28
The physiology of pregnancy
Introduction
Pregnancy brings huge physiological changes and, from a developmental point of view, it is assumed that these changes are in the fetal interest. The changes are proactive; in other words, they are not proportional to the size of the fetus, such that by the end of the first trimester, many systems are functioning at levels close to those at term. The systems are reviewed in order below.
Respiratory system
Oxygen consumption is increased by around 15–20%. The requirement for this is partly maternal – to satisfy the increase in cardiac output, renal function and other metabolic requirements, including respiratory function, breast and uterine development. Around 40% of the increased oxygen requirement is for the fetoplacental unit. To supply this increased oxygen requirement, the mother hyperventilates, increasing minute ventilation by about 40% above the normal 7 L/min. This increase in ventilation is far greater than the increase in oxygen consumption, effectively providing a safety net.
The increase is predominantly achieved by increasing tidal volume rather than respiratory rate – in other words, the mother breathes more deeply. This is more efficient than increasing the respiratory rate, as there is less dead-space movement (i.e. that air which is outwith the alveoli and hence not involved in gas exchange). Maternal serum CO2 falls, favouring CO2 transfer from the fetus to the mother. These changes are thought to be mediated by progesterone, as a smaller but similar effect is noted in patients taking progestogen-containing contraceptives.
Breathlessness is a common symptom in pregnancy. This dyspnoea is perceptual rather than a reflection of inadequate gas exchange, and is often worse at rest. In late pregnancy, the gravid uterus may restrict diaphragmatic movement, exacerbating any feelings of breathlessness. Nevertheless, it is important to consider pathological causes of breathlessness, particularly pulmonary thromboembolic disease.
Cardiovascular system
In pregnancy, there is an increase in cardiac output and a decrease in peripheral vascular resistance.
Cardiac output rises by about 40%, from around 3.5 L/min to 6 L/min, from increases in both stroke volume and cardiac rate. As with the respiratory system, these changes are disproportionately greater than required. The fall in peripheral vascular resistance mediated by vasodilatation is not quite compensated for by the increased cardiac output, so the overall effect is a slight fall in blood pressure in the second trimester, sometimes by as much as 5 mmHg systolic and 10 mmHg diastolic. The blood pressure may rise slightly again in the third trimester and it may be difficult to separate this from the pathological state of pre-eclampsia. The hyperdynamic circulation of pregnancy can often reveal functional flow murmurs, which are usually of little clinical significance.
This high blood flow maximizes PO2 on the maternal side of the placenta and maximizes oxygen transfer to the fetal circulation. The plasma volume expansion and increased cardiac output may also help heat loss by increasing blood flow through the skin, thus compensating for the increased metabolic rate of pregnancy. Peripheral vasodilatation causes a feeling of warmth and a tolerance to cold, and may be a factor in the palmar erythema and spider naevi of pregnancy.
The cardiac output may rise by a further 2 L/min in established labour; this results from an increase in heart rate and stroke volume. Following delivery the uterus contracts, reducing cardiac output to 15–25% above normal, which over the next 6 weeks gradually returns to the pre-pregnancy state.
Late in pregnancy, the mass of the uterus is liable to press on, and partially occlude, the inferior vena cava. This reduced venous return leads to a reduced cardiac output and may lead to hypotension, so-called ‘supine hypotension’. The clinical importance of this is such that women in later pregnancy and in labour should not lie flat and, ideally a wedge or other device should be employed to allow the woman to lie at a slight tilt. Supine hypotension often results in a sensation of nausea but even in the absence of this, may be associated with fetal heart rate abnormalities. All women undergoing caesarean section, elective or emergency, should be placed on an operating table capable of a 15º lateral tilt.
Blood, plasma and extracellular fluid volume
On average, the total red cell mass increases steadily throughout the pregnancy by 25%, from around 1300–1700 mL. The circulating plasma volume, however, increases by 40%, from around 2600–3700 mL. Because the plasma volume increases proportionately, more than red cell mass, there is a dilutional drop in the haemoglobin concentration and in the haematocrit, such that a haemoglobin level of 105 g/L would be normal in a healthy pregnancy.
Plasma colloid osmotic pressure falls in pregnancy; as a result, fluid shifts into the extravascular compartment, causing oedema. Around 80% of pregnant women have some degree of dependent oedema.
Blood constituents and anaemia
The typical changes in the full blood count in pregnancy are shown in Table 28.1. Iron requirements are increased (Table 28.2) to meet the requirements of the larger red cell mass, developing fetus and the placenta, and the serum ferritin level therefore falls. The fetus gains iron from maternal serum by active transport across the placenta, mostly in weeks 36–40. In the absence of iron deficiency, routine supplementation is not recommended. Nevertheless, iron deficiency is not rare particularly if iron stores are low before pregnancy. A high index of suspicion is required to aid diagnosis, particularly in at-risk populations and among resource deficient populations. The World Health Organization (WHO) has suggested iron supplementation at haemoglobin levels below 105 g/L.
Table 28.1
Blood changes in pregnancy
| Non-pregnant | Pregnant | |
| Haemoglobin (g/L) | 120–140 | 100–120 |
| Red cell count (× 1012/L) | 4.2 | 3.7 |
| Haematocrit (venous) | 40% | 34% |
| MCV (fl) | 75–99 | 80–103 |
| MCH (pg) | 27–31 | No change |
| MCHC (g/dL) | 32–36 | No change |
| White cell count (× 109/L) | 4–11 | 9–15 |
| Platelets (× 109/L) | 140–440 | 100–440 |
| ESR (mm/h) | < 10 | 30–100 |
ESR, erythrocyte sedimentation rate; MCH, mean corpuscular haemoglobin: MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume.
Table 28.2
The requirements of elemental iron during pregnancy
| Fetus and placenta | 500 mg |
| Red cell increment | 500 mg |
| Postpartum blood loss and 6 months’ lactation | 360 mg |
| Total | 1360 mg |
| Saving from amenorrhoea approximately | 360 mg |
| Net increased demand approximately | 1 g |
Folate metabolism
The daily folate requirement rises from 50 μg to 400–600 μg, and folate deficiency may occur. It is usually possible to meet this increased requirement through a normal diet, although intake in those with a poor diet is likely to be inadequate. Daily folic acid supplementation from before conception reduces the risk of neural tube defects.
Haemostasis in pregnancy
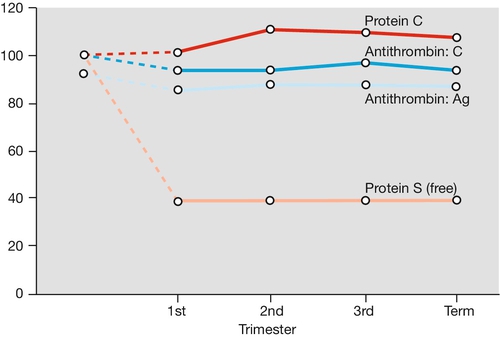
Pregnancy is a hypercoagulable state, with an increase in procoagulants (particularly fibrinogen, but also platelets, factor VIII, von Willebrand factor) and a reduction in naturally occurring anticoagulants (e.g. protein S and antithrombin). Fibrinolysis is also increased, so there is an increased net turnover of coagulation factors (Figs 28.1 and 28.2). Fibrinolytic activity returns to normal within 1 h of placental delivery, suggestive that inhibition of fibrinolysis is mediated by the placental unit.
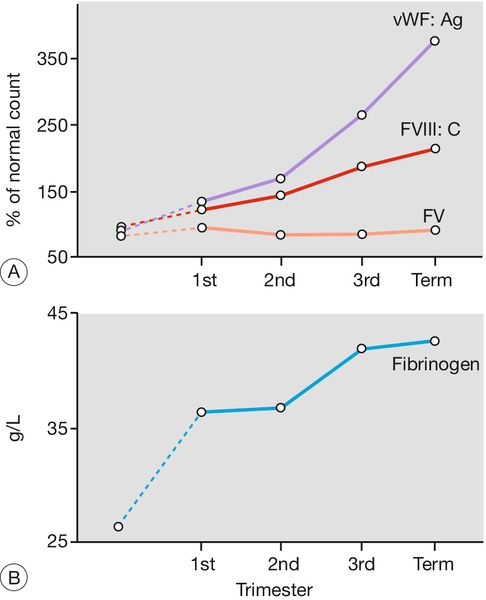
FV, factor V.
The reason for this hypercoagulable state is presumably, from a developmental point of view, to minimize blood loss at delivery, but the disadvantage is the increased risk of thromboembolic disease. Until the advent of blood transfusion, haemorrhage was a much more important cause of maternal mortality than was thromboembolic disease, and it is possible that hypercoagulability offered an evolutionary advantage.
Compared with the changes in coagulation and fibrinolysis, platelet changes are modest. The platelet count falls only slightly but there is an increase in aggregability, probably in relation to prostaglandin changes (Fig. 28.3).
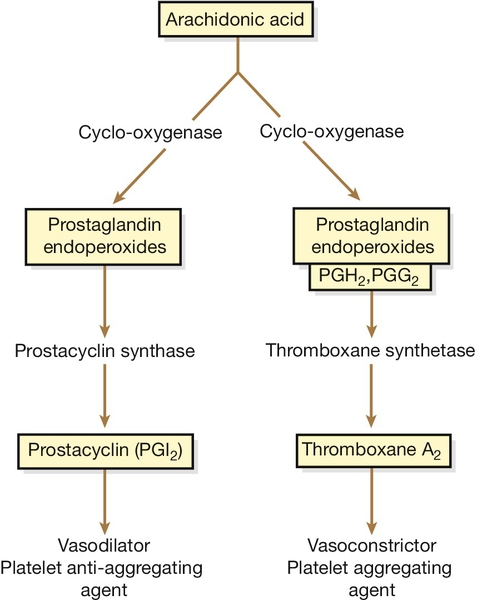
Fig. 28.3Prostaglandin metabolism.
In normal pregnancy, there is increased biosynthesis of eicosanoids – particularly prostacyclin (PGI2), a vasodilator with platelet inhibitory properties, and thromboxane A2, a vasoconstrictor with a tendency to stimulate platelet aggregation. As both usually increase in proportion to each other, there is a net neutralization and homeostasis is maintained. This homeostasis is disrupted in pre-eclampsia because of a relative deficiency in prostacyclin owing to either a decrease in its synthesis and/or an increase in the production of thromboxane A2. This imbalance leads to vasoconstriction, hypertension, and platelet stimulation.
Renal system
Renal blood flow and glomerular filtration rate (GFR) increase by about 60% from early in the first trimester to around 4 weeks postpartum. This causes a fall in plasma creatinine from around 73–47 mmol/L and urea from 4.3 to around 3.1 mmol/L. It is important to be aware of this fact when assessing renal function, as values that would appear normal pre-pregnancy may in fact indicate impairment in the pregnancy state. The increased GFR is not matched by increased tubular reabsorption and there would therefore be a tendency to lose sodium were it not for a compensatory increase in aldosterone levels.
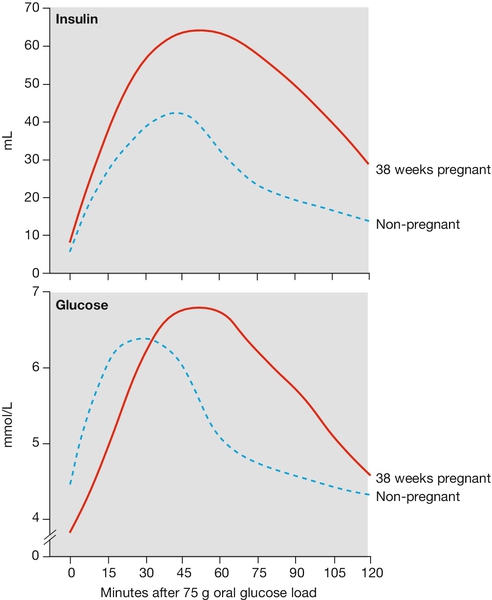
Glycosuria is common because the filtered load of glucose is greater than the tubular reabsorption capacity. Although glycosuria is often normal in pregnancy, it may still be pathological, and may represent evidence of gestational diabetes (see Fig. 28.5).
Dilatation of the renal pelvis and ureters is caused by both progesterone and local obstruction by the gravid uterus. It occurs from early in the first trimester and results in urinary stasis, which increases the likelihood of urinary tract infection; this may be further exacerbated by the presence of glycosuria.
Endocrine system
Pregnancy influences endocrine functioning in two main ways: placental hormonal production and by increased protein binding.
Placental hormonal production
The placenta produces a number of hormones, including oestrogen, progesterone (which relaxes smooth muscle), human placental lactogen (which increases maternal glucose and lipids) and human chorionic gonadotrophin (which prevents degradation of the corpus luteum in early pregnancy) (Table 28.3). The placenta also produces corticotrophin-releasing hormone, which in turn stimulates maternal production of ACTH, leading to increased aldosterone and cortisol, which in turn contribute to the maternal fluid changes described above.
Table 28.3
Roles of selected placental hormones
| Hormone | Role |
| Human chorionic gonadotrophin (hCG) | Initially maintains the corpus luteum’s secretion of progesterone and oestrogen; later it may have a role in regulating placental oestrogen secretion and in modulating the maternal immune response |
| Oestrogen | Over 90% is in the form of oestriol; it is involved in uterine growth, cervical changes, and breast development |
| Progesterone | Smooth muscle relaxation, acting on the uterus, gastrointestinal tract and ureters. Also has a role in regulating maternal physiological changes |
| Human placental lactogen (hPL) | Mobilizes maternal free fatty acids, improving glucose availability for the fetus |
Thyroid function
In pregnancy, there is increased iodine uptake activity and the total serum levels of T3 and T4 are also raised. Only the unbound portion of thyroxine is metabolically active, however, and as oestrogens also induce synthesis of thyroid-binding globulin, the levels of free T3 and T4 remain within the normal range or may even fall slightly.
Pituitary function
Oestrogen stimulates the release of thyrotrophin-releasing hormone, which in turn increases prolactin production by the anterior pituitary. Prolactin stimulates breast growth antenatally, but lactation is usually inhibited by progesterone until after delivery of the placenta, when the prolactin acts with oxytocin from the posterior pituitary to stimulate milk production. Pituitary prolactin production is increased to such an extent in pregnancy (around 10 times the pre-pregnancy level) that the pituitary increases in size by around 135%.
Gastrointestinal system
There is a general reduction in gut motility and a slowing of transit times. This may benefit the fetus by increasing the absorption of certain nutrients. Delayed gastric emptying and gastric relaxation are a feature of pregnancy, especially with highly osmotic foods (e.g. glucose) and are particularly marked in labour. This altered state becomes clinically important if the woman requires a general anaesthetic, because of the risk of aspiration pneumonia (Mendelson syndrome, see History box), and acid-reduction medications are therefore routinely prescribed prior to caesarean section.
Nausea and vomiting are common in early pregnancy. It is not clear whether they are caused by rising human chorionic gonadotrophin (hCG) or oestrogen levels or some other factor; management is discussed elsewhere. Most pregnant women report increased appetite and thirst, and many have cravings for, or aversions to, certain foods. Perhaps the most common aversions are to tea and coffee. Pica, a craving for non-food substances, such as coal, chalk or soap, is rare but well known. The cause is unknown.
Gastric acid secretion is reduced in pregnancy, which is presumably the reason why peptic ulcer disease commonly improves. In contrast, reflux oesophagitis is likely to be more severe, and results from a combination of reduced tone in the lower oesophageal sphincter and increased intra-abdominal pressure.
Many women report constipation in pregnancy and this is usually attributed to the relaxing effect of progesterone on gut smooth muscle. There is, however, little good evidence that constipation really is more common in pregnancy. If it occurs, it should be managed, as in the non-pregnant state, with increased dietary fibre and stool-bulking agents.
Rectal haemorrhoids probably result from a combination of increased straining and increased intra-abdominal pressure, and as part of the generalized vasodilatation mentioned above.
Liver and bile ducts
Normal pregnancy is a mildly ‘cholestatic’ state. Biochemical tests of liver function, however, lie within the normal range, with the exception of alkaline phosphatase levels, which are approximately doubled. Most of this increase comes from placental secretion of this enzyme, rather than from the liver. (Pathological cholestasis is discussed on page 229).
Oestrogen increases the serum cholesterol and this is translated into bile salt production, which saturates the bile. Since progesterone also reduces gall bladder emptying, pregnancy predisposes to gallstone formation.
Skin and appendages
The skin participates in the generalized increase of blood flow in pregnancy. Increased pigmentation is also seen in the nipples and in the midline of the abdomen (linea nigra), as a result of placental melanocyte-stimulating hormone production. Striae gravidarum, or stretch marks, occur in the presence of high oestrogen levels, in skin subject to stretching, such as that over the breasts and abdomen. Initially reddish/purple, they fade after delivery to a faint silvery colour. There is no effective prevention or treatment.
The cycle of hair growth is altered in pregnancy, with a greater proportion (95% vs 85%) of hairs in the actively growing phase. As a result, there are many over-aged hairs at the end of pregnancy, which fall out and lead to the common symptom of hair coming out ‘in handfuls’ postnatally. This change is temporary.
Metabolic changes
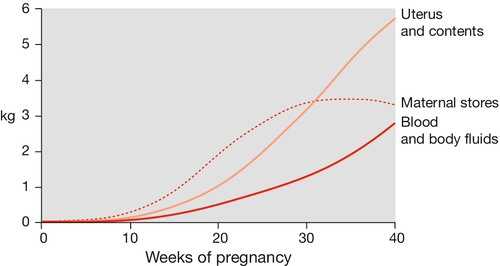
Extra energy is required not only for the developing fetus but also to fuel the increase in maternal physiological parameters. The resting metabolic rate is increased by around 20% and weight increases on average by around 12 kg (Fig. 28.4). Initially, there is an increased sensitivity to insulin, which leads to increased glycogen synthesis, increased fat deposition and an increase in amino acid transfer into cells. After mid-pregnancy there is a degree of insulin resistance. The serum glucose level at this stage may therefore rise, a change presumably in the fetal interest as fetal glucose levels will also rise. The insulin resistance also leads to increased levels of serum lipids, which can be used by the mother as an alternative energy source to glucose. Although maternal amino acid levels fall, there is increased transport across the placenta.
Pregnancy is a diabetogenic state. Cortisol, progesterone, oestrogen and human placental lactogen are all insulin antagonists, and tend to increase the glucose level. If the pancreatic islet β-cells are unable to produce sufficient insulin to balance this increase, or if there is maternal insulin resistance, the maternal glucose level may rise pathologically (Fig. 28.5). This is discussed elsewhere.
Calcium homeostasis
Fetal skeletal development requires 20–30 g of calcium, and this need is met by increasing maternal intestinal absorption. There is usually, therefore, no maternal bone demineralization. Calcium transfer across the placenta is an active process occurring against a concentration gradient and it is therefore not surprising that maternal free calcium levels are not significantly changed. Serum protein and albumin fall as part of the plasma dilution of pregnancy so that total calcium is reduced. Vitamin D deficiency is commoner in pregnancy and the UK Chief Medical Officers recommend that all pregnant and breastfeeding women should take a daily supplement containing 10 μg of vitamin D.
Placental transfer
The placenta provides a functional and immunological barrier and is an organ of:
![]() respiration
respiration
![]() nutrient transfer, also providing the mechanism for waste excretion
nutrient transfer, also providing the mechanism for waste excretion
![]() hormonal synthesis.
hormonal synthesis.
The mechanisms of transfer across the placenta are outlined in Table 28.4. The nuclei and other intracellular organelles in the syncytiotrophoblast come to lie in groups to form areas of thick metabolically active tissue, which are probably the sites of active diffusion. Between them are other areas with only a very fine layer separating maternal and fetal blood, the vasculosyncytial membrane, and these are where most passive gas transfer occurs.
Table 28.4
Some examples of the mechanism of placental transfer
| Mechanism | Substance |
| Passive diffusion | CO2, O2, water |
| Facilitated diffusion | Glucose (by a carrier molecule) |
| Active transport by enzymatic action | Amino acids, Ca, Fe, vitamins B and C, free fatty acid |
| Organelle transport by pinocytosis | IgG |
Respiration
The following changes maintain the diffusion gradient:
1. The high O2 affinity of fetal haemoglobin. The dissociation curve of fetal haemoglobin is shifted to the left so that at a given PO2, the percentage saturation is higher than for adult haemoglobin. More importantly, for the fetal interest, at a given level of oxygen transfer the PO2 will be lower (see Fig. 28.6 and Box 28.1).
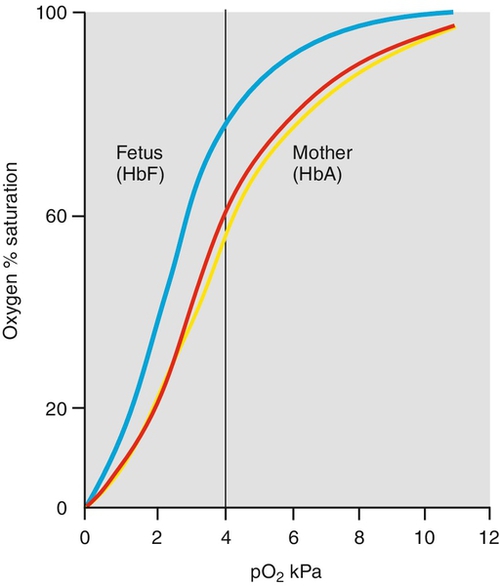
Fig. 28.6Fetal haemoglobin has a higher affinity for O2 than does maternal haemoglobin.
The dissociation curve of fetal haemoglobin (blue) is shifted to the left so that at a given PO2 the percentage saturation is higher than for adult haemoglobin. More importantly for the fetal interest, at a given level of oxygen transfer the cO2 will be lower. As CO2 passes from the fetus to the mother, so the maternal dissociation curve shifts to the right (red curve to yellow curve). This is the Bohr effect.
2. The high fetal Hb (normal 140–200 g/L) also results in a lower PO2 for a given saturation.
3. Finally, the passage of CO2 from fetus to mother helps increase maternal oxygen dissociation and fetal association. This shift of the maternal oxygen dissociation curve to the right caused by CO2 accumulation is called the Bohr effect (Fig. 28.6).
The CO2 dissociation curves are similar in fetal and maternal blood. Although most CO2 is carried as bicarbonate, bicarbonate is a charged molecule and cannot cross the placenta. Only dissolved CO2 can cross the placenta, and this is again by diffusion. This transfer is facilitated by maternal hyperventilation, which lowers maternal PCO2, and lower fetal concentrations of carbonic anhydrase, which slows the establishment of equilibrium between CO2 and 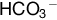 . Furthermore, as maternal Hb gives up oxygen, its CO2 affinity increases (the Haldane effect), and the opposite (reverse Haldane effect) decreases CO2 affinity of fetal haemoglobin as it in turn takes up oxygen.
. Furthermore, as maternal Hb gives up oxygen, its CO2 affinity increases (the Haldane effect), and the opposite (reverse Haldane effect) decreases CO2 affinity of fetal haemoglobin as it in turn takes up oxygen.
Nutrition
The developing fetus requires energy, largely provided by glucose, amino acids and fatty acids. These are mostly transported across the placental membrane by active processes as outlined in Table 28.4. In later pregnancy, excessive glucose is converted into glycogen and fat, such that by term, 15% of the body weight is fat. In pre-term babies and those with fetal growth restriction, these energy stores are lower.
Immunology
Most human cells have a gene on chromosome 6 that codes for a particular protein called the human leucocyte antigen or ‘HLA’. Each of us has a unique HLA gene and the protein synthesized from this gene coats the surface of all the cells in our body, allowing our immune cells to recognize ‘self’ cells. If leucocytes identify a ‘non-self’ code, they initiate a process of cell destruction. The fetus is genetically unique and will have a different HLA complement from that of either parent. If a skin graft is taken from a child and grafted onto its mother, the graft will be rejected. Yet, while the fetus is ‘grafted’ onto the lining of the maternal uterus, it is not rejected. There must therefore be some protective mechanism, or mechanisms, to prevent immunological fetal rejection.
HLA has different subtypes. The classical genes, HLA-A, -B and -C, provide the highly individual molecules coding for ‘self’, and these molecules are absent on many of the placental cells. This would render these placental cells less susceptible to maternal leucocyte recognition and therefore less liable to destruction. Some immunological reaction, however, may be important to prevent placental over-invasion, and some placental cells do express the classical genes, particularly HLA-C. Further modulation of this uneasy fetomaternal immune relationship may be modified by another HLA molecule, HLA-G, specific only to placental tissue. Much more work is required before these mechanisms are understood more clearly, particularly as it is becoming apparent that immunological disparity may lie behind recurrent miscarriage, fetal growth restriction and pre-eclampsia.
Summary
Women who are pregnant overbreathe, retain fluid and calories and increase the perfusion of most organs. In the process, many blood parameters change, and there may be anaemia, glycosuria, constipation and cholestasis. Nevertheless, most of the changes are, directly or indirectly, in the fetal interest. Minor symptoms do not usually require treatment. Blood values outwith the normal non-pregnant range should be interpreted with an awareness of altered physiology of pregnancy.