopulmonary bypass have provided the opportunity for successful organ transplants. In 1954, the first kidney was successfully transplanted. Other successful organ transplants include the first heart transplant in 1968, combined heart-lung transplant in 1981, and single-lung transplant in 1983.1
Organ transplantation has become a viable alternative to medical treatment of many conditions. Because of the success rates, many types of transplants are no longer considered experimental but are considered appropriate treatment for organ failure. As a result of improved survival, long-term outcomes are as important as short-term outcomes as a measure of successful transplantation. The number of Medicare-approved medical centers that perform lung and heart-lung transplants has grown to 50 in the United States, and there are 110 certified heart transplant centers.2 Over 2000 heart transplants and 1000 lung transplants have been performed annually since 2000. The 5-year survival rates for heart transplants of all status levels are reported as 70.9%; for lung transplants, 43.1%. Other national data on median waiting time and survival rates are presented in Table 40-1.
Table 40-1
Heart/Lung Transplantation National Data
| Heart | Lung | |
| Number of transplants, 2005-2010 | 12,660 | 8744 |
| Median waiting time (in days), 2003-2004 | ||
| Status 1A, 50 | Cystic fibrosis, 587 | |
| Status 1B, 78 | COPD, 1349 | |
| Status 2, 309 | Pulmonary fibrosis, 1115 | |
| 1-year survival (all status) | 87.3% | 83.1% |
| 5-year survival | 70.9% | 46.3% |
Data from: http://optn.transplant.hrsa.gov/latestData/step2.asp?
Organ Donation
The major cause of limitation in the number of organ transplants is the lack of organ supply with an increasing demand for organ transplantation. The number of patients who could benefit from transplant significantly exceeds the number of organs available. The Organ Procurement and Transplantation Network (OPTN) is an organization whose primary goal is to increase the availability of donated organs available and improve organ sharing. OPTN was established by the National Organ Transplant Act of the United States Congress in 1984 and is administered by the private, nonprofit organization United Network for Organ Sharing (UNOS), under contract with the U.S Department of Health and Human Services. An organ procurement organization (OPO) coordinates the management of the organ donor and family, the transplant center, and the recipient.3
The number of organs available for transplant remains well below the need for donor organs. Currently, there are over 110,000 individuals on the national waiting list for all organs, with over 3200 candidates waiting for heart transplant and approximately 1800 candidates waiting for lung transplant. A general list of criteria for patient selection for transplant is presented in Box 40-1. Names of patients on the waiting list are filed in the UNOS Organ Center, a centralized database that links transplant centers with OPOs. Once an organ donor has been identified, a computer-generated list of potential recipients is ranked, according to the criteria established for each organ. Criteria may include blood or tissue type, size of organ, medical status of the patient, and amount of time a patient has been on the waiting list. The organ procurement coordinator confers with the transplant surgeons until a potential recipient is identified. Surgical teams then travel to the donor hospital while the recipient is concurrently prepared for surgery. Heart, lung, and liver transplantation success is optimized when transplant occurs within 6 hours after removing the donated organ. Surgical methods that allow for longer travel times or changes in management of the donor organ have increased the number of available organs; however, the number of available organs still remains significantly below the need.
Policies were updated by the UNOS in 2006 to decrease the number of patients dying while waiting for transplant. These changes increased the number of transplants for the most severely ill patients awaiting heart transplant.4
Issues Associated with Transplantation
There are many ethical, psychological, and social concerns surrounding organ transplantation. Ethical issues relate to increasing potential donor sources and distributing available organs. Psychological issues include stress due to an unknown waiting time, the potential of moving from one’s home to a location near a transplant center, and possible employment disruption. The transplant process affects not only the individual patient, but the patient’s social support systems as well. This is highly significant because adequate social support is a factor in long-term transplant success and quality of life.5
Ethical Considerations
Other bioethical considerations include how to allocate the available organs to individuals waiting for transplant. All patients are screened for medical and psychological conditions that would adversely affect the outcome of transplantation. The specific acceptance criteria are different for each organ; lung transplant criteria differ from heart transplantation criteria. OPTN policies specify that each transplant center will create its own specific policies.6 In general, waiting list time remains the primary factor that determines who is next in line to receive an available organ; however, other matching factors are considered for each available organ. Patients who are current smokers are not candidates for lung transplantation, although most centers allow former smokers to qualify for a lung transplant. Patients with documented alcohol abuse are not candidates for transplantation. Criteria are used so that individual preferences of team members are not given priority. In addition, ethnicity, gender, religion, and financial status are not part of the transplant criteria.
Psychological and Social Considerations
There are many psychological issues associated with organ transplantation, such as feelings of uncertainty, upheaval associated with moving, and issues of psychological adjustment. The time spent waiting for a transplant can vary considerably. During the waiting period, patients struggle with the need to carry on their lives, knowing that at any moment they may be called to the hospital for transplant. In addition, the patient is torn between wanting to remain hopeful that a transplant will provide new life and the reality of knowing they have a terminal condition. Anxiety and depression have been identified as prevalent psychological issues related to transplant.7
The number of transplant centers in the United States is limited. Because donor organs are viable for only hours after removal from the donor, patients waiting for transplant are frequently required to live within a several-hour radius of the transplant center. Patients need information and logistics assistance for relocation to a transplant center. They may also require emotional and financial support for this transition.8 The demands of waiting and relocation to the transplant city put considerable stress on the patient and their significant others. Those patients who relocate to transplant centers may have the most difficult time psychologically, especially when they leave family and significant others behind. The psychological stresses are somewhat different for spouses. Spouses speak of the struggle to remain hopeful, yet plan for a future that may not include their loved one. If the patient and spouse move together to a new city to wait for transplant, they may find themselves with more time together than previously. In this “forced retirement” situation, new stresses are added to relationships. Transplant teams usually include a psychologist or social worker to whom patients may be referred for individual or family counseling if needed.
Other concerns include psychological adjustment and the effect of social supports on mediating coping skills. Feelings of being useless are common because patients waiting in a new city are outside of their own environment and must find some meaningful activities. Coping strategies appear to strengthen psychological outcomes. After transplant, patients are often emotionally overwhelmed with a feeling of gratefulness that they are alive and feelings of guilt that someone else’s grief has given them a chance to rejoice. A strong support system is considered an essential component of a successful transplant and improves psychosocial outcomes after transplantation.9 Therapists can assist patients by listening to feelings, being supportive, and encouraging participation in support groups. Support groups that include patients waiting for transplants as well as posttransplant patients can offer significant psychosocial support. Spouses and significant others also benefit from group support.
Rehabilitation of the Transplant Patient
Pretransplant Rehabilitation
Heart Transplant and End-Stage Cardiac Disease
Patients are referred for heart transplantation for end-stage cardiac conditions. The primary diagnoses for adults requiring a heart transplant are severe coronary artery disease (38%) and end-stage cardiomyopathy/heart failure (53%). In children under 1 year, the primary diagnosis requiring transplant is congenital cardiac abnormalities; for ages 1-10, cardiomyopathy.10
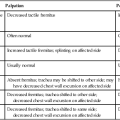
Patients with heart failure generally meet the heart transplant selection criteria if they have New York Heart Association (NYHA) class III or IV heart failure that is unresponsive to medical management. Specific criteria for recipient status vary somewhat from center to center, although International Society for Heart and Lung Transplantation (ISHLT) criteria for listing are generally followed.11 Exercise testing criteria can be used to guide transplantation. Patients who complete a maximum test may be eligible for a heart transplant if peak 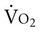 is ≤14 mL/kg/min, or ≤12 mL/kg/min if on a beta blocker.11
is ≤14 mL/kg/min, or ≤12 mL/kg/min if on a beta blocker.11
Recipients waiting for heart transplantation are classified based on severity of disease. Patients in the worst medical condition (Status IA) are placed higher on the transplant recipient list. Table 40-2 outlines the criteria for each level of heart transplant recipient status.
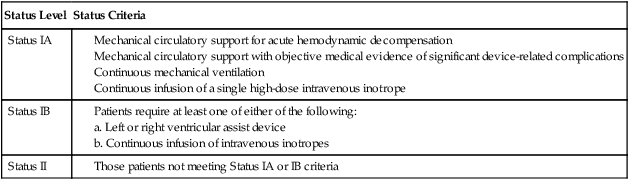
Source: http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/Policy_9.pdf
The main rehabilitation goal in the preoperative period is to prevent losses of physical function. Maintenance of range of motion, soft tissue extensibility, and muscle strength are suggested goals. Although patients with severe disease may be unable to participate in therapy, cardiac rehabilitation is an effective treatment for improving functional status of patients with significant heart failure and those with LVADs, whether or not transplantation is anticipated.12,13 Recent literature demonstrates that medically supervised exercise training is both safe and effective at improving aerobic capacity and physical function in patients with LVAD devices awaiting heart transplantation.
Lung Transplant and End-Stage Pulmonary Disease
Patients are referred for lung transplantation for end-stage pulmonary conditions. The primary diagnoses for adults requiring a lung transplant are severe chronic obstructive pulmonary disease (COPD) (44%), idiopathic pulmonary fibrosis (IPF) (17%), and cystic fibrosis (CF) (15%). Other conditions include pulmonary arterial hypertension (PAH). The guidelines for selection of patients for the lung transplant waiting list were updated by the ISHLT in 2006 and include specific criteria for the most common pulmonary conditions.14,15 Within the disease-specific listing criteria for COPD, the guidelines include a BODE (body mass index, airflow obstruction, dyspnea, and exercise capacity) score of 7-10. The BODE is more useful than measures of airflow obstruction in predicting mortality from COPD; higher scores are associated with increased mortality.16 Before 2005, patients who had accrued the most time on the waiting list were given priority for lung transplants. The change to a lung allocation score now gives priority for transplants to those patients who have a greater medical urgency for transplant. The lung allocation score is based on “(i) waitlist urgency measure (expected number of days lived without a transplant during an additional year on the waitlist), (ii) posttransplant survival measure (expected number of days lived during the first year posttransplant), and (iii) transplant benefit measure (posttransplant survival measure minus waitlist urgency measure).”17 The urgency and survival measures are based on factors that predict risk for death in patients with lung transplant and include functional status as well as physiological measures. This change has increased the number of transplants for patients with IPF and slowed the number of transplants for COPD.14
Idiopathic pulmonary fibrosis is characterized by scarring of lung parenchyma leading to decreased lung volumes and decreased diffusing capacity. Risk factors for mortality include the lung pathology, pulmonary function results, physical function from the 6-minute walk test, and respiratory failure.14 Criteria for listing of patients with IPF include a decrease in FVC of 10% or more during 6 months and pulse oximetry less than 88% during a 6-minute walk test.15 Patients with IPF waiting for lung transplant who walked less than 679 feet had higher mortality than those who walked longer distances.18
Recent advances in vasodilator therapy for patients with pulmonary artery hypertension and poor survival of patients with PAH after transplant have reduced the number of patients with PAH who receive lung transplants. The criteria for listing patients with PAH include an NYHA class III or IV status on the maximal vasodilator therapy and 6-minute walk test of less than 350 meters.15
In patients with cystic fibrosis, the predictors of mortality include declining FEV1 and chronic infection. The current criteria for transplant include FEV1 less than 30% of predicted, as well as other markers of declining pulmonary function.19
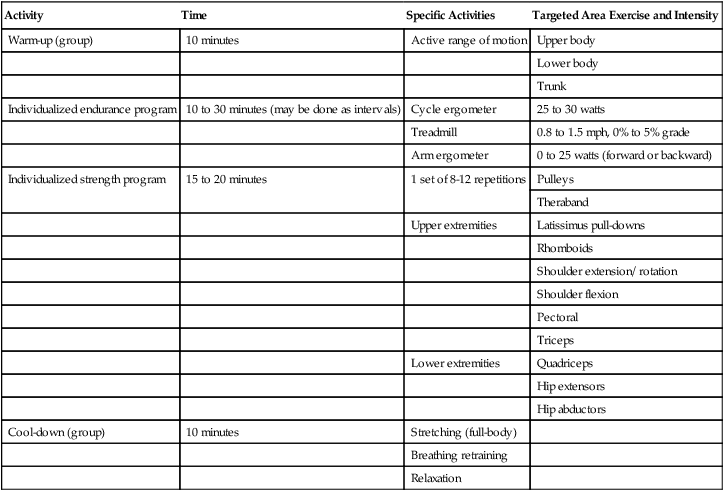
The causes of exercise limitation in people with chronic lung conditions are both ventilatory and musculoskeletal. Muscle changes in pulmonary conditions include decreased muscle oxidative capacity and muscle weakness.20 Reasonable goals for pretransplant rehabilitation include improvement in muscle strength in lower extremity musculature, functional upper extremity muscle strength and endurance, functional shoulder and chest wall range of motion, demonstration of coordinated diaphragmatic breathing with exercise and activities, and improved cardiovascular endurance. Because of the significant effect of immunosuppressive medications on muscle strength, a greater emphasis is placed on resistance training in a patient awaiting lung transplantation than in most typical pulmonary rehabilitation programs. An example of a preoperative pulmonary rehabilitation program is outlined in Table 40-3.
Table 40-3
Preoperative Pulmonary Rehabilitation
| Activity | Time | Specific Activities | Targeted Area Exercise and Intensity |
| Warm-up (group) | 10 minutes | Active range of motion | Upper body |
| Lower body | |||
| Trunk | |||
| Individualized endurance program | 10 to 30 minutes (may be done as intervals) | Cycle ergometer | 25 to 30 watts |
| Treadmill | 0.8 to 1.5 mph, 0% to 5% grade | ||
| Arm ergometer | 0 to 25 watts (forward or backward) | ||
| Individualized strength program | 15 to 20 minutes | 1 set of 8-12 repetitions | Pulleys |
| Theraband | |||
| Upper extremities | Latissimus pull-downs | ||
| Rhomboids | |||
| Shoulder extension/ rotation | |||
| Shoulder flexion | |||
| Pectoral | |||
| Triceps | |||
| Lower extremities | Quadriceps | ||
| Hip extensors | |||
| Hip abductors | |||
| Cool-down (group) | 10 minutes | Stretching (full-body) | |
| Breathing retraining | |||
| Relaxation |
Surgical Implications
Heart transplants are usually performed through a median sternotomy incision and require cardiopulmonary bypass.21 The native heart is excised, leaving a portion of the right atrium (atrial cuff), including the SA node. The left atrium of the donor heart is sutured to the recipient’s atrial cuff and the major vessels reattached, starting with the pulmonary arterial vessels and followed by the aorta. Drainage tubes are placed in the pleural and mediastinal spaces and an epicardial pacing wire is placed before closure of the sternum. Total heart ischemic time is minimized, with less than 4 hours recommended. Finally, the two halves of the sternum are wired together, the new heart defibrillated, cardiopulmonary bypass discontinued, and the patient awakened.
Double-lung transplants are performed as two single-lung transplants, in a technique referred to as bilateral sequential lung transplantation. The surgical incision extends from the midaxillary line under the submammary fold to the other midaxillary line and may or may not use a transverse sternotomy.22 In this technique, called the clamshell incision, the pectoralis major is separated from the chest wall, and the serratus anterior may be incised.23 Complications from this technique include sternal overlap if a transverse sternotomy is used.
Acute Phase Rehabilitation
Heart Transplant Acute Management
Postoperative management of the patient following heart transplantation is similar to that of other cardiac surgeries. Patients experience hemodynamic instability, dysrhythmias, pulmonary hypertension, coagulopathies, and acute rejection. Factors that can lead to a complicated postoperative course include length of ischemic time and reperfusion injury. Prolonged ischemic time is associated with graft dysfunction.24 Reperfusion injury is also associated with poor short-term outcomes.
Current guidelines for management of patients with heart transplant include a recommendation for a multidisciplinary team that meets regularly.25
Exercise Guidelines
Goals of the acute phase of rehabilitation include the following: (1) regain normal postural cardiovascular responses (no postural hypotension) and (2) increase functional activities. The former is focused on the patient being able to tolerate changes in position, increase time in upright sitting, and transfer independently. Upper and lower extremity range of motion and strength need to be adequate to perform activities of daily living. An example of a cardiac rehabilitation program is presented in Table 40-4.
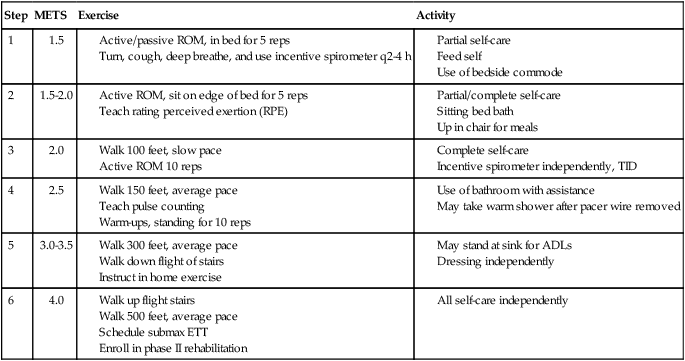
Patient HR to be 50 to 120 bpm
Exercise HR not more than 20 beats above rest
SBP <200, DBP <120 during exercise
<10 to 15 DBP drop during exercise
Patient to complete entire step before advancing to next step
In the rehabilitation process, median sternotomy precautions may need to be observed. Emerging evidence suggests that activity may be progressed more quickly than previously thought.26 Principles of inpatient cardiac rehabilitation are followed in the postoperative period. Patient activity is progressed as long as a normal physiological response in achieved.
Lung Transplant Acute Management
Exercise Guidelines
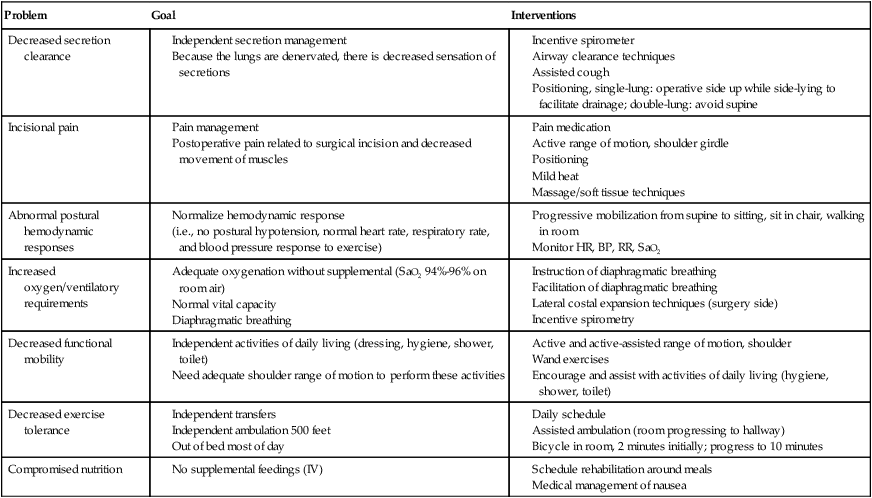
Airway clearance is an essential component of acute care management. Many techniques are considered appropriate. Breathing training that targets diaphragm function is considered an essential component of acute management of the patient following lung transplantation. Suggested goals and interventions are shown in Table 40-5.
Table 40-5
Goals and Interventions for Acute Posttransplant Rehabilitation: Lung Transplant
| Problem | Goal | Interventions |
| Decreased secretion clearance |
Posttransplant Outpatient Rehabilitation
Rejection
Heart Transplant
After heart transplant, patients continue to experience decreased aerobic capacity and endurance, muscle atrophy, and decreased physical function. In addition, immune suppression increases the risk for infection and leads to loss of muscle and bone. Rejection remains a significant risk, and heart transplant patients are likely to acquire premature atherosclerosis. Despite these problems, aerobic capacity and physical function are amenable to rehabilitation.27 Common long-term impairments are chest wall soreness, abnormal upper quarter movement patterns, and low back and knee pain from overuse. Once the patient leaves the hospital, rehabilitation looks much like phase II cardiac rehabilitation. The overall objective is to increase functional activity to within normal limits.
Cardiac and Respiratory Physiology in Heart Transplant
After transplantation, functional capacity is about 50% of the capacity of control subjects; abnormalities in heart rate, blood pressure, and ventilatory abnormalities also exist during this period. In posttransplant patients, peak heart rate is lower than in controls (156 versus 168 beats/minute),28 although heart rate kinetics are not different.29 In regard to long-term effects of heart transplantation on heart rate, some children who have received heart transplantation do acquire a normal heart rate response to exercise.30 The ventilatory response to exercise is abnormal, as demonstrated by a decreased diffusing capacity.31 However, there may be some peripheral limitations that contribute to decreased functional capacity; these include decreased capillary density in muscle.32 A summary of how cardiac variables are affected by heart transplant is shown in Table 40-6.
Table 40-6
| Heart Rate | Stroke Volume | Blood Pressure |  |
|
| Rest | Lower than normal | Lower than normal with little change when shifting positions | + | NA |
| Exercise | Lower (approximately 15 beats less) | Decreased and late onset | + | May approximate normal with exercise training program |

Exercise and the Heart Transplant Patient
Exercise training is important for reversing the physiological abnormalities observed after heart transplant, returning patients to improved functional status, and preventing disability incurred from the immunosuppressant regimen. The outpatient phase of rehabilitation resembles phase II cardiac rehabilitation, which generally continues for 8 to 12 weeks, at which time the patient is encouraged to continue exercise on his or her own or at a fitness facility. ISHLT guidelines for care address health-related issues for patients after heart transplant. Hypertension after heart transplant should be treated to achieve the same goals as the general population, and lifestyle modifications are the same as for others with cardiac risk factors.25Aerobic exercise and resistance training are recommended. Guidelines for exercise for the posttransplant cardiac patient are shown in Box 40-2.
After heart transplantation, there is no direct innervation to the heart to control heart rate response during exercise. Heart rate and stroke volume increase due to the effect of increased preload and through circulation of catecholamines. There is evidence that some sympathetic re-innervation can occur 1-2 years after transplant.33 For the patient in phase II cardiac rehabilitation, this means that the warm-up and cool-down portions of exercise should be slowly increased to 10 to 15 minutes. Exercise intensity should not be determined by heart rate alone, because of the effects of denervation. In addition, rating of perceived exertion (RPE) scales remain an unreliable method for determining exercise intensity after heart transplant, because large inter-individual variations occur in the relationship of RPE and maximal oxygen consumption.34 Although there is no strong clinical consensus about the optimal method for prescribing and monitoring exercise intensity for patients following heart transplant, ventilatory threshold, rate-pressure product (RPP), or a combination of RPE and physiological monitoring is recommended. Evidence suggests that patients with heart transplants can exercise at high intensity and achieve better functional outcomes than sedentary individuals without transplant.35 Intensity set at 10% below anaerobic threshold produced significant increases in aerobic capacity.36 Endurance and strength exercise can be performed in this rehabilitation stage, although upper extremity strengthening should not be started until after the sternum has healed. Treadmill, bike, arm ergometry, and indoor cross-country ski equipment are all appropriate choices.37
Cardiac rehabilitation can result in substantial increases in aerobic capacity with patients able to improve 20% to 50% over prerehabilitation levels. The mechanisms that contribute to increased aerobic capacity include increased maximal heart rate, improved ventilatory capacity, and improved peripheral muscle function.38 Supervised exercise programs lead to greater increases in aerobic capacity than do home exercise programs (49% versus 18% improvement, respectively).27 Resistance training has been used in patients following heart transplantation as an essential component of cardiac rehabilitation to improve physical function and prevent losses of muscle strength associated with corticosteroid use. Resistance training does appear to increase bone mineral density to nearly pretransplant levels.39 Resistance training increases oxidative capacity of muscles40 and also prevents adverse effects of corticosteroids and immune therapy on skeletal muscle.
Despite the evidence that cardiac rehabilitation is beneficial in increasing aerobic capacity and exercise capacity, scant literature exists to suggest that exercise training prevents transplant atherosclerosis. Early studies of patients after renal transplant, however, show promising results of exercise in preventing atherosclerosis.41
Lung Transplant
Cardiac and Respiratory Physiology in Lung Transplant
After lung transplant, patients continue to experience decreased aerobic capacity and endurance, muscle atrophy, and decreased physical function. In addition, immune suppression increases the risk for infection and leads to loss of muscle and bone. Rejection remains a significant risk. Despite these problems, aerobic capacity and physical function are amenable to rehabilitation. Patients in the outpatient phase of rehabilitation begin at low levels of function and progress to having few functional limitations. Therapists need to adapt and progress the rehabilitation program appropriately. Because of the debilitating side effects of immunosuppressive medications, rehabilitation needs to be aggressive and preventive in nature. Rehabilitation can occur in a group setting and/or on an individual basis. General guidelines for posttransplant lung rehabilitation are shown in Box 40-3.
After transplantation, functional capacity remains between 40% and 60% of predicted oxygen uptake, whether the patient received a single- or double-lung transplant.42 Although there are mild ventilatory abnormalities, the decreased 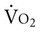 is not well explained by the decrease in ventilation or by low oxygen saturation or anemia. Instead, several articles suggest that the main cause of decreased aerobic capacity is a peripheral muscle limitation. Problems in the periphery include decreased peripheral oxygen utilization, decreased mitochondrial capacity and decreased type I fibers, as well as decreased muscle power.20,43
is not well explained by the decrease in ventilation or by low oxygen saturation or anemia. Instead, several articles suggest that the main cause of decreased aerobic capacity is a peripheral muscle limitation. Problems in the periphery include decreased peripheral oxygen utilization, decreased mitochondrial capacity and decreased type I fibers, as well as decreased muscle power.20,43
Exercise and the Lung Transplant Patient
Aerobic exercise training for patients with lung transplantation provides substantial benefits in aerobic capacity. Frequency of exercise at least three times per week, intensity of 60% to 70% of heart rate maximum or RPE of 13 to 14, and duration of at least 30 minutes created significant increases in aerobic capacity.42 Studies demonstrated an increase of aerobic capacity to 60% of prerehabilitation levels and decreased heart rate and ventilation for the same amount of submaximal exercise, indicating a training effect. After 6 to 12 weeks of training, 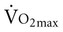 and
and 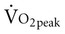 increased as much as 14% over baseline testing and increased exercise time from 20 to 30 minutes.42 The mechanisms by which aerobic capacity increases include decreased resting minute ventilation, suggesting increased efficiency of oxygen utilization. In addition, in two cohort studies, 6-MWD increased significantly.20,44
increased as much as 14% over baseline testing and increased exercise time from 20 to 30 minutes.42 The mechanisms by which aerobic capacity increases include decreased resting minute ventilation, suggesting increased efficiency of oxygen utilization. In addition, in two cohort studies, 6-MWD increased significantly.20,44
Peripheral muscle deconditioning contributes to decreased exercise ability and limited physical function. Lower extremity muscle strength can occur with resistance training. One study demonstrated a 35% increase in quadriceps force to almost 60% of that predicted after training, at 60% of 1-repetition maximum (1RM).20 Lower extremity strengthening can begin early, but upper extremity resistive training should be delayed until wound and tissue healing is complete, generally about 6 weeks because of the delays in wound healing secondary to use of prednisone. Other important aspects of rehabilitation include posture reeducation, range of motion, and upper extremity movement patterns. Guidelines for outpatient physical therapy are provided in Table 40-7.
Table 40-7
Lung Transplant: Postoperative Outpatient Physical Therapy Management
| Problem | Goal | Activity |
| Decreased aerobic capacity |
Due to deconditioning and effects of prednisone
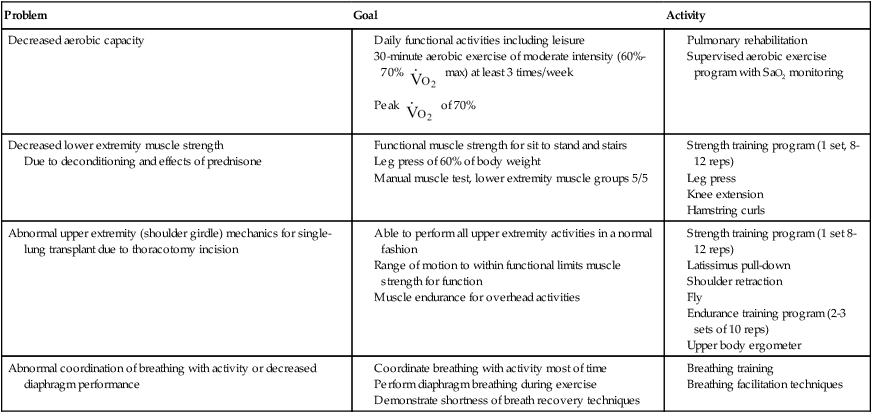
Community- or Home-Based Rehabilitation
Patients are generally followed at the transplant center until the medical staff is satisfied with patient progress. Return to home can occur at this point in time, whether or not rehabilitation goals have been met. For patients who have left their home community to wait for transplant, the transition to home frequently occurs at 3 months after transplant. The major goal of community-based rehabilitation is a return to normal function with minimal limitations. Therapists in rural settings and community hospitals may be involved with a transplant patient’s plan of care in this phase. Therapists should be comfortable treating patients in many settings, skilled in addressing the common complications, and vigilant in watching for signs of rejection. In the initial stages of posttransplant rehabilitation, a supervised program is recommended. Hospital-based phase III cardiac rehabilitation programs are appropriate, or patients may elect to exercise independently in a fitness facility. Some follow-up by the transplant center is important to encourage compliance in exercise programs. Once patients reach their desired level of fitness, a community-based program is appropriate for maintaining endurance, aerobic capacity, strength, and flexibility. Patients who participate in training can attain high levels of exercise performance, with an appropriate training regimen.45
Influence of Medications on Treatment
Rejection Issues
Types of Medications
Calcineurin Inhibitors
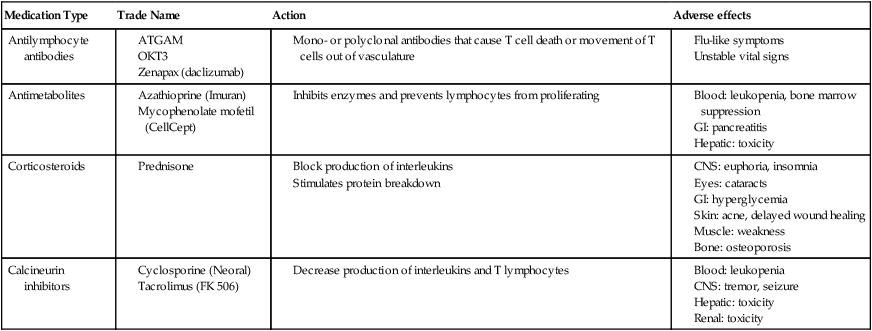
These drugs bind to proteins in the cells, decreasing the production of interleukin-2 or T lymphocytes. Cyclosporine (Neoral, Sandimmune) and tacrolimus (Prograf) are the most common of these drugs and are a main component of many immunosuppressant regimens. The main adverse effect is renal toxicity, which may be reversible if the dose is decreased. Cyclosporine is considered a nervous system irritant, leading to hand tremor and possible seizures. Therapists should be aware of these side effects. In addition, absorption of these drugs is affected by other medications and food intake. Serum concentrations of these drugs are increased with intake of grapefruit juice, as well as drugs such as erythromycin, diltiazem, and corticosteroids. Because of potential drug interactions, serum levels of these drugs are monitored closely. Table 40-8 summarizes the effects of common immunosuppressive medications.
Table 40-8
Side Effects of Immunosuppressive Medications
| Medication Type | Trade Name | Action | Adverse effects |
| Antilymphocyte antibodies |
Long-Term Complications of Transplant Management
Osteoporosis
Osteoporosis remains a prevalent and disabling problem for patients following organ transplantation surgery. Before transplant, the prevalence of osteoporosis is 37% in patients awaiting lung transplantation.46 In the first year after transplant, a high incidence of vertebral and other atraumatic fractures occurs (between 8% and 65%), which can significantly limit physical function and complicate the posttransplantation rehabilitation course. Physical therapists have a role in identifying the physical limitations of osteoporosis and in prescribing appropriate exercise programs. Osteoporosis medications are considered essential components of care, and the addition of resistance exercise to a medication regimen does improve bone mineral density.39
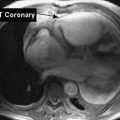
Cardiac Allograft Vasculopathy
One of the most troubling long-term complications of heart transplantation is cardiac allograft vasculopathy, an accelerated form of coronary disease affecting cardiac arteries. Blockage of the arteries occurs, and the condition may be related to occurrences of acute rejection.47 Physical therapists must continue to identify signs and symptoms of cardiac ischemia and refer patients for appropriate treatment. As preventive measures, patients may be on medications to lower cholesterol (statins) and to manage hypertension; these patients may be candidates for stenting or coronary artery bypass surgery as well. High-intensity exercise does appear to improve endothelial function and may decrease risk for cardiac events.48
Bronchiolitis Obliterans
Physical therapists working with long-term survivors of lung transplant may find that these patients demonstrate decreased lung function and symptoms of dyspnea with activity. The therapist, therefore, must continue to monitor ventilatory status and oxygen saturation in the lung transplant patient. Long-term complications of organ transplantation are summarized in Box 40-4.
Quality of Life
Although quality of life generally improves after heart and/or lung transplantation, many factors may influence that quality of life. Generally, patients report improved physical function, general health, and social functioning.49 Despite these improvements, a decline in quality of life eventually occurs in long-term survivors. It is unclear whether this downturn is due to declines in physical condition or other social or psychological factors. Patients with lung transplantation generally have increased quality of life until the onset of bronchiolitis obliterans, which then causes a decline in quality of life.

















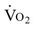 max) at least 3 times/week
max) at least 3 times/week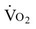 of 70%
of 70%