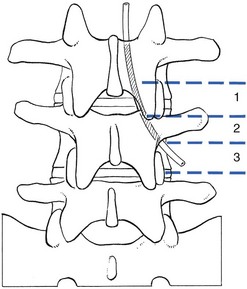
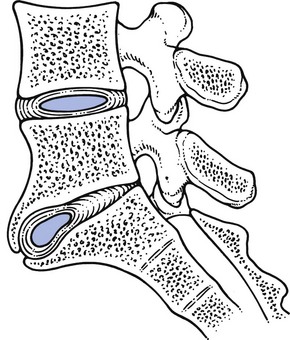
The stenotic concept
Introduction
Compression of lumbosacral nerve roots by osteoarthritic changes in the lumbar spine has been recognized since 1900, when Sachs and Fraenkel1 published the case of a Russian tailor suffering from intermittent paresis of the legs, which improved markedly after removal of the thickened, enlarged laminae of T11 and T12. Further reports on the consequences of lumbar osteoarthrosis on the function of nerve roots followed.2,3 Patients suffering from root pain in whom the only operative finding was a marked thickening of the ligamentum flavum were also recognized,4–6 and excision of the ligaments produced considerable relief of symptoms. A hypertrophied ligamentum flavum as one possible cause for backache and sciatica was suggested both in 19377 and 1945.8 By 1945, congenital narrowing of the bony canal was recognized as responsible for pressure on the cauda equina8 and, in the same decade, for nerve entrapment at the foraminal level.9 Overhanging facets were also postulated to be a cause of nerve root irritation.10 The definitive account of developmental narrowing of the lumbar vertebral canal causing radicular symptoms was given in the 1950s.11,12 In recent decades there have been many publications on the different types of ‘spinal stenosis’, and a consensus has been reached on the classification and pathophysiology of the different types of this lesion.13–17
Definitions
• Idiopathic developmental stenosis of the bony lumbar vertebral canal is a genetic disturbance that reveals its pathological effect only when growth is complete.18 The narrowing is uniform over almost the whole length of the lumbar bony canal. A mid-sagittal diameter of 10 mm or less is an absolute stenosis – the narrowing is capable of producing signs of compression of the neural content in the absence of other compressive agents. A relative stenosis has a diameter of 10–12 mm and can only lead to compression if other vertebral deformities, such as spondylitis or posterior vertebral osteophytes, compound the narrowing.
• Acquired spinal stenosis is the consequence of arthrotic changes, disc herniations, postsurgical or post-traumatic lesions, or bone diseases, including tumours.
• Degenerative stenosis, as its name implies, follows degenerative changes in the spine. Such narrowing is segmental and most marked between disc and posterior articular processes. Between the stenotic regions, the diameters of the vertebral canal are usually normal.
• Iatrogenic stenosis is the name sometimes given to stenosis resulting from surgical procedures that have caused considerable peridural fibrosis.19
• Lateral recess stenosis indicates a narrowing of the vertebral canal in its lateral portion (the lateral recess). The condition should be differentiated from narrowing of the entire vertebral canal (lumbar stenosis) caused by either a short sagittal or transverse diameter, or by a combination of both. The two cause different clinical syndromes: lumbar stenosis is related to both legs, often involving several dermatomes; the main symptom is neurogenic intermittent claudication. Lateral recess stenosis is characterized by root pain, confined to one dermatome only.
Incidence
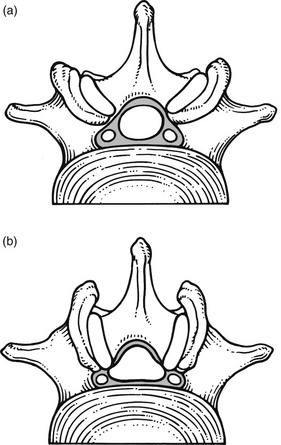
The frequency of lumbar spinal stenosis is low. A recent series of 443 lumbar spines yielded only 6 patients in 100 with relative stenosis and none with absolute stenosis.20 Lateral recess stenosis is more common. In the same series, a trefoil vertebral canal was found in 15% of the examined vertebrae and the same incidence was found in an earlier study.21
Spinal stenosis
Pathological changes
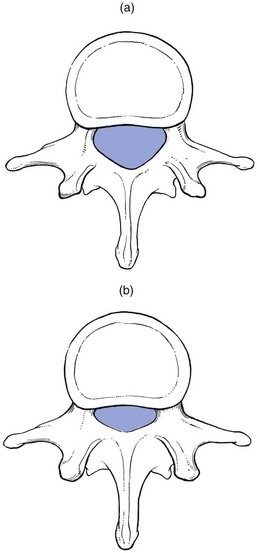
In developmental stenosis, the laminae, pedicles and posterior articular processes are increased in size (Fig. 35.1).
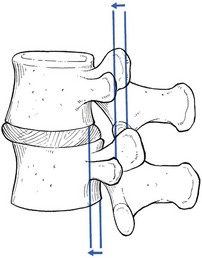
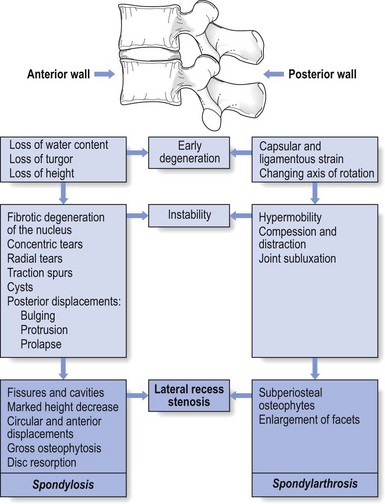
The latter is a vertebral displacement with an intact neural arch. Although the exact mechanism is not fully understood, the combination of disc degeneration,22 general joint laxity,23 increased mechanical stress24 and structural abnormalities of laminae and facet25 may precipitate forward displacement of the whole vertebra. The common denominator in the development of degenerative vertebral slippage is loss of the normal coronal orientation of the facet joints. Several studies showed a significant increase in sagittal facet joint orientation in patients with degenerative spondylolisthesis, compared with a normal population.26–28 Association with narrowing of the intervertebral joint space and development of osteoarthrosis of the facets then leads to forward vertebral subluxation.29 Later on, buckling and hypertrophy of the ligamentum flavum may supervene.30 This results in stenosis between the lamina and the posterior border of the underlying vertebra (Figs 35.2 and 35.3).31,32 The condition occurs mostly after the age of 50 years, is 4–6 times more common in women than in men, and most frequently affects the L4–L5 level.33,34 The displacement is usually moderate, less than or equal to one-third of the anteroposterior diameter of the superior border of the adjacent vertebral body.35 The displacement does not progress continuously but is arrested by further degenerative changes of the intervertebral and facet joints.36
The mechanisms that underlie the clinical symptoms in spinal stenosis are complex and poorly understood. There is pressure not only on the dural sac and nerve roots but also on the adjacent blood supply. Therefore, arterial obstruction, venous hypertension and pressure–traction on nerve roots, dura and sinuvertebral nerves are all believed to be of importance.13,37–40
Symptoms
In patients with degenerative spondylolisthesis, back pain is the most common complaint and is probably caused by overstretching of the facet capsules. Often the pain is episodic or chronic for many years. Patients usually report that their symptoms vary as a function of imposed posture and pain frequently worsens over the course of the day.41 The most significant symptom of lumbar stenosis is neurogenic intermittent claudication, or ‘pseudoclaudication’.42–44 As in true intermittent claudication, the pain is brought on by walking and relieved by rest. However, pseudoclaudication is believed to be secondary to mechanical compression of the spinal content, whereas intermittent claudication is the result of vascular insufficiency (Table 35.1).45 The two conditions can be distinguished by their symptoms.
Table 35.1
Differences between neurogenic and vascular claudication
| Neurogenic | Vascular | |
| Localization | Vague, including the back | Mostly in the calf |
| Paraesthesia | Present | Absent |
| Walking | Worse | Worse |
| Standing still | Worse | Better |
| Bending | Better | No change |
| Cycling | No change | Worse |
| Lying prone | Worse | No change |
In neurogenic claudication, leg pain is usually bilateral, poorly localized and associated with paraesthesia and numbness. Although the pain is usually provoked during walking, it does not disappear on standing still, which can even aggravate the situation.46 The pain is relieved only by adopting a stooped posture or by sitting.47,48 The main characteristic of neurogenic claudication is thus that it is generated by posture rather than by exercise. This postural mechanism explains why a patient may have the same symptoms during recumbency in the prone position and does not usually experience symptoms while riding a bike.49–51 At its most extreme, patients may report the need to sleep in the fetal position to relieve leg symptoms.
With extreme stenosis, interference with bladder and bowel control can occur but, unlike the acute and often devastating bladder and bowel symptoms of cauda equina syndrome in lumbar disk herniation, spinal stenosis often has an insidious and subtle presentation.52
Signs
The patient may have difficulty in standing erect and adopts a ‘simian stance’ with flattened lumbar lordosis and hips and knees slightly flexed.53 Extension is limited and may provoke pain in the legs, especially after the extended posture is maintained for a certain length of time. There is symmetrical and painless limitation of both side flexions. As a rule, flexion does not provoke symptoms in the legs but rather relieves the leg pain.54–56 This can be explained by the fact that extension significantly decreases both mid-sagittal and subarticular sagittal diameter, whereas flexion has the opposite effect.57,58 Straight leg raising is usually normal, as are reflexes. Because the lesion involves different levels and the compression of the roots is intermittent, there is often no demonstrable sensory deficit or muscle weakness. If there is some objective weakness, it is normally situated in the muscles supplied by the fifth lumbar and first sacral nerves.16
Radiography
Plain radiographs are sufficient to suggest the possibility of a narrow canal. Short pedicles and narrow intervertebral foramina are clearly demonstrable on a suitable lateral projection (Fig. 35.4).59 Hypertrophy of facets, disc resorption, retrolisthesis and degenerative spondylolisthesis can also be seen easily on the same views.60,61
On the plain anteroposterior projections (Fig. 35.5), hypertrophy of the pedicles (A), hypertrophic articular processes (D), small interlaminar spaces (C) and some sagittalization of the articular facets may be seen (B).62,63
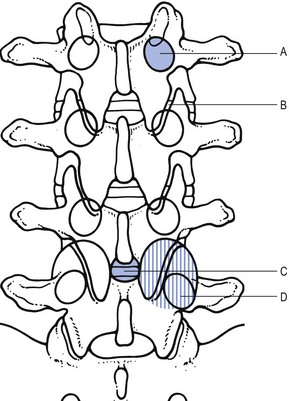
Fig 35.5 Interpretation of radiographic appearances of spinal stenosis (frontal projection): see text.
However, it is as well to remember that these radiographic findings are non-specific and can also be seen in asymptomatic individuals. In patients with clinical features, there is no correlation between symptoms and signs and the severity of the radiographic appearances.64 They are helpful only in supporting the clinical diagnosis.
In a symptomatic patient, the evaluation of spinal stenosis can best be made by magnetic resonance imaging (MRI), which has largely replaced computed tomography (CT) and CT-myelography.65 MRI is a non-invasive technique that can define the typical factors contributing to the symptoms of spinal stenosis: significant constriction of the cauda equina associated with a diminished cross-sectional area of the vertebral canal, apparent thickening and buckling of the ligamentum flavum, and hypertrophy of adjacent facet joints.66
Treatment
The natural course of spinal stenosis can vary, but in most patients it is a relatively stable disorder, with severe disability and neurological deficits developing over time and not rapidly. A recent survey reported that, in 80% of patients treated conservatively, symptoms did not worsen over 4 years.67 For patients with moderate or severe symptoms, different conservative and surgical treatment modalities are recommended.
Non-operative, conservative treatment should be used first and includes relative rest and non-steroidal anti-inflammatory drugs.68–70 Back school instruction is given in activities of daily living and pelvic tilting exercises to reduce lumbar lordosis (see p. 588).71,72 Epidural injections may be tried but reports of their efficiency are mixed.73 Some data suggest that epidural injection of corticosteroids relieves leg pain for a limited time but has no effect on functional status or the need for surgery after 1 year.74
If the patient does not respond to conservative treatment or if there is progressive deterioration, surgery is required. Decompression of the stenotic area is the usual procedure.75,76 Some advocate spinal fusion after the decompression, in order to avoid postsurgical instability.77,78 The surgical outcome is good to excellent in 45–80% of patients operated on.79–84 Patients with a preoperative duration of symptoms of less than 4 years, with no preoperative back pain and without previous back surgery tend to have a better outcome.85–88
Lateral recess stenosis
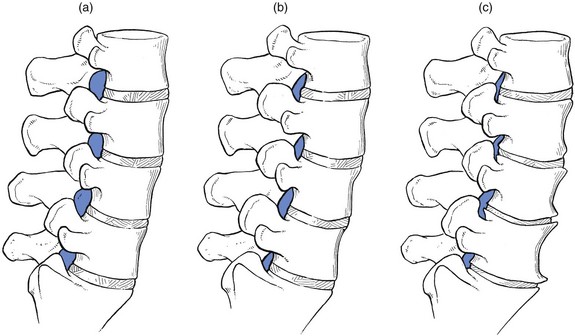
The radicular canal is at the lateral aspect of the spinal canal. It begins at the point where the nerve root sheath emerges from the dural sac and ends at the intervertebral foramen. Its posterior border is formed by the ligamentum flavum, superior articular process and lamina. The anterior border is the posterior aspect of vertebral body and disc, both covered by the posterior longitudinal ligament. The dural sac forms the medial wall and the internal aspect of the pedicle the lateral wall.89 The radicular canal can be classified into three zones (Fig. 35.6): the entrance zone is medial and anterior to the superior articular process; the midzone is located under the pars interarticularis of the lamina and below the pedicle; the exit zone is the area surrounding the intervertebral foramen.89
Pathological changes
Compression of the nerve root can be the result of different mechanisms: subarticular entrapment, pedicular kinking, or foraminal impingement due to posterior joint subluxation (MacNab90: pp. 98–104). Cyriax91 believed that the main reason for the compression lies in the fact that the posterior longitudinal ligament and the outer layers of the degenerated disc bulge in the upright position, so impinging on the nearby nerve root. Recently, stenosis as a result of surgical procedures has become more frequent.
Subarticular entrapment (Fig. 35.7)
This type of stenosis is related to the entrance zone. Emerging from the dural sac, the nerve root passes between the posterior aspect of disc and vertebral body and the anteromedial aspect of the superior articular facet of the same vertebra. Hypertrophic osteoarthrosis of the facet joint, especially involving the superior articular process, may considerably diminish the distance between both borders and thus compress the nerve root,92 although there may be an additional compression by a thickened and folded posterior longitudinal ligament91,93 (this is suggested by the observation that the pain is not constant but dependent on posture94).
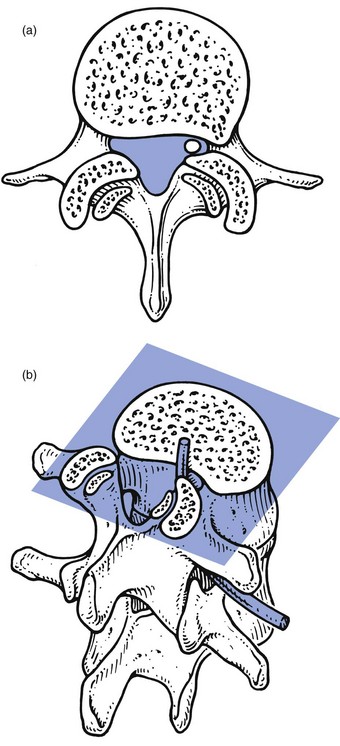
Fig 35.7 Subarticular entrapment.
The mechanism is as follows: increasing degeneration and anterior migration of discal tissue cause diminution of the intervertebral space and eventually lead to disc resorption and formation of gross anterior osteophytes. Consequently, the posterior longitudinal ligament, which spanned a joint 1 cm high, is now too long and will therefore bulge, especially in the standing or lordotic position.95 The thickened and buckling ligament can then exert pressure against the nerve root, especially if the recess is narrowed by the hypertrophic superior facet. Pain in the upright posture which disappears on sitting and bending is better explained in this way than by postulating a static subarticular entrapment. Cyriax called this mechanism the mushroom phenomenon, because of the characteristic radiographic appearances of a long-standing anterior shift of disc material, which results in a marked narrowing of the joint space and gross beak-like osteophytes (Fig. 35.8).
Pedicular kinking
This is a midzone stenosis. The mechanism is as follows: disc degeneration causes considerable narrowing of the intervertebral space and the upper vertebral body descends. The pedicle may then press on the nerve root (Fig. 35.9), especially if the narrowing is asymmetrical, and cause inflammation and oedema, which will give rise to symptoms. An alternative source of the symptoms is that the nerve root is trapped in the gutter formed by the pedicle and the posterolateral aspect of the degenerated and bulging disc.
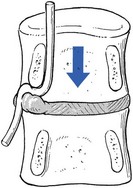
Fig 35.9 Pedicular kinking.
Foraminal encroachment
This occurs at the exit zone in the intervertebral foramen. In vitro anatomical and biomechanical studies of normal motion segments showed that the intervertebral foramen of the lumbar spine narrows significantly during extension and ipsilateral bending, and increases during flexion.96 With disc degeneration and subsequent loss of intervertebral height, the facet joints are forced into a permanent extension position: they move telescopically in relation to each other with the inferior facet moving downwards. Because of the inclination of the joints, a downward movement is accompanied by a backward one and a small retrolisthesis is produced. This further narrows the foramen, and the nerve root, which lies in close relationship to the tip of the superior articular process of the underlying vertebra, may become compressed between the tip of the subluxated (and eventually enlarged) superior facet, and the pedicle or body of the vertebra above (Fig. 35.10).
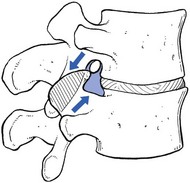
Fig 35.10 Foraminal encroachment.
Postsurgical fibrosis and stenosis
Surgical procedures on the spine can cause the formation of scar tissue around the dura and the nerve roots – a so-called laminectomy membrane.97 After laminectomy the incidence is 9%; it is approximately the same after dorsal fusion.19 Removal of a herniated disc, which decreases the intervertebral space, may also aggravate a pre-existing stenosis that has not been recognized at operation.98
Lateral compression of L5
Another, more exceptional cause of radicular entrapment is found at L5. A strong fibrous band – the corporotransverse ligament – runs from the undersurface of the fifth transverse process to the side of the vertebral body (MacNab90: p. 53). The L5 nerve root can become trapped between this and the ala of the sacrum if there is a marked downward and forward drop of L5 – for example, in degenerative spondylolisthesis at L5–S1.99 Compression can also occur in elderly patients with degenerative lumbar scoliosis, between the ala of the sacrum and the transverse process – the ‘far out’ syndrome.100,101 It has also been suggested that enlarged lumbosacral ligaments can cause extraforaminal compression of the L5 root.102
Mechanism of compression
In order to comprehend the mechanism that causes symptoms of radicular compression in a lateral recess stenosis, it is vital to understand that any impingement on the nerve root is intermittent and related to dynamic changes in the recess during changes in posture and movement. Most compression occurs when the canal is at its most narrow diameter; relief of the symptoms may be expected when the diameter increases. During an axial load on a degenerated spine, the lateral recess decreases in size103: the posterior longitudinal ligament folds and buckles in a posterior direction, the superior articular process moves forwards and upwards in relation to the vertebra above, and the pedicle tends to push downwards on the nerve root. Extension and, to a lesser degree, side flexion towards the painful side will further narrow the space, and therefore cause further compression on the root.104,105 In contrast, compression is reduced during flexion, which stretches the posterior longitudinal ligament and moves the superior articular process away from the foramen.106
Symptoms of root compression are caused not only by direct compression of the boundaries but also by inflammation and oedema in and around the nerve root.107,108 Swelling within an already confined space further increases the degree of compression.109
Lateral recess stenosis and natural history of the ageing spine
Lateral root entrapment is characteristic of elderly patients with disc degeneration, marked intervertebral narrowing and spondylarthrosis of the posterior wall (Fig. 35.11).
Symptoms
The patient is typically middle-aged or elderly (onset is seldom before the age of 50 years, and most patients are over 70).110 The complaint is of unilateral sciatica that comes on during standing and walking. Sometimes, but rather exceptionally, the root pain is bilateral. The pain does not usually start immediately on standing, only after some minutes or on walking. In the latter instance, claudication must be excluded: in vascular claudication the pain rapidly abates on standing still but in lateral recess stenosis it may persist until the patient sits. After some time, numbness and pins and needles in the foot may develop. Also bending forwards immediately relieves the pain, whereas extension movements increase it. There is sometimes nocturnal pain, caused by an increased lordosis in the prone position.111
Radiography
Various authors have noted a lack of correlation between radiographically detected stenosis and the presence and/or absence and intensity of symptoms and signs.112,113 Difficulties associated with finding such correlations include the presence of a large number of patients with radiographic changes compatible with the morphological diagnosis of lateral recess stenosis and a complete lack of symptoms, variations in canal size throughout the population and lack of an accepted system for quantifying the degree of narrowing.114,115 One should also never forget that the extent of narrowing is dynamic and likely to change with the posture of the patient (extension significantly decreases the canal area, whereas flexion has the opposite effect). Therefore, a static image of the canal dimensions may not be predictive of a patient’s symptoms. However, once a clinical diagnosis has been made, radiography can determine the affected level and is therefore a good guide in planning treatment of the lesion.
The characteristic changes on a plain lateral view are narrowing of the intervertebral space, sometimes gross, beak-like anterior osteophytes (mushroom) and subluxation of the facet joint, with anterior and superior movement of the superior articular process. On an anteroposterior view there is narrowing of the interlaminar space, which is a characteristic feature of lateral recess stenosis; the interlaminar space is encroached if there is an overgrowth of posterior facets or an abnormal configuration of the laminae.62,116,117
Myelograms may be difficult to interpret but areas of subarticular narrowing are very clearly demonstrated by CT.118 In recent decades, however, MRI has become the gold standard as the diagnostic tool in lateral recess stenosis.119 On the basis of sagittal MRI, four grades of lumbar foraminal stenosis can be distinguished120: grade 0 refers to the absence of foraminal stenosis; grade 1 refers to mild foraminal stenosis with some perineural fat obliteration; grade 2 refers to moderate foraminal stenosis showing perineural fat obliteration but no morphological changes; and grade 3 refers to severe foraminal stenosis showing nerve root collapse or morphological change.
Natural history
The natural course of lateral recess stenosis is likely to become chronic. Although symptoms tend to fluctuate considerably over time, they get worse as the years go by. Without treatment, severe disability and neurological deficits develop gradually over a longer period.121
Treatment
Occasionally, symptoms can be improved by reducing lumbar lordosis. The patient is thus instructed in the correct posture for activities of daily living (see p. 588).
Failure of response to such a programme is an indication for nerve root infiltration with 20 mg triamcinolone (see p. 588). The chief difficulty is determining where the lesion is. When there is doubt, the injection can be given at the most likely level and, if improvement does not take place after 2 weeks, it is repeated at the other level. Locating the lesion is particularly difficult after laminectomy and several attempts, at different locations, may be necessary.
It may seem surprising that infiltration around the nerve root and the sinuvertebral nerve often causes permanent relief in lateral recess stenosis.122,123 Also, prolonged relief of radicular pain after injection of a local anaesthetic around the nerve root has been reported.124–126 These excellent results can be explained by the reduction of chronic irritation in ligaments and perineural tissues.106 Triamcinolone, injected at the appropriate point, suppresses inflammation and thus reduces perineural swelling. As a result, relative narrowing of the canal is alleviated and the vicious circle of anatomical stenosis and inflammation is broken. The good and permanent results sometimes seen after infiltration of a local anaesthetic alone are explained by a chemical effect on the C fibres of the dorsal root.126
When nerve root infiltration does not afford permanent or semipermanent cure, surgery is indicated; it consists of decompression of the nerve root. Sometimes excision of an osteophyte by fenestration may be sufficient,127 but more usually laminectomy with partial excision of the enlarged facets is necessary.128 In recent years, interspinous implants (‘spacers’) have been used in the treatment of lumbar spinal stenosis. The rationale behind this minimally invasive intervention is that of de-lordosing the segment and thus widening the spinal canal in the upright position.129
References
1. Sachs, B, Fraenkel, J. Progressive ankylotic rigidity of the spine. J Nerv Ment Dis. 1900; 27:1.
2. Goldthwaite, JE. The lumbosacral articulation. An explanation of many cases of ‘lumbago’, ‘sciatica’ and paraplegia. Bost Med Surg J. 1911; 164:365.
3. Bailey, P, Casamajor, L. Osteoarthritis of the spine as a cause of compression of spinal cord and its roots. J Nerve Ment Dis. 1911; 38:588.
4. Towne, EB, Reichert, FL, Compression of the lumbosacral roots of the spinal cord by thickened ligamenta flava. Ann Surg 1931; 94:327. ![]()
5. Cramer, F. A note concerning the syndrome of cauda equina radiculitis. Bull Neurol Inst NY. 1934; 3:501.
6. Van Gelderen, C, Ein orthotisches (lordotisches) Kauda-syndrom. Acta Psychiatr Neurol 1948; 23:57. ![]()
7. Spurling, RG, Mayfield, FH, Rogers, JB. Hypertrophy of the ligamenta flava as a cause of low back pain. JAMA. 1937; 109:928.
8. Sarpyener, MA. Congenital stricture of the spinal canal. J Bone Joint Surg. 1945; 27A:70.
9. Hirsch, C, On lumbar facetectomies. Acta Orthop Scand 1948; 17:240–252. ![]()
10. Echoes, DH, Rehfeldt, FC, Failure to disclose ruptured intervertebral disks in 32 operations for sciatica. J Neurosurg 1949; 6:376–382. ![]()
11. Verbiest, H, Primaire stenose van het lumbale wervelkanaal bij volwassenen. Een nieuw ziektebeeld. Ned Tijdschr Geneeskd 1950; 94:2415–2433. ![]()
12. Verbiest, H, A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg 1954; 36B:230. ![]()
13. Arnoldi, CC, Brodsky, AE, Cauchoix, J, et al, Lumbar spinal stenosis and nerve root entrapment syndrome. Definition and classification. Clin Orthop Rel Res 1976; 115:4–5. ![]()
14. Dorwart, RH, Vogler, JB, III., Helms, CA, Spinal stenosis. Radiol Clin North Am 1983; 21:301–325. ![]()
15. Ehni, G, Significance of the small lumbar spinal canal: cauda equina syndrome due to spondylosis. Part I. Introduction. J Neurosurg 1969; 31:490–494. ![]()
16. Kirkaldy-Willis, WH, Paine, KW, Cauchoix, J, et al, Lumbar spinal stenosis. Clin Orthop Rel Res 1974; 99:30–50. ![]()
17. Paine, KWE, Clinical features of lumbar spinal stenosis. Clin Orthop 1976; 115:77–82. ![]()
18. Verbiest, H, Stenosis of the lumbar vertebral canal and sciatica. Neurosurg Rev 1980; 3:75–89. ![]()
19. Brodsky, A, Post-laminectomy and post-fusion stenosis of the lumbar spine. Clin Orthop 1976; 115:130–139. ![]()
20. Eisenstein, E, The morphometry and pathological anatomy of the lumbar spine in South African negroes and Caucasoids with specific reference to spinal stenosis. J Bone Joint Surg 1977; 59B:173–180. ![]()
21. Epstein, JA, Epstein, BS, Levine, L, Nerve root compression, associated with narrowing of the lumbar spinal canal. J Neurol Neurosurg Psychiatry 1962; 25:165–176. ![]()
22. Taillard, WF, Etiology of spondylolisthesis. Clin Orthop 1976; 117:30–39. ![]()
23. Porter, RW, Hibbert, C. Vertebral displacement in spondylolisthesis. Clin Biomech. 1989; 4:58–63.
24. Fitzgerald, JAW, Newman, PH, Degenerative spondylolisthesis. J Bone Joint Surg. 1976;58B(2):184–192. ![]()
25. Grobler, LJ, Robertson, PA, Novotny, JE, Pope, MH, Etiology of spondylolisthesis. Assessment of the role played by lumbar facet joint morphology. Spine 1993; 18:80–91. ![]()
26. Fujiwara, A, Tamai, K, An, HS, et al, Orientation and osteoarthritis of the lumbar facet joint. Clin Orthop Relat Res 2001; 385:88–94. ![]()
27. Dai, LY, Orientation and tropism of lumbar facet joints in degenerative spondylolisthesis. Int Orthop. 2001;25(1):40–42. ![]()
28. Ryan, MD, L4–L5 degenerative spondylolisthesis in monozygous twins. Spine 1994; 19:985–986. ![]()
29. Herkowitz, HN, Spine update: degenerative lumbar spondylolisthesis. Spine 1995; 20:1084–1090. ![]()
30. Hansson, T, Suzuki, N, Hebelka, H, Gaulitz, A, The narrowing of the lumbar spinal canal during loaded MRI: the effects of the disc and ligamentum flavum. Eur Spine J. 2009;18(5):679–686. ![]()
31. Junghans, H. Spondylolisthesis ohne Spalt im Zwischengelenkstuck (Pseudospondylolisthesen). Arch Orthop Trauma Surg. 1930; 29:118.
32. MacNab, I, Spondylolisthesis with an intact neural arch: the so-called pseudospondylolisthesis. J Bone Joint Surg 1950; 32B:325. ![]()
33. Newman, PH, Stenosis of the lumbar spine in spondylolisthesis. Clin Orthop 1976; 115:116–121. ![]()
34. Cauchoix, J, Benoist, M, Chassaing, V, Degenerative spondylolisthesis. Clin Orthop 1976; 115:122–129. ![]()
35. Feffer, HL, Weisel, SW, Chuckler, JW, et al, Degenerative spondylosis. To fuse or not to fuse. Spine 1985; 10:287–289. ![]()
36. Matzunaga, S, Sakou, T, Morizondo, Y, et al, Natural history of degenerative spondylolisthesis. Pathogenesis and clinical course of the slippage. Spine 1990; 15:1204–1210. ![]()
37. Kirkaldy-Willis, WH, Wedge, JH, Yong-Hing, K, et al, Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine 1978; 3:319–328. ![]()
38. Ooi, Y, Mita, F, Satah, Y, Myeloscopic study on lumbar spinal canal stenosis, with special reference to intermittent claudication. Spine 1990; 15:544–549. ![]()
39. Parke, WE, The significance of venous return impairment in ischemic radiculopathy and myelopathy. Orthop Clin North Am 1991; 22:213–221. ![]()
40. Kauppila, LI, Eustace, S, Kiel, DP, et al, Degenerative displacement of lumbar vertebrae; a 25-year follow-up study in Framingham. Spine 1998; 23:1868–1874. ![]()
41. Frymoyer, JW, Degenerative spondylolisthesis: diagnosis and treatment. J Am Acad Orthop Surg 1994; 2:9–15. ![]()
42. Auquier, L, Hirsch, JF, Paolaggi, JB, et al, Sténose du canal rachidien lombaire et claudication sciatique. Rev Rheum 1972; 39:429–437. ![]()
43. Wilson, CB, Significance of the small lumbar spine canal: cauda equina compression syndromes due to spondylosis. Part 3: intermittent claudication. J Neurosurg 1969; 31:499–506. ![]()
44. Ganz, JC, Lumbar signal stenosis: postoperative results in terms of preoperative posture-related pain. J Neurosurg 1990; 72:71–74. ![]()
45. Keenan, GF, Ashcroft, GP, Roditi, GH, et al, Measurement of lower limb blood flow in patients with neurogenic claudication using positron emission tomography. Spine 1995; 20:408–411. ![]()
46. Nicola, GC, Nizzoli, V, Claudication intermittente des membres inférieurs par sténose total du canal lombaire. Neurochirurgia 1974; 17:48–57. ![]()
47. Simkin, PA, Simian stance: a sign of spinal stenosis. Lancet 1982; ii:652–653. ![]()
48. Hall, S, Bartleson, JD, Onofrio, BM, et al, Lumbar spinal stenosis. Clinical features, diagnostic procedures and results of surgical treatment in 68 patients. Ann Intern Med 1985; 103:271–275. ![]()
49. Penning, L, Wilmink, JT, Posture-dependent bilateral compression of L4 or L5 nerve roots in facet hypertrophy, a dynamic CT-myelographic study. Spine 1987; 12:488–500. ![]()
50. Kapila, A, Chakeres, DW, Flexed sitting manoeuvre for complete lumbar myelography in patients with severe spinal stenosis and apparent block. Radiology 1986; 160:265–267. ![]()
51. Bartels, RH, Frenken, CW, Lumbale spinale stenose. Ned Tijdschr Geneeskd 1993; 137:529–532. ![]()
52. Kostuik, JP, Harrington, I, Alexander, D, et al, Cauda equina syndrome and lumbar disc herniation. J Bone Joint Surg Am 1986; 68:386–391. ![]()
53. Hai, Y. Spinal stenosis: classification, natural history and clinical evaluation. In: Herkowitz H, Dvorak J, Bell G, et al, eds. The Lumbar Spine. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:464–471.
54. Kalichman, L, Cole, R, Kim, DH, et al, Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 2009;9(7):545–550. ![]()
55. Gaskill, MF, Lukin, R, Wiot, JG, Lumbar disc disease and stenosis. Radiol Clin North Am 1991; 29:753–764. ![]()
56. Katz, JN, Dalgas, M, Stucki, G, et al, Degenerative lumbar spinal stenosis; diagnostic value of the history and physical examination. Arthritis Rheum 1995; 38:1236–1241. ![]()
57. Madsen, R, Jensen, TS, Pope, M, et al, The effect of body position and axial load on spinal canal morphology: an MRI study of central spinal stenosis. Spine (Phila Pa 1976). 2008;33(1):61–67. ![]()
58. Inufusa, A, An, HS, Lim, T, et al, Anatomic changes of the spinal canal and intervertebral foramen associated with flexion– extension movement. Spine 1996; 21:2412–2420. ![]()
59. Babin, E. Radiology of the narrow lumbar canal. In: Wackenheim A, Babin E, eds. The Narrow Lumbar Canal. Berlin: Springer, 1980.
60. Venner, RM, Crock, HV, Clinical studies on isolated disc resorption in the lumbar spine. J Bone Joint Surg 1981; 63B:491–494. ![]()
61. Butt, S, Saifuddin, A, The imaging of lumbar spondylolisthesis. Clin Radiol 2005; 60:533–546. ![]()
62. Babin, E, Capesius, P, Maitrot, D, Signes radiologiques osseux des variétés morphologiques de canaux lombaires étroits. Ann Radiol 1977; 20:491–499. ![]()
63. Jones, RAC, Thomas, JLG, The narrow lumbar canal. A clinical and radiological review. J Bone Joint Surg 1968; 50B:595–605. ![]()
64. Jönsson, B, Annertz, M, Sjöberg, C, Strömqvist, B, A prospective and consecutive study of surgically treated lumbar spinal stenosis – Part I: clinical features related to radiographic findings. Spine 1997; 22:2932–2937. ![]()
65. Bolender, N-F, Schönström, N, Spengler, D, Role of computed tomography and myelography in the diagnosis of central spinal stenosis. J Bone Joint Surg 1985; 67A:240–246. ![]()
66. Guen, YL, Joon, WL, Hee, SC, et al, A new grading system of lumbar central canal stenosis on MRI: an easy and reliable method. Skeletal Radiol. 2011;40(8):1033–1039. ![]()
67. Weinstein, JN, Tosteson, TD, Lurie, JD, et al, Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine (Phila Pa 1976). 2010;35(14):1329–1338. ![]()
68. Amundsen, T, Weber, H, Nordal, HJ, et al, Lumbar spinal stenosis: conservative or surgical management? A prospective 10-year study. Spine 2000; 25:1424–1436. ![]()
69. Simotas, AC, Dorey, FJ, Hansraj, KK, Cammisa, F, Jr., Nonoperative treatment for lumbar spinal stenosis. Clinical and outcome results and a 3-year survivorship analysis. Spine. 2000;25(2):197–203. ![]()
70. Atlas, SJ, Keller, RB, Robson, D, et al, Surgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the Maine Lumbar Spine Study. Spine. 2000;25(5):556–562. ![]()
71. Ritchie, JH, Fahnri, WH, Age changes in lumbar intervertebral discs. Can J Surg 1970; 13:65. ![]()
72. Onel, D, Sarl, H, Dinmez, C, Lumbar spinal stenosis: clinical/radiologic therapeutic evaluation in 145 patients. Conservative treatment or surgical intervention? Spine 1993; 18:291–298. ![]()
73. Botwin, K, Brown, LA, Fishman, M, Rao, S, Fluoroscopically guided caudal epidural steroid injections in degenerative lumbar spine stenosis. Pain Physician. 2007;10(4):547–558. ![]()
74. Fukusaki, M, Kobayashi, I, Hara, T, Sumikawa, K, Symptoms of spinal stenosis do not improve after epidural steroid injection. Clin J Pain. 1998;14(2):148–151. ![]()
75. Grabis, S. The treatment of spinal stenosis. J Bone Joint Surg. 1980; 62A:308–313.
76. Fast, A, Robin, GC, Floman, Y, Surgical treatment of lumbar spinal stenosis in the elderly. Arch Phys Med Rehabil 1985; 66:149–151. ![]()
77. Frymoyer, JW, Selby, OK, Segmental instability: rationale for treatment. Spine 1985; 10:280–286. ![]()
78. Postachini, F, Management of lumbar spial stenosis. J Bone Joint Surg 1996; 78B:154–164. ![]()
79. Hall, S, Bartleson, JD, Onofrio, BM, et al, Lumbar spinal stenosis, clinical features, diagnostic procedures and results of surgical treatment in 68 patients. Ann Intern Med 1985; 103:271–275. ![]()
80. Verbiest, H, Results of surgical treatment of idiopathic developmental stenosis of the lumbar vertebral canal. J Bone Joint Surg 1977; 59B:181–188. ![]()
81. Paine, KWE, Results of decompression for lumbar spinal stenosis. Clin Orthop 1976; 115:72–76. ![]()
82. Jönsson, B, Annertz, M, Sjöberg, C, Strömqvist, B, A prospective and consecutive study of surgically treated lumbar spinal stenosis – Part II: five-year follow-up by an independent observer. Spine 1997; 22:2938–2944. ![]()
83. Turner, J, Ersek, M, Herron, L, Deyo, R, Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine 1992; 17:1–8. ![]()
84. Ishac, R, Alhayek, G, Fournier, D, et al, Results of surgery for lumbar spinal stenosis in patients aged 80 years or more. A retrospective study of thirty-four cases. Rev Rhum Engl. 1996;63(3):196–200. ![]()
85. Herno, A, Airaksinen, O, Saari, T, Miettinen, H, The predictive value of preoperative myelography in lumbar spinal stenosis. Spine 1994; 19:133–1338. ![]()
86. Katz, JN, Stucki, G, Lipson, SJ, et al, Predictors of surgical outcome in degenerative lumbar spinal stenosis. Spine. 1999;24(21):2229–2233. ![]()
87. Herno, A, Airaksinen, O, Saari, T, Sihvonen, T, Surgical results of lumbar spinal stenosis. A comparison of patients with and without previous back surgery. Spine 1995; 20:964–969. ![]()
88. Aalto, TJ, Malmivaara, A, Kovacs, F, et al, Preoperative predictors for postoperative clinical outcome in lumbar spinal stenosis: systematic review. Spine (Phila Pa 1976). 2006;31(18):648–663. ![]()
89. Lee, CK, Rauschning, W, Glenn, W, Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine 1989; 13:313–320. ![]()
90. MacNab, I. Backache. Baltimore: Williams & Wilkins; 1983.
91. Cyriax, JH. Textbook of Orthopaedic Medicine, vol I. Diagnosis of Soft Tissue Lesions, 8th ed. London: Baillière Tindall; 1982.
92. Epstein, JA, Epstein, BS, Rosenthal, A, et al, Sciatica caused by nerve root entrapment in the lateral recess: the superior facet syndrome. J Neurosurg 1972; 36:584–589. ![]()
93. Cyriax, JH, Treatment of lumbar disc lesions. BMJ 1950; ii:1434. ![]()
94. Penning, L. Functionele radio-anatomie van lumbale stenose. Ned Tijdschr Man Ther. 1990; 9:36–48.
95. Beatty, RA, Sugar, O, Fox, TA, Protrusion of the posterior longitudinal ligament simulating herniated lumbar intervertebral disc. J Neurol Neurosurg Psychiatry 1968; 31:61–66. ![]()
96. Fujiwara, A, An, HS, Lim, TH, Haughton, VM, Morphologic changes in the lumbar intervertebral foramen due to flexion–extension, lateral bending, and axial rotation: an in vitro anatomic and biomechanical study. Spine (Phila Pa 1976). 2001;26(8):876–882. ![]()
97. LaRocca, H, MacNab, I, The laminectomy membrane. J Bone Joint Surg. 1974;56B(3):825–830. ![]()
98. Verbiest, H. Fallacies of the present definition, nomenclature, and classification of the stenoses of the lumbar vertebral canal. Spine. 1976; 1:217–225.
99. Transfeldt, EE, Robertson, D, Bradford, DS, Ligaments of the lumbosacral spine and their role in possible extra-foraminal spinal nerve entrapment and tethering. J Spinal Discord 1993; 6:507–512. ![]()
100. Wiltse, LL, Guyer, RD, Spencer, CW, et al, Alar transverse process impingement of the L5 spinal nerve: the far-out syndrome. Spine 1984; 9:31–41. ![]()
101. Matsumoto, M, Chiba, K, Nojiri, K, et al, Extraforaminal entrapment of the fifth lumbar spinal nerve by osteophytes of the lumbosacral spine: anatomic study and a report of four cases. Spine (Phila Pa 1976). 2002;27(6):E169–E173. ![]()
102. Olsewski, JM, Simmons, E, Kallen, FC, Mendel, FC, Evidence from cadavers suggestive of entrapment of fifth lumbar spinal nerves by lumbosacral ligaments. Spine 1991; 16:336–347. ![]()
103. Ahn, TJ, Lee, SH, Choi, G, et al, Effect of intervertebral disk degeneration on spinal stenosis during magnetic resonance imaging with axial loading. Neurol Med Chir (Tokyo). 2009;49(6):242–247. ![]()
104. Zander, DR, Lander, PH, Positionally dependent spinal stenosis: correlation of upright flexion–extension myelography and computed tomographic myelography. Can Assoc Radio J. 1998;49(4):256–261. ![]()
105. Willen, J, Danielson, B, Gaulitz, A, et al, Dynamic effects on the lumbar spinal canal: axially loaded CT-myelography and MRI in patients with sciatica and/or neurogenic claudication. Spine 1997; 22:2968–2976. ![]()
106. Nowicki, BH, Haughton, VM, Schmidt, TA, et al, Occult lumbar lateral spinal stenosis in neural foramina subjected to physiologic loading. AJNR Am J Neuroradiol. 1996;17(9):1605–1614. ![]()
107. Mumenthaler, M, Schliack, H. Läsionen peripherer Nerven. Stuttgart: Thieme; 1973.
108. Garfin, SR, Rydevik, BL, Brown, RA, Compressive neuropathy of spinal nerve roots. A mechanical or biological problem? Spine 1991; 16:162–165. ![]()
109. Rydevik, B, Brown, MD, Ehira, T, et al. Effects of graded compression and nucleus pulposus on nerve-tissue – an experimental study in rabbits. Proceedings of the Swedish Orthopaedic Association, Göteborg, Sweden, 27 August 1982. Acta Orthop Scand. 1983; 54:670–671.
110. Atlas, SJ, Delitto, A, Spinal stenosis: surgical versus nonsurgical treatment. Clin Orthop Relat Res 2006; 443:198–207. ![]()
111. Szpalski, M, Gunzburg, R, Lumbar spinal stenosis in the elderly: an overview. Eur Spine J. 2003;12(Suppl 2):170–175. ![]()
112. Amundsen, T, Weber, H, Lilleas, F, et al, Lumbar spinal stenosis. Clinical and radiologic features. Spine 1995; 20:1178–1186. ![]()
113. Sirvanci, M, Bhatia, M, Ganiyusufoglu, KA, et al, Degenerative lumbar spinal stenosis: correlation with Oswestry Disability Index and MR imaging. Eur Spine J. 2008;17(5):679–685. ![]()
114. Lohman, CM, Tallroth, K, Kettunen, JA, Lindgren, KA, Comparison of radiologic signs and clinical symptoms of spinal stenosis. Spine 2006; 31:1834–1840. ![]()
115. Jonsson, B, Annertz, M, Sjoberg, C, Stromqvist, B, A prospective and consecutive study of surgically treated lumbar spinal stenosis. Part I: clinical features related to radiographic findings. Spine 1997; 22:2932–2937. ![]()
116. Vouge, M. Interapophysolaminar spaces of the lumbar spine and their utility in the diagnosis of narrow lumbar canal. In: Wackenheim A, Babin E, eds. The Narrow Lumbar Canal. Berlin: Springer, 1980.
117. Wilmink, JT, Korte, JH, Penning, L, Dimensions of the spinal canal in individuals symptomatic and non-symptomatic for sciatica: a CT-study. Neuroradiology 1988; 30:547–550. ![]()
118. Wilmink, JT, CT morphology of intrathecal lumbosacral nerve-root compression. AJNR Am J Neuroradiol 1989; 10:233–248. ![]()
119. Fritz, JM, Delitto, A, Welch, WC, Erhard, RE, Lumbar spinal stenosis: a review of current concepts in evaluation, management, and outcome measurements. Arch Phys Med Rehabil 1998; 79:700–708. ![]()
120. Lee, S, Lee, JW, Yeom, JS, et al, A practical MRI grading system for lumbar foraminal stenosis. AJR Am J Roentgenol. 2010;194(4):1095–1098. ![]()
121. Porter, RW, Hibbert, C, Evans, C, The natural history of root entrapment syndrome. Spine 1983; 8:345–349. ![]()
122. Riew, KD, Yin, Y, Gilula, L, et al, The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am 2000; 82:1589–1593. ![]()
123. Cooper, G, Lutz, GE, Boachie-Adjei, O, Lin, J, Effectiveness of transforaminal epidural steroid injections in patients with degenerative lumbar scoliotic stenosis and radiculopathy. Pain Physician. 2004;7(3):311–317. ![]()
124. Tajima, T, Furakawa, K, Kuramochi, E, Selective lumbosacral radiculography and block. Spine 1980; 5:68–77. ![]()
125. Haueisen, DC, Smith, BS, Myers, SR, Pryce, ML, The diagnostic accuracy of spinal nerve injection studies. Clin Orthop 1985; 198:179–183. ![]()
126. Dooley, JF, McBroom, RJ, Taguchi, T, MacNab, I, Nerve root infiltration in the diagnosis of radicular pain. Spine 1988; 13:79–83. ![]()
127. Oertel, MF, Ryang, YM, Korinth, MC, et al, Long-term results of microsurgical treatment of lumbar spinal stenosis by unilateral laminotomy for bilateral decompression. Neurosurgery 2006; 59:1264–1269. ![]()
128. Kirkaldy-Willis, WH, Wedge JH. Young-Hing, K, et al, Lumbar spinal nerve lateral entrapment. Clin Orthop 1982; 169:171. ![]()
129. Zucherman, JF, Hsu, KY, Hartjen, CA, et al, A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine 2005; 30:1351–1358. ![]()