The postpartum period and lactation physiology
The postpartum period is a time of restoration and return to the nonpregnant state. This period is generally defined as the 6- to 8-week postpartum period from the delivery of the placenta to the involution and return of the reproductive organs to their nonpregnant state. The postpartum period is characterized by significant anatomic, physiologic, and endocrinologic changes related to the involution and lactation processes. It is also a time of major psychologic and social change as the new mother bonds with her infant, assumes responsibility for, and incorporates her infant into the family system. The focus of this chapter is on the physiologic and endocrinologic changes associated with return of the reproductive system to its nonpregnant state and with the anatomic, physiologic, and endocrinologic changes associated with initiation and maintenance of lactation. The physiologic and anatomic changes within other body systems as they return to the nonpregnant state are described in Chapters 8 to 20.
Involution of the reproductive organs
Uterus
Immediately following delivery of the placenta, the uterus weighs approximately 1000 g and lies with its anterior and posterior walls in close approximation in midline about halfway between the umbilicus and symphysis pubis. Over the next 12 hours the fundus of the uterus is located approximately at the level of the umbilicus. Involution of the uterus involves uterine contraction, autolysis of myometrial cells, and epithelial regeneration and proliferation. The height of the fundus continues to decrease by about 1 cm per day so that by 3 days the fundus lies 2 to 3 fingerbreadths below the umbilicus (or slightly higher in multiparous women). By 1 week, the uterus weighs about 500 g and is 4 to 5 fingerbreadths below the umbilicus. By 2 weeks, the uterus weighs about 300 g and has descended into the true pelvis and the fundus can no longer be palpated abdominally. The size of the uterus gradually decreases over the next month so that by 6 weeks the uterus has nearly returned to its nonpregnant location and size.69 Immediately following delivery of the infant, contractions of the uterine myometrium compress the blood vessels supplying the placental site, causing hemostasis and separation of the placenta from the uterine wall, leaving the basal portion of the decidua. Postpartum contractions (“afterpains”) during the first few days of postpartum may be strong, especially with increasing parity, gradually diminishing in intensity and frequency within the first week. Infant suckling also stimulates oxytocin release resulting in uterine contractions.17
Subinvolution, slowed, delayed, and/or incomplete involution, is usually due to a lack of effective uterine contraction related to various causes including uterine atony (most common), retained placental fragments, uterine inversion, lacerations, or infection, which are usually responsive to early diagnosis and treatment.17 There has been research indicating that uterine massage after placental delivery may prevent postpartum hemorrhage.31 Signs and symptoms of infection may include fever, abdominal tenderness, and foul-smelling lochia. Signs and symptoms of hemorrhage include tachycardia, low blood pressure, boggy uterus, and excessive uterine bleeding with clots, consisting of a blood loss over 500 mL post-vaginal delivery and 1000 mL or more post-cesarean delivery. Postpartum hemorrhage is most commonly associated with coagulation disorders, infection, and subinvolution and its causes, as well as other risk factors including retained placenta, failure to progress in the second stage of labor, placenta accrete, lacerations, instrumental delivery, large for gestational age infant, hypertensive disorders, induction of labor, and oxytocin-augmented labor.72 Assessment using the “four Ts” (tone of the uterus; trauma including lacerations, inversion, and rupture; tissue retention or invasion; and thrombin and coagulation disorders), along with prevention and early intervention are crucial. Management of uterine atony and hemorrhage is aimed at increasing uterine contractility by attempting bimanual uterine compression massage, and uterotonic medications such as uterotonic oxytocin, misoprostol, methylergonovine maleate, and other prostaglandins.5,83 If uterine atony and hemorrhage persist, uterine suture compression or surgical intervention may be necessary.17,37
Regeneration of the uterine epithelial lining begins 2 to 3 days postpartum with differentiation of the remaining decidua into two layers, a superficial layer and a basal layer. The superficial layer of granulation tissue, which provides a barrier to infection, is formed as leukocytes invade the remaining decidua. This layer gradually degenerates, becoming necrotic, and is sloughed off in lochia. The basal layer, containing residual endometrial glands, remains intact and contributes to the new endometrium. By about 2 to 3 weeks, the endometrium has been restored and is similar to the nonpregnant endometrium in the proliferative phase of the menstrual cycle except for remnants of hyalinized decidua with areas of leukocyte infiltration.69 Healing at the placental site takes longer with regeneration occurring gradually over 6 weeks. Following delivery, the placental site is a rough area 4 to 5 cm in diameter containing many thrombosed vessels. The large blood vessels that supplied the intervillous spaces are invaded by fibroblasts and their lumen is obscured. Some of these vessels recanalize later with smaller lumens. The placental site heals by decidual sloughing and exfoliation and by growth of endometrial tissue.69
Lochia
The process of involution and restoration of the endometrium is reflected in the characteristics of lochia, the postpartum vaginal discharge. Lochia varies in amount and color as healing progresses. Three forms of lochia are observed, generally in the following pattern: rubra, serosa, and alba. Lochia rubra is red or red-brown with a fleshy odor and is seen during the first few days or through the first week postpartum. Lochia rubra contains blood from the placental site; pieces of amnion and chorion; and cellular elements from the decidua, vernix, lanugo, and meconium. Lochia serosa is a pinkish-brown discharge lasting approximately 2 to 3 weeks. Lochia serosa contains some blood, wound exudate, erythrocytes, leukocytes, cervical mucus, microorganisms, and shreds of decidual tissue. The lochia becomes progressively lighter in color. Lochia alba is whitish-yellow in color because it primarily contains leukocytes and decidual cells which may continue for another few weeks. Overall lochia duration may last 4 to 6 weeks postpartum, which is a longer duration than traditionally described, and may vary according to breastfeeding practice and parity.51,73,78
Cervix, vagina, and perineum
Immediately following a vaginal delivery, the cervix hangs into the vagina and is dilated, bruised, and edematous, with possible lacerations. During the first 12 to 18 hours, the cervix shortens and becomes firmer (“forming up”) and, as measured by magnetic resonance imaging, has a mean length of 5.6 cm by 30 hours (versus 2.9 cm by 6 months).81 By the second or third day, the cervix is dilated 2 to 3 cm and by 1 week the cervix is approximately 1 cm dilated. By 4 weeks the external os appears as a small transverse slit, characteristic of multiparous women. Even by 6 weeks, there may still be evidence of stromal edema and round cell infiltrates, which may persist until 3 to 4 months.69 Remodeling and involution of the cervix begin immediately postpartum and occurs rapidly with an increase in fibroblasts, which play a major role in remodelling, collagen, and proteoglycans. This process may be mediated by transforming growth factor-β (TGF-β) and other cytokines.79 This process works to restore the cervical structure altered during cervical ripening and delivery.
After vaginal delivery, the vagina is edematous and relaxed, with decreased tone and absence of rugae. The vagina gradually decreases in size and regains tone, although it does not fully return to its prepregnancy state. By 3 to 4 weeks, rugae have begun to reappear, and edema and vascularity have decreased. The vaginal epithelium is generally restored by 6 to 10 weeks postpartum.69 Decreased lubrication of the vagina during this period can lead to discomfort with sexual intercourse, especially in the lactating woman, as can perineal pain related to episiotomy, laceration, and trauma.17
The use of episiotomies has significantly decreased since the 1980s and 1990s when research documented risks associated with routine use and advocated for restrictive use of episiotomies. Systematic reviews have shown that restrictive use of episiotomies is associated with less severe perineal trauma, less suturing, fewer complications, and less pain.10,27 While initial healing occurs in 2 to 3 weeks, the episiotomy site may take 4 to 6 months to completely heal. A strategy that has been found to protect the perineum and decrease the risk for performing an episiotomy is prenatal perineal massage.6,19,20
Urinary function
Diuresis occurs during the postpartum period, reversing prenatal fluid retention. Overdistention of the bladder; prolonged labor; vulvar, urethral, or bladder trauma, may result in incomplete bladder emptying and urine retention, especially when analgesics were used in labor and delivery and following cesarean delivery.44,87 Management of maternal reports of urine retention in the early postpartum period include providing privacy, positioning, and immersing the woman’s hands in water, as least invasive measures, and catheterization and medication are more invasive options.87 Urinary incontinence is associated with pregnancy and trauma during the delivery process. Research has found that pelvic floor exercises can be effective in preventing and treating prenatal and postpartum urinary incontinence.28,39 Changes in postpartum urinary function are discussed further in Chapter 11.
Breasts
The breasts or mammary glands undergo marked changes during pregnancy with development of alveolar tissue and ductal system in preparation for lactation. Following delivery, further anatomic and physiologic changes occur in breastfeeding women (see “Physiology of Lactation”).
In women who do not breastfeed, involution of the breasts occurs. Distention and stasis of the vascular and lymphatic circulation may result in primary engorgement by 2 to 4 days following delivery. The treatment of engorgement for nonlactating women includes abstaining from stimulation of milk production, abstaining from any breast stimulation, brief applications of cold compresses or cold packs, and administration of anti-inflammatory medication. Bromocriptine (Parlodel) is no longer approved by the Food and Drug Administration for lactation suppression due to numerous adverse maternal outcomes.25 Without stimulation by suckling and removal of milk, secretion of prolactin decreases and milk production ceases. Glandular tissue gradually returns to a resting state over the next few weeks, although breasts do not completely return to their prepregnant state, as new alveoli formed during pregnancy do not completely disappear.
Physical activity and sexual function
The postpartum period is often characterized by alterations in physical activity and function. Gradual resumption of safe physical exercise promotes postpartum maternal well-being, physically and psychologically, as well as facilitates postpartum weight management.4,63 Fatigue, postpartum anatomic and physiologic changes, lochia, perineal trauma, pain, decreased perineal tone, leaking or engorged breasts, the presence and stress of the new baby, adaptation to the demands of the new parental role, and psychologic factors can modify physical and sexual function. Reduced vaginal lubrication associated with the decreased estrogen levels in the postpartum period, especially in lactating women, may contribute to an alteration in sexuality and sexual discomfort.14 Masters and Johnson reported that orgasms in women for the first few months following birth tended to be shorter and less intense with greater latency of response and decreased vasocongestion of the labia majora and minora and decreased vaginal lubrication.52 The timing of resumption of sexual intercourse should depend on maternal physical restoration (cessation of bleeding and absence of discomfort) and the emotional and psychologic readiness of both partners, while accounting for traditional and cultural beliefs, attitudes, and preferences. Health care providers should be aware of, respectful of, and sensitive to cultural and religious practices associated with sexuality during the postpartum period. Interventions include education and counseling regarding postpartum physical and sexual function, contraception, use of vaginal lubricants, alternate forms of intimacy, and pelvic floor exercises to strengthen and tone the perineal muscles.
Endocrine changes
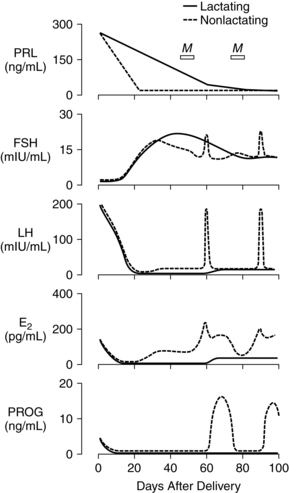
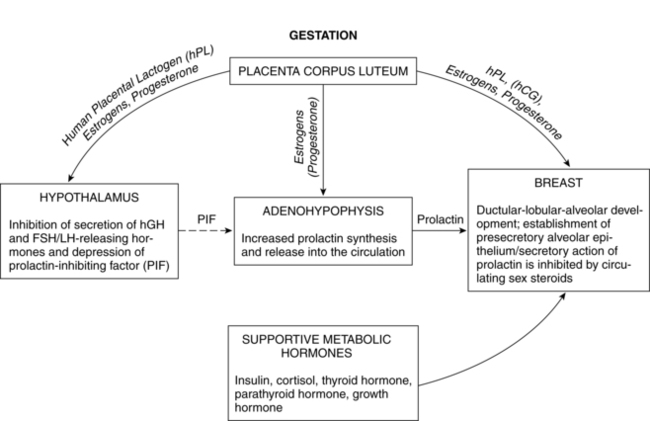
Postpartum endocrine changes primarily occur secondary to fetoplacental hormonal withdrawal related to delivery of the infant and placenta and to increased production of prolactin. In general, most peptide hormones, enzymes, and other circulating proteins of placental origin reach nonpregnant levels by 6 weeks postpartum. Removal of placental hormones alters the physiologic function of many body systems, thus initiating return of those systems to their nonpregnant state. The rate at which placental hormones disappear from the maternal system depends on the half-life phases of the particular substance in maternal blood. There are two phases. The first half-life, which is relatively short, involves hormonal removal from the intravascular system; the second half-life involves the slower removal from extravascular and intracellular space.77 For example, human placental lactogen (hPL) has a short second half-life and generally disappears by 1 to 2 days, whereas human chorionic gonadotropin (hCG) has a longer second half-life and can be detected for 3 to 4 weeks.77 Substances of fetoplacental origin, such as pregnancy-associated proteins, also disappear soon after delivery, whereas other proteins such as alpha-fetoprotein, derived from both placental and maternal sources, are present in maternal plasma for several weeks postpartum.77 Endocrine changes related to mammary development in lactating and nonlactating women are summarized in Figure 5-1.
Estrogens and progesterone
During pregnancy, estrogen promotes development of the mammary ductal system and progesterone promotes lobular and alveolar growth. The elevated levels of these hormones inhibit prolactin action thereby inhibiting lactation. Because the placenta is the major source of estrogens and progesterone, these hormones disappear rapidly following delivery. Plasma estradiol (with a first half-life of 20 minutes and second half-life of 6 to 7 hours) reaches levels that are less than 2% of pregnancy values by 24 hours. By 1 to 3 days, estradiol levels are similar to those found during the follicular phase of the menstrual cycle (less than 100 pg/mL [367 pmol/L]), and unconjugated estriol is undetectable. Although the first and second half-lives of progesterone are short, progesterone levels do not fall as rapidly as estradiol levels because the corpus luteum continues progesterone secretion during the first days following delivery. Generally progesterone falls to levels similar to the luteal phase of the menstrual cycle (2 to 25 ng/mL [6.4 to 79.5 pmol/L]) by 24 to 48 hours and to the follicular phase (less than 1 ng/mL [3.2 pmol/L]) by 3 to 7 days.77 Ovarian production of estrogens and progesterone is low during the first 2 weeks postpartum and gradually increases with resumption of gonadotropin secretion.
Pituitary gonadotropin
The pituitary-hypothalamic-ovarian axis (see Chapter 2), along with production of pituitary gonadotropin, follicle-stimulating hormone (FSH), and luteinizing hormone (LH), is suppressed during pregnancy. Serum levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) remain very low during the first 2 weeks postpartum in both lactating and nonlactating women, gradually increasing with resumption of pituitary function by 4 to 6 weeks. The basis for the initially sluggish pituitary response is unknown. Tulchinsky suggests that this phenomenon may be related to (1) suppression of the pituitary-hypothalamic-ovarian axis by the high levels of circulating estrogens during pregnancy, (2) need for time to reestablish adequate stores of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) leading to a delay in secretion of gonadotropin-releasing hormone (GnRH) by the hypothalamus, and (3) possibly inhibition of luteinizing hormone (LH) release by human chorionic gonadotropin (hCG) and prolactin.77
Prolactin
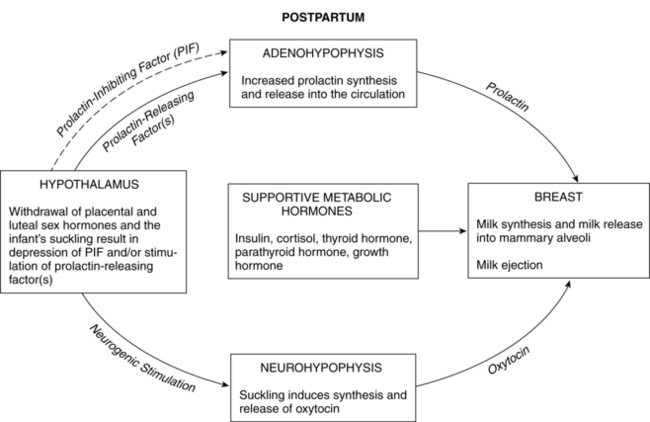
Prolactin is a single-chain peptide hormone secreted in pulses by the anterior pituitary gland. Serum levels of prolactin dramatically increase during pregnancy (from 10 ng/mL to 200 ng/mL [434.8 to 8695.6 pmol/L]), working synergistically with other hormones to promote mammary development including lobular, alveolar, and nipple growth. Throughout pregnancy, secretion of prolactin by the anterior pituitary is controlled by hypothalamic prolactin-inhibiting factor (PIF), mainly dopamine.55 A placental hormone, probably progesterone, inhibits the direct influence of prolactin on the breast during pregnancy, thereby suppressing lactation. With the expulsion of the placenta at delivery, and the significant drop in progesterone, lactogenesis II (copious milk secretion) is initiated.61 Delayed lactogenesis may result from retained placental fragments with the potential to secrete progesterone.61 Postpartum prolactin levels remain elevated due to frequent infant suckling and nipple stimulation.55 In nonlactating women, prolactin levels fall into the high end of the nonpregnant range by 7 to 14 days.77 Breastfeeding women experience a gradual decreasing trend in serum prolactin surges after 6 weeks postpartum, even with continued breastfeeding. Patterns of prolactin secretion associated with lactation are described in “Physiology of Lactation.”
Oxytocin
Oxytocin is an octapeptide hormone produced in the hypothalamus and stored and secreted by the posterior pituitary gland. The uterus becomes increasingly sensitive to oxytocin throughout pregnancy, probably as a result of increasing estrogens that mediate an increase in oxytocin receptors. When the infant suckles, nerve impulses stimulate the release of oxytocin from the posterior pituitary into maternal blood. Oxytocin stimulates the electrical and contractile activity in the myometrium by causing the myoepithelial cells of the uterus to contract and involute. Oxytocin also causes contraction of the myoepithelial cells of the breast, resulting in milk ejection. Breastfeeding the infant after delivery enhances uterine contractions and promotes involution.58
Resumption of menstruation and ovulation
The early postpartum period tends to be a period of relative infertility for many women. Resumption of menstruation and ovulation varies among individual women regardless of whether or not the woman is lactating, although there is a greater tendency for exclusively breastfeeding women to experience a longer period of anovulation and amenorrhea. Menstruation usually resumes by 6 weeks postpartum in nonlactating women, although research has found even earlier resumption of menstruation.35 The first postpartum menstrual cycle may be anovulatory, although nearly a third of these cycles are preceded by ovulation. That first cycle may also have inadequate luteal function, thereby decreasing fertility in the early postpartum period.35 Considering the possibility of resumption of ovulation in the early postpartum period, discussion of contraceptive options should be initiated prenatally or immediately postpartum.
Lactation is associated with a delay in resumption of menstruation and ovulation. In response to infant suckling during breastfeeding, there is a disruption in the normal pattern of gonadotropin-releasing hormone (GnRH) secretion from the hypothalamus, resulting in lower levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH).54 This relationship was demonstrated by a study whereby gonadotropin-releasing hormone (GnRH) infused every 90 minutes into amenorrheic lactating women resulted in stimulation of normal follicular growth.88 Although pulsatile secretion of luteinizing hormone (LH) is seen by 8 weeks postpartum, levels are low and of variable frequency with no preovulatory surge. With regular suckling, luteinizing hormone (LH) remains suppressed.58 With decreased lactation frequency, gonadotropin-releasing hormone (GnRH) secretion returns to normal and follicle-stimulating hormone (FSH) stimulates follicular growth, which in turn increases estradiol and results in luteinizing hormone (LH) release, causing follicular rupture, egg release, and formation of the corpus luteum.54 Health care providers should discuss contraceptive options with lactating women, explaining the relationship between frequency of breastfeeding and fertility, in anticipation of contraceptive needs.
The duration of lactation-suppressed ovulation, known as lactational amenorrhea, depends upon the duration and frequency of breastfeeding and milk expression, with some evidence of longer duration in women who exclusively breastfeed than in those who partially breastfeed.76 Therefore exclusive and frequent breastfeeding (often referred to as on-demand), at least 8 to 12 times per day with no supplementation, is a relatively reliable method for preventing pregnancy for the first 6 months postpartum, assuming the woman has not begun to menstruate. This method of family planning is called the lactational amenorrhea method (LAM) and has been studied and supported by research conducted since the 1988 Bellagio Consensus.42,86
Anatomy of the mammary glands
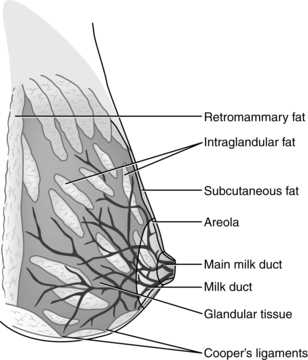
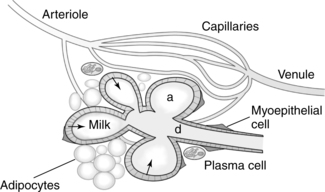
The breasts or mammary glands are modified exocrine glands consisting of epithelial glandular tissue with an extensive system of branching ducts surrounded by adipose tissue and supported by the pectoralis major muscles and the fibrous bands of the Cooper’s ligaments. Cooper’s ligaments are suspensory and support the shape of the breast. The breasts are highly innervated with rich vascular and lymphatic systems. Each breast has been found to contain a range of 4 to 18 primary milk ducts arranged in a complex network and which converge at the nipple.68 The basic glandular unit is a lobe consisting of 4 to 18 lobules, each containing clusters of alveoli that are connected to fine ducts that merge into larger ducts that merge into a primary milk duct and lead to the nipple. The alveolus is the site of milk synthesis and secretion and consists of clusters of mammary epithelial secretory cells (lactocytes) surrounded by myoepithelial cells to form smooth muscle contractile units responsible for ejecting milk into the ducts from the lumen of the alveoli.67 The ducts and alveoli are surrounded by a stroma of fibroblasts, adipocytes, blood vessels, plasma cells (B lymphocytes capable of producing immunoglobulins, especially secretory immunoglobulin A), and a few nerves.58 Research using ultrasound imaging demonstrated that there are not lactiferous sinuses under the areola rather that some women experience temporary duct dilation with milk ejection which subsides with milk flow.67,68 Ducts serve to transport milk from the alveoli toward the nipple. The anterior and posterior medical branches of the internal mammary artery and the mammary branch of the lateral thoracic artery supply most of the blood to the breast. The internal thoracic, axillary, and cephalic veins drain the breast. Lymphatic drainage is conducted through the axillary and internal mammary nodes. The 2nd through 6th intercostal nerves innervate the breast. The structure of milk production and ejection portions of the mammary glands is illustrated in Figure 5-2. Figure 5-3 illustrates the mammary alveolus. The breast may be divided into four quadrants (lower inner, upper inner, lower outer, and upper outer quadrant with the adjacent Tail of Spence) for purposes of mapping and descriptive location.
The nipple is surrounded by the areola. Both the nipple and areola are elastic and darker in pigmentation than the rest of the breast, and become even darker during pregnancy and lactation, possibly providing a visual signal for the infant to latch. When not washed off, the mother’s nipple and areola also attract the newborn infant immediately after delivery through the senses of taste and smell.80 With suckling, the nipple and much of the areola are drawn into the infant’s mouth, forming a teat. Milk is removed by the stripping action of the infant’s tongue against the hard palate along with the infant’s application of vacuum on the breast and the maternal milk ejection response.23 Smooth muscle and elastic fibers in the areola and nipple form a sphincter to prevent milk loss when the infant is not suckling.58
Physiology of lactation
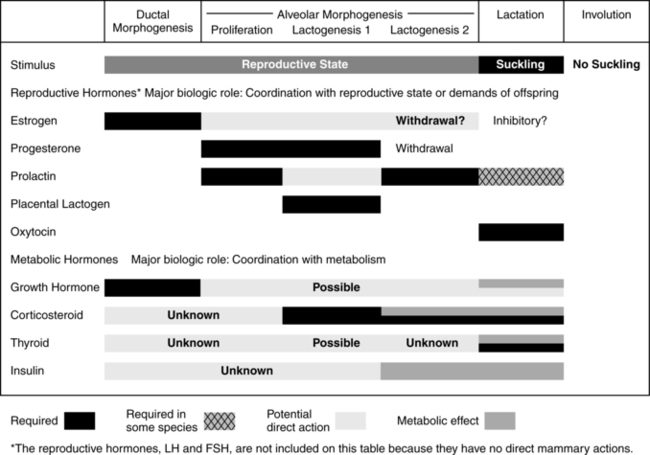
Human milk is species-specific and promotes proper growth and development of nearly all infants. Lactation is a complex physiologic process involving integration of neuronal and endocrine mechanisms. Mammary development and lactation can be divided into phases: embryogenesis, mammogenesis (mammary growth during puberty and pregnancy), lactogenesis (I and II, initiation of milk secretion), lactogenesis III (maintenance of established milk secretion), and involution (cessation of lactation).43,58 These phases are summarized in Table 5-1. Hormonal influences on lactation are summarized in Figure 5-4 andTable 5-2.
Table 5-1
| DEVELOPMENTAL STAGES | HORMONAL REGULATION | LOCAL FACTORS | DESCRIPTION |
| Embryogenesis | Unknown | Fat pad necessary for ductal extension | Epithelial bud develops in 18- to 19-week fetus, extending a short distance into mammary fat pad with blind ducts that become canalized; some milk secretion may be present at birth |
| Pubertal development prior to onset of menses | Estrogen, GH | IGF-1, HGF, TGF-β, unknown | Ductal extension into the mammary fat pad; branching morphogenesis |
| Pubertal development after onset of menses | Estrogen, progesterone, possibly PRL | Lobular development with formation of TDLU | |
| Development in pregnancy | Progesterone, PRL, hPL | Herrugulin, unknown | Alveolus formation; partial cellular differentiation |
| Transition: lactogenesis | Progesterone withdrawal, PRL, glucocorticoid | Unknown | Onset of milk secretion: Stage I: Mid-pregnancy; Stage II: Parturition |
| Lactation | PRL, oxytocin | FIL, stretch | Ongoing milk secretion, milk ejection |
| Involution | Withdrawal of prolactin | Milk stasis, FIL | Alveolar epithelium undergoes apoptosis and remodeling and gland reverts to pre-pregnant state |
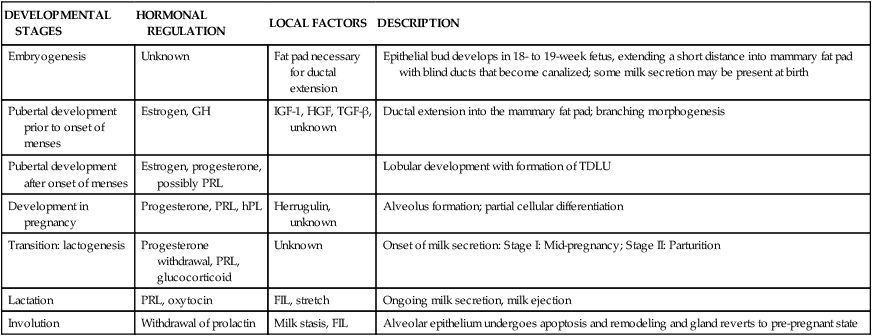
Adapted from Neville, M.C. (2001). Anatomy and Physiology of lactation. Pediatr Clin North Am, 48, 16.
Table 5-2
Hormonal Contributions to Breast Development
| HORMONE | ORIGIN | FUNCTION BEFORE AND DURING PREGNANCY | FUNCTION AFTER DELIVERY |
| Prolactin (PRL) | Anterior pituitary | Serum levels rise but estrogen suppresses its effect during pregnancy | Stimulates alveolar cells to produce milk; important in initiating and maintaining lactation; may also cause lactation infertility by suppressing release of FSH and LH from pituitary or by causing ovaries to be unresponsive to various psychogenic factors, stress, an esthesia, surgery, high serum osmolality, exercise, nipple stimulation, and sexual intercourse |
| Prolactin-inhibiting factor (PIF) | Hypothalamus | Suppresses release of PRL into blood; release stimulated by dopaminergic impulses (i.e., catecholamines) | Suppresses release of PRL from anterior pituitary; agents that increase PRL by decreasing catecholamines and thus prolactin-inhibiting factor (PIF) include phenothiazides and reserpine |
| Oxytocin | Posterior pituitary | Generally no effect on mammary function; sensitivity of myoepithelial cells to oxytocin increases during pregnancy | Causes myoepithelial cells to contract, leading to milk ejection; release is inhibited by stresses such as fear, anxiety, embarrassment, distraction; also causes uterine contraction and postpartum involution of the uterus |
| Estrogen | Ovary and placenta | Stimulates proliferation of glandular tissue and ducts in breast; probably stimulates pituitary to secrete PRL but inhibits PRL effects on breasts | Blood level drops at parturition, which aids in initiating lactation; not important to lactation thereafter |
| Progesterone | Ovary and placenta | With estrogen, stimulates proliferation of glandular tissue and ducts in breast; inhibits milk secretion | Blood level drops at parturition, which aids in initiating lactation; probably unimportant to lactation thereafter |
| Growth hormone | Anterior pituitary | May act with PRL in initiating lactation but appears to be most important in maintaining established lactation | |
| Adrenocorticotropic hormone (ACTH) | Anterior pituitary | Blood levels gradually increase during pregnancy; stimulates adrenal to release corticosteroids | High level is believed necessary for maintenance of lactation |
| Human placental lactogen (hPL) | Placenta | Like growth hormone in structure; stimulates mammary growth; associated with mobilization of free fatty acids and inhibition of peripheral glucose utilization and lactogenic action | |
| Thyroxine | Thyroid | Normally no direct effect on lactation | Appears to be important in maintaining lactation either through some direct effect on the mammary glands or by control of metabolism |
| Thyrotropin-releasing hormone | Hypothalamus | Normally no effect on lactation | Stimulates release of PRL; can be used to maintain established lactation |
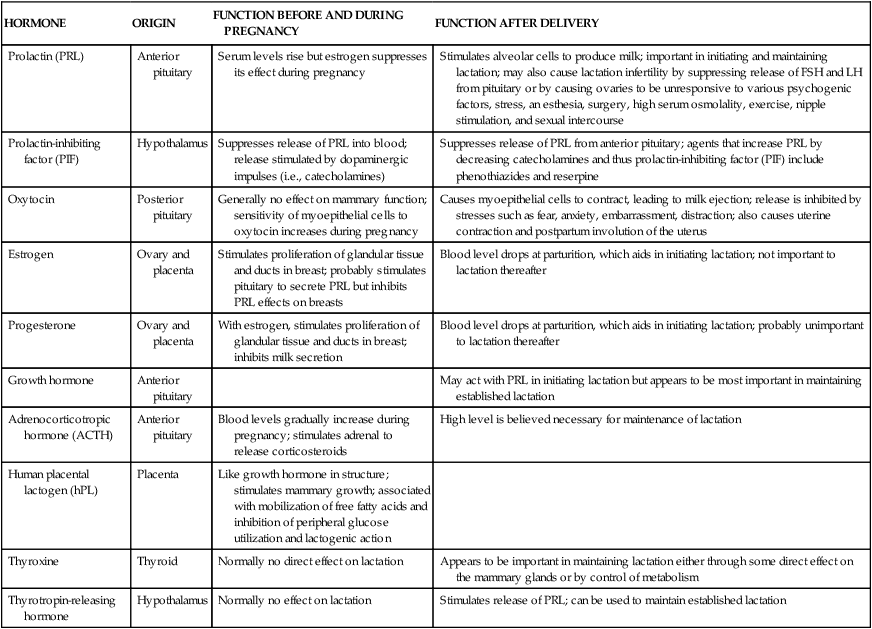
FSH, Follicle-stimulating hormone; LH, luteinizing hormone.
Adapted from Worthington-Roberts, B.S. & Williams, S.R. (1997). Nutrition in pregnancy and lactation (6th ed.). Madison, WI: Brown & Benchmark.
Embryogenesis
Mammary growth begins with development of the mammary band and streak in the fourth week of embryonic development, which progresses into the mammary ridge by the fifth week. The ectoderm continues to proliferate and undergo inward growth into the underlying mesoderm as the mammary bud is formed. From the sixth week, mammary glands, milk ducts, and lobular branching gradually develop throughout the continued embryonic growth. The continued proliferation of ingrowing ectodermal cells leads to the development of the branching mammary duct system. By 18 to 19 weeks, a bulb-shaped mammary bud is evident and extends into the mesenchyme, where fat pads are developing. The bud forms secondary buds, which will form the duct system in the mature breast. The secondary buds elongate and invade the fat pads, then branch and canalize to form the rudimentary ductal system.59 Occasionally the newborn may have transient secretions from the breast in the first few days after birth, probably due to maternal hormonal levels.
Mammogenesis
Mammogenesis involves mammary gland development that begins in fetal life, mediated by estrogen and growth hormone, and accelerates at puberty with ductal development, fatty tissue growth, lobuloalveolar development and further maturation of the breasts. Alveolar development continues under the influence of the luteal phase of the menstrual cycle and in early pregnancy. There is development of terminal duct lobular units and alveoli formation under the influence of progesterone secreted by the ovaries during the luteal phase, as well as under the influence of estrogen, and probably prolactin.59
During pregnancy, beginning soon after conception, and continuing through postpartum, breasts undergo additional changes, reaching full maturation. Lactogenesis I, characterized by mammary changes including secretory differentiation, occurs during pregnancy. External changes during pregnancy include increases in breast size and areolar pigmentation. The Montgomery tubercles enlarge and become more prominent, and the nipples become more erect. Toward the end of pregnancy, it is normal for some women to experience some leaking of colostrum while others may not (for most women, there is no special preparation of the nipples during pregnancy). The myoepithelial cells hypertrophy. The skin over the breasts appears thinner, the blood vessels are more prominent, and there is a twofold increase in blood flow to the breast. Much of the growth and development is a result of hormonal changes of the corpus luteum and placenta. During the first trimester, the ductal system proliferates and branches under the influence of estrogen and lobular formation is enhanced by progesterone. The glandular tissue of the alveoli proliferate under the influence of human placental lactogen (hPL), human chorionic gonadotropin (hCG), and prolactin (Figures 5-4 and 5-5). Growth hormone and adrenocorticotropic hormone (ACTH) act synergistically with prolactin and progesterone to promote mammogenesis.
As pregnancy progresses, the epithelial cells of the alveoli differentiate into secretory cells capable of milk production and the number of alveoli increases. Milk secretion is inhibited by the high levels of placental hormones including progesterone during pregnancy. Fat droplets accumulate in the secretory cells and concurrently breast interstitial tissue becomes infiltrated with lymphocytes, plasma cells, and eosinophils. During the second and third trimesters there is further lobular growth with formation of new alveoli and ducts and dilation of the lumens. Ductal arborization with development of extensive lobular clusters begins at mid-gestation. By the third month, prolactin stimulates production of colostrum, followed by the stimulation of its secretion by placental lactogen in the second trimester. Colostrum accumulates in the lumen. As such, a pregnant woman will produce colostrum as early as 16 weeks (see “Human Milk for the Preterm Infant”). Following birth, the alveolar epithelial cells continue to proliferate with synthesis of milk under the influence of increased levels of prolactin and the stimulus of suckling. Initial synthesis of milk components is preceded by an increase in required enzymes within the secretory cells.
Lactogenesis
Lactogenesis, or initiation of milk production, involves a complex neuroendocrine process with interaction of several hormones. Lactogenesis can be divided into three stages. Lactogenesis I occurs from early pregnancy to approximately the third postpartum day. This lactation initiation stage is not dependent upon suckling or milk removal, rather on the cascade of hormonal changes.62 Only small amounts of milk are secreted, however, as a result of the inhibitory effects of the placental hormones. During lactogenesis I, secretory differentiation begins and there is a decrease in sodium, an increase in enzymes, proteins, and immunoglobulins and an increase in the size of fat droplets in the mammary cells.61 During the first few postpartum days, colostrum has relatively high concentrations of immunoglobulin A, lactoferrin and oligosaccharide, which provide the newborn infant with protection against infection.
Lactogenesis II is the second stage, initiated by delivery of the placenta and a significant drop in progesterone along with the increasing level of prolactin. By days 2 to 4 postpartum, copious milk secretion begins, referred to as when a mother’s “milk comes in,” and plasma alpha-lactalbumin levels increase as there is a transformation in milk composition. During the first postpartum day, the infant receives less than 100 mL of breast milk which increases to approximately 500 mL by the fourth day.61 The composition of milk changes as sodium and chloride concentrations decrease and the lactose concentration increases.62 During this stage of lactogenesis II, junctions between mammary alveolar cells tighten. With frequent milk transfer, milk synthesis is increased and milk production rises. With increased vascularity and fluid congestion in the early postpartum period, many women feel breast fullness and heaviness, which resolves with efficient milk removal through infant sucking or pumping. Pathologic engorgement occurs when there is inadequate milk removal resulting in milk stasis, swelling, pain, tenderness, and inflammation. Research suggests that early and frequent suckling and pumping facilitates efficient milk production, especially as control of lactation changes from endocrine to autocrine, directed by milk removal.18 Prolonged milk stasis triggers the feedback inhibitor of lactation (FIL), the autocrine inhibitory protein that controls and suppresses milk synthesis that may lead to decreased milk production.38 Lactation research in Western populations points to the importance of early breastfeeding initiation, skin-to-skin contact, and lactation support to promote successful breastfeeding and extended breastfeeding duration, although breastfeeding may be successfully initiated and continued under many circumstances.57 For the breastfeeding woman, prevention and early treatment with encouragement to continue adequate breastfeeding and/or pumping is recommended. For treatment recommendations, see Common Breastfeeding Problems later in the chapter.
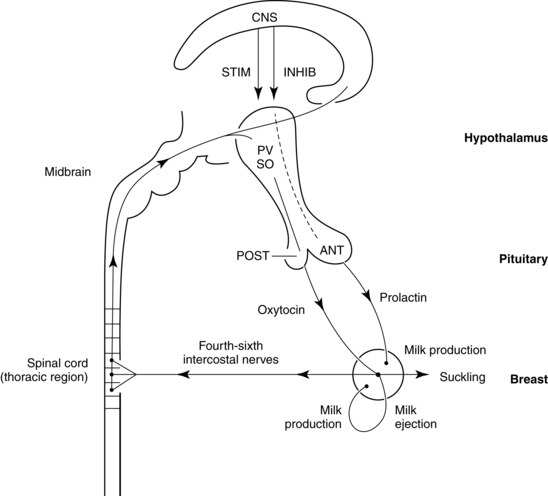
By the end of the first or second week, during the next stage, lactogenesis III (or galactopoiesis), mature milk is established and milk supply is maintained through autocrine control. Milk production rate depends upon the milk removal rate, in a manner of supply-demand response through a feedback mechanism, and the hypothalamic-pituitary axis response regulating hormonal secretion including prolactin and oxytocin. Suckling stimulates sensory nerves in the nipple and areola, sending messages to the hypothalamus to secrete prolactin and oxytocin. Prolactin stimulates milk synthesis and oxytocin stimulates contraction of the myoepithelial cells causing milk ejection or “let-down.” Other hormones enhancing lactogenesis include growth hormone, corticosteroids, thyroxine, and insulin, as demonstrated in Figures 5-5 and 5-6.60 Growth hormone and insulin are important for survival of alveolar cells and for stimulating glucose entry in the mammary epithelial cells to accelerate lipogenesis.
Extended milk stasis causes increased pressure in the breast leading to inflammation and decreased milk synthesis, thereby inhibiting lactation. The introduction of supplements is associated with decreased breastfeeding duration.33 Furthermore, the use of supplementation leads to decreased milk production as the demand placed on maternal milk production decreases. Breastfeeding on-demand and pumping when mother and infant are separated or unable to breastfeed are often effective means of maintaining adequate milk supply. If maternal milk supply is insufficient despite efforts of frequent breastfeeding or pumping, maternal use of galactagogues may augment milk production.22
Prolactin patterns during lactation
Milk production and release are controlled primarily by the effect of suckling on hormonal release via a complex neuroendocrine process. Suckling stimulates prolactin release from the anterior pituitary (Figure 5-7). Suckling also stimulates sensory nerve endings in the nipple and areola, sending impulses to the hypothalamus via the spinal cord. As a result, hypothalamic secretion of prolactin-inhibiting factor is suppressed, and adenohypophysis secretion of prolactin increases. Prolactin levels increase toward the end of a feeding, increasing the volume, fat, and protein content of milk in the next feeding.77
Prolactin, acting synergistically with insulin and cortisol, stimulates the alveolar secretory cells to produce milk proteins and fat. However, lactation must be preceded by a fall in progesterone and estrogens, removing an inhibitory effect and facilitating response to prolactin. The number of prolactin receptors in breast tissue increases markedly following delivery. The decrease in human placental lactogen (hPL) following expulsion or removal of the placenta may also facilitate prolactin action, in that human placental lactogen (hPL) competes with prolactin for the same breast tissue receptors.77 Bromocriptine suppresses lactation by decreasing the number of specific prolactin receptors, and the FDA has withdrawn its approval for such use in postpartum women, even for medically-indicated lactation suppression, due to serious adverse outcomes.25 New safer medications have been developed for lactation suppression although less medical interventions and abstaining from breast stimulation are preferred as initial efforts in indicated lactation suppression.
Serum prolactin levels are highest in the early postpartum period such that during the first month of pregnancy, baseline prolactin levels are 119 ± 19.1 μg/L and peak prolactin levels are 286 ± 22.6 μg/L in response to infant suckling stimulation.15 Baseline and peak prolactin levels steadily decline by 6 months postpartum although breastfeeding frequency and milk production may not be significantly different.15 Prolactin peaks at approximately 45 minutes from the onset of suckling stimulus, and no difference was found between the left and right breasts in prolactin concentrations.16 Prolactin circadian rhythm continues throughout lactation, manifesting higher levels at night when the rate of milk synthesis is highest and the prolactin levels in the alveoli are highest, resulting in higher prolactin levels in the first morning milk.16
While milk secretion depends upon the endocrine system such as prolactin secretion, there appears to be a more autocrine regulation of milk volume related to the rate of milk removal. Milk volume is mediated by an autocrine inhibitory protein, the feedback inhibitor of lactation (FIL).38 Feedback inhibitor of lactation (FIL) inhibits protein secretion in the alveoli, decreasing milk synthesis. Feedback inhibitor of lactation (FIL) acts upon milk stored in the secretory tissue of the alveolar lumen, not upon milk moved to the sinuses. If milk is not completely or efficiently removed from the breast, feedback inhibitor of lactation (FIL) accumulates in the alveoli and inhibits protein secretion. Furthermore, milk stasis causes reduced prolactin receptors, possibly decreasing cellular sensitivity to lactogenic hormones.38 The rate of milk synthesis may also be regulated by the stretching of the alveolar cells.62
Oxytocin release during lactation
The let-down or milk ejection reflex is a complex neuroendocrine process important for movement of milk along the duct system to the nipple. Suckling stimulates sensory nerve endings in the nipple and areola. These impulses travel via afferent neural pathways in the spinal cord to the mesencephalon and hypothalamus, stimulating oxytocin release from the posterior pituitary gland (see Figure 5-7). Oxytocin stimulates contraction of the myoepithelial cells surrounding the alveoli (as well as uterine contractions), causing the let-down reflex. Contraction of the myoepithelial cells shortens and widens the ducts, enhancing milk flow to the nipples.58 As a result, milk is ejected into the duct system and propelled to the lactiferous ducts and sinuses. Oxytocin release and the let-down reflex may also be stimulated by thinking of the infant, hearing an infant crying, and experiencing orgasm, while it may be inhibited by stress through the HPA axis mechanism.29
Human milk
Importance of human milk
Research around the world among different populations has demonstrated the benefits, health promotion, and health protection of human milk. It is superior and preferred for nearly all infants, with a few exceptions. The World Health Organization recommends exclusive breastfeeding for 6 months followed by continued breastfeeding until 2 years or mutually appropriate for the maternal-infant dyad.85 The American Academy of Pediatrics’ policy statement on breastfeeding provides an evidence-based approach to the recommendations for breastfeeding including: health promotional and beneficial for mother and infant, decreased acute and chronic diseases, promotion of infant neurodevelopment, and economic advantages.3 Longer durations of breastfeeding have been associated with decreased risk of developing diabetes for both the mother and infant, helping to prevent an increasing chronic disease.64,74 Discuss the importance of breastfeeding with the woman and her family during pregnancy and encourage continued breastfeeding during the postpartum period. Characteristics of women at risk for not breastfeeding have been noted and include cultural background and health practices, lack of health insurance, late prenatal care, lower level of maternal education, not being married, and smoking in pregnancy, to name a few.13 For the woman who is smoking, providing clear messages about the importance of reducing and eliminating smoking along with practical advice and information about resources for assistance will promote healthier outcomes in both the mother and her infant. The prenatal and postpartum periods are motivating times, considered “teachable moments,” for promoting modifiable lifestyle behaviors such as reduction of negative exposures and engagement in healthy, positive activities such as breastfeeding that are associated with improved maternal-infant health outcomes. Identify pregnant women who are at risk for not breastfeeding to target directed and appropriate messages regarding the importance of breastfeeding, barriers to breastfeeding, and resources for breastfeeding support.
Milk production and composition
The components of human milk are synthesized in the secretory cells of the alveoli (protein, fat, lactose) or extracted from maternal plasma (vitamins and minerals) by these same cells. The individual components of milk then pass into the alveolar lumen where the final milk is constituted. During lactation, milk synthesis constantly occurs at low levels and the milk is stored in the alveolar lumen until it is removed.59 Active synthesis occurs at the highest rate during infant suckling or other areolar and nipple stimulation such as with pumping.
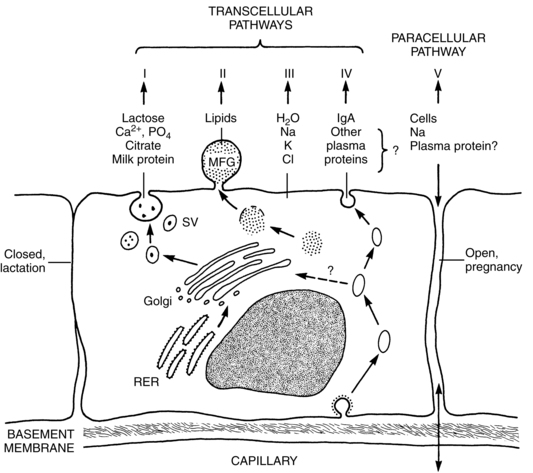
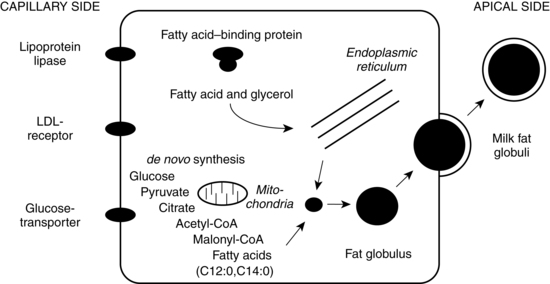
Milk synthesis depends upon four transcellular pathways and one paracellular pathway. The four transcellular pathways include exocytosis, fat synthesis and secretion, secretion of ions and water, and transcytosis of substances including immunoglobulins. The passage of substances via the paracellular pathway moves substances between epithelial cells (Figure 5-8).59 The exocytosis process begins with mRNA synthesis in the nucleus for protein synthesis. The protein molecules are transported to the endoplasmic reticulum, where they undergo modification and transportation through the Golgi system. Calcium, phosphate, and citrate from the cytoplasm are transported into the Golgi system. Lactose is then synthesized and osmotically draws water into the Golgi system. Protein, lactose, and calcium undergo exocytosis in the Golgi secretory vesicles and are discharged into the alveolar lumen.59
Lipids, specifically triglycerides, are synthesized in the endoplasmic reticulum and cytoplasm. Milk fat is secreted in milk-fat globules (Figure 5-9). These globules have a core of triglycerides encased in a membrane containing phospholipids, preventing the individual fat globules from coalescing into large globules that would be difficult to secrete. The secretion of ions and water involve direct transport across the apical membrane. The small molecules transported by this route include sodium, potassium, chloride, some monosaccharides and water. Transcytosis, the fourth pathway, is the mechanism for movement of intact proteins from the interstitial space into milk. This mechanism is used for secretion of immunoglobulins, especially IgA, proteins, hormones, and growth factors from maternal plasma into milk.59
The paracellular pathway involves transport of substances between the tight junctions of adjacent epithelial cells. The tight junctions are gasket-like structures joining the cells together and preventing substances moving from the milk back to the mother. The junctions open and leak during pregnancy, mastitis, and following involution, allowing bidirectional movement: interstitial substances move into the milk and milk components move into the plasma. In mastitis, this route helps to provide entry for anti-inflammatory components and to clear the end products of the infectious process. With involution, this route allows removal of degraded components. Mammary sodium and chloride concentrations are higher when the junctions are open, such as during pregnancy, and may help in diagnosing breastfeeding problems.59
Composition of human milk
The composition of human milk varies between women and for each woman due to gestation, stage of lactation, milk volume, and frequency of breastfeeding or milk expression, although the overall contents of human milk are fairly consistent. The primary components of mature human milk are water (87%), carbohydrates, fats, proteins, vitamins, and minerals. In a national sample of donor breast milk from 273 women at different stages of lactation, researchers found that the average mean energy content was 19 kilocalories per ounce (compared to many infant formula supplementation’s energy content of 20 kilocalories per ounce).84 The average volume of milk increases during exclusive breastfeeding from 500 mL/day by the end of the first week to at least 800 mL/day by 6 months.58 Volume is higher with multiple breastfeeding infants due to increased frequency of suckling stimulation. Milk volume is 5% to 15% higher in women with very low body fat. This is thought to be due to the decreased milk fat content and caloric density, which leads to increased infant suckling stimulation.58
Human milk is isosmotic with human plasma. Lactose is the major osmotic component of milk and is highly concentrated in human milk. In order for milk to maintain isosmolarity with plasma, concentrations of other ions—sodium and chloride, in particular—must remain proportionately lower. Levels of protein, sodium, potassium, chloride, and other ions are lower in human milk than in bovine milk, thereby maintaining a lower renal solute load and placing less stress on the kidneys (see Chapter 11). Table 5-3 presents many of the representative values of the components of human milk.
Table 5-3
Representative Values for Constituents of Human Milk
| CONSTITUENT (PER LITER)* | EARLY MILK | MATURE MILK |
| Energy (kJ) | 2730-2940 | |
| Carbohydrate | ||
| Lactose (g) | 20-30 | 67 |
| Glucose (g) | 0.2-1.0 | 0.2-0.3 |
| Oligosaccharides (g) | 22-24 | 12-14 |
| Total nitrogen (g) | 3.0 | 1.9 |
| Nonprotein nitrogen (g) | 0.5 | 0.45 |
| Protein nitrogen (g) | 2.5 | 1.45 |
| Total protein (g) | 16 | 9 |
| Casein (g) | 3.8 | 5.7 |
| β-casein (g) | 2.6 | 4.4 |
| κ-casein (g) | 1.2 | 1.3 |
| α-lactalbumin (g) | 3.62 | 3.26 |
| Lactoferrin (g) | 3.53 | 1.94 |
| Serum albumin (g) | 0.39 | 0.41 |
| sIgA (g) | 2.0 | 1.0 |
| IgM (g) | 0.12 | 0.2 |
| IgG (g) | 0.34 | 0.05 |
| Total lipids (%) | 2 | 3.5 |
| Triglyceride (% total lipids) | 97-98 | 97-98 |
| Cholesterol† (% total lipids) | 0.7-1.3 | 0.4-0.5 |
| Phospholipids (% total lipids) | 1.1 | 0.6-0.8 |
| Fatty acids (weight %) | 88 | 88 |
| Total saturated | 43-44 | 44-45 |
| C12:0 | 5 | |
| C14:0 | 6 | |
| C16:0 | 20 | |
| C18:0 | 8 | |
| Monounsaturated | 40 | |
| C18:1ω-9 | 32 | 31 |
| Polyunsaturated | 13 | 14-15 |
| Total ω-3 | 1.5 | 1.5 |
| C18:3ω-3 | 0.7 | 0.9 |
| C22:5ω-3 | 0.2 | 0.1 |
| C22:6ω-3 | 0.5 | 0.2 |
| Total ω-6 | 11.6 | 13.06 |
| C18:2ω-6 | 8.9 | 11.3 |
| C20:4ω-6 | 0.7 | 0.5 |
| C22:4ω-6 | 0.2 | 0.1 |
| Water-soluble vitamins | ||
| Ascorbic acid (mg) | 100 | |
| Thiamin (mcg) | 20 | 200 |
| Riboflavin (mcg) | 400-600 | |
| Niacin (mg) | 0.5 | 1.8-6.0 |
| Vitamin B6 (mg) | 0.09-0.31 | |
| Folate (mcg) | 80-140 | |
| Vitamin B12 (mcg) | 0.5-1.0 | |
| Pantothenic acid (mg) | 2.0-2.5 | |
| Biotin (mcg) | 5-9 | |
| Fat-soluble vitamins | ||
| Retinol (mg) | 2 | 0.3-0.6 |
| Carotenoids (mg) | 2 | 0.2-0.6 |
| Vitamin K (mcg) | 2.5 | 2-3 |
| Vitamin D (mcg) | 0.33 | |
| Vitamin E (mg) | 8-12 | 3-8 |
| Minerals | ||
| Major minerals | ||
| Calcium (mg) | 250 | 200-250 |
| Magnesium (mg) | 30-35 | 30-35 |
| Phosphorus (mg) | 120-160 | 120-140 |
| Sodium (mg) | 300-400 | 120-250 |
| Potassium (mg) | 600-700 | 400-550 |
| Chloride (mg) | 600-800 | 400-450 |
| Trace minerals | ||
| Iron (mg) | 0.5-1.0 | 0.3-0.9 |
| Zinc (mg) | 8-12 | 1-3 |
| Copper (mg) | 0.5-0.8 | 0.2-0.4 |
| Manganese (mcg) | 5-6 | 3 |
| Selenium (mcg) | 40 | 7-33 |
| Iodine (mcg) | 150 | |
| Fluoride (mcg) | 4-15 |
*All values are expressed per liter of milk with the exception of lipids that are expressed as a percentage on the basis of milk volume or weight of total lipids.
†The cholesterol content of human milk ranges from 100 to 200 mg/L in most samples of human milk after day 21 of lactation.
Data from Jensen, R.G. (1995). Handbook of milk composition. San Diego: Academic Press; Koletzko, B. & Rodriguez-Palermo, M. (1999). Polyunsaturated fatty acids in human milk and their role in early development. J Mammary Gland Biol Neoplasia 4, 269; Cuillere, M.L., Tregoat, V., Bene, M.C., et al. (1999). Changes in the kappa-casein and beta-casein concentrations in human milk during lactation. J Clin Lab Anal 13, 213; Picciano, M.F. (2001). Representative values for constituents of human milk. Pediatr Clin North Am, 48, 263.
Carbohydrate synthesis and release
The major carbohydrate found in human milk is lactose. Other carbohydrates found in small quantities include glucose, nucleotide sugars, glycolipids, glycoproteins, and oligosaccharides.65 The oligosaccharides promote development of intestinal bacterial flora (such as bifidus) and inhibit bacterial adhesion, thereby reducing the risk of gastrointestinal infections. Immunologic properties of human milk are discussed further in Chapter 13.
The concentration of lactose in human milk is stable and independent of maternal nutritional status. Lactose acts as an osmotic compound to pull water into the Golgi apparatus, thereby regulating the amount of water in milk and the volume of milk produced. If less lactose is available, less milk will be produced, maintaining a constant lactose concentration. Lactose is synthesized in the Golgi apparatus from glucose and galactose. Glucose is obtained from maternal plasma and is necessary in metabolic processes including lactose synthesis. Lactose is an important source of energy for the developing infant brain. Alpha-lactalbumin, the most plentiful protein in human milk, plays an important role in lactose synthesis, as well as provides the osmotic force in the movement of water, and serves as a source of amino acids available for the infant.34
Fat synthesis and release
The largest portion of calories in human milk comes from fat. Fats comprise approximately 4% of milk, although their concentration varies between women and within each woman, based on individual characteristics. Variation of fat content is influenced by a host of factors including gestation (higher polyunsaturated fatty acids in very preterm and preterm delivery), stage of lactation (phospholipids and cholesterol higher in early lactation), milk volume (high volume associated with lower fat), maternal nutrition (low fat maternal diet associated with lower fat), and timing within breastfeeding session (fat progressively increases during breastfeeding session, called hindmilk).65 The major constituents of milk fat are triglycerides (98%). The high proportion of lipids in human milk provides energy, short and long-chain fatty acids crucial in retinal and neural development. Human milk also contains enzymes to aid in fat digestion including lingual lipase, gastric lipase, bile salt-stimulated lipase, and pancreatic lipase that aids in fat digestion, enhancing release of energy and fat digestion and absorption.65
Most of the milk fat is derived from the maternal circulation resulting from nutrition and from stored lipids, while some of the fat is synthesized in the mammary gland from glucose metabolism.40 Maternal nutrition affects the lipid components of milk, especially some essential fatty acids. Severe maternal caloric restriction alters the fatty acid composition as does the type of fat consumed by the mother, although changes are usually buffered by maternal lipid stores. For example, if the maternal diet is altered to include more polyunsaturated fatty acids, her milk will also contain more polyunsaturated fats. Likewise, a woman who consumes a high amount of fish or who takes fish oil supplements has an increased proportion of polyunsaturated fatty acids.40
Milk fat is synthesized in the endoplasmic reticulum (see Figure 5-9) from precursors available within the secretory cell or obtained from maternal blood. Insulin stimulates glucose uptake into the mammary cells. Short-chain fatty acids are synthesized from acetate, whereas long-chain fatty acids are obtained from maternal plasma. Triglycerides are either obtained from maternal plasma or synthesized from intracellular carbohydrates (primarily glyceride and glucose). Following delivery (and possibly due to prolactin), there is an increase in two enzymes, lipoprotein lipase which hydrolizes lipids and palmitoyl-coenzyme A transferase, involved in the production and utilization of triglycerides. Lipoprotein lipase acts in the capillaries to catalyze the lipolysis of triglycerides and the uptake of glycerol and fatty acids into the epithelial cells. Palmitoyl-coenzyme A transferase catalyzes intracellular synthesis of triglyceride from glyceride.36 Fatty acids are esterified in the endoplasmic reticulum to form triglycerides. The triglycerides accumulate and coalesce to form larger fat droplets surrounded by a membrane rich in phospholipids and cholesterol. As fat droplets increase in size, they move to the membrane apex and become part of the milk fat globule.53
Protein synthesis and release
Protein components of human milk are critical to growth, development, and immunoprotection. Most proteins are synthesized in the mammary gland and some are transported from maternal circulation.46 The primary means for protein secretion by alveolar cells is through the exocytotic pathway and processing in the Golgi bodies while the transcytotic pathway is also a mechanism for the transport of proteins into human milk although the transport mechanism for amino acids is unclear to date.53 Human milk protein provides amino acids necessary for growth, protective factors such as immunoglobulins, bacteriostatic properties, bioactive factors and proteins to aid digestion and absorption, growth and promotion of beneficial gut flora, and development of a protective gastrointestinal mucosal barrier.2
Milk protein primarily consists of whey (alpha-lactalbumin; lactoferrin; serum albumin; and immunoglobulins sIgA, IgA, IgG, and IgM) and casein.41 The concentrations of whey and casein in human milk are in a 60:40 ratio versus the 20:80 ratio in bovine milk. While human milk has less total protein than bovine milk, the protein content in human milk is more bioavailable to the infant. Whey protein is easily digested, forming soft, flocculent curds. Protein content slowly decreases during the first 6 months of lactation. The protein content of colostrum is approximately double that of mature milk due to the higher concentration of essential amino acids and the increased abundance of antibodies such as secretory IgA and lactoferrin (see Chapter 13). Lactoferrin promotes anti-inflammatory function, stimulates bactericidal and antiviral activity, and may inhibit tumor growth by activating natural killer cells.26 Casein, the predominant bovine milk protein, is less easily digested and forms tough, rubbery curds. Casein requires greater energy expenditure to digest, and its digestion is more likely to be incomplete. Some casein is needed, however, to enhance the absorption of minerals by keeping them in solution in the gut. Casein may also promote the growth of beneficial gut flora and inhibit adherence of pathogenic organisms.41
Human milk also contains other nonprotein nitrogen components and other protein factors. These components are used to synthesize nonessential amino acids; enhance gut maturation, growth, and nutrient absorption; or contribute to the immunologic properties of human milk (see Chapter 13). The proteins in human milk are used for nutrition and growth as well as for other functions such as immunoprotection. The proteins in artificial formula do not contain all the proteins available in human milk and try to compensate by adding higher concentrations of total proteins, although the excess nitrogen resulting from protein metabolism may place an extra burden on the infant’s kidneys.2 Research and identification of some of the components of human milk motivates formula companies to augment their formula supplements, which implies that formula supplements cannot full copy human milk.46 Examples of additional proteins and amino acids and their function in human milk include growth factors for gastrointestinal cellular development and tissue repair, lysozyme for destruction of gram-negative bacteria and amylase for digestion and absorption, carnitine for ketogenesis and thermogenesis, taurine involved in bile acid conjugation, neurotransmission, and neuromodulation, and glutamine and glutamic acid, providing energy substrates and neurotransmission in the brain.1,2 Amino acid content is specific to the unique physiologic characteristics of the human newborn. For example, there are higher proportions of docosahexaenoic acid (DHA) in preterm mothers’ milk than in term mothers’ milk as it is needed for the less mature infants’ neurodevelopment and function.8
Human milk is rich in immunologic substances that provide passive immunity and protect the infant from infections (especially gastrointestinal and respiratory infections) (Table 5-4). It serves as an important facilitator of the initiation and development of the microflora in the infant gastrointestinal system, such as bifidus factors.24 Human milk may protect against the development of allergies—both by reducing exposure of the infant to bovine milk allergens and by supplying secretory IgA, which reduces intestinal absorption of potentially antigenic proteins before gut closure at 6 to 9 months. Cellular components of human milk include sloughed epithelial cells, macrophages, neutrophils, and lymphocytes.
Table 5-4
Protective Components in Human Milk
| IMMUNE PROTECTION | FUNCTION |
| sIgA, G, M D, E nonspecific protection |
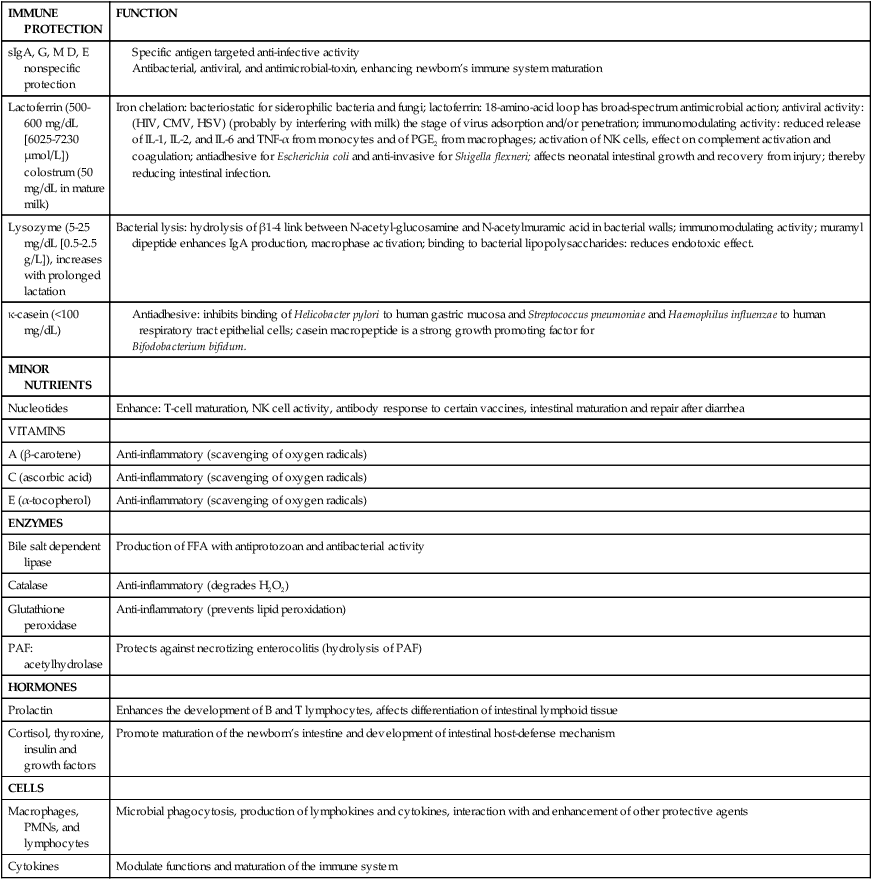
Adapted from Hamosh, M. (2001). Bioactive factors in human milk. Pediatr Clin North Am, 48, 72.
Human milk for the preterm infant
By 16 weeks’ gestation, the breast is prepared for lactation. The milk of a woman who delivers a preterm infant (less than 37 weeks) differs from that of a woman who delivers at term. Preterm breast milk has higher protein content and anti-infective properties, including secretory IgA and lactoferrin, higher fatty acids (such as docosahexaenoic acid [DHA]), oligosaccharides, fat, sodium, chloride, and iron content.8,70 Research has found that proportions of fat and fatty acids were higher in milk from mothers of preterm infants born at earlier gestations.56 The bioactive factors in human milk support the preterm infant’s immature immune system. When fed human milk rather than formula, preterm infants better tolerate feedings and have lower rates of bacterial, viral, and protozoan infections.11 Research has shown that preterm infants fed human milk have lower risk of infections including necrotizing enterocolitis (NEC), an especially threatening infection for preterm infants.71,75
Considering the high nutritional requirements of preterm infants and the lower presence of some components in preterm human milk, partially associated with the collection, storage, and delivery of human milk, it has been recommended to fortify human milk with iron, minerals, proteins, and fatty acids (to name a few fortifier components).7,70 Recent research raises some concern about human milk fortification (HMF) since the source is usually bovine rather than human milk which has higher iron supplementation which may decrease lactoferrin antibacterial activity, potentially decreasing the anti-infective properties of human milk.11 There is recent research supporting the use of human milk based human milk fortification (HMF) rather than bovine milk based human milk fortification (HMF) because of the increased risk of inhibition of antibacterial activity associated with bovine products added to human milk.12,75 Use of human milk and human milk fortifiers for feeding low-birth-weight infants is discussed in Chapter 12.
During early lactation, increasing the frequency of feedings is important in establishing adequate milk production. This is especially true for the mother of a preterm infant because the infant is often transferred to the neonatal intensive care unit and is not located with the mother. Furthermore, the infant may be unable to tolerate enteral feedings or may have difficulty with the techniques needed for breastfeeding. Early and frequent breast stimulation and milk expression is recommended to help establish and maintain milk supply.30 Researchers found that optimal milk production in mothers of preterm infants was associated with five or more milk expressions per day and pumping duration exceeding 100 min/day.32 Skin-to-skin contact (kangaroo care) has been demonstrated to improve breastfeeding outcomes.9
Nutrition during the postpartum period and lactation
Adequate nutrition is necessary to promote healing and restoration during the postpartum period. Lactation increases the nutritional demand on the breastfeeding woman. Maternal basal metabolic rate and cardiac output are greater with increased blood flow to the liver and gastrointestinal system to meet demands for specific nutrients and precursors as well as the redistribution of blood to augment the supply of nutrients and precursors to the mammary glands. For the healthy, well-nourished lactating woman, an additional 500 kcal is recommended to meet the energy requirements for milk production during the first 6 months of lactation. Approximately 100-150 kcal of the increased requirements are provided by maternal fat stores from pregnancy.21 Additional nutrition may be required for the undernourished woman or the woman breastfeeding multiple children. Once lactation is established, an overweight or obese woman may restrict her caloric intake by 500 kcal per day to facilitate postpartum weight loss without negatively impacting growth of the breastfed infant.47 Guidance and consultation on a healthy approach to postpartum weight loss in the breastfeeding woman is recommended.
Maternal diet influences water-soluble vitamins because they easily move from serum to milk.82 Intake of water-soluble vitamins is increased, as are protein (65 g), calcium (1200 mg), vitamin D (10 mcg), vitamin E (12 mcg), and folate (280 mcg).21 Fat-soluble vitamins are stored in fat and are more difficult to adjust by diet. Thus vitamin D supplementation is recommended for breastfed infants of women with insufficient vitamin D stores, especially those insufficiently exposed to ultraviolet light.82 Cultural practices such as fasting may also affect the quality of a woman’s milk and should be carefully considered and discussed in a respectful and health promotional manner.66 Women who restrict their milk and dairy intake should be assessed for possible nutrient deficiencies, especially calcium and vitamin D.50 Regarding fluid intake, lactating women should drink 600-700 mL additional healthy nutritional fluids and water per day, with extra attention to environmental and seasonal issues.48 Changes in nutritional requirements during lactation are summarized in Table 5-5.
Table 5-5
Recommended Daily Dietary Allowances for Lactation
| FIRST 6 MONTHS | SECOND 6 MONTHS | |
| Energy (kcal) | +500 | +500 |
| Protein (gm) | 65 | 62 |
| Vitamin A (RE*) | 1300 | 1200 |
| Vitamin D (mcg) | 10 | 10 |
| Vitamin E activity (mg αTE)† | 12 | 11 |
| Ascorbic acid (mg) | 95 | 90 |
| Folacin (mcg) | 280 | 260 |
| Niacin (mg)‡ | 20 | 20 |
| Riboflavin (mg) | 1.8 | 1.7 |
| Thiamin (mg) | 1.6 | 1.6 |
| Vitamin B6 (mg) | 2.1 | 2.1 |
| Vitamin B12 (mcg) | 2.6 | 2.6 |
| Calcium (mg) | 1200 | 1200 |
| Phosphorus (mg) | 1200 | 1200 |
| Iodine (mcg) | 200 | 200 |
| Iron (mg) | 15 | 15 |
| Magnesium (mg) | 355 | 340 |
| Zinc (mg) | 19 | 16 |
†α-Tocopherol equivalents: 1 mg d-α-tocopherol = 1 αTE.
‡Although allowances are expressed as niacin, it is recognized that on average, 1 mg of niacin is derived from 60 mg of dietary tryptophan.
Modified from Food and Nutrition Board, National Research Council. (1989). Recommended dietary allowances (10th ed.). Washington, DC: Government Printing Office; Worthington-Roberts, B.S. & Williams, S.R. (1997). Nutrition in pregnancy and lactation (6th ed.). Madison, WI: Brown & Benchmark.
Common breastfeeding problems
Milk stasis, due to infrequent, insufficient, or missed feedings, may lead to breast engorgement. Frequent and sufficient suckling or milk expression such as by pumping reduces the congestion and provides relief, along with proper use of antiinflammatory medication. Application of warm compresses prior to breastfeeding promotes milk flow. Brief milk expression immediately before breastfeeding reduces areolar distention related to engorgement, thereby facilitating infant latching. Milk expression between breastfeeding sessions causes overstimulation of milk production and is not recommended. Interventions such as application of cold packs and cabbage leaves have not been conclusively demonstrated to resolve engorgement or relieve symptoms and more research in the area is needed.49 Untreated and extended milk stasis causes increased pressure in the breast and decreases capillary blood flow, decreasing milk synthesis and inhibiting lactation. Extended insufficient breast stimulation, by lack of either infant suckling or milk expression, will reduce milk synthesis and inhibit lactation. Increasing frequency of breast stimulation through increased breastfeeding frequency or pumping often resolves insufficient milk production, although some women require use of galactagogues to augment milk production.22
Consultation with expert resources regarding maternal medication during lactation is important because medication may transfer into milk (see Chapter 7). The amount of drug secreted into human milk depends upon the drug’s lipid solubility, molecular weight, protein binding, ability to pass into the brain, and maternal plasma concentration.25 Consideration of alternative, safer medications for the breastfeeding mother may preserve the breastfeeding relationship when a mother is required to take medication while breastfeeding. The safety of maternal ingestion of alcohol during lactation has not been verified and should be avoided.45
Summary
The postpartum period is a time of rapid and complex change as the woman recovers from labor and delivery and undergoes reversal of the anatomic, physiologic, and endocrine changes of pregnancy, as well as changes to support lactation. These changes provide the background for the new mother’s physical function, sense of well-being, and adaptation to her new role and her infant. Understanding of the physiologic basis of lactation is essential in intervening appropriately to support the lactating woman and in assisting with problems. Clinical recommendations related to postpartum involutional changes are summarized in Table 5-6.
Table 5-6
Summary of Recommendations for Clinical Practice Related to Involutional Changes and Lactation
Recognize and monitor the progress of normal postpartum involutional changes for each of the reproductive organs (pp. 142-144).
Observe for signs of uterine subinvolution, hemorrhage, and infection (pp. 142-143).
Monitor color and characteristics of lochia flow (p. 143).
Teach postpartum women the physiologic and anatomic changes to expect during the postpartum period (pp. 142-144).
Teach women signs and symptoms of complications and infection (pp. 142-143).
Provide comfort to postpartum women experiencing afterpains (p. 142).
Counsel women regarding urinary system changes postpartum (p. 143 and Chapter 11).
Counsel women and their partners regarding changes in vaginal tone and lubrication postpartum (p.144).
Counsel women and their partners regarding resumption of postpartum sexual activity (p. 144).
Counsel women regarding the expected timing for resumption of menstruation and ovulation (pp. 145-146).
Counsel women regarding methods and risks of family planning (including lactational amenorrhea method) to reduce risk of unplanned pregnancy (pp. 145-146).
Counsel women regarding the benefits of breastfeeding and human milk (pp. 153-158).
Teach women the physiology of lactation and milk production (pp.147-158).
Encourage and facilitate early initiation of breastfeeding after delivery (pp. 153-158).
Encourage frequent breastfeeding (on demand) or milk expression (pp.153-158).
Recognize breast engorgement and implement interventions appropriate for breastfeeding and non-breastfeeding women (pp. 150-151, 159-160).
Teach women interventions to reduce the risk of milk stasis, engorgement, and infection (pp. 150-151, 159-160).
Assist women who are pumping to increase their milk volume by increasing the frequency of pumping as well as other lactation promotional activities (pp. 158-160).
Counsel women to abstain from alcohol intake during lactation and to consult with health care provider regarding medication during lactation (p.160 and Chapter 7).
Know the composition of human milk and the factors that influence composition and volume (pp. 153-160).
Know the advantages and limitations of human milk for preterm infants (pp. 157-158 and Chapter 12).
Support breastfeeding in mothers of term and preterm infants (pp. 153-160).
Assess nutritional status and counsel women regarding nutritional needs postpartum (p. 159).
Counsel lactating women regarding nutritional requirements and effects of their nutritional status on milk production (p. 159).