FIGURE 4.1 Absolute detection thresholds for sinusoidal stimuli, where it can be seen that as the vibration frequency increases, the detection thresholds decrease. (Reproduced from Bolanowski, S. J., Gescheider, G. A., Verrillo, R. T., and Checkosky, C. M., J Acoustic Soc Am, 84, 1680–1694, 1988, with the permission of the Acoustical Society of America.)
Psychophysical procedures have been traditionally employed to study the sense of touch, and as in hearing research where the sensory receptor is another type of specialized mechanoreceptor, different frequencies of vibration are used to quantify the response properties of this sensory system. Von Bekesy (7) was the first to use vibratory stimuli as an extension of his research interests in audition. In a typical experiment, participants are asked to respond with a simple button press when they can just detect the presence of a vibration presented to a digit within one of the two time periods. This two-alternative forced choice paradigm provides a threshold-tuning curve, the slopes of which provide information about a particular class of the response properties of LTMs. As can be seen from Figure 4.1, a U-shaped function is generated, with increasingly lower detection thresholds being measured as vibrotactile frequency increases to a peak at around 300 Hz, at which point the curve begins to increase again as sensitivity decreases.
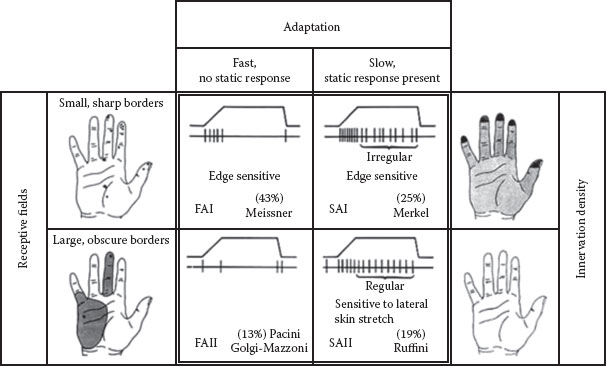
By carefully controlling the spatial configuration of the vibrating probe (i.e., its diameter and the gap between it and a static surround), the vibratory frequency, the amplitude, the stimulus duration, the skin surface temperature, and the use of various masking techniques, Verrillo et al. (8,9–12) proposed that there are four distinct psychophysical channels mediating tactile perception in the glabrous skin of the hand. This model proposes that each psychophysically determined channel is represented by one of the four anatomical end organs and nerve fiber subtypes with frequencies in the 40–500 Hz range providing a sense of vibration, transmitted by Pacinian corpuscles (Pacinian corpuscle channel or fast-adapting I [FAI]), Meissner’s corpuscles being responsible for the sense of flutter in the 2–40 Hz range (non-Pacinian I [NPI] channel or fast-adapting II [FAII]), the sense of pressure being mediated by Merkel’s discs in the 0.4–2.0 Hz range (non-Pacinian III [NPIII] or slowly adapting I [SAI]), and Ruffini end organs producing a buzzing sensation in the 100–500 Hz range (non-Pacinian III [NPII] or slowly adapting II [SAII]). Neurophysiological studies have by and large supported this model, but there is still some way to go to link the anatomy with perception (see Table 4.1 for a summary of the properties of these LTMs).
There have been relatively few studies of tactile sensitivity on hairy skin, the cat being the animal of choice for most of these studies. Mechanoreceptive afferents (Aβ fibers) have been described that are analogous to those found in human glabrous skin (FAI, FAII, SAI, and SAII), and Essick and Edin (13) have described sensory fibers with these properties in human facial skin. The relationship between these sensory fibers and tactile perception is still uncertain, and this is exemplified by the response properties of SAI afferents. Harrington and Merzenich (14) found that these afferents are responsive to levels of stimulation that are below perceptual thresholds, and Jarvilehto et al. (15) describe high levels of activity in human hairy skin SAIs that are not perceivable, in contrast to the responses of this class of afferent in glabrous skin where SAI nerve activity is directly correlated with a sense of pressure.
Sensory axons are classified according to their degree of myelination, the fatty sheath that surrounds the nerve fiber. The degree of myelination determines the speed with which the axon can conduct nerve impulses and, hence, the conduction velocity (CV) of the nerves. The largest and fastest axons are called A-α and include some of the proprioceptive neurons, such as the muscle stretch receptors. The second largest group, called A-β, includes all the discriminative touch receptors being described here. Pain and temperature include the third and fourth groups, A-δ and C fibers, and will be dealt with in the section “Temperature” (Table 4.1).
Electrophysiological studies by Vallbo and Johansson (16), on single peripheral nerve fibers innervating the human hand, have provided a generally accepted model of touch that relates the four anatomically defined types of cutaneous or subcutaneous sense organs to their neural response patterns.
TABLE 4.1
Main Characteristics of Primary Sensory Afferents Innervating Human Skin
|
Class |
Modality |
Axonal Diameter (μm) |
CV (m/s) |
|
Myelinated |
|||
|
A-α |
Proprioceptors from muscles and tendons |
20 |
120 |
|
A-β |
LTMs |
10 |
80 |
|
A-δ |
Cold, noxious, thermal |
2.5 |
12 |
|
Unmyelinated |
|||
|
C-pain |
Noxious, heat, thermal |
1 |
<1 |
|
C-tactile |
Light stroking, gentle touch |
1 |
<1 |
|
C-autonomic |
Autonomic, sweat glands, vasculature |
1 |
<1 |
The technique that they employed and developed is called microneurography and involves inserting a fine tungsten microelectrode, with tip diameter of <5 μm, through the skin of the wrist and into the underlying median nerve which innervates the thumb and the first two digits. A sensitive biological amplifier records and amplifies the spike discharges conveyed by the axons and feeds these to a loudspeaker to enable the experimenter to hear the spike activity and home-in on a single unit. The skilled manual micromanipulation of the electrode, coupled with stroking across the hand to stimulate LTMs, first results in a population response being recorded, that is, neural activity in a nerve fascicle containing hundreds of peripheral axons until finally, sometimes after many hours, a single axon is isolated. At this stage, the RF of the single unit is mapped with a Von Frey hair and the unit subtype (i.e., FA or SA) identified. Once this stage is completed, a small pulsed current of a few microamperes (typically <10 μA) is delivered to the nerve that provides a final, perceptual confirmation of the unit subtype.
FIGURE 4.2 The four types of LTMs in human glabrous skin are depicted. The four panels in the center show the nerve-firing responses to a ramp-and-hold indentation, and in percentage, the frequency of occurrence and putative morphological correlate. The black dots in the left panel show the RFs of type 1 (top) and type 2 (bottom) afferents. The right panel shows the average density of type 1 (top) and type 2 (bottom) afferents with darker area depicting higher densities. Abbreviations: LTM: low-threshold mechanoreceptor; RF: receptive field. (After Westling, G. K., Sensori-Motor Mechanisms during Precision Grip in Man, Medical dissertation, Umea University, Sweden, 1986.)
If, for example, an RA unit has been isolated, microstimulation is perceived as a flutter or vibration, depending on the frequency of the electrical pulses, and is perceptually localized to the previously mapped RF. Figure 4.2 depicts the relationships between RF, adaptation rate, and unit type from studies carried out on the human hand (17).
Temperature
The cutaneous somatosensory system detects changes in ambient temperature over an impressive range, initiated when thermal stimuli that differ from a homeostatic set point excite temperature-specific sensory nerves in the skin, and relays this information to the spinal cord and brain. It is important to recognize that these nerves code for temperature change, not absolute temperature, as a thermometer does. The system does not have specialized receptor end organs, such as those found in LTMs, but uses free nerve endings throughout the skin to sense changes in temperature. Within the innocuous thermalsensing range, there are two populations of thermosensory fibers, one that responds to warmth (warm receptors) and one that responds to cold (cold receptors), and include fibers from the A-δ and C ranges. Specific cutaneous cold and warm receptors have been defined as slowly conducting units that exhibit a steady-state discharge at constant skin temperature and a dynamic response to temperature changes (18,19). Cold- and warm-specific receptors can be distinguished from nociceptors that respond to noxious low and high temperatures (<20°C and >45°C) (20,21) and also from thermosensitive mechanoreceptors (18,22). Konietzny (22) recorded from 13 cold-specific units in humans employing the microneurography technique and measured CVs which were in the C fiber range (0.43–2.04 m/s).
Serra et al. (23) reported a number of spontaneously active fibers employing microneurography, which were sensitive to small temperature changes and that were described as cold-specific units, but all had CVs in the C fiber range (0.43–1.27 m/s). Standard medical textbooks describe the cutaneous cold sense in humans as being mediated by myelinated A fibers with CVs in the range of 12–30 m/s (24), but a work from Campero et al. (25) concludes that either human cold-specific afferent fibers are incompletely myelinated BC fibers, described by Duclaux et al. (26) as having electrophysiological and morphological properties of C fibers in their distal part and B fibers in their proximal part, or there are C as well as A cold fibers, with the C fiber group contributing little to sensation. For example, the resting discharge at room temperature (21°C) is characterized by a low-frequency discharge (~1 Hz), and this steady-state activity is suppressed by sudden warming of the RF and increased by cooling the RF.
The free nerve endings for cold- or warm-sensitive nerve fibers are located just beneath the skin surface. The terminals of an individual temperature-sensitive fiber do not branch profusely or widely. Rather, the endings of each fiber form a small, discretely sensitive point, which is separate from the sensitive points of neighboring fibers. The total area of the skin occupied by the receptor endings of a single temperature-sensitive nerve fiber is relatively small (1 mm in diameter) with the density of these thermosensitive points varying in different body regions. For example, there are up to 15–25 cold points per square centimeter in the lips, 3–5 cold points per square centimeter in the finger, and less than 1 cold point per square centimeter in some broad areas of the trunk. There are 3–10 times as many cold-sensitive points as warm-sensitive points in most areas of the body.
It is well established from physiological and psychological testing that warm- and cold-sensitive nerve fibers are distinctively different from one another in both structure and function.
Pain
Here, we consider a system of peripheral sensory nerves that innervate all cutaneous structures and whose sole purpose is to protect the skin against potential or actual damage. These primary afferents include A-δ and C fibers which respond selectively and linearly to the levels of thermal, mechanical, and chemical intensities/strengths that are tissue threatening, that is, having the potential to damage the skin. This initial encoding mechanism is termed nociception and describes the sensory process detecting any overt, or impending, tissue damage. The term pain, on the other hand, describes the perception of irritation, stinging, burning, soreness, or painful sensations arising from the skin.
It is important to recognize, especially when we are investigating an area such as sensitive skin, that the perception of pain depends not only on nociceptor inputs, but also on other processes and pathways giving information about, for example, emotional or contextual components. Pain is, therefore, described in terms of an experience rather than just a simple sensation. There are again submodalities within the nociceptive system which, at the peripheral anatomical level, are evident with respect to the degree of myelination of the nerve fibers (A-δ and C) subserving nociception (Table 4.2). A-δ fibers are thin (1–5 μm), poorly myelinated axons of mechanical nociceptors, thermal receptors, and mechanoreceptors with axon potential CVs averaging 12 m/s, and C fibers are very thin (<1 mm) slowly conducting axons (<1 m/s). Mechanical nociceptors are in the A-δ range and possess RFs distributed as 5–20 small sensitive spots over an area approximately 2–3 mm in diameter. In many cases, the activation of these spots depends upon stimuli intense enough to produce tissue damage, such as a pinprick. A-δ units with a short latency response to intense thermal stimulation in the range of 40–50°C have been described as well as other units excited by heat after a long latency—usually with thresholds in excess of 50°C. Over 50% of the unmyelinated axons (C fibers) of a peripheral nerve respond not only to intense mechanical stimulation, but also to heat and noxious chemicals, and are therefore classified as polymodal nociceptors (27) or C-mechanoheat (CMH) nociceptors (28). A subgroup of polymodal nociceptors has been reported to respond to extreme cold; however, many of these units develop an excitatory response to cooling after prior exposure to noxious heat. A small number of C fibers have mechanical thresholds in the nociceptor range with no response to heat, whereas others have been found to respond preferentially to noxious heating. RFs consist of single zones with distinct borders, and in this respect, they differ from A-δ nociceptors that have multipoint fields. Innervation densities are high, and responses have been reported to a number of irritant chemicals such as dilute acids, histamine, bradykinin, and capsaicin. By employing microneurography, Schmidt et al. (29) described not only CMH-responsive units, but also a novel class of C nociceptors responding only to mechanical stimuli (CM), units responding only to heating (CH), and units that were insensitive to mechanical and heating stimuli and also to sympathetic provocation tests (CMiCHi). Of relevance here is that some CM, CH, and CMiCHi units were sensitized to thermal and/or mechanical stimuli after topical application of skin irritants such as mustard oil or capsaicin; these units then acquired responsiveness to stimuli to which they were previously unresponsive. The recruitment of these silent nociceptors implies spatial summation to the nociceptive afferent barrage at central levels and may, therefore, contribute to primary hyperalgesia after chemical irritation and to secondary hyperalgesia as a consequence of central sensitization (detailed subsequently).
TABLE 4.2
Major Findings by Bolanowski et al. (8) and Previous Work Done by These Researchers at the Institute for Sensory Research at Syracuse University
|
Channel |
Pacinian |
NPI |
NPII |
NPIII |
|
Frequency response (Hz) |
40–80 |
3–100 |
15–400 |
<0.3 to >100 |
|
Threshold (at 1 μm) |
<−20 dB at 300 Hz |
28 dB at 3 Hz |
10 dB at 300 Hz |
28 dB at 3 Hz |
|
Sensation |
Vibration |
Flutter |
Not known |
Pressure |
|
Temporal summation |
Yes |
No |
Yes |
No |
|
Spatial summation |
Yes |
No |
Not known |
No |
|
Receptor type |
FAI Pacinian corpuscle |
FAII Meissner’s corpuscle |
SAII Ruffini end organ |
SAI Merkel’s disk |
Source: Bolanowski, S. J., Gescheider, G. A., Verrillo, R. T., and Checkosky, C. M., J Acoustic Soc Am, 84, 1680–1694, 1988; Gescheider, G. A., O’Malley, M. J., and Verrillo, R. T., J Acoustic Soc Am, 74, 474–485, 1983; Gescheider, G. A., Sklar, B. F., Van Doren, C. L., and Verrillo, R. T., J Acoust Soc Am, 78, 534–543, 1985; Gescheider, G. A., Verrillo, R. T., and Van Doren, C. L., J Acoustic Soc Am, 72, 1421–1426, 1982; Verrillo, R. T., J Acoustic Soc Am, 35, 1962–1966, 1963.
Nociceptors do not show the kinds of adaptation response found with rapidly adapting LTMs (i.e., they fire continuously to tissue damage), but pain sensation may come and go, and pain may be felt in the absence of any nociceptor discharge. They rely on chemical mediators around the nerve ending, which are released from nerve terminals and skin cells in response to tissue damage. Koltenzenburg et al. (30) showed that nerve growth factor (NGF) is an important mediator in painful inflammatory skin states, with levels increasing in inflamed tissue. Following carrageenan inflammation of rodent skin, a marked increase in the proportion of nociceptors which displayed an ongoing activity was observed, and this was reflected in a significant increase in the average ongoing discharge activity. Spontaneously active C fibers were sensitized to heat and displayed a more than twofold increase in their discharge to a standard noxious heat stimulus. Furthermore, the number of nociceptors responding to the algesic mediator bradykinin increased significantly from 28% to 58%.
In contrast, the mechanical threshold of nociceptive afferents did not change during inflammation. When the NGF-neutralizing molecule tropomyosin receptor kinase A (TrkA) immunoglobulin G (IgG) was coadministered with carrageenan at the onset of the inflammation, primary afferent nociceptors did not sensitize and displayed essentially normal response properties, although the inflammation as evidenced by tissue edema developed normally, demonstrating that NGF is a crucial component for the sensitization of primary afferent nociceptors associated with tissue inflammation.
The axon terminals of nociceptive axons possess no specialized end organ structure and, for that reason, are referred to as free nerve endings. This absence of any encapsulation renders them sensitive to chemical agents, both intrinsic and extrinsic, and inflammatory mediators released at a site of injury can initiate or modulate activity in surrounding nociceptors over an area of several millimeters leading to two kinds of sensory responses termed hyperalgesia—the phenomenon of increased sensitivity of damaged areas to painful stimuli; primary hyperalgesia occurs within the damaged area, and secondary hyperalgesia occurs in undamaged tissues surrounding this area.
One further sensation mediated by afferent C fibers is that of itch, and this is dealt with in detail in Chapter 7.
Pleasure
It is generally accepted that human tactile sensibility is solely mediated by LTMs with fast-conducting large myelinated afferents (as described earlier). However, a growing body of evidence has been accumulating, from anatomical, psychophysical, electrophysiological, and neuroimaging studies, that a further submodality of afferent slowly conducting unmyelinated C fibers exists in human hairy skin that are neither nociceptive nor pruritic but that respond preferentially to low-force, slowly moving mechanical stimuli traversing across their RFs. These nerve fibers have been classified as C-tactile afferents (CT afferents) and were first described by Nordin in 1990 (31) in the face and previously by Johansson et al. (32) in the same region, employing the technique of microneurography.
Evidence of a more general distribution of CT afferents has subsequently been found in the arm and the leg, but never in glabrous skin sites such as the palms of the hands or the soles of the feet (33–35). It is well known that the mechanoreceptive innervation of the skin of many mammals is subserved by A- and C-afferents (27,36,37), but until the observations of Nordin and Vallbo, C-mechanoreceptive afferents in human skin appeared to be lacking entirely. The functional role of CT afferents is not fully known (38), but their neurophysiological response properties, fiber class, and slow CVs preclude their role in any rapid mechanical discriminative or cognitive tasks and point to a more limbic function, particularly the emotional aspects of tactile perception (39,40). However, the central neural identification of low-threshold C-mechanoreceptors, responding specifically to light touch, and the assignment of a functional role in human skin have been achieved. In a study on a unique patient lacking large myelinated A-β fibers, it was discovered that the activation of CT afferents produced a faint sensation of pleasant touch, and functional neuroimaging showed activation in the insular cortex but no activation in the primary sensory cortex identifying CT afferents as a system for limbic touch that might underlie emotional, hormonal, and affiliative responses to skin-to-skin contacts between individuals engaged in grooming and bonding behaviors—pleasant touch (41,42). If pain is elicited via sensory C- and A-δ fibers, then it is reasonable to speculate that the same system may be alternatively modulated to deliver a sensation of pleasure. One hypothesis is that pleasant touch stimulates opioid and cannabinoid receptors on these peripheral nerve fibers (both opioids and cannabinoids also have antinociceptive and anti-inflammatory activities) and that this signal is decoded in areas of the brain such as the insular cortex, which is associated with pleasure. Further evidence of the representation of pleasant touch in the brain has been provided by Francis et al. (43), where it was shown that discriminative and affective aspects of touch are processed in different brain areas. The activation of the primary somatosensory cortex was found in the physical aspects of stimulation, whereas the orbitofrontal cortex (an area of the frontal lobes involved in emotion) was activated by pleasant aspects. This area has also been shown to represent painful as well as pleasant touch, demonstrating the relevance of this brain region for representing the emotional dimensions of skin sensitivity—the positive and the negative (44).
Work is in progress to identify this class of C fibers anatomically and histologically, and a study employing the pan-neuronal marker PGP9.5 and confocal laser microscopy has identified a population of free nerve endings located solely within the epidermis that may represent the putative anatomical substrate for this submodality (45).
Sympathetic Nerves
Although this chapter deals with the sensory aspects of skin innervation, it is important to briefly review the role of a class of efferent (motor) nerves that innervate various skin structures: (a) blood vessels, (b) cutaneous glands, and (c) unstriated muscle in the skin, for example, the erectors of the hairs. In sensitive skin conditions and some painful neuropathic states, sympathetic nerves play a role in exacerbating inflammation and irritation. The efferent sympathetic fibers that leave the CNS in connection with certain cranial and spinal nerves and end in sympathetic ganglia are known as preganglionic fibers. From these ganglia, postganglionic fibers arise and conduct nerve impulses to the different organs in the skin such as the vasoconstrictor fibers to the blood vessels, the pilomotor fibers to the hairs, and the motor fibers to the sweat glands. Most of the postganglionic neurons utilize the organic chemical noradrenalin as their neurotransmitter, which is released at the effector synapse where the neuron ends. Noradrenaline and adrenaline stimulate two types of adrenergic receptors, namely α and β receptors. Adrenaline stimulates both α and β receptors almost equally, whereas noradrenaline acts more pronouncedly on the a receptors. The stimulation of the two different types can produce different results; for example, the stimulation of the a receptors on capillaries causes vasoconstriction, whereas the stimulation of the β receptors causes vasodilation. Most of the postganglionic neurons are adrenergic; however, those which serve the sweat glands are cholinergic in their action except those on the palms of the hands, which are adrenergic.
In some cases, the sympathetic nervous system has been purported to play an important role in sustaining pain in some theories, suggesting that pain receptors in the affected part of the body become responsive to a family of nervous system messengers known as catecholamines. Animal studies indicate that noradrenaline, released from sympathetic nerves, acquires the capacity to activate pain pathways after tissue or nerve injury. Complex regional pain syndrome is a chronic pain condition that is believed to be the result of dysfunction in the CNS or the peripheral nervous systems. Typical features include dramatic changes in the color and temperature of the skin over the affected limb or body part, accompanied by intense burning pain, skin sensitivity, sweating, and swelling.
Receptors and Channels
Signaling of stimuli such as heat, pain, or chemical challenge acting on nociceptors is peripherally controlled via a complex regulation of activity in a series of ion channels. A candidate receptor for chemosensory agents such as capsaicin and menthol eluded scientific characterization until 1997 and 2002, respectively (46,47). Developments in molecular cloning of receptor types (e.g., the vanilloid receptor and associated thermo-transient receptor potential [TRP] channels—a subset of TRP ion channels) combined with electrophysiological and receptor–ligand characterization have shed new light on the understanding of how noxious stimuli are encoded at the cellular level (48). The vanilloid receptor subtype 1 (VR1, also referred to as TRPV1) is a classical cation channel and is expressed in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures (49). Interestingly, the activity for temperature (heat and cold) and pain and chemesthetic activity can all be explained in terms of the plasticity of a family of thermo-TRP cation channels (50). The development of transgenic mouse models lacking expression of the VR1 gene shows that phenotypic characteristics in VR1 null (−/−) mice support a functional role for VR1 in sensory transduction of nociceptive stimuli, although it was apparent that another unidentified receptor could partially compensate for the loss of VR1 function (51,52).
As an understanding of the process involved in sensing temperature and chemical stimulation of nociceptors has evolved, it has become apparent that there are additional non-TRP proteins and receptors which also play a role in nociception, for example, the acid-sensing ion channels and the P2X3 adenosine triphosphate receptor (53,54).
Opioids
The pain relief produced by opiates, such as morphine, derived from the opium poppy (Papaver somniferum), has been used and studied extensively for more than 5000 years. In addition to narcotic effects caused by activities within the CNS, opioids are also known for their antinociceptive and anti-inflammatory effects in the periphery. Coggeshall et al. (55) used light microscopic techniques to demonstrate the presence of μ- and δ-opioid receptors on ummyelinated afferents in human skin. In 2002, Stander et al. (56) showed a colocalization of μ-opioid receptor isoform 1A (MOR 1A) and calcitonin gene-related peptide in sensory fibers, suggesting a functional relationship for opiate agonists in terms of anti-inflammatory and antinociceptive activities. Opiates also cause vasodilatation of the skin, although this does not appear to account for a reduction in pain via a local warming mechanism; that is, the analgesic effect is clearly μ opioid receptor (MOR) mediated (57). The activity of opiates in the periphery does appear to be dependent on the extent of inflammation and local tissue damage, and this may account for many of the discrepancies reported by various authors (58–60).
A range of cell types, including neurons, keratinocytes, and immune cells, produces endogenous opioids. There are three families of peptides identified to date, each arising from alternate processing of the gene products for proopiomelanocortin (POMC), proencephalin, and prodynorphin. In the skin, the opioid, β-endorphin, is produced by posttranslational cleavage of the POMC gene and acts both on MORs on nerves and keratinocytes (61). The expression of MOR on keratinocytes and the involvement in the pathogenesis of clinical skin disease such as psoriasis suggest an additional role for opiates as immunoregulatory molecules in the skin (62).
Cannabinoids
Cannabis (Cannabis sativa L.), such as the opiates, has long been used for its narcotic effects. The discovery of specific cannabinoid receptors and endogenous ligands, produced in the periphery, has led to a new therapeutic potential as an analgesic and an anti-inflammatory molecule (63,64). To date, two G protein-coupled cannabinoid receptors, referred to as cannabinoid (CB) 1 and CB2, have been identified in both the CNS and the peripheral nervous systems (65,66). Differential localization using in situ hybridization and immunohistochemistry has shown the presence of CB receptors on both nociceptive and nonnociceptive afferents, in addition to staining on nonneuronal tissues, for example, keratinocytes and leukocytes (67,68).
Several studies have shown that both classical agonists, such as HU210, and endogenous cannabinoid (endocannabinoids) agonists, such as anandamide, have anti-inflammatory and antinociceptive benefits (69,70). The lipid metabolic pathways leading to production of endocannabinoids, their interactions with receptors, deactivation, and clearance pathways have been reviewed by Piomelli (71).
An interesting development in the understanding of the role of endocannabinoids in the skin has been the observation that they can also activate VR1. Anandamide has been shown to activate VR1 (72,73), and this may explain the ability of anandamide to act as a vasodilator, although there is still some controversy over the levels required to activate VR1 and its physiological relevance.
The Central Projections
The submodalities of skin sensory receptors and nerves that convey information to the brain about mechanical, thermal, and painful stimulations of the skin are grouped into three different pathways in the spinal cord and project to different target areas in the brain. They differ in their receptors, pathways, and targets and also in the level of decussation (crossing over) within the CNS. Most sensory systems en route to the cerebral cortex decussate at some point, as projections are mapped contralaterally. The discriminative touch system crosses in the medulla, where the spinal cord joins the brain; the pain system crosses at the point of entry into the spinal cord.
Spinal Cord
All the primary sensory neurons described earlier have their cell bodies situated outside the spinal cord in the dorsal root ganglion, there being one ganglion for every spinal nerve. Sensory neurons have a unique property in that, unlike most neurons, the nerve signal does not pass through the cell body but, as the cell body sits off to one side, the signal passes directly from the distal axon process to the proximal process, which enters the dorsal half of the spinal cord.
Tactile primary afferents, or first-order neurons, immediately turn up the spinal cord toward the brain, ascending in the dorsal white matter and forming the dorsal columns. In a cross section of the spinal cord at cervical levels, two separate tracts can be seen—the midline tracts comprise the gracile fasciculus conveying information from the lower half of the body (legs and trunk) and the outer tracts comprise the cuneate fasciculus conveying information from the upper half of the body (arms and trunk). At the medulla, situated at the top of the spinal cord, the primary tactile afferents make their first synapse with second-order neurons, where fibers from each tract synapse in a nucleus of the same name—the gracile fasciculus axons synapse in the gracile nucleus and the cuneate axons synapse in the cuneate nucleus. The neurons receiving the synapse provide the secondary afferents and immediately cross to form a new tract on the contralateral side of the brainstem—the medial lemniscus—which ascends through the brainstem to the next relay station in the midbrain, the thalamus.
As with the tactile system, pain, and thermal afferents, primary afferents synapse ipsilaterally and then the secondary afferents cross, but the crossings occur at different levels. Pain and temperature afferents enter the dorsal horn of the spinal cord and synapse within one or two segments, forming Lissauer’s tract as they do so. The dorsal horn is a radially laminar structure; the thin outermost layer is called the posterior marginalis layer, the second layer is the substantia gelatinosa, and the layer deeper to that is the nucleus proprius. The two types of pain fibers, C and A-δ, enter different layers of the dorsal horn. A-δ fibers enter the posterior marginalis and the nucleus proprius and synapse on a second set of neurons. These are the secondary afferents which will relay the signal to the thalamus. The secondary afferents from both layers cross to the opposite side of the spinal cord and ascend in the spinothalamic tract. The C fibers enter the substantia gelatinosa and synapse, but they do not synapse on secondary afferents. Instead, they synapse on interneurons—neurons which do not project out of the immediate area but relay the signal to the secondary afferents in either the posterior marginalis or the nucleus proprius. The spinothalamic tract ascends the entire length of the cord and the entire brainstem and, by the time that it reaches the midbrain, appears to be continuous with the medial lemniscus. These tracts enter the thalamus together.
It is important to note that although the bulk of afferent input adheres to the plan outlined earlier, there is degree mixing that goes on between the tracts. Some light touch information, for example, travels in the spinothalamic tract with the result that the damage to the dorsal columns does not completely remove touch and pressure sensation. Some proprioception also travels in the dorsal columns and follows the medial lemniscus all the way to the cortex, so there is conscious awareness of body position and movement. The pain and temperature system also has multiple targets in the brainstem and other areas.
We have concentrated on somatosensory inputs from the body thus far, but as facial skin is often the source of sensitive reactions to topical applications, its peripheral and central anatomy/neurophysiology will be briefly summarized here. The trigeminal nerve innervates all facial skin structures (including the oral mucosa), and just as with the spinal afferents, these neurons have their cell bodies outside of the CNS in the trigeminal ganglion with their proximal processes entering the brainstem. Just as in the spinal cord, the three modalities of touch, temperature, and pain have different receptors in the facial skin, travel along different tracts, and have different targets in the brainstem—the trigeminal nucleus, a relatively large structure that extends from the midbrain to the medulla.
The large-diameter (A-β) fibers enter directly into the main sensory nucleus of the trigeminal and, as with the somatosensory neurons of the body, synapse and then decussate, the secondary afferents joining the medial lemniscus as it projects to the thalamus. The small-diameter fibers conveying pain and temperature enter the midbrain with the main fifth cranial nerve but then descend down the brainstem to the caudal medulla where they synapse and cross. These descending axons form a tract, the spinal tract of V, and synapse in the spinal nucleus of V, so called because it reaches as far down as the upper cervical spinal cord. The spinal nucleus of V comprises three regions along its length: the subnucleus oralis, the subnucleus interpolaris, and the subnucleus caudalis. The secondary afferents from the subnucleus caudalis cross to the opposite side and join the spinothalamic tract where the somatosensory information from the face joins that from the body, entering the thalamus in a separate nucleus, the ventroposterior medial (VPM) nucleus.
Brain
The third-order thalamocortical afferents (from thalamus to cortex) travel up through the internal capsule to reach the primary somatosensory cortex, located in the postcentral gyrus, a fold of cortex just posterior to the central sulcus. The thalamocortical afferents convey all the signals, whether from ventro-postero lateral or VPM to primary somatosensory cortex where the sensory information from all body surfaces is mapped in a somatotopic (body-mapped) manner (74,75), with the legs represented medially, at the top of the head, and the face represented laterally. Within the cortex, there are thought to be nine separate areas primarily subserving somatosensation: primary somatosensory cortex, SI, comprised four subregions (2, 1, 3a, and 3b); secondary somatosensory cortex, SII, located along the superior bank of the lateral sulcus (76–80); insular cortex (81); and the posterior parietal cortex, areas 5 and 7b.
As with studies of the peripheral nervous system, the technique of microneurography has also been employed to study the relationship between skin sensory nerves and their central projections, as evidenced by the use of concurrent functional magnetic resonance imaging (fMRI). The microstimulation of individual LTM afferents, projecting to RFs on the digit, produces robust, focal, and orderly (somatotopic) hemodynamic blood oxygen level-dependent responses in both primary and secondary somatosensory cortices (82), in accordance with the findings of Penfield and Boldrey (83). It is expected that this technique will permit the study of many different topics in somatosensory neurophysiology, such as sampling from FA and SA mechanoreceptors and C fibers with neighboring or overlapping RFs on the skin and quantifying their spatial and temporal profiles in response to electrical chemical and/or mechanical stimulation of the skin areas that they innervate, as well as perceptual responses to microstimulation.
Finally, the forward projections from these primary somatosensory areas to limbic and prefrontal structures have been studied with fMRI to understand the effective representations of skin stimulation for both pain and pleasure (43,84) and it is hoped that studies of this nature will help us to better understand the emotional aspects of both negative (sensitive skin) and positive (pleasant touch) skin sensations.
REFERENCES
1. Johansson RS. Receptive field sensitivity profile of mechanosensitive units innervating the glabrous skin of the human hand. Brain Res 1976; 219:13–27.
2. Darian-Smith I. The sense of touch: Performance and peripheral neural processes. In: Brookhart JM, Mountcastle VB, eds. Handbook of Physiology: Section 1: The Nervous System. Vol. 3. Oxford: Oxford University Press, 1984:739–878.
3. Gescheider GA, Bolanowski SJ, Verrillo RT. Sensory, cognitive and response factors in the judgement of sensory magnitude. In: Algom D, ed. Psychophysical Approaches to Cognition. Amsterdam: Elsevier, 1992:575–621.
4. Greenspan JD, Lamotte RH. Cutaneous mechanoreceptors of the hand: Experimental studies and their implications for clinical testing of tactile sensation. J Hand Ther 1993; 6:75–82.
5. Vallbo AB, Hagbarth K, Torebjork E, Wallin BG. Somatosensory, proprioceptive and sympathetic activity in human peripheral nerves. Physiol Rev 1979; 59:919–957.
6. Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. 2nd ed. New York: Plenum Press, 1991.
7. von Bekesy G. Uber die Vibrationsempfindung [On the vibration sense]. Akustische Zeitschrift 1939; 4:315–334.
8. Bolanowski SJ, Gescheider GA, Verrillo RT, Checkosky CM. Four channels mediate the mechanical aspects of touch. J Acoustic Soc Am 1988; 84:1680–1694.
9. Gescheider GA, O’Malley MJ, Verrillo RT. Vibrotactile forward masking: Evidence for channel independence. J Acoustic Soc Am 1983; 74:474–485.
10. Gescheider GA, Sklar BF, Van Doren CL, Verrillo RT. Vibrotactile forward masking: Psychophysical evidence for a triplex theory of cutaneous mechanoreception. J Acoust Soc Am 1985; 78:534–543.
11. Gescheider GA, Verrillo RT, Van Doren CL. Prediction of vibrotactile masking functions. J Acoustic Soc Am 1982; 72:1421–1426.
12. Verrillo RT. Effect of contactor area on the vibrotactile threshold. J Acoustic Soc Am 1963; 35:1962–1966.
13. Essick GK, Edin BB. Receptor encoding of moving tactile stimuli in humans. II: The mean response of individual low-threshold mechanoreceptors to motion across the receptive field. J Neurosci 1995; 15:848–864.
14. Harrington T. Merzenich M. Neural coding in the sense of touch; Human sensations of skin indentation compared with responses of slowly adapting mechanoreceptive afferents innervating the hairy skin of monkeys. Exp Brain Res 1970; 10:251–264.
15. Jarvilehto T, Hamalainen H, Laurinen P. Characteristics of single mechanoreceptive fibres innervating hairy skin of the human hand. Exp Brain Res 1976; 25:45–61.
16. Vallbo AB, Johansson RS. The tactile sensory innervation of the glabrous skin of the human hand. In: Gordon G. ed. Active Touch. New York: Pergamon, 1978:29–54.
17. Westling GK. Sensori-motor mechanisms during precision grip in man. Umea University Medical dissertation. New Series 171, Umea, Sweden, 1986.
18. Hensel H. Boman KKA. Afferent impulses in cutaneous sensory nerves in human subjects. J Neurophysical 1960; 23:564–578.
19. Hensel H. Cutaneous thermoreceptors. In: Iggo, A. ed. Somatosensory System. Berlin: Spring, 1973:79–110.
20. Torebjörk, Hallin. A new method for classification of C-unit activity in intact human skin nerves. In: Bonica JJ, Albe-Fessard D, eds. Advances in Pain Research and Therapy. New York: Raven Press, 1976:29–34.
21. Campero M, Serra J, Ochoa JL. C-polymodal nociceptors activated by noxious low temperature in human skin. J Physiol 1966; 497:565–572.
22. Konietzny F. Peripheral neural correlates of temperature sensations in man. Hum Neurobiol 1984; 3:21–32.
23. Serra J. Campero M, Ochoa JL, Bostock H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. J Physiol 1999; 515:799–811.
24. Darian-Smith I, ed. Thermal sensibility. In: Handbook of Physiology, Section 1, The Nervous System, Vol. 3, Sensory Processes, Part 2. Bethesda, MD: American Physiological Society, 1984:879–913.
25. Campero M, Serra J, Bostock H, Ochoa JL. Slowly conducting afferents activated by innocuous low temperature in human skin. J Physiol 2001; 535:855–865.
26. Duclaux R, Mei N, Ranieri F. Conduction velocity along afferent vagal dendrites: A new type of fibre. J Physiol 1976; 260:487–495.
27. Bessou M, Perl ER. Response of cutaneous sensory units with unmyelinated fibres to noxious stimuli. J Neurophysiol 1969; 32:1025–1043.
28. Campbell JN, Raja SN, Cohen RH, Manning DC, Khan AA, Meyer RA. Peripheral neural mechanisms of nociception. In: Wall PD, Melzack R, eds. Textbook of Pain. New York: Churchill Livingstone, 1989:22–45.
29. Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjork E, Handwerker H. Novel classes of responsive and unresponsive C nociceptors in humans skin. J Neurosci 1995; 15:333–341.
30. Koltenzenburg M, Bennett DL, Shelton DL, McMahon SB. Neutralization of endogenous NGF prevents the sensitization of nociceptors supplying inflamed skin. Eur J Neurosci 1999; 11:1698–1704.
31. Nordin M. Low threshold mechanoreceptive and nociceptive units with unmyelinated (C) fibres in the human supraorbital nerve. J Physiol 1990; 426:229–240.
32. Johansson RS, Trulsson M, Olsson KA, Westberg KG. Mechanoreceptor activity from the human face and oral mucosa. Exp Brain Res 1988; 72:204–208.
33. Vallbo AB, Hagbarth K-E, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 1979; 59:919–957.
34. Löken L, Wessberg J, Morrison I, McGlone F, Olausson, H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci 2009; 5:547–548.
35. McGlone F, Wessberg J, Olausson H. Discriminative and Affective touch: Sensing and feeling. Neuron 2014; 82:737–755.
36. Zotermann Y. Touch, pain and tickling: An electrophysiological investigation on cutaneous sensory nerves. J Physiol 1939; 95:1–28.
37. Iggo A, Korhuber HHA. A quantitative study of C-mechanoreceptors in the hairy skin of the cat. J Physiol 1977; 271:549–565.
38. MacKenzie RA, Burke D, Skuse NF, Lethlean AK. Fibre function and perception during cutaneous nerve block. J Neurol Neurosurg Psychiatry 1975; 38:865–873.
39. Vallbo AB, Olausson H, Wessberg J, Norsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Res 1993; 628:301–304.
40. Essick G, James A, McGlone FP. Psychophysical assessment of the affective components of non-painful touch. Neuroreport 1999; 10:2083–2087.
41. Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck S, Strigo K, Worsley K, Vallbo AB, Bushnell MC. Unmyelinated tactile afferents signal touch and project to the insular cortex. Nat Neurosci 2002; 5:900–904.
42. Wessberg J, Olausson H, Fernstormm KW, Vallbo AB. Receptive field properties of unmyelinated tactile afferents in the human skin. J Neurophysiol 2003; 89:1567–1575.
43. Francis ST, Rolls ET, Bowtell R, McGlone F, O’Doherty JO, Browning A, Clare S, Smith E. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport 1999; 10:453–459.
44. Rolls ET, O’Doherty JO, Kringelbach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal cortices. Cereb Cortex 2003; 13:308–317.
45. Reilly DM, Ferdinando D, Johnston C, Shaw C, Buchanan KD, Green M. The epidermal nerve fibre network: Characterization of nerve fibres in human skin by confocal microscopy and assessment of racial variations. Br J Dermatol 1997; 137:163–170.
46. Caterina MJ, Schumaker MJ, Tominaga M, Rosen TA, Levin JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997; 389:816–824.
47. McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002; 416:52–58.
48. Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: Mechanisms of temperature sensation. Nat Rev Neurosci 2003; 4:529–538.
49. Stander S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, Brzoska T et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibres, mast cells, and epithelial cells of appendage structures. Exp Dermatol 2004; 13:129–139.
50. Montell C, Birnaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell 2002; 108:595–598.
51. Caterina MJ, Leffer A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz M, Koltzenburg M, Basbaum Ai, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000; 288:306–313.
52. Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000; 405:183–187.
53. Askwith CC, Benson CJ, Welsh MJ, Snyder PM. DEG/EnaC ion channels involved in sensory transduction are modulated by cold temperature. PNAS 2001; 98:6459–6463.
54. Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K et al. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature 2000; 407:1015–1017.
55. Coggeshall RE, Zhou S, Carlton SM. Opioid receptors on peripheral sensory axons. Brain Res 1997; 764:126–132.
56. Stander S, Gunzer M, Metze D, Luger T, Steinhoff M. Localization of m-opioid receptor 1A on sensory nerve fibres in human skin. Regul Pept 2002; 110:75–83.
57. Holland RL, Harkin NE, Coleshaw RK, Jones DA, Peck AW, Telekes A. Dipipanone and nifedipine in cold induced pain: Analgesia not due to skin warming. Br J Clin Pharmacol 1987; 24:823–826.
58. Yuge O, Matsumoto M, Kitahata LM, Collins JG, Senami M. Direct opioid application to peripheral nerves does not alter compound action potentials. Anesth Analg 1985; 64:667–671.
59. Frank GB, Sudha TS. Effects of encephalin, applied intracellularly, on action potentials in vertebrate A and C nerve fibre axons. Neuropharmacology 1987; 26:61–66.
60. Antoijevic I, Mousa SA, Schafer M, Stein C. Perineural defect and peripheral opioid analgesia during inflammation. J Neurosci 1995; 15:165–172.
61. Bigliardi PL, Bigliardi-Qi M, Buechner S, Ruffi T. Expression of m-opiate receptor in human epidermis and keratinocytes. J Invest Dermatol 1998; 111:297–301.
62. Bigliardi-Qi M, Bigliardi PL, Eberle AN, Buechner S, Ruffi T. b-Endorphin stimulates cytokeratin 16 expression and downregulates m-opiate receptor expression in human epidermis. J Invest Dermatol 1998; 114:527–532.
63. Dvorak M, Watkinson A, McGlone F, Rukweid R. Histamine-induced responses are attenuated by a cannabinoid receptor agonist in human skin. Inflamm Res 2003; 52:238–245.
64. Johanek LM, Simone DA. Activation of peripheral cannabinoid receptors attenuates cutaneous hyperalgesia produced by a heat injury. Pain 2004; 109:432–442.
65. Matsuda LA. Molecular aspects of cannabinoid receptors. Crit Rev Neurobiol 1997; 11:143–166.
66. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993; 365:61–65.
67. Price TJ, Helesic G, Parghi D, Hargreaves KM, Flores CM. The neuronal distribution of cannabinoid receptor type 1 in the trigeminal ganglion of the rat. Neuroscience 2003; 120:155–162.
68. Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocytes subpopulations. Eur J Biochem 1995; 232:54–61.
69. Rukweid R, Watkinson A, McGlone F, Dvorak M. Cannabinoid agonists attenuate capsaicin-induced responses in human skin. Pain 2003; 102:283–288.
70. Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MN. Pain modulation by release of the endogenous cannabinoid anandamide. PNAS 1999; 96:12198–12203.
71. Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 2003; 4:873–884.
72. DiMarzo V, Bisogno T, Melck D, Ross R, Brochic H, Stevenson L, Pertwee R, DePetrocellis L. Interactions between synthetic vanilloids and the endogenous cannabinoid system. FEBS Lett 1998; 436:449–454.
73. DiMarzo V, Bisogno T, Petrocellis L. Anandamide: Some like it hot. Trends Pharmacol Sci 2001; 22:346–349.
74. Penfield R, Rasmussen T. The Cerebral Cortex of Man. New York: Macmillan, 1952.
75. Maldjian JA, Gotschalk A, Patel RS, Detre JA, Alsop DC. The sensory somatotopic map of the human hand demonstrated at 4 T. Neuroimage 1999; 10:55–62.
76. Woolsey C. Second somatic receiving areas in the cerebral cortex of the cat, dog and monkey. Fed Proc 1946; 55–56.
77. Maeda K, Kakigi R, Hoshiyama M, Koyama S. Topography of the secondary somatosensory cortex in humans: A magentoencephalographic study. Neuroreport 1999; 10:301–306.
78. Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH. Distributed processing of pain and vibration by the human brain. J Neurosci 1994; 14:4095–4108.
79. Francis ST, Kelly EF, Bowtell R, Dunseath WJ, Folger SE, McGlone FP. FMRI of the responses to vibratory stimulation of digit tips. Neuroimage 2000; 11:188–202.
80. McGlone FP, Kelly EF, Trulsson M, Francis ST, Westling G, Bowtel R. Functional neuroimaging studies of human somatosensory cortex. Behav Brain Res 2002; 135:147–158.
81. Schneider RJ, Friedman DP, Mishkin M. A modality-specific somatosensory area within the insula of the rhesus monkey. Brain Res 1993; 621:116–120.
82. Trulsson M, Francis ST, Kelly EF, Westling G, Bowtell R, McGlone FP. Cortical responses to single mechanoreceptive afferent microstimulation revealed with fMRI. Neuroimage 2001; 13:613–622.
83. Penfield R, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 1937; 60:389–443.
84. Rolls E, O’Doherty J, Kringelbach M, Francis S, Bowtell R, McGlone F. Representation of pleasant and painful touch in the human orbitofrontal cortex. Cereb Cortex 2003; 10:284–294.