The Solid Organ Transplant Patient
Principles of Disease
Infection
Lifelong immunosuppression is generally necessary for all recipients of solid organ transplants, and infection is the primary cause of mortality after transplantation. Two thirds of transplant patients have at least one significant infection, most commonly nosocomial during the surgical recovery period or, subsequently, community acquired (Box 184-1).1,2
Signs of infection in this population of patients are often blunted by an impaired inflammatory response. Minor complaints or the chief complaint of a fever in an afebrile patient may herald a severe infection. Aggressive management usually translates into increased patient survival and graft function.1
First Month after Transplantation
Infections occurring in the first month after transplantation are likely to be related to the transplant procedure, stents, catheters, and intubation. In addition, the typical causes of postoperative fever should always be considered. Hospital-acquired pathogens are prominent, and management is similar for any immunosuppressed patient recently discharged.2
1 to 6 Months after Transplantation
CMV is the most important and prevalent immunomodulating virus 1 to 6 months after transplantation.1 CMV infection may produce disease in multiple systems, but pneumonitis is particularly common and may be manifested insidiously. CMV infection may be primary or due to reactivation from latent viral particles found in lymphocytes. Typically, signs of CMV infection occur at a median of 40 days after transplantation. Survival from CMV infection is improving because of aggressive use of bronchoscopy that leads to earlier diagnosis and treatment with ganciclovir and CMV-specific immune globulin. Prophylaxis with ganciclovir reduces CMV incidence and death due to infection, but routine use of ganciclovir is associated with a number of potential side effects.3 Because the risk of CMV infection is greatest during antilymphocyte therapy, some transplant centers administer ganciclovir only selectively during the treatment period, although CMV infection is still often fatal.4,5
Active CMV infection can trigger or exacerbate organ rejection. CMV is linked to a particular form of glomerulopathy in renal allograft recipients as well as acute hepatic dysfunction and the disappearing bile duct syndrome of chronic hepatic allograft dysfunction.6,7 Furthermore, both acute cardiac dysfunction and accelerated coronary artery atherosclerosis of chronic heart transplant rejection are linked to CMV.8,9
EBV infection causes clinical effects similar to those of CMV infection. EBV contributes to immunosuppression and can cause a mononucleosis-like syndrome associated with lymphadenopathy, weakness, and low-grade fevers. Because CMV and EBV often coexist, both seem to trigger graft rejection. EBV is also implicated in B-cell lymphoproliferative syndrome, which is histologically similar to polymorphic B-cell lymphoma.10
6 Months after Transplantation
Healthy Transplant.: Healthy transplant patients have no chronic immunomodulating viral infections and a functioning allograft; they are maintained with low doses of immunosuppressant medications. They have a mildly increased susceptibility to normal community-acquired infections, such as influenza, urinary tract infection, and pneumococcal pneumonia.
Chronic Viral Infection.: Progressive disease may develop as a result of the combination of viral immunomodulating infections and long-term immunosuppression. Progressive liver disease due to recurrent or acquired viral hepatitis as well as hepatocellular carcinoma may occur. B-cell lymphoproliferative disorder associated with EBV infection may also develop.1,2
Primary varicella infection may lead to rapid dissemination with pneumonia, pancreatitis, hepatitis, encephalitis, and disseminated intravascular coagulation. Patients who are varicella-zoster virus (VZV) seronegative need high doses of intravenous varicella-zoster immune globulin after any exposure, preferably before the development of the rash. Therapy can be lifesaving if it is administered early before dissemination occurs.1,2
Reactivation of latent VZV infection, manifested as cutaneous herpes zoster, affects at least 10% of solid organ transplant recipients. Fortunately, reactivation of VZV is usually confined to a single dermatome and does not disseminate. Intravenous acyclovir therapy accelerates healing but does not change the incidence of painful neuralgia. Facial zoster involving the cornea and disseminated infections in more than one dermatome are indications for admission.1 In patients who have never contracted VZV, many patients are immunized before transplantation.
Herpes simplex virus (HSV) reactivation is common after solid organ transplantation and can be manifested as oral or anogenital lesions, more often ulcers than vesicles. Some transplant centers prescribe oral acyclovir for 3 to 6 months after transplantation, whereas others choose to treat HSV immediately at the first sign of recurrence.11
Chronic Rejection.: Patients with chronic rejection require ongoing aggressive immunosuppressive treatment to protect the allograft. These patients are at the highest risk for life-threatening opportunistic infections with fungi (e.g., Candida, Cryptococcus, Coccidioides, Blastomyces, and Histoplasma), bacteria (e.g., Listeria and Nocardia), and parasites (e.g., Pneumocystis, Toxoplasma, and Strongyloides).
Fungal infections are distinct to geographic regions and typically are manifested with subacute respiratory complaints associated with fever and focal, disseminated, or miliary infiltrates on the chest radiograph.1 Invasive candidiasis and aspergillosis can also develop in the chronically immunosuppressed group. Primary infections in the lungs, gastrointestinal tract, or nasal sinuses lead to fungemia and contamination of intravenous lines and wounds. Treatment is with antifungal agents such as amphotericin B or agents such as micafungin (Mycamine).
Diverticulitis is the principal bacterial gastrointestinal infection observed in transplant patients with chronic rejection. Inflammation is suppressed with chronic steroid therapy, which leads to an increased incidence of perforation before diagnosis. Patients complain of an insidious onset of pain, change in bowel habits, and sometimes fever, but they typically lack peritoneal signs. Transplant patients are also susceptible to mucosal invasion with salmonellae (nontyphoidal) and Listeria. Infection with both organisms can be manifested as acute diarrheal syndromes that progress to bacteremia or meningitis.1,2
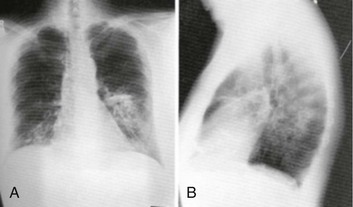
Pneumocystis jiroveci (carinii) pneumonia is often found in combination with CMV infection because CMV inhibits alveolar macrophage function (Fig. 184-1). Fortunately, its incidence is reduced with prophylactic low-dose trimethoprim-sulfamethoxazole. Without prophylaxis, infection may develop 1 to 6 months after transplantation and is manifested subacutely with fever, nonproductive cough, progressive dyspnea, and an interstitial infiltrate on the chest radiograph. Differentiation from CMV pneumonia is impossible without bronchoscopic confirmation. Treatment of Pneumocystis pneumonia includes intravenous trimethoprim-sulfamethoxazole and steroids, depending on the degree of hypoxia.
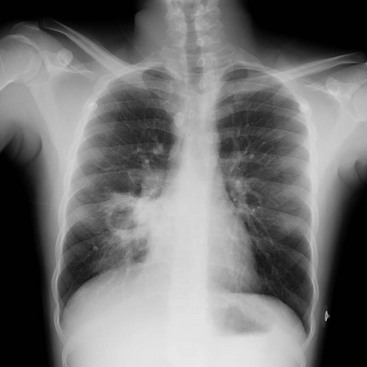
Mycobacterial disease can be manifested as opportunistic reactivation or a primary infection. Even disseminated disease can be clinically silent, with invasion of the bowel, skin, and gastrointestinal tract. Pulmonary disease can be absent, miliary, or cavitary (Fig. 184-2). Treatment is difficult because typical agents can cause graft dysfunction.1,2
Rejection
Rejection typically occurs in three phases: hyperacute, acute, and chronic. Hyperacute rejection is rare with careful donor-recipient matching. It typically occurs in the immediate perioperative period. Acute rejection occurs within the first months after transplantation. The patient presents with constitutional symptoms and signs of transplant organ insufficiency. Expeditious laboratory assessment, including possible allograft biopsy, can confirm the diagnosis of rejection, and an appropriate adjustment can be made in the immunosuppressant regimen. If immunosuppression is stopped, acute rejection may occur at any time. Chronic rejection has a time course of years and results in the gradual failure of the transplanted organ over time.12
Drug Toxicity of Immunosuppression
Pharmacology of Immunosuppression
A new term, operational tolerance, reflects long-term acceptance of transplanted organs without indefinite immunosuppression.13 Allogeneic bone marrow transplantation from the solid organ donor may facilitate operational tolerance. Successful bone marrow transplantation may revolutionize the treatment of solid organ rejection and immunosuppressive therapy.
Accommodation, an acquired resistance of an organ to immune-mediated damage, has been recognized for more than 20 years. Initially, it was identified in blood group–incompatible kidney transplants that survived and functioned normally in recipients with high titers of anti–blood group antibodies directed against antigens in the grafts. Variable sublethal graft injury induces accommodation, and that may be a dynamic condition, eventuating into tolerance on the one hand and chronic graft injury on the other.14
Because of the toxicities of current immunosuppression medications, improvement in long-term transplantation outcomes may depend on new agents with novel mechanisms of action, devoid of the toxicities.15 Inhibitors of pathways in B-cell and plasma cell activation, including belimumab and atacicept, combat the humoral immune response. Biologic agents for maintenance immunosuppression include monoclonal antibodies and fusion receptor proteins that target molecular pathways and enzymes.
Cyclosporine.: A variety of immunosuppression strategies are being tested, but the mainstay of transplant immunosuppression is cyclosporine.16,17 Cyclosporine inhibits both cellular and humoral immunity by binding to cyclophilins, which block cytokine transcription and production, thereby inhibiting lymphocyte signal transduction. The result is a potent immunosuppression of helper-inducer T cells, without affecting the suppressor T-cell subset.16,18 The helper-inducer T cells enhance antibody recognition and production by B cells.
Cyclosporine exhibits dose-related nephrotoxicity, which is additive to other nephrotoxic drugs typically used after transplantation, such as amphotericin B, aminoglycosides, and high-dose trimethoprim-sulfamethoxazole. Cyclosporine causes renal tubular injury and direct renal artery vasospasm in a dose-dependent manner, leading to systemic hypertension in many recipients.16 Hypertension should be treated aggressively with standard regimens to preserve renal function and to prevent atherosclerosis. Cyclosporine may also aggravate hyperlipidemia, leading to atherosclerosis.19 Therefore, monitoring of cyclosporine levels and renal function is routine.
Cyclosporine also causes hyperuricemia and gout in transplant patients. The nephrotoxic effects of cyclosporine complicate the management of gouty attacks in renal transplant patients.20 Traditionally, nonsteroidals are the mainstay of gout therapy, but their use is limited in transplant patients receiving cyclosporine because of a potential synergistic nephrotoxic effect.
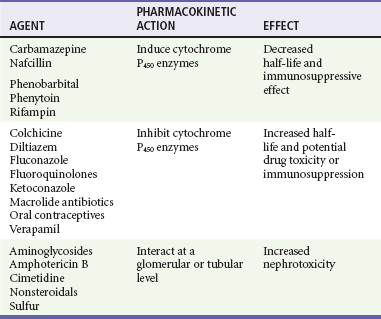
Cyclosporine levels are altered by many common post-transplantation medications (Table 184-1). Drugs that inhibit cytochrome P450 metabolism, such as erythromycin and ketoconazole, produce elevated cyclosporine levels and enhanced toxicity.21 Treatment with rifampin can increase cyclosporine metabolism, which triggers episodes of organ rejection. Cyclosporine can also cause hepatotoxicity, hyperkalemia, hirsutism, tremor, and gingival hyperplasia.16
Tacrolimus: Tacrolimus is a macrolide compound that binds to lymphocyte proteins and inhibits cytokine synthesis. Because of an improved side effect profile and more effective immunosuppression, tacrolimus is used as either primary or rescue therapy for allograft rejection and is effective for rejection in all types of solid organ transplants.22–24
Like cyclosporine, tacrolimus causes nephrotoxicity as well as neurotoxicity. In combination with steroids, tacrolimus can lead to hyperglycemia and eventual secondary diabetes. Tacrolimus can also cause anorexia, diarrhea, dyspepsia, and nausea. Macrolide antibiotics should not be prescribed to patients receiving tacrolimus because of potential toxic levels.22
Azathioprine.: Azathioprine is an antimetabolite derivative of 6-mercaptopurine. Long an important component of immunosuppressive therapy in solid organ transplants, azathioprine inhibits both deoxyribonucleic acid and ribonucleic acid synthesis, resulting in a suppression of lymphocyte proliferation. It is typically used in combination with other agents.25 Since the introduction of the calcineurin inhibitor class, the use of azathioprine has been reduced because of cyclosporine’s improved side effect profile.
Azathioprine is a bone marrow toxin, and patients exhibit dose-related neutropenia. The drug is given at the minimum effective dose to maintain a peripheral white blood cell count of 4000 to 6000 cells/mL. Azathioprine may also cause hepatic dysfunction and other gastrointestinal disturbances.25
Mycophenolate Mofetil.: Mycophenolate mofetil (MMF) prevents and suppresses organ rejection after solid organ transplantation.26,27 MMF is an antimetabolite with more potent and selective inhibition of lymphocyte proliferation. It reduces the incidence of acute rejection but with no significant change in the long-term survival of transplant recipients or their allografts.18,25
MMF has the advantage of a low side effect profile and should be used concomitantly with cyclosporine and corticosteroids when the patient can tolerate oral medication. The mostly mild effects resulting from its gastrointestinal and hematologic toxicity include abdominal pain, nausea, diarrhea, gastroenteritis, leukopenia, and thrombocytopenia. Because magnesium and aluminum antacids interfere with the absorption of MMF, care should be exercised in treatment of the gastrointestinal symptoms.27
Corticosteroids.: Corticosteroids have been used for immunosuppression since the 1960s. Glucocorticoids have a wide range of effects on the immune system, specifically the T lymphocytes. Because of the significant long-term toxic effects associated with chronic glucocorticoid administration, every effort is made to minimize glucocorticoid use. Combination therapy, in which two or more immunosuppressants are administered simultaneously, decreases the reliance on long-term, high-dose glucocorticoid administration for allograft survival.26
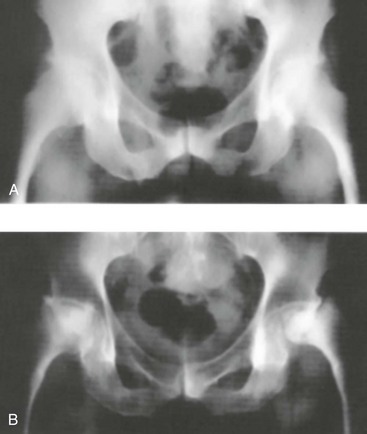
Long-term corticosteroid therapy causes osteoporosis, cataracts, gastrointestinal bleeding, glucose intolerance, skeletal myopathy, bone disease, and adrenal suppression (Fig. 184-3).28 Acute administration may lead to glucose and electrolyte abnormalities as well as altered mental status. Acute withdrawal or severe illness may lead to an addisonian crisis.
Antilymphocyte Monoclonal Antibody Preparations.: OKT3 and antithymocyte globulin are used in short courses to reverse allograft rejection.26,29 OKT3 is a mouse-derived monoclonal antibody to T cells. Chills, fever, hypotension, and headache often occur. Pulmonary edema may also develop in overhydrated, oliguric patients. OKT3 is effective in more than 90% of first rejections in most organs.18
OKT3 toxicity is common because of T-cell activation and lymphokine release caused by these agents. Patients receiving these agents are at increased risk of infection with opportunistic pathogens, particularly CMV. An increased risk of lymphoproliferative disease also exists.26,29
Specific Disorders
The pertinent clinical features of organ transplant complications are extensive. Allograft rejection symptoms are usually vague and nonspecific. Complications of the organ often result in localized symptoms and dysfunction of the allograft. The clinical features of infectious complications depend on the nature of the pathogenic organism, location of the infection, and level of immunosuppression. The resuscitation of a traumatized transplant patient should focus on standard injury patterns.30 Every complaint and finding in the organ transplant recipient should be examined in the most suspicious light to avoid missing catastrophic illness.
Management
General Approach
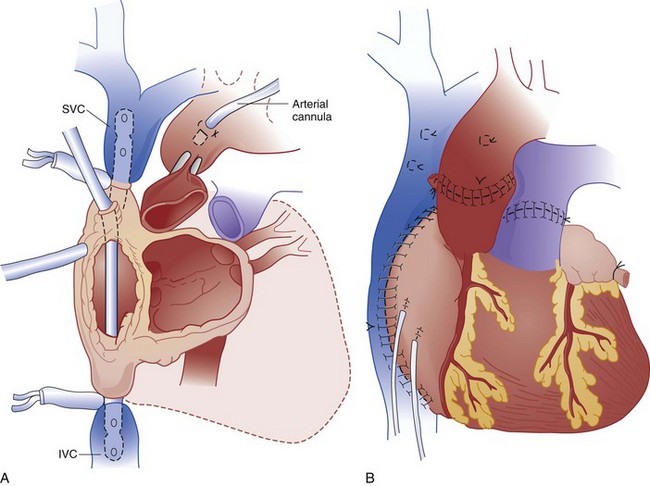
Human heart transplantation was first performed in 1967. Currently, more than 3000 procedures are done annually in the United States. The 5-year survival rates for adults and children are 72% and 80%, respectively.31 Typically, the recipient heart is removed and replaced by the donor heart (orthotopic transplant), but the recipient heart is occasionally left in place with the donor heart anastomosed to the native vessels (heterotopic transplant) (Fig. 184-4).
Patients are usually discharged from the hospital within 2 or 3 weeks. In the Stanford experience, 37% of heart transplant patients made at least one ED visit in the ensuing 3 years (range, one to six visits). The most common complaints are fever (37%) and shortness of breath (13%). Sixty percent of these patients are admitted, compared with an overall ED admission rate of 15%. The most common ED diagnoses are sepsis (18%), rejection (11%), and pneumonia (8%).19
Treatment of the traumatized transplanted heart should not differ from that of native hearts. Baseline tachycardia is expected because of denervation of the vagus nerve. Despite the absence of a pericardium, scarring and adhesions may result in clinical tamponade with pericardial fluid or blood. Blind pericardiocentesis should not be attempted.30
Infection and rejection are the leading causes of mortality in the first year. Any heart transplant patient presenting to the ED with congestive heart failure (CHF), fever (temperature above 38.0° C), shortness of breath, hypoxia, hypotension, poorly controlled hypertension, syncope, or new dysrhythmia should be admitted. Chest pain is rarely related to cardiac ischemia because the denervated heart is incapable of producing angina.32 Accelerated atherosclerosis of the graft vessels is the hallmark of chronic rejection. Ischemia is manifested as CHF, ventricular dysrhythmias, hypotension, syncope, or sudden death.33,34 CMV infections also appear to be a risk factor for accelerated atherosclerosis.9,21,35
Drug Toxicity
Lifelong immunosuppression is required; most centers use a three-drug regimen of cyclosporine, prednisone, and azathioprine. Each drug has potential for toxicity, and the combination of cyclosporine and prednisone aggravates hyperlipidemia in many patients.19,36 Rarely, cyclosporine toxicity can result in a neurotoxic syndrome of seizures, confusion, cortical blindness, and quadriplegia, progressing to coma if it is left untreated.37–39
Rejection
Of patients who have acute rejection, 75 to 85% do so within the first 3 months. Manifestations may be subtle when cyclosporine is included in the immunosuppressive regimen. Previously, rejection was obvious, with decreased QRS voltage, a new S3 heart sound, new-onset CHF, or dysrhythmias. These features are now present only during episodes of severe rejection, and the diagnosis often requires endomyocardial biopsy showing lymphocyte infiltration or myocyte necrosis.1,19 Because most episodes of early or mild rejection are asymptomatic, frequent biopsies are performed to monitor the success of the immunosuppressive regimen throughout the transplant patient’s lifetime.40,41
Acute rejection is treated with increased doses of corticosteroids (methylprednisolone, 500-1000 mg/day) and cyclosporine or with OKT3 or antithymocyte globulin. Immunosuppression is continued lifelong, with endomyocardial biopsies at least every 3 to 6 months.41,42
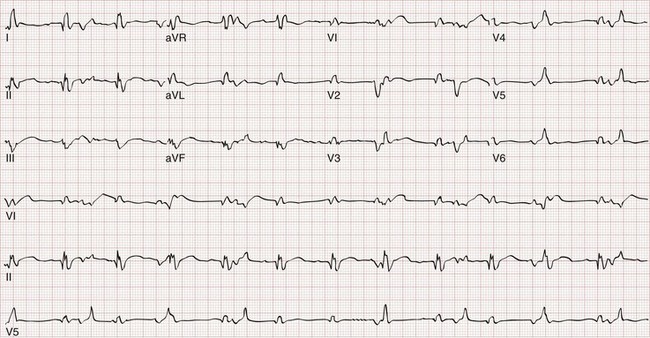
Without parasympathetic tone, the transplanted heart maintains a rate of 100 to 110 beats/minute. The electrocardiogram typically demonstrates two P waves. One wave is from the native sinus node in the posterior right atrium, which is often left in place. The second P wave is from the donor sinoatrial node, which should conduct to the ventricles as usual with a normal PR interval. In the rare heterotopic heart transplant, bizarre electrocardiographic recordings may be seen that do not conform to normal standards (Fig. 184-5).
The transplanted heart rate can increase with exercise or stress through the effects of endogenous catecholamines, up to 70% maximum for age. Exogenous pressor agents are effective in the transplanted heart. Upregulation of beta-adrenergic receptors appears to occur in the graft, with a slightly enhanced response to norepinephrine and isoproterenol.37,42–44 Typical antihypertensive agents can be used as in the nontransplant patient. Atropine is ineffective because of vagal denervation.
Infection
The most vulnerable period for infection is the first 3 months, when immunosuppression is maximal. One quarter of deaths after transplantation result from infection. Although one third of patients will have a major infection develop in the first year after transplantation, life-threatening infection after 1 year is rare.1,21
In the first month, nosocomial infection predominates, with the usual gram-positive and gram-negative bacteria. After the first 3 months, patients experience an overall 20% per year rate of infection. The most common skin infection is herpes zoster, which is treated with high-dose acyclovir. Nausea, vomiting, or diarrhea should prompt a search for CMV by culture and serologic testing.2,19,40
All heart transplant patients with fever should be aggressively evaluated unless a minor source of infection, such as an upper respiratory infection, is obvious. The diagnostic workup in the ED can include blood and urine cultures, computed tomography (CT) scan, lumbar puncture, and bronchoscopy. In addition, complete blood count, glucose concentration, serum chemistries, blood urea nitrogen and creatinine concentrations, chest radiography, and electrocardiography are recommended. In one series of 131 ED visits by such patients, 23 patients were admitted with a diagnosis of “rule out sepsis,” and an infecting organism was identified in 12 (52%).19,37,41
Any new headache with or without visual changes may be the first symptom of meningitis or brain abscess, and a CT scan of the head and a lumbar puncture should be obtained. Fever, lethargy, headache, altered mental status, and seizures are presenting signs of Listeria infection, cryptococcal meningitis, Toxoplasma gondii infection, or brain abscesses from Nocardia or Aspergillus. A more definitive diagnosis can often be made through biopsy or drainage. Aseptic meningitis may also occur in 10 to 14% of patients treated with OKT3 approximately 6 to 10 days after therapy but may be difficult to differentiate from other causes by cerebrospinal fluid analysis alone.1,21
Because of the attendant risk of endocarditis, antibiotic prophylaxis should be provided for invasive procedures likely to cause bacteremia. VZV immune globulin is recommended as soon as possible when seronegative patients are exposed to chickenpox or herpes zoster.2,19
Liver Transplant
Liver transplants have 1- and 3-year survival rates of 86% and 76%, respectively.31 Complications are common, and loss of function of the graft organ is rapidly life-threatening.
Anatomic Considerations
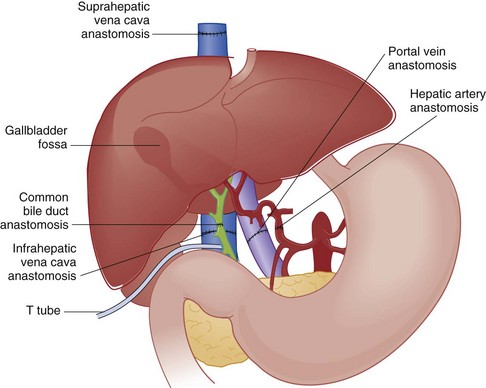
The typical liver transplant is connected to its host through five anastomoses (Fig. 184-6). The vessels are connected first, allowing organ reperfusion. The biliary system is then reconstructed and often stented with a T tube to prevent stenosis. In the early post-transplantation period, T tubes may be replaced if restenosis develops. The most common vascular complication is hepatic artery thrombosis, which most commonly occurs in the early post-transplantation period. Severe graft dysfunction is invariable, and mortality approaches 75%.45
Rejection
Liver transplant rejection is the norm despite immunosuppressive therapy. Rejection often begins 1 or 2 weeks after surgery, with fever, right upper quadrant pain, and elevated bilirubin and transaminase levels. Leukocytosis may occur but is nonspecific. Related conditions that simulate graft rejection are mechanical biliary obstruction, primary nonfunctioning graft, ischemia from thrombosis of vascular anastomoses, viral infections, drug toxicity, and recurrent primary disease.46–49
As soon as transplant rejection is suspected, hospitalization and treatment with high-dose methylprednisolone should begin. If steroids fail, OKT3 monoclonal antibodies are used in addition to polyclonal antilymphocyte globulin.46
Kidney (Renal) Transplant
With survival rates of 96% at 1 year and 91% at 3 years, kidney transplants are the most successful of the solid organs.31 Kidney transplantation provides better long-term survival than dialysis does. The best survival is in patients who receive transplants before they need dialysis, otherwise known as preemptive transplantation.50 Eventually many kidney transplant patients may avoid the sequelae of end-stage kidney disease and many of the complications of dialysis. Parenthetically, traumatic injury of the transplanted kidney is rare, despite its location in the retroperitoneal area of the anterior pelvis, where it may be at risk from direct blows and seat belt injuries.30
Infection
The classic example of bacterial infection in transplanted organs is pyelonephritis in renal allografts, which often occurs in the first month after surgery during high-dose immunosuppression. During this period, an increased risk of sepsis exists. Gram-positive organisms associated with wound infections and gram-negative organisms are the predominant causes. Pyelonephritis occurs in 35% of patients during the first 4 months, but the incidence can be reduced by prophylactic antibiotics. All transplant patients who have pyelonephritis are admitted for aggressive antibacterial therapy.51,52
Hepatitis C virus (HCV) infection is the most common cause of hepatitis in renal transplant patients. It may be transferred to the recipient by blood transfusion or through the donor organ because pretransplantation antibody testing is a poor predictor of latent HCV infection. Liver disease develops in approximately 50% of seropositive patients, occurring approximately 4 months after transplantation. Most patients have chronic hepatitis with an increased susceptibility to sepsis and spontaneous bacterial peritonitis. Pretransplantation screening is the only avenue of prevention.1,52
Rejection
With cadaveric kidney transplants, histocompatibility differences are almost universal and routinely require long-term immunosuppressive therapy. This is accomplished with a combination of azathioprine, prednisone, and cyclosporine. On diagnosis of acute rejection, high-dose intravenous methylprednisolone (500-1000 mg) is begun daily and continued for 3 days. After 6 to 12 months, many patients need only lower doses of prednisone (10-20 mg/day) to maintain immunosuppression.51,52
Clinically, renal graft rejection is manifested with fever, swelling and tenderness over the allograft, and decreased urine output. A subtle rise in serum creatinine concentration should prompt great concern. Renal ultrasound examination with color flow Doppler study should be performed to rule out obstruction, abscess, vascular thrombosis, and perirenal collections of blood, pus, or lymph.52 Early consultation with the nephrologist is prudent.
Lung Transplant
Reasonably compelling data document the beneficial impact of lung transplantation on functional status, hemodynamics, and quality of life, but the data are less compelling to prove a survival benefit.53 The lung may be transplanted alone or in combination with the heart, with 1-year survival rates of 76% and 56%, respectively.31 Lung transplants are typically done unilaterally, except for cystic fibrosis. Unequal lung sounds are to be expected. There is no published experience with lung injury after transplantation. Comparison of chest radiographs with preinjury studies is critical in the evaluation of trauma. Chest tube placement on the transplanted side may be difficult because of adhesions and loculations.30
Rejection
Although lung transplantation is still rare, it is increasing in frequency; more than 800 are performed annually in the United States.31 Most patients have early rejection, and 25 to 40% have chronic rejection. An episode of acute rejection can occur as early as a few days after transplantation or as late as several years. Clinically, the patient presents with cough, dyspnea, and fever. Rales and rhonchi are heard, with deterioration in oxygenation and pulmonary function. Early rejection is often accompanied by infiltrates on the chest radiograph. When rejection occurs more than a month after transplantation, 75% of radiographs are normal or unchanged. The diagnosis of rejection is made by transbronchial biopsy showing lymphocytic infiltration.21,54–56
Suspected episodes of acute rejection are treated with high-dose methylprednisolone (500-1000 mg/day). This is successful in reversing most episodes, but OKT3 can be used in refractory cases.55
Chronic lung transplant rejection is a leading cause of late morbidity and mortality. Antecedent acute rejection and CMV pneumonia are risk factors. On pathologic examination, vascular sclerosis and progressive limitation to airflow occur from obliterative bronchiolitis. Rejection can occur several years after transplantation, but the mean time to onset is 8 to 12 months. Clinically, this rejection mimics an upper respiratory infection or bronchitis. If dyspnea is a component of the presenting complaint, a search for transplant rejection should be initiated.55,57
Infection
Transplanted lungs are highly susceptible to pneumonia because they can be colonized by bacteria during the ventilator stage of the brain-dead donor. After transplantation, diminished mucociliary clearance, decreased cough reflex due to denervation, and defective function of alveolar macrophages are present. The most common infections are caused by gram-negative bacteria such as Pseudomonas and by Staphylococcus aureus. Antibiotic therapy should be aggressively directed toward any pathogenic bacteria cultured from the tracheobronchial tree. Community-acquired pneumonia should be considered as well. Pneumocystis pneumonia is uncommon because of routine prophylaxis with trimethoprim-sulfamethoxazole.1,55
CMV pneumonia is the most common opportunistic pulmonary infection after lung transplantation. Patients are at highest risk between 3 weeks and 4 months. Clinically, CMV infection closely resembles transplant rejection. Tissue biopsy and viral culture are required to differentiate the two entities. Treatment with ganciclovir is effective and is often required long term (>12 months). Colonization by Candida is common, but invasive disease with Aspergillus and Candida provides the most significant fungal threat to the transplanted lung. When these species are invasive, they can cause bronchiolitis obliterans syndrome by inflammation and obstruction of the bronchioles.58 Reactivation of tuberculosis is rare.55,56
Pancreas Transplant
The pancreas may be transplanted singly or in combination with a kidney. This is typically secondary to diabetes. Isolated pancreatic transplants have a high complication rate, with 1-year graft survival rates as low as 72%. Because the exocrine functions of the allograft pancreas are usually drained into the bladder, genitourinary complaints are also common. Duodenocystostomy fistulas may form in the early post-transplantation period. The clinical findings are abdominal pain, tenderness, hyperamylasemia, leukocytosis, and elevated serum creatinine. Other types of pancreatic transplant complications include urinary tract infections, hematuria, reflux pancreatitis, rejection, and pancreatic graft thrombosis.59–61
Anatomic considerations are important when these patients suffer major trauma because pancreas transplants are placed in the pelvis overlying the iliac vessels.30 Exocrine secretions are drained into the bladder for excretion, and patients have a chronic non–anion gap acidosis through loss of bicarbonate into the bladder. This should not be confused with lactic acidosis. These trauma patients should not require exogenous insulin unless the graft is injured. Positive amylase on peritoneal lavage (CT is recommended if the patient is stable) can result from either native organ or graft trauma or from a ruptured bladder. CT scanning should be done with rectal administration of contrast material, in addition to intravenous and oral agents, to better define the native and transplanted pelvic organs.30
Islet cell transplantation is under investigation as a treatment of diabetes mellitus and has entered the mainstream of therapy. Although the procedural complication rate is significantly less compared with a whole pancreas transplant, islet transplantation requires several donors for adequate islet cell mass to achieve insulin independence. In addition, there is an increased attrition of islet graft function that occurs during the first few years compared with whole pancreas transplantation. As a result, these factors have made whole pancreas transplantation the most commonly performed type of beta cell replacement therapy.62
Psychological Aspects of Organ Transplantation
Transplant programs widely use psychosocial selection criteria. In general, the cardiac programs have the most stringent criteria. The side effects of lifelong immune suppression or steroid withdrawal can include anxiety, depression, and insomnia. On the other hand, successful transplantation often improves psychological well-being. Transplant social workers can provide awareness of and access to the social network that should surround each transplant recipient. Long-term compliance with all aspects of treatment will minimize graft rejection.63
Disposition
Patients with solid organ transplants presenting to the ED have a much higher than average rate of hospitalization.31,49 The insidious nature of the diseases affecting this immunosuppressed population necessitates a thorough structured approach to evaluation. If organ rejection, infection, or drug toxicity is suspected, local transplantation specialists should be consulted. Physicians without significant transplant experience should contact the patient’s transplant center to obtain consultation and to coordinate follow-up care. Patients who are discharged require careful instructions and close follow-up.
The continuing shortfall of organs for transplantation has increased the use of donation after cardiac death (DCD). Some patients with catastrophic injuries in the ED might have potential for controlled DCD. Identification of potential donors after cardiac death in the ED with appropriate use of critical care for selected patients may contribute to a reduction in the shortfall of organs for transplantation.64 Although this area remains controversial, most states require that the local organ procurement network be contacted for any recently deceased patient.
References
1. Dummer, JS, Ho, M. Infections in solid organ transplant recipients. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Churchill Livingstone; 2005:3501–3510.
2. Rubin, RH, Fishman, JA. Infection in the organ transplant recipient. In: Ginns LC, Cosimi AB, Morris PJ, eds. Transplantation. Malden, Mass: Blackwell Science; 1999:747–770.
3. Hodson, EM, et al. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid organ transplants: A systemic review of randomized controlled trials. Lancet. 2005;365:2105.
4. Sweny, P. Infection in solid organ transplantation. Curr Opin Infect Dis. 1993;6:412.
5. Sweny, P, Burroughs, AK. Infections in solid organ transplantation. Curr Opin Infect Dis. 1994;7:436.
6. Richardson, WP, et al. Glomerulopathy associated with cytomegalovirus viremia in renal allografts. N Engl J Med. 1981;305:57.
7. O’Grady, JG, et al. Cytomegalovirus infection and donor/recipient HLA antigens: Interdependent cofactors in pathogenesis of vanishing bile duct syndrome after liver transplantation. Lancet. 1988;2:302.
8. Rubin, RH. The indirect effects of cytomegalovirus infection on the outcome of organ transplantation. JAMA. 1989;261:3607.
9. Grattan, MT, et al. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989;261:3561.
10. Benkerron, M, Durandy, A, Fischer, A. Therapy for transplant-related lymphoproliferative disease. Hematol Oncol Clin North Am. 1993;7:467.
11. Pettersson, E, et al. Prophylactic oral acyclovir after renal transplantation. Transplantation. 1985;39:279.
12. Dallman, MJ. Immunobiology of graft rejection. In: Ginns LC, Cosimi AB, Morris PJ, eds. Transplantation. Malden, Mass: Blackwell Science; 1999:23–36.
13. Bishop, GA, et al. Approaching the promise of operational tolerance in clinical transplantation. Transplantation. 2011;91:1065–1074.
14. Lynch, RJ, Platt, JL. Accommodation in renal transplantation: Unanswered questions. Curr Opin Organ Transplant. 2010;15:481–485.
15. Webber, A, Hirose, R, Vincenti, F. Novel strategies in immunosuppression: Issues in perspective. Transplantation. 2011;91:1057–1064.
16. Keown, PA. Molecular and clinical therapeutics of cyclosporine in transplantation. In: Ginns LC, Cosimi AB, Morris PJ, eds. Transplantation. Malden, Mass: Blackwell Science; 1999:101–113.
17. Harlan, DM, Kirk, AD. The future of organ and tissue transplantation: Can T-cell costimulatory pathway modifiers revolutionize the prevention of graft rejection? JAMA. 1999;282:1076.
18. Halloran, PF. Immunosuppressive agents in clinical trials in transplantation. Am J Med Sci. 1997;313:283.
19. Sternbach, GL, et al. Emergency department presentation and care of heart and heart/lung transplant recipients. Ann Emerg Med. 1992;21:1140.
20. Edwards, NL. Gout: Clinical features. In: Klippel JH, ed. Primer on the Rheumatic Diseases. 13th ed. Atlanta: Arthritis Foundation; 2001:241–250.
21. Petri, WA. Infections in heart transplant recipients. Clin Infect Dis. 1994;18:141.
22. Klintmalm, GB. Tacrolimus. In: Ginns LC, Cosimi AB, Morris PJ, eds. Transplantation. Malden, Mass: Blackwell Science; 1999:127–136.
23. Starzl, TE, Fung, JJ. Tacrolimus: Commentary. In: Ginns LC, Cosimi AB, Morris PJ, eds. Transplantation. Malden, Mass: Blackwell Science, 1999.
24. Fung, J, et al. Immunosuppression in liver transplantation: Beyond calcineurin inhibitors. Liver Transplant. 2005;11:267.
25. Calne, RY. Azathioprine. In: Ginns LC, Cosimi AB, Morris PJ, eds. Transplantation. Malden, Mass: Blackwell Science, 1999.
26. Bungardner, GL, Roberts, JP. New immunosuppressive agents. Gastroenterol Clin North Am. 1993;22:421–429.
27. Rayhill, SC, Sollinger, HW. Mycophenolate mofetil: Experimental and clinical experience. In: Ginns LC, Cosimi AB, Morris PJ, eds. Transplantation. Malden, Mass: Blackwell Science; 1999:147–162.
28. Walker, RG. Steroids and transplantation. In: Ginns LC, Cosimi AB, Morris PJ, eds. Transplantation. Malden, Mass: Blackwell Science; 1999:115–123.
29. Parlevliet, KJ, Schellekens, PT. Monoclonal antibodies in renal transplantation: A review. Transplant Int. 1992;5:234.
30. Barone, GW, et al. Trauma management in solid organ transplant recipients. J Emerg Med. 1997;15:169.
31. International Society for Heart and Lung Transplantation/United Network for Organ Sharing. International Heart and Lung Transplant Registry. http://ishlt.org/registries.
32. Gao, SZ, et al. Acute myocardial infarction in cardiac-transplant recipients. Am J Cardiol. 1989;64:1093.
33. Hosenpud, JD. Coronary artery disease after heart transplantation and its relation to cytomegalovirus. Am Heart J. 1999;138:S469.
34. Weis, M, von Scheidt, W. Cardiac allograft vasculopathy: A review. Circulation. 1997;96:2069.
35. Wagoner, LE. Management of the cardiac transplant recipient: Roles of the transplant cardiologist and primary care physician. Am J Med Sci. 1997;314:173.
36. Hunt, SA, Schroeder, JS. Managing patients after cardiac transplantation. Hosp Pract. 1989;24:83.
37. Cooper, DK, et al. Does central nervous system toxicity occur in transplant patients with hypocholesterolemia receiving cyclosporine? J Heart Transplant. 1989;8:221.
38. Rubin, AM. Transient cortical blindness and occipital seizures with cyclosporine toxicity. Transplantation. 1989;47:572.
39. Velu, T, Debusscher, L, Stryckmans, PA. Cyclosporine-associated fatal convulsions. Lancet. 1985;1:219.
40. Dec, GW. Cardiac transplantation. In: Ginns LC, Cosimi AB, Morris PJ, eds. Transplantation. Malden, Mass: Blackwell Science; 1999:438–474.
41. Taylor, AJ, Bergin, JD. Cardiac transplantation for the cardiologist not trained in transplantation. Am Heart J. 1995;129:578.
42. McCarthy, PM. Cardiac transplantation. In: Libby P, Bonow RO, Mann DL, Zipes DP, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 8th ed. Philadelphia: WB Saunders; 2007:665–683.
43. Borow, KM, et al. Cardiac and peripheral vascular responses to adrenoceptor stimulation and blockage after cardiac transplantation. J Am Coll Cardiol. 1989;14:1229.
44. Yusuf, S, et al. Increased sensitivity of the denervated transplanted human heart to isoprenaline both before and after beta-adrenergic blockade. Circulation. 1987;75:696.
45. Washington, K. Update on post–liver transplantation infections, malignancies, and surgical complications. Adv Anat Pathol. 2005;12:221.
46. Powelson, JA, Cosimi, AB. Liver transplantation. In: Ginns LC, Cosimi AB, Morris PJ, eds. Transplantation. Malden, Mass: Blackwell Science; 1999:324–374.
47. Jain, A, et al. Immunosuppressive therapy. Surg Clin North Am. 1999;79:59.
48. Munoz, SJ, et al. Long-term care of the liver transplant recipient. Clin Liver Dis. 2000;4:691.
49. Savitsky, EA, Uner, AB, Votey, SR. Evaluation of orthotopic liver transplant recipients presenting to the emergency department. Ann Emerg Med. 1998;31:507.
50. Davis, CL. Preemptive transplantation and the transplant first initiative. Curr Opin Nephrol Hypertens. 2010;19:592–597.
51. Peddi, VR, First, MR. Primary care of patients with renal transplants. Med Clin North Am. 1997;81:767.
52. Morris, PJ. Renal transplantation. In: Ginns LC, Cosimi AB, Morris PJ, eds. Transplantation. Malden, Mass: Blackwell Science; 1999:285–312.
53. Kotloff, RM. Does lung transplantation confer a survival benefit? Curr Opin Organ Transplant. 2009;14:499–503.
54. Glover, FL, et al. The past, present, and future of lung transplantation. Am J Surg. 1997;173:523.
55. Ginns, LC, Wain, JC. Lung transplantation. In: Ginns LC, Cosimi AB, Morris PJ, eds. Transplantation. Malden, Mass: Blackwell Science; 1999:490–551.
56. Midthun, DE, et al. Medical management and complications in the lung transplant recipient. Mayo Clin Proc. 1997;72:175.
57. Scott, JP, et al. Posttransplantation physiologic features of the lung and obliterative bronchiolitis. Mayo Clin Proc. 1997;72:170.
58. Lease, ED, Zaas, DW. Update on infectious complications following lung transplantation. Curr Opin Pulm Med. 2011;17:206–209.
59. Hickey, DP, et al. Urological complications of pancreatic transplantation. J Urol. 1997;157:2042.
60. Stegall, MD, et al. Pancreas transplantation for the prevention of diabetic nephropathy. Mayo Clin Proc. 2000;75:49.
61. Auchincloss, H, Shaffer, D. Pancreas transplantation. In: Ginns LC, Cosimi AB, Morris PJ, eds. Transplantation. Malden, Mass: Blackwell Science; 1999:395–422.
62. Berney, T, Johnson, PR. Donor pancreata: Evolving approaches to organ allocation for whole pancreas versus islet transplantation. Transplantation. 2010;90:238–243.
63. Craven J, Rodin GM, eds. Psychiatric Aspects of Organ Transplantation. New York: Oxford University Press, 1993.
64. Blackstock, M, McKeown, DW, Ray, DC. Controlled organ donation after cardiac death: Potential donors in the emergency department. Transplantation. 2010;89:1149–1153.