56 The Role of Dynamic Stabilization and the Aging Spine
Devices
Interspinous Spacers
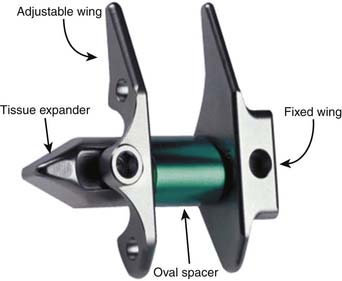
X-Stop (Kyphon)
X-Stop is a titanium alloy device that is designed to stop extension (Figure 56-1). The oval spacer conforms to the interspinous space, and the wings prevent lateral migration. It is minimally invasive and inserted laterally, thus preserving the supraspinous ligament. It is designed to be implanted under local anesthesia. In clinical trial, X-Stop was significantly better than nonoperative treatment of lumbar spinal stenosis at 1 and 2 years post-op. The observed success rate was comparable to published reports for decompressive laminectomy, but with considerably lower morbidity.1,5 X-Stop is currently approved by the U.S. Food and Drug Administration (FDA) for use in patients with claudication symptoms from lumbar stenosis. Patients with relief in flexion and who are otherwise comfortable sitting respond well.
Wallis (Zimmer Spine)
The Wallis device is a PEEK interspinous spacer secured to the spinous processes using PET bands (Figure 56-2). It is designed to block extension and control flexion. Indications include isolated lumbar intervertebral instability, such as herniated discs, Modic I degenerative lesions, degenerative disc disease at a level adjacent to a previous fusion, and spinal stenosis treated without laminectomy. In long term (13 year) OUS follow-up, the device obviated the need for arthrodesis in 80% of patients.4 The Wallis device is currently undergoing clinical evaluation and is not FDA approved.
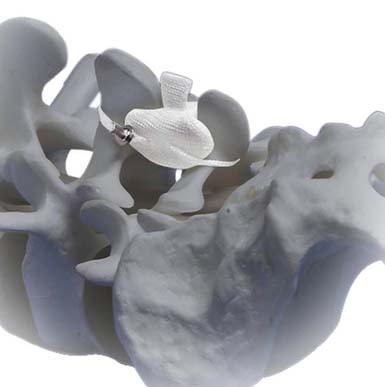
Diam (Medtronic)
Diam is an interspinous stabilizer composed of a silicone bumper, encased in polyester mesh, and secured with polyester sutures (Figure 56-3). It is inserted with minimal access and minimal tissue disruption. It is designed to resist extension and reduce intradiscal pressure. Indications include restoration of early segmental degeneration, correction of misalignment often seen in discectomy, and stenosis. Diam is currently undergoing clinical evaluation and is not FDA approved.
Coflex (Paradigm)
Coflex is a titanium alloy device whose U-shaped body conforms to the interspinous and interlaminar space (Figure 56-4). It acts as a stiff spring, dynamically stabilizing extension. Lateral migration is prevented by wings compressed onto the spinous processes. Indications include lumbar stenosis, adjacent segment disease, recurrent HNP and early symptomatic disc degeneration. In OUS implantation after decompressive laminectomy in degenerative lumbar spinal stenosis, Coflex was found to be less invasive and provided similar clinical outcome in comparison with instrumented fusion.2 Coflex is currently undergoing clinical evaluation and is not FDA approved.
ExtenSure (NuVasive)
ExtenSure is a PEEK interspinous spacer secured by geometric conformity to the anatomy and the suturing of the supraspinous ligament (Figure 56-5). It is designed to alleviate pseudoclauditory symptoms and radicular symptoms by enlarging the central canal, lateral recess, and foramina. It also decreases or eliminates low back pain by decreasing pressure on arthritic facet joints, degenerative discs, or both. It also restores disc height and foraminal volume. It is indicated in moderate to severe spinal stenosis, degenerative spondylolisthesis, and mild to moderate degenerative scoliosis. ExtenSure is currently undergoing clinical evaluation and is not FDA approved.
In-Space (Synthes)
In-Space is a laterally placed PEEK cylindrical device, secured by deployable wings (Figure 56-6). It is intended to stop segmental extension and to distract the symptomatic interspinous space. In doing so, maintenance of foraminal height, opening of the area of the spinal canal, reduction of stress on the facets, and relieving of pressure on the posterior annulus results. It is indicated in lumbar spinal stenosis, disc protrusions with discogenic low back pain, facet syndrome due to face osteoarthritis, degenerative spondylolisthesis up to grade 1, and degenerative disc disease. In-Space is not FDA approved.
Superion (Vertiflex)
Superion is a titanium alloy interspinous device with deployable wings (Figure 56-7). It is designed for percutaneous implantation in the treatment of moderate degenerative lumbar stenosis at one or two levels. Superion is currently under clinical investigation in the United States and is not FDA approved.
Facet Devices
This results in a very complex continuum of disease. Painful inflammation, osteoarthritis, stenosis, abnormal loading, and total failure of the functional spinal unit can all originate with facet disease. These challenges are not easily overcome, addressed, or surgically treated. Facet arthroplasty, a rapidly evolving subspecialty in motion preservation, strives to address these issues in the most ergonomic manner.
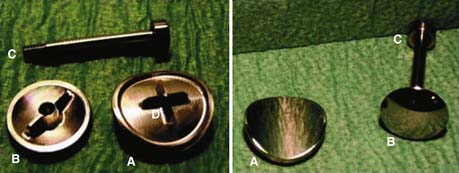
Zyre (Quantum Orthopedics)
Zyre is an interpositional arthroplasty device (Figure 56-8). It consists of a cobalt chromium intraarticular spacer, through which passes a PET cord with chromium retainers. It is minimally invasive, requires no bone resection, maintains capsular integrity, and can be implanted with or without decompression. Indications include painful degeneration of the facet with failed CMM. Advantages include minimal disruption of anatomy and multiple revision options. This concept is early technology with a paucity of clinical data. Zyre is not FDA approved.
Fenix (Gerraspine AG)
Fenix is a cobalt chromium facet joint resurfacing device. It has superior and inferior components secured with a translaminar locking screw (Figure 56-9). It is designed to eliminate the painful components, resurface the facet joint, preserve supporting structure and anatomy, and allow or restore physiologic motion. Surgery entails removal of capsule and intra-articular cartilage, with or without decompression. Indications include significant facet disease and subarticular stenosis. Fenix has had limited clinical use and is not FDA approved.
Anatomic Facet Replacement System (Facet Solutions)
Anatomic Facet Replacement System (AFRS) is a total facet replacement device composed of cobalt-chromium-molybdenum articular surfaces that uses conventional pedicle screw fixation (Figure 56-10). It is designed to reproduce facet anatomy and preserve or restore natural lumbar biomechanics. Surgical implantation is through a midline approach and entails total facetectomy. Indications include osteoarthritis of the facets causing stenosis, and low grade degenerative spondylolisthesis. Fixation with standard pedicle screws allows ready revision options. AFRS I is in clinical trial in the United States and is not FDA approved.
Total Facet Arthroplasty System (Archus)
Total Facet Arthroplasty System (TFAS) is a total facet replacement device composed of cobalt-chromium articular surfaces and titanium alloy cross arm assembly (Figure 56-11). It comprises paired cephalad bearings and paired caudal housings in a “ball in cup” motion-constraining configuration. These are supported by the titanium cross arm assembly with cemented pedicle post fixation. In situ modular assembly allows for precise adjustment to individual anatomy. Proposed indications include degenerative disease of the facets, facet instability, up to grade I spondylolisthesis with neurological impairment, central or lateral spinal stenosis, at L3-L4 or L4-L5. TFAS is being clinically evaluated in the United States and is not FDA approved.
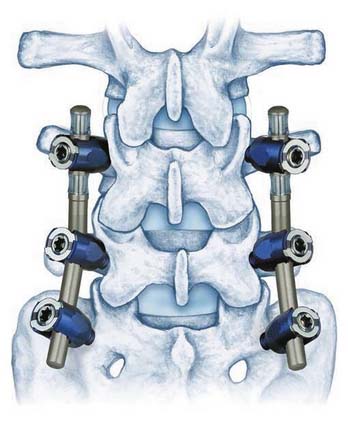
Total Posterior System (Impliant)
Total Posterior System (TOPS) is composed of opposing titanium plates with interlocking PCU. It is fixed to the spine with polyaxial pedicle screws (Figure 56-12). The entirety of the posterior elements is totally replaced. Motion is restored and constrained in all planes via polymeric dampening. Utilizing the same surgical technique as standard posterior fusion, it recreates the normal biomechanics of the spine, allowing full physiologic range of motion. Surgery occurs through a midline approach and laterally placed pedicle screws. Total facetectomy and removal of posterior elements is necessary. Implantation requires a precise jig assembly. Indications include moderate to severe spinal stenosis. TOPS is being clinically evaluated in the United States and is not FDA approved.
Pedicle-Based Dynamic Rods
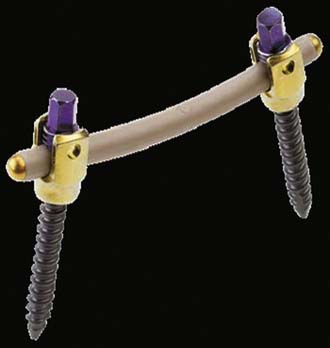
N-Hance (Synthes)
N-Hance is a flexible posterior stabilizing device that is composed of a collar of paired PCU spacers with interposed titanium ring and end caps (Figure 56-13). This unit slides over the tapered core of a 6-mm titanium rod. The construct provides elongation, compression, and angulation. N-Hance is 510K approved by the FDA as an adjunct to fusion.
Stabilimax NZ (Applied Spine)
Stabilimax NZ is an investigational posterior stabilizing system that utilizes a dual spring configuration to confer physiologic motion parameters (Figure 56-14). It is intended to provide maximum stabilization to the spine, decreasing the abnormal motion that causes pain, while maintaining physiologic motion.
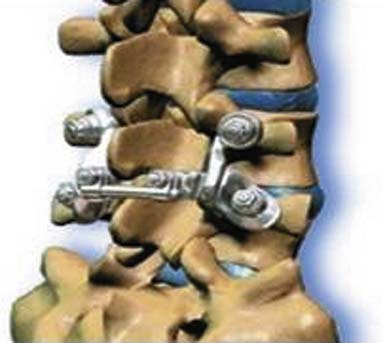
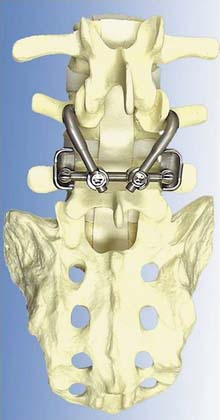
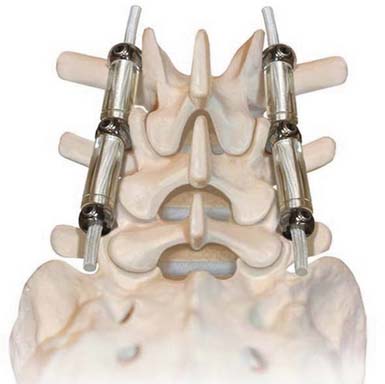
Dynesys (Zimmer Spine)
The Dynesys Dynamic Stabilization System is a posteriorly placed pedicle-based device (Figure 56-15). It is composed of titanium alloy screws, an interposed PCU spacer, and a through-passed PET cord. During implantation of the device, the cord is placed in tension and the spacer is placed in compression. These opposing vectors of force act through the pedicle screws to dynamically neutralize the spinal segment in flexion, extension, and at rest. The system is 510K approved as an adjunct to fusion. In prospective OUS clinical trial, dynamic neutralization proved to be a safe and effective alternative (to fusion) in the treatment of unstable lumbar conditions.3
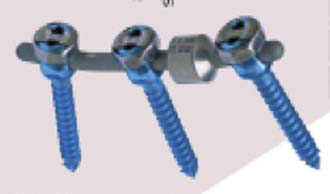
Dynamic TTL-Rod (Scient’x)
The Dynamic TTL-Rod is composed of titanium rods with an interposed damper (Figure 56-16). The damper is a series of washers contained within a bell housing. This configuration results in a posterior rod with micro motion of 2 mm. It is designed to stabilize the spinal segment in semirigid fashion with reduced forces at the bone screw interface. The Dynamic TTL-Rod is 510K approved as an adjunct to fusion.
CD Horizon Legacy Peek Rod System (Medtronic)
The CD Horizon Legacy Peek Rod System is a pedicle-based, posterior rod device (Figure 56-17). It is composed of standard polyaxial pedicle screws attached to a peek rod. It is designed to provide semirigid fixation that closely replicates the natural load distribution of the lumbar spine for patients who undergo spinal fusion surgery. The CD Horizon Legacy Peek Rod System is 510K approved as an adjunct to fusion.
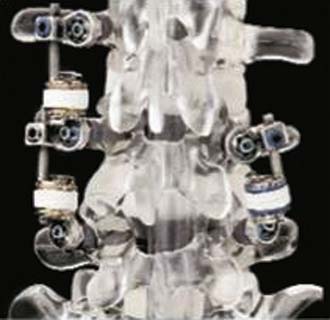
DSS Spine Stabilization System (Paradigm)
The DSS Spine Stabilization System is a pedicle-based posterior coupler device (Figure 56-18). It is an entirely modular system composed of titanium monoaxial pedicle screws, upon which are placed washers and spherical spacers. This allows polyaxial orientation of the couplers. The couplers are made of titanium. The configuration of outer spiral cut housing and inner shaft with a ball-in-socket piston confers motion is all directions with stoppage within physiologic ranges. The hemispherical screw interfaces allow further polyaxial implantation. It is designed to allow physiologic motion within an overall reduced range and to restrict the neutral zone. The DSS Spine Stabilization System is 510K approved as an adjunct to fusion.
Clinical Application
Facet
Mild capsular failure and intractable synovial inflammation may result in facet pain. Facet resurfacing technology, such as Zyre may be efficacious.
1. Anderson, et al. J. Neurosurg.. 2006 Jun;4(6):463-471.
2. Park S.C., et al. J. Korean. Neurosurg. Soc.. 2009 Oct;46(4):292-299.
3. Stoll T.M., et al. Eur. Spine J., 11 Suppl 2:2002 Oct, S170-S178.
4. Neurosurg. Rev., 32, 3:2009 Jul, 335-341, discussion 341–2