10 The thalamus and hypothalamus
Anatomy of the thalamus
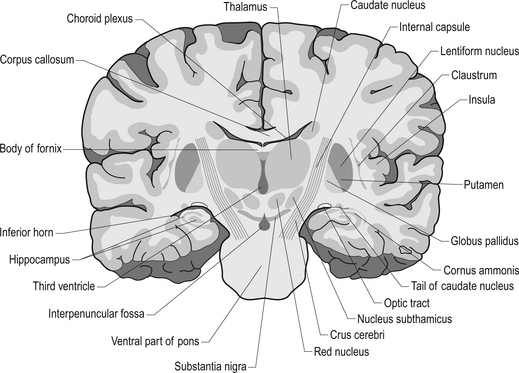
The diencephalon encloses the third ventricle and includes the thalamus with its lateral and medial geniculate bodies, the subthalamus, the epithalamus, and the hypothalamus. Each cerebral hemisphere contains a thalamus, which is a large egg-shaped mass of grey matter, in the dorsal portion of the diencephalon (Fig. 10.1). The thalamus is an important link between sensory receptors and cerebral cortex for all modalities except olfaction.
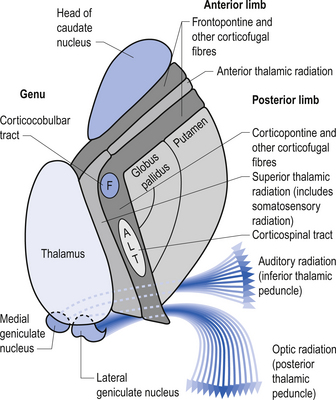
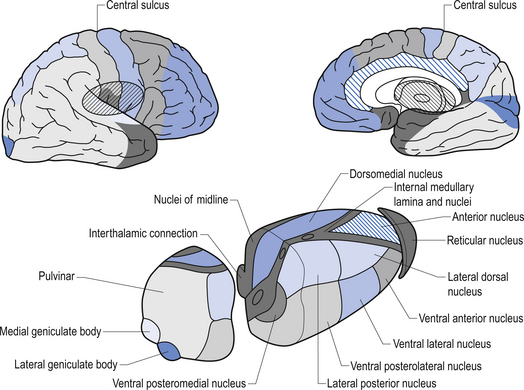
The rostral end of the thalamus, also known as the anterior tubercle, is narrower than the posterior portion of the thalamus, which contains a medial enlargement referred to as the pulvinar and a lateral enlargement referred to as the lateral geniculate body. The medial surface of the thalamus forms the lateral wall of the third ventricle and forms a connection to the medial surface of the opposite thalamus through a short communicating projection of grey matter called the massa intermedia or the central thalamic adhesion (Chusid 1982). The thalamus receives extensive projections from all of the main subcortical areas of the nervous system including spinal cord, hypothalamus, cerebellum, and the basal ganglia and forms reciprocal projections with the majority of the cerebral cortex. The connections to and from the cortex, also known as the thalamic radiations, are carried in four fibre tracts referred to as peduncles or stalks. These projections form a considerable portion of the internal capsule (Fig. 10.2). The anterior thalamic peduncle carries projection fibres from the anterior and medial thalamic nuclei to all areas of the frontal cortex. The superior peduncle carries projection fibres to and from the ventral and lateral thalamic nuclei to the pre- and postcentral gyri and premotor and presensory areas of the cortex. The posterior peduncle caries projection fibres to and from the posterior and lateral thalamic areas including the lateral geniculate body and the pulvinar to the posterior and occipital cortical areas. The inferior thalamic peduncle connects the posterior thalamic areas including the medial geniculate body to the temporal areas of cortex (Williams & Warwick 1984). The external medullary laminae are layers of myelinated fibres on the lateral surface of the thalamus immediately adjacent to the internal capsule (Fig. 10.3). The internal medullary lamina is a vertical sheet of white matter deep in the substance of the thalamus that bifurcates in the anterior portion of the thalamus to divide the substance of the thalamus into lateral, medial, and anterior segments (Fig. 10.3). The thalamus has seven groups of nuclei organised with respect to the internal medullary lamina. These include the anterior nuclear group located rostrally, the nuclei of the midline, the medial nuclei, the ventral nuclei, lateral nuclear mass which expands posteriorly to include the pulvinar, the intralaminar nuclear group, and the reticular nuclei (Fig. 10.3). Several nuclei of the thalamus are considered to be areas of singularity dependent on neural activation from the cortex to survive. These nuclei show marked transneural degeneration if the areas of cortex that project to them are damaged, understimulated, or subject to excessive inhibition (Williams & Warwick 1984). This process is an example of diaschisis, where reduced output from one area of the neuraxis results in degeneration of the downstream neuron pools.
The anterior nuclear group receives input from the ipsilateral mammillary nuclei of the hypothalamus via the mammillothalamic tract and from the presubiculum of the hippocampal formation (Fig. 10.4). Neurons in the anterior thalamic group project to regions of the cingulate and frontal cortices, mainly areas 23, 24, and 32. The anterior group of nuclei is a principal limbic component in linking the hippocampus and the hypothalamus and is involved with the modulation of memory and emotion.
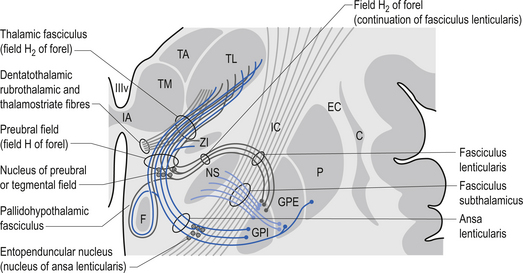
The ventral nuclear group is composed of three nuclei, the ventral anterior (VA), the ventral posterior (VP), and the ventral intermedius (VI). The ventral posterior nucleus is further divided into the functionally important ventral posterior lateral (VPL) and ventral posterior medial (VPM) nuclei. The vast majority of the fibres reaching the ventral group are from the afferent fibres of the sensory system of humans. The VPL receives projections from the contralateral cuneate and gracile nuclei via the medial lemniscal pathway and both contralateral and ipsilateral spinothalamic projections via the anterolateral system. The VPM receives projections from the trigeminal and gustatory lemnisci. These nuclei project reciprocally via the posterior limb of the internal capsule to the somatosensory areas including areas 1, 2, and 3 of cortex (see Fig. 10.2). The VI nuclei receive extensive projections from the dentate and interpositus nuclei of the cerebellum and from the basal ganglia. The VI projects to other thalamic nuclei and to the motor areas of cortex, namely areas 4 and 6. The VA nucleus receives extensive projections from the globus pallidus via the thalamic fasciculus and from the dentate nucleus of the cerebellum. The VA nucleus is therefore very important in the integration or modulation of projections from the basal ganglia and the cerebellum on the cortical areas of motor function.
The reticular nuclei form an outer shell around the lateral aspects of the thalamus. All afferent and efferent projection fibres, to and from the thalamus, pass through this reticular nuclear area. The neurons of this nucleus are predominantly GABA-ergic in nature, while other thalamic nuclei are mainly excitatory and glutaminergic. The reticular nuclei appear not to have direct projections to the cortex but only to other nuclei of the thalamus (Destexhe & Sejnowski 2003).
The lateral geniculate nucleus (LGN) appears as a swelling on the rostral surface of the pulvinar and receives afferent input from the axons of the retinal ganglion cells of the temporal half of the ipsilateral eye and the nasal half of the contralateral eye. The LGN neurons then project axons to the ipsilateral primary visual cortex via the optic radiations. The nucleus consists of six layers of nerve cells and is the terminus of about 90% of the fibres of the optic tract. The remaining 10% of fibres terminate in the pretectal areas of the mesencephalon, the superior colliculus of the tectum of the mesencephalon, and some fibres synapse directly on neurons in the hypothalamus (Snell 2001). Only 10–20% of the projections arriving in the LGN are derived directly from the retina. The remaining projections arise from the brainstem reticular formation, the pulvinar, and reciprocal projections from the striate cortex. These projections between the LGN and the striate cortex are important for a number of reasons but may play a major role in the process of physiological completion or ‘fill-in’ that occurs during visual processing in the cortex.
The medial geniculate nucleus (MGN) or body is the tonotopically organised auditory input to the superior temporal gyrus. It appears as a swelling on the posterior surface of the pulvinar. Afferent fibres arriving in the medial geniculate body from the inferior colliculus form the inferior brachium. The inferior colliculus receives projections from the lateral lemniscus. The MGN receives auditory information from both ears but predominantly from the contralateral ear. The efferent projection fibres of the MGN form the auditory radiations that terminate in the auditory cortex of the superior temporal gyrus (Snell 2001).
The physiological ‘blind spot’
It is expected that when one eye is closed the visual field should now have an area not represented by visual input, and the absence of vision over the area of the blind spot should be apparent. However, this does not occur. The cortical neurons responsible for the area of the blind spot must receive stimulus from other neurons that create the illusion that the blind spot is not there. This is indeed the case and is accomplished by a series of horizontal projecting neurons located in the visual striate cortex that allow for neighbouring hypercolumns to activate one another. The horizontal connections between these hypercolumns allow for perceptual completion or ‘fill-in’ to occur (Gilbert & Wiesel 1989; McGuire et al. 1991).
Perceptual completion refers to the process whereby the brain fills in the region of the visual field that corresponds to a lack of visual receptors. This explains why one generally is not aware of the blind spot in everyday experience. The size and shape of the blind spots can be mapped utilising simple procedures as outlined in Chapter 4.
Therefore, muscle stretch and joint mechanoreceptor potentials will alter the FOF of primary afferents that may have an effect on visual neurons associated with the cortical receptive field of the blind spot when visual afferents are in a steady state of firing. Professor Carrick proposed that ‘A change in the frequency of firing of one receptor-based neural system should effect the central integration of neurons that share synaptic relationships between other environmental modalities, resulting in an increase or decrease of cortical neuronal expression that is generally associated with a single modality’ (Carrick 1997).
• Multiple evanescent white dot syndrome;
• Acute macular neuroretinopathy;
• Acute idiopathic blind spot enlargement (AIBSE) syndrome;
• Pseudo-presumed ocular histoplasmosis;
An ophthalmoscopic examination is therefore an important component of the functional neurological examination. There are several other valuable ophthalmoscopic findings discussed in Chapter 4 that can assist with estimating the CIS of various neuronal pools.
Functions of the thalamus
Thalamic dysfunction may result in profound effects including sensory loss, thalamic pain, and involuntary movements
Spontaneous, contralateral, pain that is often excessive in nature to the stimulus may follow thalamic lesions such as infarction. This type of pain is referred to as thalamic pain and is usually not responsive to even powerful doses of analgesic drugs.
Processing of thalamic input
Sensory input from all modalities except olfaction do not reach the cerebral cortex directly but first synapse on thalamocortical relay neurons in specific regions of the thalamus. These relay neurons in turn project to their respective areas of sensory cortex via reciprocal pathways that result in a topographically organised thalamocortical loop projection system (Jones 1985). The thalamocortical relay neurons also form reciprocal connections with thalamic reticular neurons which are inhibitory. These reticular neurons also receive projections from all other afferent or efferent projections coming into or leaving the thalamus. This network thus receives bidirectional excitatory stimulus from the thalamocortical and corticothalamic loops and inhibitory input from the reticular collaterals. In addition to relaying sensory input the thalamic relay neurons also have intrinsic properties that allow them to generate endogenous threshold activity and exhibit complex firing patterns (Sherman 2001). They relay information to the cortex in the usual integrate and fire pattern unless they have recently undergone a period of inhibition. Following a period of inhibition stimulus, in certain circumstances they can produce bursts of low-threshold spike action potentials referred to as post-inhibitory rebound bursts. This activity seems to be generated endogenously and may be responsible for production of a portion of the activation of the thalamocortical loop pathways thought to be detected in encephalographic recordings of cortical activity captured by electroencephalograms (EEG) (Destexhe & Sejnowski 2003). In addition to displaying integrate and fire and burst and tonic modes of behaviour, the relay neurons can also generate sustained oscillation activity in the delta frequency range of 0.5–4 Hz (Curro Dossi et al. 1992). The thalamic reticular neurons also produce oscillatory activity but in the range 8–12 Hz (Contreras 1996). The control of the thalamic neuronal oscillations appears to be under the modulation of the cortex (Blumenfeld & McCormick 2000). In fact, it appears that cortical feedback is necessary to maintain the thalamic oscillations. One theory suggests that the thalamic oscillations are utilised by a variety of structures in the brain to promote neuroplastic change through the constant repetition of synaptic stimulation that would result from the periods of oscillations in a neural circuit. One such example would be in the formation of long-term memory. The hippocampus recalls events that have happened throughout the day and presents them to the cortex. The cortex could then stimulate the thalamus to form oscillatory excitation patterns that would result in synaptic plasticity that may constitute long-term memory (Destexhe & Sejnowski 2001).
Anatomy of the hypothalamus
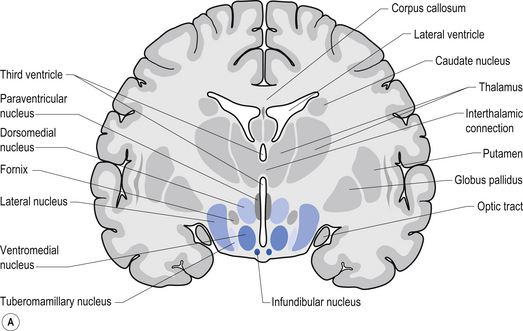
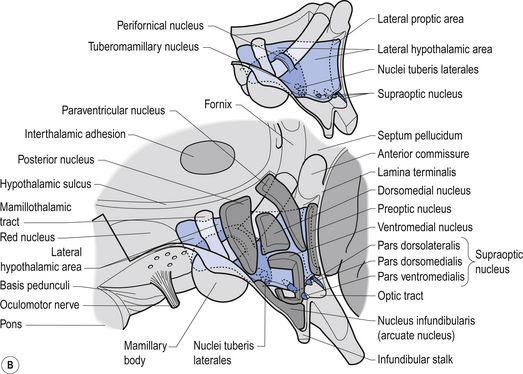
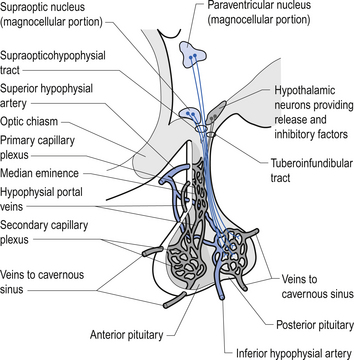
The hypothalamus is composed of a number of structures including the mammillary bodies, the tuber cinereum, the infundibulum which arises from the tuber cinereum and continues inferiorly as the pituitary stalk, the optic chiasm, and a number of nuclear groups of neurons (Chusid 1982). The nuclear groups of the hypothalamus are divided by the fornix and the mammillothalamic tract into medial and lateral zones. The medial zone contains eight distinct groups of nuclei including the preoptic nucleus, the anterior nucleus, a section of the suprachiasmatic nucleus, the paraventricular nucleus, the dorsal medial nucleus, the ventromedial nucleus, the infundibular or arcuate nucleus, and the posterior nucleus (Fig. 10.5).
The hypothalamus receives information from the rest of the body in a variety of ways that include information from the nervous system, information from the blood stream, and information from the cerebrospinal fluid (Fig. 10.5).
Afferent inputs to the hypothalamus
• The tegmentum and periaqueductal grey area of the mesencephalon;
• Areas of the anterior olfactory cortex;
• The amygdaloid nuclear complex;
• The pontomedullary reticular formation;
• Collaterals from the lemniscal somatic afferents; and
• Certain regions of the limbic cortex including the orbital frontal cortex, insular cortex, anterior cingulate cortex, and areas of the temporal cortex.
The hypothalamus receives a number of prominent projections from limbic system structures
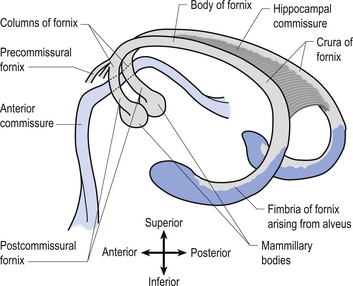
The fornix is a fibre bundle that projects from the hippocampal formation to the mammillary bodies. The fornix receives collateral contributions from the cingulate gyrus and many of the septal nuclei as it curves ventrally towards the anterior commissural area. The fornix divides into two columns or crura at the anterior commissural area (Fig. 10.6). The hippocampal commissure is a collection of transverse fibres connecting the two crura throughout most of the length of the fornix. Before the anterior commissure intersects with the crural fibres the fornix gives rise to precommissural projections to the preoptic regions of the hypothalamus. The postcommissural fornix gives rise to projections to the dorsal, lateral, and periventricular regions of the hypothalamus before terminating in the mammillary bodies of the hypothalamus.
The amygdaloid complex projects to the preoptic regions and to a variety of other hypothalamic nuclei via the amygdalohypothalamic fibres that arise from two different pathways, the stria terminalis and the ventral amygdalofugal tract (Fig. 10.7).
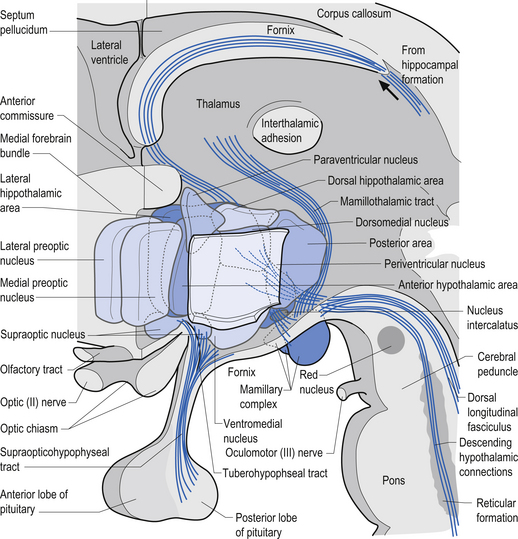
The medial forebrain bundle constitutes the main longitudinal pathway of the hypothalamus and contains both afferent and efferent fibres. Fibres from the septal nuclei, the olfactory cortex, and orbitofrontal cortex descend in this tract to the hypothalamic nuclei. Fibres from the pontomedullary reticular formation, the ventral tegmental cholinergic and noradrenergic projection systems, and mesolimbic dopamine projection system ascend in the medial forebrain bundle (Fig. 10.7).
Efferent projections of the hypothalamus
The three major efferent projection systems of the hypothalamus include:
Functions of the hypothalamus
Hypothalamic projections that originate mainly from the paraventricular and dorsal medial nuclei influence both parasympathetic and sympathetic divisions of the autonomic nervous system. These descending fibres initially travel in the medial forebrain bundle and then divide to travel in both the periaqueductal grey areas and the dorsal lateral areas of the brainstem and spinal cord. They finally terminate on the neurons of the parasympathetic preganglionic nuclei of the brainstem, the neurons in the intermediate grey areas of the sacral spinal cord usually beginning below the L2 spinal cord level, and the neurons in the intermediolateral cell column of the thoracolumbar spinal cord which usually habitate the spinal cord between the levels of T1 and L2. Descending autonomic modulatory pathways also arise from the nucleus solitarius, noradrenergic nuclei of the locus ceruleus, raphe nuclei, and the pontomedullary reticular formation.
The hypothalamus may play an important function in the emotional modulation of autonomic pathways and immune system function through influences of the limbic system projections it receives (Beck 2005).
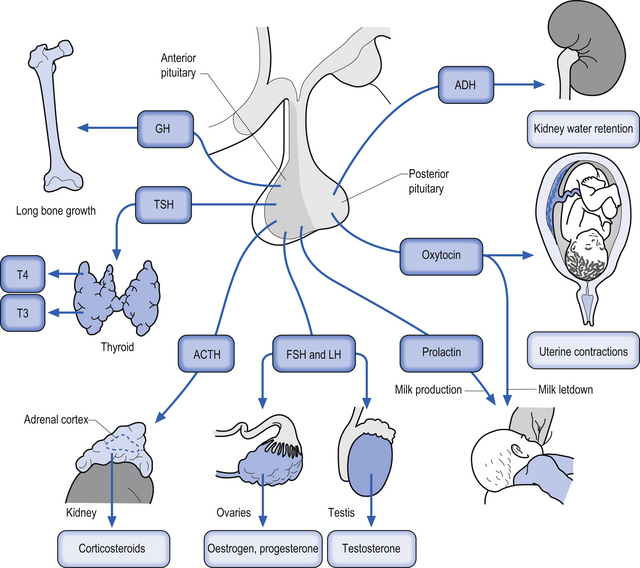
A variety of homeostatic functions are also modulated by the hypothalamus. The suprachiasmatic nucleus regulates circadian rhythms; the lateral hypothalamus regulates appetite and body weight set points; the ventromedial nucleus inhibits appetite, where dysfunctions in this nucleus can result in obesity; the anterior regions of the hypothalamus regulate thirst, and both anterior and posterior hypothalamic regions are involved in thermoregulation. Sexual desire and other complex emotional states are also modulated by hypothalamic nuclei. Neuroendocrine control mechanisms operate mainly through the pituitary. Parvocellular neurons project to the median eminence to control the anterior pituitary gland. The hypothalamus does this indirectly via release of neurotransmitters and peptides into the highly fenestrated portal venous system and promotes the release of ‘releasing hormones’ and ‘release-inhibiting hormones’. Magnocellular neurons continue down the stalk to the posterior pituitary gland, directly into its general circulation. The hypothalamus promotes the release of oxytocin and vasopressin (Figs 10.8 and 10.9). The intimate relationship between the hypothalamus, the pituitary gland, and the adrenal gland, which is modulated by hormones released by the pituitary gland, is referred to as the hypothalamus–pituitary–adrenal axis. This system is responsible for numerous homeostatic responses of the neuraxis and has been implicated in the negative aspects of the stress response. When a disturbance in the homeostatic state is detected, both the sympathetic nervous system and the hypothalamus–pituitary–adrenal axial system become activated in the attempt to restore homeostasis via the resulting increase in both systemic (adrenal) and peripheral (postganglionic activation) levels of catecholamines and glucocorticoids. In the 1930s, Hans Selye described this series of events or reactions as the general adaptation syndrome or generalised stress response (Selye 1936). Centrally, two principal mechanisms are involved in this general stress response; these are the production and release of corticotrophin-releasing hormone produced in the paraventricular nucleus of the hypothalamus and increased norepinephrine release from the locus ceruleus norepinephrine-releasing system in the brainstem. Functionally, these two systems cause mutual activation of each other through reciprocal innervation pathways (Chrousos & Gold 1992). Activation of the locus ceruleus results in an increased release of catecholamines, of which the majority is norepinephrine, to wide areas of cerebral cortex and subthalamic and hypothalamic areas. The activation of these areas results in an increased release of catecholamines from the postganglionic sympathetic fibres as well as from the adrenal medulla.
This results in a number of catecholamine-mediated responses such as increased heart rate, increased blood pressure, and increased glucose release into the blood (see Chapter 8 for a more complete list of responses).
Case 10.2
10.2.1
The blind spot has been found to increase in size because of the following conditions:
• Multiple evanescent white dot syndrome;
• Acute macular neuroretinopathy;
• Acute idiopathic blind spot enlargement (AIBSE) syndrome.
• Pseudo-presumed ocular histoplasmosis;
• Peripapillary retinal dysfunction;
• Systemic vascular disease; and
• Decreased cortical activity contralaterally to the enlarged blind spot.