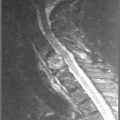
3 The thoracic spine
Introduction
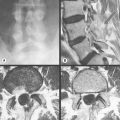
Perhaps more than any other area, pain in the thoracic spine and its associated rib articulations requires differentiation from referred visceral pain and pathology, which can easily mimic musculoskeletal problems.1 However, the thoracic spine is one of the most difficult areas to image with plain film radiography. On an x-ray, the thoracic cage is superimposed over the vertebral bodies; the discrepancy in tissue density between the osseous thoracic spine and the lung parenchyma creates a confluence of densities that precludes the acquisition of high-quality radiographic images.2 This is particularly true for the lower thoracic spine, owing to the superimposition of the diaphragm, and the upper regions of the thoracic spine, owing to the soft tissues of the shoulder.
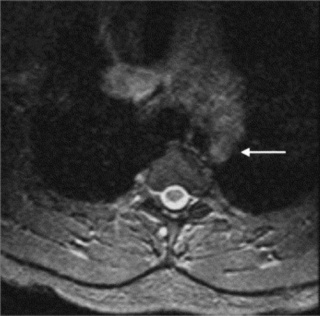
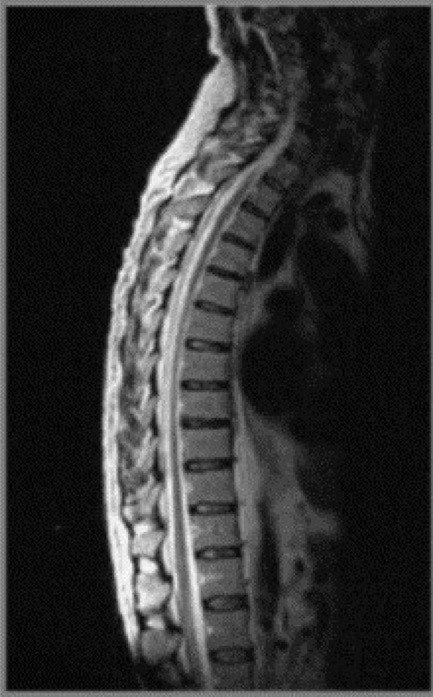
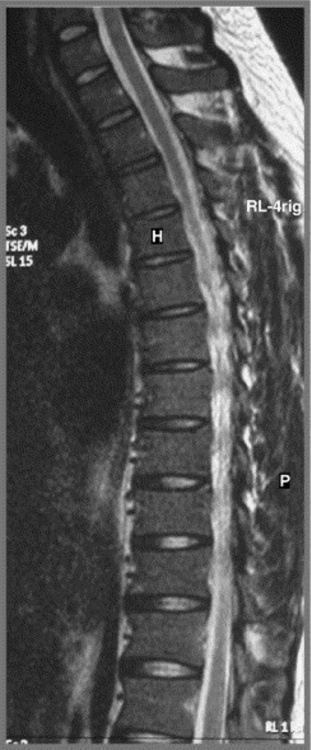
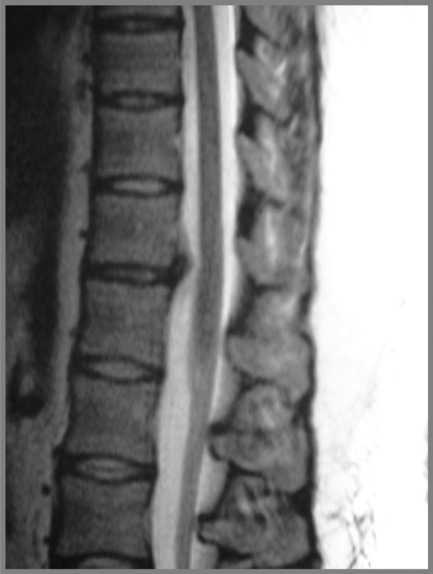
Cross-sectional imaging of the spine, spinal cord and surrounding anatomical structures has therefore contributed substantially to the detection and characterization of disorders affecting the thoracic spine; however, magnetic resonance (MR) imaging of this area is not without its own complications, which mainly arise from motion artifacts. Image quality can be affected by the pulsation of the heart, the respiratory cycle, and the vertical pulsatile movement of the blood and the cerebrospinal fluid through the thoracic region (Figure 3.01).3 It is therefore important to recognize that the method of imaging the thoracic spine has been designed to reduce the effect of motion artifacts by utilizing techniques such as peripheral gating or saturation pulses.4,5
MR imaging of the thoracic spine is often performed to determine the presence of a disc lesion. Although such lesions tend to be less common and also smaller in volume than those in other spinal areas, because the dimension of the thoracic spinal canal is relatively smaller, the risk of neurological compression is correspondingly higher.6,7
MR imaging has superior imaging quality of epidural and bone marrow involvement compared to other general imaging modalities, providing a means for the differentiation of normal haematopoietic marrow from infiltrative processes such as metastatic disease. MR imaging, by virtue of the differing signal intensities, allows for the diagnosis and evaluation of disorders affecting the epidural fat, spinal cord, subarachnoid and intramedullary regions.8
By contrast, CT provides beautiful detail of bony structures but is somewhat limited in its depiction of canal lesions. MR imaging also allows the depiction of the soft tissue structures around the spine, including paraspinal soft tissue and neural structures; this is useful in conditions such as paraspinal abscess formation and neural tumours.9,10
History, examination, diagnosis and indications for imaging
Examination of the thoracic spine is considerably more difficult than that of the cervical and lumbar areas. There is a dearth of orthopaedic tests of diagnostic significance; the dermatomes overlap to the point whereby a single root lesion remains occult; there are no reliable myotomal indicators and no deep tendon reflexes, although the umbilical reflexes can sometimes indicate a radicular lesion and long tract signs can also be an important indicator of central cord pathology.6,11
This makes the history even more important: apart from a thorough review of the cardiovascular and respiratory systems, the thoracic spine also contains the sympathetic trunk and so a generalized screen of the patient’s systemic health is also essential.12,13
Observation of the patient can provide important clues. Scoliosis is a condition that commonly affects the thoracic spine, and which is easily seen.14 Hyperkyphosis can also be associated with Scheuermann’s disease (juvenile discogenic disease), ankylosing spondylitis and compression fractures, most commonly secondarily to osteoporosis; the age and sex of the patient is therefore important in indicating the differential diagnosis (Figure 3.02).15
Palpation can also be suggestive of conditions such as thoracic facet injury, costovertebral syndrome and costochondritis (Tietze’s syndrome), all common causes of thoracic pain; however, because of the difficulty involved in definitive testing, diagnostic imaging is all the more important.16,17
Protocol
MR imaging of the thoracic spine requires the patient to lie supine during the examination; usually the patient enters the magnet headfirst. The complete MR examination lasts approximately 30–45 minutes, depending on the sequences required. It is, however, possible for patients to move between the various sequences, each of which commonly lasts for around 2 to 3 minutes. A spinal coil is placed under the patient to increase the quality of the image and a triangular foam pad is frequently placed under the knees, which may affect the normal spinal curvature. A positioning light is centred over the sternum in the midline.4,5
The field of view in MR images of the thoracic spine is approximately 16 cm in the sagittal plane and 14 cm in the axial plane. Slice thickness is usually 3–4 mm.4,5 The initial sequence is the coronal localizer (scout view); this is taken to verify the patient positioning and ordinarily includes C2 in order to correctly identify the thoracic levels. Imaging slices may be acquired either from the left to right field borders or from right to left of the vertebral bodies and it is important to verify this by noting the order of the slice numbers on the localizer view.
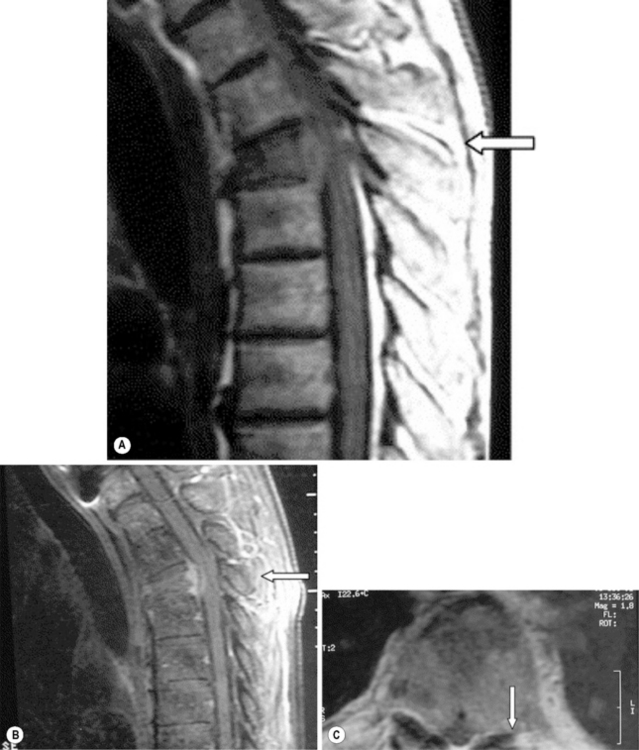
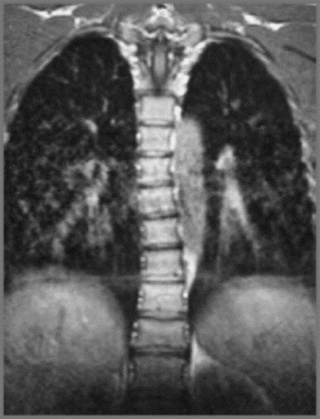
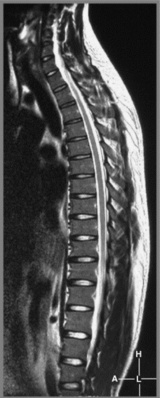
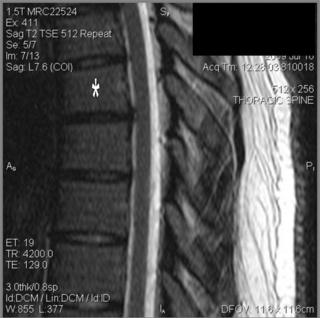
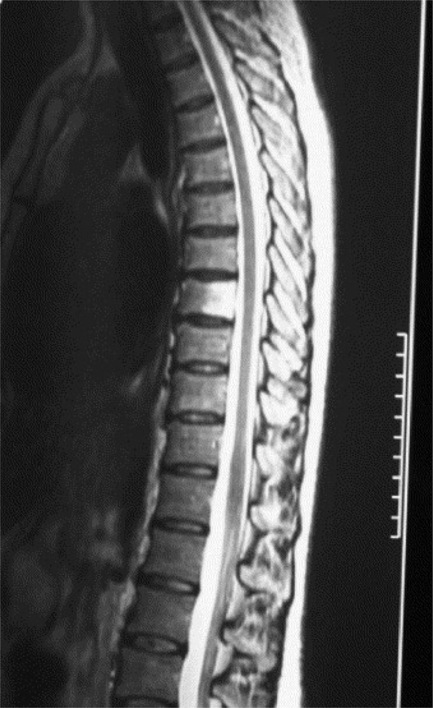
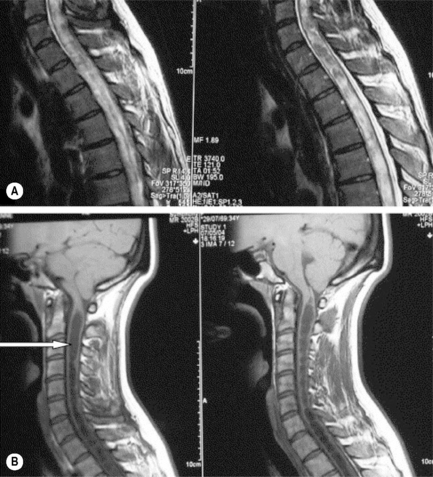
MR imaging of the thoracic spine commonly consists of T1-weighted and fast spin echo (FSE) T2-weighted sequences in the sagittal plane (Figures 3.03, 3.04). In the axial/axial oblique plane, T2-weighted FSE (or intermediate weighted axial FSE) images parallel to the disc are acquired (Figure 3.01). The oblique plane is very useful to acquire information at the level of the intervertebral disc and in cases of scoliosis; the coronal/frontal plane may also be included, again particularly in scoliotic patients (Figure 3.05).
Specific protocols may be used in particular clinical circumstances. Most MR imaging thoracic spine protocols for metastases include T1- and T2-weighted sagittal and axial images with repetition of the T1-weighted images following the addition of gadolinium.18 Alternative imaging sequences may include short tau inversion recovery (STIR) and fat suppression. The use of STIR is especially useful in increasing the sensitivity of lesion detection in the posterior elements of the vertebrae.19
Normal anatomy and variants
Intervertebral disc
The thoracic intervertebral disc is important to evaluate owing to the relatively smaller spinal canal diameter in this area of the spine, which predisposes to a higher risk of neurological involvement in thoracic disc herniations.7 The hydrated nucleus of the normal intervertebral disc should be appreciated, together with its relationship to the cerebrospinal fluid of the anterior subarachnoid space. In degenerative disc disease, a reduction in hydration is demonstrated as hypointensity in the disc on T2-weighted sequences.20
Symptomatic thoracic disc lesions, such as extrusions, are uncommon, and present a particular diagnostic challenge to the clinician.21 Sagittal, T2-weighted FSE sequences are excellent for displaying indentation of the ventral thecal sac and effacement of the spinal cord by a thoracic disc extrusion. Axial images help delineate lateralization of the disc lesion and evaluate impingement of the traversing nerve roots.
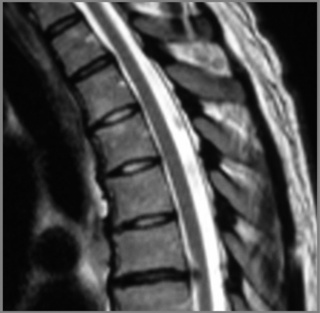
Because of the relatively smaller size of thoracic discs, they may appear hypointense on T2-weighted sequences compared to discs in other areas of the spine (Figures 3.03B, 3.04).
Vertebral body
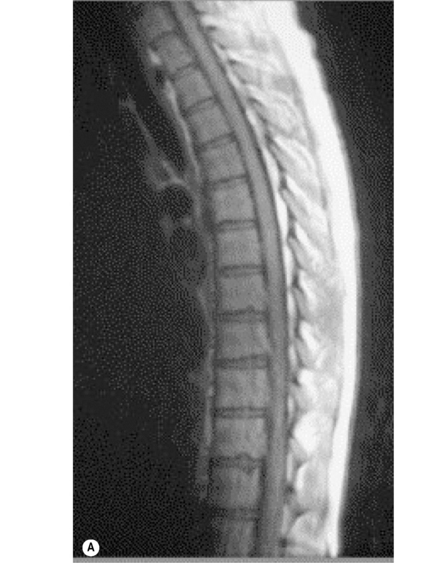
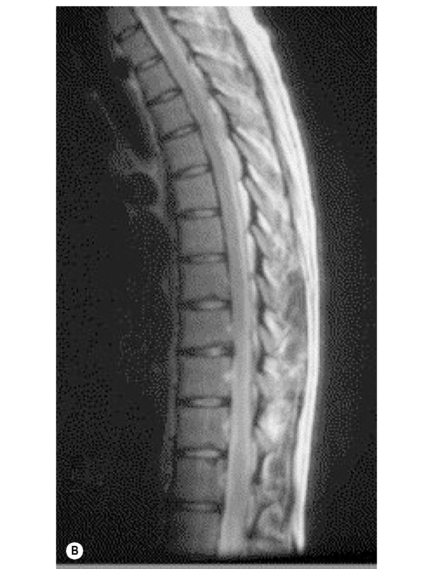
The individual osseous components of the vertebral bodies are evaluated well using MR imaging; this is particularly true of the bone marrow. Cortical bone should appear as low signal intensity owing to the lack of mobile protons, especially on T1-weighted images (Figure 3.06). There should be no evidence of change in the contour of the vertebral bodies, which should have a homogeneous signal intensity on T1-weighted sequences (Figure 3.07).22 Because of the higher fat content of the marrow, the vertebral body will be of intermediate or higher signal intensity relative to the adjacent disc.
The normal appearance of the vertebral body on T2-weighted sequences is that of homogeneous low signal intensity when compared with that of the intervertebral discs and cerebrospinal fluid (Figure 3.08).
A variation of the normal MR imaging appearance of bone marrow may include the presence of small foci of high signal (relative to the surrounding bone marrow) on T1-weighted imaging, which reduces on the T2-weighted sequences. This most likely represents an intraosseous lipomatous deposit, a normal variation.4,5
Another common phenomenon is high signal intensity in the bone marrow on both T1-weighted and T2-weighted sequences, representative of an intraosseous haemangioma. This condition does not usually have clinical consequences for the patient and is almost invariably an incidental finding. In less than 1% of patients, haemangiomas may become symptomatic – particularly if they begin to expand, and the expansion is posterior. Such expansion is determined by evaluating the posterior wall of the vertebral body for evidence of slight posterior bulging of the cortical margin; this may suggest the presence of expansion before there is frank clinical evidence.23,24
By contrast, if high signal intensity is evident in the bone marrow on T2-weighted sequences and decreased signal intensity on T1-weighted sequences, this should be a cause for concern, since this pattern is suggestive of an infiltrative process such as metastatic disease, and should be followed with more specific sequences such as STIR or out-of-phase GRE MR imaging, and T1-weighted images with the addition of gadolinium.4
Spinal cord
The spinal cord should appear as intermediate (grey) signal intensity on all pulse sequences (Figure 3.09), although on T2-weighted sequences it may be possible to distinguish the white and grey matter of the spinal cord. The spinal cord should be evaluated for shape, size, symmetry and internal organization to exclude the presence of disorders such as spinal cord impingement or syrinx formation.
The anterior, lateral and posterior columns (white matter) are demonstrated as hypointense signal intensity on the T2-weighted sequences. The cerebrospinal fluid is visible on MR imaging, visualized well on T2-weighted sequences as hyperintense signal and on T1-weighted sequences as hypointense signal, compared to the bone marrow signal intensity.5
Intervertebral foramen
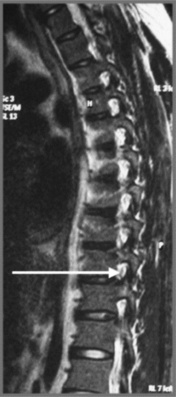
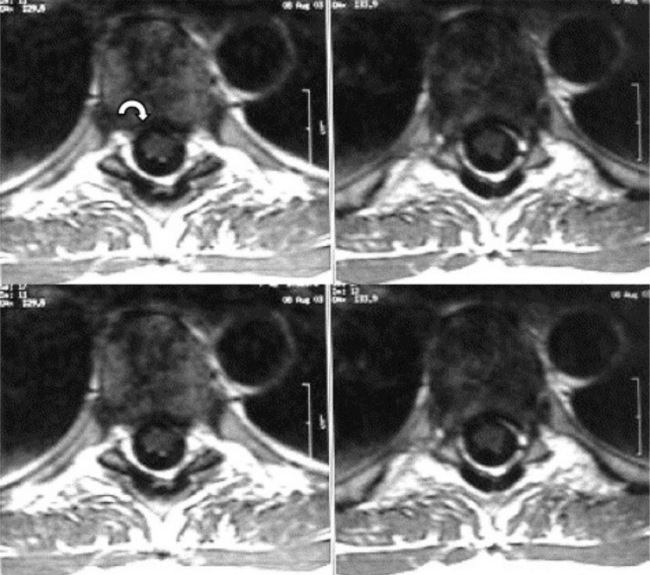
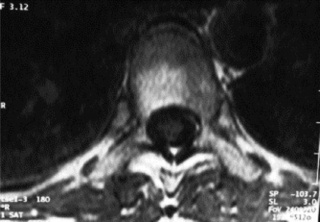
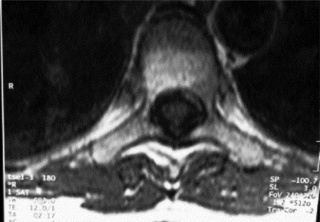
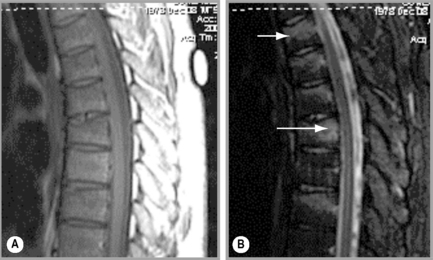
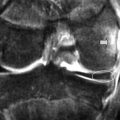
MR imaging allows for definition of the size and morphology of the intervertebral foramen as well as its contents. The thoracic foramina are best visualized on the axial and sagittal sequences (Figure 3.10). Identification of the exiting nerve roots surrounded by their perineural fat may be possible. Disorders that can involve the IVF in the thoracic spine include nerve root tumours, such as neurinomas, or abnormalities affecting the nerve root sleeve such as meningocoele or diverticulum formation (Figure 3.11).25
Pathological conditions
Thoracic disc lesions
The articulations with the ribcage make the thoracic spine the least mobile of the spinal regions; injuries to the thoracic spine and intervertebral discs are, therefore, less common than to the adjacent cervical and lumbar regions. There are, however, some factors that may predispose individual patients to disc lesions in the thoracic spine. Owing to the thoracic kyphosis, the spinal cord lies anteriorly in the spinal canal, approximating to the posterior longitudinal ligament and providing a greater propensity for ventral cord compression.26 The thoracic spinal canal is also smaller than its cervical and lumbar counterparts and, although the spinal cord too is less massive, the size relative to the canal is still greater than in other areas of the spine. The clinical consequences of this are that although thoracic disc lesions may be less common, there is a higher chance of them being symptomatic, as the neural structures are more prone to compressive or irritative pathology.13
Though not a common entity, thoracic disc lesions are, nevertheless, considerably less rare than once thought. Due to the paucity of associated clinical findings, thoracic disc lesions were difficult to diagnose before the advent of MR imaging and they were once thought to represent fewer than 5% of all disc lesions.21 The incidence of symptomatic thoracic disc lesions is still thought to be low (although this is difficult to ascertain and there is often no way to accurately determine whether a patient’s symptoms relate to discal pathology by clinical examination alone), whilst the presence of asymptomatic thoracic disc lesions may be as high as 37%21; certainly, clinicians should not readily correlate disc bulges identified by MR with their patient’s pain syndromes unless there is compelling clinical evidence to the contrary, such as a history of significant trauma causing spinal compressive impaction or indications of a space-occupying lesion such as a positive Valsalva’s sign/manoeuvre. Disc lesions in the lower thoracic spine are more likely to produce symptoms; however, thoracic disc lesions can also spontaneously reduce in size and will often spontaneously resolve.27
The mechanism of discal injury in the thoracic spine is somewhat different to that affecting the cervical and lumbar spine, principally due to the differing anatomy and biomechanical demands in this area. The thoracic region, with the thoracic cavity and rib attachments, is afforded a greater degree of functional rigidity and, to some degree, protection from the shearing forces that can progressively damage the annular fibres; however, there does appear to be some correlation with torsional movement such as that found in a golfer’s swing. The predominant mode of disc injury in the thoracic spine, however, involves vertical compressive forces, usually related to trauma such as ‘spearing’ injuries.26
When present at all, the symptoms of thoracic disc lesions are varied and often vague, increasing the difficulty of an accurate and timely diagnosis. In addition to the local thoracic spine, the pain may be referred to the abdomen or chest, providing further confusion as to the origin of the pain. Upper thoracic lesions may present with symptoms similar to thoracic outlet compression syndrome type complaints, neck pain, and sensory or motor changes. Horner’s syndrome has also been reported.28
In the lower thoracic spine, it becomes even more difficult to correlate symptomatology with a specific spinal level (Box 3.01).29 Additionally, sympathetic trunk compression can cause visceral symptoms that may be easily confused with cardiac and abdominal disorders or with postherpetic neuralgia.30
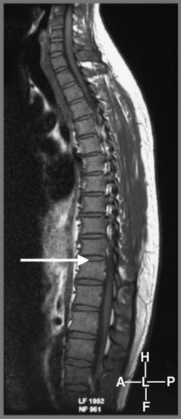
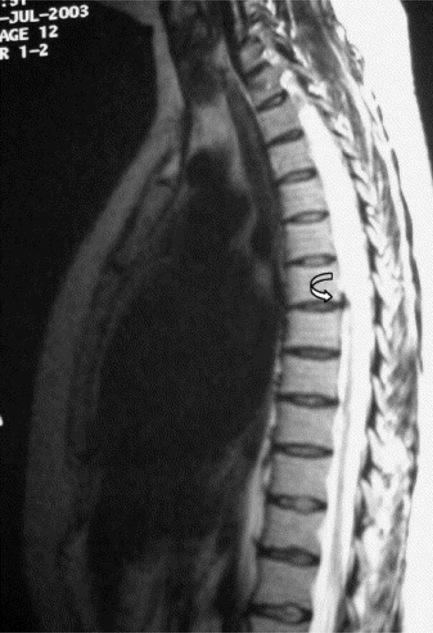
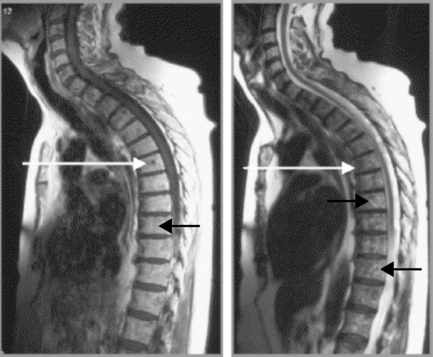
The reason for this plethora of neurologically diverse symptomatology relates to the increased chance of thoracic spinal cord compression discussed above and the fact that the lumbar and sacral nerves are, of course, traversing the thoracic canal; this can give rise to a thoracic spinal myelopathy.31 Since the spinal cord/conus medullaris terminates at L1–L2 in most adult patients, the segments of the spinal cord do not match vertebral body levels below T4.32 Lesions of the thoracolumbar region are described as epiconus syndrome, involving the L4–S2 cord segments at the T12 vertebral body level, and conus syndrome, involving cord segments S3–S5 at the T12/L1 intervertebral disc level. These syndromes can mimic cauda equina syndrome (Figures 3.12, 3.13).29
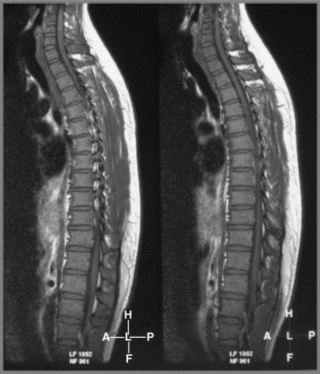
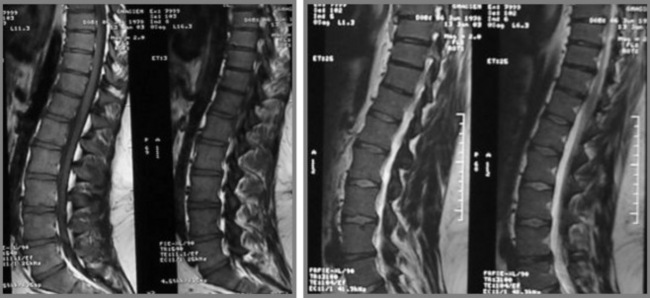
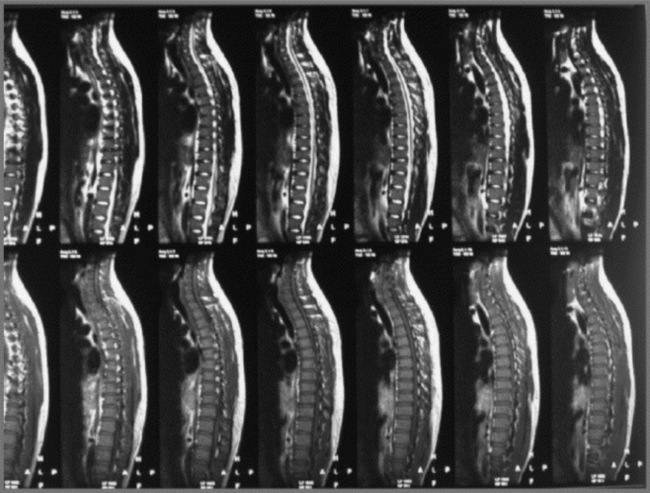
The presence of myelopathy is an indication for surgical intervention. Given the anatomy of the thoracic region, surgical intervention is complex and is considered controversial for pain relief alone.10 Owing to the high incidence of asymptomatic thoracic disc lesions, their identification should be carefully correlated with the patient’s clinical history (Figures 3.14, 3.15). The classification of a thoracic disc lesion should be related to its anatomical level, just as in the cervical and lumbar spine; however, as we have seen, this can be clinically challenging, if not impossible, even with the aid of MR imaging, which may correlate poorly to clinical or, indeed, represent incidental findings.
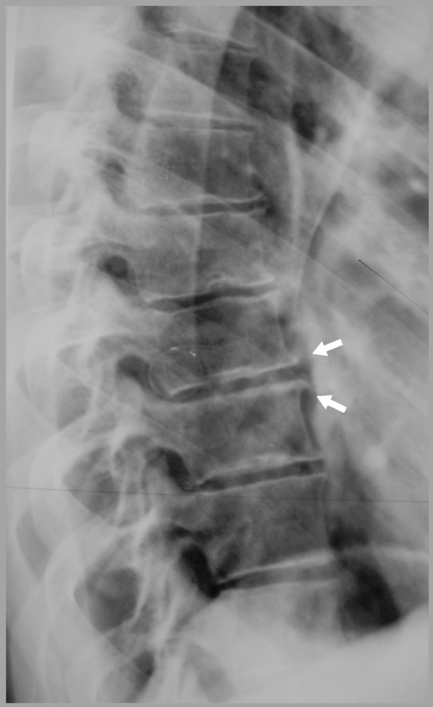
Intraosseous disc lesions are more commonly termed Schmorl’s nodes. This lesion is frequently encountered in clinical practice, where it is easily identifiable on plain film x-ray (Figures 3.16–3.18); it is usually asymptomatic. Whilst the routine radiograph will demonstrate the focal indentation along the superior or inferior endplate of one or multiple levels of the thoracic spine (though these may also be encountered in the lumbar spine too), special imaging may also detect these lesions. Often, degenerative disc disease is noted to be associated with the intraosseous disc lesion. Although MR imaging is not the imaging modality of choice for the diagnosis of these lesions, routine radiography being sufficient to make the diagnosis, it is useful to determine the imaging appearance on MR imaging that may have been performed to determine the origin of the patient’s complaint.
Scheuermann’s disease
Multiple intraosseous disc lesions may be associated with Scheuermann’s disease (also occasionally termed juvenile kyphosis or, when seen in the lumbar spine, juvenile discogenic disease), a condition affecting the development of the endplates during teenage skeletal growth and characterized by hyperkyphosis, anterior vertebral body wedging, endplate irregularities and the development of Schmorl’s nodes. It may be asymptomatic and is found commonly on thoracic spine radiographs. When symptomatic, Scheuermann’s disease typically produces an ‘aching’ type of pain. In addition to the changes seen on plain film, MR imaging may show the presence of associated intervertebral disc lesions (Figure 3.19).
The condition appears to arise from a combination of genetic factors, rendering the vertebral endplates weaker than normal because of a fragile blood supply, and the application of trauma, usually vertical compressive forces along the normal thoracic physiological curve.33 The result is the development of multiple, intraosseous herniations together with irregularity of the endplates, a cuneiform aspect to the shape of the vertebral bodies with anterior wedging, and an apparent increase in the sagittal dimension of the vertebral body. The latter finding is due to the disproportionate growth in the sagittal dimension relative to the vertical height following an interruption of the normal growth pattern of the endplates. Degenerative disc disease is often associated, with a decrease in the disc height and osteophyte formation.
Annular tears
As well as identifying the location of the thoracic disc lesion and its anatomical relationship with surrounding structures, it is also useful to determine whether there is evidence of any tear in the annulus. Annular tears or fissures, often termed HIZ (high intensity zones, owing to their MR appearance seen in Figure 3.20), may, in the thoracic spine, be associated with pain in the local area, and can therefore be a diagnostically useful finding. A thoracic annular tear or fissure is best identified on the axial or sagittal slices, and noted on the T2-weighted sequence as a small region of increased signal intensity, usually in the outer one-third of the annulus along the posterolateral margin.
Disc calcification
Additional findings in disc lesions in the thoracic spine may include calcification. In general, calcification of disc lesions is relatively uncommon, although children have a higher likelihood of disc calcification than do adults. Although usually asymptomatic, it can be associated with torticollis in the cervical and upper thoracic spine; however, this tends to be self-limiting.34–36 Whilst disc calcification is a relatively unusual phenomenon in the adult population, it may be more prevalent in the thoracic spine.37 Although detectable on routine radiographs, if MR imaging of the thoracic spine is the only modality that has been provided, it is useful to recognize calcification as a region of low signal intensity at the level of the disc both on T1- and T2-weighted sequences.
Tumours
Vertebral body haemangioma
Haemangiomas are benign, primary, vascular neoplasms that may be regarded as an abnormality in the development of bone; they are considered the most common benign neoplasm of the spine. Most lesions are asymptomatic and are considered incidental findings when seen on plain film radiography; however, occasionally, they may expand into the spinal canal, producing central canal stenosis.38,39
Haemangiomas are vascular malformation anomalies that predominate in the spine (they may also be located in soft tissues) and most frequently occur in the thoracic region. On biopsy, haemangiomas are full of thin-walled vascular channels lined with flattened endothelial cells, interspersed with adipose tissue. These, together with sinuses, lie between vertically oriented trabeculae, giving the bone its classical striated appearance on radiographs, known as a ‘corduroy cloth’ vertebra.40
MR imaging can detect very small haemangiomas not evident on plain film radiography. The location of these small haemangiomas is characteristic: on the sagittal, T2-weighted images there will often be a small linear region of high signal intensity along the posterior vertebral body; this represents the entrance of the basivertebral vein. Where this vein terminates anteriorly, a focal region of circular increase in signal intensity is often noted, which is often of the same form and signal intensity on the T1-weighted image; this region represents a small haemangioma (Figures 3.21–3.23).
This increased signal intensity on both T1- and T2-weighted sequences differentiates haemangiomas from most other tumours, which often show decreased signal intensity on T1-weighted sequences owing to their water content. However, if the haemangioma is predominantly vascular – thus with a correspondingly lower level of adipose – a less intense signal is noted on the T1-weighted sequence (the increased signal intensity of haemangiomas on T1-weighted sequences is thought to be due to the fatty components of these lesions, whereas the increased signal intensity on T2-weighted sequences is due to the water content of the tumour).38,40
In most cases, haemangiomas affect the vertebral body rather than the posterior arch. They may be of differing sizes, located in the centre of the vertebral body only or occupying the totality of the vertebral body in question. Having determined the presence of any osseous haemangioma, it is important to ascertain whether there is evidence of bony expansion, particularly posteriorly. Normally, there is a slight concavity in the posterior wall (this is not to be confused with posterior scalloping, which is a clinically significant accentuation of this normal concavity). If there is loss of this concavity or any appearance of a bulge (deformation posterior of the posterior wall), this is suggestive of posterior expansion of the haemangioma, which may provoke not only local pain but also the development of neurological symptoms and signs, dependent on the level involved.4,24 Highly vascular/low adipose haemangiomas (the ones with lower signal intensity on T1-weighted images) are also suggestive of lesions that are more likely to be associated with focal pain.
Symptoms from haemangiomas are most commonly produced from pathological fractures, haematomas, extension of the lesion into the epidural space, or from bony expansion.38,40,41 Symptomatic lesions are more likely to involve the entire vertebral body and appear more irregular and less well defined.40 Previously, treatment for symptomatic haemangiomas has included radiotherapy, embolization, and surgical excision. Vertebroplasty, the injection of methylmethacrylate (bone cement) into the vertebral body, has been used and can produce both symptomatic relief and bone strengthening. Injection of the lesion with ethanol represents another method of treatment, the ethanol acting as a sclerosing agent that shrinks the tumour.42
Metastasis
Aggressive tumour processes may also affect the thoracic spine; by far the most commonly encountered is metastatic disease. Metastasis tends to affect the older population (over the age of 50 years), although if there is a history of a childhood tumour – for example a Wilms’ tumour – the patient may develop metastatic disease at a younger age.43,44 Osseous metastatic deposits tend to affect the axial skeleton in the older patient, since the red marrow located in this region acts as the nutrient bed. In an adult, red marrow is located primarily in the axial skeleton and the proximal halves of the femurs and humeri. Where there is a clinical suspicion of metastatic disease, then, depending on local protocols, imaging may comprise routine radiography, bone scanning and then cross-sectional imaging.2
MR imaging is particularly valuable in the assessment of metastatic disease as well as the response to treatment; however, with the development of newer techniques, future evaluation may include the use of positron emission tomography (PET) as part of the routine assessment of the patient.45,46
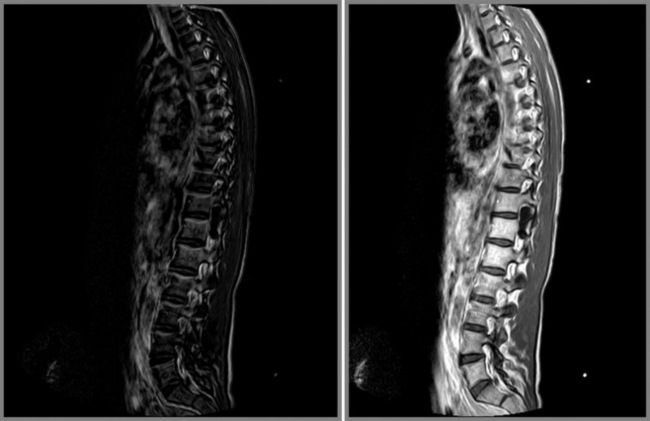
In order to recognize the presence of metastatic disease in the thoracic spine, it is essential to be thoroughly familiar with normal radiographic anatomical appearance. As we saw earlier in the chapter, the bony marrow in the thoracic spine should be of a relatively high signal on the T1-weighted images and, moreover, should be homogeneous in appearance. On the fluid-sensitive, T2-weighted sequences, the vertebral marrow should be of a lower signal intensity compared with that on the T1-weighted sequence, and again should be homogeneous (Figures 3.07, 3.08). There are, however, a number of factors that can affect the normal appearance of the marrow, including age, general health status, smoking, and anaemia.47,48
If there is metastatic disease in the thoracic spine, a focal area of increased signal intensity will initially be seen on T2-weighted sequences, usually in the vertebral body although the posterior arch can also be affected. Over time, depending on the patient’s treatment, the area of increased signal intensity may enlarge and be noted at multiple levels. On the T1-weighted sequences, the corresponding region will, in general, have low signal intensity (Figure 3.24). This represents the commonest appearance of metastatic disease affecting the bony marrow and corresponds to the osteolytic form of the condition. By contrast, osteoblastic lesions will cause a decrease in signal intensity on both the T1- and T2-weighted sequences.4,24
Vertebral body fracture
Simple compression fractures, be they due to the generalized demineralization related to senile osteoporosis or to trauma, will tend to have a preservation of the posterior vertebral body height and a reduction of the anterior height. By contrast, a pathological fracture commonly causes a global reduction in vertebral body height, both posteriorly and anteriorly (see Figure 3.11).2 Osteoporotic compression fractures are by far the commonest form of spinal fracture, affecting at least one million women every year in the USA alone, and they are particularly prevalent in the thoracic spine.49
In the case of a simple compression fracture, the majority of the increased signal intensity on the T2-weighted sequence will be located immediately underneath and parallel to the superior endplate. This is known as the zone of condensation, and is produced by the overlapping, compressed trabeculae. On the T1-weighted sequence, this appears as a region of grey, or as a slightly decreased signal (corresponding to oedema) with a hypointense signal just underneath the superior endplate (corresponding to the fracture).4
In a pathologic fracture, there may be diffuse or patchy alteration in signal intensity that may involve a significant portion of the vertebral body. This will manifest as a focus of increased signal on the T2-weighted sequence and decreased signal on the T1-weighted sequence. Furthermore, with a simple compression fracture, no additional deformation of the vertebral body will be noted posteriorly, whereas with the pathologic fracture, posterior deformation may be apparent with perhaps evidence of a soft tissue mass extending into the spinal canal (Figure 3.25).
Syrinx
A syrinx is a cyst-like expansion of the spinal cord. The term ‘syringomyelia’ is used for cysts that are lined with glial cells, whilst the term ‘hydromyelia’ implies that the cyst is lined with ependymal cells. The former usually represents an acquired cavity, whilst the latter is typically of congenital origin.50 Patients with a syrinx may present with symptoms of back pain, headaches and paresis and have altered reflexes.33 The clinical signs may also consist of a loss of pain and temperature sensations owing to interruption of the spinothalamic tracts; muscular weakness due to involvement of the anterior horn cells; and hyperreflexia due to involvement of the upper motor neurons.6
There appears to be an association between syringomyelia and atypical scoliosis patterns.33,51,52 In particular, the presence of a left-sided scoliosis and hyperkyphosis have been associated with cord syrinx, and also with Arnold-Chiari type I malformations. Syringohydromyelia may be associated with additional abnormalities of the nervous and osseous systems, including Arnold-Chiari malformation, Klippel–Feil syndrome or occipitalization of the atlas. Cord syrinxes can also occur following spinal trauma or can arise as a postsurgical complication.51
The syrinx is usually imaged in both the sagittal and axial planes (imaging of the coronal plane is not commonly performed unless scoliosis is suspected). The author’s preference is to use the sagittal T2-weighted sequence to initially determine the extent of the syrinx in the longitudinal dimension; the sagittal plane will also give an idea of the loculations associated with the syrinx. The axial slices are then useful to determine the extent of spinal cord displacement and direction of expansion (see Figure 3.26).
The MR imaging appearance of syringomyelia is that of low signal on T1-weighted sequences and high signal on T2-weighted sequences, consistent with the imaging characteristics of CSF (Figure 3.27). Following the initial identification of the syrinx, it is important to determine if there are other important associated findings, for example the presence of Arnold-Chiari malformation (see Chapter II) whereby the cerebellar tonsils will be noted to be engaged, ectopic or frankly displaced inferiorly. When symptomatic, a syrinx may be treated with surgical shunting to reduce the collection of CSF; however, syrinxes may recur and, if symptomatic, may need to be re-drained.
1 Gatterman M.I., editor. Chiropractic Management of Spine Related Disorders. Baltimore: Williams and Wilkins, 1990.
2 Rowe L., Yochum T. Principles of radiological interpretation. In: Yochum T., Rowe L., editors. Essentials of Skeletal Radiology. 2nd ed. Baltimore: Williams and Wilkins; 1996:547-585.
3 Henry-Feugeas M.C., Idy-Peretti I., Baledent O., et al. Origin of subarachnoid cerebrospinal fluid pulsations: a phase-contrast MR analysis. Magn Reson Imaging. 2000;18(4):387-395.
4 Helms C., Major N., Anderson M.W., et al. Spine. In Musculoskeletal MRI, 2nd ed, Philadelphia: Elsevier Saunders; 2009:273-323.
5 Wessely M. Magnetic resonance imaging of the thoracic spine: Part 1: normal imaging. Clinical Chiropractic. 2008;7(4):187-195.
6 Lindsay K., Bone I., Callander R. Localised neurological disease and its management. In: Neurology and Neurosurgery Illustrated. Edinburgh: Elsevier; 1991:213-466.
7 Standring S., editor. Gray’s Anatomy – The back (Section 45). Edinburgh: Elsevier, 2009.
8 Wade A. Imaging with magnetic resonance. In: Fundamental Physics for Probing and Imaging. Oxford: Oxford University Press; 2006:207-232.
9 Wade A. Medical imaging and therapy with ionising radiation. In: Fundamental Physics for Probing and Imaging. Oxford: Oxford University Press; 2006:233-266.
10 DeVries R.M., Wessely M. Magnetic resonance imaging of the thoracic spine: Part 2: common disorders. Clinical Chiropractic. 2008;8(1):33-40.
11 Bannister R. Disorders of the nerve roots and peripheral nerves. In Brain and Bannister’s Clinical Neurology, 7th ed, Oxford: Oxford University Press; 1992:420-458.
12 Green J., Silver P. The vertebral column and the back. In: An Introduction to Human Anatomy. Oxford: Oxford University Press; 1981:52-68.
13 Moore K. The back. In Clinically Oriented Anatomy, 2nd ed, Baltimore: Williams and Wilkins; 1985:565-625.
14 Wong H.K., Hui J.H., Rajan U., Chia H.P. Idiopathic scoliosis in Singapore schoolchildren: a prevalence study 15 years into the screening program. Spine. 2005;30(10):1188-1196.
15 Bates B. The musculoskeletal system. In: Bates B., editor. A Guide to Physical Examination. 3rd ed. Philadelphia: JB Lippincott; 1983:324-370.
16 Beers M., Berkow R. Musculoskeletal abnormalities. In The Merck Manual, 17th ed, West Point, PA: Merck & Co; 1999:2198-2241.
17 Berkow R. Neck, shoulder and upper limb pain. In The Merck Manual, 16th ed, West Point, PA: Merck & Co; 1992:1362.
18 Kim H.J., Ryu K.N., Choi W.S., et al. Spinal involvement of hematopoietic malignancies and metastasis: differentiation using MR imaging. Clin Imaging. 1999;23(2):125-133.
19 Ghanem N., Altehoefer C., Hogerle S., et al. Comparative diagnostic value and therapeutic relevance of magnetic resonance imaging and bone marrow scintigraphy in patients with metastatic solid tumors of the axial skeleton. Eur J Radiol. 2002;43(3):256-261.
20 Arana E., Marti-Bonmati L., Molla E., Costa S. Upper thoracic-spine disc degeneration in patients with cervical pain. Skeletal Radiol. 2004;33(1):29-33.
21 Wood K.B., Garvey T.A., Gundry C., Heithoff K.B. Magnetic resonance imaging of the thoracic spine. Evaluation of asymptomatic individuals. J Bone Joint Surg Am. 1995;77(11):1631-1638.
22 Goh S., Price R.I., Song S., et al. Magnetic resonance-based vertebral morphometry of the thoracic spine: age, gender and level-specific influences. Clin Biomech (Bristol, Avon). 2000;15(6):417-425.
23 Stoller D. Magnetic Resonance Imaging in Orthopaedics and Sports Medicine, 2nd ed. Philadelphia: Lippincott Williams and Wilkins, 1996.
24 Berquist T. MRI of the Musculoskeletal System, 4th ed. Philadelphia: Lippincott Williams and Wilkins, 2000.
25 Lecouvet F., Cosnard G. Thoraco-lumbar disc disease with nerve root impingement and differential diagnosis. J Radiol. 2002;83(9 Pt 2):1181-1189.
26 Eustace S., Johnston C., O’Neill P., O’Byrne J. Sports Injuries: Examination, Imaging and Management. Edinburgh: Elsevier Churchill Livingstone, 2007.
27 Wood K.B., Blair J.M., Aepple D.M., et al. The natural history of asymptomatic thoracic disc herniations. Spine. 1997;22(5):525-529. discussion 9–30
28 Caner H., Kilincoglu B.F., Benli S., et al. Magnetic resonance image findings and surgical considerations in T1–2 disc herniation. Can J Neurol Sci. 2003;30(2):152-154.
29 Tokuhashi Y., Matsuzaki H., Uematsu Y., Oda H. Symptoms of thoracolumbar junction disc herniation. Spine. 2001;26(22):E512-E518.
30 Wilke A., Wolf U., Lageard P., Griss P. Thoracic disc herniation: a diagnostic challenge. Man Ther 2000:(3):181-184
31 Dimar J.R.2nd, Bratcher K.R., Glassman S.D., et al. Identification and surgical treatment of primary thoracic spinal stenosis. Am J Orthop. 2008;37(11):564-568.
32 Clemente C.D. Anatomy: A Regional Atlas of the Human Body. Baltimore: Urban and Schwarzenberg, 1983.
33 Whitaker C., Schoenecker P.L., Lenke L.G. Hyperkyphosis as an indicator of syringomyelia in idiopathic scoliosis: a case report. Spine. 2003;28(1):E16-E20.
34 Beluffi G., Fiori P., Sileo C. Intervertebral disc calcifications in children. Radiol Med. 2009;114(2):331-341.
35 Dhammi I.K., Arora A., Monga J. Calcified thoracic intervertebral disc at two levels as a cause of mid-back pain in a child: a case report. J Orthop Sci. 2002;7(5):587-589.
36 Bagatur A.E., Zorer G., Centel T. Natural history of paediatric intervertebral disc calcification. Arch Orthop Trauma Surg. 2001;121(10):601-603.
37 Bazzi J., Dimar J.R., Glassman S.D. Acute calcific discitis in adults. Am J Orthop. 2002;31(3):141-145.
38 Ross J.S., Masaryk T.J., Modic M.T., et al. Vertebral hemangiomas: MR imaging. Radiology. 1987;165(1):165-169.
39 Yochum T.R., Lile R.L., Schultz G.D., et al. Acquired spinal stenosis secondary to an expanding thoracic vertebral hemangioma. Spine. 1993;18(2):299-305.
40 Friedman D.P. Symptomatic vertebral hemangiomas: MR findings. Am J Roentgenol. 1996;167(2):359-364.
41 Doppman J.L., Oldfield E.H., Heiss J.D. Symptomatic vertebral hemangiomas: treatment by means of direct intralesional injection of ethanol. Radiology. 2000;214(2):341-348.
42 Bas T., Aparisi F., Bas J.L. Efficacy and safety of ethanol injections in 18 cases of vertebral hemangioma: a mean follow-up of 2 years. Spine. 2001;26(14):1577-1582.
43 Sikorski C.W., Pytel P., Rubin C.M., Yamini B. Intradural spinal Wilm’s tumor metastasis: case report. Neurosurgery. 2006;59(4):E942-E943. discussion E3
44 Klein S.L., Sanford R.A., Muhlbauer M.S. Pediatric spinal epidural metastases. J Neurosurg. 1991;74(1):70-75.
45 Young M.F. Diagnostic imaging. In: Essential Physics for Musculoskeletal Medicine. Edinburgh: Elsevier; 2010.
46 Allison W. Imaging with magnetic resonance. In: Fundamental Physics for Probing and Imaging. Oxford: Oxford University Press; 2006:207-226.
47 Tall M.A., Thompson A.K., Vertinsky T., Palka P.S. MR imaging of the spinal bone marrow. Magn Reson Imaging Clin N Am. 2007;15(2):175-198. vi
48 Vande Berg B.C., Malghem J., Lecouvet F.E., Maldague B. Magnetic resonance imaging of normal bone marrow. Eur Radiol. 1998;8(8):1327-1334.
49 Kaplan P., Helms C., Dussault R., Anderson M. Musculoskeletal MRI. Philadelphia: WB Saunders, 2001.
50 Resnick D. Diagnosis of Bone and Joint Disorders, 3rd ed. Philadelphia: WB Saunders, 1995.
51 Spiegel D.A., Flynn J.M., Stasikelis P.J., et al. Scoliotic curve patterns in patients with Chiari I malformation and/or syringomyelia. Spine. 2003;28(18):2139-2146.
52 Ouellet J.A., LaPlaza J., Erickson M.A., et al. Sagittal plane deformity in the thoracic spine: a clue to the presence of syringomyelia as a cause of scoliosis. Spine. 2003;28(18):2147-2151.