The Perianesthesia Patient
Advances in anesthetic agents and monitoring have resulted in more precise and safer delivery of anesthetic agents. Caring for the critically ill patient who is emerging from anesthesia requires diligent monitoring of the patient’s physical and psychologic status to prevent potential complications that may occur as a result of the anesthetic agents or techniques. To provide safe and competent patient care, the critical care nurse needs knowledge of anesthetic agents and techniques and the physiologic and psychologic responses of patients who receive anesthesia.1,2
Selection of Anesthesia
The complex structure of the anesthetic agents, combined with potential medication interactions and the patient’s physical condition, can make it difficult to predict the patient’s response when emerging from anesthesia. Knowledge of the general principles of anesthesia prepares the nurse for the most commonly expected outcomes.2,3 The American Society of Anesthesiologists’ physical status classification is widely accepted as a method of preoperative patient evaluation.4 It guides communication of clinical conditions and predicts risks for anesthesia (Box 42-1). Preoperative evaluation allows the anesthesia care provider to individualize and modify care for patients at high risk for surgery.4
The type of anesthesia used for surgery may be local, regional, or general. Local and regional anesthetics eliminate the sensation of pain to a specific part of the body without loss of protective reflexes or consciousness. Many patients also receive intravenous sedation with benzodiazepines to relieve anxiety, provide amnesia, and promote relaxation. Local anesthesia with sedation may be defined as moderate or procedural sedation. Another term used is monitored anesthesia care. Depending on the sedation given and the patient’s response, the level of consciousness can range from light to deep. Further information on sedation is provided in Chapter 10. Regional anesthesia includes peripheral and central neuraxial (spinal, epidural, caudal) blocks. The blocks include single injection and continuous infusion techniques, typically used for postoperative or postprocedural analgesia. Spinal anesthesia involves injecting local anesthetic into cerebrospinal fluid contained within the subarachnoid space, below L1 in the adult and L3 in the child.5 Epidural anesthesia involves injecting the epidural space, which lies within the vertebral canal but outside the dural sac, with local anesthetics. Epidurals may be performed at all levels of the neuraxis. Spinal and epidural anesthesia cause sensory and motor anesthesia. The advantages of epidural over spinal anesthesia are a decreased incidence of spinal headache, less incidence of systemic hypotension, ability to provide a segmental sensory block, and increased ability to provide postoperative pain management.6
General anesthesia is a controlled, reversible state of unconsciousness: the patient is not arousable, there is partial or complete loss of protective reflexes, and the airway and ventilation needs to be continuously monitored and maintained. An endotracheal tube is commonly used for airway maintenance for general anesthesia, although a laryngeal mask airway (LMA) may also be used.7
Several factors influence the choice of anesthetic agent and the mode of delivery, including age and coexisting diseases, site of surgery, position of patient during surgery, status of surgery (i.e. elective, emergent), duration of the procedure, the skills of the anesthesia care provider and surgeon, and patient preference.8
General Anesthesia
The goals of general anesthesia are analgesia, amnesia/hypnosis, suppression of autonomic and sensory reflexes, and skeletal muscle relaxation.9 Stages of anesthesia, defined by Guedel around World War I, were initially used to identify the patient’s physiologic state and monitor anesthetic depth. These stages are less relevant today due to technologic advances in monitoring and anesthetic agents with faster onset and elimination, limiting the usefulness of the classic signs and symptoms associated with the stages to assessment and care of the patient after surgery.10
During general anesthesia, the goal is to keep the patient insensate, immobile, and safe. Recall or awareness during surgery can occur when the depth of anesthesia is inadequate (Box 42-2). Excessive anesthesia may stress the patient and increase emergence and recovery time. The level of anesthesia is monitored by continual assessment of the patient’s clinical presentation. Basic anesthetic monitoring standards adopted by the American Society of Anesthesiologists (ASA) mandate the use of pulse oximetry, capnography, an oxygen analyzer, disconnect alarms, body temperature measurements, and a visual display of an electrocardiogram (ECG) during the intraoperative period in all patients undergoing anesthesia.11 The standard may be exceeded as warranted by the patient’s condition and additional monitors used. External monitoring devices used to assess levels of anesthesia include lower esophageal contractility, heart rate variability, surface electromyogram, spontaneous electroencephalographic activity monitors, and evoked potentials.
Anesthetic Agents
Typically two or more anesthetic agents are used in combination to achieve the desired level of anesthesia. To anticipate the patient’s response, it is important for the nurse to have knowledge of the anesthetic agents that are used and their usual physiologic effects.2 The characteristics of ideal anesthetic agents and adjuncts are listed in Box 42-3.
Inhalation Agents
Inhalation agents are used for induction and maintenance of anesthesia or, in combination with other anesthetic agents, to maintain surgical anesthesia. They can be classified as volatile or gaseous. Volatile agents are further classified as halogenated hydrocarbons or ethers, are liquid at room temperature, and have a boiling point of 20° C. Gaseous agents are gases at room temperature. Inhaled anesthetics are delivered through the respiratory tract and are absorbed into the circulation through the alveoli. The effects of inhalation agents depend on alveolar ventilation, the ventilation-perfusion ratio, co-administered gases, gas flow, and the physicochemical properties of the gas. Their exact mechanism of action is unknown, but all cause central nervous system (CNS) depression and a state of unconsciousness that is deep enough to allow surgery. Table 42-1 lists the inhalation anesthetics presently used and their chief characteristics, effects, and nursing implications.10
TABLE 42-1
| MEDICATION | CHARACTERISTICS | EFFECTS | CONSIDERATIONS |
| Nitrous oxide | Light anesthetic; inorganic gas; nontoxic, nonirritating; carrier for other inhalation agents; always given with oxygen | Anesthetic and analgesic; minimal pulmonary, cardiac, or CNS effects; increases intracranial pressure; amnesia | Eliminated by ventilation; postoperative nausea and vomiting; diffusion hypoxia; mild myocardial depression |
| Isoflurane (Forane) | Pungent, ether-like odor; low potential for toxicity; volatile agent | Higher cardiovascular stability; potentiates muscle relaxants | Mild depression of spontaneous ventilation; may trigger malignant hypothermia; postoperative shivering; fewer dysrhythmias noted |
| Desflurane (Suprane) | Strong, pungent odor; rapid onset; volatile agent; requires warmed vaporizer for administration | Minimal metabolism; respiratory depression; cardiovascular depression; no lingering analgesia | Observe for breath holding; coughing and laryngospasm; may trigger malignant hypothermia; needs immediate postoperative analgesia |
| Sevoflurane (Ultane) | Nonirritating to airway; volatile agent; choice for pediatric anesthetic | Minimal airway irritation; rapid elimination; great precision and control over anesthetic depth; decreases SVR | May trigger malignant hypothermia; observe for breath holding; needs immediate postoperative analgesia |
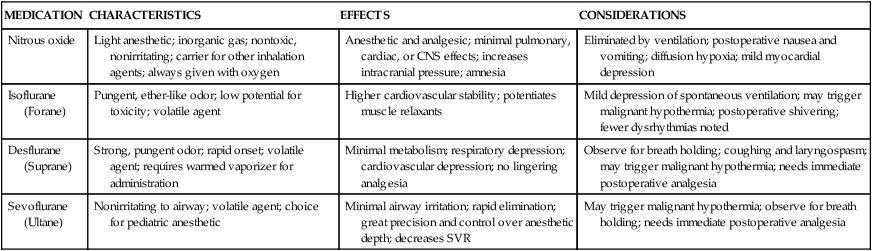
CNS, Central nervous system; SVR, systemic vascular resistance.
Intravenous Anesthetics
Because inhalation anesthetics can produce adverse effects such as vasodilation, hypotension, dysrhythmias, and myocardial depression, other medications and methods of delivery have been sought to provide general anesthesia. Intravenous anesthetics are commonly used in the perioperative period. Intravenous anesthetics are grouped by their primary pharmacologic action as nonopioid or opioid intravenous agents.12–14 The nonopioid agents are further divided into the barbiturates, nonbarbiturates, and sedatives. These medications can be administered by intermittent intravenous push dosing to induce anesthesia or by continuous intravenous drip to maintain anesthesia.
Nonopioid Intravenous Anesthetics
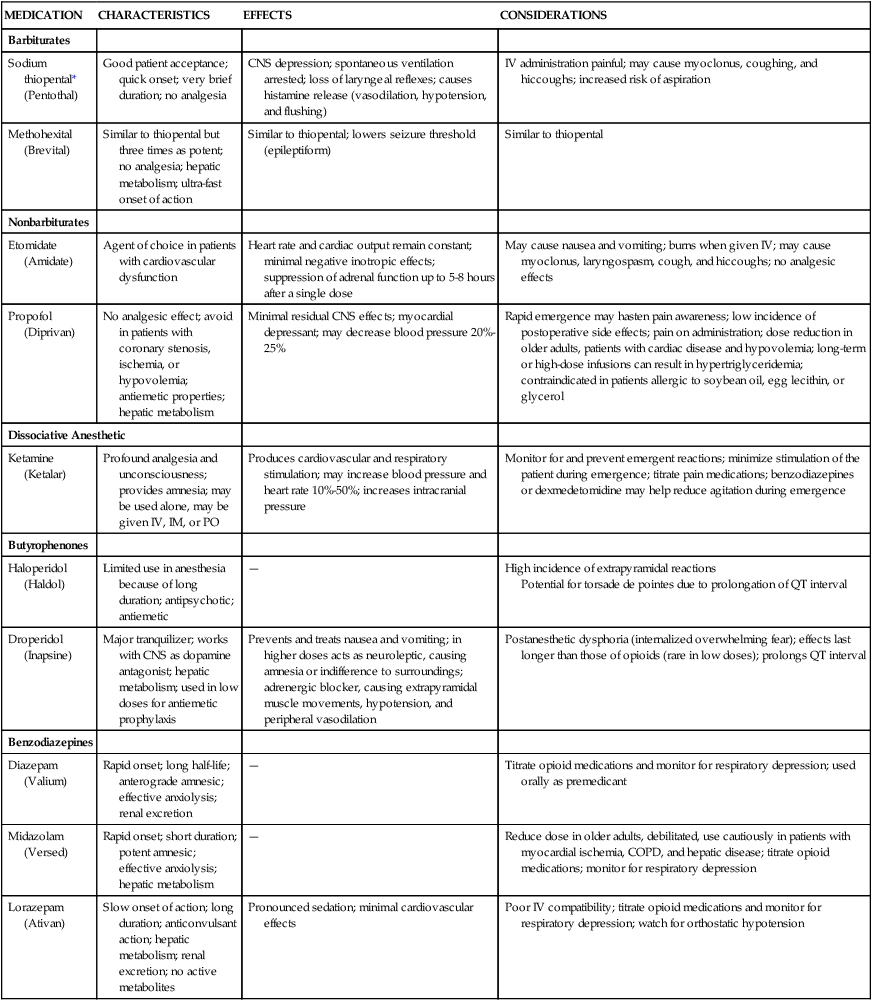
Gamma-aminobutyric acid (GABA) is an inhibitory neurotransmitter. The nonopioid medications appear to interact with GABA in the brain. Activation of the GABA receptors inhibits the postsynaptic neurons and results in a loss of consciousness. Barbiturates bind to GABA postsynaptic receptors, inhibiting neuronal activity and causing a loss of consciousness. Sedatives such as benzodiazepines potentiate the action of GABA, leading to inhibition of neuronal activity. Nonbarbiturate induction agents, such as etomidate, antagonize the muscarinic receptors in the CNS and work as opioid agonists, resulting in a hypnotic state and loss of consciousness. Table 42-2 presents the nonopioid intravenous anesthetics and their effects and nursing considerations.12
TABLE 42-2
NONOPIOID INTRAVENOUS ANESTHETICS
| MEDICATION | CHARACTERISTICS | EFFECTS | CONSIDERATIONS |
| Barbiturates | |||
| Sodium thiopental* (Pentothal) | Good patient acceptance; quick onset; very brief duration; no analgesia | CNS depression; spontaneous ventilation arrested; loss of laryngeal reflexes; causes histamine release (vasodilation, hypotension, and flushing) | IV administration painful; may cause myoclonus, coughing, and hiccoughs; increased risk of aspiration |
| Methohexital (Brevital) | Similar to thiopental but three times as potent; no analgesia; hepatic metabolism; ultra-fast onset of action | Similar to thiopental; lowers seizure threshold (epileptiform) | Similar to thiopental |
| Nonbarbiturates | |||
| Etomidate (Amidate) | Agent of choice in patients with cardiovascular dysfunction | Heart rate and cardiac output remain constant; minimal negative inotropic effects; suppression of adrenal function up to 5-8 hours after a single dose | May cause nausea and vomiting; burns when given IV; may cause myoclonus, laryngospasm, cough, and hiccoughs; no analgesic effects |
| Propofol (Diprivan) | No analgesic effect; avoid in patients with coronary stenosis, ischemia, or hypovolemia; antiemetic properties; hepatic metabolism | Minimal residual CNS effects; myocardial depressant; may decrease blood pressure 20%-25% | Rapid emergence may hasten pain awareness; low incidence of postoperative side effects; pain on administration; dose reduction in older adults, patients with cardiac disease and hypovolemia; long-term or high-dose infusions can result in hypertriglyceridemia; contraindicated in patients allergic to soybean oil, egg lecithin, or glycerol |
| Dissociative Anesthetic | |||
| Ketamine (Ketalar) | Profound analgesia and unconsciousness; provides amnesia; may be used alone, may be given IV, IM, or PO | Produces cardiovascular and respiratory stimulation; may increase blood pressure and heart rate 10%-50%; increases intracranial pressure | Monitor for and prevent emergent reactions; minimize stimulation of the patient during emergence; titrate pain medications; benzodiazepines or dexmedetomidine may help reduce agitation during emergence |
| Butyrophenones | |||
| Haloperidol (Haldol) | Limited use in anesthesia because of long duration; antipsychotic; antiemetic | — | High incidence of extrapyramidal reactions Potential for torsade de pointes due to prolongation of QT interval |
| Droperidol (Inapsine) | Major tranquilizer; works with CNS as dopamine antagonist; hepatic metabolism; used in low doses for antiemetic prophylaxis | Prevents and treats nausea and vomiting; in higher doses acts as neuroleptic, causing amnesia or indifference to surroundings; adrenergic blocker, causing extrapyramidal muscle movements, hypotension, and peripheral vasodilation | Postanesthetic dysphoria (internalized overwhelming fear); effects last longer than those of opioids (rare in low doses); prolongs QT interval |
| Benzodiazepines | |||
| Diazepam (Valium) | Rapid onset; long half-life; anterograde amnesic; effective anxiolysis; renal excretion | — | Titrate opioid medications and monitor for respiratory depression; used orally as premedicant |
| Midazolam (Versed) | Rapid onset; short duration; potent amnesic; effective anxiolysis; hepatic metabolism | — | Reduce dose in older adults, debilitated, use cautiously in patients with myocardial ischemia, COPD, and hepatic disease; titrate opioid medications; monitor for respiratory depression |
| Lorazepam (Ativan) | Slow onset of action; long duration; anticonvulsant action; hepatic metabolism; renal excretion; no active metabolites | Pronounced sedation; minimal cardiovascular effects | Poor IV compatibility; titrate opioid medications and monitor for respiratory depression; watch for orthostatic hypotension |
Benzodiazepine Antagonists
Flumazenil (Romazicon) reverses the sedative, amnesic, respiratory depressant, and muscle-relaxant effects of benzodiazepines.14 This medication is specific for the benzodiazepine receptors and does not reverse the effects of barbiturates or opiates. It should be used with caution in patients who have a history of seizures or chronic benzodiazepine use, as it can precipitate seizures. Because flumazenil has a shorter duration of action than most of the benzodiazepines, the risk of resedation can occur after the initial dose starts to wear off, especially when high doses of benzodiazepines are administered. The patient must be monitored for resedation and other residual effects. If the patient develops signs of resedation, flumazenil is repeated at 20-minute intervals. Flumazenil has proved to be a valuable asset in the care of the patient who has received an excessive dose of a benzodiazepine such as midazolam or lorazepam. Consequently, flumazenil is very useful intraoperatively, postoperatively, and in the critical care unit.
Opioid Intravenous Anesthetics
Intravenous opioid anesthetics play an important role in clinical anesthesia care. These medications enhance the effectiveness of inhalation agents by providing the analgesic portion of the anesthetic process. Intravenous opioids blunt the sympathetic response to painful stimuli during anesthesia. The use of opioids allows for reduction in the concentration of the inhalation agent to be administered, increasing safety. Opioids bind to specific receptors and produce a morphine-like or opioid agonist effect. Opioids are used to manage acute and chronic pain and are administered for general anesthesia, sedation, and pain relief during regional anesthesia; they are important in all phases of the perioperative experience. Table 42-3 presents a summary of clinical uses and nursing implications for the most frequently used opioids.13
TABLE 42-3
| CLINICAL USES | IMPLICATIONS | CONSIDERATIONS |
| Preoperative sedation | Monitor for hypotension | Keep naloxone (Narcan) available |
| Induction of anesthesia | Monitor for bradycardia | Keep resuscitation equipment available |
| Maintenance of anesthesia | Monitor for respiratory depression | Respiratory depressant effect may outlast analgesia |
| Postoperative pain management | May cause nausea and vomiting |
*Agents include alfentanil (Alfenta), fentanyl (Sublimaze), ketorolac (Toradol), morphine, sufentanil (Sufenta), remifentanil (Ultiva), and hydromorphone (Dilaudid).
Opioid Antagonists
Opioid antagonists are used to reverse the effects of opioids, particularly respiratory depression, restoring spontaneous ventilation in patients who are breathing inadequately. The medication of choice in perianesthesia care is naloxone (Narcan). Naloxone competes with and displaces the opioid on the receptor site; it therefore reverses respiratory depressant and analgesic effects of opioids. Naloxone is diluted and then titrated to the patient’s response, minimizing the risk of rapid reversal and subsequent adverse effects. The onset of action is 1 to 2 minutes and the duration of action is 1 to 4 hours. If adequate reversal has not been achieved after 3 to 5 minutes, naloxone administration is repeated until reversal is complete.15
Neuromuscular Blocking Agents
Neuromuscular blocking agents (NMBAs), or muscle relaxants, interrupt the transmission of impulses from the nerve to the muscle, causing a decrease in muscle activity. Decreasing muscle activity allows the surgeon to operate in a quiet field and decreases the need for deep anesthesia. These medications have contributed greatly to clinical anesthesia care. Use of NMBAs is not limited to the operating room; they are used to facilitate endotracheal intubation, to terminate laryngospasm, to eliminate chest wall rigidity that may occur after the rapid injection of potent opioids, and to facilitate mechanical ventilation by producing total paralysis of the respiratory muscles.15
Table 42-4 presents a pharmacologic overview of the commonly used skeletal muscle relaxants.16 A number of factors can potentiate or antagonize the effects of nondepolarizing NMBAs; these factors are listed in Box 42-4.17
TABLE 42-4
NEUROMUSCULAR BLOCKING AGENTS*
| IMPLICATIONS | ||
| CHARACTERISTICS | DEPOLARIZING | NONDEPOLARIZING |
| Compete with acetylcholine at the myoneural junction | Nonreversible | Reversible with time and anticholinesterases |
| Use cautiously in patients with neuromuscular disease, such as myasthenia gravis or muscular dystrophy; contraindicated for pediatric use except in emergencies | Use cautiously in patients with hepatic or renal disease, obesity, asthma, or COPD | |
| Shorter-acting agents are most appropriate for anesthesia | Adverse effects include bradycardia, tachycardia, ventricular dysrhythmias, asystole, hypertension, hyperkalemia | Adverse effects include tachycardia, hypertension, hypotension, bronchospasm, and flushing |
| Provide surgical relaxation Facilitate intubation | Increases intraocular, intracranial, and intragastric pressure | |
| Assist in ventilatory support | Precipitates muscle fasciculations and pain | |
| Prolongs respiratory depression | ||
| Histamine release causes hypotension | ||
| Use cautiously in patients with head injury, cerebral edema, trauma, burns, electrolyte imbalances, and renal or hepatic disease | ||
| Monitor for clinical signs of malignant hyperthermia | ||
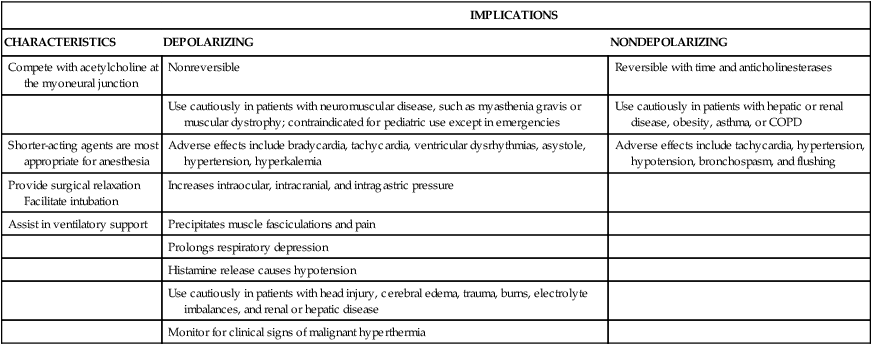
*Long-acting agents include pancuronium (Pavulon); intermediate-acting agents include atracurium (Tracrium), vecuronium (Norcuron), and cisatracurium (Nimbex); short-acting agents include rocuronium (Zemuron) and succinylcholine (Anectine), which is a depolarizing agent.
Neuromuscular Blocking Agent Antagonists
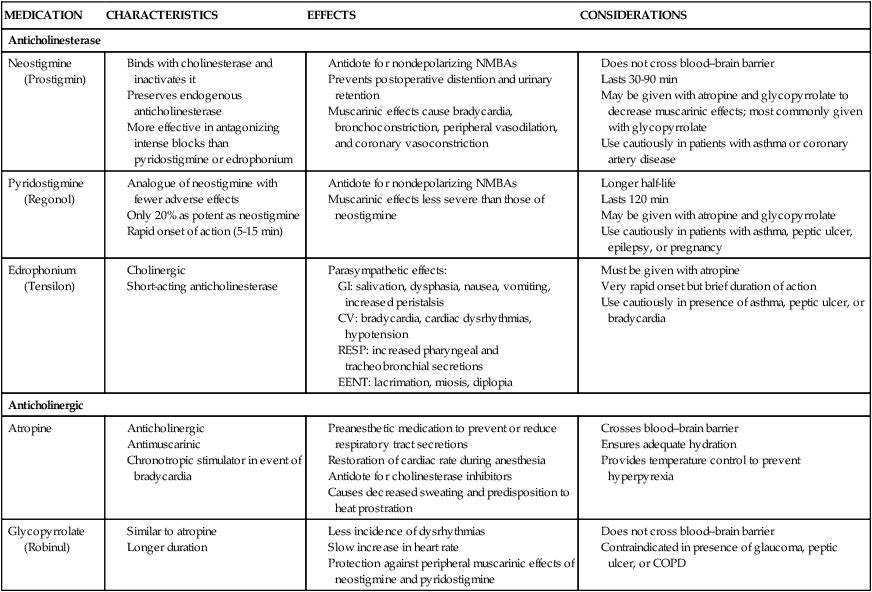
The pharmacologic actions of nondepolarizing NMBAs can be reversed by anticholinesterase medications such as neostigmine (Prostigmin). These medications increase the amount of acetylcholine available at the receptor sites by preventing its destruction by acetylcholinesterase. This promotes more effective competition of acetylcholine with the nondepolarizing skeletal muscle relaxant that is occupying the receptor sites. Because of the increased availability and mobilization of the acetylcholine, the concentration gradient favors acetylcholine and the removal of the nondepolarizing agent from the receptors, resulting in the return of normal skeletal muscle depolarization and contraction.18 These medications also produce undesired side effects by increasing the level of acetylcholine at receptor sites in the heart, the lungs, the eyes, and gastrointestinal tract, which can lead to bradycardia, bronchospasm, miosis, and increased peristalsis and secretion. To prevent or minimize these effects, anticholinergic agents such as atropine or glycopyrrolate (Robinul) are given with the anticholinesterase agent. Table 42-5 outlines the common NMBA reversal agents used in anesthesia and their nursing implications.18
TABLE 42-5
| MEDICATION | CHARACTERISTICS | EFFECTS | CONSIDERATIONS |
| Anticholinesterase | |||
| Neostigmine (Prostigmin) | |||
Perianesthesia Assessment and Care
The goal of management in the immediate postoperative period is the recognition and immediate treatment of any problems, to eliminate or lessen complications that may occur. This requires the collaborative effort of the nurse, the anesthesiologist, and the surgeon. Physical assessment of the postanesthesia patient begins immediately on admission to the unit. This initial assessment focuses on airway, ventilation, and circulation (heart rate, rhythm, and blood pressure). Following the brief initial assessment, the nurse admitting the patient receives a verbal report from the anesthesia care provider and surgeon. The report includes information about the patient’s general condition, significant past history or co-morbidities, the operation performed, the type of anesthesia administered, estimated blood loss, total intake and output during surgery, and any problems or complications encountered in the operating room.19
Respiratory Function
Pulse oximetry, a noninvasive technique, measures oxygen saturation of functional hemoglobin. Pulse oximetry can be used to identify hypoxemia, and its use should be standard on all postoperative patients. If the patient is intubated and mechanically ventilated, capnography can be used to assess for adequate ventilation. Arterial blood gas measurements are appropriate to definitively confirm abnormal pulse oximetry or capnography values. Normal pulse oximetry values are 97% to 99%; however, preanesthetic baseline values must be noted. Some patients normally have lower saturation values on room air for a variety of reasons, and attempting to maintain higher oxygen saturation levels may result in prolonged oxygen therapy.20
For spontaneously breathing patients, supplemental oxygen may be needed, typically using nasal cannula (prongs) or simple facemask. Surgery and anesthesia often interrupt the normal functioning of the nose, so humidification or nebulization with oxygen delivery may be needed. Humidifiers convert water from the liquid to the gaseous state, whereas nebulizers produce tiny water particles. This is especially helpful at higher flow rates.21
Patients may require mechanical ventilation following anesthesia. Various modes, such as positive end-expiratory pressure (PEEP), continuous positive airway pressure (CPAP), and synchronized intermittent mandatory ventilation (SIMV), are used to improve the respiratory status of the patient.21
Stir-Up Regimen
A significant aspect of perianesthesia nursing management is the stir-up regimen.22 The regimen is aimed at the prevention of complications, primarily atelectasis and venous stasis. The stir-up regimen consists of five major activities—deep-breathing exercises, coughing, positioning, mobilization, and pain management.
Deep-Breathing Exercises.
The sustained maximal inspiration (SMI) maneuver is a method to enhance the lung volumes of postoperative patients.22 The SMI maneuver consists of having the patient inhale as close to lung capacity as possible and, at the peak of inspiration, hold that volume for 3 to 5 seconds before exhaling it. This maneuver is more effective than simple deep breathing in preventing reduced lung volumes in the immediate postanesthesia periods. If the patient’s vital capacity is inadequate or if anesthesia respiratory depression is prolonged, deep breathing and the SMI maneuver may be augmented with a manual resuscitation bag connected to an oxygen source or with an intermittent positive-pressure breathing apparatus.
Coughing.
A purposeful cough is the most effective method to clear the air passages of obstructive secretions.22 For the patient recovering from anesthesia, the cascade cough is the most effective coughing maneuver. Instruct the patient to take a rapid, deep inhalation. This will increase the volume of air in the lungs and dilate the airways, allowing air to pass behind the retained secretions. On exhalation, have the patient perform multiple coughs. With each cough the length of the airways increases, enhancing the effectiveness of the cough.
Pain Management.
Pain accelerates the cardiovascular system by activating the sympathetic nervous system and the adrenal system. Normally, this causes an increase in heart rate and blood pressure. However, anesthesia and some cardiac medications can blunt the sympathetic response; asking the patient his or her pain level is the most valid method of assessing pain levels (see Chapter 9). It has been suggested that pain, especially at upper abdominal and thoracic sites, decreases or eliminates the normal sighing (yawning) mechanism. The absence of an appropriate sigh leads to reduced lung volumes and, ultimately, to atelectasis and pneumonia. Appropriate pain relief in these patients reduces the postoperative incidence of atelectasis and pneumonia.23
After the assessment has been completed and it has been determined that the patient is experiencing acute postoperative pain, certain interventions are suggested. If the patient has received an inhalation anesthetic, such as isoflurane, and demonstrates manifestations of acute pain, relief is instituted early in the postanesthetic period. Similarly, patients undergoing a nitrous oxide–opioid technique are medicated early in the immediate postoperative period, particularly if the intraoperative opioids were of short duration. If medications such as sufentanil and morphine were used intraoperatively, opioids must be administered with caution to avoid respiratory depression as a result of the synergistic action of the intraoperative and postoperative opioid agonists. Because of the synergistic effects of the medications, it may be necessary to decrease the amount of opioids administered during the first 24 hours after administration of anesthesia, but this decision must always be based on the patient’s report of pain and clinical status.24–27
Cardiovascular Function
Evaluation of the cardiovascular system involves assessment of the heart, circulating blood, and the arteriovenous system. These three basic components control CO. Because tissue perfusion depends on a satisfactory CO, most of the assessment is aimed at evaluating this component.28
In most patients, the nurse uses physical assessment skills to evaluate cardiovascular function.29 The patient’s overall condition is observed, especially skin color and turgor. Peripheral cyanosis, edema, jugular venous distention, shortness of breath, and many other findings may be indicative of cardiovascular problems. All operative and access sites are checked for bleeding, and the amount of blood lost during surgery and the patient’s most recent hemoglobin level are noted.
The patient’s blood pressure must be assessed and correlated to the preoperative assessment, intraoperative course, and anesthetic course. The major component of systolic pressure is stroke volume, and the major component of diastolic pressure is systemic vascular resistance (SVR). Changes in the patient’s systolic or diastolic pressure or narrowing of the pulse pressure may indicate cardiovascular comprominse.29 Peripheral pulses are assessed bilaterally. The rate, character, and any irregularities are documented and reported to the physician if clinically indicated.29 Electrocardiographic (ECG) monitoring is also essential in the immediate postoperative recovery period. Dysrhythmias of any type may occur at any time and in any patient during the postoperative period.29,30
Central Nervous System Function
Assessment of the CNS in the immediate postanesthesia period typically involves gross evaluation of behavior, level of consciousness, intellectual performance, and emotional status. Anesthetic agents are usually reversed before the patient leaves the operating room, and the nurse should anticipate that the patient will be responsive. However, even if anesthesia is not reversed, most patients can respond within 60 to 90 minutes from time of admission to the unit.31 Perioperative stroke can occur in up to 7% of patients following noncardiac and non-neurosurgical procedures.32 As a result of this risk, the nurse caring for the postanesthesia patient following general anesthesia should complete a neurologic assessment that includes stroke assessment (facial symmetry, vocalization to assess for slurred speech, strength, and movement of extremities), and pupillary check. In addition, Glasgow coma scale assessment may be completed. For patients who have undergone neurosurgical procedures, a more detailed assessment of the CNS is necessary.
Occasionally, a patient becomes agitated and thrashes about; this behavior when extreme is referred to as emergence delirium. It occurs more often in children, older adults, and patients with histories of drug dependency or psychiatric disorder.30 Emergence delirium also tends to occur more commonly in patients who have undergone breast and abdominal procedures and procedures of longer duration.33
Thermal Balance
Measurement of the patient’s body temperature in the immediate postanesthesia recovery period is particularly important. Factors influencing the body temperature include type of anesthesia, preoperative medication, age of patient, site and temperature of intravenous fluids, body surface exposure, temperature of irrigation solutions, temperature of the ambient air, and vasoconstriction (from blood loss or anesthetic agents). Hypothermia (temperature less than 36° C) and hyperthermia (temperature greater than 38° C) are associated with physiologic alterations that may interfere with recovery.34
The body maintains its temperature in a narrow range, between 36° C and 38° C. This is accomplished by a balance of heat production and heat loss that is controlled by the thermoregulation mechanisms in the CNS. These mechanisms receive input from various thermoreceptors located in the skin, nose, oral cavity, thoracic viscera, and spinal cord. They then send sensory information in hierarchic order to the spinal cord, the reticular formation, and the primary control center in the hypothalamic region of the brain.35
The central temperature controls maintain body temperature through physiologic and behavioral responses. The physiologic thermoregulatory responses consist of sweating, shivering, and alterations in peripheral vasomotor tone. These responses fine-control the regulatory process of body temperature; heat is conserved by vasoconstriction and lost by vasodilation and sweating. The physiologic responses also can lower the metabolic rate to decrease heat production or increase muscle tone and shivering to increase heat production. Behavioral thermoregulation is accomplished by subjective feelings of discomfort or comfort. For example, in a hot environment a person seeks air conditioning, and in a cold environment a person seeks heat. Behavioral thermoregulation is a stronger response mechanism, but it cannot fine-tune body temperature as the physiologic responses can.35
Fluid and Electrolyte Balance
Normal output in the average adult results from urinary output and insensible losses, including evaporation of water from the skin and exhalation during respiration.36
A lower-than-normal urinary output can be expected in the immediate postanesthesia recovery period as a result of the body’s normal reaction to stress and anesthetics. External losses from vomiting, nasogastric tubes, T-tubes, and wound drainage are assessed and monitored. Accurate measurement and recording of all intake and output is vital in the assessment of the patient’s fluid and electrolyte status.36
In deciding the type of fluid to use in the postanesthesia recovery period, the clinician can differentiate between crystalloids and colloids, between maintenance and replacement fluids, and among fluids of differing tonicity. Because of the large variety of fluid solutions, some general guidelines are recommended in clinical practice.36 Crystalloids are typically used as maintenance fluids to compensate for insensible fluid losses and as replacement fluids to correct body fluid deficits (i.e., treatment of specific fluid and electrolyte disturbances). Maintenance fluid requirements are calculated according to body weight and are used to replace insensible losses from the lungs, skin, urine, and feces. Adults typically require 1.5 to 2 mL/kg/hour. When crystalloids are used to replace blood loss, the replacement factor is 3 mL of crystalloid for each 1 mL of blood loss.37 Isotonic solutions, 0.9% normal saline, or lactated Ringer solution are usually administered in the immediate postoperative period. Colloids typically are used for fluid replacement associated with severe hypotension or shock resuscitation. In general, they do not leave the intravascular space and therefore require lower infusion volumes to achieve volume replacement.
Blood and blood components are reserved for specific patient situations.37 Red blood cells are indicated to increase oxygen-carrying capacity in patients with anemia. Platelets are used to treat bleeding associated with deficiencies in platelet number or function. Fresh-frozen plasma is transfused to increase clotting factor levels in patients with demonstrated deficiencies. A good understanding of the fluid types available, a systematic approach to evaluating fluid depletion, and awareness of the indications for blood component therapy allow the nurse to make appropriate decisions when implementing fluid therapy in the immediate postanesthesia period.
Psychosocial Status
Assessment of the patient’s psychosocial and emotional well-being is an important component of perianesthesia care. As with any other assessment, this must be made in the context of the whole patient. Almost all patients experience a degree of anxiety about anesthesia and the surgical procedure and a fear of postoperative pain.38 The physical manifestations include increased heart rate and blood pressure; pale, cool skin; increased respiratory rate; increased muscle tone; restlessness; agitation; and dilated pupils.
Other Nursing Considerations
In the postanesthesia care unit (PACU), assessment and nursing care are based on the complexity and acuity of the patient’s condition.39 Concerns surround the use of the PACU as an overflow unit for critically ill patients.40 Issues surrounding these concerns are competencies of staff; adequacy of equipment, medications, supplies, and technology; and appropriate nurse staffing. Refer to Box 42-5 for guidelines for the overflow of critical care patients into the PACU.40
General Comfort Management
Postanesthesia care includes general comfort and safety measures.22 Whenever patients are recovering, at least two nurses (one of whom is a registered nurse competent in Phase I perianesthesia nursing) must always be present in the same room or unit for safety.41 Patients should not be left alone, especially when unconscious and emerging from anesthesia. Side rails should be in the up position and locked when care is not being provided. Wheel locks prevent sliding of the bed.
Management of Postanesthesia Problems and Emergencies
In the immediate postoperative period, significant physiologic changes occur as the patient emerges from the effects of anesthesia. Factors that influence the development of problems are listed in Box 42-6.42
Respiratory Complications and Emergencies
Respiratory complications occur with some regularity in the postanesthesia period. All general anesthetic agents and opioid analgesic medications have respiratory depressant effects. Acute pain also impairs the ability to breathe deeply. Most respiratory complications are related to upper airway obstruction, although other problems can occur, including acute respiratory failure, aspiration, pulmonary edema, and respiratory arrest.30
Airway Obstruction
Relaxed nasal or oropharyngeal muscles, rigid neck muscles, or secretions in the upper respiratory tract can cause obstruction of the airway. Soft tissue obstruction occurs when the pharynx is blocked and air cannot flow in and out. The most common cause of soft tissue obstruction is the tongue. Clinical manifestations of an airway obstruction include snoring, stridor, flaring of the nostrils, retractions at the intercostal spaces and the suprasternal notch, abnormal use of accessory muscles, asynchronous movements of the chest and abdomen, increased pulse rate, decreased oxygen saturation level, and decreased breath sounds.43
Management of an airway obstruction begins with immediate recognition. Stimulation may be all that is necessary to relieve the obstruction and obtain a patent airway. With the nonreactive patient, the head tilt–chin lift maneuver or elevation of the mandible at its angles (jaw thrust) can be used to displace the tongue and open the airway. If patency of the airway cannot be achieved by either of these methods, an oropharyngeal or nasopharyngeal airway is inserted. A nasopharyngeal airway is usually better tolerated, although it can occasionally cause nasal bleeding. Oropharyngeal airways should be used only for an unconscious patients, because they can cause gagging, vomiting, and laryngospasm in the awake patient. The patient can also be turned on his or her side to a lateral position, which facilitates displacement of the tongue and drainage of secretions. If the obstruction is still unrelieved, positive-pressure mask ventilation, intubation, tracheotomy, or cricothyrotomy may be required.4,30,43
Laryngeal Edema
Management consists of placing the patient in the upright position; using cool, humidified oxygen; and administering nebulized racemic epinephrine. If the laryngeal edema is a result of an allergic reaction, the reaction must be managed with epinephrine, bronchodilators, and antihistamines. Reintubation is performed only if the patient’s symptoms cannot be controlled by an inhalation treatment within 30 minutes, if hypercarbia persists, or if the patient appears to be in respiratory distress. If reintubation is done, the endotracheal tube must be at least one size smaller than the previous tube used, and an air leak must be present around the cuff.30,43
Laryngospasm
After the spasm, the patient continues to receive supplemental oxygen until stable. It is also important at that time for the nurse to reassure the patient that the spasm has resolved. The patient’s feelings of being unable to breathe during laryngospasm are intense, and emotional support from the nurse is imperative.30,44
Bronchospasm
Bronchospasm is treated initially by removal of any possible irritants or medications. The first line of therapy consists of inhaled bronchodilators. These inhalants cause fewer cardiovascular side effects than systemically administered medications. Common inhalant medications used are albuterol, metaproterenol, and beclomethasone. Systemic bronchodilators and anti-inflammatory agents may also be required at times. Epinephrine is occasionally needed as a continuous infusion. Intravenous methylprednisolone manages the inflammatory aspect of bronchospasm. Cholinergics have been given by nebulizer to decrease secretions.30,44
Noncardiogenic Pulmonary Edema
Pulmonary edema may be defined simply as increased total lung water. Fluid can accumulate in the interstitial spaces or in the alveoli as the result of cardiogenic or pulmonary capillary processes. Noncardiogenic pulmonary edema, also known as postobstructive pulmonary edema, in the postanesthesia recovery period can result from pulmonary aspiration, blood transfusion reaction, allergic medication reaction, upper airway obstruction, or sepsis.45 The most common causative factor seems to be an upper airway obstruction, usually laryngospasm. Often, a short episode of airway obstruction occurred in the operating room. Noncardiogenic pulmonary edema has also been reported after the administration of naloxone to patients who have received general anesthesia. Reversal of the analgesics causes a rise in the level of adrenal catecholamines, which can lead to pulmonary hypertension and probably increased pulmonary vascular permeability.
Aspiration
If aspiration is suspected, management begins with lowering the patient’s head, if possible. The patient is positioned to the side or the head is turned to the side to permit gravity to pull secretions from the trachea. Management centers on promoting tissue oxygenation by maintaining arterial oxygenation by means of CPAP and supplemental oxygen. Positive-pressure ventilation by mask can be applied if the patient is awake and can protect his or her airway or by an endotracheal tube if the patient cannot tolerate the mask or requires higher levels of airway pressure.30,44
Hypoxemia
Administration of oxygen by facemask or nasal cannula to recovering patients may be needed to prevent hypoxemia in the postoperative patient, based on oxygen saturation levels that may be assessed by noninvasively by pulse oximetry. Respiratory depressant effects of residual agents may result in a shallow breathing pattern with an increased ratio of dead space to tidal volume. This is even more evident in patients after upper abdominal surgery, and the reduction in the effective ventilation can have serious effects on oxygenation.42
The most common cause of hypoxemia is ventilation perfusion mismatching. Atelectasis often occurs as a result of bronchial obstruction by secretions or blood. Reduction in FRC is caused by the effects of anesthesia and, in the case of upper abdominal surgery, by the surgical procedure. When FRC falls below closing capacity, dependent alveoli occlude, leading to increased mismatching. Impairment of hypoxic pulmonary vasoconstriction by inhalation agents and some vasoactive medications potentiates this effect.30
Hypoventilation
Hypercarbia resulting from postoperative hypoventilation may cause hypertension and tachycardia, increasing the risk of myocardial ischemia in susceptible individuals. Hypoventilation, by itself or in combination with the other factors previously discussed, can cause hypoxemia. Very high levels of carbon dioxide may have sedative effects. Evaluation of suspected hypoventilation requires measurement of arterial blood gases. Capnographic monitoring may be especially useful in patients at high risk for hypoxemia.30
Careful titration of opioid antagonists, such as naloxone, may be effective in improving ventilation without compromising pain relief. Planning ahead to provide adequate postoperative analgesia is essential for maintaining ventilation, particularly in patients undergoing abdominal or thoracic procedures. Placing obese patients in a semi-Fowler’s position and relieving the effects of tight dressings and casts can also be important. Hypoventilation that cannot be improved sufficiently by noninvasive means requires intubation and mechanical ventilation until the patient can maintain adequate ventilation.44
Cardiovascular Problems and Emergencies
In the immediate postoperative period, cardiovascular complications causing an alteration in CO can occur. These include anesthetic effects on cardiac function, myocardial dysfunction, dysrhythmias, hypertension, and hypotension. These conditions may occur individually or in combination.30,46
Effects of Anesthesia on Cardiac Function
In the immediate postoperative period, the residual effects of anesthetic agents and their adjuncts must be considered in the evaluation of a patient who has cardiac dysfunction. Volatile anesthetic agents such as sevoflurane and isoflurane can cause a dose-related reduction in myocardial function. The actions of these agents may be observed for several hours after the conclusion of surgery.4 Nitrous oxide is an inhalation agent that demonstrates insignificant cardiac depression. However, the combined action of opioids given during emergence from nitrous oxide can result in marked cardiovascular depression.3
Therapy directed toward mitigating the myocardial depressant effects of inhalation anesthetic agents primarily focuses on increasing preload. Elevation of the legs and a crystalloid fluid bolus are commonly sufficient treatment, but ephedrine or other positive inotropic agents may be needed.24
Individually, most opioids and benzodiazepines only moderately depress cardiac function. Opioids reduce the sympathetic response and enhance vagal and parasympathetic tone. This results in vasodilation and a decrease in SVR. Benzodiazepines also cause vasodilation and a decrease in the SVR. Used in combination, these medications can have a significant effect on the cardiovascular system. Specifically, the overall reaction may include a lowered SVR, heart rate, ventricular contractility, catecholamine level, baroreceptor reflex, CO, and blood pressure. Aggressive administration of crystalloid solutions may be required to counteract these effects. High-dose opioids combined with vecuronium produce a negative inotropic and chronotropic effect. Patients may require short-term vascular support until these medications dissipate.30,47
Barbiturates depress the activity of the vasomotor center, causing peripheral vasodilation and hypotension. These actions are dose related and are more marked in the presence of underlying cardiovascular disease. Ketamine has a direct myocardial depressant effect that is usually counterbalanced by its indirect effect on the autonomic nervous system to increase heart rate and blood pressure. In patients who are unable to mount a sympathetic response to stress, ketamine causes a net decrease in CO. Propofol causes a dose-dependent decrease in blood pressure primarily because of a decrease in SVR. This must be considered when caring for patients who are hypovolemic or have minimal cardiac reserves.44,48
Local anesthetic agents can cause cardiovascular toxicity if inadvertently injected into the systemic circulation or if an excessive dosage of the agent is used. Decreased myocardial contractility, reduced SVR, and diminished CO have resulted from these agents. Cardiovascular compromise appears to occur in a dose-related fashion. Management of the cardiovascular complications associated with local anesthetic agents includes measures to increase preload, namely elevation of legs and fluid administration. In refractory cases, ephedrine may also be needed.4,30
Myocardial Dysfunction
Myocardial ischemia results from an imbalance of oxygen supply and demand. Commonly, ischemia results from a decrease in myocardial blood flow caused by atherosclerosis, vasospasm, or hypotension. In the postoperative period, the stress of surgery and the actions of certain anesthetic agents can increase myocardial ischemia.30,46
Dysrhythmias
In the immediate postanesthetic period, patients are predisposed to a variety of cardiac dysrhythmias. The most common dysrhythmias are sinus tachycardia, sinus bradycardia, premature ventricular contractions, supraventricular tachydysrhythmias, and ventricular tachycardia. A variety of causes can result in dysrhythmias, including circulatory instability, pre-existing heart disease, an increase in vagal tone, medications, pain, electrolytic disturbances, and hypoxemia.49
The hemodynamic effects of the dysrhythmia also should be thoroughly assessed. The clinical presentation of the dysrhythmia determines the severity of underlying cardiac disease and the type of treatment. Tachydysrhythmias shorten diastolic filling time and interfere with coronary artery perfusion. These two effects, coupled with an increase in myocardial oxygen consumption, may produce cardiac decompensation. Bradycardia can produce a clinically significant decrease in CO if stroke volume is limited by underlying cardiac disease or if the venous return is reduced. The cause of the rhythm disturbance should be considered.48
Endogenous catecholamine levels in postoperative patients are elevated because of the pain and stress of surgery. Increased catecholamine levels increase sinus and AV node rates, as well as atrial and ventricular irritability. The direct result is tachydysrhythmias and atrial or ventricular premature contractions.30,46,48
Postoperative Hypotension
Prolonged hypotension can lead to serious ischemic organ damage. Prompt treatment is essential. If the underlying cause is not immediately apparent, the first treatment is an attempt to increase preload by elevating the patient’s legs and infusing fluids. Examination of the ECG monitor for dysrhythmias or evidence of ischemia may help guide therapy. The lungs are examined for evidence of pulmonary edema or tension pneumothorax. Vasopressors may be administered to maintain perfusion while additional monitoring modalities are evaluated and established. Insertion of a central venous catheter allows measurement of right ventricular filling pressure and may be used to guide fluid administration in patients with normal myocardial function. In the presence of left ventricular dysfunction or when the cause remains unclear, a pulmonary artery catheter may be inserted to guide therapy through evaluation of left-sided filling pressure, CO, and SVR. The use of additional fluids, inotropic agents, or vasoconstrictor or vasodilator medications is determined by these measurements.49
Thermoregulatory Problems and Emergencies
Hypothermia
Hypothermia is a common occurrence intraoperatively and postoperatively, because the conditions associated with surgery and anesthesia typically inhibit the body’s heat-generating mechanisms and favor its heat-loss mechanisms.50 Hypothermia occurs when systemic heat loss lowers core body temperature below 36° C. Causes include wound and skin exposure, respiratory gas exchange, fluid and blood administration, use of mechanical warming or cooling devices, chemical reactions, alterations in body temperature regulation, and disease states. Clinical manifestations of hypothermia include bluish tint to the skin (peripheral cyanosis); shivering and an increase in metabolic rate (early sign); dysrhythmias; and a decrease in metabolic rate (late sign), oxygen consumption, muscle tone, heart rate, and level of consciousness.35
Hypothermia has several adverse effects, including discomfort, vasoconstriction, and shivering. It depresses the myocardium and increases susceptibility to ventricular dysrhythmias. Significant hypothermia slows metabolic processes, leading to reduced medication biotransformation and impaired renal transport. This may prolong medication effects and delay emergence.35,50
Shivering
Shivering may be a result of the compensatory response to hypothermia or the effects of anesthetic agents, and it can produce an increase in the metabolic rate. Under these conditions, increased oxygen consumption and greater carbon dioxide production can increase the ventilatory requirements. If these requirements are not met, the Paco2 increases and the Pao2 can decrease, especially if any significant intrapulmonary shunting coexists. The demand for blood flow by the diaphragm can increase sharply, requiring CO and myocardial workload to increase and causing an increase in myocardial oxygen consumption. This can result in myocardial ischemia, particularly in older adult patients and patients with coronary artery disease.35,50
Vasoconstriction, a particularly deleterious consequence of postoperative hypothermia, may be responsible for unexplained hypertension in the postanesthesia period. Because vasoconstriction can increase SVR and myocardial workload, the potential for myocardial ischemia exists. Vasoconstriction can mask hypovolemia, and sudden reductions in blood pressure can occur as the patient warms and vasodilates.51 Delayed medication clearance as a consequence of hypothermia is particularly significant in older adult patients who may already have impaired medication-clearing mechanisms and a decreased metabolic rate. For example, the maximal rate of renal excretion of a medication can decline by 10% for every 0.6° C drop in body temperature. Older adult hypothermic patients are more likely to have residual paralysis because of muscle relaxants that are difficult to reverse pharmacologically.52
Management of the hypothermic patient is directed toward the restoration of normothermia. Rewarming prevents the thermoregulatory responses to cold, such as shivering. Management depends on the degree of temperature loss. If the patient’s body temperature is between 36° C and 37° C, the patient can simply be covered with warmed blankets, and heat lamps can be used to keep the patient adequately warm. If the patient’s body temperature is less than 36° C, rapid rewarming is required to decrease the possible complications of hypothermia and the postanesthesia recovery time. Convective warming devices provide a safe and effective means of rewarming the patient.34 In patients with a normal metabolic rate, a setting of “low” or “medium” increases the mean body temperature by about 1° C per hour. A “high” setting increases the mean body temperature by about 1.5° C per hour.4 Other methods of rewarming are thermal mattresses, fluid and blood warming, and environmental warming. Supplemental oxygen may be needed to meet the increased metabolic demand in shivering patients. Patients who are shivering may respond to a small dose of meperidine (Demerol).51
Hyperthermia
By definition, hyperthermia occurs at any core body temperature above normal. Severe, clinically significant hyperthermia results when core temperature exceeds 40° C. Although it is not as common perioperatively as hypothermia, hyperthermia is nevertheless a serious complication of surgery. Postoperative temperature elevations may be caused by accidental overwarming of the patient during surgery, infection, sepsis, or transfusion reactions. Because an elevated temperature increases oxygen demand and, subsequently, the ventilatory and cardiac workload, a hyperthermia patient with poor cardiac reserve suffers serious consequences.53
Postoperative fever must be distinguished from other hyperthermic syndromes. A fundamental difference exists between fever and specific hyperthermic states. In non–fever-related hyperthermic states, body temperature rises above normal despite the body’s heat-dissipating mechanisms (e.g., vasodilation, sweating). Excessive heat gain from internal or external factors exceeds the body’s cooling capabilities, with a consequent rise in body temperature.2,4
In contrast, fever results from a resetting to a higher temperature from the normal set point. Until the body reaches its new set point temperature, heat-generating mechanisms (i.e., vasoconstriction and shivering) are activated. After the new set point temperature is reached, there is an equilibrium between heat generation and heat loss. Unlike hyperthermia, with fever there is no physiologic activity to bring body temperature back to normal. Factors that can contribute to raising core body temperature and fever during the perioperative period are listed in Box 42-7.50
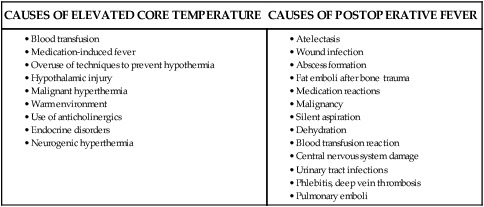
Primary therapy for hyperthermia includes cooling and decreasing thermogenesis. Cooling by evaporative or direct external methods has proved effective. This includes ice packs, a cool environment, and cooling blankets. Gastric and bladder lavage have also proved effective. Although physical cooling is an appropriate therapy for other hyperthermias, attempts to cool a febrile patient may be resisted by the thermoregulatory system. Consequently, the first course of action is to restore normothermia with the use of antipyretic medications. Antipyretics are useful because they have the ability to prevent prostaglandin synthesis in the hypothalamus. Measures to manage the febrile patient include using antipyretics (as indicated), providing a sponge bath with tepid water, keeping the environment cool, using a cooling blanket for sustained fever, and monitoring fluid and electrolyte balance as fluid needs increase during fever. The possibility of malignant hyperthermia (MH) must always be considered.53
Malignant Hyperthermia
Malignant hyperthermia is a genetically determined condition. MH is precipitated by certain general inhalation anesthetics, depolarizing skeletal muscle relaxants, local anesthetics, and stress in susceptible patients. The incidence of MH ranges from 1 in 15,000 in children to 1 in 50,000 in adults. The onset of MH usually occurs during induction of anesthesia but may occur within the first few hours of recovery from anesthesia.3 Because successful management of MH depends on early assessment and prompt intervention, the nurse must be knowledgeable about the pathophysiology and treatment of this syndrome.
Identification before anesthesia of patients who may be susceptible to MH is of major therapeutic importance.54 Patient history or genealogy going back two generations may be positive. Physical examination may reveal myopathies such as cryptorchidism, pectus carinatum, kyphosis, lordosis, ptosis, or hypoplastic mandible. Electromyographic changes are seen in fewer than half of MH-susceptible patients.
The most definitive test for detecting MH susceptibility is a biopsy of skeletal muscle.54 Samples are obtained from the quadriceps muscles and are subjected to isometric contractor testing. The skeletal muscle of the MH-susceptible patient has an increased isometric tension when exposed to caffeine or halothane. Genetic testing for the presence of a ryanodine mutation that predicts MH susceptibility may ultimately replace the invasive diagnostic tests.3
When a susceptible patient is exposed to a triggering agent for MH, such as isoflurane, the clinical features are produced by an excess of calcium ions in the myoplasm. With an elevated calcium ion concentration in the myoplasm, skeletal muscle contraction is intense and prolonged, leading to a hypermetabolic state of acid and heat production. More specifically, heat is produced by the accelerated and continued synthesis and use of adenosine triphosphate (ATP) during glycolysis. The metabolic byproduct of glycolysis, lactic acid, is transported to the liver and then back to the metabolically active muscle, where the cycle repeats. Respiratory acidosis and metabolic acidosis develop because of this hypermetabolic state, and symptoms such as tachycardia, tachypnea, ventricular dysrhythmias, and unstable blood pressure appear. Because of intense vasoconstriction, the skin is mottled and cyanotic.55 Box 42-8 lists the clinical manifestations of MH.
Elevated body temperature can actually be a late sign of MH. For this reason, the nurse must not prolong the assessment of the patient on the assumption that the patient’s temperature must be significantly elevated before intervention is attempted. After the patient’s temperature begins to rise, it may increase at a rate of 1° C every 3 to 5 minutes and may approach levels as high as 46° C.30
Muscle rigidity occurs in about 75% of the patients who experience MH. This is especially true in MH-susceptible patients after the administration of succinylcholine. Muscle rigidity may be so severe that the nurse cannot open the patient’s mouth to insert an airway. The onset of skeletal muscle rigidity after the administration of succinylcholine could be a sign of impending development of MH.53,55
Various environmental and pharmacologic agents can stimulate an acute episode of MH (Box 42-9).54 Fatigue, emotional upset, or very hot and humid weather can trigger a waking febrile episode. The anesthetic agents that may trigger MH seem to affect the sarcoplasmic reticulum. Because of their widespread use, volatile anesthetic agents (e.g., isoflurane) and succinylcholine are the most common triggering agents. In MH-susceptible patients and in patients who have had an episode of MH in the operating room, all possible triggering agents must be stringently avoided. As another precaution, because emotional upsets trigger MH, nurses must provide a stress-free environment for the MH-susceptible patient.
Neurologic Problems and Emergencies
Almost all patients exhibit some level of arousal within 15 minutes after anesthesia is completed.3 Although many factors are known to prolong anesthetic effects, most reports of delayed emergence and emergence delirium are anecdotal.
Delayed Emergence
Delayed awakening after general anesthesia occurs occasionally, and is defined as a failure to regain consciousness within 20 to 30 minutes after the anesthetic ends.56 It can usually be attributed to prolonged action of anesthetic medications, metabolic causes, or neurologic injury.30 In most cases, prolonged sedation is the result of residual general anesthetic. Hypoventilation resulting from a high concentration of inhaled anesthetic limits exhalation of the agent and prolongs its retention. Opioids used as adjunct therapy may contribute to hypercarbia and sedation as well. Hypothermia, advanced age, hepatic dysfunction, and renal disease may contribute to prolonged recovery from anesthetics by heightening sensitivity or delaying elimination, or both. The use of premedication may also prolong recovery, especially if opioids or benzodiazepines, particularly lorazepam, are used.57
In addition to experiencing a slowing elimination of potent inhalation anesthetic, the hypoventilating patient may develop hypoxia and hypercapnia. The hypercapnia may cause significant narcosis and may potentiate the depressive effects of the anesthetics. In the diabetic patient, fasting or excessive insulin may cause electrolyte abnormalities, leading to unconsciousness, or even coma.56
Severe electrolyte disturbances are most commonly seen after excessive water absorption during transurethral prostate surgery. The subsequent dilution hyponatremia may manifest as sedation, coma, or hemiparesis. Dilution hyponatremia may also be seen after the inappropriate release of antidiuretic hormone. Hypocalcemia after parathyroid surgery may result in delayed awakening. High magnesium levels after prolonged administration of magnesium sulfate to the eclamptic or pre-eclamptic patient may also result in prolonged postoperative sedation and in muscle weakness after cesarean section under general anesthesia.36
Neurologic injury and subsequent unconsciousness may be the result of an unsuspected cerebral vascular accident. Intracranial hemorrhage may result from hypertensive responses to anesthetic agents or surgical manipulations, especially in the patient receiving anticoagulant therapy. Paradoxic air emboli may cross a patent foramen ovale in the presence of a right-to-left shunt. Direct emboli from cardiac valves, intracardiac thrombi, and atherosclerotic vessels may also be a threat. Fat emboli can occur after massive long-bone or tissue damage and may not appear until during or after surgical manipulation or reduction of the fracture. Deliberate, induced hypotension in normal patients is not usually associated with neurologic damage. However, uncontrolled intraoperative hypotension may result in ischemia, especially in the patient with hypertension or carotid occlusive disease.57
The successful management of delayed arousal depends on careful consideration of the differential diagnosis. A thorough review of the patient’s preoperative medical condition and the intraoperative course (surgical and anesthetic) usually points to a cause. If the cause of the sedation is not immediately obvious, the first consideration must be assessing the patient’s oxygenation and ensuring adequate gas exchange. Pulse oximetry, end-tidal carbon dioxide measurement, and arterial blood gas analysis can give an estimate of any ventilatory depression and rule out ongoing hypoxia and hypercarbia factors.57,58
If the cause is thought to be residual inhalation anesthetic, maintenance of adequate ventilation should be sufficient treatment. Residual opioids can be reversed by naloxone, and anticholinergic CNS depression can be reversed by physostigmine. The benzodiazepine antagonist flumazenil has been shown to directly antagonize the CNS sedative and amnesic effects of the benzodiazepines.4
Body temperature is measured, and warming is instituted if hypothermia exists. Serum electrolytes and magnesium and calcium levels are checked if ion disturbance is suspected. Blood for a serum glucose assay may also be drawn, but the simple fingerstick glucose determination is faster and accurate enough to exclude hypoglycemia from consideration if the result is normal. Other laboratory tests may be useful if hepatic or renal disease is being considered. Unless perioperative events point specifically to it, neurologic injury is usually a diagnosis of exclusion. If other causes of prolonged arousal have been excluded, a thorough neurologic consultation is obtained.57
Emergence Delirium
Most patients emerge from general anesthesia in a calm, tranquil manner. Some patients, however, emerge in a state of excitement, a condition characterized by restlessness, disorientation, crying, moaning, or irrational talking. In the extreme form of excitement, which is referred to as emergence delirium, the patient screams, shouts, and thrashes about wildly. This condition is seen most frequently after tonsillectomy, thyroid surgery, circumcision, hysterectomy, and perineal and abdominal wall procedures.30,58
Hypoxia with resulting air hunger and hypercarbia may appear as restlessness, disorientation, slurred speech, and agitation. Hyponatremia, hypochloremia, and acid–base changes can all be seen during the immediate postoperative period and can be the cause of mental confusion. Pain is a common cause of restlessness and is frequently seen postoperatively. Urinary bladder and gastric distention, which can cause considerable discomfort, are easily overlooked.30,58
Medication reactions are commonly implicated as the cause of postoperative agitation. Such reactions are less common than they once were, because the use of offending medications is less prevalent. The most frequently implicated medications are the anticholinergics, most notably scopolamine and atropine. They have been shown to have CNS-toxic effects that can include psychotic behavior, delirium, and motor disturbance. Ketamine is also associated with a high incidence of unpleasant agitation. Neuroleptic medications such as droperidol, especially in rarely used high doses, may be associated with development of dyskinesia and involuntary muscle activity and with postoperative confusion.57
The patient’s state of apprehension or anxiety can have a marked effect on emergence agitation; it is especially notable in apprehensive patients and, conversely, in those who are seemingly unconcerned about forthcoming major surgery. Factors such as fear of disfigurement (e.g., caused by cancer surgery) and feelings of suffocation also increase the likelihood of emergence excitement. Young patients tend to have an increased incidence of postoperative excitation, as do patients undergoing emergency procedures. Several psychiatric factors have been shown to increase the incidence of postoperative delirium, including a history of alcoholism, insomnia, depression, or debility.30,58
If emergence delirium occurs, the patient’s status is thoroughly evaluated. Management includes determining a cause, initiating specific therapy, and protecting patients from injuring themselves. When the nurse encounters a restless, confused postoperative patient, the first measure is to ensure that the agitation is not the result of hypoxia. Presuming pain to be the cause of agitation in a hypoxic patient and treating the excitement with opioids or sedatives can have disastrous consequences. Hypoxia must be quickly excluded from the differential diagnosis by pulse oximetry or arterial blood gas determination, or both.2,59
Hyponatremia may be suspected in the confused patient after prostate surgery, especially if the surgery was prolonged. A serum electrolyte determination can confirm the diagnosis. The treatment usually consists of fluid restriction and, rarely, hypertonic saline administration. Severe acid–base disturbances can be diagnosed and therapy directed by arterial blood gas determination. If pain appears to be the diagnosis after exclusion of hypoxia, intravenous opioids may be administered in small increments. The CNS effects of the anticholinergics are usually dramatically reversed by the administration of physostigmine. The incidence of hallucinations with ketamine may be decreased by benzodiazepine administration. Gastric distention may be relieved by nasogastric aspiration, and urinary bladder distention is easily treated with catheterization.4
Occasionally, the nurse is faced with a restless postoperative patient who does not seem to be getting relief from opioid administration. Small intravenous doses of a short-acting benzodiazepine such as midazolam may be warranted. The anxiolytics are administered in reduced doses, because their respiratory depressant effects are cumulative with those of existing opioids, sedatives, and residual general anesthetics. If overt psychotic behavior is apparent despite adequate treatment, psychiatric consultation should be obtained.58
The patient’s respiratory function and airway patency are checked first, because restlessness is a well-known manifestation of hypoxia. Other causes of emergence delirium include a full bladder, cramped or sore muscles and joints from prolonged abnormal positioning on the operating table, pain, incomplete reversal of NMBAs, withdrawal from alcohol and other medications, acid–base disturbances, and electrolyte abnormalities. The restless patient requires constant, careful observation. Gentle physical restraint may be required to prevent injury. Several nurses or other personnel may be needed. If hypoxia, pain, and a full bladder are ruled out, a change in positioning may have a quieting effect.2
Nausea and Vomiting
Nausea and vomiting, although usually not life-threatening, are probably the most unpleasant and lasting memories many patients have of their anesthesia.60 Although the incidence has decreased in recent years, nausea and vomiting still result in significant postoperative morbidity and patient discomfort. Nausea is described as a subjective, unpleasant mental experience that usually leads to vomiting. Retching is the rhythmic muscular activity that usually precedes vomiting. Vomiting is defined as the forceful expulsion of gastrointestinal contents through the mouth.61
Decidedly unpleasant, vomiting can also be significant.62 The physical exertion may increase postoperative bleeding and disrupt delicate suture lines. Tearing or rupture of the esophagus is probably rare but must be a concern in patients with a history of esophageal pathology. Aspiration of emesis is a life-threatening complication in a patient whose airway-protective reflexes are blunted by residual anesthetic or sedative medications or damaged by surgical activity. If the vomiting is protracted, dangerous hypokalemia, hypochloremia, hyponatremia, and dehydration may develop. Nausea and vomiting are also the leading causes of unexpected hospitalization after surgery.61,63
Risk factors most strongly associated with postoperative nausea and vomiting include female gender, history of PONV, history of motion sickness (subjective as reported by patient), nonsmoker, postoperative use/administration of opioids, use of volatile anesthetics, and use of nitrous oxide.64 Age, duration of surgery, and type of surgery are supported as risk factors by weak or conflicting evidence.
The most effective treatment of postoperative nausea and vomiting is prevention.62 Several maneuvers merit mention as being fairly simple to perform and effective in their usefulness. Avoidance of gastric insufflation is paramount. Swallowing of blood should be prevented during oral, pharyngeal, or nasal surgery. If distention is suspected, it should be decompressed intraoperatively.4,52 A nasogastric tube is placed to empty fluid and gases from the stomach, but its presence may trigger gagging and subsequent vomiting. An oral airway may elicit the same response in a partially conscious patient and is removed at the first signs of gagging to prevent vomiting and subsequent aspiration.65
Many medications have been shown to possess antiemetic qualities, and prophylactic antiemetics and combination medications have been shown to be effective in decreasing or preventing postoperative nausea and vomiting. Available antiemetics include anticholinergics, phenothiazines, antihistamines, butyrophenones, and antidopaminergics. Complementary therapies, including acupuncture and aromatherapy with peppermint oil, have also been shown to be effective in relieving symptoms.66 It is important to remember the supportive care of the nauseated and vomiting patient. If vomiting is severe, electrolyte replacement must be considered. Prolonged vomiting may result in hypovolemia. Intravenous fluids may need to be increased to compensate for fluid losses. Decreased opioid doses may be needed to reduce the risk of nausea and vomiting; adding nonopioids, such as nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen, may allow for adequate analgesia with less risk of nausea.67
Summary
Selection of Anesthesia
• The type of anesthesia used for surgery may be local, regional, or general.
• Local and regional anesthetics eliminate the sensation of pain to a specific part of the body without loss of protective reflexes or consciousness.
• General anesthesia is a controlled state of unconsciousness; the patient is not arousable, there is partial or complete loss of protective reflexes, and the airway needs to be continuously monitored and maintained.
Perianesthesia Assessment and Care
• Caring for the critically ill patient who is emerging from anesthesia requires monitoring of the patient’s physical and psychologic status to prevent potential complications that may occur as a result of anesthesia or surgical procedures.
• Knowledge of the preoperative history is important in evaluating and managing postoperative care.
• Communication and hand-off among the anesthesia care provider, the perioperative nurse, and the registered nurse caring for the patient in the immediate postoperative period is important for continuity and safe patient care.
• The goal of patient management in the immediate postoperative period is recognition and immediate treatment of any problems to eliminate or lessen complications.
• Assessment of the cardiopulmonary system is the immediate priority.
• The stir-up regimen is probably the most important aspect of perianesthesia nursing management and consists of five major activities: deep-breathing exercises, coughing, positioning, mobilization, and pain management.
Management of Postanesthesia Problems and Emergencies
• The most frequent complications include respiratory compromise, hypovolemia, hypothermia, and cardiac dysrhythmias.
• The most common adverse reaction is postoperative nausea and vomiting.
• The most lethal complication is malignant hyperthermia.
• Respiratory issues include upper airway obstruction, laryngeal edema, laryngospasm, bronchospasm, acute respiratory failure, aspiration, pulmonary edema, and respiratory arrest.
• Pain management is a priority; inadequate analgesia can interfere with respiratory function and adequate ventilation, affect hemodynamic status, and have a prolonged psychologic impact.