8 The shoulder
Introduction
Upper extremity pain and, more specifically, shoulder pain is a common complaint in the general population1,2 and is a familiar presentation to any physician in primary care.2–4 The incidence of new episodes of shoulder pain is approximately 11 per 1000 presentations in general practice,2 making it responsible for 16% of all musculoskeletal complaints.1 The complex arrangements of soft tissue structures in the shoulder joint, as well as its proximity to significant neurovascular anatomy, renders it a difficult joint to assess, while the combination of the joint’s instability and mobility subject it to an increased probability of injury.5,6
A multitude of pain generators can be responsible for shoulder pain and these are not merely limited to local structures; referred pain from spinal structures is also a common cause of shoulder pain.7,8 This myriad of clinical factors requires the primary care physician to have an advanced understanding of shoulder joint anatomy and pathophysiology, as well as a more than cursory knowledge of appropriate physical examination and diagnostic imaging procedures to correctly diagnose an injury.
Evaluation of the shoulder joint typically includes plain film radiography; however, many common shoulder complaints involve soft tissue structures not detectable using radiographs. Magnetic resonance (MR) imaging offers unmatched anatomical detail, relative ease of assessment and high accuracy and allows multiplanar assessment.9,10 These attributes have made MR imaging the procedure of choice for evaluation of occult fractures, articular structures and soft tissues of the shoulder including tendons, ligaments, muscles and capsulolabral structures11 despite its relatively high cost and occasional limited availability in some regions.12
History and examination
In assessing the painful shoulder, it is necessary to evaluate all possible pain generators and contributing conditions. While many shoulder disorders have their signature clinical presentations, many also tend to precipitate secondary conditions or are common comorbidities; it is relatively uncommon for an isolated condition to be the sole cause of shoulder pain in a patient.13
Of key importance to the diagnostic process is an accurate history of both the present complaint and previous episodes; occupational- and sports-related factors; epidemiological data; injury pathomechanics; exacerbating activities; and other diagnosed conditions that may (or may not) directly affect the shoulder joint. In most cases of shoulder pain, a careful, well-directed history will lead to a correct diagnosis.14,15
Physical examination procedures including specific provocative tests are vital to the diagnostic picture and play a large role in directing imaging and, consequently, management decisions. Both active and passive range of motion and muscle testing will provide important information to the clinician, although many traumatic injuries may limit the ability to perform them. It is important not to mistake muscle failure resulting from the pain induced by provocation testing with muscle weakness. Palpation will help to localize the site of pain as well as identify palpable deformities related to dislocations or separations. The differential diagnosis can be further refined by conducting special tests; these tests, their associated clinical findings and significance are discussed later in conjunction with the conditions to which they pertain in order to allow the physician to correlate their imaging findings with their investigative procedures.14
Assessment of shoulder pain also necessitates evaluation of the cervical spine owing to the frequency of complaints coexisting in both areas.16,17 Pain related to the majority of common shoulder conditions typically does not extend beyond the elbow; this finding should direct the clinician towards a cervical, brachial plexus or peripheral nerve lesion.8
Differential diagnosis
The range of common conditions that can mimic shoulder injury is detailed and discussed in Table 8.01.
| Condition | Effect on shoulder | Clinical features |
|---|---|---|
| Biliary disease | Right-sided pain |
Clinical indications for diagnostic imaging
If the history and examination are sufficiently proficient, then often the diagnosis will be self-evident and no diagnostic imaging required. However, in the event of significant trauma; if the patient’s pain is uncontrolled or precludes adequate physical examination; or in the event that the patient fails to respond to conservative therapy, there is an occasionally confusing wealth of imaging possibilities available to the physician.18
Plain radiographs are often still the first step in diagnostic imaging and can reveal fractures, dislocation and neoplastic osseous lesions; pathology in the thoracic outlet/inlet; acromioclavicular joint changes; calcification of soft tissues; and degenerative joint disease. The most common protocol for the shoulder is anteroposterior views with the shoulder in internal and then external rotation and ‘baby arm’ (neutral abduction); these views may be supplemented by transaxillary, scapular outlet or ‘Y’ (Lamy) views if indicated by the clinical findings.19–21 The physician also must consider whether acromioclavicular, cervical spine or chest x-rays are required.
Ultrasound, as a diagnostic modality, has developed markedly over recent years to the point where it can rival MR imaging in its depiction of soft tissue pathology in certain instances; these include the principal shoulder tendons. Although it is unlikely to supplant MRI as the primary means of evaluating tendon pathology and is dependent on operator skill, ultrasound plays an important role in the evaluation of the rotator cuff and offers a number of obvious advantages, including lack of ionizing radiation, portability in the office setting, high patient acceptance, low cost, and lack of medical contraindications.22,23
MR imaging has become the gold standard for diagnostic imaging of the shoulder, particularly with regard to injuries of the soft tissues. It is non-invasive and offers a high degree of resolution, enabling the evaluation of multiple potential pathological processes. It should be considered by the clinician whenever further evaluation of non-osseous structures is required, or as a follow-up to inconclusive plain film radiographs.18
Techniques and protocols
MR imaging of the shoulder should enable visualization of the anatomy surrounding the glenohumeral and acromioclavicular joints in the axial, and oblique sagittal and coronal planes. Whilst the neurovascular bundle can be visualized on typical shoulder MR images, separate MR neurography studies are required to assess the brachial plexus.10 MR imaging of the shoulder customarily includes images in all three planes with T1-weighted, T2-weighted and proton density (PD) sequences. Fat saturation techniques are usually performed only upon request and are becoming more popular during faster acquisition sequences (fast spin echo and gradient echo); their utility will be discussed later.
The localizer, or ‘scout’, is a single axial view through the glenohumeral joint, used to plan all the subsequent acquisitions (Figure 8.01). This view allows for the sagittal slices to be obtained parallel to the glenoid fossa; these are termed sagittal obliques, owing to their non-parallel relationship to the true anatomical plane. Based on the scout view, the coronal oblique slices are obtained perpendicular to the glenoid fossa, parallel to the supraspinatus tendon. The fibres of the supraspinatus tendon, as well as the glenohumeral joint, are slightly oblique to the true coronal plane, hence the images are orientated to reflect this, and the resultant images are termed coronal obliques. If true or direct coronal images were to be obtained, the supraspinatus muscle and tendon would appear discontinuous and shortened, mimicking tears.10,24,25
Patient placement and cooperation are critical during the procedure. The patient should lie supine with the involved arm at their side, supported by sponges, in neutral to slight external rotation. A surface coil will most likely be used to ensure greater image quality. Because the shoulder joint will be lateral to the isocentre of the magnet, the diagnostic area of the magnetic field will be inhomogeneous, which may lead to artifacts and lower-quality images without the use of a coil.10,25
Extreme rotation of the arm is not recommended, even if it allows good visualization of the glenoid labrum. This position is difficult to maintain for patients, causing pain, distorting the biceps tendon and increasing the chance for motion artifacts (figure motion artifact). In this position, the synovium can also become redundant and may mimic a soft tissue mass.24,25 If the arm is placed in internal rotation, the supraspinatus tendon curves anteriorly and leaves the oblique coronal plane and the capsule will appear lax.26 Increased overlap of supraspinatus and infraspinatus tendons, as well as signal changes at the infraspinatus insertion, may mimic tears. It is important to note that a small percentage of shoulders will demonstrate these findings when imaged in the neutral position as well,27 making it necessary to evaluate suspected findings in all planes and with all sequences.
Some authors have also suggested image acquisition during complete abduction and external rotation of the arm, termed the ABER manoeuvre (Figure 8.02). Studies have shown better visualization of partial cuff tears and labrum tears utilizing this position in conjunction with arthrography than images obtained with arthrography only.28–30 This position is, however, difficult to maintain for long periods of time, decreasing patient compliance and increasing pain level and motion artifacts.10,24,25 Even experienced technicians require additional time for patient placement, positioning and image acquisition, adding substantial time to an already time-consuming procedure. These images will usually be obtained during MR arthrography and are typically reserved for inconclusive findings on conventional MR or arthrography.31
As a general guide, T1-weighted sequences have the highest level of anatomical detail. Bone marrow and peri-articular fat both display high signal intensity. On T2-weighted images, soft tissue oedema or other fluid collections, such as that seen in bursitis, are depicted as high intensity zones. These sequences are also better suited for identification of pathologies and may help in the assessment of artifacts found on T1-weighted images, such as the ‘magic angle’ phenomenon: when a structure is oriented at 55° to the main magnetic field, it will appear as an area of hyperintensity on T1-weighted sequences, mimicking pathology.32 This angle has been termed the ‘magic’ angle and can appear in MR imaging of various body regions, including, in the shoulder, the supraspinatus tendon, glenoid labrum and biceps tendon. Muscle, ligaments and tendons will appear as areas of low signal intensity on both sequences. Proton density-weighted sequences use a relatively long relaxation time (TR) and short echo time (TE) and have shown a high sensitivity for detection of injury to the rotator cuff and glenoid labrum and capsular complex. Fast spin echo (FSE) sequences have more recently been used to decrease imaging time and improve signal-to-noise ratio. Fat will appear brighter with this technique, which may obscure small lesions adjacent to lipid structures, including subtle defects of the rotator cuff tendons and pathological bursal fluid.33 Fat saturation techniques can minimize these effects and help with the differentiation between fluid and fat at their interface; this can increase the sensitivity for detecting partial tears.34
Use of intra-articular contrast (arthrography) may enhance partial articular surface tears of the rotator cuff muscles or increase conspicuity of the capsulolabral anatomy;35–37 however, it is usually performed only on unresponsive patients or after an inconclusive non-contrast study. To perform the procedure, a needle is inserted into the glenohumeral joint under fluoroscopy. In order to verify intra-articular positioning, a small amount of iodinated contrast is injected and an image is taken to confirm correct placement. Following this, 10 to 16 ml of dilute gadopentetate dimeglumine is injected in to the intra-articular space, avoiding both the introduction of air and overdistension of the joint.38 An alternative procedure involves injection of saline solution followed by gentle shoulder mobilization (within patient tolerance) prior to the FSE MR imaging study.31 The use of local anaesthetic has the advantage of not masking the area if an aberrant injection is made.38 The procedure is typically painful to the patient and is associated with the typical risks of a mildly invasive procedure; expectations should be discussed prior to the procedure. Utilization of MR arthrography for specific conditions will be discussed individually throughout the chapter.
Normal anatomy
Osseous structures
Biomechanically, the shoulder is the most complicated articulation in the body. It comprises the glenohumeral, acromioclavicular and sternoclavicular joints, which, together with the articulation between the scapula and the true ribs, form a closed-loop kinematic chain.39–41 The shoulder has multitudinous muscle attachments, supporting ligaments and bursae, many of which demonstrate interrelated comorbidities when the shoulder is affected by internal derangements.5,6 The major advantage of MR imaging, as compared with plain film radiography, is the visualization of these soft tissue structures and their pathoanatomical interrelationships, the most common source of pain when addressing shoulder complaints.2,42
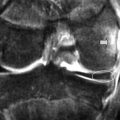
The sternoclavicular joint is a synovial ‘saddle’ joint (sellaris) and represents the only point of contact between the pectoral girdle and axial skeleton.6,41 The glenohumeral joint is a ‘ball and socket’ (spheroidal) synovial joint, allowing three degrees of freedom.6,41 It occurs between the glenoid fossa of the scapula, which is shallow and lined with hyaline cartilage, as is the reciprocal articulating surface of the humeral head. Hyaline cartilage, unlike fibrocartilage, will show up as an area of intermediate signal intensity on both T1- and T2-weighted sequences. The fossa is rimmed by a fibrocartilaginous disc known as the glenoid labrum (Figures 8.03, 8.04), which will show up as a low intensity area on most sequences. Six labral variants have been noted, with more variability in the anterior labrum (Table 8.02).
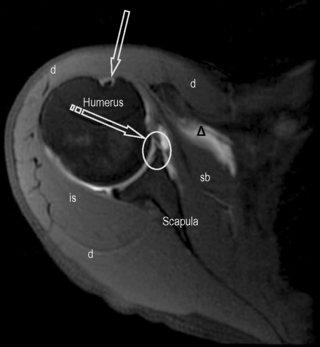
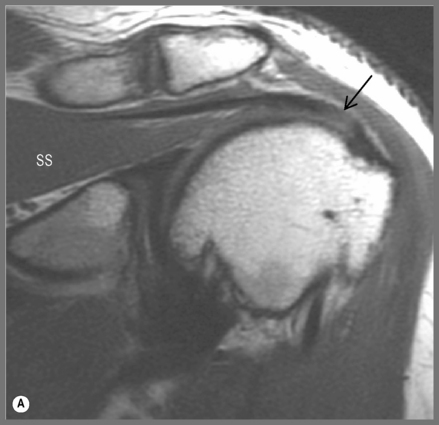
Figure 8.03 • Axial, T2-weighted, fat-suppressed MR image through the mid-portion of the glenohumeral joint, below the supraspinatus muscle. The biceps tendon is well seen, outlined by the fluid-filled synovial sheath (arrow). The subscapularis bursa is visible and identified by the delta symbol ( ). It is of high signal intensity, or fluid-filled, due to its continuity with the articular capsule. Only the subscapularis and infraspinatus bursae should be visualized in a non-inflamed shoulder. A small cleft is visible between the glenoid labrum and the scapula, representing a normal variant, often mistaken for glenoid tears (striped arrow and oval). The key to the legend is detailed in Box 8.01.
). It is of high signal intensity, or fluid-filled, due to its continuity with the articular capsule. Only the subscapularis and infraspinatus bursae should be visualized in a non-inflamed shoulder. A small cleft is visible between the glenoid labrum and the scapula, representing a normal variant, often mistaken for glenoid tears (striped arrow and oval). The key to the legend is detailed in Box 8.01.
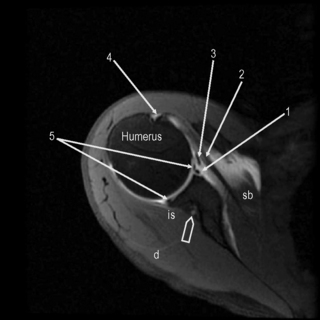
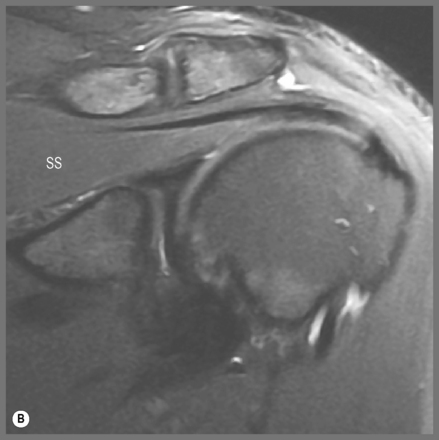
Figure 8.04 • An axial, T2-weighted, fat-suppressed MR image through the glenohumeral joint (slightly superior to Figure 8.03) through the plane of the subscapularis tendon. The suprascapular neurovascular bundle is demonstrated by the arrowhead. 1 = anterior capsular reflection; 2 = subscapularis tendon; 3 = middle glenohumeral ligament; 4 = bicipital tendon and synovial sheath; 5 = anterior and posterior glenoid labrum.
| Variant | Frequency anterior | Frequency posterior |
|---|---|---|
| Triangular | 45% | 71% |
| Round | 19% | 12% |
| Cleft | 15% | 0% |
| Notched | 8% | 0% |
| Flat | 7% | 6% |
| Absent | 6% | 8% |
It is important to note these variations so as not to confuse them with pathology and to be aware of the lack of variability in the posterior labrum, where cleaved or notched patterns should raise suspicion of a tear. Of additional note is posterosuperior labral absence, which is considered a normal variant.43 The labrum is the site of the fibrous attachment of the glenohumeral ligaments and the joint capsule to the scapula. In most patients, the hyaline cartilage of the glenoid fossa will extend beneath the labrum, creating an area of increased intensity (referred to by some as undercutting) that may be confused with a tear.25,44,45 Increased signal intensity has been identified in both the posterosuperior and anteroinferior labrum without a tear, owing to the ‘magic angle’ phenomenon.46 It is useful to be aware that posterosuperior labrum tears are more common in athletes involved in throwing and there should be increased clinical suspicion in the physician diagnosing this population.47
The articular capsule extends from the glenoid labrum to the humeral head. Three proximal capsular attachment variants have been described and can occur at the anterior labrum. Type I capsules insert at the tip or base of the labrum, whilst type II insert no more than 1 cm medial to the labrum. Both will appear with approximately the same frequency at the anterior labrum. Type III capsules occur in about 4% of shoulders, insert more than 1 cm medially and are usually indistinguishable from congenital synovial pouches or capsular stripping. If type II or III capsules are found posteriorly, this should raise clinical suspicion of injury.43 The synovial lining of the capsule extends to form a sheath around the proximal aspect of the long head of the biceps muscle.41
The coracohumeral and the three glenohumeral ligaments may be difficult to evaluate separately from the capsule; indeed, they are usually regarded as capsular folds or thickenings.10,24,48,49 Of these, the inferior glenohumeral ligament is the largest and most important; it forms the axillary recess of the capsule. The superior glenohumeral ligament is the smallest and its function is not well understood; in 3–10% of the population, it is congenitally absent.24 In many of these instances, the medial glenohumeral ligament is thickened and cord-like and attaches directly to the superior labrum at the base of the biceps tendon. The combination of the two anomalies is referred to as the Buford complex and has been reported to occur in 1.5% of the population.50
Another variation of the capsular-ligamentous complex is a labrum foramen, a hole in which a small detachment of the anterosuperior corner of the labrum is present; this occurs in 12% of the population.51 In as many as three-quarters of the population, a sublabral recess/sulcus may form between the bicipito-labral complex and the superior portion of the glenoid fossa by a synovial reflection.52 All three of these variants can easily be mistaken for labral tears.
Anatomical variants in bone marrow may be confused with pathologies on MR. On T1-weighted MR sequences, red (haematopoietic) marrow appears as an area of hypointensity, whilst yellow (fatty) marrow appears as an area of hyperintensity. As individuals age, red marrow is converted to yellow marrow, starting distally and moving towards the appendicular skeleton. Adult marrow patterns are typically realized by the age of 18–21 years, although residual red marrow is a common finding. It is important to note that an area of hypointensity, usually visualized as a subcortical curvilinear distribution in the medial humeral head, is a typical location for residual or reconverted marrow.53,54 This should not be mistaken for marrow disease and will occur in the absence of soft tissue mass, cortical destruction or medullary expansion.31 The finding will be relatively symmetrical and imaging of the contralateral shoulder may help with differentiation.
The head of the humerus has a normal anatomical flattening that occurs underneath the path of the teres minor muscle as it inserts on the lateral portion of the humeral head. It is important to differentiate this normal finding from a Hill–Sachs impaction fracture (Figure 8.05). Differentiation on individual axial images cannot be made with confidence by either the size of the indentation or its location in the axial plane. The most accurate way to distinguish the two entities is by their position on the long axis of the humerus. Hill–Sachs lesions will be visible within the superior 5 mm of the humeral head and typically extend up to 18 mm from the top of the humeral head. They should be visible within the first two transaxial sections. In contrast, normal flattening occurs 20 mm or more caudal to the humeral head.55
Whilst it may be possible for lesions to extend further caudally and overlap the normal groove, they can be differentiated by viewing the more cephalic slices. In addition, in a new or recent Hill–Sachs lesion, associated bone marrow oedema will be noted surrounding the depression of the superolateral aspect of the humeral head. Often, the Hill–Sachs lesion is associated with additional lesions, particularly the Bankart lesion (either cartilaginous or osteocartilaginous).56,57
The acromioclavicular joint is regarded as a ‘gliding’ (plane) synovial joint although in reality a number of variations exist that can affect either articular surface, though in a reciprocal manner.41 Acromial morphology was first categorized by Bigliani58 into three shapes:
The last two, non-planar variations are associated with a higher predisposition to degenerative change.59 A fourth acromial configuration, with a convex undersurface, has since been identified60 but it is considered uncommon and no correlation has been made between this shape and impingement.31 The prevalence of each acromial type in the general population and in subjects with painful shoulders remains uncertain.58,61,62
Both joint surfaces are covered by fibrocartilage, sometimes mixed with the hyaline cartilage more typically associated with synovial articulations.41,63 The joint contains a disc, which is highly variable: true discs are relatively rare but, when present, can divide the joint in two; more commonly, the disc is a meniscus, whose fibrocartilage structure can be difficult to differentiate from the articular surfaces of the joint. The disc is frequently degenerate and can be congenitally absent.63,64
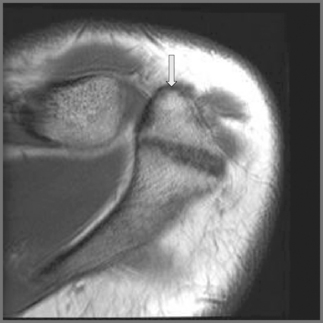
When viewing the acromion in the coronal oblique plane, a common finding is an area of hypointensity projecting inferolaterally from the lateral aspect of the acromion, termed a ‘pseudospur’; this is the normal inferior tendon slip of the deltoid insertion on the acromion and should not be confused with a spur.45 If any doubt exists, correlation with findings on other imaging planes or plain film radiography will be helpful (Figure 8.07).
Muscles
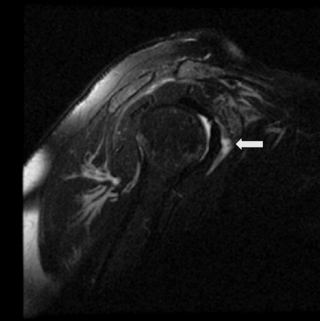
MR imaging also allows for a good assessment of the major muscle groups of the shoulder and their tendons, in particular the rotator cuff group, the biceps and deltoid muscles. Muscle and tendon should appear hypointense on T1- and T2-weighted sequences (Figures 8.03, 8.04, 8.08–8.12; Box 8.01). When observing the supraspinatus tendon on T1-weighted sequences, any areas of increased signal intensity must be correlated with T2-weighted images to avoid misdiagnosis due to the magic angle phenomenon (Figure 8.13).30 This most commonly occurs at the critical zone, where the tendon angles anteriorly and where tendon pathology is most commonly located.45
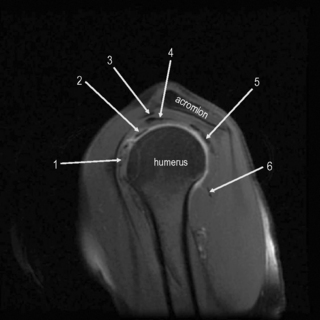
Figure 8.11 • Sagittal oblique, T2-weighted, fat-suppressed MR image through the middle of the humerus, several slices lateral to Figure 8.10. This slice allows for visualization of the tendons of the main muscle groups. These structures are most at risk for impingement, owing to their proximity to the undersurface of the coracoacromial arch. 1 = subscapularis tendon and muscle; 2 = long head of the biceps; 3 = coracoacromial ligament; 4 = supraspinatus tendon; 5 = infraspinatus tendon; 6 = teres minor muscle and tendon.
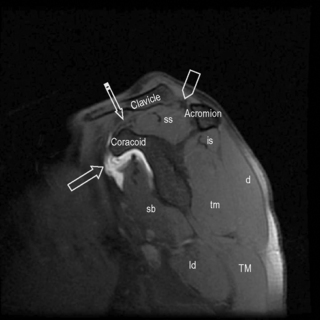
Figure 8.12 • Sagittal oblique, T2-weighted, fat-suppressed MR image though the medial scapula demonstrating the coracoacromial arch and main muscle groups. This image is slightly medial to Figure 8.05. The high intensity zone adjacent to the coracoid process represents the subscapularis bursa (arrow). Note the position of the supraspinatus muscle and tendon, occupying the entire space between the clavicle and scapula. The coracoclavicular ligament is shown by the striped arrow and the acromioclavicular ligament by the arrowhead.
On axial slices, the biceps tendon is well visualized within the bicipital groove; it is maintained there in part by the transverse ligament of the humerus, an expansion of fibres contributed by the subscapularis tendon. Because the biceps tendon sheath communicates with the glenohumeral joint space, a small amount of fluid in the sheath is normal and should be located posterior to the tendon (Figure 8.03). A round, focal fluid collection just lateral to the biceps tendon may also be noted; anatomical studies have identified this as the anterolateral branch of the anterior circumflex humeral vessels, and not fluid collection within the sheath. In normal shoulders, fluid should not encircle the tendon and such a finding indicates tendon injury, inflammation or a glenohumeral joint effusion.45
Bursae
The subscapular bursa is the only bursa communicating directly with the glenohumeral joint and represents an extension of the capsular synovial sheath. Communication occurs mainly between the superior and middle glenohumeral ligaments. It is considered that the purpose of this bursa is to protect the subscapularis tendon as it travels beneath the coracoid process and over the scapular neck.65
The most clinically important bursal structures are the subdeltoid and subacromial bursae. They do not communicate with the joint; however, in most individuals, they are contiguous structures although are variable in size and configuration. Superiorly, the bursae are bordered by the acromion and inferiorly by the rotator cuff muscles and tendons. The medial border stretches medially beyond the acromioclavicular joint, and the lateral portion to the greater tuberosity. Anteriorly, the bursa covers portions of the bicipital groove and posteriorly it lies between the deltoid and rotator cuff muscles. This ensures proper gliding motion between the rotator cuff and coracoacromial arch. Inflammation of these bursae can be related to impingement or rotator cuff diseases.65,66
A subcoracoid bursa is common and does not communicate with the glenohumeral joint but has been reported to communicate with the subdeltoid–subacromial bursa in anywhere from 11% to 55% of subjects.67 Both calcific and non-calcific subcoracoid bursitis is an infrequent cause of isolated anterior shoulder pain.68
The MR imaging appearance of all bursae should be isointense to muscle. If increased signal is observed on T2-weighted sequences, it should be considered a sign of effusion. Fluid should only be observed in the joint space as a fine line between the two articular surfaces, in communicating bursae or surrounding the long head of the biceps tendon. Increased signal intensity in the bursa can be due to a rotator cuff tear, inflammation or inadvertent injection into the bursa on arthrography (Figure 8.14).66
Pathological conditions
Rotator cuff disease
The term rotator cuff disease covers a wide range of conditions involving the muscles and tendons of the rotator cuff and associated structures. The rotator cuff is responsible for shoulder movement, dynamic stabilization and muscular balance about the glenohumeral joint. Two important actions of the group are depression of the humeral head by counteracting the force of the deltoid and compression of the humeral head into the glenoid fossa, reinforcing the joint capsule.6 Controversy still exists regarding the pathophysiology of rotator cuff disease, although several mechanisms have been described. An understanding of the anatomy of the region as well as the kinematics of the joints is necessary to determine which mechanism is responsible for the cuff pathology.69–71
Impingement syndromes
Subacromial impingement syndrome (SIS) is a frequent cause of rotator cuff disease and occurs most commonly when the rotator cuff tendons, long head of the biceps brachialis or subacromial bursa are compressed and inflamed.72 Put more simply, any structure travelling through the subacromial space that is subject to inflammation or injury can be affected by any number of obstructions in the space.
Primary, extrinsic impingement involves the anatomical structures of the coracoacromial arch. This has been attributed to forward elevation of the shoulder73 as well as to variant acromial morphology, as discussed earlier.58–60 Type I acromion has the least correlation to impingement syndrome (3%) while the type III acromion (Figure 8.06) has the highest correlation (70%–80%). Variable frequencies of each of the three types of acromion have been reported in the population. It is important to note that symmetrical morphology is frequent (70%) and that type III acromion is more common in males.62 Acromial slope and position have also been implicated in impingement syndrome74 as they can alter the shape and size of the outlet, although this remains controversial.75–77 A low-lying acromion has been correlated to degenerative changes74 and may be due to instability of the acromioclavicular joint.
Osteophytes of the acromioclavicular joint and the undersurface of the acromion have both been implicated in impingement (Figure 8.15).31 An os acromiale, the unfused apophysis of one or more of the three ossification centres of the acromion, has been reported to occur in 1%–15% of the population (Figure 8.16). The presence of osteophytic spurring or instability of the os acromiale may lead to supraspinatus impingement.78 The subacromial space may also be narrowed by a thickened coracoacromial ligament.79
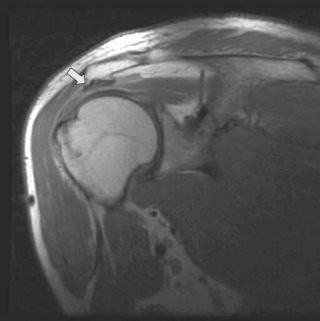
Figure 8.15 • Coronal oblique, T1-weighted MR image with a subacromial degenerative spur pointing inferior (arrow).
Secondary, extrinsic impingement usually occurs without any abnormalities of the coracoacromial arch. Instead, the impingement syndrome is related to glenohumeral instability (Table 8.03). This dynamic instability is created when the arm is abducted and externally rotated, causing impingement against the acromion and coracoacromial ligament; rotator cuff pathology is typically less advanced in these individuals.80,81 Scapulothoracic instability has also been implicated in secondary impingement, where abnormal scapular motion can create a narrowed subacromial space.82,83 Clinically, differentiation between these two causes of impingement is vital to allow treatment to address the underlying instability.84
Table 8.03 Classification factors in glenohumeral instability
| Factor | Range |
|---|---|
| Frequency |
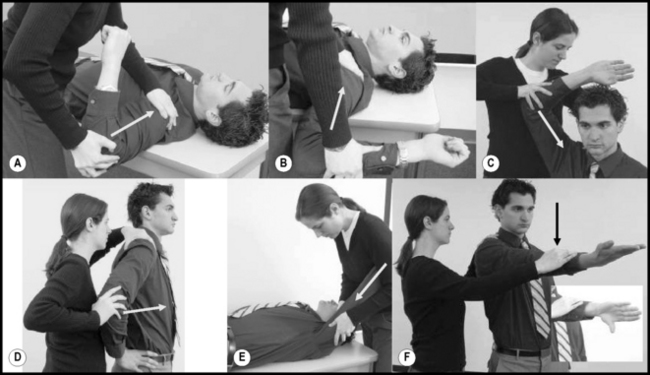
Primary impingement generally occurs in the post-35-year-old population and presents with positive impingement tests without shoulder instability. Secondary impingement is due to anterior instability from repetitive trauma or the excessive demands of throwing and overhead activities. It generally affects a younger, athletic population, under the age of 35 years, and presents with positive impingement and stability tests. Neer and Hawkins tests (Figure 8.17) have demonstrated high sensitivities and positive predictive values for subacromial impingement only, which is useful considering this is the most common location.72
More recently described lesions involve the articular side of the cuff tendon. Impingement between the posterosuperior glenoid rim and the humeral head is termed posterior internal impingement and presents with posterior shoulder pain that occurs when placing the shoulder in 90° of abduction and external rotation.85,86 Anterior internal impingement can occur when the articular side of the tendon contacts the anterosuperior labrum. Patients will present with anterior shoulder pain with positive impingement signs in the absence of instability (Figure 8.18).87
Subcoracoid impingement is a rare yet clinically important entity as patients may present following surgical intervention without resolution of their pain. It occurs when the interval between the coracoid process and the humerus is decreased, placing the subscapularis tendon, the tendon of the long head of the biceps, and the middle glenohumeral ligament at risk of impingement.88 Clinically, patients present with isolated anterior shoulder pain that can be reproduced by assuming a position of adduction, internal rotation and flexion. While the subcoracoid–humeral interval can be evaluated by MR imaging, findings are of poor predictive value, making the diagnosis mostly clinical.89
Another consideration, especially in athletes, is muscle hypertrophy or overdevelopment.10,24 This can be seen as an indentation of the muscle or tendon adjacent to the acromioclavicular joint on the coronal or sagittal slices. Patients typically present with the same symptoms as primary subacromial impingement syndrome, exacerbated by shoulder abduction. This will typically occur without changes in coracoacromial outlet size.
As well as extrinsic causes of rotator cuff disease, intrinsic cuff degeneration must also be considered as an aetiology. The distal supraspinatus tendon has an area of decreased vascularity 1 cm medial to its insertion; this is termed the ‘critical zone’.42,90 It is known to be a key component of rotator cuff disease as the majority of cuff tears begin on the articular side of the tendon; if extrinsic bony impingement was the cause, the tears should occur on the bursal surface.42,91
In order to explain the degenerative processes that accompany rotator cuff disease, the following theory has been proposed: following tendinopathy and failure of the supraspinatus muscle, superior migration of the humeral head causes the degenerative spurring that is seen in these patients. This can be shown by the lack of degenerative spurring in patients with rotator cuff tendinopathy.92 Proponents of this theory feel that for rotator cuff disease caused by an intrinsic mechanism treatment should focus on debridement of the supraspinatus tendon and avoidance of an unnecessary acromioplasty.
Extrinsic impingement may present with several bone changes, including acromial joint osteophytes, subacromial spurs, and sclerosis and cysts of the humeral head. The subacromial bursa may appear thickened and compressed by the anterior acromion. An area of increased signal intensity will be seen in the supraspinatus tendon without tendon retraction. This is indicative of tendinopathy or cuff disruption without tendon retraction. Intensity approaching fluid may be evident in the subdeltoid bursa, representing inflammation.93 Coracoacromial ligament thickening may contribute to a decrease in supraspinatus outlet size and can best be viewed on sagittal oblique images.94
Trauma
Trauma can play either a primary or secondary role in the development of rotator cuff tears. Severe trauma such as a car accident or a fall may result in an anterior dislocation with a tear of the supraspinatus muscle. More commonly, trauma can worsen an already torn or degenerated tendon. In an older population, history of minor trauma may often be elicited; in these patients, the trauma probably plays a secondary role in an ongoing degenerative process.95 Typically, there will be tears of more than one tendon. Younger patients tend to present without a history of trauma but instead a history of pain related to athletics.31
Tears will typically involve one tendon and are related to instability and impingement with a final episode of traumatic symptomatology. True traumatic tendon rupture is uncommon and occurs in the absence of tendon degeneration.96 The patient is typically younger and has no symptoms prior to the injury48; the injury will be substantial, such as a fall on an outstretched hand (FOOSH).96,97 In these traumatic tears, two entities should be investigated using MR imaging: greater tuberosity fractures in patients under 40 years of age and subscapularis tears in patients over the age of 40.96
Tendon tears
Rotator cuff tears have been classified by a number of different systems including the size or shape of the tear, the number of involved tendons, the location of the tear, the age of the injury or the aetiology, as described above.31,98 Most of these classification systems are important only when determining the efficacy of surgical intervention or the appropriateness of specific surgical approaches. Of importance to the physician is the differentiation between complete and partial tears, which tendons are involved and the location of the tear (bursal or articular) as these factors alter the clinical picture.
A complete, or full thickness, tear is one that extends from the bursal to the articular surface and allows direct communication between the subdeltoid bursa and the joint cavity (Figure 8.19).48 Partial thickness tears involve either the bursal or the articular side of the tendon (Figure 8.20).
The typical presentation of rotator cuff tears is the complaint of pain with overhead activity and weakness on lifting the arm. Partial thickness tears usually present with pain, less severe weakness and posterior capsule tightness99; full thickness tears are likely to present with weakness on resisted isometric contraction with the shoulder in the ‘full can’ testing position (Figure 8.21).48,99
Superior glenohumeral instability has been described in conjunction with supraspinatus tears; in these instances, it is usually unclear if the tear is a result of instability and secondary impingement or the chronic rotator cuff dysfunction was the cause of the instability as described above.99 Differentiation requires a complete understanding of the mechanism of injury and its temporal progression. Another presentation of a supraspinatus lesion is a subacromial abrasion, which presents with crepitus as the humerus rotates under the acromion in the absence of pain or weakness.99
These orthopaedic tests are generally good at either ruling out tendinopathies (high sensitivity) or confirming tendinopathies (high specificity) but never both. The ‘empty can’ and ‘full can’ tests are better at ruling out pathology and are more accurate for complete rupture (positive is weakness) as opposed to tendinopathy (positive is pain).100 The Codman’s drop arm or, more simply, drop arm test is highly specific for full thickness tears but has a low sensitivity.99 No conclusive evidence exists for the decisive diagnostic ability of one test over another.101
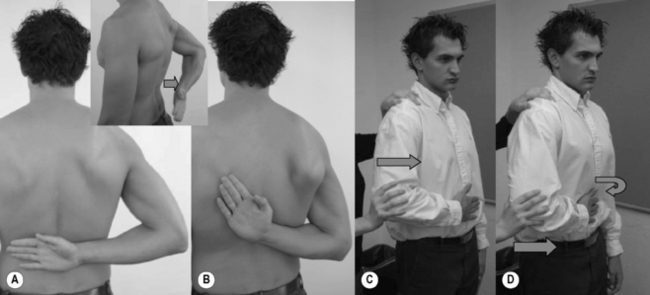
Subscapularis tendon tears can occur following anterior shoulder dislocation in an older patient or following trauma with external rotation of the arm at the side or extension of the humerus.102 Patients will usually report a traumatic injury, suffer from recurrent anterior instability and are generally younger than those who present with degenerative supraspinatus tears.48 Anterior shoulder pain, night pain due to anatomical location in relation to sleep position and weakness are common presentations.102 Clinicians should be aware of limitations in activities of daily living (ADLs), such as the inability to tuck in the back of a shirt, fasten a brassiere or place a wallet in the back pocket, which could point to a tear.102 Physical examination reveals weakness on internal rotation and increased external rotation. If the patient has full range of motion, the Gerber99 and Modified Gerber tests103 should be performed. Lack of abduction or internal rotation could create a false positive and necessitates use of the Napoleon sign (Figure 8.22).99,102
Both the teres minor and infraspinatus are frequently overlooked when evaluating the rotator cuff. Because most tears of these tendons occur in the presence of other rotator cuff tears, suspicion of any tear necessitates their assessment. Infraspinatus tears present with severe weakness, impairment and a positive dropping sign. Teres minor tears present with an inability to externally rotate the shoulder, and full thickness tears present with positive Hornblower’s sign, in which the shoulder is externally rotated with 90° of abduction with the examiner supporting the arm in the scapular plane. The elbow is flexed to 90° and the patient is asked to rotate the arm externally against the resistance; a positive sign is the inability to maintain the externally rotated position with the arm dropping back to a neutral position.104
Once clinical suspicion is established, MR imaging is often the procedure most appropriate for further evaluation. This modality has demonstrated high sensitivity and specificity of approximately 90% in multiple studies.105–107 Although MR arthrography has shown higher figures, it is not routinely employed, being reserved for difficult patients or when aggressive management options are considered.24,108 MR imaging can show the size, orientation and degree of retraction of the tears as well as the quality of the torn edges, with good correlation on surgical evaluation; it can also demonstrate associated muscle and osseous changes.109–112 Even with the high level of confidence that these imaging modalities provide, it is important to note that patients still may suffer from rotator cuff tears with a negative study; clinical correlation is always key.113
The normal signal pattern for the rotator cuff tendon is low to intermediate intensity on most common sequences, including T1-weighted, T2-weighted and fat-suppressed.10,24,48,114 Variations in the signal intensity can be attributed to pathological changes such as tears or degeneration but also to technical factors.10,24,30 The magic angle phenomenon, the focal change in signal intensity when a structure is oriented 55° to the main magnetic field, occurs only on sequences with short echo time (T1 and spin density). If a tear is suspected, one should look for secondary signs of tears and for correlation with the T2-weighted sequences, where this phenomenon does not occur.10,24,30 A full thickness tear is present when there is complete disruption of the fibres in the superior to inferior direction, creating an area of communication between the capsule and the bursa, however small it may be. Some tendon fibres may, however, still be intact, especially at the posterior aspect.
Tears occur most commonly at the anterior, distal portion of the supraspinatus tendon near the insertion of the greater tuberosity. They also occur at the critical zone, an area of the tendon located 1 cm proximal to the insertion.10,11,115 Discontinuity of the tendon fibres is the best evidence to indicate the presence of a tear. The gap between the fibres will be filled with fluid, which will appear as high intensity zones on the T1-weighted sequence with contrast and on T2-weighted sequences. It is possible for the tendon gap to be obscured by granulomatous tissue or debris, making the tear difficult to identify. If clinical evidence points to a tear but the defect cannot be visualized, there are secondary signs that can be useful in establishing a diagnosis.10,24,111 These include focal thinning, blurred tendon margins or tendon displacement and may all be indicative of a tear.
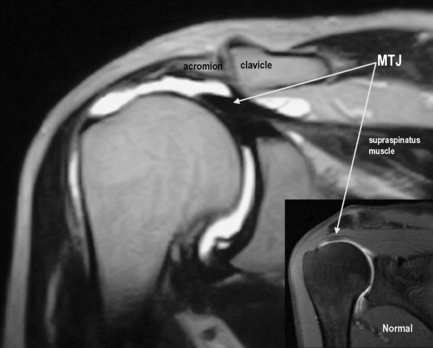
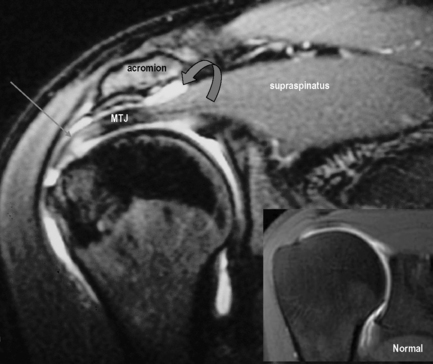
The normal position of the musculotendinous junction of the supraspinatus is just lateral to the level of the acromioclavicular joint. Medial displacement, indicating retraction, is a useful sign. High signal intensity on T2-weighted sequences (fluid) in the subacromial/subdeltoid bursa is always abnormal and is another sign of a tear. The fluid indicates communication between the bursa and the articular capsule due to a tear in the tendon (Figure 8.12). This finding is not specific, however, since it can also be present with partial tear or bursitis. The last indirect sign is muscle atrophy, seen as loss of muscle bulk, change of shape and streaks of increased T1 signal, associated with fatty infiltration.10,24 The presence of these signs is useful not only for detection of tears but also for evaluation of the patient’s prognosis. With severe atrophy and fatty infiltration, a surgical repair will likely not be undertaken, owing to the poor chances of success; the muscle would likely not be able to perform its function even if reattached.116
Partial or incomplete tears are more frequent but slightly more difficult to detect. An area in the tendon of increased signal intensity on T2-weighted images, with or without fat suppression, is the best sign, but in many cases will not be present. Often, the defect will appear as isointense to muscle, or an area of intermediate signal surrounded by the normal low signal intensity of the rest of the tendon. The edges of the defect may also be smooth and regular, making the tendon appear thinned, as opposed to torn. The location will indicate whether the damage involves the articular surface (most common), the bursal surface or is intratendinous (Figure 8.20).
On T1-weighted images, the involved area will be of low to intermediate signal intensity.10,24 Tendinosis or degenerative changes usually demonstrate similar signal on both types of sequences, allowing some differentiation between the two conditions.10
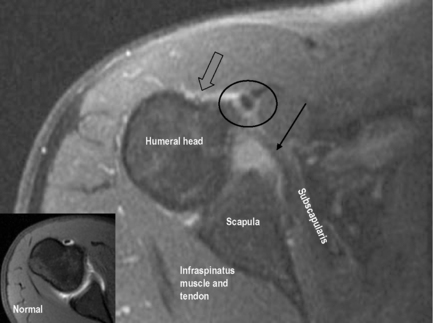
Evaluation of subscapularis tears is best achieved on axial or sagittal oblique images, in which the tendon can be visualized in its entirety.117,118 Discontinuity of the fibres or intrasubstance signal change, as with the supraspinatus, is the best evidence of tears (Figure 8.23). The tears will be areas of bright signal on T1-weighting with contrast and on T2-weighted sequences. Fatty infiltration of the muscle belly, abnormal tendon position or shape, and fluid leak under the tendon insertion on the lesser tuberosity (subscapularis recess) are all supporting or secondary signs of tears.
Anomalies of the long head of the biceps are usually associated with subscapularis tears. Tears in both tendons are frequently missed, making assessment of secondary signs exceedingly important. Seventy per cent of patients also have a concurrent supraspinatus tear, glenoid labral tear or subcoracoid bursitis.117,118 The imaging findings for complete or partial tears of the infraspinatus and teres minor are similar to the other rotator cuff pathologies. Intrasubstance signal change and displacement are the most obvious signs.
Glenohumeral instability
Owing to the mobile nature of the shoulder joint and the range of motion that it allows, instability is a common entity in the shoulder. Instability is clinically defined as only occurring in the presence of pain, as the humeral head may move beyond the boundaries of the glenoid in the absence of symptoms.119 It is important to differentiate generalized joint laxity from glenohumeral instability, although laxity may be a predisposing factor and the two may, therefore, coexist. Classification systems of instability involve several factors; authors tend to utilize different systems depending on whether their focus is clinical or surgical.31,48,49
The acronyms TUBS and AMBRI encompass the ends of a spectrum of instability. TUBS stands for traumatic instability, unidirectional in nature, Bankart lesion present, responds to surgery. AMBRI stands for atraumatic aetiology, multidirectional bilateral involvement, responds to rehabilitation and rarely requires an inferior capsule shift (although this may become necessary if conservative measures fail). A third group has recently been added to include persons with microtraumatic instability; AIOS stands for acquired instability related to overstress of the joint and responds to surgery. This group consists mainly of athletes involved in overhead activities.120
Glenohumeral stability is provided by a combination of static and dynamic mechanisms, failure of which may produce instability. Static factors include the labrum, the glenohumeral ligaments, the long head of the biceps, the posterior capsule and the negative intra-articular pressure in the joint. Dynamic stability is accomplished in coordination with the static stabilizers. Balanced function of the rotator cuff, biceps and scapulothoracic musculature provides compression of the humerus into the glenoid as well as positioning the glenoid for optimal articular contact.119 Disruption of static or dynamic shoulder stabilizers may lead to instability and it is important to assess these structures in determining the aetiology of the instability.
It is necessary to perform a thorough history when assessing a patient for instability. As noted above, the multifactorial nature of instability requires detailed information about any previous trauma, including arm position and severity of the event. A force applied to an abducted, externally rotated and extended arm will point towards anterior instability, while forward flexion adduction and internal rotation are more likely in posterior instability (Figure 8.09).31 Anterior instability is the most common and can occur with or without a history of trauma. Posterior instability is infrequent and is typically associated with severe trauma, seizures or electrical shock. Atraumatic posterior instability can be attributed to athletic activities that repeatedly require the arm to be placed in flexion, adduction and internal rotation. Severe traumatic dislocation in the young has a high incidence of recurrence, whilst the same event in the elderly is associated with rotator cuff tears.31 Physician-assisted relocation of traumatic dislocation will typically have a higher incidence of labral pathology. Pain localization is harder in patients with instability owing to its dynamic nature and should be correlated with specific movements. Anterior shoulder pain associated with abduction and external rotation, such as the cocking phase of a throw, indicates anterior instability. Superior pain is generally related to rotator cuff pathology or osteoarthritis. Posterior pain experienced in adduction and internal rotation, such as punching, can be related to posterior instability. Pain while lifting with the arm at the side is identified in inferior instability.121 An important clinical entity is the voluntary dislocator. This individual will typically dislocate at will for social approval and will typically not be forthcoming with information regarding history of dislocation.
When assessing the patient, it is important to evaluate for generalized joint laxity; this is most commonly assessed using the Beighton scale.121 Shoulder hypermobility comprising laxity without pain is not instability. Shoulder range of motion in patients with instability is typically normal to increased. Apprehension or discomfort in any range of motion may point to instability. Increases in external rotation are associated with laxity of the inferior glenohumeral ligamentous complex or a tear of the subscapularis, as discussed previously. An increase in internal rotation points to stretching of the posterior capsule. Abnormal scapulothoracic motion will affect dynamic stabilization of the glenohumeral joint. Tests to identify altered biomechanics of the scapula include ‘winging’, tested with a pushup against a wall, which indicates long thoracic nerve palsy.83
Specific tests for instability are used to assess the static stabilizers by passive end range motion.122 Each test should be performed bilaterally with specific attention paid to the degree of humeral head translation and pain provocation.123 The ‘sulcus test’ is used to evaluate inferior instability: inferior-directed traction is placed on the arm while it is at the side. The force must be applied superior to the elbow so as not to involve the biceps. A positive sulcus sign will usually point to multidirectional instability.122 Numerous tests have been identified for clinically addressing unidirectional anterior or posterior instability, including the apprehension tests, drawer tests, load and shift tests and the posterior stress test.101,102 What the examiner is identifying with any of these tests is movement beyond the glenoid accompanied by pain. Instability alone is not diagnostic and pain alone may point to another involved structure, such as the rotator cuff (Figure 8.18).120,121 The compression rotation, clunk, crank and anterior slide tests as well as the O’Brien sign have been extensively studied and have relatively strong predictive value (Figure 8.24). Reproduction of pain, clicking and clunking during the manoeuvres are good indicators of tears.99 Combinations of these tests have been found to have greater sensitivity than MRI without contrast for detecting glenoid labrum tear.4,124,125
Initial evaluation of shoulder instability should include plain radiography, although bony defects associated with instability are difficult to identify and appear in a minority of instability patients.122 Specialized views such as the apical oblique view will help to identify osseous Bankart injuries of the glenoid, whilst the Stryker notch view and AP internal rotation can help identify a Hill–Sachs deformity. Plain radiography will typically be normal, as the above-listed lesions only occur with traumatic or repetitive dislocations. A very specific finding on the anteroposterior film with a humeral avulsion of the glenohumeral ligaments (HAGL lesion) is scalloping of the medial side of the neck of the humerus and/or a small bone fragment medial to it; this is discussed in more detail later. While these findings are very specific to the lesion, they occur in a minority of cases.126
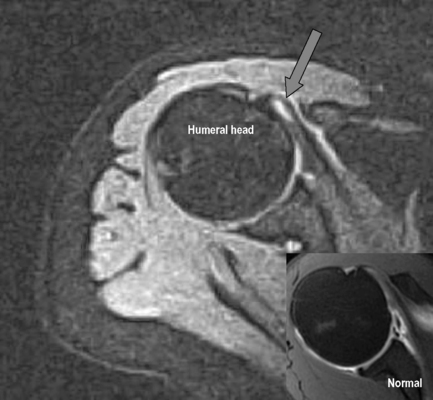
Anterior instability is the most common form and presents following one or multiple episodes of dislocation or subluxation. Dislocations may occur posteriorly, inferiorly or anteriorly, with the last-mentioned occurring up to 98% of the time.127 The mechanism for anterior dislocation is abduction, extension and external rotation of the shoulder. Recurrent subluxation or dislocation is very common in those under the age of 30, occurring in up to 90% of patients, and is more common in men. Recurrence rates drop as low as 15% or less after the age of 40. Anterior subluxation does not require a history of dislocation or injury to occur; repetitive microtrauma or overuse has been implicated in the development of anterior glenohumeral instability.31 Injuries to the bone, labrum, capsule and ligaments about the glenohumeral joint have all been implicated in anterior instability. The two most common bone abnormalities identified are Hill–Sachs and osseous Bankart lesions. A Hill–Sachs lesion, as previously described, is a posterolateral notch defect of the humeral head caused by contact between the posterolateral portion of the humeral head and the anteroinferior glenoid rim. This finding is specific to a prior anterior inferior glenohumeral dislocation and has been shown to occur in 47% to 100% of dislocated shoulders.62 The defect is best viewed on transverse images at the level of the coracoid; remember that a normal anatomical groove will be found caudal to this location. Acute cases with marrow oedema can best be viewed utilizing fat-suppressed T2-weighted or STIR sequences. The accuracy of MR diagnosis of this defect has been placed as high as 94%.128 A bony Bankart lesion involves the avulsion of the anteroinferior capsulolabral complex – a Bankart lesion, with a fracture of the adjacent glenoid rim.129 Fragment size will affect treatment course, as larger defects can give rise to recurrent instability even following surgical soft tissue repair. These defects will require fragment refixation or bone grafting. Smaller defects may be overlooked on MR and may necessitate CT with or without contrast. Larger defects are well demonstrated on MR imaging and may alter glenoid geometry, creating an inverse pear shape.62 Look to sagittal oblique images to evaluate glenoid shape as well as cystic changes, sclerosis and the marrow changes associated with osseous Bankart lesions.31
Injuries to the labrum, capsule and glenohumeral ligaments have been correlated with recurrent anterior subluxation and dislocation. Soft tissue lesions associated with anterior instability have recently come under the term avulsion/injury of the anteroinferior labroligamentous complex.130 This complex and, most notably, the anterior band of the inferior glenohumeral ligament will most frequently fail at its glenoid insertion, causing an avulsion of the labrum resulting in variations of the Bankart lesion. Failure less frequently occurs at the humeral insertion site or intrasubstance.122
The Bankart lesion has been found in a large majority (87%–100%) of traumatic anterior instability cases.131 It has also been reported in microtraumatic and atraumatic instability,130 although labral fraying and degeneration is more common in the latter.119 The lesion is an avulsion of the labroligamentous complex (capsule, inferior glenohumeral ligament and labrum) from the anteroinferior portion of the glenoid, termed the 3 to 6 o’clock position.31 The lesion and the displaced fragment may also extend superiorly, beyond the typically described location. Disruption of the scapular periosteum occurs in all Bankart variants. When attempting to identify this lesion, variants and pitfalls need to be ruled out. The labrum is a common site of the magic angle phenomenon, undercutting and anatomical variation which could resemble a tear (as discussed previously). On MR imaging, the normal glenoid labrum will appear as a uniform signal hypointensity. Tears of the labrum are visualized on unenhanced MR imaging as an area of hyperintensity (less than fluid on T2-weighted images). The Bankart lesion will extend to the surface of the lesion and a detached fragment may or may not be present, while internal degeneration will appear only as a diffuse area of hyperintensity.57
One presentation of the Bankart lesion, observed in chronic instability cases, is a thick, low intensity, detached anterior labral fragment. It is named after its description on MR: glenoid labrum ovoid mass (GLOM).132 Unenhanced MR has a high specificity and sensitivity for anterior labral tears, similar to that of MR arthrography.133 The sensitivity and specificity for unenhanced MR imaging decreases when evaluating superior and posterior tears.31 When arthrography is employed, contrast will extend into the labral defect and will highlight labral fraying better than will MR imaging. Identification of these injuries will be enhanced with ABER position as well, but this painful position should be employed only following equivocal MR arthrography.28,29
Bankart variants are typically identified utilizing MR arthrograms and specialized positions. The Perthes lesion has been defined as a non-displaced Bankart, whereby the periosteum remains intact but is stripped.31,57 In these cases, the labrum can rest in its normal position and heal, leaving only the redundant periosteum.31 During traditional MR arthrography, contrast may not extend into a healed fragment, making diagnosis difficult.119 Even with arthrography, these lesions can be hard to identify, particularly without prior knowledge of the lesion.134 The ABER position may help to separate the labrum from the glenoid, allowing better visualization.57,119,134
The anterior labroligamentous periosteal sleeve avulsion (ALPSA) is a medially displaced avulsion of the anterior labrum.135 In this lesion, the periosteum will remain intact, like the Perthes variant. The labroligamentous complex will strip down the scapular neck, resembling the rolling up of a shirtsleeve, and will cause incompetence of the inferior glenohumeral ligament. Common terms to describe the lesion are ‘peel back lesion’ and ‘medialized Bankart’ lesion. This lesion is more common in recurrent dislocation than in first-time traumatic dislocation.136
It is important not to mistake a slightly displaced labrum with a congenital labral variant.136 The typical finding on MR arthrography is a cleft between the glenoid and the nodular-shaped fibrous tissue.137 There is some doubt as to the value of unenhanced MR in detecting Bankart and variant lesions; most of these lesions have only been described utilizing contrast-enhanced MR.137 It is important for the clinician to remember that a negative test does not indicate the absence of a lesion, which should be investigated fully if clinical suspicion remains.
A second classification of anterior instability lesion does not involve the labroligamentous complex at its glenoid insertion. The humeral avulsion of the glenohumeral ligaments (HAGL) lesion is an isolated tear of the inferior glenohumeral ligament at its humeral insertion and has been correlated with instability. The typical presentation is an older, male patient suffering a violent dislocation. In the absence of a Bankart lesion in a patient with anterior instability, the incidence of a HAGL lesion is 27%.126 Overall, in cases of anterior instability, the incidence of such lesions has been reported as being between 7% and 9%. The majority of patients with a HAGL lesion will have associated injuries such as labral tears, Bankart lesions, Hill–Sachs lesions or rotator cuff tears.120 Whilst identification on conventional MR is possible, it requires joint effusion to be present. On coronal oblique T2-weighted images, the lesion can be identified by the conversion of the inferior glenohumeral ligament from a U-shaped, fluid-distended structure to a J-shaped structure with fluid extravasation beyond the humeral attachment of the ligament.120 HAGL lesions have been missed on arthroscopic Bankart repair and have been identified as the cause of continued complaints post surgery96,109; this makes presurgical identification important for appropriate management.120,126
In approximately 20% of HAGL cases, a bony avulsion from the humeral attachment will occur (BHAGL). This can typically be seen on plain film radiography and may resemble a fragment from an osseous Bankart. Medial scalloping of the humeral neck is very specific to the BHAGL and can help differentiate the two lesions.126
A final Bankart variation is the ‘floating’ anterior band of the inferior glenohumeral ligament (AIGHL). This lesion occurs when there is a classic Bankart lesion in conjunction with a humeral avulsion. It is a rare occurrence and will be treated surgically.57
‘SLAP’ lesions are injuries to the glenoid labrum involving the insertion of the tendon of the long head of the biceps muscle. When the arm is bent inward forcefully at the shoulder, the superior portion of the glenoid labrum is torn away from the cavity in an anterior to posterior direction.4,124 The acronym ‘SLAP’ refers to the location and direction of the tear: Superior Labrum, Anterior to Posterior lesion. Patients with labral tears usually present with painful locking of the shoulder through specific movement patterns and signs of anterior shoulder instability. The latter may include pain, apprehension, loss of function and aggravation of symptoms in overhead and abduction/external rotation positions.4,138
SLAP lesions were originally classified by Snyder into four types: type I, fraying of the free edge of the superior labrum (11%); type II, avulsion of the labrum and the biceps tendon anchor (41%); type III, bucket-handle tear without involvement of the biceps tendon anchor (33%); type IV, bucket-handle tears with extension into the biceps anchor (15%).139 More recent delineation of the above classifications have been made and more categories have been added to further classify the lesions. On conventional MR, identification of SLAP lesions can be difficult. Cartland et al have identified the following findings in their study. Type I lesions exhibit minimal irregularity of the articulating labral contours. Type II lesions exhibit globular high signal interposed between the superior part of the glenoid labrum and the superior part of the glenoid fossa. Type III lesions exhibit areas of high signal intensity within the superior section of the labrum separate from the normal superior part of the labral recess. Type IV lesions exhibit diffuse high signal intensity within the glenoid labrum with marked abnormal high signal intensity extending into the proximal biceps tendons.140 In more difficult cases, arthrography may be necessary to visualize a SLAP lesion.
Posterior instability is a very rare entity, accounting for only 2%–4% of all cases of shoulder instability56,141; however, the majority of these cases can be treated conservatively with strengthening exercises, and surgical intervention is rare.142 Despite its infrequency, posterior instability can present a clinical challenge due to the inconsistency of its aetiology and lack of distinct signs. Bilateral posterior dislocation is nearly always caused by electric shock or epileptic convulsion and does not commonly recur. Unilateral traumatic posterior dislocation will usually occur after a violent force is applied to an adducted internally rotated arm, and is prone to recurrence, especially in the presence of large bony defects. The most common clinical lesion involves repeated posterior subluxation due to repetitive microtrauma in the adducted flexed and internally rotated position.
There are no definitive lesions of posterior instability. Prior violent dislocation may result in an osseous impaction of the anteromedial humeral head on the posterior glenoid rim: a reverse Hill–Sachs lesion. Abnormalities of the bony glenoid include fractures in traumatic dislocation and marrow oedema, sclerosis or cystic lesions with repetitive impaction.31 In cases of atraumatic posterior instability, glenoid rim insufficiency and retroversion and humeral retroversion have been implicated.141 Posterior labral tears are less indicative of posterior instability than anterior tears are of anterior instability; in fact, tears of the posterior labrum have been found in cases of anterior instability – a consequence of repetitive dislocation – and in cases of clinically stable shoulders.141,143
The posterior labrocapsular periosteal sleeve lesion (POLPSA) can occur when the capsule strips the labrum from the scapular periosteum. Isolated labral tears are uncommon and may be due to trauma or unidirectional or multidirectional instability. Posterior labral tears have been identified in contact-sport athletes, such as offensive linemen in American football, who engage their opponents with their arms in front of the body.144 MR studies will exhibit high signal outlining the lesions, which will brighten on T2-weighting and become more defined with fat suppression. Arthrography will allow contrast material to extravasate beneath lesions, allowing improved delineation.
A very specific injury to the posterior labrum in overhead throwers has been termed the Bennett lesion. Traction placed on the inferior glenohumeral ligament, caused by the deceleration and follow-through phases of pitching, can create an avulsion injury of the posterior capsule.145 These forces create enthesophytes, which, when mineralized, can be seen using conventional radiographs, CT or MR imaging as a crescent shape at the posterior inferior glenoid rim. The associated soft tissue injuries to the rotator cuff, labrum and capsule can be identified on MR imaging.
Biceps tendinopathy
Biceps tendinopathies consist of tendinosis, instability and rupture and typically affect young or middle-aged individuals with a history of overhead arm activity.2 Imaging findings for bicipital tendon tears will be similar to those for other tendon tears, with increased intrasubstance high-signal changes and alteration in the course of the tendon. Tears unrelated to impingement occur distal to the musculoskeletal junction. The ‘empty bicipital groove sign’ or ‘naked humerus sign’ occurs when there is distal retraction of the tendon and muscle and can be seen in both types of tear (Figure 8.25).52 It is, however, important to scrutinize both the axial and the parasagittal oblique slices, since the findings can be subtle.55
Primary tendinopathy is caused by eccentric overload, hypovascularity or bicipital groove abnormalities, which lead to tendon attrition. Tendinopathy can also occur secondary to glenohumeral joint instability or rotator cuff impingement. Approximately 95%–98% of patients diagnosed with biceps tendinopathy have impingement primarily, with the secondary tendinopathy as the pain generator.58 Pain is typically present in the proximal, anterior aspect of the shoulder, sometimes extending into the biceps muscle belly and rarely radiating into the neck or beyond the biceps.2 Instability of the tendon, although a common diagnosis, occurs infrequently and has been attributed to tears of the transverse humeral ligament60; however, even with complete transection of this ligament, subluxation of the tendon is nearly impossible with an intact rotator cuff.61
When instability is suspected, the rotator cuff should be examined for incompetence to determine whether there is a primary problem therein (in cases where the rotator cuff is incompetent or dysfunctional, the ‘subluxation’ of the tendon is a secondary phenomenon). Patients with instability typically present with the same pattern as those with tendinopathy, although a palpable snap is evident on motion.2 Isolated biceps tendon rupture can be due to acute trauma or the end result of any of the above-listed tendon disorders. Most ruptures are associated with rotator cuff tears and necessitate evaluation of these tendons. Ruptures present with acute pain and may have a palpable snap. Because of its attachment, the biceps anchor can be injured along with the glenoid labrum, which can cause a click or snap.
1 Urwin M., Symmons D., Allison T., et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis. 1998;57(11):649-655.
2 van der Windt D.A., Koes B.W., de Jong B.A., Bouter L.M. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis. 1995;54(12):959-964.
3 Berkow R. Neck, shoulder and upper limb pain. In The Merck Manual, 16th ed, West Point, PA: Merck & Co; 1992:1362.
4 Souza T. Differential Diagnosis and Management for the Chiropractor, 2nd ed. Riverwoods, IL: Aspen, 2000.
5 Moore K. The upper limb. In Clinically Oriented Anatomy, 2nd ed, Baltimore: Williams and Wilkins; 1985:626-793.
6 Palastanga N., Field D., Soames R. The upper limb. In Anatomy and Human Movement: Structure and Function, 5th ed, Edinburgh: Elsevier Butterworth Heinemann; 2006:43-234.
7 Travell J., Simons D. Myofascial Pain and Dysfunction. Baltimore: Williams and Wilkins, 1992.
8 Lindsay K., Bone I., Callander R. Localised neurological disease and its management. In: Neurology and Neurosurgery Illustrated. Edinburgh: Elsevier; 1991:213-466.
9 Stoller D. Magnetic Resonance Imaging in Orthopaedics and Sports Medicine, 2nd ed. Philadephia: Lippincott Williams and Wilkins, 2006.
10 Berquist T. MRI of the Musculoskeletal System, 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins, 2000.
11 Shahabpour M., Kichouh M., Laridon E., et al. The effectiveness of diagnostic imaging methods for the assessment of soft tissue and articular disorders of the shoulder and elbow. Eur J Radiol. 2008;65(2):194-200.
12 Fotiadou A.N., Vlychou M., Papadopoulos P., et al. Ultrasonography of symptomatic rotator cuff tears compared with MR imaging and surgery. Eur J Radiol. 2008;68(1):174-179.
13 Harryman D.T.2nd, Hettrich C.M., Smith K.L., et al. A prospective multipractice investigation of patients with full-thickness rotator cuff tears: the importance of comorbidities, practice, and other covariables on self-assessed shoulder function and health status. J Bone Joint Surg Am. 2003;85-A(4):690-696.
14 Bates B. The musculoskeletal system. In: Bates B., editor. A Guide to Physical Examination. 3rd ed. Philadelphia: JB Lippincott; 1983:324-370.
15 Bates B., Hoekelman R. Interviewing and the health history. In: Bates B., editor. A Guide to Physical Examination. 3rd ed. Philadelphia: JB Lippincott; 1983:1-27.
16 Ndetan H.T., Rupert R.L., Bae S., Singh K.P. Prevalence of musculoskeletal injuries sustained by students while attending a chiropractic college. J Manipulative Physiol Ther. 2009;32(2):140-148.
17 Abbassian A., Giddins G.E. Subacromial impingement in patients with whiplash injury to the cervical spine. J Orthop Surg. 2008;3:25.
18 Stevenson J.H., Trojian T. Evaluation of shoulder pain. J Fam Pract. 2002;51(7):605-611.
19 Rowe L., Yochum T. Radiographic positioning and normal anatomy. In: Yochum T., Rowe L., editors. Essentials of Skeletal Radiology. Baltimore: Williams and Wilkins; 1987:1-93.
20 Bianchi S., Prato N., Martinoli L., Derchi E. Shoulder radiography. In: Davies M., Hodler J., editors. Imaging of the Shoulder: Techniques and Applications. London: Springer; 2003:3-20.
21 Blum A., Carrillon Y., Railhac J.-J., et al. Instability. In: Davies M., Hodler J., editors. Imaging of the Shoulder: Techniques and Applications. London: Springer; 2003:161-190.
22 Parker B.J., Zlatkin M.B., Newman J.S., Rathur S.K. Imaging of shoulder injuries in sports medicine: current protocols and concepts. Clin Sports Med. 2008;27(4):579-606.
23 Prickett W.D., Teefey S.A., Galatz L.M., et al. Accuracy of ultrasound imaging of the rotator cuff in shoulders that are painful postoperatively. J Bone Joint Surg Am. 2003;85-A(6):1084-1089.
24 Stoller D. Magnetic Resonance Imaging in Orthopaedics and Sports Medicine, Ist ed. Philadelphia: Lippincott Williams and Wilkins, 1996.
25 Mirowitz S. Pitfalls, Variants and Artifacts in Body MR Imaging. St Louis: Mosby, 1996.
26 Bloem J., Sartoris D. MRI and CT of the Musculoskeletal System: A Text-Atlas. Baltimore: Williams and Wilkins, 1992.
27 Davis S.J., Teresi L.M., Bradley W.G., et al. Effect of arm rotation on MR imaging of the rotator cuff. Radiology. 1991;181(1):265-268.
28 Cvitanic O., Tirman P.F., Feller J.F., et al. Using abduction and external rotation of the shoulder to increase the sensitivity of MR arthrography in revealing tears of the anterior glenoid labrum. Am J Roentgenol. 1997;169(3):837-844.
29 Choi J.A., Suh S.I., Kim B.H., et al. Comparison between conventional MR arthrography and abduction and external rotation MR arthrography in revealing tears of the antero-inferior glenoid labrum. Korean J Radiol. 2001;2(4):216-221.
30 Tirman P.F., Bost F.W., Garvin G.J., et al. Posterosuperior glenoid impingement of the shoulder: findings at MR imaging and MR arthrography with arthroscopic correlation. Radiology. 1994;193(2):431-436.
31 Zlatkin M.B. MRI of the postoperative shoulder. Skeletal Radiol. 2002;31(2):63-80.
32 Timins M.E., Erickson S.J., Estkowski L.D., et al. Increased signal in the normal supraspinatus tendon on MR imaging: diagnostic pitfall caused by the magic-angle effect. Am J Roentgenol. 1995;165(1):109-114.
33 Kijowski R., Farber J.M., Medina J., et al. Comparison of fat-suppressed T2-weighted fast spin-echo sequence and modified STIR sequence in the evaluation of the rotator cuff tendon. Am J Roentgenol. 2005;185(2):371-378.
34 Reinus W.R., Shady K.L., Mirowitz S.A., Totty W.G. MR diagnosis of rotator cuff tears of the shoulder: value of using T2-weighted fat-saturated images. Am J Roentgenol. 1995;164(6):1451-1455.
35 Palmer W.E., Brown J.H., Rosenthal D.I. Rotator cuff: evaluation with fat-suppressed MR arthrography. Radiology. 1993;188(3):683-687.
36 Palmer W.E., Caslowitz P.L., Chew F.S. MR arthrography of the shoulder: normal intraarticular structures and common abnormalities. Am J Roentgenol. 1995;164(1):141-146.
37 Chandnani V.P., Yeager T.D., DeBerardino T., et al. Glenoid labral tears: prospective evaluation with MRI imaging, MR arthrography, and CT arthrography. Am J Roentgenol. 1993;161(6):1229-1235.
38 Jacobson J.A., Lin J., Jamadar D.A., Hayes C.W. Aids to successful shoulder arthrography performed with a fluoroscopically guided anterior approach. Radiographics. 2003;23(2):373-378. discussion 9
39 Young M.F. The physics of anatomy. In: Essential Physics for Muscloskeletal Medicine. Edinburgh: Elsevier; 2009.
40 Standring S., editor. Gray’s Anatomy – Pectoral girdle and upper limb: General organization and surface anatomy of the upper limb (Section 48). Edinburgh: Elsevier, 2009.
41 Standring S., editor. Gray’s Anatomy – Pectoral girdle and upper limb: Pectoral girdle, shoulder region and axilla (Section 49). Edinburgh: Elsevier, 2009.
42 Uhthoff H.K., Sarkar K. An algorithm for shoulder pain caused by soft-tissue disorders. Clin Orthop Relat Res. 1990:254:121-127.
43 Neumann C.H., Petersen S.A., Jahnke A.H.Jr, et al. MRI in the evaluation of patients with suspected instability of the shoulder joint including a comparison with CT-arthrography. Rofo. 1991;154(6):593-600.
44 Liou J.T., Wilson A.J., Totty W.G., Brown J.J. The normal shoulder: common variations that simulate pathologic conditions at MR imaging. Radiology. 1993;186(2):435-441.
45 Kaplan P.A., Bryans K.C., Davick J.P., et al. MR imaging of the normal shoulder: variants and pitfalls. Radiology. 1992;184(2):519-524.
46 Sasaki T., Yodono H., Prado G.L., et al. Increased signal intensity in the normal glenoid labrum in MR imaging: diagnostic pitfalls caused by the magic-angle effect. Magn Reson Med Sci. 2002;1(3):149-156.
47 Roger B., Skaf A., Hooper A.W., et al. Imaging findings in the dominant shoulder of throwing athletes: comparison of radiography, arthrography, CT arthrography, and MR arthrography with arthroscopic correlation. Am J Roentgenol. 1999;172(5):1371-1380.
48 Rockwood C., Matsen F.A.3rd. The Shoulder, 2nd ed. Philadelphia: WB Saunders, 1998.
49 Ianotti J., Williams G. Disorders of the Shoulder: Diagnosis and Management. Baltimore: Lippincott: Williams and Wilkins, 1999.
50 Williams M.M., Snyder S.J., Buford D.Jr. The Buford complex—the “cord-like” middle glenohumeral ligament and absent anterosuperior labrum complex: a normal anatomic capsulolabral variant. Arthroscopy. 1994;10(3):241-247.
51 Kwak S.M., Brown R.R., Resnick D., et al. Anatomy, anatomic variations, and pathology of the 11- to 3-o’clock position of the glenoid labrum: findings on MR arthrography and anatomic sections. Am J Roentgenol. 1998;171(1):235-238.
52 Smith D.K., Chopp T.M., Aufdemorte T.B., et al. Sublabral recess of the superior glenoid labrum: study of cadavers with conventional nonenhanced MR imaging, MR arthrography, anatomic dissection, and limited histologic examination. Radiology. 1996;201(1):251-256.
53 Richardson M.L., Patten R.M. Age-related changes in marrow distribution in the shoulder: MR imaging findings. Radiology. 1994;192(1):209-215.
54 Mirowitz S.A. Hematopoietic bone marrow within the proximal humeral epiphysis in normal adults: investigation with MR imaging. Radiology. 1993;188(3):689-693.
55 Richards R.D., Sartoris D.J., Pathria M.N., Resnick D. Hill-Sachs lesion and normal humeral groove: MR imaging features allowing their differentiation. Radiology. 1994;190(3):665-668.
56 Bui-Mansfield L.T., Taylor D.C., Uhorchak J.M., Tenuta J.J. Humeral avulsions of the glenohumeral ligament: imaging features and a review of the literature. Am J Roentgenol. 2002;179(3):649-655.
57 Takase K., Yamamoto K. Intraarticular lesions in traumatic anterior shoulder instability: a study based on the results of diagnostic imaging. Acta Orthop. 2005;76(6):854-857.
58 Bigliani L., Morrison D., April E. The morphology of the acromion and its relationship to rotator cuff tears. Orthop Trans. 1986;10:216.
59 Shah N.N., Bayliss N.C., Malcolm A. Shape of the acromion: congenital or acquired—a macroscopic, radiographic, and microscopic study of acromion. J Shoulder Elbow Surg. 2001;10(4):309-316.
60 Vanarthos W.J., Monu J.U. Type 4 acromion: a new classification. Contemp Orthop. 1995;30(3):227-229.
61 Epstein R.E., Schweitzer M.E., Frieman B.G., et al. Hooked acromion: prevalence on MR images of painful shoulders. Radiology. 1993;187(2):479-481.
62 Getz J.D., Recht M.P., Piraino D.W., et al. Acromial morphology: relation to sex, age, symmetry, and subacromial enthesophytes. Radiology. 1996;199(3):737-742.
63 Heers G., Gotz J., Schubert T., et al. MR imaging of the intraarticular disk of the acromioclavicular joint: a comparison with anatomical, histological and in-vivo findings. Skeletal Radiol. 2007;36(1):23-28.
64 Salter E.G.Jr, Nasca R.J., Shelley B.S. Anatomical observations on the acromioclavicular joint and supporting ligaments. Am J Sports Med. 1987;15(3):199-206.
65 Duranthon L.D., Gagey O.J. Anatomy and function of the subdeltoid bursa. Surg Radiol Anat. 2001;23(1):23-25.
66 White E.A., Schweitzer M.E., Haims A.H. Range of normal and abnormal subacromial/subdeltoid bursa fluid. J Comput Assist Tomogr. 2006;30(2):316-320.
67 Grainger A.J., Tirman P.F., Elliott J.M., et al. MR anatomy of the subcoracoid bursa and the association of subcoracoid effusion with tears of the anterior rotator cuff and the rotator interval. Am J Roentgenol. 2000;174(5):1377-1380.
68 Schraner A.B., Major N.M. MR imaging of the subcoracoid bursa. Am J Roentgenol. 1999;172(6):1567-1571.
69 Nho S.J., Yadav H., Shindle M.K., Macgillivray J.D. Rotator cuff degeneration: etiology and pathogenesis. Am J Sports Med. 2008;36(5):987-993.
70 Budoff J.E., Nirschl R.P., Ilahi O.A., Rodin D.M. Internal impingement in the etiology of rotator cuff tendinosis revisited. Arthroscopy. 2003;19(8):810-814.
71 Tang K.L., Habermeryer P., Li Q.H., et al. Etiology, classification and clinical evaluation of partial-thickness tears of rotator cuff. Chin J Traumatol. 2003;6(5):309-317.
72 Calis M., Akgun K., Birtane M., et al. Diagnostic values of clinical diagnostic tests in subacromial impingement syndrome. Ann Rheum Dis. 2000;59(1):44-47.
73 Neer C.S.2nd. Rotator cuff tears associated with os acromiale. J Bone Joint Surg Am. 1984;66(8):1320-1321.
74 Edelson J.G., Taitz C. Anatomy of the coraco-acromial arch. Relation to degeneration of the acromion. J Bone Joint Surg Br. 1992;74(4):589-594.
75 Moses D.A., Chang E.Y., Schweitzer M.E. The scapuloacromial angle: a 3D analysis of acromial slope and its relationship with shoulder impingement. J Magn Reson Imaging. 2006;24(6):1371-1377.
76 Yao L., Lee H.Y., Gentili A., Shapiro M.M. Lateral down-sloping of the acromion: a useful MR sign? Clin Radiol. 1996;51(12):869-872.
77 Chang E.Y., Moses D.A., Babb J.S., Schweitzer M.E. Shoulder impingement: objective 3D shape analysis of acromial morphologic features. Radiology. 2006;239(2):497-505.
78 Park J.G., Lee J.K., Phelps C.T. Os acromiale associated with rotator cuff impingement: MR imaging of the shoulder. Radiology. 1994;193(1):255-257.
79 Gallino M., Battiston B., Annaratone G., Terragnoli F. Coracoacromial ligament: a comparative arthroscopic and anatomic study. Arthroscopy. 1995;11(5):564-567.
80 Ticker J.B., Fealy S., Fu F.H. Instability and impingement in the athlete’s shoulder. Sports Med. 1995;19(6):418-426.
81 Jobe F.W., Kvitne R.S., Giangarra C.E. Shoulder pain in the overhand or throwing athlete. The relationship of anterior instability and rotator cuff impingement. Orthop Rev. 1989;18(9):963-975.
82 Michener L.A., McClure P.W., Karduna A.R. Anatomical and biomechanical mechanisms of subacromial impingement syndrome. Clin Biomech (Bristol, Avon). 2003;18(5):369-379.
83 Voight M.L., Thomson B.C. The role of the scapula in the rehabilitation of shoulder injuries. J Athl Train. 2000;35(3):364-372.
84 Hebert L.J., Moffet H., McFadyen B.J., Dionne C.E. Scapular behavior in shoulder impingement syndrome. Arch Phys Med Rehabil. 2002;83(1):60-69.
85 Jobe C.M. Posterior superior glenoid impingement: expanded spectrum. Arthroscopy. 1995;11(5):530-536.
86 Edelson G., Teitz C. Internal impingement in the shoulder. J Shoulder Elbow Surg. 2000;9(4):308-315.
87 Struhl S. Anterior internal impingement: An arthroscopic observation. Arthroscopy. 2002;18(1):2-7.
88 Dines D.M., Warren R.F., Inglis A.E., Pavlov H. The coracoid impingement syndrome. J Bone Joint Surg Br. 1990;72(2):314-316.
89 Giaroli E.L., Major N.M., Lemley D.E., Lee J. Coracohumeral interval imaging in subcoracoid impingement syndrome on MRI. Am J Roentgenol. 2006;186(1):242-246.
90 Fuduka H. Shoulder impingement and rotator cuff disease. Curr Orthop. 1990;4:225-232.
91 Ozaki J., Fujimoto S., Nakagawa Y., et al. Tears of the rotator cuff of the shoulder associated with pathological changes in the acromion. A study in cadavers. J Bone Joint Surg Am. 1988;70(8):1224-1230.
92 Budoff J.E., Nirschl R.P., Guidi E.J. Debridement of partial-thickness tears of the rotator cuff without acromioplasty. Long-term follow-up and review of the literature. J Bone Joint Surg Am. 1998;80(5):733-748.
93 Seeger L.L., Gold R.H., Bassett L.W., Ellman H. Shoulder impingement syndrome: MR findings in 53 shoulders. Am J Roentgenol. 1988;150(2):343-347.
94 Brossmann J., Preidler K.W., Pedowitz R.A., et al. Shoulder impingement syndrome: influence of shoulder position on rotator cuff impingement—an anatomic study. Am J Roentgenol. 1996;167(6):1511-1515.
95 Norwood L.A., Barrack R., Jacobson K.E. Clinical presentation of complete tears of the rotator cuff. J Bone Joint Surg Am. 1989;71(4):499-505.
96 Zanetti M., Weishaupt D., Jost B., et al. MR imaging for traumatic tears of the rotator cuff: high prevalence of greater tuberosity fractures and subscapularis tendon tears. Am J Roentgenol. 1999;172(2):463-467.
97 Quillen D.M., Wuchner M., Hatch R.L. Acute shoulder injuries. Am Fam Physician. 2004;70(10):1947-1954.
98 Morag Y., Jacobson J.A., Miller B., et al. MR imaging of rotator cuff injury: what the clinician needs to know. Radiographics. 2006;26(4):1045-1065.
99 Ellenbecker T. Clinical Examination of the Shoulder. Philadelphia: Elsevier WB Saunders, 2004.
100 Itoi E., Kido T., Sano A., et al. Which is more useful, the “full can test” or the “empty can test”, in detecting the torn supraspinatus tendon? Am J Sports Med. 1999;27(1):65-68.
101 Dinnes J., Loveman E., McIntyre L., Waugh N. The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review. Health Technol Assess. 2003;7(29):1-166. iii
102 Kelly J. Shoulder pain and weakness. Physician Sportsmed. 2004;32:11.
103 Stefko J.M., Jobe F.W., VanderWilde R.S., et al. Electromyographic and nerve block analysis of the subscapularis liftoff test. J Shoulder Elbow Surg. 1997;6(4):347-355.
104 Walch G., Boulahia A., Calderone S., Robinson A.H. The ‘dropping’ and ‘hornblower’s’ signs in evaluation of rotator-cuff tears. J Bone Joint Surg Br. 1998;80(4):624-628.
105 Sahin-Akyar G., Miller T.T., Staron R.B., et al. Gradient-echo versus fat-suppressed fast spin-echo MR imaging of rotator cuff tears. Am J Roentgenol. 1998;171(1):223-227.
106 Schibany N., Zehetgruber H., Kainberger F., et al. Rotator cuff tears in asymptomatic individuals: a clinical and ultrasonographic screening study. Eur J Radiol. 2004;51(3):263-268.
107 Martin-Hervas C., Romero J., Navas-Acien A., et al. Ultrasonographic and magnetic resonance images of rotator cuff lesions compared with arthroscopy or open surgery findings. J Shoulder Elbow Surg. 2001;10(5):410-415.
108 Magee T., Williams D., Mani N. Shoulder MR arthrography: which patient group benefits most? Am J Roentgenol. 2004;183(4):969-974.
109 Bryant L., Shnier R., Bryant C., Murrell G.A. A comparison of clinical estimation, ultrasonography, magnetic resonance imaging, and arthroscopy in determining the size of rotator cuff tears. J Shoulder Elbow Surg. 2002;11(3):219-224.
110 Motamedi A.R., Urrea L.H., Hancock R.E., et al. Accuracy of magnetic resonance imaging in determining the presence and size of recurrent rotator cuff tears. J Shoulder Elbow Surg. 2002;11(1):6-10.
111 Rafii M., Firooznia H., Sherman O., et al. Rotator cuff lesions: signal patterns at MR imaging. Radiology. 1990;177(3):817-823.
112 Teefey S.A., Rubin D.A., Middleton W.D., et al. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance imaging, and arthroscopic findings in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
113 Nakatani T., Fujita K., Iwasaki Y., et al. MRI-negative rotator cuff tears. Magn Reson Imaging. 2003;21(1):41-45.
114 Mirowitz S.A. Normal rotator cuff: MR imaging with conventional and fat-suppression techniques. Radiology. 1991;180(3):735-740.
115 Tuite M.J., Turnbull J.R., Orwin J.F. Anterior versus posterior, and rim-rent rotator cuff tears: prevalence and MR sensitivity. Skeletal Radiol. 1998;27(5):237-243.
116 Lam F., Mok D. Open repair of massive rotator cuff tears in patients aged sixty-five years or over: is it worthwhile? J Shoulder Elbow Surg. 2004;13(5):517-521.
117 Tung G.A., Yoo D.C., Levine S.M., et al. Subscapularis tendon tear: primary and associated signs on MRI. J Comput Assist Tomogr. 2001;25(3):417-424.
118 Li X.X., Schweitzer M.E., Bifano J.A., et al. MR evaluation of subscapularis tears. J Comput Assist Tomogr. 1999;23(5):713-717.
119 McCluskey G.M., Getz B.A. Pathophysiology of anterior shoulder instability. J Athl Train. 2000;35(3):268-272.
120 Woertler K., Waldt S. MR imaging in sports-related glenohumeral instability. Eur Radiol. 2006;16(12):2622-2636.
121 Cordasco F.A. Understanding multidirectional instability of the shoulder. J Athl Train. 2000;35(3):278-285.
122 Gerber C., Ganz R. Clinical assessment of instability of the shoulder. With special reference to anterior and posterior drawer tests. J Bone Joint Surg Br. 1984;66(4):551-556.
123 Ellenbecker T.S. Rehabilitation of shoulder and elbow injuries in tennis players. Clin Sports Med. 1995;14(1):87-110.
124 Liu S.H., Henry M.H., Nuccion S., et al. Diagnosis of glenoid labral tears: a comparison between magnetic imaging and clinical examinations. Am J Sports Med. 1996;24:149-154.
125 O’Brien S.J., Pagnani M.J., Fealy S., et al. The active compression test: a new and effective test for diagnosing labral tears and acromion-clavicular joint abnormality. Am J Sports Med. 1998;26:610-613.
126 Palmer W.E., Caslowitz P.L. Anterior shoulder instability: diagnostic criteria determined from prospective analysis of 121 MR arthrograms. Radiology. 1995;197(3):819-825.
127 Bokor D.J., Conboy V.B., Olson C. Anterior instability of the glenohumeral joint with humeral avulsion of the glenohumeral ligament. A review of 41 cases. J Bone Joint Surg Br. 1999;81(1):93-96.
128 Chalidis B., Sachinis N., Dimitriou C., et al. Has the management of shoulder dislocation changed over time? Int Orthop. 2007;31(3):385-389.
129 Workman T.L., Burkhard T.K., Resnick D., et al. Hill-Sachs lesion: comparison of detection with MR imaging, radiography, and arthroscopy. Radiology. 1992;185(3):847-852.
130 Itoi E., Lee S.B., Berglund L.J., et al. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
131 Massengill A.D., Seeger L.L., Yao L., et al. Labrocapsular ligamentous complex of the shoulder: normal anatomy, anatomic variation, and pitfalls of MR imaging and MR arthrography. Radiographics. 1994;14(6):1211-1223.
132 Ly J.Q., Beall D.P., Sanders T.G. MR imaging of glenohumeral instability. Am J Roentgenol. 2003;181(1):203-213.
133 Legan J.M., Burkhard T.K., Goff W.B.2nd, et al. Tears of the glenoid labrum: MR imaging of 88 arthroscopically confirmed cases. Radiology. 1991;179(1):241-246.
134 Gusmer P.B., Potter H.G., Schatz J.A., et al. Labral injuries: accuracy of detection with unenhanced MR imaging of the shoulder. Radiology. 1996;200(2):519-524.
135 Wischer T.K., Bredella M.A., Genant H.K., et al. Perthes lesion (a variant of the Bankart lesion): MR imaging and MR arthrographic findings with surgical correlation. Am J Roentgenol. 2002;178(1):233-237.
136 Waldt S., Burkart A., Imhoff A.B., et al. Anterior shoulder instability: accuracy of MR arthrography in the classification of anteroinferior labroligamentous injuries. Radiology. 2005;237(2):578-583.
137 Neviaser T.J. The GLAD lesion: another cause of anterior shoulder pain. Arthroscopy. 1993;9(1):22-23.
138 Warren R.F., Craig E.V., Altchek D.W. The Unstable Shoulder. Philadelphia: Lippincott Raven, 1999.
139 Snyder S.J., Karzel R.P., Delpizzo W., et al. SLAP lesions of the shoulder. Arthroscopy. 1990;6:274-279.
140 Cartland J.P., Crues J.V.3rd, Stauffer A., et al. MR imaging in the evaluation of SLAP injuries of the shoulder: findings in 10 patients. Am J Roentgenol. 1992;159(4):787-792.
141 Hottya G.A., Tirman P.F., Bost F.W., et al. Tear of the posterior shoulder stabilizers after posterior dislocation: MR imaging and MR arthrographic findings with arthroscopic correlation. Am J Roentgenol. 1998;171(3):763-768.
142 Tung G.A., Hou D.D. MR arthrography of the posterior labrocapsular complex: relationship with glenohumeral joint alignment and clinical posterior instability. Am J Roentgenol. 2003;180(2):369-375.
143 Shin R.D., Polatsch D.B., Rokito A.S., Zuckerman J.D. Posterior capsulorrhaphy for treatment of recurrent posterior glenohumeral instability. Bull Hosp Jt Dis. 2005;63(1–2):9-12.
144 Minkoff J., Cavaliere G. Glenohumeral instabilities and the role of magnetic resonance imaging techniques. The orthopedic surgeon’s perspective. Magn Reson Imaging Clin N Am. 1993;1(1):105-123.
145 Escobedo E.M., Richardson M.L., Schulz Y.B., et al. Increased risk of posterior glenoid labrum tears in football players. Am J Roentgenol. 2007;188(1):193-197.

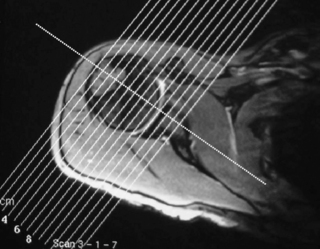
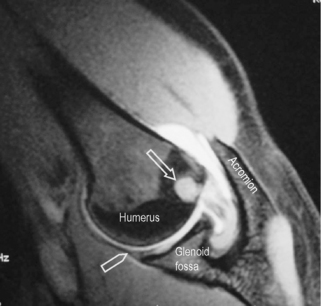
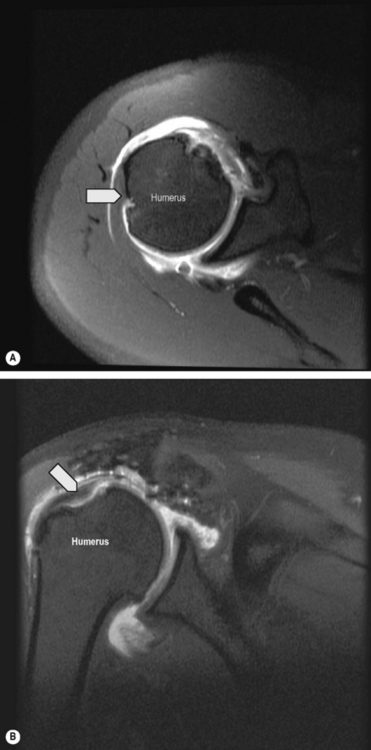
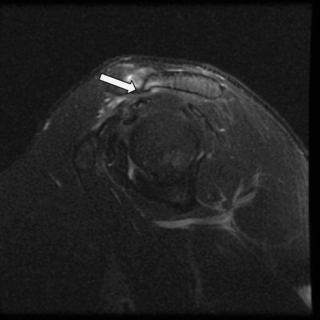
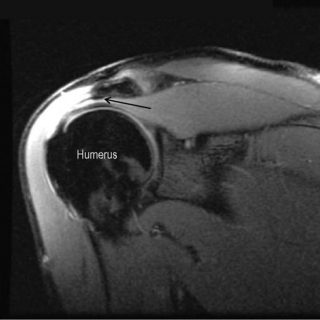
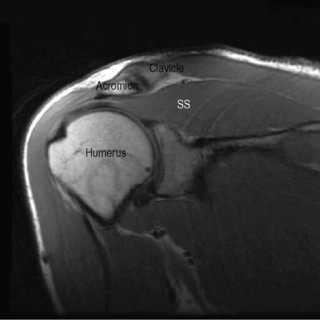
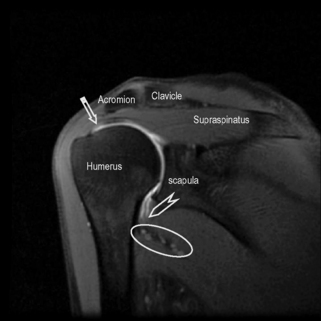
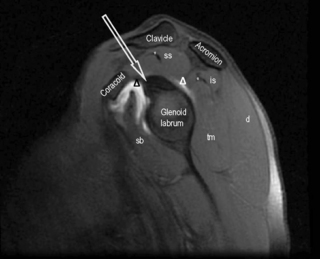
 ).
).