13 The renal system
Acetonuria: The appearance of acetone in the urine. Acetonuria is present when excessive fats are consumed or when an inadequate amount of carbohydrates is metabolized.
Albuminuria: The presence of protein in the urine, also called proteinuria. Albumin is the most common protein found in the urine. This condition usually indicates a malfunction in glomerular filtration.
Anuria: Lack of urine production, less than 50 mL/day.
Azotemia: The presence of nitrogenous products in the blood, usually because of decreased kidney function.
Cystitis: An inflammation of the bladder.
Dysuria: Painful or difficult urination.
Enuresis: Involuntary discharge of urine.
Glomerular Filtration Rate: Volume of fluid filtered through the kidney.
Glycosuria: The presence of glucose in the urine.
Hematuria: The presence of blood in the urine.
Nephritis: Inflammation of the kidney.
Nephrosis: Degeneration of the kidney without the occurrence of inflammation.
Oliguria: A decreased urine formation of less than 500 mL/day or 0.5 mL/kg/h in an adult.
Pyelonephritis: An inflammation of the renal pelvis and calices.
Stricture: An abnormal narrowing; in the urinary tract, a narrowing of the ureter or urethra.
Uremia: The presence of nitrogenous substances in the blood.
Urinary Incontinence: The inability to retain urine in the bladder.
Anatomy of the kidneys
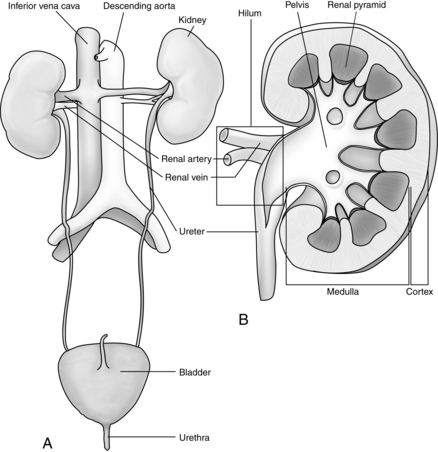
The kidneys are two bean-shaped organs in the retroperitoneal spaces at the level of the twelfth thoracic to third lumbar vertebrae. The right kidney is slightly lower than the left. Each kidney weighs approximately 150 g. The notched portion of the kidney is called the hilum, which is where the ureter, the renal vein, and the renal artery enter the kidney (Fig. 13-1). The kidney is divided into an outer cortex and an inner medulla.
Blood is supplied to each kidney by a renal artery arising from each side of the abdominal aorta. The rate of blood flow through both kidneys of a man who weighs 70 kg is approximately 1200 mL/min, or approximately 21% of the cardiac output. As the renal artery enters the kidney at the hilum, it divides into the interlobar arteries. Branches from the interlobar arteries divide into afferent arteries that supply the capillaries of the nephrons. The capillaries form the efferent arterioles and divide to form the peritubular capillaries that help to supply the nephron. (Figs. 13-2 and 13-3).
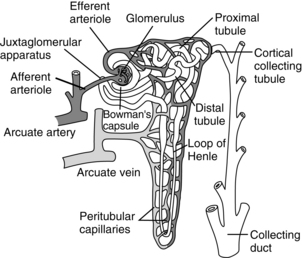
FIG. 13-2 Functional nephron.
(From Hall J: Guyton and Hall textbook of medical physiology, ed 12, Philadelphia, 2011, Saunders.)
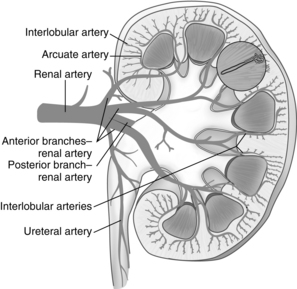
FIG. 13-3 Arterial supply to kidney. Soon after entering the hilum of the kidney, the renal artery divides into several anterior and posterior branches. Branches divide into interlobar arteries, which give off arcuate arteries that course between cortex and medulla.
The nephron is the functional unit of the kidney. Together the kidneys contain approximately 2.4 million closely packed nephrons. Each nephron consists of a glomerulus, a proximal convoluted tubule, a loop of Henle, a distal convoluted tubule, and collecting ducts. The blood enters the afferent arteriole and goes into the glomerulus located in the cortex. The glomerulus is a compact network of capillaries encased in a double-layered capsule, the Bowman capsule. Filtered blood flows out of the glomerulus into the efferent arteriole. The portion of the blood that is filtered drains into the proximal convoluted tubule. The renal tubules begin in the Bowman capsule. The pressure gradient, caused by renal artery blood flow, forces fluid to leave the glomerulus and enter the Bowman capsule. The filtrate flows into the proximal convoluted tubule, which is still in the cortex of the kidney, and then into the loop of Henle. The proximal loop of Henle is thick walled, but becomes thin at the distal segment in the medulla of the kidney. The filtrate then flows into the distal convoluted tubule, located in the cortex of the kidney, and passes into the collecting ducts. The collecting ducts traverse the cortex to the medulla, where they merge into the renal pelvis by way of the renal calyces. In the collecting ducts, the filtrate is termed urine.
Kidney physiology
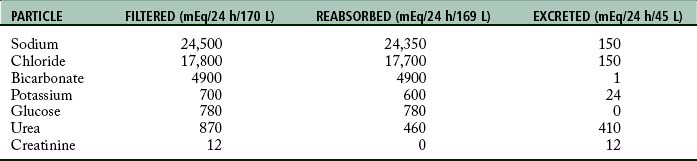
Urine is formed by processes of filtration, reabsorption, and secretion. Filtration occurs as the blood passes through the glomerulus. The force of filtration is a pressure gradient that pushes fluid through the glomerular membrane. Approximately 180 L of water every 24 hours along with other substances is filtered out of plasma by the glomeruli (Table 13-1). Blood cells and heavy particles including proteins are retained in the blood because they are too large to pass through the glomerular epithelium. The presence of red blood cells or protein in the urine usually indicates a pathologic process in the kidney.
Regulation of kidney function
Countercurrent mechanism
The countercurrent mechanism is used by the kidneys to concentrate urine. The vasa recta are special capillaries that, with the loop of Henle, form the countercurrent mechanism. Fluids flow in opposite directions between the ascending and descending loops and sections of the vasa recta. The osmolality or weight of particles in solution of the interstitial fluid increases deeper into the medulla. The increase in osmolality results in active transport of particles or solutes into the interstitial fluid. The countercurrent mechanism is useful when the body needs to excrete a large amount of waste products yet reabsorb the normal amount of solutes and when the water in the body needs to be conserved, while waste products are eliminated.
Hormonal control
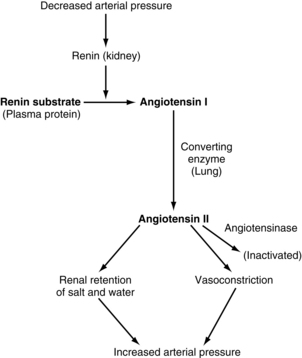
The juxtaglomerular complex is a group of cells, located just before the glomerulus and in close proximity to the distal tubule, which contain granules of inactive renin. Renin is released in response to reduced arterial blood pressure entering the afferent arteriole of the kidney or a low concentration of sodium in the distal tubule. The released renin acts as an enzyme to convert angiotensinogen to angiotensin I. Angiotensin I is converted to angiotension II by the angiotensin-converting enzyme, which leads to angiotensin II. Angiotensin II stimulates the adrenal cortex to release aldosterone. Aldosterone signals the kidney tubules to increase the reabsorption of sodium and retention of water. Because the renin-angiotensin system causes this reabsorption of water and sodium, it plays a role in the control of arterial blood pressure and is important in the conservation of sodium and control of fluid volume in hypotensive states (Fig. 13-4).
Regulation of body homeostasis
The extracellular fluid volume is controlled indirectly by the kidneys as blood volume is controlled. The relative ratio of the extracellular fluid volume to blood volume depends on the physical properties of the circulation and of the interstitial spaces, including compliances and dynamics. The kidney maintains the osmolality of the extracellular fluid mainly by regulating the extracellular sodium concentration. Extracellular sodium controls 90% to 95% of the effective osmotic pressure of extracellular fluid. The extracellular concentration of other electrolytes, including potassium, calcium, magnesium, and phosphate ions, is also under renal control.
Components of urine
The end product of filtration, absorption, and excretion is urine, composed of 95% water and 5% solids. The solids, approximately 60 g/L of urine, are listed in Table 13-2. Urea is derived from the breakdown of amino acids. Uric acid is an end product of purine metabolism, formed from purines ingested as food and from those formed in the body. Creatinine is an end product of creatine, of which 95% is found in muscle tissue. Creatinine is produced during the production of muscle energy.
| CONSTITUENTS | AMOUNT (g/L) |
|---|---|
| Organic | |
| Urea | 20-300 |
| Uric acid | 0.6-0.75 |
| Creatinine | 1.5 |
| Others | 2.6 |
| Inorganic | |
| Sodium chloride | 9.0 |
| Potassium chloride | 2.5 |
| Sulfuric acid | 1.8 |
| Phosphoric acid | 1.8 |
| Ammonia | 0.5-15 |
| Calcium | 0.2 |
| Magnesium | 0.2 |
Acid-base balance
The kidneys correct alkalosis by decreasing the bicarbonate in the extracellular fluid, which occurs because fewer hydrogen ions enter the tubules because of a low carbon dioxide concentration and because a high bicarbonate concentration exists in the tubules. The bicarbonate cannot be reabsorbed without first combining with the hydrogen; therefore the excess bicarbonate ions are lost to the urine as are other positive ions such as sodium and hydrogen. Cellular potassium can exchange with the sodium instead of the cellular hydrogen to conserve the hydrogen, which can help to return the pH to normal limits.
Diuretic therapy
Potassium-sparing diuretics
A second type of potassium-sparing diuretic is also an aldosterone agonist. Spironolactone acts on the aldosterone receptors in the conducting ducts antagonizing the reabsorption of sodium and chloride by effects of aldosterone. When spironolactone is administered, sodium and chloride reabsorption are increased and potassium excretion is decreased. Because of the decrease in potassium excretion in the conducting ducts, hyperkalemia is a serious side effect of the drug. Spironolactone is indicated for patients with fluid overload from cirrhosis of the liver, nephrotic syndrome, and heart failure. The disadvantage of spironolactone is a slow onset of action and slow metabolism, requiring several days for an effect.
Effects of drugs in patients with compromised kidney function
Kidney disease affects approximately 5% of adults in the United States. Patients with end-stage kidney disease exhibit anemia, body fluid shifts, and alterations in blood albumin and electrolyte levels. Patients may be debilitated and deconditioned. If uremia is present, the CNS may be depressed. When the renal impaired patient exhibits CNS depression, the actions of opioids are intensified and prolonged. Diazepam, which has a 24-hour half-life, is also not a good choice because of its additive effect on the CNS. Drugs that are not metabolized in the body but are typically excreted unchanged by the kidneys are avoided in patients with renal disease. Such drugs include long-acting barbiturates such as barbital and phenobarbital, skeletal muscle relaxants decamethonium and gallamine, and cardiac glycosides digoxin and lanatoside C. Because half the administered dose of the belladonna alkaloids, atropine and hyoscyamine, are excreted unchanged, the dosage of these drugs should be modified by the degree of renal impairment.
Acute kidney failure
A sudden decrease in renal function is the hallmark of acute kidney failure. Acute kidney failure will occur in 1 of 100 surgical patients. Patient risk factors include gender (male), age, and diabetes. Additional risk factors include a history of heart failure, hypertension, ascites or preoperative renal insufficiency.1 Emergency surgery increases the risk of acute kidney failure by 100%, whereas intraperitoneal surgery increases risk by a factor of 3. Acute kidney failure can be due to prerenal, intrarenal, or postrenal causes.
Assessment of kidney function
Urine output, usually associated with adequate renal function, is also not a reliable marker of kidney injury. Anuria can be a sign of severe kidney dysfunction, unless the cause is postrenal obstruction. Anuria always requires further assessment. The PACU nurse is more likely to detect persistent oliguria, less than 0.5 mL/kg/h or 25 mL of urine per hour for more than 2 hours. Low urine output can have numerous causes, but constitutes a medical emergency with the surgeon notified immediately.
Preexisiting kidney failure
Patients with stage 4 or stage 5 kidney disease require creation of an arteriovenous fistula or placement of a temporary vascular shunt to allow hemodialysis. Any patient with CKD can require surgery though their morbidity and mortality risk is substantially increased. In the postoperative period they can be hemodynamically labile. Venous access is difficult because of preexisting vascular disease and the presence of a fistula or shunt. An intravenous catheter is never placed in the same extremity as the fistula or shunt; a blood pressure cuff is never placed above, on, or below the fistula or shunt. Patients with ESRD are scheduled to receive dialysis the day before surgery to optimize volume and electrolyte status. Intravenous fluids are minimized during minor surgery.2 If extensive surgery is planned, intraoperative dialysis may be required. Patients with ESRD have anemia and coagulation abnormalities because of a lack of erythropoietin and altered platelet function. Bleeding during the PACU stay can be a surgical emergency.
Summary
A review of the complex anatomy and physiology of the renal system was presented to demonstrate the role the kidney plays in regulating and controlling body systems. Pharmacologic agents administered during the perioperative period must be carefully monitored to avoid kidney damage. Maintaining blood volume and renal perfusion is the key to preserving kidney function. The PACU nurse must be aware of the patient’s fluid volume status and carefully monitor the adequacy of urine output. Patients with chronic kidney failure present additional challenges for post anesthesia recovery. Care of the patient requiring genitourinary surgery including renal transplantation is presented in Chapter 41.
1. Kheterpal S, et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology.2009;110:505–515.
2. Wagener G, Brentjens T. Anesthetic concerns in patients presenting with renal failure. Anesthesiol Clin. 2010;28:39–54.
Aitkenhead A, et al. Textbook of anaesthesia, ed 5. Philadelphia: Churchill Livingstone; 2007.
Atlee J. Complications in anesthesia, ed 2. Philadelphia: Saunders; 2007.
Barash P, et al. Clinical anesthesia, ed 6. Philadelphia: Lippincott Williams & Wilkins; 2009.
Brunton L, et al. Goodman and Gilman’s the pharmacological basis of therapeutics, ed 12. New York: McGraw-Hill; 2011.
Miller R, et al. Miller’s anesthesia, ed 7, Philadelphia: Churchill Livingstone, 2010.
Porth C. Essentials of pathophysiology, ed 3. Philadelphia: Wolters Kluwer; 2011.
Stoelting R, Miller R. Basics of anesthesia, ed 5. Philadelphia: Churchill Livingstone; 2007.