58 The Role for Biologics in the Aging Spine
Introduction
Currently, there are over 36 million people over the age of 65 years in the United States. This number is projected to increase to 71 million (nearly 20%) by 2030.1 As the population ages there will be a similar increase in age-related diseases, such as degenerative disorders of the spine. The elderly are also living a more active lifestyle than at any other time, and thus the demand for addressing issues in this population will only increase over time. However, treating degenerative conditions of the aging spine poses particularly challenging situations to surgeons. Not only must the spinal surgeon address the spinal pathology, but these patients often have serious comorbidities that may affect the treatment options.2,3 Therefore, patients, families, and physicians will have to weigh the additional risks of operative treatment against the benefits of reducing disabling pain and improving quality of life.
Currently, iliac crest autologous bone (autograft) remains the gold standard bone graft material for achieving spinal fusion in all ages. It is an excellent choice because it is the only bone graft option that provides all of the components necessary for arthrodesis: osteoconductive matrix, osteoinductive proteins, and osteogenic cells. However, there are significant problems with using autograft including the limited supply of available bone and subjecting the patient to a secondary invasive procedure to harvest the autologous bone which is, in itself, associated with potential morbidity, such as infection, fracture, and intractable pain.4,5 This consideration is particularly relevant for the elderly population who, because of their medical comorbidities, often have longer recovery times and more complications following surgeries of any kind. As a result, there has been significant work to develop materials to supplement or even replace iliac crest bone graft in the hope of minimizing surgical morbidities while ensuring that the treatment objectives are still achieved.
Bone Morphogenetic Proteins
Bone morphogenetic proteins (BMPs), members of the transforming growth factor-beta superfamily, have gradually become better understood since their initial identification by Marshall Urist6 in the 1960s.7 They function by binding to the cell membrane of undifferentiated mesenchymal type of cells to promote the induction of bone formation. Although more than 12 BMPs have been identified, only a few have been explored for potential clinical use.
In a prospective, randomized trial comparing anterior lumbar interbody fusion with either rhBMP-2 implanted on a collagen sponge in a tapered fusion cage or autogenous iliac crest bone graft, there was a 94.5% fusion rate in the BMP group versus an 88.7% fusion rate in the control group, as determined radiographically.8 Moreover, 5.9% of the subjects in the autograft control group experienced adverse events related to their bone grafting procedures, and 32% reported persistent pain at the donor site at the time of final follow-up. Back, leg, and neurologic pain scores improved in both groups to a similar extent. Nonetheless, this procedure is less commonly considered than posterior procedures in the aged population because of the increase in complication rate and recovery time associated with anterior approaches.
Posterior lumbar fusion with BMP has significant challenges including limited surface area for healing, distractive forces, and the large gap between transverse processes needing to be bridged. Furthermore, studies have demonstrated the need for a bulking agent in posterior procedures. One recent retrospective study comparing instrumented posterolateral fusion with rhBMP-2 versus iliac crest autograft demonstrated equivalent fusion masses between the two groups at 2-year follow-up.9 Nonunion rate was 6.6% in the BMP group compared to 11.1% in the control group. No significant differences in the bulking agent used (local bone, allograft bone, demineralized bone matrix, and ceramic) were noted. Demonstrated outcomes when rhBMP-7 (OP-1) is used are comparable to those of autograft when used in uninstrumented posterolateral fusion for the treatment of degenerative spondylolisthesis.10
Recently Glassman and colleagues11 reported on the utility of rhBMP-2 with an absorbable collagen sponge (ACS) compared to autograft in an elderly population (older than 60 years of age). They assessed the clinical, radiographic, and economic outcomes at 2-year follow-up for 102 patients treated by posterolateral lumbar fusion with iliac crest autograft versus rhBMP-2/ACS. They found no increased rates of complications due to the rhBMP-2 (8 of the 50 patients) in this population. In fact, there were increased perioperative complications in the autograft group (23 of the 52 patients). These complications included donor site infections, cardiac issues, pain, urinary tract infections, and neurologic deficit. Additionally, they found that the rhBMP-2 group had significantly better fusion grades on radiographic imaging and equivalent improvements in health-related quality of life outcomes for both groups.
Though BMPs provide substantial benefits in spinal arthrodesis, their use is not always without complication.12 Potential complications include the formation of ectopic bone, hematoma and seroma formation, bone resorption and graft subsidence, antibody formation, and possible carcinogenicity. While some of the effects may be dose or carrier related, many of the applications currently considered for their use are off-label and their use has to be considered carefully. Further, the cost-to-benefit considerations remain to be elucidated.
Other Bone Graft Alternatives
Allograft
Allograft bone provides an osteoconductive scaffold for new bone formation. Various allograft bone types are available. Successful use of allograft bone in spinal surgery is largely dependent on its placement. When structural allograft is implanted in the anterior column, it is associated with relatively high fusion rates, both in the cervical and the thoracolumbar regions of the spine.13,14 However, when nonstructural allograft bone is placed under tension, as in the posterior spine, it incorporates at a slower rate than autograft and leads to lower rates of arthrodesis when used alone.15,16
A recent study by Anderson and associates17 evaluated the use of morcelized femoral head allograft with and without instrumentation in the elderly. The study did not harvest autograft from any of the patients. The demonstrated successful fusion, determined by radiographs, is 68% with allograft alone and 81% with allograft plus instrumentation. Additionally, there were 15 of the 94 patients treated who required revision surgeries, a revision rate that is similar to what is seen when using autograft alone. This study demonstrated superior outcomes with allograft plus instrumentation for posterolateral fusion surgeries. However, more importantly for this review, it is one of the few studies to specifically evaluate allograft in an elderly population.
Demineralized Bone Matrix
Demineralized bone matrix is allograft bone that has been decalcified to produce a product of collagen and noncollagenous proteins. Multiple products are available, but there are relatively limited data available regarding their efficacy, as limited regulatory requirements have been established for these minimally manipulated tissue products. Further, studies have questioned the osteoinductive nature of one product versus another, as well as lot-to-lot variability.18,19
There are few randomized controlled clinical trials evaluating the use of DBMs in humans. In one series, 77 patients underwent one, two, or three level noninstrumented anterior cervical discectomy and fusion procedures, using freeze-dried structural allografts filled with a DBM or autogenous iliac crest bone graft alone.20 At a minimum of 1-year follow-up, fusion rates were 54% and 74% for the allograft/DBM and autograft groups, respectively. Despite this relatively easy fusion environment, inferior fusion rates were observed in the experimental study arm.
In contrast, another study, involving 50 subjects who received lumbar interbody fusions, reported a 96% success rate after implanting titanium mesh cages packed with a DBM and a coralline hydroxyapatite carrier.21 The authors performed circumferential fusions with posterior instrumentation and autograft but they did not have a control group to compare against. However, they only used the DBM anteriorly. Again, this study only used the DMB in the anterior spinal column where fusion is generally more easily achieved. This is an excellent example of how DBM could be used as a bone graft extender by eliminating the need for iliac crest bone graft (ICBG) in the anterior fusion construct.
Synthetic Materials (Ceramics)
One of the inherent disadvantages of ceramics is that they are relatively weak and brittle. When ceramics are introduced into the anterior spinal column they must be protected from the significant compressive forces by internal fixation until the graft is incorporated into the new bone growth.13 Like allograft, ceramics are also inferior to autogenous bone when placed under tension.22 Other biologically active materials, such as local autograft, demineralized bone matrices (DBMs), or osteoinductive growth factors, may be combined with ceramic osteoconductive matrices to form a composite graft that can then lead to increased amounts of bone formation.23,24
Bone Marrow Aspirates
Autologous bone marrow represents another source of osteogenic cells and osteoinductive proteins for spinal fusion. The most significant advantage of this technique is that aspiration of bone marrow has much less morbidity than the procurement of iliac crest autograft. However, it must be used in combination with an osteoconductive matrix, bone marrow aspirate to form a composite graft. The major limitation to this technique is that unfractionated bone marrow has only moderate osteogenic potential. Even in healthy adults, it is estimated that only 1 out of every 50,000 nucleated bone marrow cells is capable of undergoing differentiation into an osteoblast.25 Additionally, it has been shown that the number of viable bone marrow cells decreases significantly with age, which may limit utility in an elderly population.26
One recent study demonstrated comparable fusion rates to autograft in an instrumented posterolateral fusion study.27 Both allograft and DBM can be combined with the osteogenic elements of bone marrow aspirate to help promote fusion. By adding bone marrow aspirate, osteogenic elements may be added to help promote fusion. Studies have shown improved fusion rates with the use of bone marrow aspirate from iliac crest when used in conjunction with autologous autograft in a bone paucity model.28 Recently, aspirates from the vertebral bodies accessed during pedicle screw instrumentation have been shown to be an excellent source of osteogenic cells with only modest depletion with serial aspirations.29 However, there was a reduction in the cellularity of the specimens in older individuals.
Other Potential Application of Biologics in the Aging Spine
Vertebral Body Augmentation in Vertebral Body Compression Fractures
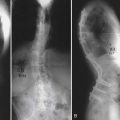
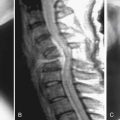
Unique to elderly patients, vertebral compression fractures are the most common type of osteoporotic fragility fracture that can occur from low energy trauma, such as a fall from standing height or less. Although many are asymptomatic, the possible consequences of such injuries can be serious, resulting in limited ambulation, chronic pain, depression, and loss of independence. The primary goals for treatment include pain control and treatment, surgical or nonsurgical, aimed at returning the patient to full activity as quickly as possible to avoid long-term sequelae of deconditioning and muscle weakness.
Surgical options for vertebral body compression fractures include vertebroplasty and kyphoplasty to inject bone cement into the collapsed vertebrae with or without the use of an inflatable balloon to elevate the endplates prior to injecting the cement. Both procedures provide short-term improvement in pain and correction of the deformity, and long-term benefits for functional capacity and prevention of recurrent pain.30
A recent meta-analysis30 comparing kyphoplasty and vertebroplasty found that both treatments resulted in significant improvements in pain and functionality compared to baseline. Compared to medical treatment there was improvement in function, but not pain. Comparing the two surgical treatments, this meta-analysis concluded that balloon kyphoplasty provided better correction of the kyphotic angle and vertebral height while also having less complications from cement extravasation and pulmonary embolism. This study concluded that balloon kyphoplasty is likely superior to traditional vertebroplasty for compression fractures refractory to conventional medical therapy; however they based their assessments on level III data because no randomized control trials were available to appropriately evaluate these treatments.30
Newer alternatives to the traditional polymethylmethacrylate (PMMA) bone cement used for these procedures are also being evaluated. The current cement material has a number of shortcomings including a strong exothermic polymerization reaction, which could damage surrounding tissues, lack of bioavailability and osteoconductivity, toxicity of the cement, and extremely limited resorption over time.31,32 Thus newer cements are being developed to address some of these issues. A recent study evaluating the use of two novel variants (aluminum-free and zinc-based glass polyalkenoate cement) in an in vitro model of osteoporotic compression fractures found them to have similar material properties (injectability, radiopacity, uniaxial compression strength, and biaxial flexural modulus) to traditional cement and performed well over serial compression tests in the lab.33
Clinical trials for some of these new cement alternatives have begun with differing results. One study of calcium phosphate cement in balloon kyphoplasty demonstrated improvement in pain and disability outcomes, but poor resistance to flexural, tractive, and shear forces, which resulted in radiographic loss of correction.34 The authors ultimately recommended against the routine use of this cement alternative to traditional PMMA.
Nonfusion Applications: Addressing Disc Degeneration Directly
Much of low back pain in the aging spine is believed to be a result of disc pathology. Various studies have attempted to identify changes in disc anatomy that may play a role in the generation of back discomfort. Recent studies have demonstrated that there is an overall decrease in cellular density within the disc as well as a reduction in the production of cartilage-specific extracellular matrix components.35 As a result, there is an overall loss of water-binding capacity and thus an alteration in the biomechanics of the disc. Despite the paucity of cells within the disc, it plays an integral role in the maintenance of matrix proteins. The reduction in cells during aging is believed to be attributable to both apoptotic and necrotic processes. A focus of recent scientific work has been to induce or augment these cells.36 Cellular transplantation offers potential promise in the treatment of degenerative disc disease; other therapies that have been proposed are the injection of biomaterials into the disc to augment the nucleus pulposus.
1. Federal Interagency Forum on Aging-Related Statistics. Older Americans update 2006: key indicators of well-being. In: Federal Interagency Forum on Aging-Related Statistics. Washington, DC: U.S. Government Printing Office; 2006.
2. Cloyd J.M., Acosta F.L.Jr., Ames C.P. Complications and outcomes of lumbar spine surgery in elderly people: a review of the literature. J. Am. Geriatr. Soc. 2008;56–7:1318-1327.
3. Hart R.A., Prendergast M.A. Spine surgery for lumbar degenerative disease in elderly and osteoporotic patients. Instr. Course Lect. 2007;56:257-272.
4. Gupta A.R., Shah N.R., Patel T.C. Perioperative and long-term complications of iliac crest bone graft harvest for spinal surgery: a quantitative review of the literature. Int. Med. 2001;8:163-166.
5. Steinmann J.C., Herkowitz H.N. Pseudarthrosis of the spine. Clin. Orthop. Relat. Res. 1992;284:80-90.
6. Urist M.R. Bone: Formation by autoinduction,. Science. 1965;150:893-899.
7. Carlisle E., Fischgrund J.S. Bone morphogenetic proteins for spinal fusion. Spine J. 2005;5–6(Suppl):240S-249S.
8. Burkus J.K., Gornet M.F., Dickman C.A., Zdeblick T.A. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J. Spinal Disord. Tech. 2002;15–5:337-349.
9. Glassman S.D., Carreon L., Djurasovic M., Campbell M.J., Puno R.M., Johnson J.R., Dimar J.R. Posterolateral lumbar spine fusion with INFUSE bone graft. Spine J. 2007;7–1:44-49.
10. Brandoff J.F., Silber J.S., Vaccaro A.R. Contemporary alternatives to synthetic bone grafts for spine surgery. Am. J. Orthop. 2008;37–8:410-414.
11. Glassman S.D., Carreon L.Y., Djurasovic M., Campbell M.J., Puno R.M., Johnson J.R., Dimar J.R. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion: a randomized, controlled trial in patients over sixty years of age. Spine (Phila Pa 1976). 2008;33–26:2843-2849.
12. Benglis D., Wang M.Y., Levi A.D. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery. 62–5(Suppl 2), 2008. ONS423-31; discussion ONS31
13. Malloy K.M., Hilibrand A.S. Autograft versus allograft in degenerative cervical disease. Clin. Orthop. Relat. Res. 2002;394:27-38.
14. Vaccaro A.R., Chiba K., Heller J.G., Patel T., Thalgott J.S., Truumees E., Fischgrund J.S., Craig M.R., Berta S.C., Wang J.C. Bone grafting alternatives in spinal surgery. Spine J. 2002;2–3:206-215.
15. Jorgenson S.S., Lowe T.G., France J., Sabin J. A prospective analysis of autograft versus allograft in posterolateral lumbar fusion in the same patient. A minimum of 1-year follow-up in 144 patients. Spine (Phila Pa 1976). 1994;19–18:2048-2053.
16. Nugent P.J., Dawson E.G. Intertransverse process lumbar arthrodesis with allogeneic fresh-frozen bone graft. Clin. Orthop. Relat. Res. 1993;287:107-111.
17. Andersen T., Christensen F.B., Niedermann B., Helmig P., Hoy K., Hansen E.S., Bunger C. Impact of instrumentation in lumbar spinal fusion in elderly patients. Acta Orthop. 2009:445-450.
18. Bae H.W., Zhao L., Kanim L.E., Wong P., Delamarter R.B., Dawson E.G. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine (Phila Pa 1976). 2006;31-12:1299-1306. discussion 307–8
19. Peterson B., Whang P.G., Iglesias R., Wang J.C., Lieberman J.R. Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J. Bone Joint Surg. Am. 2004;86-A-10:2243-2250.
20. An H.S., Simpson J.M., Glover J.M., Stephany J. Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. A prospective multicenter study. Spine (Phila Pa 1976). 1995;20–20:2211-2216.
21. Thalgott J.S., Giuffre J.M., Klezl Z., Timlin M. Anterior lumbar interbody fusion with titanium mesh cages, coralline hydroxyapatite, and demineralized bone matrix as part of a circumferential fusion. Spine J. 2002;2–1:63-69.
22. Bucholz R.W., Carlton A., Holmes R.E. Hydroxyapatite and tricalcium phosphate bone graft substitutes. Orthop. Clin. North Am. 1987;18–2:323-334.
23. Damien C.J., Parsons J.R., Prewett A.B., Huismans F., Shors E.C., Holmes R.E. Effect of demineralized bone matrix on bone growth within a porous HA material: a histologic and histometric study. J. Biomater. Appl. 1995;9–3:275-288.
24. Kania R.E., Meunier A., Hamadouche M., Sedel L., Petite H. Addition of fibrin sealant to ceramic promotes bone repair: long-term study in rabbit femoral defect model. J. Biomed. Mater. Res. 1998;43–1:38-45.
25. Burwell R.G. The function of bone marrow in the incorporation of a bone graft. Clin. Orthop. Relat. Res. 1985;200:125-141.
26. Muschler G.F., Nitto H., Boehm C.A., Easley K.A. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J. Orthop. Res. 2001;19–1:117-125.
27. Bridwell K.H., Anderson P.A., Boden S.D., Vaccaro A.R., Wang J.C. What’s new in spine surgery. J. Bone Joint Surg Am. 2008;90–7:1609-1619.
28. Curylo L.J., Johnstone B., Petersilge C.A., Janicki J.A., Yoo J.U. Augmentation of spinal arthrodesis with autologous bone marrow in a rabbit posterolateral spine fusion model. Spine (Phila Pa 1976). 1999;24-5:434-438. discussion 8–9
29. McLain R.F., Boehm C.A., Rufo-Smith C., Muschler G.F. Transpedicular aspiration of osteoprogenitor cells from the vertebral body: progenitor cell concentrations affected by serial aspiration. Spine J. 2009;9–12:995-1002.
30. Taylor R.S., Taylor R.J., Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine (Phila Pa 1976). 2006;31–23:2747-2755.
31. Lewis G. Injectable bone cements for use in vertebroplasty and kyphoplasty: state-of-the-art review. J. Biomed. Mater. Res. B. Appl. Biomater. 2006;76–2:456-468.
32. Lewis G. Properties of acrylic bone cement: state of the art review. J. Biomed. Mater. Res. 1997;38–2:155-182.
33. Lewis G., Towler M.R., Boyd D., German M.J., Wren A.W., Clarkin O.M., Yates A. Evaluation of two novel aluminum-free, zinc-based glass polyalkenoate cements as alternatives to PMMA bone cement for use in vertebroplasty and balloon kyphoplasty. J. Mater. Sci. Mater. Med. 2009.
34. Blattert T.R., Jestaedt L., Weckbach A. Suitability of a calcium phosphate cement in osteoporotic vertebral body fracture augmentation: a controlled, randomized, clinical trial of balloon kyphoplasty comparing calcium phosphate versus polymethylmethacrylate. Spine (Phila Pa 1976). 2009;34–2:108-114.
35. Hohaus C., Ganey T.M., Minkus Y., Meisel H.J. Cell transplantation in lumbar spine disc degeneration disease. Eur. Spine J. 2008;4(17 Suppl):492-503.
36. Boyd L.M., Carter A.J. Injectable biomaterials and vertebral endplate treatment for repair and regeneration of the intervertebral disc. Eur. Spine J. 2006;3(15 Suppl):S414-S421.