11 The temporomandibular articulation
Introduction
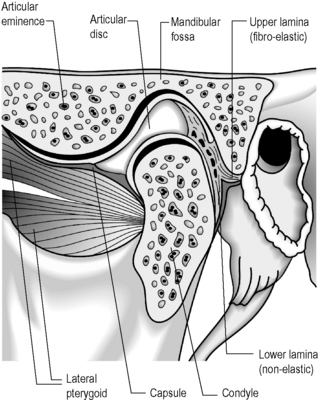
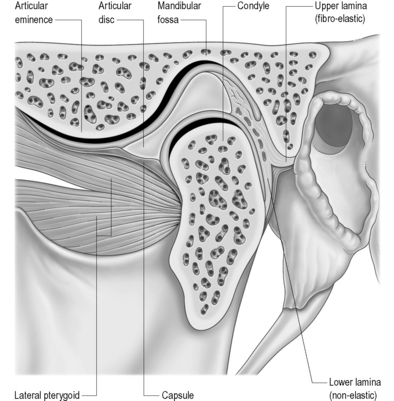
The temporomandibular articulation (TMA) is formed between the ovoid mandibular condyle and its complementary elliptical articulating surface on the glenoid fossa of the temporal bone (Figure 11.01).1,2 Although a true synovial joint, it eludes traditional classification and different authors will place it in different groups.
Many simpler texts will term it a hinge joint (ginglymus)3; however, this overlooks the gliding phase of movement, typical of arthrodial joints – for this reason, some authors classify the joint in a unique category, terming it ginglymoarthrodial.4 Although this describes the joint from a functional standpoint, structurally it is more commonly classified as a bicondylar joint; that is an ellipsoidal (or condylar) joint – one in which an ovoid head of one bone moves in an elliptical cavity of another (permitting all movements except axial rotation) with the additional feature of a meniscus between the articular surfaces. This feature also defines it as a complex joint.5–7
Regardless of the semantics over the joint’s classification (it is most probably easiest to regard it as unique; there is no directly comparable joint), the important thing for the clinician to understand is that it is composed of a series of complicated, interrelated osseous and soft tissue structures, each of which may be implicated in clinical syndromes. As such, the joint can be responsible for a significant level of morbidity with local symptoms including pain, restriction, stiffness, crepitus and clicking.8 It can also be responsible for referred pain syndromes and is an often overlooked cause of headache9–11; facial pain12,13; vertigo, tinnitus and ear symptoms14–18; and interrelated dysfunction elsewhere in the stomatognathic system (the dentition, mandible, cranium, spine and pelvis).19–21
The osseous structures centre around the articulating surfaces and the adjacent coronoid process; the soft tissue elements include the articular disc and its various attachments, a multitude of delicate ligaments, muscular insertion points and synovial and capsular components.2,3 There is considerable dispute about the contribution of these various elements to the symptoms of temporomandibular disorder (TMD),8 and diagnosis always needs to be carefully correlated with history and clinical findings; however, magnetic resonance (MR) imaging offers the clinician the chance to evaluate the articular disc in great detail. It is essential, therefore, to be able to differentiate the well-documented physiological, age-related changes from pathological conditions, which include internal derangement, tears, abnormal displacement (with or without reduction), dislocation and perforation. These, in turn, must be differentiated from internal signal abnormalities.22
History and examination
Because of the sequelae to and comorbidity with TMD, it is important to elicit a basic dental history from all patients. This should cover several key points (Box 11.01).23,24 As with any clinical history, it is also necessary to undertake a systems review and to explore psychological history, which is particularly pertinent in chronic cases.24,25
Box 11.01 The key points in eliciting a basic dental history from patients with temporomandibular disorder
Owing to the interrelated complexity of head and face pain disorders, it is necessary to evaluate non-masticatory structures in order to properly establish a differential diagnosis. This screening should comprise examination of the eyes, ears, cervical spine and cranial nerves.26–28
The examination of the temporomandibular joint should cover the muscles of mastication, the joint itself and the teeth.24 Whereas this last is more properly the province of the dentist or orthodontist, it is perfectly simple for any competent clinician to note the absence or significant decay of teeth, excessive wear or obvious malocclusion – all of which can be primary causative factors in TMD29–33 – and, if necessary, refer for an expert opinion and reconstructional or orthodontic intervention.
The examination of myofascial structures should start with observation of facial symmetry and evidence of muscle atrophy. Digital palpation should then be used to elicit the presence of myofascial trigger points in the muscles of mastication – temporalis, masseter and the medial and lateral pterygoids – plus associated muscles, including the digastric, suprahyoid, sternocleidomastoid and the intrinsic and extrinsic muscles of the cervical spine.34
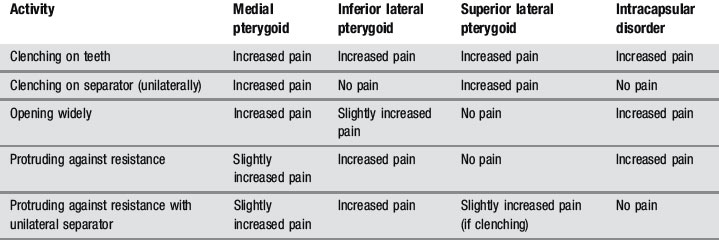
Because the pterygoids can be hard to palpate, functional manipulation can be used to assess these muscles. This is accomplished by contracting the muscles by clenching, both against the opposing teeth and a separator, and by protruding against resistance and then stretching by passive and active opening of the mouth. An increase in pain is regarded as a positive finding, although this needs to be carefully differentiated from intracapsular pain and restriction (Table 11.01).24,35
When evaluating temporomandibular gait, any lateral deviation on opening and closing should be noted, as well as the smoothness of transition from swing to glide phases. Trismus (popping, clicking or grating sounds from within the joint) can often be felt more easily than heard if the fingers are placed lightly over the joints during gait; alternatively, a stethoscope may be used. When the mouth is fully open, the maximum interincisal distance should be between 53 and 58 mm in an adult, equivalent to the width of the middle three fingers.36–39
Differential diagnosis
Temporomandibular disorders need to be differentiated from other facial pain syndromes, including: osseous disease; dental conditions; salivary and lymphatic disorders; neuropathic pain and headache; and miscellaneous conditions such as atypical facial pain.40 This is usually easy to achieve by competent history-taking and examination without recourse to diagnostic imaging.8
The different aetiologies of TMD also need to be differentiated, as the underlying cause will obviously affect both management and prognosis. The classification of TMD is detailed in Box 11.02; diagnostic imaging is of particular use in differentiating types II and IV TMD.8
Clinical indications for diagnostic imaging
Temporomandibular disorders can be classified as extra-articular or intra-articular, or can of course be a combination of both. Extra-articular causes of TMD are usually muscular in origin and are a common clinical entity presenting to a wide variety of primary healthcare practitioners. Aetiological factors include bruxism, often exacerbated by stress or anxiety, trauma, and occlusal disruption.41
The intra-articular causes of temporomandibular disorders, also known as arthrogenous TMD, are most commonly attributed to abnormalities affecting the meniscus: displacement, either temporary or chronic, degenerative joint disease, inflammatory disorders such as rheumatoid arthritis, infection or, rarely, tumour processes.8,42,43 Discal pathology is where MR imaging may be most useful, to determine the resting position of the meniscus and assess its movement during temporomandibular gait.44 Some meniscal dysfunction is also related to myopathology; for example, anterior disc displacement in the closed position, which reduces with opening, has been linked with a lateral pterygoid spasm, evidence of which may be detectable with MR imaging.45,46
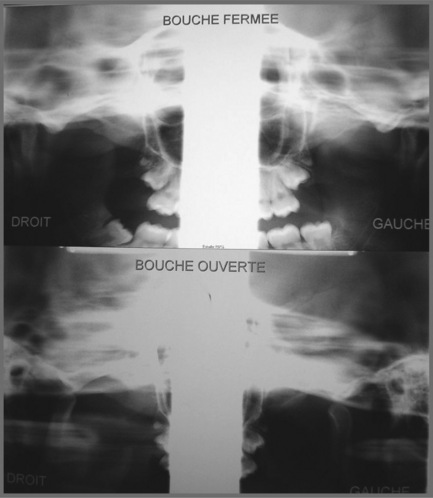
In former years, imaging of the TMA consisted of routine radiography, including the panoramic views which can provide some information regarding the shape, size and general state of the mandibular condyles (Figure 11.02); conventional tomography was also used to assess the open and closed mouth (Figure 11.03).47 High-resolution ultrasound may also be sensitive enough to detect gross internal derangement affecting the TM joint.48–50 Although computed tomography (CT) has also provided detail regarding the bony parameters of the TMA, MR imaging excels in the evaluation of the soft tissue components of the TMA, so often the origin of the patient’s complaint.49,51,52 Current knowledge of TMA pathologies has largely relied on both the clinical presentations of a range of conditions as well as the use of basic, invasive and not always helpful imaging modalities. With the advent of MR imaging, the understanding of the osseous component of articulations has been furthered and the soft tissues in and about the articulation have also moved firmly into the spotlight.22,47,49
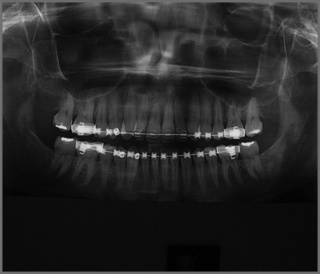
Figure 11.02 • Panoramic digital imaging of the mandible illustrating the typical appearance of this image.
The distinct advantage of MR imaging and MR arthrography is that they allow for the excellent visualization of both bony and soft tissue anatomy using a technique that is only minimally invasive (Figures 11.04, 11.05). The advancement of the technology of imaging has led to significant improvement in the understanding of the complex nature of the anatomy and hence the pathology encountered in the TMA.53–55
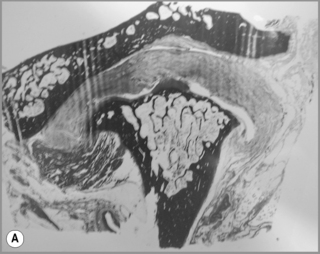
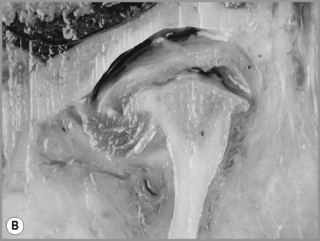
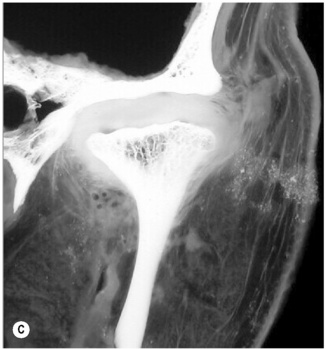
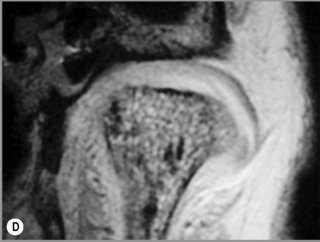
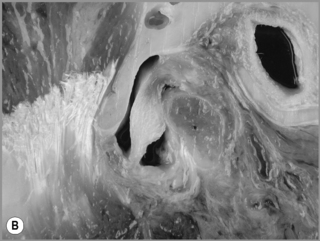
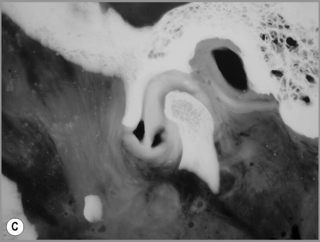
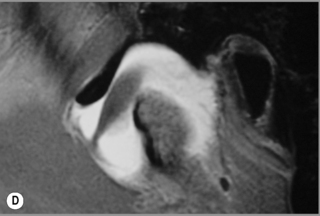
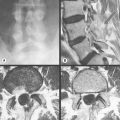
Figure 11.05 • Images of the same male cadaver as Figure 11.04. (A) Coronal slice through the right TMA at a microscopic level (right is lateral). Note the irregularities of the mandibular condyle, which represents a normal variation of the condylar surface, not usually appreciated on plain radiographs but which can be appreciated on special imaging. (B) The same specimen seen with the naked eye, allowing appreciation of the relationship between the previous image and the gross anatomy. (C) Faxitron image (radiograph) performed in the AP view demonstrating the relationship between the meniscus, mandibular condyle and the glenoid fossa of the temporal bone. (D) MR T1-weighted arthrogram showing how the meniscus is draped over the mandibular condyle, which is somewhat irregular.
Protocol
Normally, the patient is imaged in the axial plane to determine the orientation of the anterior condyles. From this, the sagittal oblique sequences are planned, with the slices perpendicular to this line, in both the closed- and then the open-mouth position (Figures 11.06, 11.07), noting in particular the location of the meniscus in both positions. Coronal oblique images may also be performed and are planned off the initial axial sequence along the long axis of the condyles. Both T1-weighted and T2-weighted images may be performed, in both the closed- and open-mouth positions, depending on the preference of the imaging centre and based on the type of MR machine.56–58
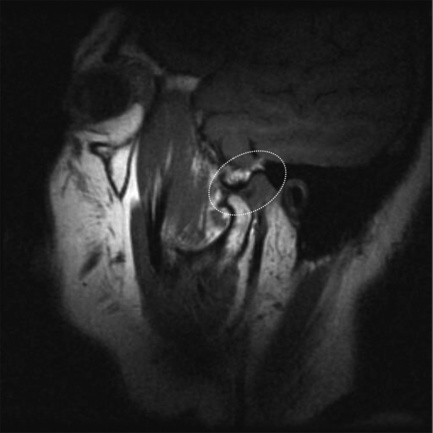
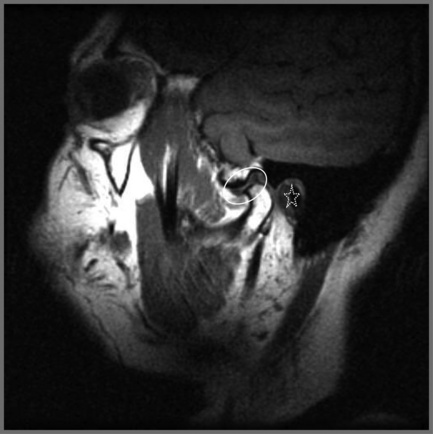
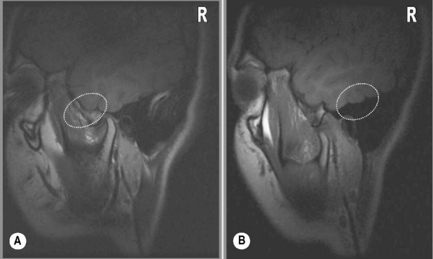
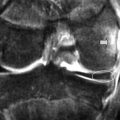
Figure 11.07 • Sagittal oblique, T1-weighted image of a normal temporomandibular articulation in the open-mouth position. Note the altered position of the mandibular condyle with respect to the glenoid fossa of the temporal bone (Figure 11.02). Anterior translation of the mandibular condyle has occurred, with an alteration of the relationship with the meniscus (oval).
Normal imaging anatomy
Osseous structures
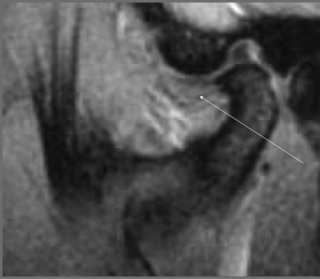
Normally, on the sagittal image, the condyles should be oval in shape and covered by a thin layer of cortical bone (Figure 11.08), which on the MR image will appear with low signal intensity. The signal intensity should be homogeneous and, on the fluid-sensitive sequences, should be intermediate to low signal. On the T1-weighted sequence, the signal intensity should be brighter than on T2-weighting and be the same as that in the remaining normal bone marrow visualized elsewhere on the image (Figure 11.09).
Articular disc
The articular disc is a lozenge or elongated oval shape and has a superior and inferior surface. The superior surface is concavo-convex from anterior to posterior to conform to the contours of the articular fossa and condyle. The inferior surface is moulded to the condyle of the mandible and is, therefore, concave (Figure 11.08).1,59 The disc completely divides the TMA into two distinct compartments – superior and inferior, both of which are usually compressed – and, ordinarily, there is no interconnection; however, in the older population there can be a significant degree of degeneration in the region of the disc. This may result in partial or complete perforation of the disc, thus allowing communication between the superior and inferior joint spaces.2,60,61
The disc is attached both to the fibrous capsule and to the lateral pterygoid muscle anteriorly. There are extensions that pass medial and laterally, which head towards and insert into the mandibular condyle. The posterior portion of the disc divides into superior and inferior portions, or lamellae. The upper, or superior, lamella inserts into the posterior margin of the mandibular fossa and is composed of fibroelastic tissue. The lower, or inferior, lamella is attached to the posterior condyle but is not elastic and is composed of white fibres.53
The thickest portion of the disc is just posterior to the central portion at a position that corresponds with the deepest part of the mandibular fossa. The disc has been further divided into various parts according to the anatomical appearance – anterior extension, anterior band, intermediate zone, posterior band and the bilaminar zone (upper and lower lamellae).1
Morphological evaluation of the superior and inferior surface of the disc reveals some distinct differences in the nature of pathological changes. The inferior surface is by far the more commonly affected in patients, though without discal symptoms, suggesting that there may be no correlation between the presence or absence of disc derangement and the degree of change to disc morphology. Although the exact function and related biomechanical properties of the disc have not been fully determined clearly, the highly ordered crimped nature of the collagen indicates its viscoelastic ability to be loaded, and to become stiffer with time.62,63 There is also a change in the properties of the collagen of the disc with time; it becomes stiffer.63 This loss of proteoglycans and increased water content probably makes the disc more susceptible to mechanical stresses and increases the risk of disc disruption.
On the microscopic level, fibres extend from anterior to posterior and also in the vertical direction. The chief component of the disc is the glycosaminoglycan chondroitin sulphate. There is a direct relationship between the disc morphology with respect to its length and degree of displacement. As the degree of displacement increases, so the convexity of the posterior slope of the articular eminence decreases. As the disc displacement increases, so do the inferior, superior and posterior joint spaces.64
The meniscus of the TMA is visible in all planes on MR imaging: sagittal oblique, axial and coronal oblique. The appearance of a normal meniscus is that of a biconcave structure of low signal intensity covering the mandibular condyle (Figures 11.04, 11.05). The signal intensity within the meniscus is usually homogeneous, although this depends somewhat on the age of the patient; in the older patient, small alterations in intrameniscal signal intensity may be considered normal.
In the sagittal oblique plane, the meniscus should be directly superior to the mandibular condyle in the closed-mouth position. On the T2-weighted (fluid-sensitive) sequences, the meniscus is outlined by the fluid located in the superior and inferior compartments; this is particularly pronounced on the sagittal oblique slices (Figure 11.09). There is neither means to quantify the volume of fluid that is considered normal or abnormal nor agreement as to what defines these terms; indeed, some authors consider that any fluid noted on the fluid-sensitive sequences should be considered evidence of a joint effusion. The assessment is, therefore, largely down to the experience of the individual radiologist.30,65–69
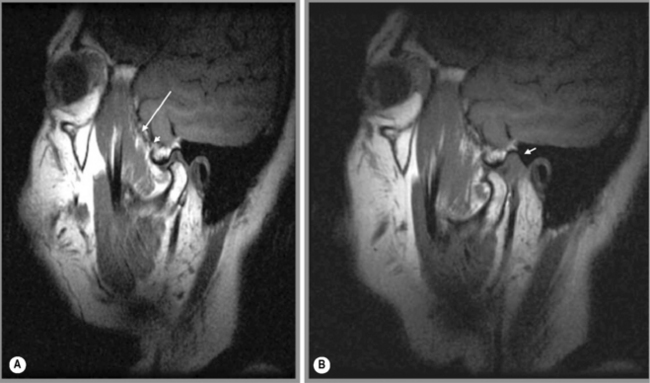
The meniscus is attached posteriorly to the articular capsule. The posterior meniscal attachment may be anatomically divided into three sections: from anterior to posterior, the temporal posterior attachment, the intermediate posterior attachment and the condylar posterior attachment. This last cannot be seen on MR imaging due to the current lack of resolution; however, the first two regions can be identified.70,71 The attachment is best identified on the sagittal oblique view, where a small band or line of low signal intensity is noted to connect the posterior part of the meniscus to the articular capsule. This can be noted in the closed-mouth and then also in the open-mouth position (Figure 11.10): it should be visible in both positions and demonstrate complete continuity with the meniscus. More specifically, the temporal posterior attachment should be visible in the closed-mouth position as a structure of low signal intensity located posterior to the mandibular condyle. It should be a thin, slightly curved region of tissue. In the open-mouth position, the temporal posterior attachment becomes more arched and is usually noted in the normal patient, placed more superiorly, towards the glenoid fossa.
Using MR imaging and this knowledge of normal discal anatomy, the disc can be assessed for disorders involving its displacement, which may reduce in the open-mouth position, remain unreduced in the open-mouth position or be displaced and/or perforated, the latter of which is the more clinically severe.72 Symptoms related to disc dysfunction may include pain, tenderness and/or noise over the articulation, limited ability to open the mouth, earache and headache.8
Muscles (Figures 11.11–11.16)
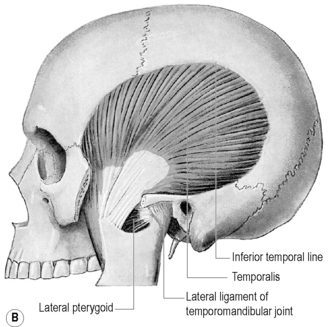
Of the muscles of mastication, the lateral pterygoid muscle has received the most attention, is the most intimately related to the TMA and has been implicated as an aetiological factor in disc pathology46,70,73; however, the medial pterygoid, masseter and temporalis muscles can also be aetiological factors and are frequently comorbid in TMD.
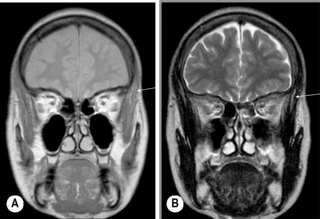
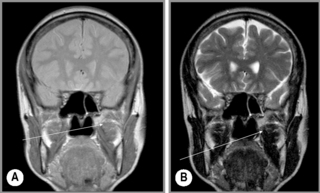
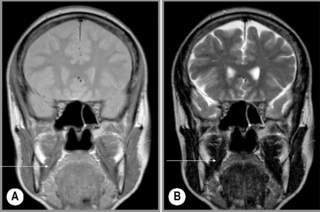
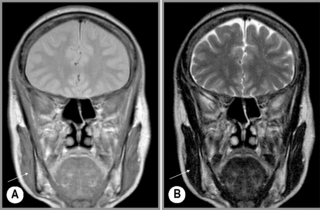
Figure 11.16 • Coronal, T1-weighted (A) and T2-weighted (B) MR imaging of the TMA demonstrating the position of the temporalis muscle (arrow), a much more diminutive muscle as compared with the masseter (Figure 11.15).
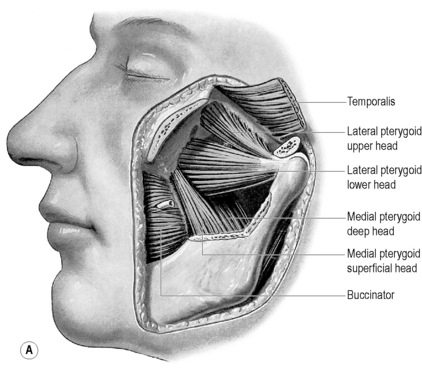
Lateral pterygoid
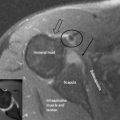
The lateral pterygoid muscle is divided into two heads: the upper or superior head and the lower or inferior head (Figures 11.11, 11.13). The superior head originates from the sphenoid bone and the infratemporal surface and crest of the greater wing of the sphenoid bone. The inferior head originates from the lateral surface of the lateral pterygoid plate. The buccal nerve and the maxillary artery pass between the two heads, both of which extend posteriorly to insert into the pterygoid fovea, inferior to the condyloid process of the mandible and into the articular capsule of the TM joint. Some controversy exists as to whether there is also an insertion into the meniscus; although this is often touted as established fact,1,2,4,74,75 actual cadaveric studies show that there is usually only an attachment to the capsule, which, in turn, has connection to the disc.70,76,77
The action of the two discrete elements of the lateral pterygoid muscle is complex and has only recently been fully appreciated. When the muscles contract bilaterally, the condyle is pulled anteriorly and inferiorly, assisting the digastric and geniohyoid muscles in opening the jaw. If only one lateral pterygoid contracts, the jaw rotates about a vertical axis passing roughly through the opposite condyle and is pulled contralaterally. This action, together with that of the medial pterygoid (see below), provides most of the medially directed force used when grinding food between teeth of the same side. The superior head is inactive during jaw opening and most active when the jaws are clenched, although most of the power generated is due to contraction of masseter and temporalis. The latter muscle also acts to potentially pull the condyle posteriorly, and the action is prevented by the simultaneous contraction of the superior head of lateral pterygoid.1,4
Medial pterygoid
As with the lateral pterygoid, its medial counterpart has two heads (Figures 11.11, 11.14). The deep medial pterygoid forms the major part of the muscle and arises from the medial surface of the lateral pterygoid plate of the sphenoid, deep to the lower lateral head of lateral pterygoid. The smaller, superficial medial pterygoid arises from the pyramidal process of the palatine bone and the maxillary tuberosity. The insertion of both heads is the medial, posteroinferior surface of the ramus and angle of the mandible.1,4 The muscles act to elevate and protrude the mandible. When acting independently, they pull the chin to the opposite side; used alternately, they can therefore produce a grinding motion.2
Masseter
The masseter muscle comprises three layers, the fibres of which blend anteriorly. The largest of these layers is the superficial masseter, which arises from the maxillary process of the zygoma and the anterior two-thirds of the inferior border of the zygomatic arch. Its fibres pass posteriorly and inferiorly, to insert into the angle and lower posterior half of the lateral surface of the mandibular ramus. The middle masseter arises from the anteromedial two-thirds and posteroinferior one-third of the zygomatic arch, inserting into the central part of the ramus of the mandible. The deep masseter arises from the deep surface of the zygomatic arch and inserts into the upper part of the mandibular ramus and its coronoid process (Figure 11.15).1 There is still debate as to whether fibres of masseter are attached to the anterolateral part of the articular disc of the temporomandibular joint; however, cadaveric studies suggest that the actual attachment is to an area of pseudodiscal material termed the premeniscal lamina.78,79
Temporalis
The largest masticatory muscle arises from the temporal fossa and the deep surface of the temporal fascia. Its fibres converge and descend into a tendon that attaches to the medial surface, apex, anterior and posterior borders of the coronoid process and to the anterior border of the mandibular ramus. Fibres of temporalis may occasionally have attachment to the articular disc (Figures 11.12, 11.16).70,78,79
Ligaments
The ligamentous capsule of the TMA extends superiorly from the articular tubercle of the temporal bone anteriorly to the region of the squamotympanic fissure posteriorly. In between these two extremes, the capsule has fibres that conform to the contour of the mandibular fossa. Inferiorly, the capsule descends to the level of the neck of the mandible. In the closed-mouth position, the superior portion of the capsule is loose, in contrast to the inferior portion, which is taut.1,75
Apart from the joint capsule, the ligaments of the TMA are small and rarely injured in isolation. The most frequent cause of ligamentous injury in this joint is dislocation, which can often be associated with a fracture of the mandible.80,81 There are three ligaments to note (Figure 11.11):
The sphenomandibular ligament lies medial to the joint capsule, with which it can sometimes blend. It is a flat, thin band that descends from the spine of the sphenoid, widening as it reaches the lingula of the mandibular foramen. Some fibres traverse the medial end of the petrotympanic fissure and attach to the anterior malleolar process.82,83
The (lateral) temporomandibular ligament is a broad ligament that arises from the zygomatic process of the temporal bone and extends inferiorly and posteriorly to attach to the lateral surface and posterior border of the neck of the condyle.84,85
The stylomandibular ligament is a thickened band of deep cervical fascia stretching from the apex and anterior surface of the styloid process of the temporal bone to the angle and posterior border of the mandible. Its function as a ligament has been debated and it can offer little in the way of biomechanical support to the joint; however, it has been postulated that it has a proprioceptive role, and injury to the ligament can cause considerable pain and dysfunction: Ernest syndrome.1,86,87
Normal variants and diagnostic pitfalls
Osseous variants
There is an interesting difference between the male and female adolescent population with respect to the contour of the articular surface of the TMA. Statistically significant differences in joint space and contour of the eminence-loading surface have been noted with a normal disc position. In addition, when an anterior disc position in the closed mouth was noted, a noticeable difference in the anatomy of the surrounding bone relationships was also seen; perhaps this goes part of the way to explain the slightly higher prevalence of TMA dysfunction in females.88
Muscular variants
There can be considerable variation in the muscles of mastication and these can be detected on MR imaging. This appears to be most clinically significant in the lateral pterygoid muscle, where two types of insertion have been described. In type 1, the superior head of the lateral pterygoid muscle appears to be attached to the meniscus and the inferior head of the lateral pterygoid muscle to the condyle. In type 2, fibres of the lateral pterygoid muscle appear to be attached to both disc and capsule whilst those of the inferior head again attach to the condyle. It has been postulated that, in type 1, the meniscus may be able to displace more easily, which in turn leads to dysfunction in the lateral pterygoid muscle, resulting in muscle atrophy. Equally, hypertonicity of this muscle in type 1 patients may predispose to meniscal displacement.46
Pathological conditions
Meniscus
The most common mechanical lesion to affect the TMA is that of discal derangement and this structure needs to be fully examined in the closed- and then open-mouth position, with the possible addition of contrast as deemed necessary.89,90
The meniscus can frequently develop a tear within it, often as part of the degenerative process in the older patient, and this may be symptomatic. The easiest way of detecting such a lesion is by assessing the signal intensity of the meniscus on the fluid-sensitive sequences. Normally, the meniscus should be a low signal intensity structure with attachments anteriorly and posteriorly.42,91
In a degenerative meniscal tear, a small collection of bright or high signal areas will be located within the body of the meniscus. With meniscal tears that are associated with trauma, in addition to these areas of high signal, joint effusion may be noted. This may act as a natural capsule dilator, accentuating the appearance of the meniscal tear and making it easier to identify. In a traumatic meniscal lesion, associated findings may occur in the attachment of the meniscus to the anterior and, more commonly, the posterior attachments to the articular capsule, which need to be assessed. Although the attachments may not be visible along the whole of their length, a close evaluation on all the slices needs to be performed in order to ensure that the meniscus is intact.92
Following the assessment of the signal intensity of the meniscus, the positional relationship needs to be evaluated with respect to the mandibular condyle and articular eminence, or glenoid fossa. Although MR techniques have even been developed to assess the TMA during small incremental degrees of opening, the normal protocol is to assess the patient with the mouth closed, establishing the position of the meniscus, and then having the mouth opened in a secured position to assess for any positional change in the meniscus, which should remain associated with the mandibular condyle, although some slight superior migration is regarded as normal.93,94
Most frequently, meniscal displacement occurs anteriorly; this is best assessed on the sagittal images. However, the meniscus can displace medially or laterally, and, in these cases, the coronal or axial images will offer the best visualization.95,96
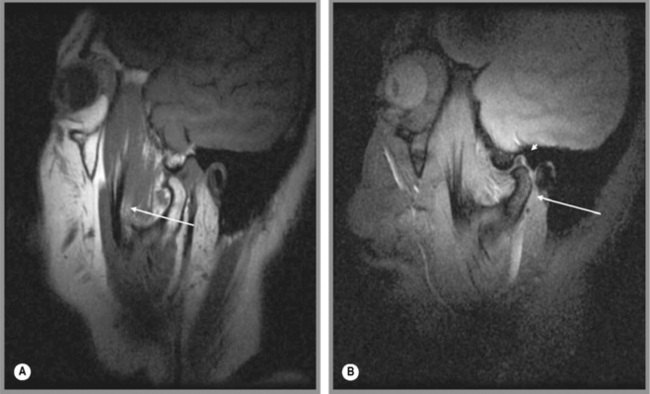
Regardless of the direction of translation of the disc, meniscal displacement can occur in two ways. Either the displacement will occur with meniscal reduction during opening or closing, or the meniscus will remain displaced; what is referred to as non-reducing displacement (Figures 11.17, 11.18). The latter condition is the more challenging to manage and, in general, is associated with a higher level of symptomatology. Whilst assessing the position of the meniscus, it is also useful to look for evidence of degenerative joint disease of the TMA; there is an association between the presence of meniscal displacement and the presence of degenerative joint disease.93,97,98
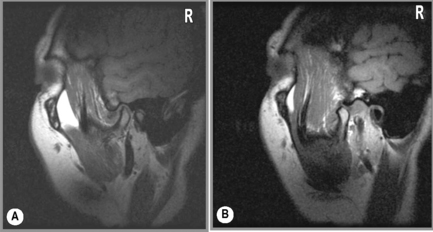
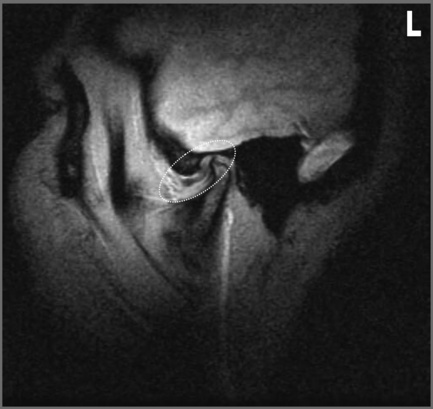
Figure 11.18 • Sagittal oblique, T1-weighted MR images of a 40-year-old female with right jaw pain. In the closed-mouth position (A), the meniscus is completely dislocated anteriorly. In the open-mouth position (B), the meniscus does regain some engagement with the mandibular condyle, representing a degree of recapture; however, this is markedly less than in Figure 11.17B.
Articular conditions
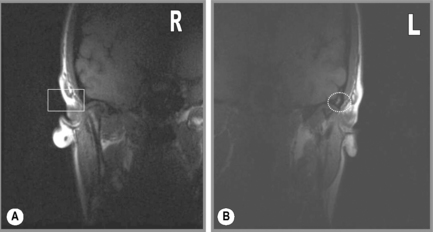
The mandibular condyles can be affected by various disorders, causing a change in their morphology or the signal intensity therefrom (Figure 11.12). In certain conditions, such as juvenile chronic arthritis, there is an underdevelopment of the mandibular condyles during skeletal growth, which results in bilateral hypoplasia. In other patients, the hypoplasia can be unilateral, resulting from clinical situations such as direct trauma to the TMA (Figure 11.19). Mandibular hypoplasia will be noted on both routine and special imaging as an underdevelopment of the mandibular condyle; the challenge for the clinician is to correlate the findings to the patient’s presentation and history to determine whether it is congenital, developmental or acquired. Associated internal derangements, particularly meniscal lesions, may be found with mandibular hypoplasia, presumably because of the altered biomechanical stress placed upon the articulation.99–101
The signal intensity of the mandibular condyle may be altered due to either an articular disorder, leading to the development of abnormality in the subchondral bone, or disorders affecting the bony marrow. Articular disorders affecting the mandibular condyle include degenerative joint disease, rheumatoid arthritis and less common conditions such as gout and calcium pyrophosphate crystal deposition disease (CPPD).98 The synovial membrane that covers the majority of the TMA may also be affected by local processes (including infection and degenerative joint disease) or by systemic synovial-based processes, including rheumatoid arthritis, tuberculosis, gout and pigmented villonodular synovitis.63,102
Degenerative joint disease (DJD) will manifest as irregularity of the articular surface, with thinning of the articular cartilage, osteophyte formation, and subchondral cysts. Although routine radiography can be used to evaluate the TMA for DJD, if internal derangements are suspected, special imaging may be more appropriate. Degenerative changes to the articular surfaces of the TMA are found most often in the lateral third of the joint. Remodelling occurs where there is an alteration of the normal articular contours of the joint and is commonly seen as thinning of the cartilage, possibly as a physiological response to the biomechanical stresses placed upon it. If a contrast study has been performed during the MR imaging, the articular cartilage may be visible and demonstrate this thinning, which can be variable in extent. Degenerative changes are associated with perforation of the disc as well as more classical signs, including osteophyte formation, subchondral cysts and denudation of the articular cartilage (Figure 11.20).60,97
In rheumatoid arthritis, marginal erosions may be seen, with thinning of the articular cartilage particularly prominent. In addition, secondary DJD findings will need to be assessed and synovial effusion may also be detectable. With the use of intravenous gadolinium, active synovitis may be detectable. Internal derangements of the TMA are often associated with rheumatoid arthritis and will be visible on MR imaging.103,104
The bony marrow of the mandibular condyle may be affected by osseous pathology. Avascular necrosis of the mandible is a phenomenon that has a well-documented association with medications used to treat osteoporosis; this makes evaluation of the mandibular condyle a particularly important target for clinicians with patients who are being managed with such pharmacological interventions. Patients may complain of discomfort about the TMA, with limitation in the normal range of movement and pain during particular movements that solicit the articulation; the physical examination may also suggest abnormality of the joint. Routine radiography may be able to detect avascular necrosis; however, the sensitivity and specificity of MR imaging in the detection of avascular necrosis, particularly at an early stage, is well documented.105,106 Avascular necrosis of the mandibular condyle is shown as a region of high signal on the fluid-sensitive sequences, in a somewhat heterogeneous appearance in the subchondral bone, which may extend to the neck of the mandible. On the T1-weighted image, irregular heterogeneous regions of low signal will be noticed interspersed in the subchondral bone. Associated lesions of the menisci may be noted.100,107,108
1 Standring S., editor. Gray’s Anatomy – Infratemporal region and temporomandibular joint. Edinburgh: Elsevier, 2009. (Section 30)
2 Moore K. The head. In Clinically Oriented Anatomy, 2nd ed, Baltimore: Williams and Wilkins; 1985:794-982.
3 Green J., Silver P. The scalp and face. In: An Introduction to Human Anatomy. Oxford: Oxford University Press; 1981:L294-L308.
4 Okeson J. Functional anatomy and biomechanics of the masticatory system. In Management of Temporomandibular Disorders and Occlusion, 6th ed, St Louis: Elsevier Mosby; 2008:2-24.
5 Young M.F. The physics of anatomy. In: Essential Physics for Musculoskeletal Medicine. Edinburgh: Elsevier, 2009.
6 Koenigsberg R., editor. Churchill’s Illustrated Medical Dictionary. New York: Churchill Livingstone, 1989.
7 Standring S., editor. Functional anatomy of the musculoskeletal system. In: Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 39th ed, Edinburgh: Elsevier, 2005.
8 Okeson J. Signs and symptoms of temporomandibular disorders. In Management of Temporomandibular Disorders and Occlusion, 6th ed, St Louis: Elsevier Mosby; 2008:164-215.
9 Taub D., Stiles A., Tucke A.G. Hemicrania continua presenting as temporomandibular joint pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):e35-e37.
10 Mongini F. Temporomandibular disorders and tension-type headache. Curr Pain Headache Rep. 2007;11(6):465-470.
11 Lupoli T.A., Lockey R.F. Temporomandibular dysfunction: an often overlooked cause of chronic headaches. Ann Allergy Asthma Immunol. 2007;99(4):314-318.
12 Isong U., Gansky S.A., Plesh O. Temporomandibular joint and muscle disorder-type pain in U.S. adults: the National Health Interview Survey. J Orofac Pain. 2008;22(4):317-322.
13 Janal M.N., Raphael K.G., Nayak S., Klausner J. Prevalence of myofascial temporomandibular disorder in US community women. J Oral Rehabil. 2008;35(11):801-809.
14 Bjorne A. Assessment of temporomandibular and cervical spine disorders in tinnitus patients. Prog Brain Res. 2007;166:215-219.
15 Bjorne A., Agerberg G. Reduction in sick leave and costs to society of patients with Meniere’s disease after treatment of temporomandibular and cervical spine disorders: a controlled six-year cost-benefit study. Cranio. 2003;21(2):136-143.
16 Bjorne A., Agerberg G. Symptom relief after treatment of temporomandibular and cervical spine disorders in patients with Meniere’s disease: a three-year follow-up. Cranio. 2003;21(1):50-60.
17 Wright E.F. Otologic symptom improvement through TMD therapy. Quintessence Int. 2007;38(9):e564-e571.
18 Tuz H.H., Onder E.M., Kisnisci R.S. Prevalence of otologic complaints in patients with temporomandibular disorder. Am J Orthod Dentofacial Orthop. 2003;123(6):620-623.
19 Howat J. Chiropractic: Anatomy and Physiology of Sacro Occipital Technique. Oxford: Cranial Communication Systems, 1999.
20 Chaitow L., Commeaux Z. Cranial Manipulation. Edinburgh: Elsevier, 2005.
21 Alcantara J., Plaugher G., Klemp D.D., Salem C. Chiropractic care of a patient with temporomandibular disorder and atlas subluxation. J Manipulative Physiol Ther. 2002;25(1):63-70.
22 Wessely M., Young M.F. Magnetic resonance imaging of the temporomandibular joint. Clinical Chiropractic. 2008;11(1):37-44.
23 Bates B. The musculoskeletal system. In: Bates B., editor. A Guide to Physical Examination. 3rd ed. Philadelphia: JB Lippincott; 1983:324-370.
24 Okeson J. History and examination for temporomandibular disorders. In Management of Temporomandibular Disorders and Occlusion, 6th ed, St Louis: Elsevier Mosby; 2008:216-285.
25 Yatani H., Studts J., Cordova M., et al. Comparison of sleep quality and clinical and psychologic characteristics in patients with temporomandibular disorders. J Orofac Pain. 2002;16(3):221-228.
26 Drum R.K., Fornadley J.A., Schnapf D.J. Malignant lesions presenting as symptoms of craniomandibular dysfunction. J Orofac Pain. 1993;7(3):294-299.
27 Yoon S.Z., Lee S.I., Choi S.U., et al. A case of facial myofascial pain syndrome presenting as trigeminal neuralgia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(3):e29-e31.
28 Foreman P.A. Chronic orofacial pain: a clinical challenge. N Z Dent J. 2008;104(2):44-48.
29 Kanehira H., Agariguchi A., Kato H., et al. Association between stress and temporomandibular disorder. Nihon Hotetsu Shika Gakkai Zasshi. 2008;52(3):375-380.
30 Costa A.L., D’Abreu A., Cendes F. Temporomandibular joint internal derangement: association with headache, joint effusion, bruxism, and joint pain. J Contemp Dent Pract. 2008;9(6):9-16.
31 Huang G.J., Drangsholt M.T., Rue T.C., et al. Age and third molar extraction as risk factors for temporomandibular disorder. J Dent Res. 2008;87(3):283-287.
32 Huang G.J., Rue T.C. Third-molar extraction as a risk factor for temporomandibular disorder. J Am Dent Assoc. 2006;137(11):1547-1554.
33 Mackie A., Lyons K. The role of occlusion in temporomandibular disorders—a review of the literature. N Z Dent J. 2008;104(2):54-59.
34 Travell J., Simons D. Myofascial Pain and Dysfunction. Baltimore: Williams and Wilkins, 1992.
35 Thomas C.A., Okeson J.P. Evaluation of lateral pterygoid muscle symptoms using a common palpation technique and a method of functional manipulation. Cranio. 1987;5(2):125-129.
36 Agerberg G. Maximal mandibular movements in teen-agers. Acta Morphol Neerl Scand. 1974;12(2):79-102.
37 Agerberg G., Osterberg T. Maximal mandibular movements and symptoms of mandibular dysfunction in 70-year old men and women. Sven Tandlak Tidskr. 1974;67(3):147-163.
38 Agerberg G. Maximal mandibular movements in young men and women. Sven Tandlak Tidskr. 1974;67(2):81-100.
39 Agerberg G. Maximal mandibular movements in children. Acta Odontol Scand. 1974;32(3):147-159.
40 Suarez P., Clark G. Oral conditions of 1,049 patients referred to a university-based oral medicine and orofacial pain center. Spec Care Dentist. 2007;27(5):191-195.
41 Okeson J. Etiology of functional disturbances in the masticatory system. In Management of Temporomandibular Disorders and Occlusion, 6th ed, St Louis: Elsevier Mosby; 2008:130-163.
42 El-Essawy M.T., Al-Nakshabandi N.A., Al-Boukai A.A. Magnetic resonance imaging evaluation of temporomandibular joint derangement in symptomatic and asymptomatic patients. Saudi Med J. 2008;29(10):1448-1452.
43 Jirman R., Fricova M., Horak Z., et al. Analyses of the temporomandibular disc. Prague Med Rep. 2007;108(4):368-379.
44 Okeson J. Diagnosis of temporomandibular disorders. In Management of Temporomandibular Disorders and Occlusion, 6th ed, St Louis: Elsevier Mosby; 2008:285-332.
45 Bakke M., Moller E., Werdelin L.M., et al. Treatment of severe temporomandibular joint clicking with botulinum toxin in the lateral pterygoid muscle in two cases of anterior disc displacement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(6):693-700.
46 Taskaya-Yilmaz N., Ceylan G., Incesu L., Muglali M. A possible etiology of the internal derangement of the temporomandibular joint based on the MRI observations of the lateral pterygoid muscle. Surg Radiol Anat. 2005;27(1):19-24.
47 Lewis E.L., Dolwick M.F., Abramowicz S., Reeder S.L. Contemporary imaging of the temporomandibular joint. Dent Clin North Am. 2008;52(4):875-890. viii
48 Pereira L.J., Gaviao M.B., Bonjardim L.R., Castelo P.M. Ultrasound and tomographic evaluation of temporomandibular joints in adolescents with and without signs and symptoms of temporomandibular disorders: a pilot study. Dentomaxillofac Radiol. 2007;36(7):402-408.
49 Tvrdy P. Methods of imaging in the diagnosis of temporomandibular joint disorders. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151(1):133-136.
50 Vilanova J.C., Barcelo J., Puig J., et al. Diagnostic imaging: magnetic resonance imaging, computed tomography, and ultrasound. Semin Ultrasound CT MR. 2007;28(3):184-191.
51 Gonzalez Y.M., Greene C.S., Mohl N.D. Technological devices in the diagnosis of temporomandibular disorders. Oral Maxillofac Surg Clin North Am. 2008;20(2):211-220. vi
52 Hussain A.M., Packota G., Major P.W., Flores-Mir C. Role of different imaging modalities in assessment of temporomandibular joint erosions and osteophytes: a systematic review. Dentomaxillofac Radiol. 2008;37(2):63-71.
53 Murakami K., Nishida M., Bessho K., et al. MRI evidence of high signal intensity and temporomandibular arthralgia and relating pain. Does the high signal correlate to the pain? Br J Oral Maxillofac Surg. 1996;34(3):220-224.
54 Westesson P.L., Brooks S.L. Temporomandibular joint: relationship between MR evidence of effusion and the presence of pain and disk displacement. Am J Roentgenol. 1992;159(3):559-563.
55 Haley D.P., Schiffman E.L., Lindgren B.R., et al. The relationship between clinical and MRI findings in patients with unilateral temporomandibular joint pain. J Am Dent Assoc. 2001;132(4):476-481.
56 Kaplan P., Helms C., Dussault R., Anderson M. Temporomandibular joint. In: Musculoskeletal MRI. Philadelphia: WB Saunders; 2001:169-173.
57 Taskaya-Yilmaz N., Ogutcen-Toller M. Magnetic resonance imaging evaluation of temporomandibular joint disc deformities in relation to type of disc displacement. J Oral Maxillofac Surg. 2001;59(8):860-865. discussion 5–6
58 Gynther G.W., Holmlund A.B., Reinholt F.P., Lindblad S. Temporomandibular joint involvement in generalized osteoarthritis and rheumatoid arthritis: a clinical, arthroscopic, histologic, and immunohistochemical study. Int J Oral Maxillofac Surg. 1997;26(1):10-16.
59 Joseph J. Locomotor system: temporomandibular joint. In: Hamilton W., editor. Textbook of Human Anatomy. 2nd ed. London: MacMillan; 1976:82-83.
60 Stratmann U., Schaarschmidt K., Santamaria P. Morphometric investigation of condylar cartilage and disc thickness in the human temporomandibular joint: significance for the definition of osteoarthrotic changes. J Oral Pathol Med. 1996;25(5):200-205.
61 Akerman S., Kopp S., Rohlin M. Histological changes in temporomandibular joints from elderly individuals. An autopsy study. Acta Odontol Scand. 1986;44(4):231-239.
62 Berkovitz B.K. Crimping of collagen in the intra-articular disc of the temporomandibular joint: a comparative study. J Oral Rehabil. 2000;27(7):608-613.
63 Berkovitz B.K. Collagen crimping in the intra-articular disc and articular surfaces of the human temporomandibular joint. Arch Oral Biol. 2000;45(9):749-756.
64 Smith H.J., Larheim T.A., Aspestrand F. Rheumatic and nonrheumatic disease in the temporomandibular joint: gadolinium-enhanced MR imaging. Radiology. 1992;185(1):229-234.
65 Lee S.H., Yoon H.J. MRI findings of patients with temporomandibular joint internal derangement: before and after performance of arthrocentesis and stabilization splint. J Oral Maxillofac Surg. 2009;67(2):314-317.
66 Emshoff R., Rudisch A. Temporomandibular joint internal derangement and osteoarthrosis: are effusion and bone marrow edema prognostic indicators for arthrocentesis and hydraulic distention? J Oral Maxillofac Surg. 2007;65(1):66-73.
67 Emshoff R., Brandlmaier I., Gerhard S., et al. Magnetic resonance imaging predictors of temporomandibular joint pain. J Am Dent Assoc. 2003;134(6):705-714.
68 Emshoff R., Brandimaier I., Bertram S., Rudisch A. Magnetic resonance imaging findings of osteoarthrosis and effusion in patients with unilateral temporomandibular joint pain. Int J Oral Maxillofac Surg. 2002;31(6):598-602.
69 Rudisch A., Innerhofer K., Bertram S., Emshoff R. Magnetic resonance imaging findings of internal derangement and effusion in patients with unilateral temporomandibular joint pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(5):566-571.
70 Christo J.E., Bennett S., Wilkinson T.M., Townsend G.C. Discal attachments of the human temporomandibular joint. Aust Dent J. 2005;50(3):152-160.
71 Sindelar B.J., Herring S.W. Soft tissue mechanics of the temporomandibular joint. Cells Tissues Organs. 2005;180(1):36-43.
72 Suenaga S., Ogura T., Matsuda T., Noikura T. Severity of synovium and bone marrow abnormalities of the temporomandibular joint in early rheumatoid arthritis: role of gadolinium-enhanced fat-suppressed T1-weighted spin echo MRI. J Comput Assist Tomogr. 2000;24(3):461-465.
73 Usui A., Akita K., Yamaguchi K. An anatomic study of the divisions of the lateral pterygoid muscle based on the findings of the origins and insertions. Surg Radiol Anat. 2008;30(4):327-333.
74 Joseph J. Locomotor system: muscles of the head. In: Hamilton W., editor. Textbook of Human Anatomy. 2nd ed. London: MacMillan; 1976:154-158.
75 Palastanga N., Field D., Soames R. Head and brain. In Anatomy and Human Movement: Structure and Function, 5th ed, Edinburgh: Elsevier Butterworth Heinemann; 2006:635-694.
76 Heylings D.J., Nielsen I.L., McNeill C. Lateral pterygoid muscle and the temporomandibular disc. J Orofac Pain. 1995;9(1):9-16.
77 Zhang L., Sun L., Ma X. [A macroscopic and microscopic study of the relationship between the superior lateral pterygoid muscle and the disc of the temporomandibular joint]. Zhonghua Kou Qiang Yi Xue Za Zhi. 1998;33(5):267-269.
78 Le Toux G., Duval J.M., Darnault P. The human temporo-mandibular joint: current anatomic and physiologic status. Surg Radiol Anat. 1989;11(4):283-288.
79 Merida Velasco J.R., Rodriguez Vazquez J.F., Jimenez Collado J. The relationships between the temporomandibular joint disc and related masticatory muscles in humans. J Oral Maxillofac Surg. 1993;51(4):390-395. discussion 5–6
80 Schwartz A.J. Dislocation of the mandible: a case report. AANA J. 2000;68(6):507-513.
81 Umstadt H.E., Ellers M., Muller H.H., Austermann K.H. Functional reconstruction of the TM joint in cases of severely displaced fractures and fracture dislocation. J Craniomaxillofac Surg. 2000;28(2):97-105.
82 Sencimen M., Yalcin B., Dogan N., et al. Anatomical and functional aspects of ligaments between the malleus and the temporomandibular joint. Int J Oral Maxillofac Surg. 2008;37(10):943-947.
83 Shiozaki H., Abe S., Tsumori N., et al. Macroscopic anatomy of the sphenomandibular ligament related to the inferior alveolar nerve block. Cranio. 2007;25(3):160-165.
84 Alomar X., Medrano J., Cabratosa J., et al. Anatomy of the temporomandibular joint. Semin Ultrasound CT MR. 2007;28(3):170-183.
85 Tomas X., Pomes J., Berenguer J., et al. Temporomandibular joint soft-tissue pathology, II: Nondisc abnormalities. Semin Ultrasound CT MR. 2007;28(3):205-212.
86 Brown C.R. Ernest syndrome: insertion tendinosis of the stylomandibular ligament. Pract Periodontics Aesthet Dent. 1996;8(8):762.
87 Shankland W.E.2nd. Ernest syndrome as a consequence of stylomandibular ligament injury: a report of 68 patients. J Prosthet Dent. 1987;57(4):501-506.
88 Major P.W., Kinniburgh R.D., Nebbe B., et al. Tomographic assessment of temporomandibular joint osseous articular surface contour and spatial relationships associated with disc displacement and disc length. Am J Orthod Dentofacial Orthop. 2002;121(2):152-161.
89 Helms C., Major N., Anderson M.W., et al. The temporomandibular joint. In Musculoskeletal MRI, 2nd ed, Philadelphia: Elsevier Saunders; 2009:172-176.
90 Ribeiro R.F., Tallents R.H., Katzberg R.W., et al. The prevalence of disc displacement in symptomatic and asymptomatic volunteers aged 6 to 25 years. J Orofac Pain. 1997;11(1):37-47.
91 Katzberg R.W., Westesson P.L., Tallents R.H., Drake C.M. Anatomic disorders of the temporomandibular joint disc in asymptomatic subjects. J Oral Maxillofac Surg. 1996;54(2):147-153. discussion 53–55
92 Tomas X., Pomes J., Berenguer J., et al. MR imaging of temporomandibular joint dysfunction: a pictorial review. Radiographics. 2006;26(3):765-781.
93 Burnett K.R., Davis C.L., Read J. Dynamic display of the temporomandibular joint meniscus by using “fast-scan” MR imaging. Am J Roentgenol. 1987;149(5):959-962.
94 Okeson J. Mechanics of mandibular movement. In Management of Temporomandibular Disorders and Occlusion, 6th ed, St Louis: Elsevier Mosby; 2008:81-94.
95 Nebbe B., Major P.W. Prevalence of TMA disc displacement in a pre-orthodontic adolescent sample. Angle Orthod. 2000;70(6):454-463.
96 Milano V., Desiate A., Bellino R., Garofalo T. Magnetic resonance imaging of temporomandibular disorders: classification, prevalence and interpretation of disc displacement and deformation. Dentomaxillofac Radiol. 2000;29(6):352-361.
97 Kondoh T., Westesson P.L., Takahashi T., Seto K. Prevalence of morphological changes in the surfaces of the temporomandibular joint disc associated with internal derangement. J Oral Maxillofac Surg. 1998;56(3):339-343. discussion 43–44
98 Beers M., Berkow R. Temporomandibular disorders. In The Merck Manual, 17th ed, Merck & Co: West Point, PA; 1999:772-776.
99 Pirttiniemi P., Peltomaki T., Muller L., Luder H.U. Abnormal mandibular growth and the condylar cartilage. Eur J Orthod. 2009;31(1):1-11.
100 Larheim T.A. Role of magnetic resonance imaging in the clinical diagnosis of the temporomandibular joint. Cells Tissues Organs. 2005;180(1):6-21.
101 Singh D.J., Bartlett S.P. Congenital mandibular hypoplasia: analysis and classification. J Craniofac Surg. 2005;16(2):291-300.
102 Larheim T.A., Katzberg R.W., Westesson P.L., et al. MR evidence of temporomandibular joint fluid and condyle marrow alterations: occurrence in asymptomatic volunteers and symptomatic patients. Int J Oral Maxillofac Surg. 2001;30(2):113-117.
103 Scutellari P.N., Orzincolo C. Rheumatoid arthritis: sequences. Eur J Radiol. 1998;27(suppl 1):S31-S38.
104 Ozcan I., Ozcan K.M., Keskin D., et al. Temporomandibular joint involvement in rheumatoid arthritis: correlation of clinical, laboratory and magnetic resonance imaging findings. B-ENT. 2008;4(1):19-24.
105 Kaplan P., Helms C., Dussault R., Anderson M. Musculoskeletal MRI. Philadelphia: WB Saunders, 2001.
106 Rowe L., Yochum T. Hematological and vascular disorders. In: Yochum T., Rowe L., editors. Essentials of Skeletal Radiology. 2nd ed. Baltimore: Williams and Wilkins; 1996:1243-1326.
107 Fu K.Y., Li Y.W., Zhang Z.K., Ma X.C. Osteonecrosis of the mandibular condyle as a precursor to osteoarthrosis: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(1):e34-e38.
108 Campos P.S., Freitas C.E., Pena N., et al. Osteochondritis dissecans of the temporomandibular joint. Dentomaxillofac Radiol. 2005;34(3):193-197.