The Spirochetes
1. Describe the bacterial agents discussed in this chapter in terms of morphology, taxonomy, and growth conditions.
2. Identify the four stages of syphilis (i.e., primary, secondary, latent, and tertiary) according to clinical symptoms, antibody production, transmission, and infectivity.
3. Explain congenital syphilis, including transmission and clinical manifestations.
4. Define reagin, cardiolipin, and biologic false positive.
5. Differentiate reagin and treponemal antibodies, including specificity and association with disease.
6. Identify the various serologic methods that utilize specific treponemal or nonspecific nontreponemal antigens.
7. Describe the basic principles for the RPR, VDRL, FTA-ABS, TP-PA, and MHA-TP assays.
8. Compare Borrelia spp. to the other spirochetes discussed in this chapter, including morphology and growth conditions.
9. Describe the pathogenesis for relapsing fever and Lyme disease, including the routes of transmission, vector, and disease presentation.
10. Explain the methodology and clinical significance for using a two-step diagnostic procedure for Borrelia spp. infections.
11. Describe the pathogenesis associated with leptospirosis, including the two major stages of the disease and the recommended clinical specimens.
12. Describe Brachyspira spp., including potential pathogenesis, appropriate specimen, transmission, and clinical significance.
13. Correlate patient signs and symptoms with laboratory data to identify the most likely etiologic agent.
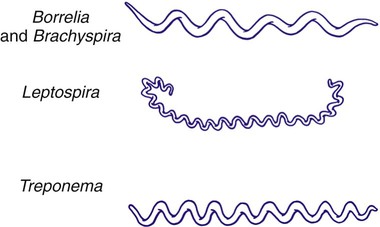
The spirochetes are all long, slender, helically curved, gram-negative bacilli, with the unusual morphologic features of axial fibrils and an outer sheath. These fibrils, or axial filaments, are flagella-like organelles that wrap around the bacteria’s cell walls, are enclosed within the outer sheath, and facilitate motility of the organisms. The fibrils are attached within the cell wall by platelike structures, called insertion disks, located near the ends of the cells. The protoplasmic cylinder gyrates around the fibrils, causing bacterial movement to appear as a corkscrew-like winding. Differentiation of genera within the family Spirochaetaceae is based on the number of axial fibrils, the number of insertion disks present (Table 46-1), and biochemical and metabolic features. The spirochetes also fall into genera based loosely on their morphology (Figure 46-1): Treponema appear as slender with tight coils; Borrelia are somewhat thicker with fewer and looser coils; and Leptospira resemble Borrelia except for their hooked ends. Brachyspira are comma-shaped or helical, with tapered ends with four flagella at each end.
TABLE 46-1
Spirochetes Pathogenic for Humans
| Genus | Axial Filaments | Insertion Disks |
| Treponema | 6 to 10 | 1 |
| Borrelia | 30 to 40 | 2 |
| Leptospira | 2 | 3 to 5 |
Treponema
General Characteristics
Epidemiology and Pathogenesis
Key features of the epidemiology of diseases caused by the pathogenic treponemes are summarized in Table 46-2. In general, these organisms enter the host by either penetrating intact mucous membranes (as is the case for T. pallidum subsp. pallidum—hereafter referred to as T. pallidum) or entering through breaks in the skin. T. pallidum is transmitted by sexual contact and vertically from mother to the unborn fetus. After penetration, T. pallidum subsequently invades the bloodstream and spreads to other body sites. Although the mechanisms by which damage is done to the host are unclear, T. pallidum has a remarkable tropism (attraction) to arterioles; infection ultimately leads to endarteritis (inflammation of the lining of arteries) and subsequent progressive tissue destruction.
TABLE 46-2
Epidemiology and Spectrum of Disease of the Treponemes Pathogenic for Humans
| Agent | Transmission | Geographic Location | Disease | Clinical Manifestations* | Age Group |
| T. pallidum subsp. pallidum | Sexual contact or congenital (mother to fetus) | Worldwide | Venereal syphilis† | Refer to text in this chapter | All ages |
| T. pallidum subsp. pertenue | Traumatized skin comes in contact with an infected lesion (person-to-person contact) | Humid, warm climates: Africa, South and Central America, Pacific Islands | Yaws | Skin—papules,† nodules, ulcers | Children |
| Primary lesion (mother yaw), disseminated lesions (frambesia) | |||||
| May progress to latent stage and late infection involving destructive lesions to bone and cartilage | |||||
| T. pallidum subsp. endemicum | Mouth to mouth by utensils, (person-to-person contact) | Arid, warm climates: North Africa, Southeast Asia, Middle East | Endemic nonvenereal syphilis | Skin/mucous patches, papules, macules, ulcers, scars† | Children or adults; rarely congenital |
| May progress to disseminated oropharyngeal with generalized lymphadenopathy | |||||
| May demonstrate a latent stage, and late syphilis destructive to skin, bone, and cartilage | |||||
| T. carateum | Traumatized skin comes in contact with an infected lesion (person-to-person contact) | Semiarid, warm climates: Central and South America, Mexico | Pinta | Skin papules, macules. Hyperkeratotic pigmented may lead to disseminated skin lesions and lymphadenopathy; late stage may result in pigmentary changes in skin (hyper- or hypopigmentation) | All ages but primarily children and adolescents |
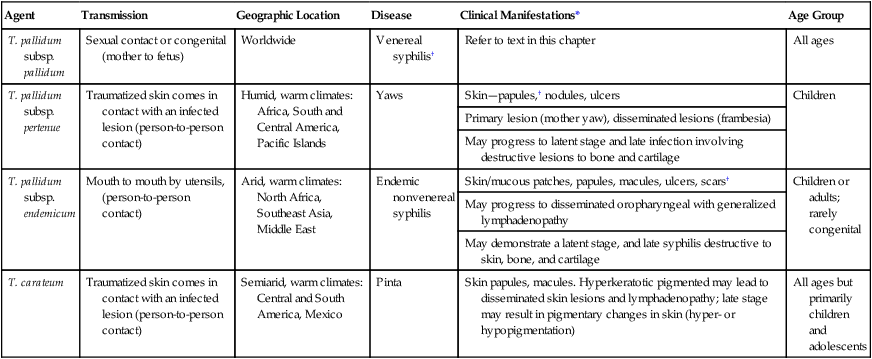
*All diseases have a relapsing clinical course and prominent cutaneous manifestations.
†If untreated, organisms can disseminate to other parts of the body such as bone.
Spectrum of Disease
The additional pathogenic treponemes are major health concerns in developing countries. Although morphologically and antigenically similar, these agents differ epidemiologically and with respect to their clinical presentation from T. pallidum. The diseases caused by these treponemes are summarized in Table 46-2.
Laboratory Diagnosis
Specimen Collection
Direct Detection
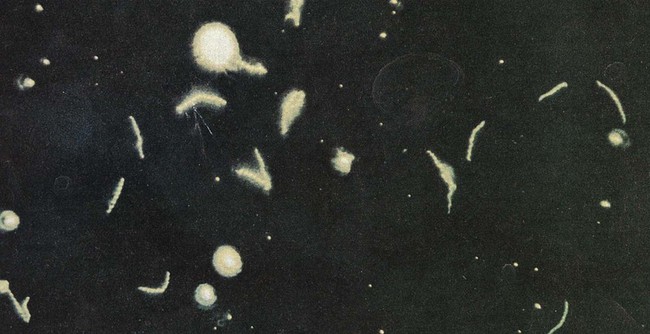
Material for dark-field examination is examined immediately under 400× high-dry magnification for the presence of motile spirochetes. Treponemes are long (8 to 10 µm, slightly larger than a red blood cell) and consist of 8 to 14 tightly coiled, even spirals (Figure 46-2). Once seen, characteristic forms should be verified by examination under oil immersion magnification (1000×). Although the darkfield examination depends greatly on technical expertise and the numbers of organisms in the lesion, it can be highly specific when performed on genital lesions.
Serodiagnosis
The two most widely used nontreponemal serologic tests are the Venereal Disease Research Laboratory (VDRL) and rapid plasma reagin (RPR) tests. Each of these tests is a flocculation (or agglutination) test, in which soluble antigen particles are coalesced to form larger particles that are visible as clumps when they are aggregated in the presence of antibody. The VDRL is used as a quantitative test and may be performed on serum or CSF in suspected cases of neurosyphilis. See Procedures 46-1 and 46-2 on the Evolve site for details and limitations for the VDRL and RPR.
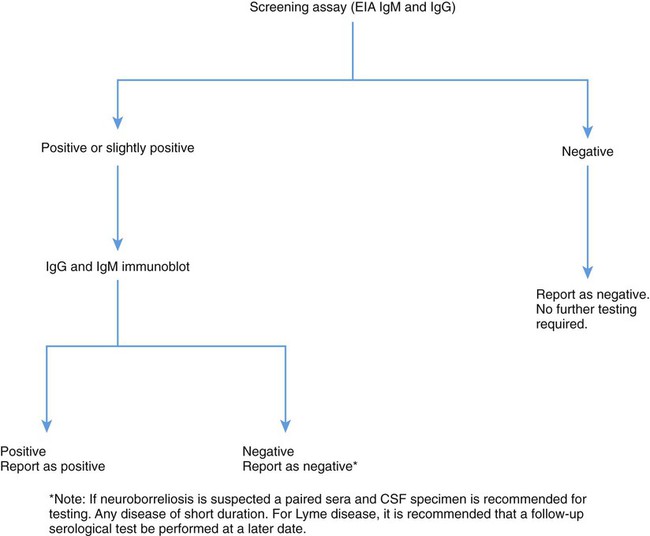
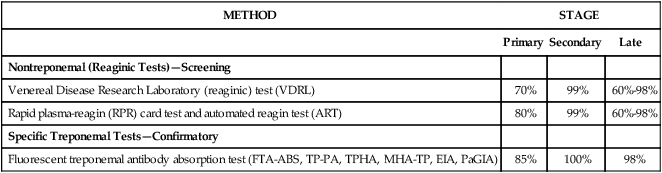
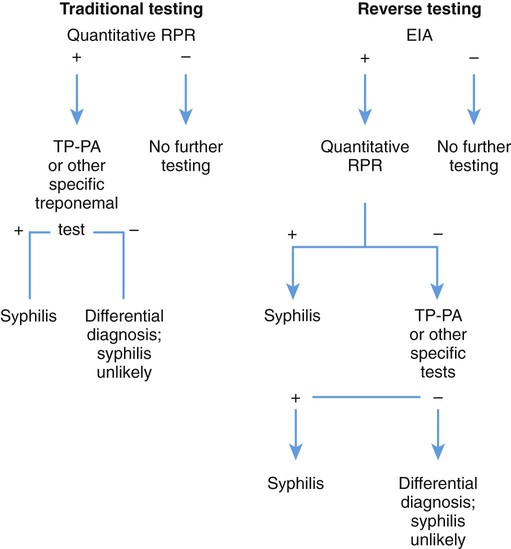
The nontreponemal serologic tests for syphilis can be used to determine antibody quantitative titers, which are useful to follow the patient’s response to therapy. The relative sensitivity of each test is shown in Table 46-3 to confirm that a positive nontreponemal test result is due to syphilis rather than to one of the other infections or biologic false-positive conditions previously mentioned. Traditional diagnosis for syphilis is useful in active infections. However, early or treated infections may be incorrectly diagnosed. In addition, primary testing using RPR or VDRL may result in a high rate of false-positives. The Centers for Disease Control has recommended a reverse algorithm to detect early primary or treated infections that may be missed using traditional nonspecific screening methods. Reverse testing suggests the use of specific antibody testing for syphilis, using enzyme-linked immunoassay (EIA) for IgM and IgG or a similar technique. T. pallidum antibodies persist for many years following infection. Specific tests may then be followed by nonspecific screening tests, which become less reactive over time. However, reverse testing is not currently widely accepted, and more data are needed to resolve clinical diagnostic discrepancies (Figure 46-3).
TABLE 46-3
Sensitivity of Commonly Used Serologic Tests for Syphilis
| METHOD | STAGE | ||
| Primary | Secondary | Late | |
| Nontreponemal (Reaginic Tests)—Screening | |||
| Venereal Disease Research Laboratory (reaginic) test (VDRL) | 70% | 99% | 60%-98% |
| Rapid plasma-reagin (RPR) card test and automated reagin test (ART) | 80% | 99% | 60%-98% |
| Specific Treponemal Tests—Confirmatory | |||
| Fluorescent treponemal antibody absorption test (FTA-ABS, TP-PA, TPHA, MHA-TP, EIA, PaGIA) | 85% | 100% | 98% |
Borrelia
General Characteristics
Borreliosis is considered a relapsing fever that is transmitted by a human-specific body louse or a tick. Organisms belonging to the genus Borrelia are composed of 3 to 10 loose coils (see Figure 46-1) and are actively motile. They contain endoflagella located beneath the outer membrane. The cells contain a protoplasmic cylinder that is composed of a peptidoglycan layer and an inner membrane. In contrast to the treponemes, Borrelia spp. stain well with Giemsa’s stain. Species that have been grown in vitro are microaerophilic or anaerobic.
Spectrum of Disease
Relapsing Fever
Lyme Disease
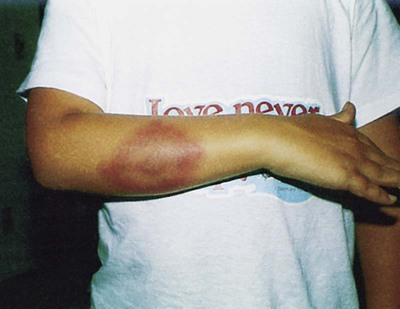
Lyme disease is characterized by three stages, not all of which occur in any given patient. The first stage, erythema migrans (EM), is the characteristic red, ring-shaped skin lesion with a central clearing that first appears at the site of the tick bite but may develop at distant sites as well (Figure 46-4). Patients may experience headache, fever, muscle and joint pain, and malaise during this stage. The second stage, beginning weeks to months after infection, may include arthritis, but the most important features are neurologic disorders (i.e., meningitis, neurologic deficits) and carditis. This is a result of the hematogenous spread of spirochetes to organs and tissues. In addition, neurologic symptoms and infection may occur in the meninges, spinal cord, peripheral nerves, and brain. The third stage is usually characterized by chronic arthritis or acrodermatitis chronica atrophicans (ACA), a diffuse skin rash, and may continue for years. There is an association between Borrelia species and distinct clinical manifestations. For example, B. garinii has been associated with up to 72% of European cases of neuroborreliosis.
Laboratory Diagnosis
Specimen Collection, Transport, and Processing
Serodiagnosis
Lyme Disease.
Numerous serologic tests are commercially available; however, these tests have not yet been standardized, and their performance characteristics vary greatly. The most common of these tests are the indirect immunofluorescence assay (IFA), the enzyme-linked immunosorbent assay (ELISA), and Western blot. Measuring antibody by enzyme-linked immunosorbent assay (ELISA) is the primary screening method because it is quick, reproducible, and relatively inexpensive. However, false-positive rates are high, mainly as a result of cross-reactivity. The specificity of IFA may be improved by adsorption of serum with Treponema phagedenis sonicate (IFA-ABS). Patients with syphilis, HIV infection, leptospirosis, mononucleosis, parvovirus infection, rheumatoid arthritis, and other autoimmune diseases commonly show positive results. Capture EIAs have been developed to avoid false positive reactions with rheumatoid factor. In addition, this may be overcome by pretreatment of the patient’s sera with anti-IgG. For the United States, the CDC recommends a two-step approach to the serologic diagnosis of Lyme disease. The first step is to use a sensitive screening test such as an ELISA or IFA; if this test is positive or equivocal, the result must be confirmed by immunoblotting (Figure 46-5). In certain clinical situations, results of serologic tests must be interpreted with caution. For example, patients with Lyme arthritis frequently remain antibody-positive despite treatment but do not necessarily have persistent infection. Conversely, patients with a localized EM may be seronegative. Because of these limitations and others, the Food and Drug Administration (FDA) and the American College of Physicians have published guidelines regarding the use of laboratory tests for Lyme disease diagnosis. Of paramount importance is the clinician’s determination before ordering serologic tests of the pretest probability of Lyme disease based on clinical symptoms and the incidence of Lyme disease in the population represented by the patient.
Leptospira
General Characteristics
Epidemiology and Pathogenesis
Pathogenic leptospires rapidly invade the bloodstream after entry and spread throughout all sites in the body such as the central nervous system and kidneys. Virulent strains show chemotaxis toward hemoglobin as well as the ability to migrate through host tissues. A number of potential virulence factors that might facilitate this process are shown in Box 46-1. Precisely how L. interrogans causes disease is not completely understood, but it appears that the presence of endotoxin and other toxins may play a role in which hemostasis pathways are activated as is an autoimmune response in the human host.