Gastrointestinal and hepatic systems and perinatal nutrition
The gastrointestinal (GI) system consists of processes involved in intake, digestion, and absorption of nutrients and elimination of by-products in bile and stool.249 Utilization of nutrients for production of energy and other vital functions is discussed in Chapters 16 and 17. This chapter focuses on the processes involved in preparing nutrients for absorption across the intestinal villi.
Maternal nutrition is one of the most important factors affecting pregnancy outcome. The maternal GI tract must digest and absorb nutrients needed for fetal and placental growth and development and to meet the altered demands of maternal metabolism as well as eliminate unneeded by-products and waste materials from both the woman and the fetus. Structural and physiologic immaturity of the neonate’s GI tract can result in alterations in neonatal nutritional status and increase the risk of malabsorption and dehydration. This chapter reviews GI and hepatic function in the pregnant woman, fetus, and neonate and implications for clinical practice. Hepatic function related to drug metabolism is discussed in Chapter 7.
Maternal physiologic adaptations
Antepartum period
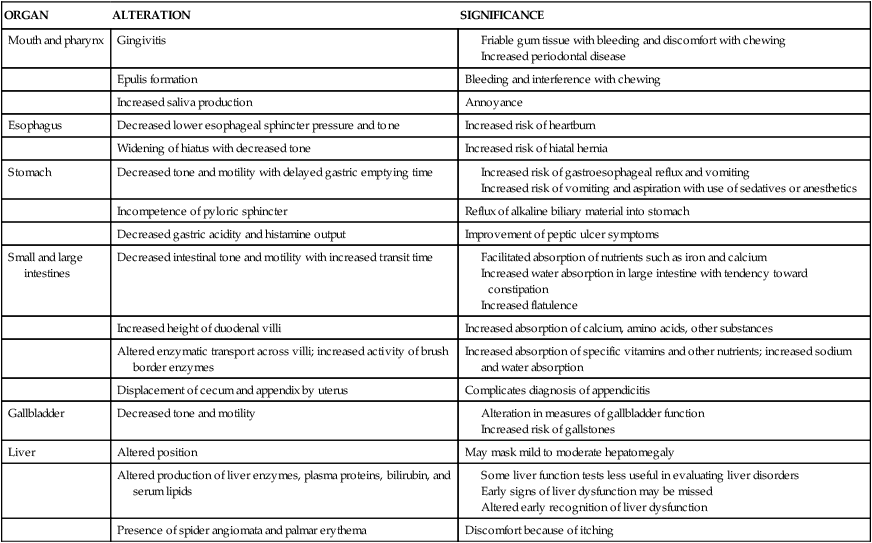
The antepartum period is characterized by anatomic and physiologic changes in all the organs of the GI system. These changes and their implications are summarized in Table 12-1. Pregnancy is associated with increased appetite; increased consumption of food; and alterations in the types of food desired, including cravings, avoidance of certain foods, and, rarely, pica (craving for nonnutrient substances). Specific changes in food consumption and the types of foods craved or avoided are strongly influenced by cultural and economic factors. Food consumption has been reported to increase 15% to 20% beginning in early pregnancy, peaking at mid-gestation and decreasing near term.207 Alterations in food intake and appetite create a positive energy balance during pregnancy to meet the needs of the pregnant woman and fetus and to prepare for lactation (see Chapter 16).128 Changes in maternal caloric intake do not parallel changes in basal metabolism or fetal growth.
Table 12-1
Alterations in the Gastrointestinal System during Pregnancy
| ORGAN | ALTERATION | SIGNIFICANCE |
| Mouth and pharynx | Gingivitis |
The basis for changes in patterns of food intake is unclear but may be a response to the movement of glucose and other nutrients to the fetus, alterations in taste threshold and acuity, and hormonal changes. Estrogen acts as an appetite suppressant and progesterone as an appetite stimulant. Influences of estrogen and progesterone on patterns of food intake are supported by similar changes during the menstrual cycle. Decreased appetite and food intake have been reported during the follicular phase of the menstrual cycle (when estrogen peaks), and with increased appetite during the luteal phase (when progesterone peaks).249 During pregnancy, alterations in insulin and glucagon combine with estrogen and progesterone to influence food intake.249 Leptin serum levels parallel changes in body mass index (BMI) during pregnancy and may also mediate maternal appetite changes. The increased food intake with increased leptin levels suggests the development of leptin insensitivity or resistance, which is mediated by human placental lactogen.128
Mouth and pharynx
Contrary to the old wives’ tale regarding the loss of a tooth per baby, pregnancy does not result in demineralization of the woman’s teeth. Fetal calcium needs are drawn from maternal body stores, not from the teeth (see Chapter 17). The major component of tooth enamel (hydroxyapatite crystals) is not reduced by the biochemical or hormonal changes of pregnancy.156,167,214 As a result of gingival alterations, however, the pregnant woman may become more aware of preexisting or newly developed dental caries. Changes in saliva and the nausea and vomiting of pregnancy may increase the risk of caries during pregnancy although this has not been well studied.167 Dental plaque, calculus, and debris deposits increase during pregnancy and are associated with gingivitis.21,214 In addition, there may be a transient increase in tooth mobility.214
Pregnancy may exacerbate existing periodontal disease with an increase in periodontal pocket depth during gestation.165 Periodontal disease has also been associated with intrauterine infection and an increased risk of preterm birth and low–birth weight risk in some but not all studies.2,82,150,191,248,261 The mechanism is unclear but may be due to either alterations in maternal and fetal immune responses or translocation of oral bacteria into the uterus with colonization and inflammation of the placenta.95 Increased prostaglandin (PG) synthesis mediated by proinflammatory cytokines from inflamed gingival tissues or via release of bacterial endotoxins could then initiate labor onset (see Chapter 4).150,261 These studies lack consistent definitions of periodontal disease with lack of control for confounding variables such as socioeconomic status and smoking in some studies.261 Several recent meta-analyses concluded that oral prophylaxis and treatment of periodontal disease may reduce preterm and low–birth weight rates and that further evaluation is needed; another analysis did not find an association.82,191,248
Gingivitis occurs in 30% to 80% of pregnant women, generally beginning around the second month and peaking in the middle of the third trimester.156,167,214 The anterior region of the mouth is usually the most affected site.21 Gingival tissue contains both estrogen and progesterone receptors.21 Estrogen increases blood flow to the oral cavity and accelerates turnover of gum epithelial lining cells. The gums become highly vascularized (with proliferation of small blood vessels and connective tissue), hyperplastic, and edematous.21,62,156 Progesterone and estradiol may stimulate located inflammation via production of PGs and decreased levels of inflammatory inhibitors.21 Development of gingivitis may be related to these alterations in the inflammatory process during pregnancy (see Chapter 13), with increased intensity of localized irritation, or to changes in connective tissue metabolism.21,143 These changes, along with the decreased thickness of the gingival epithelial surface, result in friable gum tissues that may bleed easily or cause discomfort with chewing. Bleeding with brushing occurs more frequently during pregnancy. The incidence of gingivitis is higher with increasing maternal age and parity, preexisting periodontal disease, and poor dentition.156,208
In up to 5% of pregnant women, a specific angiogranuloma known as an epulis or pregnancy tumor develops.214,249 Epulis formation generally occurs between the second and third month, but can occur later.21,167 Epulis may gradually increase in size, but rarely is larger than 2 cm in diameter. The etiology is unknown but is thought to relate to hormonal changes (epulis tissue has estrogen and progesterone receptors) and inflammation.21 Epulis formation is characterized by gingivitis that is advanced and severe. There is a hyperplastic outgrowth that is generally found along the maxillary gingiva and often appears between the upper anterior maxillary teeth.21 This mass is purplish red to dark purple, very friable, bleeds easily, and often interferes with chewing.21 Epulis is usually painless, but may ulcerate and become painful in some women.21,167 Epulis usually regresses spontaneously after delivery, but may recur in the same locations with subsequent pregnancies. Occasionally these growths may need excising during pregnancy due to bleeding, interference with chewing, or increasing periodontal disease.156,214
Saliva becomes more acidic during pregnancy, with alterations in electrolyte content and microorganism load, but it usually does not increase in volume.167 Some women may experience a sense of increased saliva production due to difficulty in swallowing saliva during the period of nausea and vomiting in early pregnancy.156 A few women do experience excessive salivation (ptyalism). This uncommon disorder begins as early as 2 to 3 weeks and ceases with delivery. The excessive salivation seems to occur primarily during the day.62 The pathogenesis of ptyalism is unknown, but it is thought to be due to increased saliva, the inability to swallow due to nausea, or activation of the esophagosalivary reflex during gastroesophageal reflux (GER).214
Esophagus
Lower esophageal sphincter (LES) tone decreases. This decrease is thought to be primarily due to the smooth muscle relaxant activity of progesterone.61,117 The LES is a pressure barrier between the stomach and the esophagus, acting as a protective mechanism to prevent or minimize GER. Resting LES pressure decreases during pregnancy with decreased responsiveness to hormonal and physiological stimuli.61,62,117 At the beginning of the second trimester, basal LES tone is unchanged, although a marked decrease in the normal rise in LES pressure in response to stimulation with a protein meal has been reported.62,203 This suggests an inhibitory effect and may signal the loss of an important protective response—that is, the ability to modify LES pressure in response to increased intragastric pressure so that reflux is prevented.62 LES pressure gradually falls by 33% to 50%, with most of the decrease occurring in the third trimester and reaching a nadir at about 36 weeks.203
Changes in the LES in pregnancy are similar to changes seen during the ovarian cycle and in women on oral contraceptives, supporting the theory of a hormonal cause for this alteration. An increased incidence of acid reflux with heartburn, which is associated with decreased LES pressure, is seen in nonpregnant women during the luteal phase of the ovarian cycle, when progesterone levels are highest.203,249 After delivery or discontinuance of oral contraceptives, LES function returns to normal.62,76,249 Alterations in LES tone and pressure are etiologic factors in the development of heartburn during pregnancy.
Other changes in the esophagus during pregnancy include an increase in secondary peristalsis and nonpropulsive peristalsis and increased incidence of hiatal hernia. Flattening of the hemidiaphragm causes a loss of the normal acute esophageal-gastric angle, which may also lead to reflux.62
Stomach
The stomach of the pregnant woman tends to be hypotonic with decreased motility due to actions of progesterone. GI motility is decreased, with prolonged small intestinal transit time. Incompetence of the pyloric sphincter may result in alkaline reflux of duodenal contents into the stomach.62,117 Gastric emptying time is thought to be unchanged.61,76,204,214,228,242,259
The effect of pregnancy on gastric acid secretion is unclear. In several studies, gastric volume was not increased nor was gastric pH decreased during early pregnancy.117,242 Others have reported a decrease in acidity during the first and second trimesters along with normal gastrin levels, with an increase in acidity to greater than nonpregnant values during the third trimester, accompanied by an increase in gastrin.214 In general there seems to be a tendency for decreased gastric acidity in pregnancy, especially during the first and second trimesters, with an increase in the third along with small but statistically significant decreases in both basal and histamine-stimulated acid output.62 Women with peptic ulcers tend to have fewer symptoms during pregnancy, partly because of these changes (see Pregnancy in Women with Peptic Ulcer Disease).117
Secretion of pepsin parallels changes in gastric acid output.61 Decreased gastric acidity is thought to result from hormonal influences (particularly estrogen) and increased levels of placental histaminase.62,208 Placental histaminase is thought to mediate acid and pepsin secretion by reducing parietal cell responsiveness to endogenous histamine.62 Gastrin levels are normal during most of pregnancy, with marked increases late in the third trimester, at delivery, and immediately after delivery. The additional gastrin is probably of placental origin.
Small and large intestines
The action of progesterone on smooth muscles decreases intestinal tone and motility. The decreased motility observed in pregnancy may not necessarily be a direct effect of progesterone however, but rather due to inhibition by plasma motilin.228 Decreased GI tone leads to prolonged intestinal transit time, especially during the second and third trimesters. Alterations in transit time increase with advancing gestation, paralleling the increase in progesterone.
Intestinal transit times during stages of pregnancy have been compared with phases of the ovarian cycle in nonpregnant women. Intestinal motility is altered and transit time prolonged during late pregnancy and the luteal phase of the ovarian cycle when progesterone secretion is elevated. The prolonged transit time in late pregnancy is due to an increase in small bowel transit secondary to inhibition of smooth muscle contraction and not to delayed gastric emptying time. The woman may experience a sense of “bloating” and abdominal distention secondary to the delay in intestinal transit times.214
The height of the duodenal villi increases (hypertrophies) during pregnancy, which in turn increases absorptive capacity.117 This change, along with the influences of progesterone on intestinal transit time and increased activity of brush border enzymes, increases the absorptive capacity for substances such as calcium, lysine, valine, glycine, proline, glucose, sodium, chloride, and water.117,249 Progesterone also increases lactase and maltase activity. Absorption of other nutrients (including niacin, riboflavin, and vitamin B6) is reduced, perhaps due to the influence of progesterone on enzymatic transport mechanisms.117,214,249 Duodenal absorption of iron increases nearly twofold by late pregnancy, probably in response to a reduction of maternal circulating iron stores due to uptake by the placenta and fetus.214 As a result of the decreased intestinal motility, nutrients and fluids tend to remain in the intestinal lumen for longer periods of time. This may facilitate absorption of nutrients such as iron and calcium. The amount and efficiency of intestinal calcium absorption increase, mediated primarily by increased 1,25-dihydroxyvitamin D (see Chapter 17).
Progesterone may also enhance absorption of calcium, sodium, and water and increase net secretion of potassium.214,249 The reduced motility and increased transit time in the large intestine increase water and sodium absorption in the colon.117 Stools are smaller with lower water content, which contributes to development of constipation during pregnancy. Increased flatulence may also occur due to decreased motility along with compression of the bowel by the growing uterus. The appendix and cecum are displaced superiorly by the growing uterus, so that by term the appendix tends to be located along the right costal margin.
Pancreas
The pancreas contains estrogen receptors, which in the rich estrogen environment of pregnancy may increase the risk of pancreatitis.249 Serum amylase and lipase decrease during the first trimester. The significance of this change is unclear. Changes in the islet cells and the increased production and secretion of insulin are discussed in Chapter 16.
Gallbladder
Muscle tone and motility of the gallbladder decrease during pregnancy, probably due to the effects of progesterone on smooth muscle. As a result, gallbladder volume is increased and emptying rate decreased, especially in the second and third timesters.61,214 Most measures of gallbladder function are altered during pregnancy, especially after 14 weeks. Some studies have reported that fasting and residual volumes increase to about 20 weeks’ gestation, then remain high to term, paralleling the increase in progesterone.76 The residual gallbladder volume after fasting and emptying is nearly twice as large in the pregnant woman as in nonpregnant women who are not taking oral contraceptives. The increased fasting volume may also be due to decreased water absorption by the mucosa of the gallbladder. This change results from reduced activity of the sodium pump in the mucosal epithelium secondary to estrogens. As a result, bile is more dilute, with a decreased ability to solubilize cholesterol. The sequestered cholesterol may precipitate to form crystals and stones, increasing the tendency to form cholesterol-based gallstones in the second and third trimesters.61,76,142 In the third trimester, bile is supersaturated with lithogenic cholesterol, which, in conjunction with biliary stasis and sludging, increases the risk of gallstones (see Cholelithiasis and Pregnancy).76,142 Alterations in gallbladder tone also lead to a tendency to retain bile salts, which can lead to pruritus.
Liver
During pregnancy the enlarging uterus displaces the liver superiorly, posteriorly, and anteriorly. Hepatic blood flow per se is not significantly altered despite marked changes in total blood volume and cardiac output. This is because much of the increased cardiac output is sent to the uteroplacental circulation. As a result, the proportion of cardiac output delivered to the liver remains constant at 25% to 35%.121 Histologically, only minor nonspecific changes in the liver such as increased fat and glycogen storage and variations in cell size have been reported. The size of the liver does not increase.258
Liver production of plasma proteins, bilirubin, serum enzymes, and serum lipids is altered. These changes arise primarily from estrogen, which increases the rough endoplasmic reticulum and liver protein synthesis, and in some cases from hemodilution.258 Progesterone increases proliferation of the smooth endoplasmic reticulum and cytochrome P450 isoenzymes.258 Changes in liver products during pregnancy and their significance are summarized in Table 12-2.
Table 12-2
Liver Function Tests in Normal Pregnancy and Postpartum
| SUBSTANCE | PREGNANCY EFFECT | TRIMESTER OF MAXIMUM CHANGE | RETURN TO NONPREGNANT LEVEL | BASIS AND IMPLICATION |
| Albumin | ↓ 20%-40% | 2 | ? | Result of hemodilution and increased catabolism; leads to decreased protein for binding and increased concentrations of free substances |
| γ-Globulin | N to sl ↓ | 3 | ? | Transfer of immunoglobulin G to fetus in third trimester for protection of fetus from infection |
| α-Globulin | sl ↑ | 3 | 3 weeks | Facilitation of transport of lipids and carbohydrates to the placenta, as well as transport of increased maternal thyroid hormones |
| β-Globulin | sl ↑ | 3 | 3 weeks | Facilitation of transport of lipids, carbohydrates, and iron to placenta, as well as transport of hormones |
| Total protein | ↓ 20% | 2 | ? | Primarily related to a fall in albumin; decreases protein-bound substances and increases concentrations of free protein for transport across the placenta |
| TBG | ↑ 2- to 3-fold | 1-3 | Decreases with removal of placenta and decreased estrogen | Increases beginning within a few weeks after fertilization and plateaus from midgestation to delivery; alters concentration of free versus bound thyroid hormone |
| CBG | ↑ 2- to 3-fold | Decreases with removal of placenta and decreased estrogen | Alters concentration of free versus bound cortisol | |
| Fibrinogen | ↑ 50% | 2 | 2-3 weeks | Protection against excessive blood loss at delivery by facilitating clotting |
| Ceruloplasmin | ↑ | 3 | 3 weeks | Involved in the transport of most of the body copper needed by mother, fetus, and placenta |
| Transferrin | ↑ | 3 | ? | Involved in the binding and transport of iron to meet increased maternal and fetal needs |
| Bilirubin | N | — | — | May be a slight increase in bilirubin clearance due to maternal clearance of bilirubin from fetus |
| BSP | N to sl ↑ | 3 | Soon after delivery | Increased removal associated with decreased albumin; alterations may reflect the mild cholestasis seen in pregnancy |
| AST (SGOT) and ALT (SGPT) in pregnancy | sl ↓ in upper limit | — | —* | Do not change significantly during pregnancy, so these enzymes can be used as indicators of liver or other organ damage during pregnancy |
| AST (SGOT) and ALT (SGPT) in labor | ↑ | In labor | By 2-3 weeks* | Increase during labor and delivery, perhaps reflecting the effects of the mechanical forces of labor |
| GGT | sl ↓ in upper limit | 3 | ?* | Important in synthesis of amino acids for maternal and fetal use |
| Alkaline phosphatase | 2- to 4-fold ↑ | 3 | Usually by 3 weeks | Much of increase is probably a result of increased production by the fetus and placenta rather than the maternal liver |
| Lactic dehydrogenase in pregnancy | sl ↑ | 3 | ? | Enzyme associated with tissue injury that catalyzes lactic acid to pyruvic acid |
| Lactic dehydrogenase in labor | ↑ | In labor | By 2-3 weeks | Increases further during labor, perhaps reflecting the effects of the mechanical forces of labor |
| Cholesterol | 1½- to 2-fold ↑ | 2-3 | By 10 days with significant decrease within 24 hours | Essential precursor for many lipid substances; needed for alterations in lipid metabolism and increased demands for lipids during pregnancy including production of estrogens and progesterone by the placenta |
*May rise for up to 10 days postpartum, especially after cesarean delivery.
Modified from Monheit, A.G., Cousins, L., & Resnik, R. (1980). The puerperium: Anatomic and physiologic adjustments. Clin Obstet Gynecol, 23, 973.
Although liver function is not impaired during pregnancy, most of the changes in liver function tests are in the same direction as seen in individuals with liver disorders. Some liver function tests are less useful in evaluating liver disorders during pregnancy; other tests such as aspartate aminotransferase (AST, or serum glutamic-oxaloacetic transaminase [SGOT]), alanine aminotransferase (ALT, or serum glutamic-pyruvic transaminase [SGPT]), and bilirubin are only slightly lower than nonpregnant values.61,110,209 Changes in hepatic metabolism of drugs during pregnancy are discussed in Chapter 7.
Spider angiomas (also called spider nevi) and palmar erythema (common findings in many liver disorders) are thought to be caused by estrogens and are seen in many pregnant women (see Chapter 14). These findings tend to develop between the second and fifth months and disappear or diminish following delivery. Increases in the size of previously existing spider angiomas may also be noted.
Weight gain during pregnancy
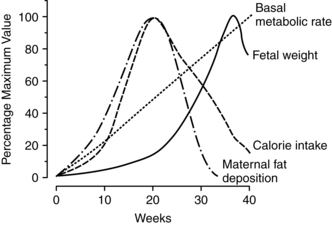
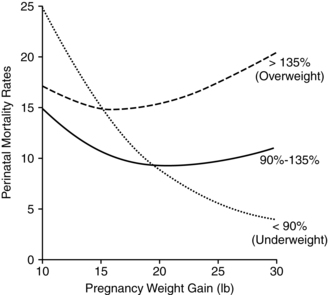
Weight gain during pregnancy reflects increased maternal stores as well as those of the developing fetus and placenta (Figure 12-1). Approximately 62% of the gain is water, 30% fat, and 8% protein. About 25% of the total gain is attributable to the fetus, 11% to the placenta and amniotic fluid, and the remainder to the mother.101 Optimal weight gain during pregnancy varies with maternal prepregnancy weight; greater weight gain is generally recommended in women who are underweight, and a lower total weight gain is preferable for women who are obese. Maternal weight gain per se lacks sensitivity and specificity as a predictor of pregnancy outcome, in that many women with good pregnancy outcomes have weight gains outside the recommended range.37,115 However, higher or lower than usual weight gains do increase the risk of maternal and fetal complications and perinatal mortality rates (Figure 12-2).1,93,215
Body mass index (BMI) and energy expenditure (see Chapter 16) must also be considered. Prepregnancy BMI and adiposity also affect perinatal outcome. Underweight women have an increased risk of preterm and small-for-gestational-age (SGA) infants with decreased macrosomia, preeclampsia and cesarean birth; overweight women have an increase in spontaneous abortion, congenital anomalies, intrauterine death, gestational diabetes, hypertensive disorders, preeclampsia, thromboembolic complications and cesarean birth, and decreased risk of SGA and growth-restricted infants.215
The Institute of Medicine (IOM) guidelines for weight gain and the pattern of gain based on prepregnancy weight for height were published in 1990.103 These guidelines generated controversy, particularly in relation to the weight gain range for obese women, and concern that using them would increase the number of large-for-gestational-age (LGA) infants and maternal and fetal complications.37,115,202,215 Abrams and colleagues reviewed pregnancy outcomes from studies using the IOM guidelines and found that weight gain within these recommendations was associated with the best maternal and fetal outcome.1 However, they noted that in most women, weight gain was not within the IOM guidelines.1 In other studies, only about one third of pregnant women gained weight within the recommended limits, with most gaining more than recommended.37,38 Chu et al. found that using the 1990 IOM guidelines 30% of normal weight women and 60% of overweight women gained more than recommended; excessive gestational weight gain was correlated with weight retention postpartum and later obesity.52 Others have found similar outcomes.178
In response to concerns of excessive weight gain and later development of obesity, the IOM recently revised these guidelines (see Table 12-3). The revised guidelines also added recommended gain for three subcategories of obesity (class I, II, and III). The current IOM guidelines assume a 1.1- to 4-lb weight gain during the first trimester, followed by a recommended 0.8 to 1 lb (0.36 to 0.45 kg) per week for the second and third trimesters in normal BMI women, with higher gains of 1 to 1.3 lb (0.45 to 0.59 kg) per week in underweight and less per week in overweight women (0.6 to 0.7 lb or 0.23 to 0.32 kg). Obese women are recommended to restrict weight gain during the first trimester to 0.4 to 0.6 lb (0.18 to 0.27 kg).181,198,199
Table 12-3
| PREPREGNANCY BMI | BMI* (kg/m2) | TOTAL WEIGHT GAIN (lb)+ | 2nd AND 3rd TRIMESTER MEANS (RANGE) GAIN (lb)/WEEK |
| Underweight | <18.5 | 28-40 | 1 (1-1.3) |
| Normal weight | 18.5-24.9 | 25-35 | 1 (0.8-1) |
| Overweight | 25.0-29.9 | 15-25 | 0.6 (0.5-0.7) |
| Obese (includes all categories) | >30.0 | 11-20 | 0.5 (0.4-0.6) |
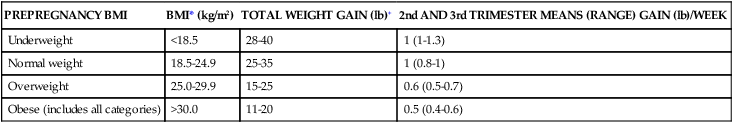
*BMI is calculated using metric units (BMI = kg/m2 × 100).
+Calculations assume a 0.5-2 kg (1.1-4.4 lb) weight gain in the first trimester.
From Rasmussen, K.M. & Yaktine, A.L. (2009). Weight gain during pregnancy: reexamining the guidelines. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM pregnancy weight guidelines. Washington, DC: National Academies Press.
Weight gain during the first trimester goes primarily toward maternal fat stores (see Figure 12-1). Weight gained during the second half of pregnancy goes toward growth of the fetus and maternal supportive tissues. Marked or persistent deviations from these patterns should be evaluated.198,199 Women whose weight gain significantly exceeds these limits or deviates from the expected pattern require similar evaluation. For example, if a woman gains 30 lb in the first 20 weeks of pregnancy, she has added approximately 25 lb (11.33 kg) of fat to her stores (and additional fat is often difficult to lose postpartum). This woman will still need to gain approximately 20 lb (9.07 kg) during the second half of pregnancy to ensure adequate growth of the fetus and her own tissues.208 Greater gains are needed in women with multifetal pregnancies.109,181 These women also have increased nutritional needs, including higher energy, vitamin D, linolenic and linoleic acids, calcium, and other minerals.44
Intrapartum period
Labor is accompanied by delays in gastric emptying times that differ from both third-trimester and nonpregnant values.61,259 Opioids may be the main factor in delayed gastric emptying during labor. Opioids also delay gastric emptying and increase the risk of aspiration if used in conjunction with general anesthesia. Reduced use of general anesthesia for delivery has reduced the numbers of women at risk for intrapartum aspiration. However, any use of general anesthesia with a pregnant woman, whether for delivery or nonobstetric surgery, requires careful monitoring and use of interventions to prevent vomiting and aspiration.
During labor, alkaline phosphatase levels, which double during pregnancy, increase further. Serum aminotransferases (AST and ALT), which only change slightly during pregnancy, and lactic dehydrogenase increase up to twice normal values.185 These enzymes are associated with tissue injury and may reflect the stresses of labor on the mother and placenta.
Postpartum period
During the postpartum period, the anatomic and physiologic changes within the GI and hepatic systems gradually return to their prepregnant state. Delivery results in an average weight loss of 4.5 to 5.8 kg (10 to 13 lb).60 Findings regarding postpartum weight loss are inconsistent. Some women have further weight loss during the first week; others have no change or gain weight. The increased adrenocortical hormone and arginine vasopressin activity associated with the stress of labor tends to lead to water and sodium retention that may prevent weight loss or lead to a gain. In general, after 4 days, most women begin to show some additional loss, averaging another 2.3 to 3.6 kg (5 to 8 lb) due to diuresis and 0.9 to 1.4 kg (2 to 3 lb) from involution and lochia by the end of the first week.58 Alterations in energy utilization may alter losses in nonlactating women.60
Most women lose weight steadily over the first 3 to 6 months postpartum, with the greatest loss in the first 3 months. Weight loss occurs sooner and to a greater degree in women of lower parity, age, and prepregnant weight.60 Lactation may facilitate postpartum weight and body fat loss as maternal tissue stores are catabolized to use as energy for milk production.60 Most women do not lose all of the pregnancy weight gain, with an average retention of 1 kg (2.2 lb) with each pregnancy; 10% retain more than 15 lb (6.8 kg).60 Women who are overweight or normal weight before pregnancy are more likely to retain weight following pregnancy than women who were underweight.60 Abrams and colleagues found no evidence that weight gain during pregnancy within the IOM recommendations increased the risk of weight retention following birth.1 However, weight gain above these recommendations was common and associated with an increased risk of further weight gain during the first postpartum year and perhaps later development of obesity.180,221 Most studies have small sample sizes and often do not control for activity, diet, and psychosocial factors.
Gingivitis often disappears after delivery but may last for up to 6 months postpartum.214 Epulis regresses and also usually disappears postpartum, but a scarred area may remain.214 LES pressure and tone return to normal levels by 4 to 8 weeks postpartum.203,214 Gallbladder volume returns to normal by 2 weeks postpartum.76 Gallbladder contractility is enhanced postpartum, enabling the previously atonic gallbladder to empty a larger proportion of its volume and expel microgallstones that developed during pregnancy.249 Expulsion of these stones can lead to a gallstone pancreatitis.
Most of the liver enzymes return to nonpregnant levels within 3 weeks of delivery.201 Fatty acids, cholesterol, triglycerides, and lipoproteins tend to reach normal levels by about 10 days.201 AST, ALT, and serum γ-glutamyl transferase (GGT) (see Table 12-2), which rose during the intrapartum period, may continue to rise for up to 10 days postpartum, especially following cesarean birth.201 These parameters generally reach nonpregnant levels by 2 to 3 weeks. Alkaline phosphatase decreases after delivery and usually returns to nonpregnant levels by 20 days, but may remain elevated for up to 6 weeks.185,201
The appendix returns to its usual position by 10 days postpartum.228 GI muscle tone and motility are decreased during the intrapartum and early postpartum periods. Decreased gastric motility along with relaxation of the abdominal musculature can result in gaseous distention 2 to 3 days postpartum. Decreased intestinal motility can lead to postpartum ileus and constipation. Bowel movements usually resume 2 to 3 days after birth, with resumption of normal bowel patterns by 8 to 14 days.
Clinical implications for the pregnant woman and her fetus
Nutritional requirements of pregnancy
The physical and physiologic demands of pregnancy on the mother, along with fetal needs for nutrients, significantly increase nutritional requirements during pregnancy (Table 12-4). Nutrient needs are also altered postpartum and during lactation (see Chapter 5). During pregnancy an additional 300 to 340 kcal per day are needed in the second trimester and up to 450 kcal per day are needed in the third trimester to meet energy and growth demands of the mother and fetus and to conserve protein for cell growth.181 Energy needs vary considerably from woman to woman with adaptations in individual metabolism to spare energy for fetal growth. Thus the diet should be individualized based on maternal age, trimester, BMI and activity.116 The woman meets the energy demands of pregnancy by increasing intake, decreasing activity, or limiting maternal fat storage (see Chapter 16).120 The total minimum energy costs of pregnancy average 80,000 kcal, although some women may need up to 120,000 kcal.215 Protein requirements increase to 60 g or slightly more to provide nitrogen for maternal, fetal, and placental tissue synthesis and growth.108,188 Sources of protein should contain all the essential amino acids. Adequate maternal intake of the essential fatty acids, linoleic acid, and α-linolenic acid (an omega-3 fatty acid) is important for fetal growth and development, especially brain and vision development.215
Table 12-4
Basis for Increased Nutrient Needs in Pregnancy
| NUTRIENT | REASON FOR INCREASED NEED IN PREGNANCY |
| Protein |
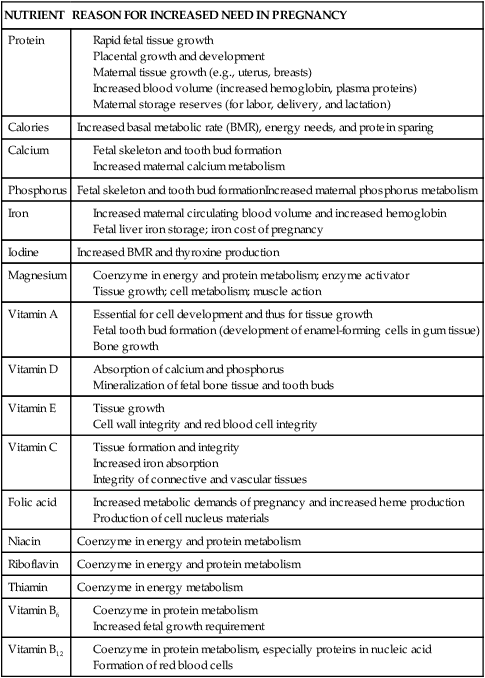
Adapted from Worthington-Roberts, B.S. & Williams, S.R. (1996). Nutrition in pregnancy and lactation. New York: McGraw-Hill.
Maternal plasma levels of water-soluble vitamins and many minerals gradually decline during gestation. This decline is probably primarily due to the effects of hemodilution rather than to greater fetal and maternal demands.207 With the exception of vitamin D (see Chapter 17) and iron (see Chapter 8), little is known about the effects of pregnancy on metabolism of most vitamins and minerals. Recommended dietary reference intakes (DRIs) for childbearing-age, pregnant, and lactating women are available from the Food and Nutrition Board of the National Academy of Science’s Institute of Medicine.104–108
During pregnancy, recommended dietary reference intakes for many vitamins are increased by 20% to 100%, including vitamin E, vitamin C, thiamin, riboflavin, niacin, vitamin B6, and vitamin B12.2,105,106,188,215 Vitamin E is essential during pregnancy for tissue growth and integrity of cell and red blood cell membranes. Vitamin C increases iron absorption and is needed for collagen formation and tissue formation and integrity. Thiamin, niacin, riboflavin, and vitamins B6 and B12 serve as coenzymes for protein and energy metabolism, which are increased in the pregnant woman.233 Zinc supplementation has been reported to increase birth weight in undernourished women with low serum zinc levels and decrease the risk of fetal growth restriction.8 Requirements for minerals and other vitamins are discussed in Chapter 8 (iron and folate), Chapter 17 (calcium, phosphorus, magnesium, and vitamin D), and Chapter 19 (iodine). Absorption of some minerals, including calcium, iron, zinc, and selenium, increases during pregnancy.8,121 Excessive intake or marked deficiency of specific vitamins and minerals has been reported to be associated with adverse pregnancy outcome, although the number of observations is limited. Excessive vitamin A (retinol) is associated with an increase in birth defects.8,159,188 The reader is referred to texts on nutrition during pregnancy for further discussion of nutritional assessment and requirements.103,207,233
The benefits of routine multivitamin supplementation other than for specific supplements such as folic acid and iron have not been clearly documented.215,217,223 The IOM recommendations indicate that pregnant women with balanced diets do not need routine multivitamin and mineral supplementation, except iron, and even routine iron supplementation is not without controversy (see Chapter 8).55,103 Folic acid supplementation is recommended for all women of childbearing age to reduce the incidence of neural tube defects (see Chapter 15).67 Additional multivitamin and mineral supplementation or supplementation of specific nutrients may be needed by women whose diet is inadequate (which may apply to women both below and above the poverty level) or who have a multiple pregnancy, smoke, or are alcohol or drug abusers. Micronutrient supplementation during pregnancy has been reported to decrease low birth weight but not to affect preterm birth rates or perinatal or neonatal mortality.51
Fetal nutritional needs
The fetus is dependent on the mother and placenta for transfer of nutrients essential for normal fetal growth and development. (Fetal growth and alterations are discussed in Development of the Gastrointestinal and Hepatic Systems in the Fetus.) Maternal nutrition during pregnancy, whether adequate, inadequate, or excessive, leads to “programming” of fetal tissues and may have long-term consequences for neurobehavioral outcomes and later health of offspring, including risks of hypertension, obesity, and cardiovascular disease.144
Nutritional needs of the fetus are met by three mechanisms depending on the stage of development. Before implantation, the blastocyst absorbs nutrients from its surrounding tissues and from fluids within the fallopian tube and uterus. Between implantation and placental development, nutrients are absorbed via a sinusoidal space between maternal and fetal tissues. With formation of the placenta, nutrients are transferred across this structure from mother to fetus via a variety of mechanisms (see Chapter 3). The energy needs of the fetus near term are met through carbohydrates (80%) and amino acids (20%).207 Fats are not used as a primary energy source by the fetus due to immaturity of fat metabolism. The major fetal energy source is glucose from the mother; free fatty acids are used as an alternative energy source and a substrate for lipid formation (see Chapter 16). Amino acids are actively transported from mother to fetus, and imbalances in maternal plasma amino acid concentrations can result in excessive fetal concentrations and subsequent damage as can occur in women with phenylketonuria (see Chapter 1).
Fetal needs for most vitamins and minerals can be met if maternal intake follows recommended DRIs.103 Lipid-soluble vitamins (A, D, E, and K) cross the placenta more readily than water-soluble vitamins and with increasing ease with advancing gestation. The vitamins most likely to be associated with deficiencies during pregnancy are folate and B6 (see Chapter 8).
Calcium and phosphorus are actively transported across the placenta, which allows accumulation of calcium and calcification of the fetal skeleton (see Chapter 17). The fetus needs micronutrients such as zinc, copper, chromium, iodine, magnesium, and manganese. Maternal dietary intake of these elements is usually sufficient. Iron supplementation is recommended to enhance maternal iron stores (see Chapter 8).
Heartburn and gastroesophageal reflux
Heartburn (reflux esophagitis with retrosternal burning) arises from reflux of gastric acids into the lower esophagus. Heartburn has been reported in up to 80% of women at some point during pregnancy, with an increased frequency seen in the third trimester.21,203,214,117,228 Heartburn usually begins during the second trimester, although about 25% experience heartburn in the first trimester. Heartburn intensifies with advancing gestation and disappears following delivery.203 Interventions are summarized in Table 12-5.
Table 12-5
Recommendations for Common Problems during Pregnancy Related to the Gastrointestinal System
Drink hot or cold liquids (especially on an empty stomach)
Eat high-fiber/bulk laxative foods such as fruits and raw vegetables
Eat high-fiber bran and wheat foods
Encourage regular light exercise during pregnancy
Use bulk-forming fiber-containing agents (which are not absorbed and are therefore safest)
Use stimulant laxatives with caution and avoid long-term use
Avoid use of mineral oil in pregnancy (absorbs fat-soluble vitamins including vitamin K)
Monitor for side effects if laxatives are prescribed (fluid accumulation, sodium retention and edema, cramping)
Monitor for drug interactions if laxatives are prescribed (decreased serum K with diuretics, decreased effectiveness of anticoagulants and salicylates)
Eat small, frequent, high-carbohydrate, low-fat meals and snacks
Avoid strong odors, fatty or spicy foods, and cold liquids
Consume dry crackers or toast before arising
Consume ginger (e.g., soda, tea, cookies, supplement)
Try elastic wrist bands (SeaBands)
Lie down when first experiencing symptoms
Practice relaxation techniques
Avoid factors and situations that precipitate symptoms
Monitor for side effects of pharmacologic agents (see pp. 404)

The pathogenesis of heartburn during pregnancy is multifactorial. The major etiologic factor is relaxation of the LES along with alterations in pressure gradients across the sphincter. In nonpregnant women, LES tone increases in response to elevations in intragastric pressure as a protective mechanism to prevent or minimize reflux. Alterations in LES tone during pregnancy eliminate or significantly reduce this protective mechanism.61,117,228 Pregnant women without heartburn tend to have LES pressures sufficient to maintain the normal pressure gradient across the gastroesophageal junction, whereas women with heartburn do not demonstrate this compensatory mechanism.208,228 Pregnant women (regardless of whether or not they experience heartburn) have increased nonpropulsive esophageal motor activity with decreased wave amplitude and slower spread of peristaltic waves, with a reduction in secondary peristalsis.208 These findings are suggestive of reflux and are more prominent in women who experience symptoms of heartburn during pregnancy.
Pressure from the growing uterus increases intragastric pressure and, along with flattening of the hemidiaphragm, causes anatomic distortion of the stomach and decreases the acuteness of the angle at the gastroesophageal junction.62,117 Elevations in intragastric and intraabdominal pressure are intensified by multiple pregnancy, hydramnios, obesity, lithotomy position, bending over, or application of fundal pressure.228 The tendency toward reflux is increased by the decreased GI tone and relaxation of the cardiac sphincter.62,246 As a result of gastric stasis and pyloric incompetence, the refluxed material may be alkaline or acidic. Prolonged reflux of normal pH or alkaline duodenal material can lead to esophagitis.62 If pharmacologic therapy is needed, histamine2-receptor antagonists, which are Food and Drug Administration (FDA) class B agents, are usually recommended, with cimetidine and ranitidine having the greatest use.203,228 For more severe GER, proton pump inhibitors, which are FDA class C agents, may sometimes be used. Data on use of these agents during pregnancy are limited, but several studies have reported that use of omeprazole was not associated with major teratogenic risks, especially with use after the first trimester.177
The frequency of hiatal hernia is also increased, occurring in 15% to 20% of pregnant women primarily after 7 to 8 months’ gestation. This disorder arises from alterations in muscle tone and pressure with widening of the hiatus. Interventions are similar to those for heartburn (see Table 12-5).
Constipation
Constipation occurs in 10% to 30% of women and tends to be worse in the first and third trimesters.214,228,246 Constipation probably arises primarily from alterations in water transport and reabsorption in the large intestine. The smooth muscle relaxant effects of progesterone decrease intestinal motility and prolong transit time, which increases electrolyte and subsequently water absorption in the large intestine. Progesterone may also inhibit motilin (a stimulating GI hormone) release.228 Other predisposing factors are compression of the rectosigmoid area by the enlarging uterus and changes in dietary habits and activity and exercise patterns. Interventions for women with constipation are summarized in Table 12-5.
Hemorrhoids
Hemorrhoids arise more frequently during pregnancy and are aggravated by constipation. Factors that contribute to hemorrhoid formation during pregnancy include poor support for hemorrhoidal veins in the anorectal area; lack of valves in these vessels, leading to reversal in the direction of blood flow and stasis; gravity; pressure of the expanding uterus; increased venous pressure in the pelvic veins; venous congestion and engorgement; and enlargement of the hemorrhoidal veins.18 Interventions for women with hemorrhoids are summarized in Table 12-5.
Nausea and vomiting
Nausea with or without vomiting is a self-limiting event experienced by up to 70% to 90% of pregnant women in Western cultures.35,64,138,204 Vomiting occurs in 30% to 45% of these women, but is uncommon in many other cultures.138,214 Nausea and vomiting in pregnancy (NVP) generally begins between 4 and 6 weeks, but may occur as early as 2 to 3 weeks after the last menstrual period, and peaks at 8 to 12 weeks.228 NVP usually resolves by 10 to 12 weeks, although a few women (less than 10%) may experience symptoms to term.228,252 NVP incidence and severity are often linked to dietary cravings and aversions.54 The most frequent food aversions are to meat, fish, poultry, and eggs.79 NVP is often thought to occur most prominently before rising in the morning and ingestion of food (hence the term morning sickness), but many women experience symptoms in the afternoon, evening, or throughout the day.64,117 Although NVP usually disappears by 10 to 12 weeks it persists to 14 weeks in 40% of women, 16 weeks in less than 20%, and 20 weeks in less than 10%.208,214
The exact cause and function of nausea and vomiting is unknown. Many theories have been proposed, focusing on mechanical, endocrinologic, allergic, metabolic, genetic, and psychosomatic etiologies, but none has substantial research support.90,100,129,138,208,214,228 An adaptive and protective mechanism for NVP has also been postulated.79,100 NVP likely results from a combination of metabolic and endocrine factors, many of them placental in origin, mediated by other factors.138
The most common hormonal theories are related to rapidly increasing and high levels of estrogen, human chorionic gonadotropin (hCG), and possibly thyroxine. Support for a hormonal basis comes from studies documenting nausea in women on estrogen medications or combined oral contraceptive pills, the high correlation between women who experience nausea with both oral contraceptive use and pregnancy, and parallels between hCG patterns and the timing of symptom appearance and disappearance in NVP.100,138 However, studies examining the correlation of hCG levels with symptom appearance and intensity in individual women have produced inconsistent results.90 Increased NVP is seen in women with multiple and molar pregnancies, both of which are characterized by increased hCG.138 Perhaps a combination of endocrine factors leads to NVP, and individual women may have different sensitivities to these substances. The prevalence of specific hCG isoforms or alterations in hCG receptors may account for differences in NVP prevalence among various populations.138 NVP has been associated with favorable pregnancy outcomes such as decreased miscarriage rates, low birth weight, and perinatal mortality, although some researchers have found no differences in perinatal mortality and low birth weight.13,54,79,100,117,231,256 Few studies have been done to document a psychogenic basis to NVP, and many available reports are case studies of women in psychotherapy. Studies that have been done on more representative populations present conflicting findings.
Another hypothesis is that NVP may have an adaptive function to protect the embryo from potentially toxic substances in foods, such as animal products that might contain parasites and other pathogens if not handled correctly, caffeinated beverages, and alcohol.100 A study of 20 societies in which women experience NVP and 7 in which NVP is rare found that the societies in which NVP was rare were more likely to have plants (corn) as the primary staple rather than animal products.79
Decreased energy intake in early pregnancy is correlated with increased placental weight in animal and human studies.79 Huxley suggests that hCG activates the thyroid, thus increasing thyroxine secretion, which stimulates placental growth. As noted above, hCG (and thyroxine) levels correlate with severity and onset of NVP in some but not all studies. Huxley postulates that NVP reduces maternal energy intake. Maternal levels of anabolic hormones, insulin, and insulin-like growth factor I (IGF-I) are lowered as is maternal tissue synthesis, favoring early placental growth and development, and later fetal growth.100 Underweight women tend to experience less severe NVP than women with normal preconceptional BMI.100
Interventions for women experiencing NVP are summarized in Table 12-5. Pharmacologic treatment may occasionally be required due to severity of symptoms or interference with the woman’s responsibilities. Use of pharmacologic agents is fraught with potential problems, in that NVP and thus the administration of any drugs occur during the period of embryonic organogenesis. Until the early 1980s, Bendectin (which combined an antihistamine and pyridoxine) was commonly used to treat NVP. Litigation over the relationship of Bendectin and congenital defects resulted in removal of this drug from the market, although a causal relationship has never been documented.253 Alternative therapies have been investigated.114,151,253 Ginger has been reported to be effective in reducing nausea in most studies, although there continue to be concerns about high doses.35,36,192,252,253 Ginger may work by increasing gastric tone and peristalsis via anticholinergic and antiserotonin actions.252 Vitamin B1 has also been shown to reduce NVP.176 However, studies on the use of acupuncture for NVP have had equivocal findings.114,151
Hyperemesis gravidarum, an uncommon disorder seen in 0.3% to 1% of pregnant women, is intractable vomiting associated with alterations in nutritional status, dehydration, electrolyte imbalance, significant weight loss (greater than 5%), ketosis, and acetonuria.13,61,117,228,252 These women often require hospitalization. Risk factors include primiparas, multiple gestation, family history or hyperemesis in previous pregnancies.117,138 Hyperemesis has been linked to alterations in thyroid hormones (see Chapter 19) and endocrine changes (especially progesterone and human chorionic gonadotropin), immunologic changes, and metabolic alterations, and is associated with Helicobacter pylori.61,138,228,252 Hyperemesis usually begins by 4 to 10 weeks and resolves by 20 weeks, but persists to delivery in about 10%.252
Food and fluid intake in labor
For many years most hospitals in the United States did not allow women in active labor to eat or consume beverages other than ice chips or clear liquids. These prohibitions developed during the period when general anesthesia was commonly used during the second stage of labor; accordingly, there were concerns regarding the risk of aspiration should the anesthetized woman vomit. These constraints have been questioned, with many practitioners advocating more liberal food and fluid policies during labor.122 Potential benefits include meeting maternal energy needs and reducing maternal stress and the risks of ketosis, hyponatremia, and vomiting.218 There is little documentation supporting either the benefits of this therapy or the risk of oral intake for most women (see Chapter 11).145 Most studies have not reported adverse effects of oral intake during labor in low-risk women (studies of high-risk women are lacking).148,183,218,243 O’Sullivan and associates compared a light diet versus water during labor and found no differences in labor duration, cesarean section rate, incidence of vomiting, or outcomes.183 A Cochrane review examined oral fluid or food intake during labor and concluded that there was no evidence to support restrictions for low-risk women.224 Current guidelines from professional groups also support oral intake during labor in low-risk women.12,122,260
Individuals opposed to a more liberal food and fluid policy in labor argue that, although rare, aspiration has devastating consequences and can still occur with an endotracheal tube in place or use of regional anesthesia. Pregnant women are at particular risk for pulmonary aspiration because of delayed gastric emptying time in late pregnancy, increased levels of gastrin (resulting in increased gastric volume), and decreased LES tone (which allows stomach contents in an unconscious woman to passively move into the pharynx and into the lungs). Those advocating relaxation of restrictions note that (1) general anesthesia has been replaced by regional anesthesia; (2) the incidence of maternal mortality from aspiration of stomach contents in normal labor is rare; (3) gastric emptying may not be significantly altered in normal women who have not received narcotics; (4) use of intravenous fluid administration is increased; and (5) prolonged fasting during labor has physiologic and psychologic effects.40,41,145 Sharts-Hopko noted that maternal aspiration “is so rare that a randomized control trial to see if oral intake related to maternal mortality is not even feasible.”218,p.198 General anesthesia, if used, is safer than in the past as the result of changes in anesthetic agents and administrative techniques, such as the use of endotracheal tubes, which prevent aspiration of vomitus. Potential physiologic effects of fasting include increased ketones and fatty acids with decreased alanine, glucose, and insulin. Psychologic effects include increased anxiety and stress.41 Use of intravenous fluids has been associated with maternal and infant fluid and electrolyte problems (see Chapter 11). Low-risk women who deliver at home or in alternative settings and women in other cultures often consume food and beverages during labor with few complications.
Effects of altered maternal nutrition
Adequate nutrition during pregnancy is essential for optimal fetal growth and development. Most studies demonstrate a correlation between maternal weight gain and birth weight even when other variables that influence birth weight (gestational age, maternal height, and birth order) are held constant. The relationship between weight gain during pregnancy and perinatal mortality varies with maternal prepregnancy weight and pregnancy weight gain (see Figure 12-2). Alterations in maternal nutrition can influence both fetal growth and later outcomes of offspring. For example, undernourished women, especially those with low prepregnancy BMI and low weight gain during pregnancy, have an increased risk of SGA and fetal growth restricted infants. These infants are at risk for later developing obesity and type 2 diabetes.128,140,215 Infants born to obese women are at later risk of developing obesity and type I diabetes.140 Specific effects of altered maternal nutrition are listed in Table 12-6. Effects of maternal nutrition on the fetus are discussed further in Fetal Growth.
Table 12-6
Expected Consequences of Inadequate Nutrition in Women during a Reproductive Cycle
| Deficient Nutrition | ||
| PREPREGNANCY | PREGNANCY | POSTPREGNANCY |
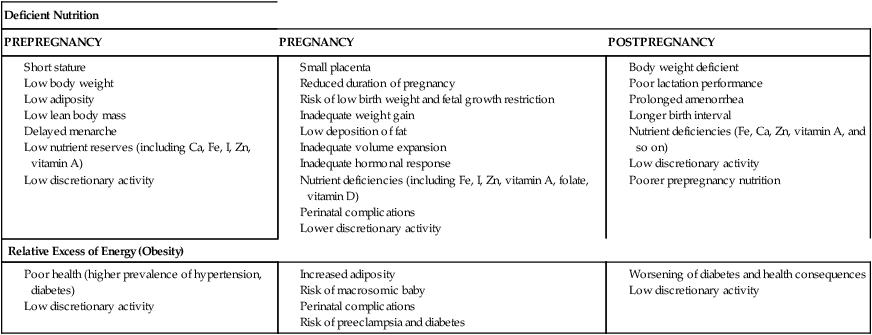
IUGR, Intrauterine growth restriction.
Adapted from Viteri, F.E., Schumacher, L., & Silliman, K. (1989). Maternal malnutrition and the fetus. Semin Perinatol, 13, 236.
Undernutrition and pregnancy
Women who are underweight have a higher incidence of pregnancy loss and SGA infants. These women may fail to gain adequate weight during pregnancy, further increasing the risk of fetal growth restriction and maternal nutritional anemia and malnutrition. Maternal undernutrition can also alter fetal metabolic programming, increasing the risk of later disorders (see “Fetal Growth”).47,78
Kristal and Rush and others have reviewed studies examining the effects of maternal undernutrition on fetal growth and concluded the following: (1) limitations in the overall amount of maternal food intake from either starvation or iatrogenic limitations lead to a consistent depression in birth weight (up to 550 g); (2) relief of acute undernutrition up to the beginning of the third trimester is associated with return of birth weight to previous levels; (3) results of supplementation studies with pregnant women at risk nutritionally (in developed and developing countries) are consistent, with increases in birth weight of 40 to 60 g; (4) there seems to be a limit in the amount of supplementation a given individual can tolerate and to the effect of these supplements; and (5) a consistent depression in birth weight is seen with use of high-density protein supplements in which more than 20% of calories supplied are protein.125,126 High-density protein diets have also been associated with an increased incidence of SGA infants and possibly increased neonatal morbidity in some populations.125 Both caloric and protein deprivation affect the fetus, although it is controversial as to which is most detrimental to fetal growth and development. Human and animal studies suggest that restrictions in caloric intake have the most marked effects on fetal growth, whereas protein deprivation leads to alterations in later development.93 Providing undernourished women with balanced protein energy supplementation reduces the risk of fetal growth restriction.102 Studies of supplementation with micronutrients of undernourished women have been sparse (and tend to focus on a single nutrient), although some have reported improvements in maternal and fetal weight.51
Maternal obesity and pregnancy
Maternal obesity is one of the most common risk factors in pregnant women.128 Maternal obesity is associated with an increased incidence of macrosomia, LGA infants, delivery complications, and perinatal mortality.28,128,168 These LGA infants are usually larger than expected in weight but not length due to increased deposition of adipose tissue.93 The woman’s excess adipose tissue reserves may be supplying some of the fuel needed for fetal growth. Cord blood triglyceride levels are elevated, reflecting enhanced fat synthesis by the fetal liver and adipose tissue.93 Because obese women tend to have LGA infants even when pregnancy weight gain is inadequate, may be difficult to determine whether their infants are growth restricted.
Many of the metabolic changes seen in pregnancy (e.g., increased circulating insulin, insulin resistance) are similar to those seen in obese women.93 Obese women tend to have more problems during delivery due to increased fetal growth and macrosomia. The incidence of preeclampsia and chronic hypertension, thrombophlebitis, varicose veins, and diabetes mellitus is increased in obese women.28,93,140,181 These women may gain excess weight during pregnancy, which can be difficult to lose later.52,178,181 Maternal obesity also has been reported to be a risk factor for neural tube defects.181
Caloric restriction during pregnancy is generally not recommended because of potential adverse effects on the fetus. Severe caloric restriction can significantly reduce the availability of glucose (the major fetal energy substrate) and increase maternal serum amino acid and ketone levels. Maternal ketosis has been associated with poor neurologic development in offspring, although some studies have not confirmed this finding.93 However, caloric restriction may be indicated in the obese pregnant woman to maintain a weight gain that has been associated with an improved pregnancy outcome.
Pregnancy and gastrointestinal disorders
The physiologic and anatomic changes of the GI tract during pregnancy have varying effects on disorders of this system. The course of some disorders is minimally affected by pregnancy. Approximately 1/500 to 1/735 pregnant women require nonobstetric surgery.206 Appendicitis and cholelithiasis are the most common reasons for nonobstetric surgery during pregnancy.226
Pregnancy and acute appendicitis
Appendicitis is not more common during pregnancy, but may be more severe due to delayed diagnosis.117 Diagnosis of appendicitis during pregnancy is complicated by anatomic and physiologic changes of pregnancy. Because the appendix is displaced upward and laterally to the right, the point of maximal tenderness may be as high as the right costal margin. By the second trimester, the appendix lies above the iliac crest. As a result, radiated pain associated with suppuration or perforation tends to be felt at the point where the appendix abuts the peritoneum, which becomes higher and more lateral as gestation progresses.14,226,231
Guarding and rebound tenderness are often milder and less well localized, in that the uterus is between the appendix and the parietal peritoneum.228 Nausea is common in the first trimester, and changes in white blood cell counts associated with appendicitis are similar to changes in pregnancy. Suidan and Young suggest that one way to differentiate uterine from appendiceal pain is to turn the woman onto her left side while pressing the point of maximal tenderness. If the pain decreases or ceases, the pain is probably uterine in origin; if not, appendicitis should be suspected.231 Appendicitis increases the risk of spontaneous abortion and preterm labor, especially if accompanied by perforation and peritonitis.117,228 The risk of perforation is greatest in the third trimester.228
Pregnancy in women with inflammatory bowel disease
One of the more common GI disorders occurring in the childbearing population is inflammatory bowel disease (IBD), which includes ulcerative colitis and Crohn’s disease. Most recent studies report that fertility rates in women with IBD are similar to the general population; however, fertility is reduced after surgical intervention.27 If these disorders are quiescent at the time of pregnancy the outcome for both mother and fetus is usually good, although these women have an increased risk of preterm delivery (most ≥35 weeks), low birth weight, cesarean delivery, and spontaneous abortion.27,59,117,147,164,228 If the disorder is active at the time of conception, there is a greater risk of these complications.27,117,147,164,228 The risk of exacerbation during pregnancy is similar to the risk in nonpregnant women.27 Risk of exacerbation is highest during the first trimester and postpartum. Long-term detrimental effects of pregnancy on the course of IBD have not been reported.27
Pregnancy in women with peptic ulcer disease
PUD in women is seen more often after menopause and is uncommon during the childbearing years. The risk of PUD is greatest with use of nonsteroidal anti-inflammatory agents in combination with colonization with Helicobacter pylori.228 In women of childbearing age, estrogen may protect the gastric lining from ulcer formation, perhaps by increasing gastric and duodenal mucous secretion.249 Pregnancy has a further protective effect on the development and progression of PUD.117,208 Up to 80% of women with PUD im prove during pregnancy, although 50% experience recurrence of symptoms by 3 months postpartum and almost all by 2 years after delivery.62,208 Women with persistent symptoms during pregnancy usually have other problems such as hyperemesis gravidarum and albuminuria.208
The basis for improvement during pregnancy may be due to the normal GI changes that accompany pregnancy, including decreased gastric acidity and motility and increased mucous secretion.61 Production of hydrochloric acid (both basal and in response to histamine) decreases in pregnancy, with a tendency to return to normal or increased levels of acidity in the third trimester.228 Decreased gastric acidity, increased plasma histaminase (thought to mediate acid and pepsin secretion by reducing parietal cell responsiveness to endogenous histamine), increased prostaglandins (protective of gastric mucosa), and increased mucous secretion (which protects the gastric mucosa from the effects of acid) may all contribute to improvement in peptic ulcer symptoms.25,62,214 Gastrin levels are normal during most of pregnancy, with marked increases late in the third trimester, at delivery, and immediately after delivery. Thus, by late pregnancy, gastric pH and pepsin output have returned to nonpregnant levels. It is at this time that peptic ulcer symptoms tend to recur.62,208
Cholelithiasis and pregnancy
The incidence of cholelithiasis, which is more common in women, is increased further during pregnancy and in women taking oral contraceptive agents.76,142,258 Cholelithiasis is the second most common nonobstetric surgical problem (acute appendicitis being the most common) during pregnancy. A hormonal basis for the risk of gallstones in women has been suggested, in that the increased risk is seen primarily between menarche and menopause. Elevated estrogen and progesterone levels during pregnancy may further aggravate the tendency toward cholelithiasis, as does the increased bile stasis and sludging during pregnancy.142,258
There are three forms of gallstones: cholesterol, pigment, and mixed (composed primarily of calcium bilirubinate). The increased incidence of gallstones associated with females and pregnancy is seen primarily with cholesterol gallstones. The process of gallstone development involves (1) production of bile supersaturated with cholesterol; (2) nucleation and crystallization of cholesterol, which initiates stone formation; and (3) growth of the stone.204 Supersaturation of bile with cholesterol occurs when cholesterol secretion is high or bile acid secretion is low (concentrated bile is more likely to hold cholesterol in solution). Cholesterol production increases during pregnancy. In addition, estrogens and progesterone increase biliary cholesterol saturation and estrogen decreases the proportion of chenodeoxycholic acid. This acid is a component of the bile acid pool that dissolves gallstones by decreasing biliary cholesterol secretion. Altered gallbladder tone during pregnancy, with incomplete emptying and increased fasting and residual volumes, may also increase the risk of gallstone formation by sequestering cholesterol crystals.76,142,204 Cholelithiasis during pregnancy may increase the risk of chronic gallbladder disease.142
Pregnancy and liver disease
Liver disease in pregnancy can be divided into two categories: (1) disorders seen only in pregnancy and associated with jaundice and abnormal liver tests (intrahepatic cholestasis, preeclampsia, and fatty liver of pregnancy); and (2) liver diseases that may occur during and are affected by pregnancy.185 Fetal fatty acid oxidation defects may increase the risk of maternal liver disease, especially acute fatty liver of pregnancy and HELLP syndrome, which includes hemolysis (H); elevated serum levels of liver enzymes, especially AST and ALT (EL); and low platelets (LP).38,43 The reason for this risk is postulated to be due to accumulation and deposition of fetal 3-hydroxy fatty acid acylcarnitine intermediary metabolites.43
The most common liver disease seen in pregnant women is viral hepatitis.185 Major effects of pregnancy on liver disorders are potential difficulties with diagnosis, increased fetal risk and, especially with hepatitis B, transmission to the fetus. Alterations in some liver function tests during pregnancy can make diagnosis of liver disorders more difficult, although jaundice is always abnormal. Jaundice and liver disorders during pregnancy are discussed in Chapter 18.
Severe pruritus and jaundice characterize intrahepatic cholestasis (see Table 14-2). The risk of postpartum hemorrhage, gallstones, fetal distress, stillbirth, and prematurity is increased.175 The basis for this disorder is unclear, although it may have a genetic basis or hormonal cause, in that a similar syndrome occurs with oral contraceptive use.204 Fatty liver of pregnancy is a rare disorder of unknown cause that usually appears during the third trimester, often in association with preeclampsia, with high fetal and maternal mortality rates. Delivery of the infant results in rapid improvement.204
Liver function and histologic changes are associated with preeclampsia (see Table 9-5), with alterations in liver function tests (AST, ALT, GGTP, and bilirubin) reported in some women. The degree of liver abnormality tends to parallel the severity of preeclampsia. The most significant involvement is in women who develop HELLP syndrome (see Chapter 9).204,258
Summary
The pregnant woman experiences changes in GI and hepatic function that enhance absorption of nutrients for herself and her fetus. These changes are associated with common experiences and discomforts of pregnancy such as heartburn, constipation, nausea, and vomiting. A major component of interconceptional and prenatal care is nutritional assessment and counseling. Maternal nutrition before and during pregnancy is critical for optimal growth and development of the fetus and prevention of maternal, fetal, and neonatal disorders. Table 12-7 summarizes recommendations for clinical practice related to the GI system and perinatal nutrition.
Table 12-7
Counsel women regarding changes in appetite, food preferences, and intake during pregnancy (p. 392).
Counsel women regarding gingival changes during pregnancy and the need for dental hygiene (pp. 393-395).
Counsel women regarding common problems (heartburn, nausea and vomiting, constipation, hemorrhoids) associated with the gastrointestinal (GI) system (pp. 395, 402-404 and Table 12-1).
Implement interventions to reduce or relieve heartburn, nausea and vomiting, constipation, and hemorrhoids (pp. 395, 402-404 and Table 12-5).
Counsel women regarding the presence of spider angiomas and palmar erythema (p. 396 and Chapter 14).
Recognize the usual parameters for liver function tests and patterns of change during pregnancy and the postpartum period (pp. 396, 407-408 and Table 12-2).
Know the expected parameters for weight gain during pregnancy and monitor maternal patterns (pp. 396, 398-399 and Table 12-3).
Know the recommended nutritional requirements during pregnancy (pp. 400-401 and Table 12-4).
Assess maternal nutritional status and provide nutritional counseling (pp. 400-402 and Table 12-4).
Recognize potential maternal and fetal/neonatal complications associated with undernutrition and obesity during pregnancy (pp. 405-406 and Table 12-6).
Monitor fluid, food, and caloric intake during labor and the early postpartum period (pp. 404-406).
Counsel women regarding weight loss patterns following delivery (p. 400).
Recommend postpartum exercises to enhance weight loss and return of abdominal and perineal tone (p. 400).
Evaluate GI function postpartum (p. 400).
Recognize factors that increase the risk of gallstone formation and recognize signs of cholelithiasis (pp. 396, 407).
Counsel women with GI and liver problems regarding the effect of their disorder on pregnancy and of pregnancy on the disorder (pp. 406-407).
Recognize signs of appendicitis during pregnancy (pp. 406-407).
Recognize signs of liver disorders that are unique to pregnancy (p. 407 and Chapter 18).
Counsel women with phenylketonuria and other metabolic disorders regarding risks to their infant and need for dietary restrictions (p. 407, Chapter 1).
Know fetal nutritional requirements and counsel women regarding fetal needs and growth patterns (pp. 401-402, 416-417).
Know factors that can alter fetal growth and monitor fetal growth patterns (pp. 401-402, 416-417 and Table 12-6).
Counsel women regarding changes in GI function with use of oral contraceptive agents (pp. 395, 402, 407).
Development of the gastrointestinal and hepatic systems in the fetus
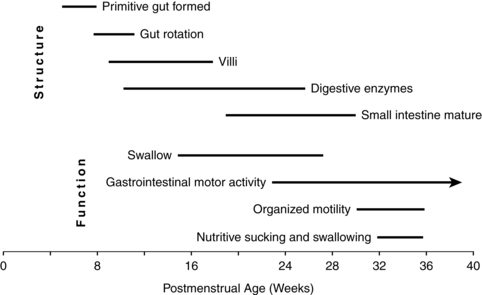
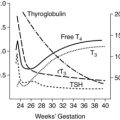
The development of the gastrointestinal (GI) system can be divided into three phases. During early gestation, anatomic development gives rise to the organs and other structures of this system. During middle to late gestation, functional components such as hormones, enzymes, and reflexes develop (Figure 12-3). Finally, after birth, coordinated function develops with interaction of hormones and enzymes in the digestion of food substances along with maturation of suck-swallow coordination.
The enteric nervous system (ENS) consists of neurons in the wall of the GI tract that modulate motility, microcirculation, secretion, and immune responses. The ENS develops from neural crest cells that colonize the gut by 13 weeks. Failure of migration and colonization can lead to disorders such as Hirschsprung disease.43 Characteristics and timing of other common GI anomalies are described in the next section and are summarized in Table 12-8. GI anomalies may occur as isolated malformations or in association with malformations of other systems, most frequently the skeletal, cardiovascular, or urogenital systems.235 Fetal growth restriction is seen in approximately one third of infants with GI malformations.235
Table 12-8
Incidence, Time of Occurrence, and Associated Defects of Various Gastrointestinal Anomalies
| ANOMALY | INCIDENCE (PER LIVE BIRTHS) | FETAL AGE AT WHICH DEFECT OCCURS | PRESENCE OF HYDRAMNIOS | OTHER CONCURRENT DEFECTS |
| Diaphragmatic hernia | 1:4000 | 8th-10th week of fetal life | >75% | Lung hypoplasia, malrotation of bowel, PDA, coarctation of aorta, and neurologic malformations |
| Tracheoesophageal fistula and esophageal atresias |
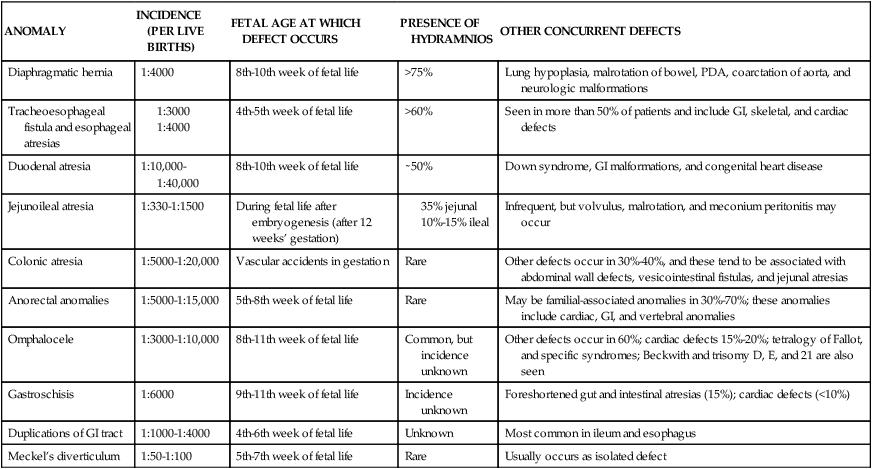
GI, Gastrointestinal; PDA, patent ductus arteriosus.
From Sunshine, P. (1990). Fetal gastrointestinal physiology. In R.D. Eden & F.H. Boehm (Eds.), Assessment and care of the fetus. Norwalk, CT: Appleton & Lange.
Anatomic development
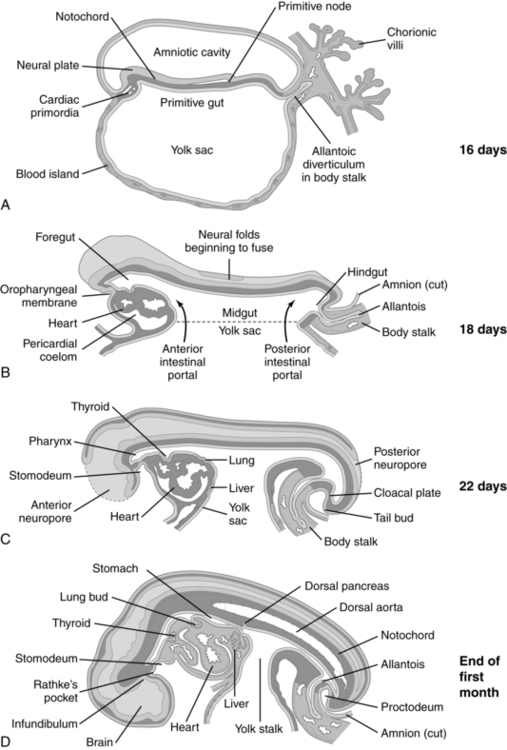
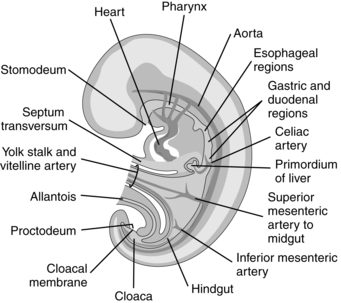
Anatomic development of the GI system begins during the fourth week with partitioning of the yolk sac into intraembryonic and extraembryonic portions. Initially the cranial portion of the GI system develops concurrently with the respiratory system (see Chapter 10). The epithelium of the trachea, the bronchi, and the lungs and digestive tract arise from the primitive gut, a derivation of the yolk sac. The GI system develops in a cranial-to-caudal direction. The yolk sac arises at 8 days and by the fourth week has divided into two parts. The extraembryonic or secondary yolk sac provides for nutrition of the embryo, before development of the mature placenta, and then is assimilated into the umbilical cord by 3 to 4 months. The intraembryonic portion is incorporated into the embryo as the primitive gut (Figure 12-4).
The primitive gut is initially closed at both ends by membranes. The cranial (buccopharyngeal) membrane is reabsorbed during the third week, forming the stomodeum (future site of the mouth); the caudal (cloacal) membrane is absorbed during the ninth week. The midgut remains temporarily connected to the yolk sac by the vitelline duct. Development of the primitive gut and its derivatives can be divided into four sections: pharyngeal gut, foregut, midgut, and hindgut.159
Development of the pharyngeal gut
The pharyngeal gut extends from the buccopharyngeal membrane (which becomes the stomodeum) to the tracheobronchial diverticulum, forming the pharynx and its derivative, lower respiratory tract, and upper esophagus (Figure 12-5). The pharyngeal area develops from bands of mesenchymal tissue (branchial or pharyngeal arches) separated by deep clefts (branchial or pharyngeal clefts) on the exterior of the embryo. A series of indentations (pharyngeal pouches) appear on the lateral walls of the pharyngeal gut and penetrate into the surrounding mesenchyme but do not communicate with the external clefts.210 The pharyngeal arches form the muscular and skeletal components of the pharyngeal area, aortic arch, and nerve networks; the mandible; the dorsal portion of the maxillary process; the hyoid bone; the thyroid bone; the laryngeal cartilage; and associated vascular and nerve supplies. The pharyngeal pouches form the eustachian tubes, tonsils, thymus, parathyroid, and part of the thyroid.159,210
Development of the foregut and common anomalies
The foregut extends from the tracheobronchial diverticulum to the upper part of the duodenum. Structures formed from the foregut (lower esophagus, stomach, liver, upper portion of the duodenum to the entry of the common bile duct, liver, biliary tree, and pancreas) are all supplied by the celiac artery.159
Esophagus.
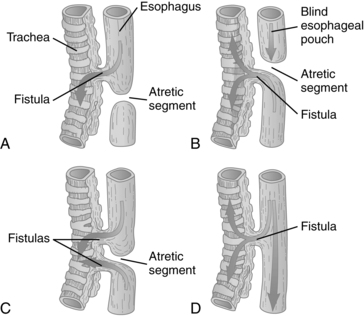
Incomplete division of the foregut into respiratory and digestive portions at 4 to 5 weeks leads to tracheoesophageal fistula with or without esophageal atresia (Figure 12-6; also see Table 12-8). This malformation probably arises from posterior deviation or unequal development of the septum developing between the primitive trachea and esophagus due to genetic and environmental factors.65,77 Failure of the lumen to recanalize during the eighth week leads to esophageal stenosis or atresia.
Stomach, duodenum, and pancreas.
The stomach arises during the fourth week as a spindle-shaped dilation in the caudal area of the foregut (see Figure 12-5), and its structure is well established by 6 weeks. The stomach dilates and enlarges, rotating around a longitudinal and an anteroposterior axis. During the longitudinal rotation, the stomach rotates 90 degrees clockwise, ending with the left side facing anteriorly and the right posteriorly. The subsequent greater growth of the posterior wall in comparison with the anterior wall leads to the lesser and greater curvatures of the stomach. Initially the cephalic and caudal ends of the stomach are in midline. During the anteroposterior rotation of the stomach, the caudal (pyloric) portion moves right and upward, and the cephalic (cardiac) portion moves left and slightly downward.159,210 Embryonic anomalies of the stomach are rare, probably because stomach development is relatively simple. The most common stomach anomaly is pyloric stenosis, which is thought to be genetic in origin.
The pancreas appears at about 5 weeks as dorsal and ventral buds in the duodenal area. As the duodenum rotates to the right and becomes C-shaped, the ventral pancreatic bud migrates toward the lower end of the common bile duct. The two pancreatic buds meet and fuse to form the final pancreas by 7 weeks. In individuals with an annular pancreas, the ventral bud encircles the duodenum and may cause obstruction.210 All pancreatic cell types are seen by 9 to 10 weeks.
Liver and gallbladder.
The liver appears during the third week as a ventral thickening (liver bud or hepatic diverticulum) consisting of rapidly proliferating strands of cells at the distal end of the foregut (see Figure 12-5). The hepatic diverticulum divides into a large cranial portion, which forms the hepatic parenchyma and main bile duct, and a smaller caudal portion, from which the gallbladder arises.210 The liver initially grows into the septum transversum, a thick mesodermal plate separating the yolk sac and the thoracic cavity. The liver grows rapidly, eventually bulging into the caudal part of the abdominal cavity and stretching the mesoderm of the septum transversum until it becomes a thin membrane. The ventral portion of this membrane forms the falciform ligament; the dorsal portion forms the lesser omentum. The cranial portion of the septum transversum forms part of the diaphragm. Further growth of the liver promotes closure of the pleuroperitoneal canals (two large openings on either side of the foregut). Hepatocytes develop as long cords, 3 to 5 cells thick, that insert into the liver stroma.26
The lumina of the gallbladder and the intrahepatic and extrahepatic bile ducts are initially open, becoming temporarily obliterated by proliferating epithelium and later recanalizing. Biliary atresia can arise from failure of recanalization. With complete failure, the ducts are narrow nonfunctional fibrous cords. Failure of part of a bile duct to recanalize results in partial obstruction or atresia of that duct, with distention of the gallbladder and hepatic duct proximal to the atretic area.159,210
Development of the midgut and common anomalies
Development of the midgut is characterized by rapid elongation of the gut and associated mesentery.159 The midgut begins caudal to the liver and gives rise to the small intestine (except for the upper duodenum), cecum, appendix, ascending colon, and proximal portion of the transverse colon. These structures are supplied by the superior mesenteric artery.159 The intestines increase in length 1000-fold during gestation, doubling in length in the last 15 weeks (mean length is 275 cm at term).171
Development of the midgut involves four steps: herniation, rotation, retraction, and fixation (Figure 12-7).159,210 The midgut initially elongates and forms a U-shaped loop, which projects (herniates) into the proximal umbilical cord (see Figure 12-7, A). The cranial limb of this loop grows rapidly, forming coils characteristic of the small intestine with little change in the caudal portion except for appearance of the cecal bud (see Figure 12-7, B).
The midgut rotates a total of 270 degrees in a counterclockwise direction around an axis formed by the superior mesenteric artery. Midgut rotation occurs in two stages. The initial 90-degree rotation occurs while the midgut is in the umbilical cord (see Figure 12-7, A and B); the second rotation (180 degrees) takes place as the gut returns to the abdomen at 10 weeks (see Figure 12-7, B and C). The initial 90-degree rotation is in a counterclockwise direction. As a result, the cranial limb moves to the right and down and the caudal limb moves to the left and up (see Figure 12-7, B). The lumen of the intestines becomes temporarily obliterated by rapid epithelial growth, with later recanalization.
Retraction or return of the midgut to the abdominal cavity occurs rapidly during the tenth week. The stimulus for this return is unknown, but it occurs as the rate of liver growth slows, the relative size of the kidney decreases, and the abdominal cavity enlarges. As the midgut reenters the abdominal cavity, the gut rotates 180 degrees counterclockwise. The jejunum returns first and the area of the cecal bud last; the cecum and appendix end up near the liver in the right upper quadrant (see Figure 12-7, D).210
The final step in development of the midgut is fixation (see Figure 12-7, E). The cecum and appendix descend into the lower right quadrant. The proximal colon lengthens, becoming the ascending colon. The mesenteries are pressed against the posterior abdominal wall and fuse with the wall. In some regions of the midgut, the mesenteries also fuse with the parietal peritoneum so that the ascending colon is rectoperitoneal.
Common anomalies of the midgut.
Congenital anomalies of the midgut include omphalocele, gastroschisis, umbilical hernia, intestinal stenosis and atresia, and malrotation (see Table 12-8). Omphalocele arises at 8 to 11 weeks’ gestation from a developmental arrest at the stage of herniation of the midgut into the umbilical cord with failure of all or part of the gut to return to the abdominal cavity (results in bowel-containing omphalocele). There is often an associated defect in development of the abdominal musculature at the junction of the umbilical cord (results in liver-containing omphalocele). This defect results from a primary failure in the formation of the lateral folds, which along with the cephalic and caudal folds form the abdominal wall.159 The size of the defect influences the size of the omphalocele, which can range from a single loop of intestine to a mass containing most of the intestines and parts of the liver, bladder, and other organs. The omphalocele is covered by a thin, avascular membrane (derived from amnion) that may be intact or ruptured. The umbilical cord generally inserts into the apex of the omphalocele sac. Omphalocele is associated with Beckwith-Wiedemann syndrome, congenital heart disease, trisomy 13, trisomy 18, and urinary tract problems.123
In gastroschisis, the extrusion of the intestines is due to a defect in the anterior abdominal wall that probably arises between 9 and 11 weeks but may occur as early as 5 to 6 weeks. This defect is usually to the right of, and not necessarily continuous with, the umbilical ring. Because there is usually no hernial sac present, the intestines extrude into the amniotic cavity and are embedded in a gelatinous mass. Gastroschisis arises secondary to a paraumbilical abdominal wall defect that may be a result of (1) failure of differentiation of the lateral fold somatopleure after the bowel has returned to the peritoneal cavity and the umbilical ring has formed; (2) failure in formation of the umbilical coelom with rupture of the amniotic membrane at the base of the umbilical cord; (3) intrauterine rupture of an incarcerated hernia into the cord; or (4) weakness in the abdominal wall arising from alterations in the normal involution of the second umbilical vein or ischemic damage.94
Intestinal stenoses and atresias can arise as primary or secondary defects. Primary stenosis or atresia arises at 8 to 10 weeks, and perhaps as early as 6 to 7 weeks, as a result of partial or complete failure of the intestinal lumen to recanalize. Secondary stenosis or atresia is a result of (1) fetal vascular accidents or infarction (with interruption of blood supply to part of the intestines), or (2) secondary to twisting or inflammatory changes.91 Vascular accidents and infarction are common causes of jejunal and ileal atresias and probably occur after 12 weeks (see Table 12-8).
Alterations in midgut development can also lead to malrotation. Three of the more common forms are nonrotation, mixed malrotation, and reverse rotation. With nonrotation, the midgut rotates 90 degrees instead of 270 degrees, without the 180-degree rotation that normally occurs upon reentry of the gut into the abdominal cavity. As a result, the colon enters the abdomen first instead of last so that the colon ends up on the left and the small intestine on the right. This form of malrotation is sometimes referred to as left-sided colon. In mixed malrotation, the midgut rotates only 180 degrees, so that the terminal ileum reenters first. The cecum is subpyloric and fixed to the abdominal wall, which may compress the duodenum. In reverse rotation, the initial 90-degree rotation is clockwise instead of counterclockwise, resulting in placement of the transverse colon behind the duodenum. Malrotation increases the risk of volvulus, with twisting of the intestinal loops and abnormal fixation of the mesenteries, resulting in excessive mobility of the bowel. This can lead to kinking of the bowel and blood vessels and necrosis.159,210
Development of the hindgut and common anomalies
Development of the hindgut and urogenital systems is interrelated. The cloaca is the expanded terminal end of the gut; the hindgut ends at the cloacal membrane (Figure 12-8). The hindgut gives rise to the distal transverse colon, descending and sigmoid colons, rectum, upper anal canal, bladder, and urethra, which are supplied by the inferior mesenteric artery.159
At 5 to 7 weeks, the cloaca is divided into two parts by the urorectal septum, a wedge of downward-growing mesenchymal tissue. During weeks 6 to 7, the urorectal septum reaches and fuses with the cloacal membrane, forming the perineum. The area of fusion is the perineal body. This fusion divides the cloacal membrane into two parts. The ventral urogenital membrane is incorporated in the terminal portion of the urogenital system (see Chapters 1 and 11); the dorsal part becomes the anal membrane. A pit that develops in the anal membrane ruptures at 8 to 9 weeks, resulting in an open communication between the rectum and the body exterior. The lower portion of the anal canal develops from ectodermal tissue around the site of the anal pit.210
Functional development
Anatomically the fetal GI tract develops to the stage seen in the newborn by about 20 weeks.136,124 Functional development begins during fetal life with development of digestive and liver enzyme systems and the absorptive surfaces of the intestine (Table 12-9; see Figure 12-3) and continues into the postbirth period. Most of the processes needed for adequate enteral nutrition are in place by 33 to 34 weeks’ gestation.124 Although the placenta takes care of the nutrient needs of the fetus, function of the fetal GI tract is important in amniotic fluid homeostasis (see Chapter 3). Amniotic fluid in turn contains nutrients, hormones, and growth factors that stimulate secretion of hormones and regulatory peptides and enhance growth and maturation of the gut.71,124,250
Table 12-9
Development of the Gastrointestinal Tract in the Fetus
| DEVELOPMENT | GESTATION (WEEKS) |
| ANATOMIC DEVELOPMENT | |
| Esophagus | |
| Superficial glands develop | 20 |
| Squamous cells appear | 28 |
| Stomach | |
| Gastric glands form | 14 |
| Pylorus and fundus defined | 14 |
| Pancreas | |
| Differentiation of endocrine and exocrine tissue | 14 |
| Liver | |
| Lobules form | 11 |
| Small Intestine | |
| Crypt-villi develop | 14 |
| Lymph nodes appear | 14 |
| Colon | |
| Diameter increases | 20 |
| FUNCTIONAL DEVELOPMENT | |
| Sucking and Swallowing | |
| Swallowing begins | 10-14 |
| Only mouthing | 28 |
| Immature suck-swallow | 33-36 |
| Stomach | |
| Gastric motility and secretion | 20 |
| Pancreas | |
| Zymogen granules | 20 |
| Liver | |
| Bile metabolism | 11 |
| Bile secretion | 22 |
| Small Intestine | |
| Active transport of amino acids | 14 |
| Glucose transport | 18 |
| Fatty acid absorption | 24 |
| Enzymes | |
| α-Glucosidase | 10 |
| Dipeptidases | 10 |
| Lactase | 10 |
| Enterokinase | 26 |
From Lebenthal, A. & Lebenthal, E. (1999). The ontology of the small intestinal epithelium. J Parenter Enteral Nutr, 23, S5.
The major gut-regulating polypeptides, including gastrin, motilin, and somatostatin, are all present by the end of the first trimester and act as local inducing agents regulating growth and development of the gut.19,174,250 Initially, cells that produce these substances are more widely distributed than in the adult, but they reach adult distribution by 24 weeks. Gastric epidermal growth factor (EGF) receptors appear by 18 weeks. EGF enhances growth and development of the gut and may protect the stomach from hydrochloric acid. Transport of amino acids begins by 14 weeks, glucose transport by 18 weeks, and fatty acids by 24 weeks (see Table 12-9).134
The intestinal villi begin to develop around 7 weeks and are present throughout the small intestine by 14 to 16 weeks, with well-developed villi and crypts seen by 19 weeks.25,171 Intestinal motility and peristalsis develop gradually and mature during the third trimester.25 Meconium is first found at 10 to 12 weeks and moves into the colon by 16 weeks.6 Small amounts of meconium may enter the amniotic fluid in the second trimester before development of anal sphincter function at 20 to 22 weeks’ gestation.6
Fetuses by 13 to 15 weeks respond to oral stimulation with tongue protrusion, rooting, and sucking.98 Older fetuses have been noted on ultrasound to suck reflexively on their fingers.14 Nonnutritive sucking begins by 20 weeks.71 Swallowing begins at 10 to 14 weeks, and by 16 weeks the fetus swallows 2 to 6 mL of amniotic fluid per day, increasing to 200 to 600 mL/day (average, 450 mL/day) by term. About 20% of the fluid swallowed by the fetus is lung fluid not amniotic fluid.75 Swallowing is important for amniotic fluid homeostasis, regulation of amniotic fluid volume, GI development, and somatic growth.75 Swallowing may also be important in fetal thirst and appetite programming. About 10% of fetal protein intake comes from swallowed amniotic fluid.75 Failure of the fetus to swallow amniotic fluid is associated with GI obstruction and polyhydramnios (see Chapter 3).
Most of the metabolic functions of the fetal liver are handled by the maternal liver. The fetal liver is primarily a hematopoietic organ until the latter part of gestation, when bone marrow erythropoiesis and liver metabolic activity increase. Many liver enzyme systems are still immature at birth. Fetal hepatic metabolism of drugs is discussed in Chapter 7.
GI enzymes involved in protein digestion and absorption develop early in gestation and may be important in fetal life to prevent bowel obstruction by cellular debris.83 Glucose from the mother is the major source of fetal energy (see Chapter 16). Disaccharidase enzymes are present by 9 to 10 weeks, increase rapidly after 20 weeks, and become very active after 27 to 28 weeks (except for lactase, which does not reach mature levels until 36 to 40 weeks).123,135 Pancreatic amylase activity is minimal in the fetus.73 Salivary amylase is present by 16 to 18 weeks in amniotic fluid and by 20 weeks in the fetus, but remains low.73,174 Pancreatic lipase develops by 32 weeks, but remains low; lingual and gastric lipase are present by 26 weeks and increase to term.73 Proteolytic enzymes are found by 20 to 25 weeks.73 Trypsin achieves 90% of childhood values by term.73 Enterokinase activity appears at 26 weeks. Bile acids can be detected in the liver and gallbladder by 14 to 16 weeks and in the intestines by 22 weeks; however, the bile acid pool remains low even at term. The fetal jejunum and liver have decreased capacity for reabsorbing bile acids and poorer enterohepatic recirculation of taurine-conjugated bile acids, the major bile acid at birth.94,124 Lipase is present by 10 to 12 weeks.174
Fetal growth
Fetal growth is dependent on factors such as genetic determinants, general maternal health and nutrition, availability of growth substrates, presence of fetal growth–promoting hormones, and vascular support via changes in plasma volume during pregnancy and the maternal blood supply to the placenta.49,86 Availability of growth substrates depends on perfusion of the intervillous spaces and availability of glucose, amino acids, and fats in maternal blood (see Chapter 16). Fetal growth does not seem to be greatly dependent on hormones such as growth hormone, thyroid hormones, glucocorticoids, and sex steroids that are critical for postnatal growth. Hormones and peptide growth factors believed to be necessary for fetal growth include insulin, human placental lactogen (hPL), insulin-like growth factors I and II (IGF-I and IGF-II), epidermal growth factor, platelet-derived growth factor, leptin, and transforming growth factor–α.188 Insulin and the IGFs (especially IGF-I and IGF-II) are critical in regulating fetal growth, although insulin probably has a permissive rather than a direct effect on fetal growth by stimulating nutrient uptake and utilization.85 Growth factors appear early in gestation, beginning at the four- to eight-cell stage. “Disruption of the IGF1, IGF2, or IGF1R [receptor] gene retards fetal growth, whereas disruption of IGF2R or overexpressing IGF2 [genes] enhances fetal growth.”85
Many of the genes involved in growth regulation are imprinted (see Box 1-2 on p. 9) including IGF-II (paternally imprinted) and its receptor (maternally imprinted).86,157 Loss of paternal imprinting with reactivation of the maternal allele (gene form) is seen in infants with Beckwith-Wiedemann syndrome, which is characterized by abnormal fetal and postnatal growth.157
Early in gestation, placental and embryonic growth are regulated primarily by IGF-II. IGF-II is unaffected by nutrient availability and enhances placental growth and nutrient transfer.85 Thus embryo and placental growth are maintained even if maternal energy intake is decreased, as often occurs with NVP.100 During the second and third trimesters, fetal growth becomes dependent on IGF-I, which is sensitive to nutrient status.86,100 Another factor that is thought to have a role in fetal growth is placental leptin, which is secreted into both maternal and fetal circulations. Leptin (see Chapter 16) is thought to act on the hypothalamus to regulate food intake and satiety. During pregnancy, placental leptin may signal satiety to the maternal hypothalamus, thus resulting in reduced food intake and energy intake, which stimulates placental growth.20,100 Dysregulation of leptin is seen with fetal growth restriction.130 Leptin can be found in cord blood by 18 weeks’ gestation.184
Growth is slow during the first 2 months (period of organ formation), and then accelerates rapidly. Maximum growth rate is achieved from the fourth to eighth months, when the fetus grows at the rate of 5% to 9% per week. Most of the fetal weight is gained from 20 weeks to term, increasing from about 5 g/day at 15 weeks, to 15 to 20 g/day at 20 weeks, and 30 to 35 g/day after 32 to 34 weeks.157,202 At the cellular level, growth occurs through hypertrophy (increased cell size) or hyperplasia (increased cell numbers). The human fetus undergoes primarily hyperplastic growth from conception to 20 weeks (similar increases in deoxyribonucleic acid [DNA] and organ protein content), followed by a period of simultaneous hyperplasia and hypertrophy from 20 to 28 weeks. From 28 weeks on, growth is predominantly hypertrophic with rapid increases in cell size and accumulation of fat, muscle, and connective tissue.157,265
Fetal growth is altered in approximately 15% of pregnancies, with either fetal under- or overgrowth. Fetal overgrowth and macrosomia are discussed in Chapter 16; fetal undergrowth is discussed in the next section. The effects of maternal nutrient restriction or excess depend on the stage of pregnancy, length of restriction, and type of restriction. For example, if maternal nutrients are restricted throughout pregnancy, fetal growth restriction develops. If the restriction is only during the first trimester, infant birth weights tend to be within normal limits, with increased placental weight. Increased food intake in early gestation tends to be associated with infants with lower birth and placental weights. High carbohydrate intake in early pregnancy is associated with lower placental and birth weights. Restricted protein intake (and thus restricted intake of essential amino acids) in early pregnancy has a detrimental effect on both fetal and placental development.100
The “developmental origins of health and disease” hypothesis proposes an association between an abnormal fetal environment and later disorders.22,23,39,47,87 Developmental programming in utero is mediated by nutrients and hormones. Fetal or developmental programming refers to effects of the fetal environment on susceptibility to later disorders. The nutritional and hormonal status during fetal and early post birth life can alter organ development including the hypothalamus and other endocrine structures (see Chapter 19). The mechanisms by which the fetal environment influence later status are not well understood, but are thought to be related to placental adaptive responses to the intrauterine environment. After birth these adaptations may no longer be appropriate for the extrauterine environment and increase the risk for adult onset disorders such as insulin resistance and type 2 diabetes (due to altered endocrine programming or changes in glucose-insulin metabolism); obesity (due to altered leptin and endocrine programming), hypertensive disorders, coronary artery disease (due to alterations in vascular development and lipid metabolism), and osteoporosis (due to alterations in leptin and bone development).22,23,47,68,74,78,87,88
In infants with fetal growth restriction and “placental insufficiency,” nutritional delivery is decreased due to both decreased blood flow and a smaller placental size, but also down-regulation of nutrient transporters.111 Conversely with fetal overgrowth, as occurs with maternal diabetes, placental nutritional transporters are up-regulated.111 Altered programming can influence later metabolic control and these changes may be mediated in part by adipocytokines (hormones secreted by white adipose tissue that modulate metabolism, energy homeostasis, and growth).39,74,78,119 Adipocytokines are secreted by placental and fetal tissues and include dentin, adiponectin, resistin, vistatin, and apelin, all of which exert effects on fat, muscle, and liver cells in early life.39 Under- or over-provision of nutrients in utero can program adipose tissue function and amount.39,87 As noted above, adverse in utero nutrition may have long-term effects on the infants, with an increased risk of altered neurologic development, increased childhood mortality rates, and a predisposition to chronic disease such as hypertension, coronary artery disease, and type 1 (with excess growth) or 2 (with fetal undernutrition) diabetes in adult life.74,78,86,88,128,140,157,233
Fetal growth restriction
Fetal growth restriction is the failure of a fetus to achieve its genetic growth potential in utero.157 In practice this is stated as measures of absolute (i.e., low birth weight [LBW]) and relative (i.e., small-for-gestational-age) size. Factors that cause alterations in growth, such as malnutrition during the period of hyperplasia, can decrease the rate of cell division and result in organs or a fetus that is smaller in size with fewer cells. This symmetric form of growth alteration is not reversible after the time when hyperplastic cell growth would normally have ceased. Malnutrition during the period of hypertrophy results in organs or a fetus that are smaller in size (due to reduction in cell size) but has a normal number of cells. This is asymmetric growth restriction, which is seen in about 75% of infants with fetal growth restriction.157 Hypertrophic growth alterations may be reversible with adequate nutrition. Because fetal tissues are undergoing hyperplastic growth throughout gestation, the fetus is especially vulnerable to irreversible changes at the cellular level.249
Factors altering fetal growth can be intrinsic or extrinsic.157,205,262 Intrinsic factors are those within the fetus arising from chromosomal or genetic abnormalities, infectious agents, or other teratogens that alter the normal process of cell division and usually result in symmetric growth restriction. Extrinsic factors include maternal preeclampsia, placental alterations or insufficiency, and fetal malnutrition. Pathologic changes characteristic of reduction in placental blood flow are seen in placentas of growth-restricted infants.251 Placental insufficiency due to maternal, fetal, or placental factors often results in caloric restriction to the fetus (inadequate glucose transported to meet fetal growth needs) and tends to occur later in gestation. Caloric restriction usually leads to an asymmetric growth failure in which brain growth is spared. Fetal malnutrition due to maternal protein restriction (arising from maternal malnutrition or significantly protein-reduced diets) generally results in symmetric fetal growth failure in which brain growth is not necessarily spared.249 Management of fetal growth restriction varies with the underlying cause. Strategies range from fetal therapies to prepregnancy counseling and preventive interventions such as cessation of smoking and alcohol use, promotion of adequate nutrition, and early identification of and interventions for pathologic problems such as preeclampsia. The growth-restricted fetus and neonate are at risk for hypoxic-ischemic events, meconium aspiration, hypoglycemia, and other problems (Table 12-10) and later development of type 2 diabetes, obesity and cardiovascular disease (see previous section).15,22,23,47,68,111,112,158,220,227,238,257
Table 12-10
Perinatal Adaptive Problems of Small-for-Gestational-Age Infants
| PROBLEM | PATHOGENESIS | PREVENTION |
| Hypoxic-ischemic events |
DIC, Disseminated intravascular coagulation.
Adapted from Kliegman, R.M. (2006). Intrauterine growth restriction. In R.J. Martin, A.A. Fanaroff, & M.C. Walsh (Eds.), Fanaroff and Martin’s neonatal-perinatal medicine: Diseases of the fetus and infant (8th ed.). Philadelphia: Mosby.