21
The Practical Conduct of Anaesthesia
The conduct of anaesthesia is planned after details concerning the surgical procedure and the medical condition of the patient have been obtained at the preoperative visit. Preoperative assessment and selection of appropriate premedication are discussed in Chapter 17.
PREPARATION FOR ANAESTHESIA
The anaesthetic machine must be tested before use for leaks, misconnections and proper function. A checklist, e.g. that published by the Association of Anaesthetists of Great Britain and Ireland (AAGBI 2004), is recommended. This is discussed in Chapter 20. The breathing system to be used should be new for each patient, or a new filter of appropriate size for each patient should be placed between the patient and the system, according to the AAGBI recommendations (2008).
The availability and function of all anaesthetic equipment should be checked before starting (see Table 21.1). The anaesthetist should be satisfied that the correct operation is being performed upon the correct patient and that consent has been given. Surgical Safety checklists are available for all the theatre team. The patient must be on a tilting bed or trolley and the anaesthetist should have a competent, trained assistant.
TABLE 21.1
Equipment Required for Tracheal Intubation
Correct size of laryngoscope and spare (in case of light failure)
Tracheal tube of correct size + an alternative smaller size
Tracheal tube connector
Wire stilette
Gum elastic bougies
Magill forceps
Cuff-inflating syringe
Artery forceps
Securing tape or bandage
Catheter mount(s)
Local anaesthetic spray – 4% lidocaine
Cocaine spray/gel for nasal intubation
Tracheal tube lubricant
Throat packs
Anaesthetic breathing system and face masks – tested with O2 to ensure no leaks present
INDUCTION OF ANAESTHESIA
Anaesthesia is induced using one of the following techniques:
Inhalational Induction
The most common indications for inhalational induction of anaesthesia are listed in Table 21.2.
TABLE 21.2
Indications for Inhalational Induction
Young children
Upper airway obstruction, e.g. epiglottitis
Lower airway obstruction with foreign body
Bronchopleural fistula or empyema
No accessible veins
Intravenous Induction
Doses of the common i.v. agents are shown in Table 21.3. The induction dose varies with the patient’s weight, age, state of nutrition, circulatory status, pre-medication and any concurrent medication. A small test dose is commonly administered and its effects are observed. Slow injection is recommended in the aged and in those with a slow circulation time (e.g. shock, hypovolaemia, cardiovascular disease) while the effects of the drug on the cardiovascular and respiratory systems are assessed.
TABLE 21.3
| Agent | Induction Dose (mg kg–1) |
| Thiopental | 3–5 |
| Etomidate | 0.3 |
| Propofol | 1.5–2.5 |
| Ketamine | 2 |
A rapid-sequence induction technique is indicated for patients undergoing emergency surgery and for those with potential for vomiting or regurgitation. After i.v. induction, a rapid transition to stage 3 anaesthesia (see below) is achieved; this is maintained by the introduction of an inhalational agent or by repeated bolus injections or a continuous infusion of an i.v. anaesthetic agent. Emergency anaesthesia is discussed fully in Chapter 37.
Complications and Difficulties
Histamine release. Thiopental in particular may cause release of histamine with subsequent formation of typical wheals. Severe reactions may occur to individual agents, and appropriate drugs and fluids should be available in the anaesthetic room for treatment. Guidelines for emergency management of acute major anaphylaxis are available (AAGBI) and may be displayed in the anaesthetic room. This is discussed further in Chapter 43.
POSITION OF PATIENT FOR SURGERY
Some commonly used positions are shown in Figure 21.1. Each may have adverse effects in terms of skeletal, neurological, ventilatory and circulatory effects.
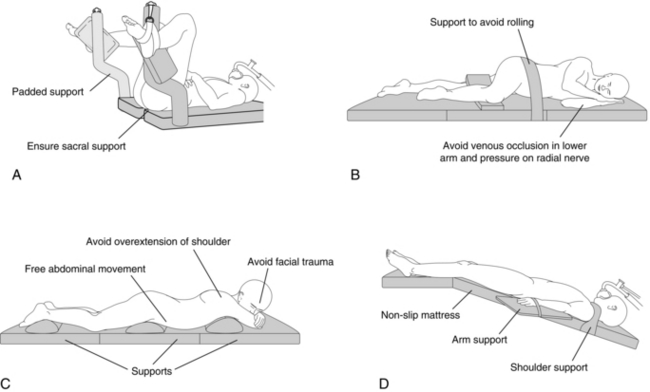
FIGURE 21.1 Positions on the operating table. (A) Lithotomy position. (B) Lateral position. (C) Prone position. (D) Trendelenburg position.
The supine position carries the risk of the supine hypotensive syndrome during pregnancy (see Ch 35) or in patients with a large abdominal mass.
Positioning during anaesthesia is discussed extensively by Martin & Warner (1997).
MAINTENANCE OF ANAESTHESIA
Inhalational Anaesthesia with Spontaneous Ventilation
Minimum Alveolar Concentration
Minimum alveolar concentration (MAC) is the minimum alveolar concentration of an inhaled anaesthetic agent which prevents reflex movement in response to surgical incision in 50% of subjects. MAC values of commonly used inhalational agents are shown in Appendix C. MAC varies little with metabolic factors but is reduced by opioid medication and in the presence of hypothermia. MAC is higher in neonates and is reduced in the elderly (see Ch 2). The effects of inhalational anaesthetics are additive: thus 1 MAC-equivalent could be achieved by producing an alveolar concentration of 70% nitrous oxide (0.67 MAC) and 0.4% isoflurane (0.33 MAC).
The rate at which MAC is attained may be increased by raising the inspired concentration and by avoidance of airway obstruction. Increasing ventilation at a constant inspired concentration produces more rapid equilibration between inspired and alveolar concentrations. The time taken for equilibration increases with the blood/gas solubility coefficient of the agent; those with a high blood/gas solubility coefficient (e.g. halothane) do not reach equilibrium for several hours (see Ch 2). It follows, therefore, that the inspired concentration must be considerably higher than MAC to produce an adequate alveolar concentration when such agents are used.
Signs of Anaesthesia
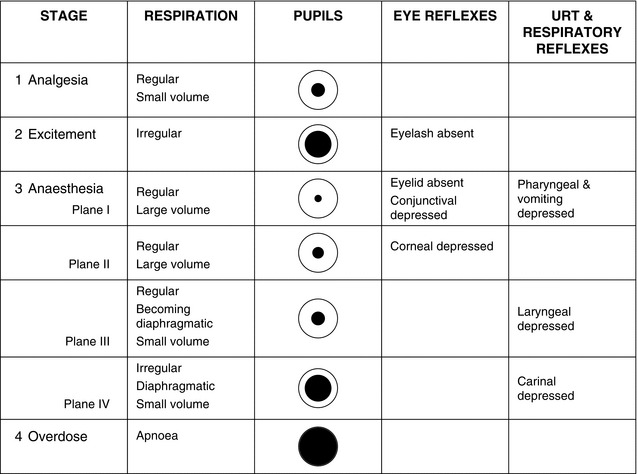
Guedel’s classic signs of anaesthesia are those seen in patients premedicated with morphine and atropine and breathing ether in air. The clinical signs associated with anaesthesia produced by other inhalational agents follow a similar course, but the divisions between the stages and planes are less precise (Fig. 21.2).
Stage 1: the stage of analgesia. This is the stage attained when using nitrous oxide 50% in oxygen, as used in the technique of relative analgesia (see Ch 29).
Complications and Difficulties
Malignant hyperthermia. Volatile agents, succinylcholine or amide-type local anaesthetic agents may trigger this syndrome in susceptible individuals (see Ch 43).
Atmospheric pollution. The use of the appropriate scavenging apparatus helps to reduce levels of theatre pollution by volatile and gaseous agents (see Ch 20).
Delivery of Inhalational Agents – Airway Maintenance
Use of the Face Mask: Inhalational anaesthesia usually involves the use of a face mask. The face mask has many variants of type and size, and selection of the correct fit is important to provide a gas-tight seal.
Use of the Laryngeal Mask Airway and Other Supraglottic Airway Devices:
 A patient with a ‘full stomach’ or with any condition leading to delayed gastric emptying.
A patient with a ‘full stomach’ or with any condition leading to delayed gastric emptying.
 A patient in whom the risk of regurgitation of gastric contents into the oesophagus is increased (e.g. hiatus hernia).
A patient in whom the risk of regurgitation of gastric contents into the oesophagus is increased (e.g. hiatus hernia).
 Where surgical access (e.g. to the pharynx) is impeded by the cuff of the LMA.
Where surgical access (e.g. to the pharynx) is impeded by the cuff of the LMA.
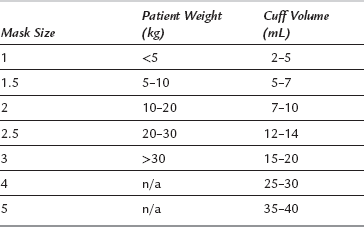
Conduct of LMA insertion. An appropriate depth of anaesthesia is required for successful insertion of the LMA. Fewer difficulties are encountered after i.v. induction of anaesthesia with propofol than with thiopental because of the greater tendency of the former to suppress pharyngeal reflexes. The appropriate size of LMA is chosen according to the weight of the patient (Table 21.4). In general, the largest size possible is used to create a seal with a cuff inflation less than the maximum. In adults, the larger sizes are used according to inspection. The patient’s head is extended, the mouth is opened and, if necessary, the mandible can be held down by an assistant. The LMA cuff is evacuated and the LMA is inserted into the pharynx in a direction along the axis of the hard palate so that the cuff encounters the posterior pharyngeal wall and is swept distally into the laryngopharynx. This may be assisted by use of the gloved fingers in the ‘classic’ technique. The cuff then lies posterior to the larynx. Air is injected into the cuff and the breathing system is attached via a catheter mount to the 22-mm proximal connector. The LMA is secured in place with tape or a bandage after confirmation of correct placement by observation of movement of the reservoir bag, or of the chest after a gentle manual inflation of the lungs. The reinforced LMA may be useful when the standard LMA may hinder surgical access or be prone to kinking.
Alternative SADs comprise the Igel, Pro-seal LMA, supreme LMA (SLMA), and the intubating LMA (ILMA) which may be used to facilitate tracheal intubation. These are described in Chapter 15. The anaesthetist should gain clinical experience with these types in order to appreciate the differences in insertion technique from that of the classic LMA.
Tracheal Intubation
 Provision of a clear airway, e.g. anticipated difficulty in using mask anaesthesia in the edentulous patient.
Provision of a clear airway, e.g. anticipated difficulty in using mask anaesthesia in the edentulous patient.
 An ‘unusual’ and prolonged position, e.g. prone or sitting. A reinforced non-kinking tube may be necessary.
An ‘unusual’ and prolonged position, e.g. prone or sitting. A reinforced non-kinking tube may be necessary.
 Operations on the head and neck, e.g. ENT, dental. A nasotracheal tube may be required.
Operations on the head and neck, e.g. ENT, dental. A nasotracheal tube may be required.
 Protection of the respiratory tract, e.g. from blood during upper respiratory tract or oral surgery and from inhalation of gastric contents in emergency surgery or patients with oesophageal obstruction. The use of a cuffed tube for adults is mandatory in these circumstances.
Protection of the respiratory tract, e.g. from blood during upper respiratory tract or oral surgery and from inhalation of gastric contents in emergency surgery or patients with oesophageal obstruction. The use of a cuffed tube for adults is mandatory in these circumstances.
 During anaesthesia using IPPV and muscle relaxants.
During anaesthesia using IPPV and muscle relaxants.
Preparation
Before starting, the anaesthetist must check the availability and function of the necessary equipment. He or she should have a ‘dedicated’, trained, experienced assistant. Laryngoscopes of the correct size are chosen and the function of bulb and batteries checked, the patency of the tracheal tube is checked and the integrity of the cuff ensured. Various aids to intubation must also be present (see Table 21.1).
Choice of Equipment
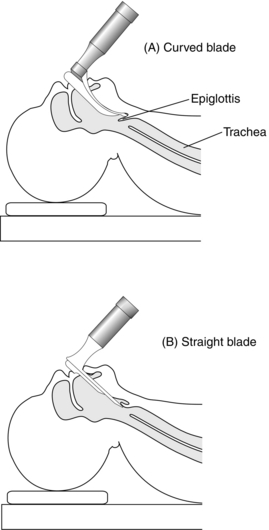
Laryngoscopes: Laryngoscopes are manufactured in many shapes and sizes. There are two basic types of blade – straight or curved. Straight-blade laryngoscopes (e.g. Magill) are favoured for children, in whom the epiglottis is floppy, and are designed to pass posterior to the epiglottis and to lift it anteriorly, exposing the larynx. The curved blade (e.g. Macintosh) is designed so that the tip lies anterior to the epiglottis in the vallecula, pressing on the hyoepiglottic ligament and moving it anteriorly to expose the larynx and vocal cords (Fig. 21.3). The McCoy blade incorporates a movable distal tip to facilitate a view of the glottis in appropriate patients.
Tracheal Tubes: Modern tracheal tubes are disposable and made from PVC which is ‘implant tested’ for its inert effect upon the tissues. In some circumstances, e.g. head and neck or throat surgery, the tracheal tube may be subject to direct or indirect pressure and standard tubes may kink or become compressed. It may be appropriate to use a tube which is reinforced with a nylon or steel spiral in such cases. Tracheal tubes are introduced usually through the mouth, although it may be preferable to pass the tube through the nose, particularly for oral surgery. The supplied length of disposable tubes exceeds that required normally for oral intubation and the tube should be cut to the appropriate length before use. During thoracic surgery, it may be necessary to ventilate the lungs independently and a bronchial tube, bronchial blocker or double-lumen tube is required (see Ch 33).
Anaesthesia for Tracheal Intubation
Inhalational Technique for Intubation: Adequate depth of anaesthesia is necessary to depress the laryngeal reflexes and provide a degree of relaxation of the laryngeal and pharyngeal muscles. Sevoflurane 8% provides rapid attainment of the necessary depth, which can be judged from the pattern of respiration with predominance of diaphragmatic breathing (a useful sign in children is the ‘dissociation’ of the thoracic and abdominal excursion). The mask is removed and laryngoscopy and intubation performed. The anaesthetic circuit is then connected to the tracheal tube and anaesthesia maintained at a depth appropriate for surgery.
Relaxant Anaesthesia for Intubation: After i.v. or inhalational induction of anaesthesia, the short-acting depolarizing muscle relaxant succinylcholine may be used to provide relaxation for tracheal intubation. After loss of consciousness, the patient breathes 100% oxygen or 50% nitrous oxide in oxygen and succinylcholine is administered in a dose of 1–1.5 mg kg–1. Assisted ventilation is maintained via the face mask until muscle relaxation occurs (except in emergency patients and those likely to regurgitate) and laryngoscopy and intubation are performed. Inhalational anaesthesia may be continued with manual ventilation until the effects of the relaxant have ceased, whereupon spontaneous ventilation is resumed. Alternatively, non-depolarizing neuromuscular blockade is produced and ventilation controlled.
Conduct of Laryngoscopy
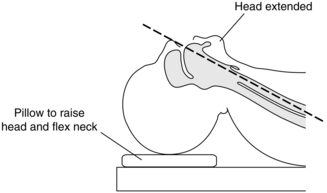
The position of the patient’s head and neck is important. The neck should be flexed and the head extended with the support of a pillow; thus, the oral, pharyngeal and tracheal axes are brought into alignment (Fig. 21.4). The laryngoscope is designed for left-hand use and is introduced into the right side of the mouth while the right hand opens the mouth, parting the lips to avoid interposing them between laryngoscope and teeth. The teeth may be protected from blade trauma with the fingers or the use of a plastic ‘guard’. The laryngoscope blade deflects the tongue to the left and the length of the blade is passed over the contour of the tongue. The laryngoscope is lifted upwards and forwards, avoiding a levering movement which can damage the upper anterior teeth. Using a straight blade, the tip is passed posterior to the epiglottis, which is lifted anteriorly, and the vocal cords are seen. With a curved blade, the tip is inserted into the vallecula and pressure on the hyoepiglottic ligament moves the epiglottis to expose the vocal cords. External pressure on the thyroid cartilage by an assistant may aid laryngeal vision at this stage. Alternatively, using the McCoy adaptation of the Macintosh blade, the distal lever may be used to elevate the epiglottis to assist in viewing the larynx.
Conduct of Intubation
The tube cuff is inflated sufficiently to abolish audible gas leaks on inflation of the lungs. The correct position of the tube must now be confirmed. If the tube has been seen clearly at laryngoscopy to pass through the vocal cords into the trachea, then equal movement of both sides of the chest during ventilation should be confirmed and auscultation in each axilla for breath sounds should be performed to ensure that the tip of the tracheal tube has not passed too far distally to enter, or occlude, one of the main bronchi (see Ch 43); if there is unilateral air entry, the tube should be withdrawn slowly and carefully until air entry is equal in both lungs. If the tube has not been seen clearly to enter the trachea, or if there is any reason to suspect that its distal end is not in the trachea, then the steps outlined in Chapter 43 must be undertaken immediately to identify possible oesophageal intubation.
Difficult Intubation
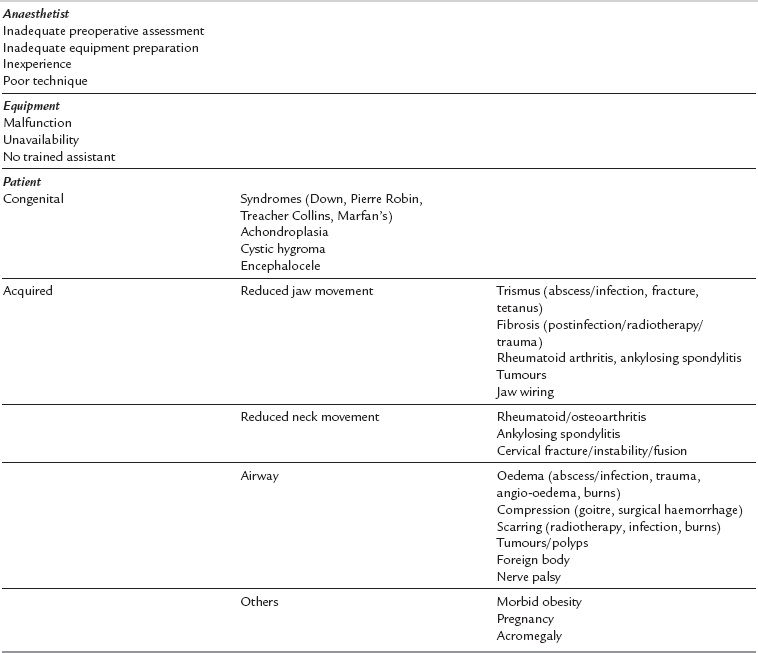
Aetiology: Table 21.5 shows the common causes of difficult intubation. The single most important cause is an inexperienced or inadequately prepared anaesthetist, often complicated by equipment malfunction. There are numerous causes of difficult laryngoscopy related to patient factors. The anatomical features associated with difficult laryngoscopy are listed in Table 21.6. Of these, the atlanto-occipital distance is the best predictor of difficulty but requires an X-ray examination. Many of these factors are normal anatomical variations; they may also be congenital or acquired.
TABLE 21.6
Anatomical Factors Associated with Difficult Laryngoscopy
Short, muscular neck
Protruding incisors (buck teeth)
Long, high arched palate
Receding lower jaw
Poor mobility of mandible
Increased anterior depth of mandible
Increased posterior depth of mandible (reduces jaw opening, requires X-ray)
Decreased atlanto-occipital distance (reduces neck extension, requires X-ray)
Management: Preoperative assessment. Preoperative examination of the airway (Table 21.7) is essential. Identifying patients with a potentially difficult airway (see Tables 21.5 and 21.6) allows time for planning an appropriate anaesthetic technique. Previous anaesthetic records should always be consulted. However, a past record of normal tracheal intubation is no guarantee for future anaesthesia as airway anatomy may be altered. Pregnancy is a common example. The presence of stridor or a hoarse voice is a warning sign for the anaesthetist. As it is impossible to identify all patients with a difficult airway during preoperative assessment, the anaesthetist must be prepared to manage the unexpected difficult laryngoscopy.
TABLE 21.7
Preoperative Assessment of the Airway
General appearance of neck, face, maxilla and mandible
Jaw movement
Head extension and neck movement
Teeth and oropharynx
Soft tissue of neck
Recent chest and cervical spine X-rays
Previous anaesthetic records
Many additional clinical tests to predict difficult laryngoscopy have been described. None of these tests is totally reliable, but their use may complement routine examination of the airway. The ‘Mallampati’ test is a widely used and simple classification of the pharyngeal view obtained during maximal mouth opening and tongue protrusion (Fig. 21.5). In practice, this test suggests a higher incidence of difficult laryngoscopy if the posterior pharyngeal wall is not seen. The predictive value of this test may be strengthened if the thyromental distance (thyroid cartilage prominence to the bony point of the chin during full head extension) is less than 6.5 cm. The Mallampati classification correlates with the view obtained at laryngoscopy (Fig. 21.6). The difficulty associated with a ‘grade 3’ laryngoscopy may usually be overcome by posterior laryngeal displacement and/or the use of a suitable bougie. A patient whose epiglottis is not visible at laryngoscopy (‘grade 4’) usually has obvious preoperative anatomical abnormalities. Management of these patients requires the use of special techniques such as fibreoptic laryngoscopy (see Ch 15).
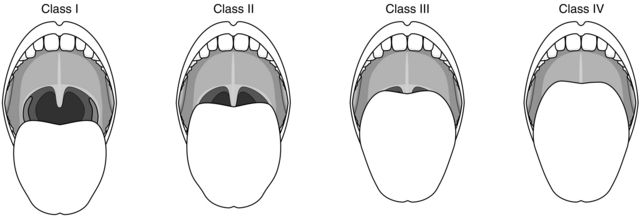
FIGURE 21.5 Classification of the pharyngeal view when performing the Mallampati test. The patient must fully extend the tongue during maximal mouth opening. Class I: pharyngeal pillars, soft palate, uvula visible. Class II: only soft palate, uvula visible. Class III: only soft palate visible. Class IV: soft palate not visible.
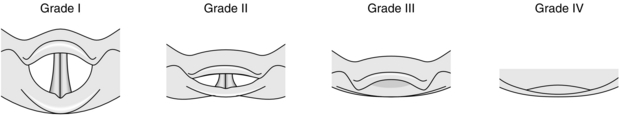
FIGURE 21.6 Grading of the laryngoscopic view. Grade I: vocal cords visible. Grade II: arytenoid cartilages and posterior part of vocal cords visible. Grade III: epiglottis visible. Grade IV: epiglottis not visible. Note: the pharyngeal view (Fig. 21.5) is a clinical guide to the likely laryngoscopic view.
1. Patients with an increased risk of regurgitation and aspiration (e.g. full stomach, intra-abdominal pathology, pregnancy). An inhalational induction is inappropriate in these patients. Regional anaesthesia is preferable in the parturient (see Ch 35). Preoxygenation and a rapid sequence induction with succinylcholine can be used if there is little anticipated difficulty. If intubation is unsuccessful, no further doses of neuromuscular blocking drug should be used, the patient allowed to wake and further assistance sought. If there is a high degree of anticipated difficulty, an awake technique is recommended (see below).
2. Patients with little anticipated difficulty and no airway obstruction (e.g. mild reduction of jaw or neck movement). After a sleep dose of intravenous induction agent and confirmation of the ability to ventilate the lungs manually by mask, succinylcholine may be given to provide the best conditions for tracheal intubation. If difficulty is encountered, the patient is allowed to wake up and the procedure replanned. Where appropriate, anaesthesia is deepened by spontaneous ventilation using a volatile agent and alternative techniques to facilitate tracheal intubation used (see below).
3. Patients with severe anticipated difficulty and no airway obstruction (e.g. severe reduction of jaw or neck movement). Appropriate techniques include inhalational induction with sevoflurane or the use of fibreoptic laryngoscopy either in the awake patient or after inhalational induction. Neuromuscular blocking drugs must not be used until the ability to ventilate the lungs manually and view the vocal cords is confirmed.
4. Patients with airway obstruction (e.g. burns, infection, trauma). An inhalational induction may be used; otherwise an awake technique should be considered. Neuromuscular blocking drugs should not be used until tracheal intubation is confirmed.
5. Extreme clinical situations. Tracheostomy performed under local anaesthesia may be the safest technique.
Inhalational Induction: Premedication with an antisialagogue is desirable. Depth of anaesthesia is increased carefully by spontaneous ventilation of increasing concentrations of a volatile agent in 100% oxygen until laryngoscopy may be performed safely. Halothane may still be the agent of choice for this purpose. If the larynx is viewed easily intubation may be performed with or without succinylcholine. If the view is limited, the use of a suitable bougie assists passage of the tracheal tube through the larynx. This is confirmed by detecting tracheal rings or resistance when the smaller bronchi are encountered. The tracheal tube is then ‘railroaded’ over the bougie into the trachea, often made easier by rotating the tracheal tube through 90° in an anticlockwise direction to align the bevel as it passes through the larynx. If this is unsuccessful, anaesthesia may be maintained and the use of fibre-optic laryngoscopy can be considered.
Awake Intubation: Fibreoptic laryngoscopy and intubation require special equipment, skill and time. The procedure may be performed by the nasal or oral route after topical anaesthesia is achieved by spraying the nasal and oropharyngeal mucosa and/or gargling viscous preparations. The injection of 3–5 mL of lidocaine 2% through the cricothyroid membrane induces coughing and anaesthetizes the tracheal and laryngeal mucosa. Conventional laryngoscopy may also be performed in awake patients. After cricothyroid injection of lidocaine, laryngoscopy is performed in stages. The oropharynx is anaesthetized progressively with lidocaine spray until the patient tolerates deep insertion of the laryngoscope, enabling the larynx to be viewed.
Complications of Tracheal Intubation
Complications may be mechanical, respiratory or cardiovascular and may occur early or late.
Early Complications: Trauma may occur to lips and teeth or dental crowns. Jaw dislocation and dislocation of arytenoids may be produced. Trauma during intubation may result in damage to larynx and vocal cords. Nasal intubation may produce epistaxis, trauma to the pharyngeal wall or dislodgement of adenoid tissue. Obstruction or kinking of the tube may occur and carinal stimulation or bronchial intubation may take place if the tube is too long. Laryngeal trauma may produce postoperative croup, bronchospasm or laryngospasm, especially in children. Mechanical complications may be avoided with a careful technique. Broken teeth must be retrieved and the event documented. Immediate postoperative respiratory complications may be minimized by humidification of inspired gases. Cardiovascular complications of intubation include arrhythmias and hypertension, especially in untreated hypertensive patients.
Late Complications: These are more common after long-term intubation. Tracheal stenosis is rare, but damage to tracheal mucosa from a cuffed tube may be related to its design; high-volume, low-pressure cuffs may be preferred for long-term intubation. Trauma to vocal cords may result in ulceration or granulomata which may require surgical removal. Cord trauma may be more common in the presence of an upper respiratory tract infection.
Anaesthesia Using Neuromuscular Blocking Drugs
Conduct of Relaxant Anaesthesia
After induction of anaesthesia, neuromuscular blockade is produced by using either (a) a depolarizing neuromuscular blocker (NMB) (succinylcholine) followed, after its action has subsided, by a non-depolarizing NMB, or (b) in the case of an elective fasting patient with normal gastric emptying and no history of hiatus hernia or regurgitation, an intubating dose of a non-depolarizing NMB (see Ch 6). The choice of agent depends upon operative indications or the patient’s condition (e.g. vecuronium and rocuronium produce little cardiovascular depression). The airway is then secured with a tracheal tube.
Controlled ventilation is commenced, first manually by compression of the reservoir bag and then by a mechanical ventilator delivering the appropriate tidal and minute volumes (see Appendix C). Anaesthesia and analgesia are provided by nitrous oxide/oxygen or air/oxygen, together with a volatile agent and i.v. analgesic. The inspired and end-expired concentrations of volatile agents should be monitored. Analgesia may also be supplemented by opioid pre-medication or by use of regional or local anaesthetic techniques.
Assessment of Relaxant Anaesthesia: Light anaesthesia with preservation of reflexes permits the use of physical signs for the continued assessment of the adequacy of anaesthesia.
Adequacy of muscle relaxation. Clinical signs of return of muscle tone include retraction of the wound edges during abdominal operations and abdominal muscle, diaphragmatic or facial movement. An increase in airway pressure (with a time- or volume-cycled ventilator) may indicate a return of muscle tone. Quantitative estimation of neuromuscular status may be obtained with a peripheral nerve stimulator (see Ch 6). Small increments (e.g. 25–35% of the original dose) of NMB may be given to maintain relaxation; alternatively, an i.v. infusion may be a more convenient method of administration, but the use of a peripheral nerve stimulator is mandatory with this technique.
CONDUCT OF EXTUBATION
This may take place with the patient supine if the anaesthetist is satisfied that airway patency can be maintained by the patient in this position and there is no risk of regurgitation. In patients at risk of regurgitation and potential aspiration, the lateral position is preferred. However, it is safer to use the lateral recovery position after extubation (Fig. 21.7). Return of respiratory reflexes is signified by coughing and resistance to the presence of the tracheal tube.
EMERGENCE AND RECOVERY
After completion of surgery, anaesthetic agents are withdrawn and oxygen 100% is delivered. Following removal of the tracheal tube or LMA, the patient’s airway is supported until respiratory reflexes are intact. The patient’s muscle power and coordination are assessed by testing hand grip, tongue protrusion or a sustained head lift from the pillow in response to command. Adequacy of neuromuscular transmission may also be assessed before the patient is conscious (see Ch 6). Return of adequate muscle power must be ensured before the patient leaves theatre. Full monitoring of the patient should not be discontinued before recovery of consciousness.
The patient is then ready for transfer from the operating table to a bed or trolley. Oxygen is delivered by face mask during transport, and further recovery takes place in a recovery area of theatre or in the recovery ward (see Ch 40).
The lateral recovery position (see Fig. 21.7) is adopted unless the anaesthetist is satisfied that this is unnecessary. The patient is turned on one side, upper leg flexed and lower extended; the head is on one side and the tongue falls forward under gravity, thus avoiding airway obstruction.
REFERENCES AND FURTHER READING
AAGBI (Association of Anaesthetists of Great Britain and Ireland). Suspected anaphylactic reactions associated with anaesthesia. London: AAGBI; 2003.
AAGBI (Association of Anaesthetists of Great Britain and Ireland). Checking anaesthetic equipment. London: AAGBI; 2004.
AAGBI (Association of Anaesthetists of Great Britain and Ireland). Infection control in Anaesthesia 2. London: AAGBI; 2008.
Ahmed, I., Russell, W. Jaw thrust: are we applying it correctly? Pediatr. Anesth. 2010;20:107–108.
Brimacombe, J.R., Brain, A.I.J., Berry, A.M. The laryngeal mask airway instruction manual, third ed. Pangbourne: Intavent; 1996.
Department of Health. Protecting the breathing circuit in anaesthesia – Report of an expert group on blocked anaesthetic tubing. http://webarchive.nationalarchives.gov.uk/20130107105354/, http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4081825, 2004.
Henderson, J.J., Popat, M.T., Latto, I.P., Pearce, A.C. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia. 2004;59:675–694.
Latto, I.P., Vaughan, S. Difficulties in tracheal intubation, second ed. London: WB Saunders; 1997.
Martin, J.T., Warner, M.A. Positioning in anesthesia and surgery, third ed. Philadelphia: WB Saunders; 1997.
WHO Surgical Safety Checklist. http://www.nrls.npsa.nhs.uk/resources/clinical-specialty/surgery/?entryid45=59860