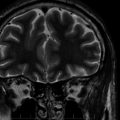
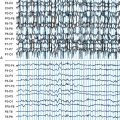
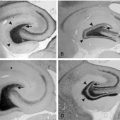
Chapter 19 The Surgery of Temporal Lobe Epilepsy I—Historical Development, Patient Selection, and Seizure Outcome
Introduction
Surgery for temporal lobe epilepsy (TLE) is widely practiced in the developed world. Its efficacy in comparison to medical treatment for intractable epilepsy has not been seriously doubted by many workers and has now been confirmed in a randomized, controlled study of 80 patients, half with ongoing medical treatment versus half subjected to surgery. Fifty-eight percent in the surgical group and 8% in the medical group (P <0.001) had fewer seizures at 1 year.1 The current authors examined 72 papers on the outcome of surgery for TLE published between 1956 and 2000 (references not given). It was possible to reasonably amalgamate the outcome data in 26 papers (737 patients): 491 (67%) had excellent and 162 (22%) had good outcomes (excellent: UCLA classification I; good: UCLA classification II and III; for the UCLA classification see Vickrey et al., 19872).
This is an extensive and complex field encompassing many workers with varying approaches to preoperative assessment and operation types and encompassing the gamut of investigational modalities from the well established and widely available to the more cutting edge. For the purpose of orientation, therefore, we have divided this chapter into two sections. The first provides a brief summary of the historical development of epilepsy surgery and the second a summary of aspects of the outcome, in terms of seizure control, of temporal lobe surgery in contemporary practice. In the following chapter, we address the outcome in terms of adverse effects and complications.
History
From scarification to colectomy and nasal polypectomy, the list of physical treatments visited on patients with epilepsy appears, at least in retrospect, bizarre and misguided. Postauricular arteriotomy, first recorded in ancient Greece, was still used in the late 1800s, and in the following century, Charles Brown-Séquard advocated cauterization and William Gower circumcision; thumb amputation was performed as late as the 1920s.3,4
Some instances of prehistoric trephination (excision of a piece of the calvarium) may have been attempts to cure posttraumatic epilepsy.5 Otherwise, prior to the late nineteenth century, brain surgery was largely confined to the drainage, cleaning, and closure of open head injuries in a military setting. In Europe and the United States, trephination was sometimes performed at the site of head injuries to relieve seizures, but its popularity fluctuated.5,6 Success was limited, mortality high: “Nearly all the patients perished within the first week from inflammation of the brain and its envelopes” (Samuel Gross, 1872).7 There were also sporadic reports of trephination for cerebral abscess drainage, but the results were poor and, toward the end of the eighteenth century, many surgeons began to eschew it. Pierre Joseph Desault, a prominent French surgeon, “proscribed it entirely on the double reason of its danger and ordinary inutility.”8
As the development of modern general surgery flourished following the introduction of antisepsis and anaesthesia (ether and chloroform 1846 and 1847, respectively; antisepsis, 1867), brain surgery continued to be shunned.6 In 1886, Horsley remarked: “It is notorious that, for the last thirty or forty years … trephining has been in exceedingly evil repute, owing to the very high mortality which followed its practice.”9 The advent of modern neurosurgery required a third factor—the birth of modern neurology, with its emerging concepts of anatomical localization in the cerebral cortex.
Prominent landmarks included the identification of an expressive language area by Pierre Broca,10 the demonstration of the canine motor cortex by Gustav Fritsch and Eduard Hitzig,11 and the ethically reprehensible work of Robert Bartholow, a Cincinnati physician, on one unfortunate Mary Rafferty.12 A rodent ulcer had left much of her cerebral cortex exposed but “without any interruptions of its functions.” Bartholow, making use of an electrotherapeutics room that he had previously established at the Good Samaritan Hospital, subjected Mary Rafferty to repeated electrostimulatory experiments, clearly demonstrating contralateral movements with hemispheric stimulation. The final experiment induced status epilepticus that led to death within a few days. A full historical account has been given by Morgan.13
John Hughlings Jackson’s work was of particular importance. He began to use cerebral localization to explain the semiology of epileptic seizures, classically the “the Jacksonian march,” which he related to a lesion in the motor area.6,14 This was directly applied in 1884 when Rickman Godlee and Alexander Bennett found and excised a glioma from the right precentral gyrus based on an inference that the patient’s focal motor seizures arose there.15 This operation was observed by Horsley, Jackson, and David Ferrier.16 These workers, based at the National Hospital for Paralysis and Epilepsy, Queen Square in London, went on to make by far the greatest contribution to the early development of neurosurgery.
Horsley initiated brain surgery at the National Hospital in 1886 and, within 1 year, published a case series of 10 operations. These cases were mostly similar in concept to Godlee and Bennett’s operation (i.e., clinical localization was used to identify and pursue a structural lesion). However, one case (O.S.H.), a “moral imbecile” with “seizures beginning at the left angle of the mouth” stands out. Finding no cortical abnormality, Horsley used electrical faradic cortical stimulation to map out the “facial center,” which he excised.17 This appears to be the first instance of a stimulation-guided corticectomy (some have, incorrectly, attributed it to Fedor Krause6). The contribution of William Macewan in Glasgow should also be remembered, although of less direct relevance to epilepsy surgery rather than general neurosurgery. In 1888, William Macewan published a series of 21, mostly successful, operations for brain abscesses.18
Horsley had demonstrated the technical feasibility of brain operations, firmly basing his surgical technique on animal experiment and anatomical observation. In addition to the application of neurological localization, he greatly developed surgical technique. Horsley’s advocacy of brain surgery was evidently emboldened, rebutting opponents’ “vague statements which one sees paraded on our journals … even were the language in which they are couched worthy of notice”; urging that “no one … need hesitate to follow the dictates of reason and common sense, and proceed to operate.”9 In Germany, Krause took up focal anterior cortical excisions for Jacksonian epilepsy in 1893 and, in 1910, reported on 29 patients, 8 with a marked improvement in seizures (although with a mortality of 10%).6 Some cases lacked surgical pathology, and corticectomies were based on stimulation (galvanic and, later, safer monopolar faradic).
Thus, neurosurgery became increasingly accepted: in 1899, Otto Binswanger identified 50 reports and seven theses on epilepsy surgery between 1894–1898.3 However, surgery in this period was almost entirely restricted to the vicinity of the primary motor area of the frontal lobe. The importance of the temporal lobe in epilepsy had not been appreciated.
In the first few decades of the 20th century, epilepsy surgery largely centered on the excision of cortical scars, fueled by the First World War. In Breslau, Otfrid Förster, neurologist turned neurosurgeon, ventured beyond the motor strip, performing excisions in all lobes. He developed the use of simulation and was the first to use intraoperative electrocorticography (ECoG).7 Following on from Förster, Wilder Penfield initiated the epilepsy surgery program at the Royal Victoria Hospital (and then the Montreal Neurological Institute), Canada, in 1928.19 Penfield’s program was chiefly concerned with the excision of, mostly extratemporal, cortical scars in addition to expanding, in collaboration with Jaspers, work on cortical localization.19 Penfield emphasized the importance of surgical pathology—structural lesions and gyral atrophy—in determining resections. However, ECoG and cortical stimulation were usually employed and, whereas Penfield, on the one hand, counseled against the resection of normal appearing cortex, he did also describe extending resections into surrounding normal cortex based on the ECoG findings. In addition, electrodes were used to locate areas of potentially abnormal cortex not visible without dissection or manipulation (“the diviner’s rod”). Later, Penfield increasingly performed temporal lobe operations, but mainly on the neocortex. Of 68 cases between 1939 and 1949, the excisions were focused on the anterior and lateral temporal lobe, the uncus being excised in ten cases (15%) and the hippocampus in only two (3%).20,21 Only following the work of Morris, Bailey, and Gibbs (see later discussion) did his attention turn to the mesial structure.
The most important development underpinning the further development of epilepsy surgery arose from the electroencephalographic work of Herbert Jaspers and then Pearce Bailey and Frederick Gibbs that, in the 1940s, crystallized the concept of psychomotor seizures arising from the temporal lobe.22,23 Previously, the importance of the temporal lobe in epilepsy had not been widely emphasized, even though macroscopic and microscopic descriptions of sclerosis of the mesial temporal lobe in association with epilepsy had been published in 1825 and 1880, respectivley,24 and Jackson had essentially characterized psychomotor seizures (“the uncinate group of fits”) and localized them to the medial temporal lobe.25 However, the primary nature of mesial temporal sclerosis was opposed by prominent clinicians and neuropathologists, and it was widely held that the pathological changes were the consequence rather than the cause of epilepsy.
Bailey and Gibbs quickly translated their findings into surgical practice, initiating temporal lobe operations at the Illinois College of Medicine in 1947.26 Their first 19 operations were limited excisions determined by ECoG, but the success rate was low, prompting more radical excisions, “radical lobectomy”: all tissue between the Sylvian fissure and the occipitotemporal sulcus, extending posteriorly at least to the level of the central sulcus and, in some cases, depending on the ECoG findings, up to one centimeter posterior to it. The hippocampus and insula were spared for fear of producing the neuropsychological deficits reported in primates following bilateral ablation of the medial temporal lobe. The results of the radical procedure were superior (“… very good to date”), and Bailey and Gibbs urged its adoption in all cases.
Simultaneously, although with much less acclaim, Morris at Georgetown University School of Medicine developed a similar radical resection but, remarkably, including the uncus, amygdala, and 2 to 4 cm of the anterior end of the hippocampus. Morris invariably observed diffuse temporal lobe epileptiform activity on ECoG (as Bailey and Gibbs did), and consequently, he took the bold step of abandoning intraoperative electrophysiological studies, simply performing “standard temporal lobectomy” in all patients—with good results.27
In the 3 years subsequent to 1949, Penfield performed 81 temporal lobe operations in contrast to 68 in the preceding 10 years. Furthermore, the uncus, amygdala, and hippocampus were routinely removed as well as the anterolateral temporal lobe anterior to the vein of Labbé. Penfield ascribed this development in his practice to the recognition of incisural sclerosis (i.e., hippocampal sclerosis, henceforth referred to as mesial temporal sclerosis [MTS] in this chapter) as the commonest cause of TLE arising from his earlier work.19 Additionally, Penfield reoperated on “a number” of his earlier temporal lobe patients to excise the hippocampus, sometimes with conversion of failure to success.28
Murray Falconer at the Maudsley Hospital, London, perused the approach of removal of the lateral and media structures, developing the technique of “anterior temporal lobectomy,” using an en bloc excision. In addition to the medial structures, the whole temporal lobe was amputated 5.5 to 8 cm (most commonly 6 cm) posterior to its tip, although sparing the superior temporal gyrus other than its anterior 1 to 2 cm; this modification was introduced to avoid dysphasia.29 The en bloc method allowed pathological study and revealed, in many cases, gliosis and atrophy in the hippocampus. As these abnormalities often extended into the contiguous gray matter, the term “mesial temporal sclerosis” was coined.30 Falconer’s finding that seizure outcome was superior in cases where the resection included definitely pathological tissue, particularly MTS, as opposed to no or nonspecific abnormalities, led him to the belief that “the removal of diseased brain tissue rather than the interruption of neuronal circuits” was essential to successful outcome.29,31
The past 50 or so years have not seen any further major conceptual evolution in temporal lobe surgery, although some practical improvements have been made to surgical method. The main change has been to reduce the volume of temporal neocortex resected, and one modification (the Spencer operation) in which more posterior cortex is preserved is in wide usage. More selective methods of amygdala-hippocampectomy have also been devised, which minimize the resection of lateral cortex, but these carry more risk, and these techniques are now not widely employed, These more selective operations were developed with the aim of minimizing neuropsychological deficit, but it is not clear whether this goal was attained. The seizure outcome of the Spencer operation is probably equivalent to that of the standard temporal lobectomy, but that of the highly selective amygdala-hippocampectomy is generally less good. Indeed, seizure outcome has remained static as evidenced by an examination of the literature (see Figure 19-1). Admittedly, however, it is difficult to compare reports of outcome with great confidence (see following discussion); although we examined 72 papers reporting outcome, only 13 included data to allow a comparison of outcome over time, and even then outcome had to be broadly defined. The major advances in temporal lobe surgery have come from the use of magnetic resonance imaging (MRI) in the field of patient selection, which has allowed what amounts to preoperative in vivo visualization of hippocampal sclerosis. This has refined patient selection and increased the confidence of physicians to refer patients for surgery.
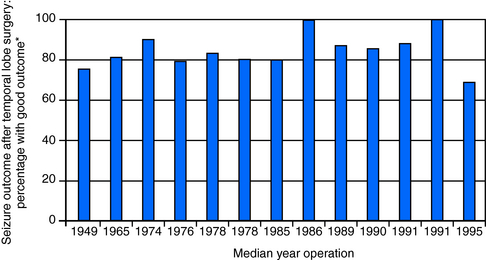
Figure 19–1 To analyze any change in the seizure outcome of temporal lobe surgery for intractable epilepsy over the time that the operation had been applied, a literature search was performed to identify suitable papers. Of 72 potentially suitable papers, 13 contained sufficient data to allow determination of the range of operation year and classification of seizure outcome according to the UCLA classification (see Vickrey et al., 19872); each bar represents the percentage of patients with excellent or good outcome (UCLA classification I to III).27,70–81 Each bar represents a single paper, and the median year of operation in the paper is given on the x-axis.
Current Practice
Epilepsy surgery is now carried out widely around the world. The commonest operation is the modified anterior temporal lobectomy with mesial temporal structures included in the resection, for TLE. The commonest pathological substrate is hippocampal sclerosis. Additionally, a miscellany of structural lesions (including dysembryoplastic neuroepithelial tumor, cavernous hemangioma, ganglioglioma) may cause TLE and may be addressed by lesionectomy, with or without corticectomy around the lesion, or by anterior temporal lobectomy with amygdalohippocampectomy. The latter is particularly indicated where the structural lesion is accompanied by MTS (dual pathology). Temporal lobe surgery is also an option in some patients with focal cortical dysplasia, particularly of the Taylor type, and with no pathology evident on MRI. For patients with no resective option, a number of nonresective procedures are also current (intra- and extracranial neural stimulation, radiosurgery, deep brain simulation, lobotomy).
Preoperative Assessment
It is universally agreed that surgery is most likely to be successful where different classes of data are concordant in their identification of the epileptogenic region. Ictal and interictal EEG and seizure semiology determined by videotelemetry, MRI, and neuropsychometry are generally held to be of principal importance. This, of course, is a rather banal theoretical basis for selection, but has proved to have practical utility. These data must, of course, be analyzed in the context of a detailed clinical history and examination. Additional localizing tools, particularly PET, SPECT, and MEG have also been widely employed, although it has proved difficult to assess their importance systematically, and the sensitivity and specificity of individual tests is not known. A more sophisticated approach to presurgical evaluation has not been developed and would require multicenter outcome assessment.
The preoperative assessment must also address the safety of surgery with respect to the risk of neurological and psychological deficits, and this is of equal importance to the identification of the seizure focus (the complications and adverse effects of surgery are discussed in the following chapter). The Wada test is widely used to assess memory function. A short-acting barbiturate is injected sequentially into the circulation of each hemisphere (or less commonly, a more selective injection to target more specifically the proposed region of resection) via catheter before briefly testing certain cognitive functions. This is widely used to predict any postoperative deficit. The applicability and value of the Wada test remains controversial, with some centers performing it on all patients, and others being more selective.32 More recently, alternative approaches, particular functional MRI, have been explored.33
Neuropsychiatric assessment is essential because psychiatric disorders, principally depression with or without anxiety, are of increased prevalence in intractable TLE.34 Ictus-related psychoses are generally held not to contraindicate surgery, as they will resolve in parallel with seizures after successful surgery.
What Factors Predict the Seizure Outcome of Temporal Lobectomy?
Over the decades, a large number of papers have examined the relationship between preoperative data and seizure outcome. Epilepsy surgery is an intensive endeavor, and therefore, even publications from institutions with the largest programs report on relatively small numbers of patients. This has inevitably prompted meta-analyses, but the approach is fraught with difficulty in this field, such that, in the authors’ view, it is in general possible to merely survey the literature; any attempts at combining data from different centers are at best semiquantitative. The main obstacle is significant variation in the battery of investigations (and even where they are nominally equivalent, it is usually problematic to compare data from different institutions) and the criteria used to determine which patients have operations. Additionally, the postoperative follow-up periods vary widely, making meaningful comparison difficult, as there are significant changes in seizure outcome with increasing time from surgery.35–42 Moreover, many different outcome classification systems are used, some standardized, some idiosyncratic. Finally, there is the problem of the variation in the factors examined in different reports and differences in the statistical presentation of data, such as categorical as opposed to continuous.
Many preoperative factors have been examined as potential positive predictors of good seizure outcome. These include IQ, electrocorticographic findings, hippocampal atrophy as determined by MRI volumetric analysis, resection side, intracarotid amobarbital testing, age at onset of epilepsy, age at surgery, duration of epilepsy at surgery, seizure frequency, occurrence of secondary generalized seizures, and history of febrile seizures.43–46 Tonini et al. did attempt a meta-analysis that included 47 papers and identified a number of factors that predicted good seizure outcome: febrile seizures (OR 0.48; 95% CI, 0.27–0.83), mesial temporal sclerosis (OR 0.47; 95% CI 0.35–0.64), tumors (OR 0.58; 95% CI 0.42–0.80), abnormal MRI (OR 0.44; 95% CI 0.29–0.65), EEG/MRI concordance (OR 0.52; 95% CI 0.32–0.83), and extensive surgical resection (OR 0.24; 95% CI 0.16–0.36).45 They also identified two negative predictors: postoperative discharges (OR 2.41; 95% CI 1.37–4.27) and intracranial monitoring (OR 2.72; 95% CI 1.60–4.60). Firm conclusions could not be made for the extent of resection, EEG/MRI concordance and postoperative discharges. Neuromigrational defects, CNS infections, vascular lesions, interictal spikes, and side of resection did not affect the seizure outcome.
The anatomical details of the resection used have been scrutinized throughout the history of temporal lobe resections (see earlier discussion). The standard temporal lobectomy remains the most widely used operation, but may variations have emerged. Again, attempting to objectively compare operations is problematic. Surgical technique varies, and stated degree of temporal lobe resection, although intended, is not always actually achieved. Some centers still use corticography and intraoperative EEG to guide resections. However, most reported studies relating outcome to degree of resection have been essentially qualitative. Among these, some workers have reported greater success with more complete hippocampal resection, whereas others have found no difference between resection of only the lateral cortex as compared to lateral plus mesial structures.47–56
It is difficult to draw any conclusions from these studies. Any convincing comparison of different standard procedures should rest on an accurate anatomical description of resections. Early attempts at this were based on intraoperative assessment, but due to several limitations identified by Awad et al., they did not provide objective, standardized data.53 Quantitative studies of the surgical specimen were equally unsatisfactory.53 MRI appears to offer more promising strategies, particularly where high-resolution, volume acquisition MRI with spatial reformatted and serial scans registration allows comparison of an individual brain at different times. Imaging may be performed at any time postoperatively, allowing for the resolution of tissue changes that cause distortion and difficulties in interpretation of the signal characteristics of tissues.54 However, these techniques remain developmental, and there are particular issues with respect to the satisfactory definition of anatomical structures, especially postoperatively, when structures are partially resected.58
Nonlesional Temporal Lobe Epilepsy
As outlined earlier, in general, where there is concordance between different modalities in identifying the epileptogenic zone, surgical success tends to be high. Throughout the history of epilepsy surgery (see earlier discussion), the finding that it is most successful where the resection encompasses a defined structural abnormality has been reiterated such that this has become axiomatic to some workers. The introduction of MRI has vastly increased the ability to determine the presence of such pathology, principally MTS, to be identified preoperatively. In the absence of a significant lesion, seizure freedom at 5 years has been reported to be as low as 21%.59 Conversely, some centers report good outcomes in this situation; for example, Alarcón et al. found no difference in favorable outcome between MRI-negative and MRI-positive patients after temporal resections (92% vs. 80%).60 This apparently paradoxical disparity may be explained by differences in patient populations and the emphasis placed on investigation modalities, particularly imaging versus electrophysiology. For example, it is possible that MRI-negative patients might not be considered so if postprocessing techniques such as hippocampal volumetry are applied. A normal MRI should not, therefore, preclude patients from consideration for surgery. However, it is clear that in this situation most or all patients will require more extensive preoperative workup, including intracranial EEG (all patients in Alarcón’s study). The risks of invasive monitoring have to be considered, and this is probably best achieved on a case-by-case basis.
Is Surgery Underutilized?
It has been suggested that epilepsy surgery is underutilized in developed countries. Duncan has estimated that 4500 individuals in the U.K. were unrecognized candidates in 2007 and that the median interval from onset of epilepsy to surgery is around 15 years, whereas intractability is almost always apparent much earlier. A proportion of this is due to patients declining consideration for surgery, but there is also under-referral to surgical centers due lack of awareness of a surgical option at all or that the patient is sufficiently intractable to warrant its consideration. In the latter regard, it should be recognized that the prognosis for seizure control in patients in whom two drugs have failed is very poor, and it has, therefore, been suggested that a surgical referral should be considered at that point.16
Unsurprisingly, in developing countries, the availability of epilepsy surgery is very limited because of the high cost and level of expertise required. To a degree, this might be addressed by simplifying the preoperative workup to identify patients with the most straightforward clinical profiles. Engel has estimated that 50% of individuals with intractable epilepsy have TLE with MTS: “the prototype of a surgically remediable syndrome”. Based on historical considerations (see earlier discussion) and recent studies (e.g., Özkara et al., 200062), it is very likely that the preoperative assessment might be limited to noninvasive studies and interictal EEG recording in many of these patients. There is much to be gained, both in improvement in quality of life and economically through medical cost savings and return of individuals to productivity.63
Nonresective Surgery
In recent decades, radiosurgery, vagal nerve stimulation, and deep brain simulation have been applied to intractable TLE. At this point, however, resective surgery remains the gold standard. Gamma-knife radiosurgery (GKS) appears attractive in avoiding the morbidity and mortality of major anaesthesia and craniotomy. However, there is a risk of radionecrosis and cerebral edema, and it seems that any decrease in seizure frequency is delayed. There is evidence, however, that radiation doses with a low risk of tissue necrosis may be effective in disrupting seizure circuits.64 A prospective, multicenter European study of GKS for MTS found similar efficacy as for conventional surgery at 2 years.65 However, the modality remains unproven, and some unsatisfactory outcomes have been reported. For example Srikijvilaikul et al. reported five consecutive patients, none of whom were seizure free after GKS and two were dead as a result of ongoing seizures.66 Currently, it is our view that radiosurgery for TLE should be considered only in the context of a clinical trial.
Vagal nerve simulation is usually performed by precordial implantation of a stimulator that is connected to the ipsilateral cervical vagus nerve. It is a palliative procedure and should only be considered in patients unsuitable for resective surgery. The published results vary, but for TLE it seems that there is a mean seizure frequency reduction of around 50%, and a small proportion of patients become seizure free.67
Human brain stimulation has its roots in the nineteenth century (see earlier discussion) and was pioneered in the 1970s as a treatment for epilepsy. Over the last decade or so, interest in deep brain stimulation (DBS) for epilepsy has been rekindled, the main targets being thalamus, subthalamic nucleus, the caudate nucleus, cerebellum, and hippocampus.68,69 There are several positive series in the literature, but all are on small numbers of patients. Larger studies are required. Currently, DBS may be considered in intractable patients where there is no good resective option.
1. Wiebe S, Blume WT, et al. for the Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311-318.
2. Vickrey BG, Hays RD, Engel J, et al. Outcome assessment for epilepsy surgery: the impact of measuring health-related quality of life. Ann Neurol. 1995;37:158-166.
3. Wolf LS. The history of surgical treatment of epilepsy in Europe. In: Lüders HO, editor. Epilepsy Surgery. New York: Raven Press; 1992:9-17.
4. Meyers R. The surgical treatment of temporal lobe epilepsy: an inquiry into current premises, their implementation and the criteria employed in reporting results. Epilepsia. 1954;3:9-36.
5. Horsley WV. Brain surgery in the stone age. BMJ. 1887:582.
6. Feindel W, Leblanc R, Villemure J. History of the surgical treatment of epilepsy. In: Greenblatt SH, Dagi TF, Epstein MH, editors. A History of Neurosurgery. New York: American Association of Neurological Surgeons,; 1997:1-24.
7. Penfield W, Jasper H. Surgical therapy. In: Penfield W, Jasper H, editors. Epilepsy and the Functional Anatomy of the Human Brain. London: J & A Churchill Ltd; 1954:739-817.
8. Sachs E. The seventeenth and eighteenth centuries. In: Sachs E, editor. The History and Development of Neurological Surgery. London, Toronto, Melbourne, Sydney & Wellington: Cassell and Company Ltd; 1952:44-52.
9. Horsley V. Brain-surgery. BMJ. 1886;2:670-675.
10. Broca PP. Perte de la parole, ramollisement chronique et destruction partielle du lobe antérieure gauche du cerveau. Bull Soc Anthropol. 1861;2:235-238.
11. Fritsch G, Hitzig E. Uber die elektrishe Erregbakeit des Grosshirns. Arch Anat Physiol Wiss Med. 1870;37:239-275.
12. Bartholow R. Experimental investigations into the functions of the human brain. Am J Med Sci. 1874;67:305-313.
13. Morgan JP. The first reported case of electrical stimulation of the human brain. J Hist Med Allied Sci. 1982;XXXVII:51-64.
14. Jackson JH. On epilepsy and epileptiform convulsions. In: James Taylor, ed. (with the advice and assistance of Gordon Holmes and FMR Walshe). Selected Writings of John Hughlings Jackson.: Vol 1. London, Hodder and Stoughton; 1931.
15. Bennett AH, Godlee RJ. Case of cerebral tumor. The surgical treatment. BMJ. 1885;i:988-989.
16. Marsh WR. Epilepsy surgery. Neuroimaging Clin N Am. 1995;5:729-738.
17. Horsley V. Ten consecutive cases of operations upon the brain and cranial cavity to illustrate the details and safety of the method employed. BMJ. 1887;2:863-866.
18. Macewan W. Pyogenic Infective Disease of the Brain and Spinal Cord. Meningitis, Abscess of the Brain, Infective Sinus Thrombosis. Glasgow: Hanes Maclehose and Sons, 1893.
19. Penfield W, Jasper H. In: Epilepsy and the Functional Anatomy of the Human Brain. London: J. & A. Churchill Ltd, 1954;v-vii.
20. Penfield W, Flanigin H. Surgical therapy of temporal lobe seizures. Arch Neurol Psychiatry. 1950;64:491-500.
21. Penfield W, Paine K. Results of surgical therapy for focal epileptic seizures. Can Med Assoc. 1955;73:515-521.
22. De Almeida AN, Teixeira MJ, Feindelo WH. From lateral to mesial: the quest for a surgical cure for temporal lobe epilepsy. Epilepsia. 2008;49(1):98-107.
23. Gibbs EL, Gibbs FA, Fuster B. Psychomotor epilepsy. Arch Neurol Psychiatry. 1948;60:331-339.
24. Sommer W. Erkrankung des Ammonshorns als aetiologisches Moment der Epilepsie. Arch Psychiatr Nervenkr. 1880;10:631-675.
25. Jackson JH, Colman WS. Case of epilepsy with tasting movements and “dreamy state”—very small patch of softening in the left uncinate gyrus. Brain. 1898;21:580-590.
26. Bailey P, Gibbs FA. The surgical treatment of psychomotor epilepsy. JAMA. 1951;145:365-370.
27. Morris AA. Temporal lobectomy with removal of uncus, hippocampus and amygdala. Arch Neurol Psychiatry. 1956;79:479-496.
28. Earle KA, Baldwin M, Penfield W. Incisural sclerosis and temporal lobe seizures produced by hippocampal herniation at birth. Arch Neurol Psychiatry. 1953;69:27-42.
29. Falconer M, Hill D, Pampliglione G. Discussion on the surgery of temporal lobe epilepsy. Proc R Soc Med. 1953;46:965-976.
30. Falconer MA. Mesial temporal (Ammon’s Horn) sclerosis as a common cause of epilepsy. Aetiology, treatment and prevention. Lancet. 1974;2:767-770.
31. Falconer MA, Serafetinides EA, Corsellis JAN. Etiology and pathogenesis of temporal lobe epilepsy. Arch Neurol. 1964;10:233-248.
32. Baxendale S, Thompson P, Duncan J, Richardson M. Is it time to replace the Wada test? Neurology. 2002;23:60-61. 59
33. Richardson MP, Strange BA, Duncan JS, Dolan RJ. Memory fMRI in left hippocampal sclerosis: optimizing the approach to predicting postsurgical memory. Neurology. 2006;66:699-705.
34. Fong J, Flugel D. Psychiatric outcome of surgery for temporal lobe epilepsy and presurgical considerations. Epilepsy Res. 2007;75:84-96.
35. Gleissner U, Johanson K, Helmstaedter C, et al. Surgical outcome in a group of low-IQ patients with focal epilepsy. Epilepsia. 1999;40:553-559.
36. Lüders H, Murphy D, Awad I, et al. Quantitative analysis of seizure frequency 1 week and 6, 12, and 24 months after surgery of epilepsy. Epilepsia. 1994;35:1174-1178.
37. Polkey CE, Scarano P. The durability of the result of anterior temporal lobectomy for epilepsy. J Neurosurg Sci. 1993;37:141-148.
38. So EL, Radhakrishnan K, Silbert PL, et al. Assessing changes over time in temporal lobectomy: outcome by scoring seizure frequency. Epilepsy Res. 1997;27:119-125.
39. Malla BR, O’Brien TJ, Cascino GD, et al. Acute postoperative seizures following anterior temporal lobectomy for intractable partial epilepsy. J Neurosurg. 1998;89:177-182.
40. Ficker DM, So EL, Mosewich RK, et al. Improvement and deterioration of seizure control during the postsurgical course of epilepsy surgery patients. Epilepsia. 1999;40:62-67.
41. Rougier A, Dartigues JF, Commenges D, et al. A longitudinal assessment of seizure outcome and overall benefit from 100 cortectomies for epilepsy. J Neurol Neurosurg Psychiatry. 1992;55:762-767.
42. Salanova V, Andermann F, Rasmussen T, et al. The running down phenomenon in temporal lobe epilepsy. Brain. 1996;119:989-996.
43. Tran TA, Spencer SS, Marks D, et al. Significance of spikes recorded on electrocorticography in nonlesional medial temporal lobe epilepsy. Ann Neurol. 1995;38:763-770.
44. Arruda F, Cendes F, Andermann F, et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40:446-450.
45. Grigsby J, Kramer RE, Schneiders JL, et al. Predicting outcome of anterior temporal lobectomy using simulated neural networks. Epilepsia. 1998;39:61-66.
46. Armon C, Radtke RA, Friedman AH, Dawson DV. Predictors of outcome of epilepsy surgery: multivariate analysis with validation. Epilepsia. 1996;37:814-821.
47. Tonini C, Beghi E, Berg AT, Bogliun G, et al. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Res. 2004:75-87.
48. Keogan M, McMackin D, Peng S, et al. Temporal neocorticectomy in management of intractable epilepsy: long-term outcome and predictive factors. Epilepsia. 1992;33:852-861.
49. Wyler AR, Hermann BP, Somes G. Extent of medial temporal resection on outcome from anterior temporal lobectomy: a randomized prospective study. Neurosurgery. 1995;37:982-990.
50. Arruda F, Cendes F, Andermann F, et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40:446-450.
51. Rasmussen T, Feindel W. Temporal lobectomy with major hippocampectomy: review of 100 cases. Can J Neurol Sci. 1991;18:S601-S602.
52. Bengzon ARA, Gloor P, Dussault J, et al. Prognostic factors in the surgical treatment of temporal lobe epilepsies. Neurology. 1968;18:717-731.
53. Awad IA, Katz A, Hahn JF, et al. Extent of resection in temporal lobectomy for epilepsy: I. Interobserver analysis and correlation with seizure outcome. Epilepsia. 1989;30:756-762.
54. Kitchen ND, Thomas DG, Shorvon SD, et al. Volumetric analysis of epilepsy surgery resections using high resolution magnetic imaging: technical report. Br J Neurosurg. 1993;7:651-656.
55. Jack CR, Sharbrough FW, Marsh WR. Use of MR imaging for quantitative evaluation of resection for temporal lobe epilepsy. Radiology. 1988;169:463-468.
56. Awad IA, Katz A, Lüders H, Weinstein M. Quantification of temporal lobe resections: a new approach. Cleve Clin J Med. 1989;56:833-836.
57. Kitchen ND, Cook MJ, Shorvon SD, et al. Image guided audit of surgery for temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1994;57:1221-1227.
58. Moran NF, Lemieux L, Maudgil D, Kitchen ND, Fish DR, Shorvon SD. Analysis of temporal lobe resections in MR images. Epilepsia. 1999;40:1077-1081.
59. Berkovic SF, McIntosh AM, Kalnins RM, et al. Preoperative MRI predicts outcome of temporal lobectomy: an actuarial analysis. Neurology. 1995;45:1358-1363.
60. Alarcón G, Valentín A, Watt C, et al. Is it worth pursuing surgery for epilepsy in patients with normal neuroimaging? J Neurol Neurosurg Psychiatry. 2006;77:474-480.
61. Duncan JS. Epilepsy surgery. Clin Med. 2007;7:137-142.
62. Özkara C, Özyurt E, Hanoglu L, et al. Surgical outcome of epilepsy patients evaluated with a noninvasive protocol. Epilepsia. 2000;41(S4):S41-S44.
63. Campos MG, Godoy J, Mesa MT, Torrealba G, Gejman R, Huete I. Temporal lobe epilepsy surgery with limited resources: results and economic considerations. Epilepsia. 2000;41(S4):S18-S21.
64. Dunoyer C, Ragheb J, Resnick T, et al. The use of stereotactic radiosurgery to treat intractable childhood partial epilepsy. Epilepsia. 2002;43:292-300.
65. Srikijvilaikul T, Najm I, Foldvary-Schaefer N, et al. Failure of gamma knife radiosurgery for mesial temporal lobe epilepsy: report of five cases. Neurosurgery. 2004;54:1395-1402.
66. Regis J, Rey M, Bartolomei F, Vladyka V, et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004;45:504-515.
67. Boon P, Vonck K, Vandekerckhove T, et al. Vagus nerve stimulation for medically refractory epilepsy; efficacy and cost-benefit analysis. Acta Neurochir (Wien). 1999;141:447-452.
68. Cooper IS, Amin I, Upton A, Riklan M, Watkins S, McLellan L. Safety and efficacy of chronic stimulation. Neurosurgery. 1977;1:203-205.
69. Boon P, Vonck K, De Herdt V, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48:1551-1560.
70. Cahan LD, Sutherling W, McCullough MA, et al. Review of the 20-year UCLA experience with surgery for epilepsy. Cleve Clin Q. 1984;51:313-318.
71. Walczak TS, Radtke RA, McNamara JO, et al. Anterior temporal lobectomy for complex partial seizures: evaluation, results, and long-term follow-up in 100 cases. Neurology. 1990;40:413-418.
72. Duncan JS, Sagar HJ. Seizure characteristics, pathology, and outcome after temporal lobectomy. Neurology. 1987;37:405-409.
73. Goldring S, Edwards I, Harding GW, Bernardo KL. Results of anterior temporal lobectomy that spares the amygdala in patients with complex partial seizures. J Neurosurg. 1992;77:185-193.
74. Swartz BE, Tomiyasu U, Delgado-Escueta AV, et al. Neuroimaging in temporal lobe epilepsy: test sensitivity and relationships to pathology and postoperative outcome. Epilepsia. 1992;33:624-634.
75. Radtke RA, Hanson MW, Hoffman JM, et al. Temporal lobe hypometabolism on PET: predictor of seizure control after temporal lobectomy. Neurology. 1993;43:1088-1092.
76. Chee MW, Morris HH, Antar MA, et al. Presurgical evaluation of temporal lobe epilepsy using interictal temporal spikes and positron emission tomography. Arch Neurol. 1993;50:45-48.
77. Cascino GD, Trenerry MR, Jack CRJr, et al. Electrocorticography and temporal lobe epilepsy: relationship to quantitative MRI and operative outcome. Epilepsia. 1995;36:692-696.
78. Wheelock I, Peterson C, Buchtel HA. Presurgery expectations, postsurgery satisfaction, and psychosocial adjustment after epilepsy surgery. Epilepsia. 1998;39:487-494.
79. Gilliam F, Bowling S, Bilir E, et al. Association of combined MRI, interictal EEG, and ictal EEG results with outcome and pathology after temporal lobectomy. Epilepsia. 1997;38:1315-1320.
80. Polkey CE. Selection of patients with chronic drug-resistant epilepsy for resective surgery: 5 years experience. J R Soc Med. 1981;74:574-579.
81. Jensen I, Vaernet K. Temporal lobe epilepsy. Follow -up investigation of 74 temporal lobe resected patients. Acta Neurochirurgica. 1977;37:173-200.