The Pancreas
Embryology, Anatomy, and Physiology
Developmental deviation from this embryologic pattern can give rise to variants. The usual ductal configuration is most commonly bifid, formed by the ducts of Wirsung and Santorini (60% of cases). Less common configurations include a rudimentary duct of Santorini (30%); a dominant duct of Santorini (1%); and ansa pancreatica, in which the duct of Santorini curves as it courses to the duct of Wirsung. Ductal narrowing can be seen at the site of fusion of the dorsal and ventral ducts. The absence of proximal dilation allows differentiation of this normal variant from a true stricture. Duodenal obstruction, pancreatobiliary maljunction pancreatitis, and biliary cysts occur secondary to developmental variants. Pancreatobiliary maljunction is associated with congenital common bile duct webs.1
The pancreas grows substantially in the first year of life, and growth slows from year 1 through 18. The gland is relatively larger in children than in adults, and the overall ratio of gland size to patient body size decreases with age (Table 96-1). The pancreatic head is more prominent in children compared with the body and tail. The diameter of the pancreatic duct also varies with age (Table 96-2). Enlarged ducts (>1.5 mm at 1 to 6 years, >1.9 mm at 7 to 9 years, and >2.2 mm at 13 to 18 years) are associated with pancreatitis.
Table 96-1
Normal Sonographic and Computed Tomographic Dimensions of the Pancreas
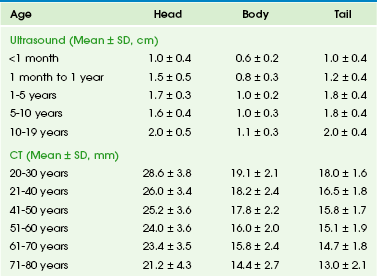
Modified from Heuck A, Maubach PA, Reiser M, et al. Age-related morphology of the normal pancreas on computed tomography. Gastrointest Radiol. 1987;12:18-22; and Siegel MJ, Martin KW, Worthington JL. Normal and abnormal pancreas in children: US studies. Radiology. 1987;165:15-18.
Table 96-2
Normal Diameter of the Pancreatic Duct by Ultrasound and Computed Tomography
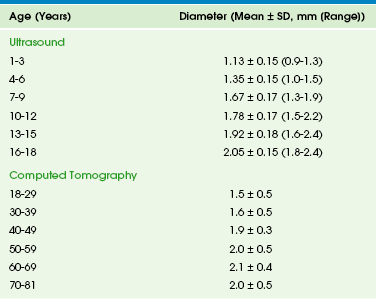
Modified from Siegel MJ, Martin KW, Worthington JL. Normal and abnormal pancreas in children: US studies. Radiology. 1987;165:15-18; Heuck A, Maubach PA, Reiser M, et al: Age-related morphology of the normal pancreas on computed tomography. Gastrointest Radiol. 1987;12:18-22; Chao HC, Lin SJ, Kong MS, et al. Sonographic evaluation of the pancreatic duct in normal children and children with pancreatitis. J Ultrasound Med. 2000;19:757-763; and Glaser J, Hogemann B, Krummenerl T, et al. Sonographic imaging of the pancreatic duct. New diagnostic possibilities using secretin stimulation. Dig Dis Sci. 1987;32:1075-1081.
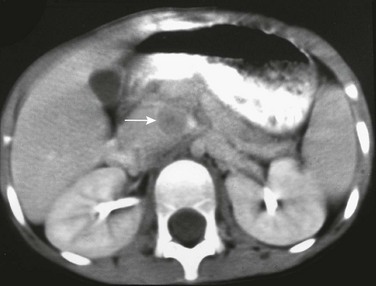
The pancreas lies transversely in the retroperitoneum. It is divided into the head, body, and tail (Fig. 96-1). The head is to the right of midline, situated within the “C-loop” of the duodenum. At the junction of the inferior and left margins of the pancreatic head is an extension of the gland called the uncinate process. The anterior surface of the pancreatic head is in contact with the transverse colon, gastroduodenal artery, and loops of small intestine. The anterior surface of the uncinate process is in contact with the superior mesenteric artery and vein. The posterior surface of the head is adjacent to the inferior vena cava, common bile duct, renal veins, and the abdominal aorta.
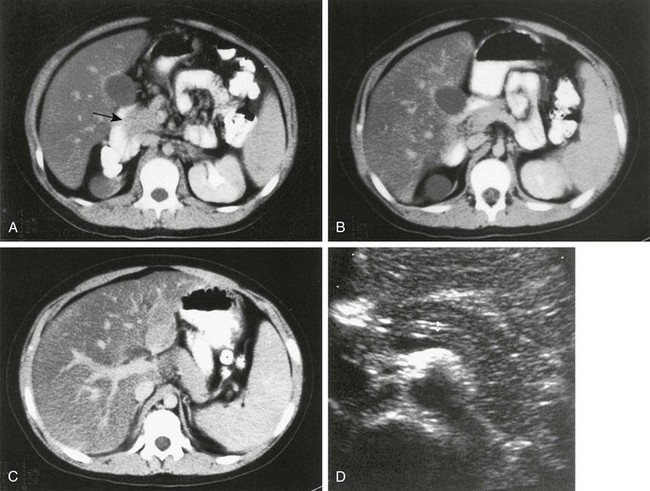
Figure 96-1 Normal pancreas in an 11-year-old boy.
A to C, Computed tomography (CT) sections of the pancreas. The head of the pancreas (arrow in A) is slightly bulbous and distinct from the contrast-filled duodenal sweep. The body of the pancreas (B) is narrower than the head or tail and is seen anterior to the aorta, from which the superior mesenteric artery arises. The tail of the pancreas (C) is thicker in children than in adults and extends to the spleen. D, Transverse ultrasound shows the double track of a normal pancreatic duct.
Imaging the Pancreas in Children
The pancreas itself is not seen on plain radiographs, although calcifications in patients with chronic pancreatitis or cystic fibrosis may be identified on abdominal radiographs (Fig. 96-2). In acute pancreatitis, dilated loops of bowel and fluid levels within the upper midabdomen may suggest localized ileus. A pancreatic mass may be sufficiently large to displace adjacent gas-filled portions of the gastrointestinal (GI) tract.2
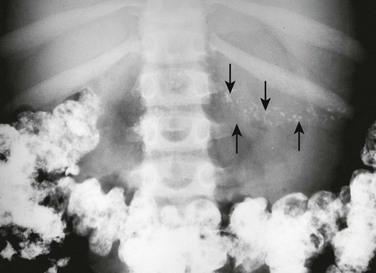
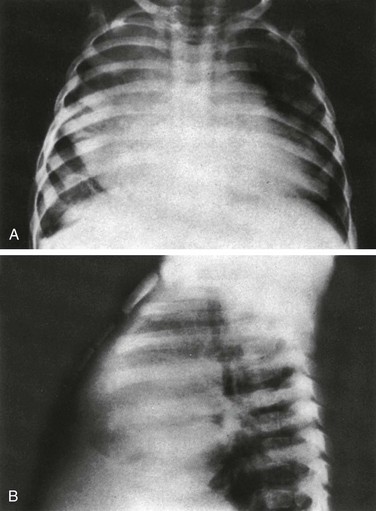
Figure 96-2 Cystic fibrosis in a 9-year-old girl.
Multiple pancreatic calcifications (arrows) are seen on radiography.
Ultrasonography (US) is the primary screening tool to evaluate the pediatric pancreas.3 The pancreas is most easily seen if the stomach and duodenum are not distended with gas. Ingestion of water devoid of gas bubbles may improve visualization. The distal body and tail may also be imaged in the prone position using the left kidney as an acoustic window. Age-matched normal dimensions of the pancreas are given in Table 96-1.3 Pancreatic size is best measured at its body, but individual variation is sufficient to warrant caution when determining pancreatic size. Enlargement of the pancreas should be diagnosed when the anteroposterior dimension of the pancreatic body is greater than 1.5 cm.4 The normal duct may be seen as a single- or double-track echogenic line anterior to the junction of the splenic and mesenteric veins (see Fig. 96-1). The pancreas has a spectrum of echogenicity relative to that of the liver, but in most children, the pancreas is hypoechoic or nearly isoechoic with the liver. However, in neonates, particularly in premature infants, the pancreatic gland is more echogenic.5 US is also helpful for image-guided biopsy and in aligning radiotherapy.6
Computed tomography (CT) of the pancreas7 is indicated less frequently than US, but it is valuable in certain conditions, particularly pancreatitis, tumors, and pseudocysts with uncommon features. The pancreas is best visualized during bolus injection of intravenous (IV) contrast material, which readily identifies the adjacent vessels, and with meticulous administration of GI contrast to opacify the adjacent stomach and duodenum. The pancreas is hypodense compared with the liver, both with and without intravenous contrast. The contours of the pancreas are commonly smooth but may be slightly lobulated. Because the pancreas in children is oblique to the axial plane, multiple thin sections may be necessary for optimal visualization; in-plane reconstructions, particularly from axial volumetric data obtained with multidetector equipment, can image the pancreas in its own oblique plane. Imaging of the pancreatic head may be optimized by scanning the patient in the right lateral decubitus position soon after ingestion of GI contrast material. With this technique, the optimally opacified duodenal C-loop outlines the pancreatic head, and opacified proximal jejunal loops outline the remainder of the gland. CT is the best modality for assessing neoplasms, pancreatic trauma, and pancreatitis and its complications and for further evaluation of abnormalities on US. Thin collimation volumetric CT coupled with curved planar reformations produces quality imaging of pancreatic and peripancreatic tissues.8
Magnetic resonance imaging (MRI) of the pancreas9,10 is more difficult in children than in adults because of adjacent gas-filled loops of intestine and motion artifact from peristalsis and respiration.11 Nevertheless, MRI is a powerful tool for imaging pediatric developmental abnormalities. The pancreas normally has signal intensity equal to that of liver on T1- and T2-weighted spin echo images with midfield strength magnets. Pancreatic images produced with high-field strength magnets may have greater signal intensity than those of the liver. To some degree, signal varies with age. Although normal children do not have as much intrapancreatic macroscopic fat as adults, adolescents have more fat in the pancreatic septa than do preadolescent children, and the amount of intrapancreatic fat may be increased in children with cystic fibrosis. The value of MRI is enhanced by the use of breath-holding techniques (generally not possible in younger children), fat suppression, contrast enhancement, and respiratory gating.
Because of its noninvasive nature, magnetic resonance cholangiopancreatography (MRCP)12 may be more useful than endoscopic retrograde cholangiopancreatography (ERCP)13,14 in children (Fig. 96-3). Reported sensitivity, specificity, and accuracy are 87%, 90%, and 89% respectively for stones; 100%, 98%, and 98% for cholangitis; 92%, 97%, and 96% for bile duct tumors; and 89%, 96%, and 95% for periampullary stenosis.15 MRCP is also useful in certain congenital abnormalities, such as pancreas divisum, and after pancreatic trauma to identify duct of Wirsung transections. Although intravenous CT cholangiography is superior to MRCP in delineating postoperative anatomy after choledochal cyst repair, MRCP is highly accurate (84%) in depicting the anastomotic site, intrahepatic biliary tree, and reconstructed bowel, and it clearly demonstrates pancreatobiliary maljunction, residual distal common bile duct, common channel,16 and pancreatic duct.17 Similarly, MRCP accurately depicts the postoperative anatomy and complications after orthotopic liver transplantation.18 A normal MRCP may obviate the need for ERCP or percutaneous transhepatic cholangiography, and abnormalities visualized with MRCP can direct the modality and route for further intervention. An overview of MRCP pitfalls has been provided by Van Hoe and colleagues.19
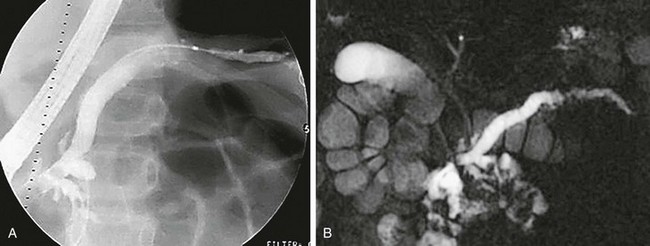
Figure 96-3 Endoscopic retrograde cholangiopancreatography (ERCP) and magnetic resonance cholangiopancreatography (MRCP) in a 9-year-old with recurrent pancreatitis and stones.
A, Anteroposterior image from ERCP shows abnormal dilation of the proximal pancreatic duct and narrowing, irregularity, beading, and an undulating caliber distally. B, Anterior MRCP image of the same patient similarly defines the abnormal caliber and irregularity of the pancreatic duct, consistent with recurrent pancreatitis. The common bile duct is normal. (Courtesy Dr. Kimberly Applegate, Indianapolis, IN.)
Secretin stimulation with MRCP further enhances the imaging information obtained, because it gives additional, valuable, functional and anatomic information about the pancreatic duct and pancreatic excretory capacity. Secretin-enhanced MRCP has been described in detail in recent years20–23 and has been found useful for detection and diagnosis of a variety of congenital, inflammatory, and neoplastic pancreatic conditions.24 Secretin causes temporary dilation of pancreatic ducts, principally by increasing pancreatic exocrine secretions, thus it allows better visualization of the ducts during MRCP.
Congenital and Hereditary Pancreatic Abnormalities
Congenital Pancreatic Abnormalities25,26
Pancreas Divisum
Overview: Pancreas divisum occurs when the dorsal and ventral ducts fail to fuse, although the pancreas is otherwise anatomically normal. This anomaly has been described in 4% to 14% of the population, depending on the method of evaluation.
Clinical Presentation: In such cases, the accessory ampulla drains the major portion of the gland. It has been suggested that the incidence of pancreatitis is higher, although recent literature refutes this.
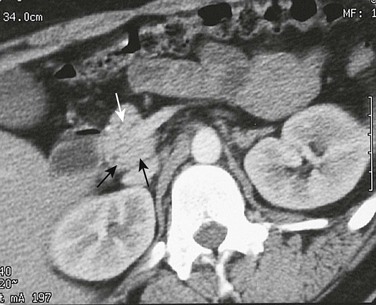
Imaging: CT in children with pancreas divisum and pancreatitis demonstrates enlargement of both ducts, in addition to the characteristic findings of pancreatitis. Further enlargement of the ducts can be provoked with secretin stimulation.27,28 Increased thickness of the pancreatic head has also been described.29 Zeman and colleagues30 reported that thin-section CT demonstrated the unfused ducts in 5 of 12 patients (Fig. 96-4), and two distinct pancreatic moieties separated by a fat cleft was seen in 4 patients. Pancreas divisum may be associated with minor papilla adenoma beyond the childhood years.
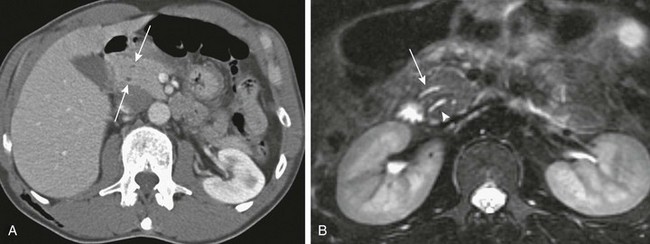
Figure 96-4 Pancreas divisum.
A, Axial contrast-enhanced computed tomography shows two pancreatic ducts (arrows). B, Axial fast spin-echo T2-weighted magnetic resonance image from a teenager with recurrent pancreatitis shows separate ducts of Santorini (arrow) and Wirsung (arrowhead) in the pancreatic head. (Courtesy Dr. Marilyn Siegel, St. Louis, MO.)
Congenital Short Pancreas
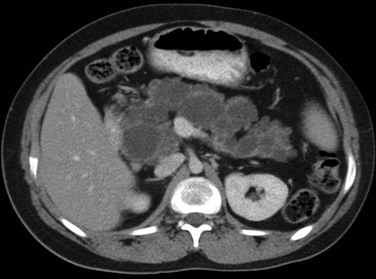
Overview: Congenital short pancreas, also known as agenesis of the dorsal pancreatic anlage,32 occurs when the portion of the pancreas derived from the dorsal embryonic bud is absent, and only the smaller portion, derived from the ventral anlage, is present. Thus, the pancreatic neck, body, and tail are absent. This anomaly has been described in patients with polysplenia syndrome, or it may be a sporadic finding.
Clinical Presentation: Variable symptoms of abdominal pain and epigastric discomfort and diabetes may be present.33
Imaging: Only a globular pancreatic head can be identified on CT (Fig. 96-5). Size is variable, and some patients show an enlarged or prominent pancreatic head, whereas others may show a normal sized or even mildly atrophic and small pancreatic head. The diagnosis of agenesis of the dorsal pancreas is inconclusive without demonstration of the absence of the dorsal pancreatic duct, either with MRCP or ERCP. Patients with this abnormality have an increased risk of developing diabetes mellitus34 because of the paucity of islet cells, most of which are located in the distal pancreas. This condition may also be associated in later life with pancreatic tumors35 such as intraductal papillary mucinous neoplasms.
Ectopic Pancreas
Overview: Ectopic pancreatic tissue36 is an aberrant rest of normal pancreatic tissue remote from the pancreatic body that occurs in 1% to 13% of the population. The vast majority of pancreatic rests (about 70%) are located in the stomach,37 duodenum, and jejunum, but they can occur elsewhere, such as omphaloenteric duct rest.38 An association with Beckwith-Weidemann syndrome has been reported.39
Annular Pancreas
Overview: Several theories of embryonic dysgenesis have been proposed for annular pancreas, but most suggest some form of rotational anomaly of the ventral bud, which may be bifid. The pancreatic annulus, or the portion surrounding the duodenum, frequently has a separate duct entering the duodenum opposite the ampulla of Vater. Duodenal contents may reflux through this duct into the annulus. Affected patients may have associated duodenal stenosis or atresia and may come to medical attention with duodenal obstruction in infancy. Many other associated abnormalities have been described, the most common being intestinal malrotation, tracheoesophageal fistula, anal atresia, and cardiac abnormalities.
Clinical Presentation: Annular pancreas is frequently diagnosed in infancy because of associated duodenal obstruction. However, in approximately half the cases, the diagnosis is made beyond infancy (Fig. 96-6). The associated abnormalities described above are most common in patients who also have trisomy 21. Annular pancreas has also been described in de Lange syndrome, with heterotaxy, and as a cause of extrahepatic biliary obstruction.41 Pancreatitis that solely affects the annulus of an annular pancreas has been reported in adults.14
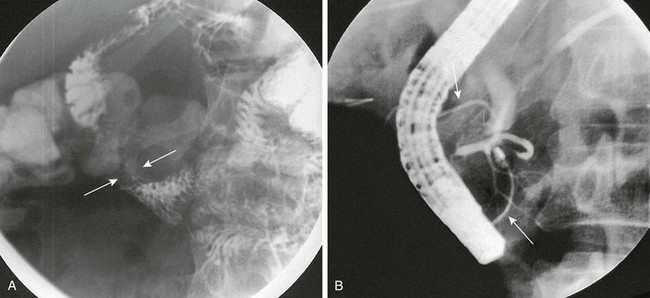
Figure 96-6 Annular pancreas.
A, Oblique view during an upper gastrointestinal series shows extrinsic narrowing of the duodenal C-loop (arrows). B, Endoscopic retrograde cholangiopancreatography confirms the presence of small ducts (arrows) encircling the duodenum, consistent with an annular pancreas. (Courtesy Dr. George Taylor, Boston, MA.)
Imaging: MRI has advantages over CT in the diagnosis of annular pancreas, because with MRI it is easier to detect and characterize the tissue surrounding the duodenum as pancreatic. Diagnosis by US has also been described.42 ERCP and MRCP are used to investigate ductal anatomy. Coincidence of congenital short and annular pancreas with gallbladder agenesis and splenic malrotation is rare.43
Congenital Pancreatic Cysts
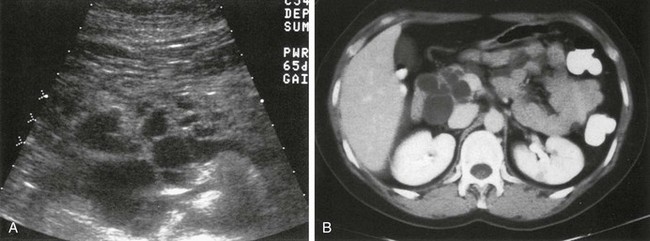
Overview: Congenital cysts of the pancreas are rare and are often confused with choledochal,45 omental, or mesenteric cysts (e-Fig. 96-7). Single congenital pancreatic cysts are very rare and occur predominantly in females.46
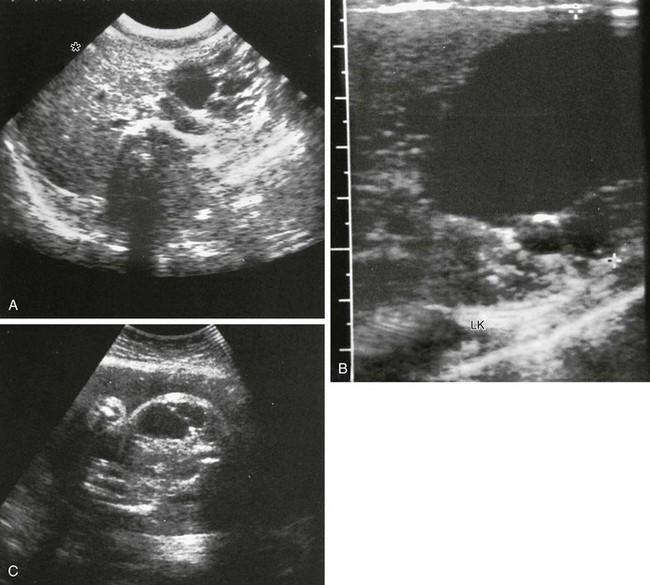
e-Figure 96-7 Congenital pancreatic cyst.
A-B, Transverse sonograms of the midabdomen in a newborn girl reveal a large anechoic mass. C, Antenatal ultrasound shows the cyst in the fetus. At surgery, a large congenital pancreatic cyst was found. LK, left kidney. (C, Courtesy Dr. D. McCallum, Palo Alto, CA; from Baker LL, Hartman GE, Northway WH Jr. Sonographic detection of congenital pancreatic cysts in the newborn: report of a case and review of the literature. Pediatr Radiol. 1990;20:488.)
Clinical Presentation: Congential pancreatic cysts are usually asymptomatic, but when symptoms do occur, they are related to mass effect and compression of adjacent structures.
Imaging: Congenital cysts are anechoic by US; they are usually unilocular, located in the pancreatic tail, and range in size from microscopic to 5 cm.47 Rarely, they may communicate with the ductal system. In contrast to single congenital pancreatic cysts, multiple congenital cysts may be associated with a polycystic disorder such as von Hippel–Lindau disease.48 Juxtapancreatic GI duplication cysts occur as abnormalities of the developing foregut and therefore usually have an alimentary tract epithelial lining. Most of these cysts arise from the stomach or duodenum but may rarely be sequestered within the pancreas.49
Hereditary Systemic Conditions with Pancreatic Involvement
Overview: Cystic fibrosis (CF) leads to exocrine pancreatic insufficiency in 80% of affected patients. The pancreatic ductules contain goblet cells that produce abnormally thick mucus, leading to obstruction of enzyme egress pathways and associated pancreatic changes.
Clinical Presentation: Patients have pancreatic insufficiency, mainly exocrine, with failure to thrive, abdominal distension, steatorrhea, and occasional rectal prolapse. Patients with pancreatic cystosis (see below) are usually asymptomatic.
Imaging: In young patients with cystic fibrosis (CF), US50 shows a normal pancreas or pancreatic enlargement, but chronic obstruction ultimately results in shrinkage of the gland with fatty infiltration and fibrosis. On US, these histopathologic changes are visualized as increased echogenicity of the gland. CT shows a shrunken pancreas with reduced attenuation secondary to fatty infiltration. Fibrosis without fatty infiltration is found infrequently. Unenhanced scans may show pancreatic calcifications, ductal dilation, and pancreatic cysts (Fig. 96-8).51 MRI findings are variable but can accurately depict the changes of fatty infiltration, fibrosis, and atrophy.52–54
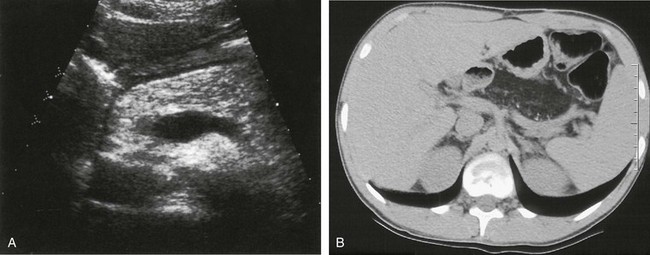
Figure 96-8 Cystic fibrosis (CF) with diffuse fatty replacement of the pancreas.
A, Markedly hyperechoic pancreas on US in an 18-year-old woman with CF and fatty infiltration of the pancreas. B, Axial noncontrast abdominal computed tomography shows diffuse fatty replacement of the pancreas with multiple tiny calcifications. (Courtesy Dr. Robert Kaufman, Memphis, TN.)
Cystic transformation of the pancreas, or pancreatic cystosis, in children and young adults with CF has been described.55,56 This is an unusual form of pancreatic involvement with CF, in that the pancreas is replaced by macrocysts that are rarely more than 1 cm in diameter. This can be imaged with US, CT (Fig. 96-9), and MRI. These are true, epithelium-lined cysts that result from the accumulation of inspissated mucus, produced as a result of residual exocrine secretory function in the acinar cells, proximal to ducts obstructed from inflammation.
Shwachman-Diamond Syndrome
Overview and Clinical Presentation: Shwachman-Diamond syndrome is an autosomal-recessive disorder that results in short stature, exocrine pancreatic insufficiency, metaphyseal chondrodysplasia, and bone marrow dysfunction.
Imaging: US and CT evaluations of the pancreas demonstrate fatty replacement (pancreatic lipomatosis)57 as described earlier in patients with CF (e-Fig. 96-10). Other causes of pancreatic lipomatosis include chronic pancreatitis, prolonged steroid use, obesity, Cushing syndrome, hemochromatosis, obstruction of the duct of Wirsung, and Johanson-Blizzard syndrome.
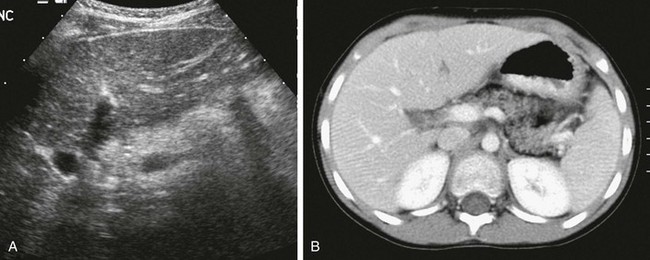
e-Figure 96-10 Shwachman-Diamond syndrome in an 8-year-old boy with anemia.
A, Transverse ultrasound through the pancreas shows increased echogenicity consistent with fatty infiltration. B, Corresponding axial contrast-enhanced computed tomography confirms partial fatty replacement of the pancreas. The patient also had coxa valga (not shown).
von Hippel–Lindau Disease
Overview and Clinical Presentation: von Hippel–Lindau disease (VHL) is an autosomal-dominant disorder characterized by hemangioblastoma in multiple organs, especially the retina and central nervous system; skin lesions and cysts of numerous organs, including the pancreas. Pancreatic involvement is seen in approximately 21% of patients.58 Up to 75% of these multiple pancreatic lesions (e-Fig. 96-11) likely originate from progenitor cells, not mature endocrine cells as previously thought.59
Imaging: VHL-associated cysts are typically anechoic on US and have reduced attenuation on CT compared with the surrounding pancreatic tissue. Multiple cysts—as well as serous and mucinous cystadenomas of the pancreas, carcinoma, adenocarcinoma, and islet cell tumors—are also associated with VHL.60 Adenocarcinoma occurs in affected adults, and pancreatic calcifications can be seen on unenhanced CT scans.
Autosomal-Dominant Polycystic Disease
Overview: Autosomal dominant polycystic disease is a hereditary disorder with 100% penetrance but variable expressivity.
Clinical Presentation: Symptoms of large pancreatic cysts include abdominal pain, jaundice, and fever.
Beckwith-Wiedemann Syndrome
Overview and Clinical Presentation: Beckwith-Wiedemann syndrome (BWS) is a relatively frequent overgrowth syndrome with an incidence estimated at 1 in 14,000 births. It is probably an autosomal-dominant disorder with variable transmission. The BWS gene has been identified on the short arm of chromosome 11 (11p15.5). The syndrome is characterized by visceromegaly, hemihypertrophy, and development of malignant tumors in 10% to 15% of affected patients; benign tumors are also found.
Hemochromatosis
Overview: Hemochromatosis is an autosomal-recessive disorder that manifests as iron accumulation mainly in the liver, pancreas, and heart secondary to excessive iron reabsorption from the intestine, which leads to organ dysfunction.
Clinical Presentation: Symptoms referable to the pancreas may be absent or nonspecific, such as malaise and fatigue, or they may be those of insulin resistance that result from pancreatic damage from iron deposition and culminates in diabetes.
Imaging: The pancreatic changes from hemochromatosis are better appreciated with MRI, rather than CT, because of the magnetic susceptibility effects of iron. The liver and pancreas appear diffusely hypointense on T2*- and T2-weighted sequences. Distinction should be made from acquired transfusional iron overload (hemosiderosis), in which iron accumulates in the reticuloendothelial cells and leads to T2 hypointensity in the liver and spleen; with the exception of very severe cases, the pancreas is usually normal.
Pancreatitis
Overview: Acute pancreatitis manifests as mild, moderate, severe, or necrotizing disease. It is uncommon in childhood, possibly because the most common predisposing factors in adults, alcohol and cholelithiasis, are seldom encountered in children. In a series of 61 children with acute pancreatitis, the most common cause was multisystem disease, including Reye syndrome, which is now uncommon; sepsis; shock; hemolytic uremic syndrome; and viral infection, specifically with mumps.62 Other causes included blunt trauma in 15% of patients, congenital anatomic abnormalities in 10%, metabolic diseases in 10%, and drug toxicity in 3%. No cause was identified in 25% of patients. MRCP is useful in identifying unsuspected abnormal ductal anatomy in patients with idiopathic pancreatitis.63
Anatomic abnormalities associated with pancreatitis include pancreas divisum, congenital choledochal dilation, cysts with pancreatobiliary malunion/maljunction (40% to 50%),64 duodenal web, and congenital pancreatic cyst. An anomalous pancreatobiliary ductal junction may also cause pancreatitis, because the abnormal insertion of the common bile duct into the pancreatic duct may facilitate reflux of bile into the pancreas.65–67 The frequency of acute pancreatitis in children with a choledochal cyst is reportedly as high as 68%. Associated metabolic disorders include hypercalcemia, hyperlipidemia, and CF; the drugs implicated most frequently are L-asparaginase (e-Fig. 96-12), steroids, and acetaminophen. A reported increased incidence of biliary sludge in adult patients with pancreatitis suggests that biliary sludge may be the probable cause in as many as 70% of patients with idiopathic pancreatitis.68
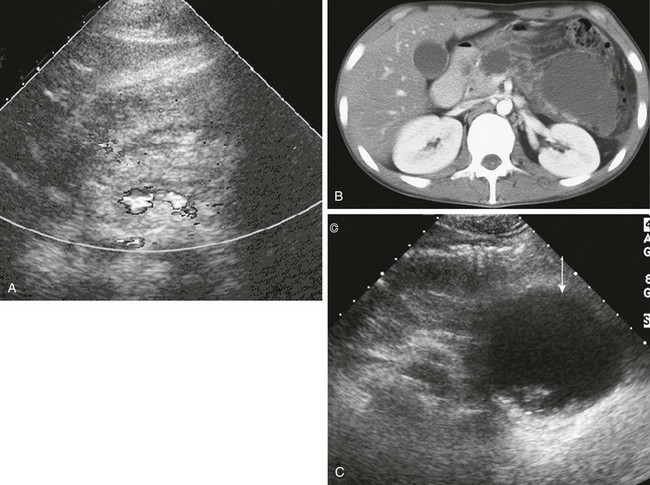
e-Figure 96-12 Asparaginase-induced pancreatitis and pseudocyst formation.
A, Transverse ultrasound in a 17-year-old boy undergoing treatment for acute lymphoblastic leukemia who developed acute pancreatitis. The body of the pancreas is enlarged and mildly hyperechoic with indistinct margins. Doppler signal indicates the splenic vein dorsal to the pancreas. B, Axial computed tomography scan 2 days later shows peripancreatic edema and two pseudocysts in the body and tail. C, Additional follow-up with ultrasound shows a large pseudocyst (arrow) that involves the pancreatic tail and contains complex debris.
Clinical Presentation: Patients with nontraumatic acute pancreatitis typically come to medical attention with abdominal pain, most frequently in the epigastrium. Nausea and vomiting are common associated symptoms. Elevation of serum concentrations of pancreatic enzymes—amylase, lipase, and trypsinogen—is common. Although abnormal laboratory values are typically considerably more sensitive than imaging in identifying pancreatitis, imaging studies may be useful to confirm the diagnosis and to determine the extent of associated inflammatory changes and complications. Very rarely, pancreatitis may be the presenting symptom of a pancreatic malignancy in children and adolescents.
Imaging: Abdominal radiographic findings are nonspecific, but certain findings are suggestive. Reactive ileus or “sentinel loops” from nearby GI structures may lead to abnormal air-fluid levels in the stomach and duodenum, focal dilation of the duodenal sweep, and dilation of the transverse colon that ends abruptly at the splenic flexure; left pleural effusion may also occur. Although ascites is common, the amount is rarely sufficient to be appreciated on abdominal radiographs.
US may be the initial imaging procedure for the evaluation of possible pancreatitis. Semierect and coronal scans, as well as the standard scanning planes, may improve evaluation of an abnormal pancreas.69 The edema that accompanies acute pancreatitis often results in a diffusely enlarged hypoechoic gland. A minority of affected patients have increased pancreatic echogenicity (see e-Fig. 96-12), and some have a normal-appearing pancreas. The pancreatic duct may be dilated,70 but this is an inconsistent finding; this is especially true when the gland is markedly swollen, because this causes compression of the duct. When the duct is dilated, there is a correlation with serum lipase in the acute and healing phases of the disease. Masses may be identified in the pancreas that represent focal areas of fluid, hemorrhage, or phlegmon formation, seen as a focal, inflammatory, hypoechoic mass. Ascites is usually identified on US.
The presence of peripancreatic fluid collections is evidence of acute pancreatitis. The most commonly involved areas are the lesser sac, anterior pararenal space,71 transverse mesocolon, and perirenal space. US is excellent in demonstrating these fluid collections. Fluid collections may be found as far from the pancreas as the mediastinum and the inguinal regions, and inflammation may involve the adjacent splenic vein and may result in thrombosis.
CT may show pancreatic abnormalities to better advantage than US. The findings mirror those seen with US and include pancreatic swelling, ductal dilation, mass effect from phlegmon or hemorrhage, peripancreatic fluid collections, thickening of adjacent fascial planes, and ascites. Abscesses and necrosis are particularly well delineated on CT, especially with dynamic CT.72 Patients with necrosis have higher rates of morbidity, mortality, and complications.
ERCP is seldom needed in children, but it is useful to evaluate complicated or recurrent pancreatitis73 and in cases of unusual pseudocyst formation. The findings range from mild irregularity of the duct to ductal narrowing with wall ectasia and acinar enlargement, which has been likened to a “string of beads.” Marked ductal ectasia is usually not seen in acute pancreatitis. MRCP74 may replace ERCP in the evaluation of childhood pancreatitis because of its noninvasive nature (see Fig. 96-3). Secretin administration may help to optimize MRCP visualization of the pancreatic duct and its radicles, and it increases the sensitivity for identifying structural abnormalities.
Pseudocyst formation is a potential complication of pancreatitis, regardless of cause. Although most pseudocysts are in the region of the pancreas itself (Fig. 96-13; see e-Fig. 96-12), they can appear nearly anywhere in the abdomen and in the mediastinum (e-Figs. 96-14 and 96-15).75,76 In adults, approximately 5% of patients with acute pancreatitis develop pseudocysts. Although most pseudocysts resolve spontaneously within an average of 5 months,77 some persist and require intervention.78,79 Features associated with spontaneous resolution include a pseudocyst diameter less than 7.5 cm, absence of internal debris, and total pseudocyst volume less than 250 mL.
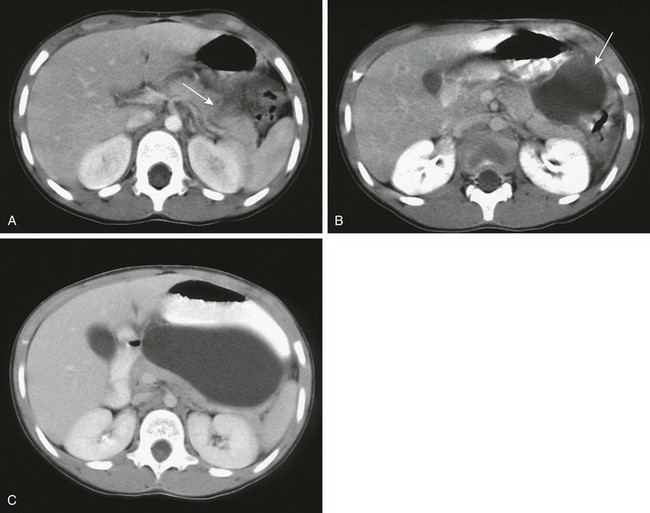
Figure 96-13 Posttraumatic pseudocyst in a 7-year-old.
Ten days after blunt epigastric trauma, the patient continued to have vomiting and rising amylase levels. A, Axial contrast-enhanced abdominal computed tomography shows a pancreatic laceration (arrow) in the midbody. Pseudocyst formation (arrow) is shown on follow-up imaging 10 days (B) and 20 days (C) after the initial study. (Courtesy Dr. Robert Kaufman, Memphis, TN.)
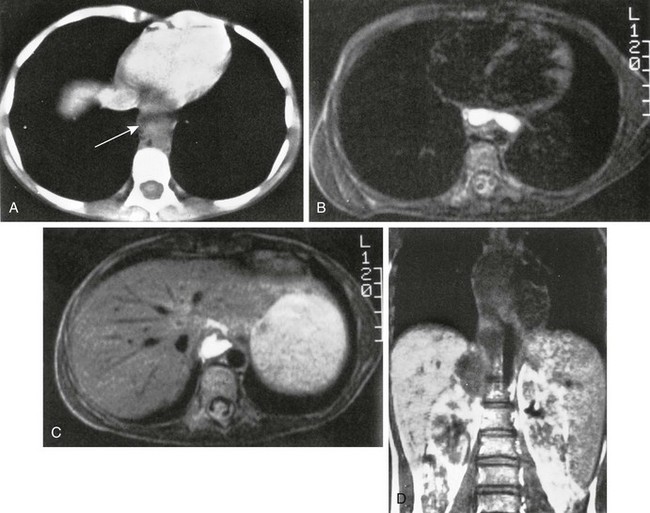
e-Figure 96-15 Mediastinal infiltration of a pancreatic pseudocyst.
A, Computed tomographic image through the lower chest shows a fluid-filled mass (arrow) anterior to the descending aorta. Axial T2-weighted magnetic resonance (MR) images through the lower mediastinum (B) and thoracolumbar spine (C) show the fluid content of the mass. D, Coronal T1-weighted MR imaging shows the low signal intensity mass extending from a position superior to the right kidney into the posterior mediastinum. Longitudinal (E) and transverse (F) sonograms of the midabdomen show marked dilation of the pancreatic duct. G, Endoscopic retrograde cholangiopancreatography shows a dilated pancreatic duct characteristic of chronic pancreatitis.
Imaging is indicated when a pseudocyst is suspected. They frequently cause mass effect on adjacent structures, especially the stomach and duodenum, and this may be seen on radiographs or upper GI studies performed for unexplained abdominal pain. Pseudocysts are typically anechoic, although some may contain debris. Their effect on adjacent organs may be identified on US but is seen to better advantage with CT. ERCP usually shows the irregular ductal dilation of chronic inflammation (see Fig. 96-3, A, and e-Fig. 96-15, G). Skeletal changes, particularly bone marrow infarcts, have long been recognized as a complication of pancreatitis, possibly related to increased levels of circulating lipase and to generalized enzymatic dysfunction of the pancreas.80
Treatment: Primary treatment includes analgesia and bowel rest with parenteral nutrition.81–85 Postpyloric enteral feeds may be begun prior to oral refeeding to prevent relapse and to provide nutritional support while preventing gut mucosal atrophy. Antibiotic use is recommended for necrotic pancreatitis, and ERCP can reduce morbidity and mortality in select cases. Surgery and interventional procedures are indicated for infected necrotic pancreatitis and complications such as pseudocyst and abscess formation, splenic artery and vein thrombosis, hemorrhage, and pseudoaneurysms.
Chronic Pancreatitis
Overview: Chronic pancreatitis in children is less common than acute pancreatitis. Although it occurs as a sequela of the acute disease, chronic pancreatitis can also be found in association with other entities, such as CF. Familial hereditary pancreatitis is an autosomal-dominant disease that usually presents in childhood or during the teenage years.
Clinical Presentation: Patients with chronic pancreatitis usually come to medical attention with persistent abdominal pain; some have constant, debilitating pain, whereas others have pain related to food intake, especially fats and protein-rich meals. Steatorrhea as a result of fat malabsorption is common, and weight loss is attributed to malabsorption and a reduction in food intake secondary to pain and food aversion. Diabetes may result from chronic pancreatic damage, and chronic pancreatitis may result in obstructive jaundice secondary to biliary strictures.86
Imaging: Ductal dilation, pseudocysts, and calcifications are the most common imaging abnormalities in chronic hereditary pancreatitis,87 and pancreatic atrophy may also occur. Chronic fibrosing pancreatitis is characterized by bands of collagen enclosing normal acini88; the resulting mass simulates a tumor.
Trauma
Overview: Pediatric pancreatic trauma manifests in several ways besides the acute pancreatitis discussed above.
Imaging: At US, posttraumatic contusions may appear as a focal or diffusely enlarged, hypoechoic gland. However, because abdominal pain may be due to the trauma itself and associated acute pancreatitis, US may be limited. CT is the best imaging modality in these cases and demonstrates focal or diffuse hypoattenuation and enlargement of the gland, heterogeneous attenuation, peripancreatic fat stranding or frank fluid collections, abscesses, and pseudocyst formation. Fluid between the pancreas and splenic vein is a secondary sign of pancreatic injury. Pancreatic laceration, transection, and comminution are direct signs of injury and appear as hypoechoic or hypoattenuating areas that may be subtle in the early stages of injury. Duodenal hematomas are often associated and can serve as pointers to pancreatic trauma. These and pancreatic hematomas also may obstruct the pancreatic duct and biliary tree. CT may demonstrate pancreatic hypoenhancement, and associated CT features of shock bowel and the hypoperfusion complex may also be present. ERCP is the gold standard for evaluation of the ductal system in trauma, particularly if stent placement is anticipated. Noninvasive MRCP may be used preceding ERCP but is often limited because of distorted anatomy from posttraumatic edema and hematoma.
Treatment: Treatment is supportive and directed at the dominant pancreatic abnormality resulting from the trauma. Disruption of the pancreatic duct is treated surgically or by therapeutic endoscopy with stent placement, whereas injuries without duct involvement are usually treated nonsurgically.91–93
Pancreatic Neoplasms
Primary pancreatic tumors, both benign and malignant, are very rare in childhood and adolescence (Table 96-3). Pancreatic tumors that occur in pediatric patients include solid-cystic pseudopapillary tumor (Frantz tumor), pancreatoblastoma, and islet cell tumors; carcinomas are rare. Other tumors that typically occur elsewhere, but can arise within the pancreas, include lymphoma94,95 and rhabdomyosarcoma and rare cases of pancreatic neuroblastoma. Secondary involvement of the pancreas by adjacent tumor, especially neuroblastoma, may be difficult to distinguish from a primary pancreatic tumor.
Table 96-3
Classification of Common Pancreatic Tumors of Childhood
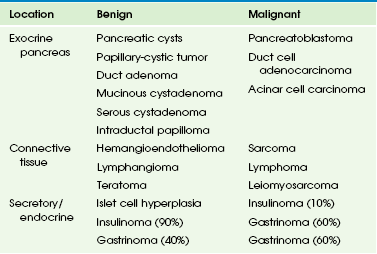
Modified from Enríquez G, Vázquez E, Aso C, et al. Pediatric pancreas: an overview. Eur Radiol. 1998;8:1236-1244.
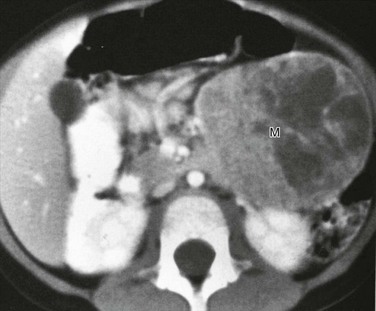
Overview: Solid-cystic papillary tumor is also known by many other terms: Frantz tumor, solid pseudopapillary tumor, papillary epithelial neoplasm (PEN), solid and papillary epithelial neoplasm (SPEN), solid and cystic acinar cell tumor, papillary and solid neoplasm, papillary-cystic epithelial neoplasm, papillary-cystic carcinoma, solid and cystic tumor, solid and papillary neoplasm, papillary cystic tumor, and low-grade papillary neoplasm.96 Solid-cystic papillary tumor is histologically low grade, with a reported 5 year survival of 97%. It accounts for about 0.2% to 2.7% of all nonendocrine pancreatic tumors.
Clinical Presentation: This tumor seems to have a predilection for women97 and for persons of Asian extraction; it is probably the most common pancreatic tumor in Asian children. Although the median age at diagnosis is 26 years, approximately 20% of cases have been reported in children. Children usually have a better prognosis than adults, owing to less frequent metastatic disease. Abdominal pain is the presenting symptom in about one third of cases, and a palpable abdominal mass is typically present; jaundice is extremely rare.
Imaging: CT findings include a large, well-defined, solid mass with varying degrees of cystic components that usually represent necrosis but are unrelated to tumor size (Figs. 96-16 and 96-17).98–100 Calcifications may also be present. On T1-weighted MR sequences, a low signal rim may represent either a fibrous capsule or compressed pancreatic parenchyma; central high-signal areas that represent debris or hemorrhagic necrosis have also been reported. Almost half of all lesions occur in the pancreatic head. Invasion of adjacent structures occurs and is often associated with liver and lymph node metastases.101–103
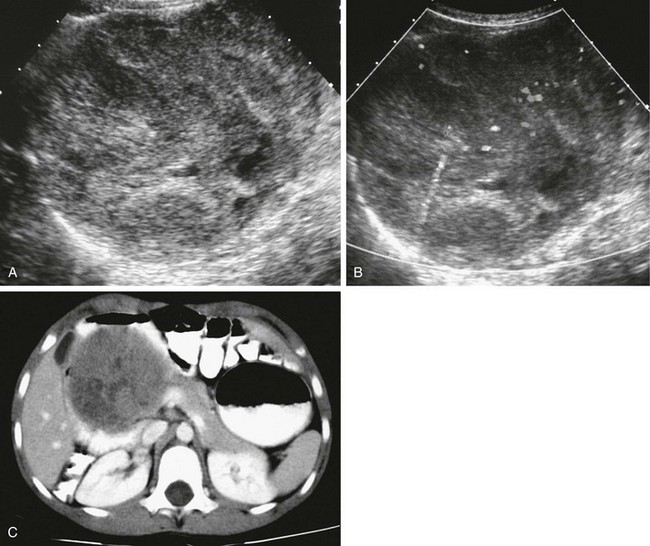
Figure 96-16 Solid-cystic papillary tumor (Frantz tumor).
A, Axial ultrasound through the midabdomen shows a large, heterogeneous, solid mass that arises from the pancreatic head. B, The mass is mildly vascular, as shown with gray scale depiction of the color Doppler image. C, Axial contrast-enhanced computed tomography through the midabdomen shows the mildly heterogeneous enhancement pattern of the mass and its origin from the head of the pancreas, widening and displacing the duodenal loop.
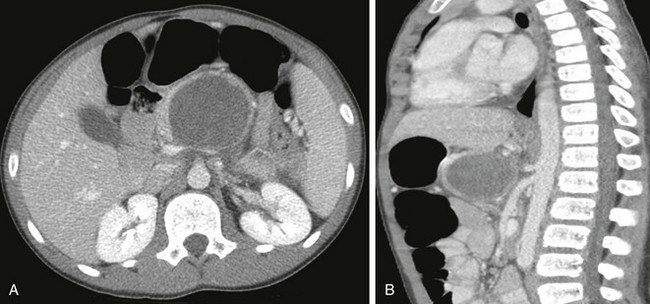
Figure 96-17 A solid-cystic papillary tumor that was causing abdominal pain in a 12-year-old boy.
A, Axial computed tomography through the upper abdomen shows a large, centrally necrotic, peripherally solid enhancing mass centered in the pancreatic body. Axial (A) and sagittal (B) images demonstrate that the mass abuts the celiac axis posteriorly and protrudes into the lesser sac anteriorly.
Pancreatoblastoma
Overview: Pancreatoblastoma, or pancreaticoblastoma,104,105 arises from the pancreatic acinar cells, usually in the head or tail of the gland. The cells of these tumors represent persistence of the fetal anlage of the pancreatic acinar cells. Pancreatoblastoma is one of the most common exocrine tumors in pediatric patients and represents about 0.5% of all pancreatic epithelial tumors.
Clinical Presentation: These tumors are found in boys twice as often as in girls. Incidence of pancreatoblastoma in East Asia is relatively high. Serum α-fetoprotein is elevated in 25% to 55% of cases, and an association with Beckwith-Wiedeman Syndrome has been reported. A review of 153 patients with pancreatoblastoma found that the median age at presentation was 5 years, although this tumor has been diagnosed in patients as old as 68 years.106 The liver is the most common site of metastatic disease (88% of metastatic sites), which was found in 17% of the patients in this series. Factors associated with a worse prognosis include metastatic or nonresectable disease and age older than 16 years at diagnosis. Pancreatoblastomas are often large at presentation, up to 12 cm, and areas of central necrosis are sometimes present.
Imaging: US and CT findings of pancreatoblastoma are often indistinguishable from those of pancreatic adenocarcinoma. Masses are typically hypoechoic and heterogeneous on US.106 On CT, pancreatoblastoma is hypodense and appears to be multiloculated with enhancing septa (Fig. 96-18). Calcifications are not uncommon. When vascular encasement is present, it usually involves the inferior vena cava or mesenteric vessels. Despite the typically large size of pancreatoblastomas, obstruction of the biliary system is infrequent. Although variable, MRI characteristics include typically low signal intensity compared with the liver on T1-weighted spin echo images and isointensity to hyperintensity on T2-weighted images; enhancement varies.107 Although findings are nonspecific as to tumor type, imaging suggests the malignant nature of the tumor and can clearly exclude the kidney and adrenal glands as organs of origin.
Islet Cell Tumors
Overview: Hormonally active tumors arise from the islet cells and may be benign or malignant. Islet cell tumors are named after the hormone produced, and insulinoma is the most common islet cell tumor in children (Fig. 96-19 and e-Fig. 96-20). Diffuse adenomatosis (nesidioblastosis) refers to diffuse adenomatous islet cell hyperplasia, and focal adenomatous hyperplasia (nesidioblastoma) refers to focal involvement.108
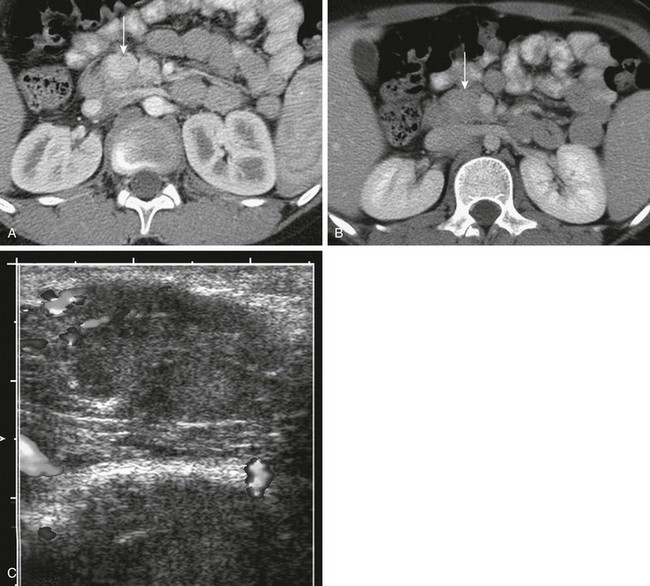
Figure 96-19 Insulinoma.
Axial contrast-enhanced computed tomographic images through the pancreas during the arterial (A) and venous (B) phases show a well-defined, ovoid, enhancing mass at the junction of the head and body of the pancreas (to the left of the superior mesenteric vein; arrows) that is particularly conspicuous on the arterial phase. C, Intraoperative ultrasound confirms the mass as a well-defined lesion of intermediate echogenicity within the pancreas. (Courtesy Dr. George Taylor, Boston, MA.)
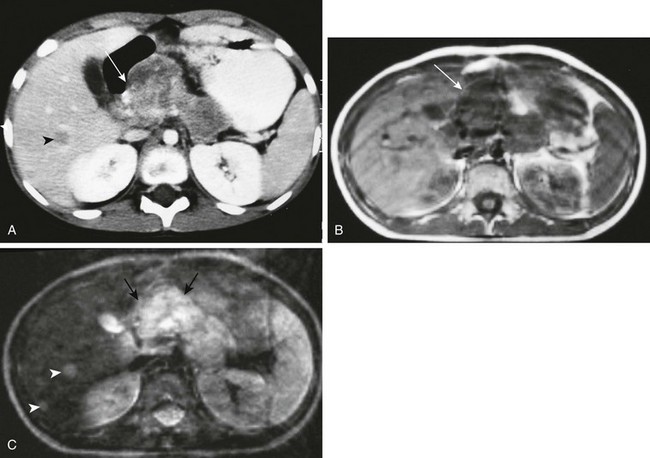
e-Figure 96-20 Insulinoma.
A, Axial contrast-enhanced computed tomography through the pancreas of a 10-year-old girl with hypoglycemia. A large, heterogeneously enhancing mass involves the pancreatic head (arrow). The remainder of the pancreas is also somewhat enlarged. Note the low-density hepatic metastasis (arrowhead). B, Axial T1-weighted magnetic resonance image at the same level shows the intermediate-intensity pancreatic mass (arrow). C, T2-weighted sequence shows intensely increased signal (arrows). Note also the conspicuity of the liver metastases on T2 weighting and a second, smaller metastatic focus located more peripherally (arrowheads).
Clinical Presentation: Patients with insulinoma are seen initially with hypoglycemia, which typically manifests in children as erratic behavior and seizures. Patients with nesidioblastosis can also be seen initially with hypoglycemia. In a series of 12 pediatric patients, only 3 patients with profound hypoglycemia had identifiable islet cell tumors; the others had islet cell hyperplasia or nesidioblastosis on histologic examination of specimens obtained after partial pancreatectomy.108 Tumors that secrete vasoactive intestinal polypeptide (VIP), so-called VIPomas, are associated with the syndrome of secretory diarrhea, hypokalemia, and achlorhydria. VIPomas are rare, functioning tumors with an estimated annual incidence of 0.2 to 0.5 per million population.
Imaging: The most common site for islet cell tumors is in the pancreatic head.109 These tumors are round or oval and are well circumscribed on US. They are hypoechoic but may have a hyperechoic rim; isoechoic and hyperechoic lesions have also been described in children and young adults. Tumors may be located superficially or may be deep within the pancreas. On CT, contrast may cause marked tumor enhancement, particularly in the arterial phase (see Fig. 96-19). Because these tumors are hypervascular, arteriography may be necessary in high-risk patients in whom US and CT are nondiagnostic, although magnetic resonance angiography (MRA) may replace this invasive technique. Intraoperative US has been used successfully to locate functioning islet cell tumors in children.110,111 Selective venous sampling in children with hyperinsulinism can also help diagnose and localize tumors.112 Indium-111-pentetreotide (Octreoscan) scintigraphy may also be useful in the diagnosis of primary or metastatic tumors.109
Other islet cell tumors are rare in children, but they may be found in association with tumors in other organs as part of the multiple endocrine neoplasia (MEN) syndromes. Type 1 (MEN 1) is an inherited condition characterized by synchronous or metachronous tumors of the parathyroid glands, anterior pituitary, pancreas, GI tract, and other less commonly involved organs. Patients usually seek medical attention in their twenties and thirties, and rarely, in familial cases, in childhood. Pancreatic involvement is in the form of multiple islet cell tumors.108 Gastrinomas may be found in children with Zollinger-Ellison syndrome. In one series, 2 of 56 reported cases of VIP-producing tumors in children were islet cell tumors; neurogenic tumors generated the hormone in the other patients.113 Glucagonomas and somatostatinomas have not been reported in children.
Other Pancreatic Tumors
Overview: The exocrine tissues of the pancreas give rise to benign and malignant tumors that are hormonally inactive, such as cystadenoma, adenocarcinoma, and adenosarcoma.
Clinical Presentation: The most common presenting symptoms include abdominal pain in 55.8%, nausea or vomiting in 32.6%, fatigue in 25.6%, and an abdominal mass in 23.3%.114 Cystadenomas and adenocarcinomas of the pancreas occur in children and have been described in infants as well.115 Pancreatic adenocarcinoma has been described in an adolescent boy with Peutz-Jeghers syndrome,116 and there is a hundredfold increased risk of pancreatic adenocarcinoma in patients with this syndrome.117
Imaging: On US, solid tumors are typically hyperechoic, and cystic lesions are anechoic or hypoechoic. Adenocarcinomas may have cystic or hemorrhagic areas that result in mixed echogenicity. CT usually identifies a pancreatic mass of variable size, often causing biliary obstruction. In a recent study, pancreatic duct adenocarcinomas were identified in only 3 patients younger than 20 years among a total cohort of 439 cases, corresponding to an incidence of 0.1% in this age group.118 Such tumors are often associated with a genetic predisposition. Because the diagnosis of pancreatic carcinoma is so rare in children (only about 50 cases have been reported), imaging evaluation is often delayed, and vascular invasion and metastases to lymph nodes and liver may be noted on imaging.
Rhabdomyosarcoma may arise primarily in the pancreas (e-Fig. 96-21), as may lymphoma (Fig. 96-22). Neuroblastoma has been reported in the pancreas either as a primary (e-Fig. 96-23) or secondary to direct extension. A single case of abdominal desmoid tumor presenting in the pancreas was reported in a 17-year-old boy with familial adenomatous polyposis syndrome.119
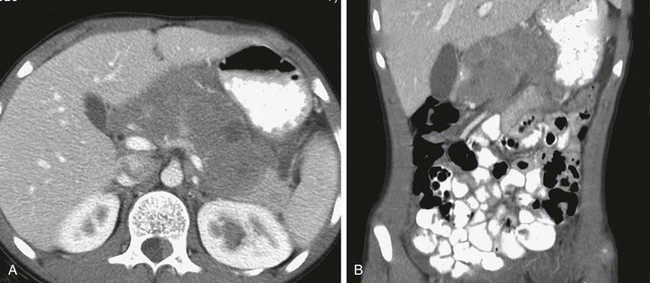
Figure 96-22 Primary pancreatic non-Hodgkin lymphoma.
Axial (A) and reconstructed coronal (B) contrast-enhanced computed tomographic images through the abdomen show diffuse enlargement of the pancreas with focal low-density regions suggestive of necrosis. Mass effect on the stomach from this primary pancreatic Burkitt lymphoma is best seen on the coronal reconstruction. (Courtesy Dr. George Taylor, Boston, MA.)
Lymphatic malformations of the pancreas are extremely rare and account for less than 1% of cases.120 They may occur in any portion of the pancreas at any age and are more frequent in females. On imaging, lymphatic malformations appear as septated, fluid-filled masses. Clinical presentation is nonspecific and includes nausea, vomiting, vague abdominal pain, and a palpable mass.
Exceptionally rare tumors of the pancreas include anaplastic large cell lymphoma,94 infantile myofibromatosis,121 and mature cystic teratoma.122 Cystic teratoma of the pancreas has been reported in at least seven pediatric patients, who ranged in age from 2 to 16 years. These tumors arise from pluripotent cells of ectodermal cell lines and, like other extragonadal teratomas, likely originate from aberrant germ cells. Such tumors may be indistinguishable from other cystic abdominal masses.
Pancreatic involvement with metastatic disease is also rare but includes malignant melanoma, lymphoma (e-Fig. 96-24), rhabdomyosarcoma (Fig. 96-25), acute lymphoblastic leukemia, and osteosarcoma (e-Fig. 96-26).123
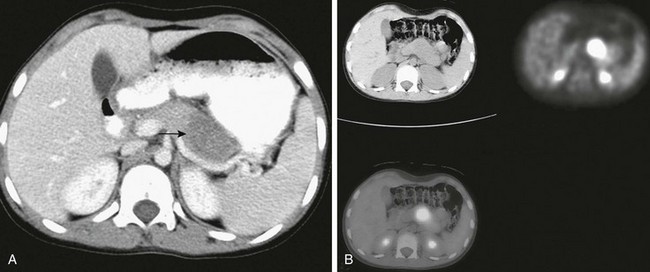
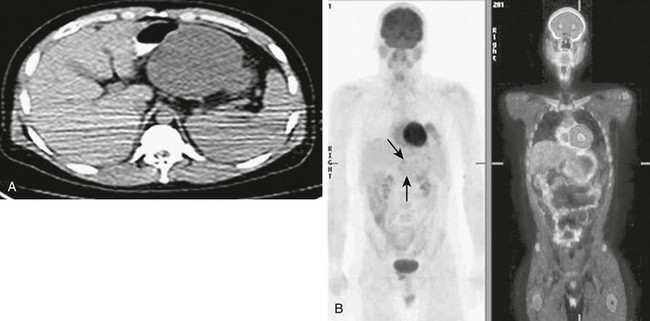
Figure 96-25 Recurrent rhabdomyosarcoma metastatic to the pancreas.
Three years earlier, this 10-year-old girl had been treated for alveolar rhabdomyosarcoma of the calf. A, Axial contrast-enhanced abdominal computed tomography (CT) shows a well-defined low-density mass in the pancreas (arrow). B, Axial fluorodeoxyglucose positron emission tomography CT image shows the lesion as a focal mass with intense uptake of radiopharmaceutical in the midbody of the pancreas.
Treatment of Pancreatic Neoplasms: Most pancreatic tumors are treated primarily with pancreatic resection and pancreatoduodenectomy. Resection is complete for aggressive and advanced tumors; partial pancreatectomy124 and enucleation may be indicated for less aggressive or small neoplasms. Radiotherapy or chemotherapy is less frequently indicated in the pediatric population.108 Supportive medical therapy may be indicated, such as IV glucose for insulinoma- or adenomatosis-induced hypoglycemia and for gastrinoma-induced Zollinger-Ellison syndrome.
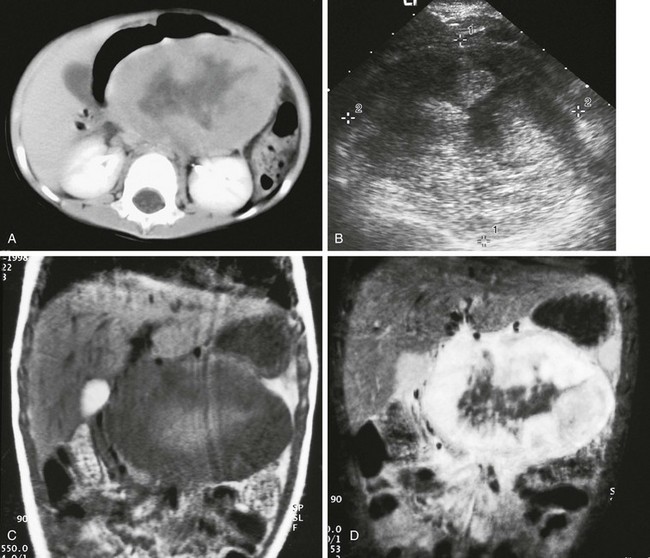
e-Figure 96-21 Primary alveolar rhabdomyosarcoma of the pancreas in a 3-year-old boy.
A, Axial, contrast-enhanced abdominal computed tomography shows a large midabdominal mass that arises from the pancreatic body with a central hypodense area. B, Axial ultrasound through the upper abdomen shows the mass to be of heterogeneous echogenicity. C, Coronal, noncontrast, T1-weighted magnetic resonance image shows irregular high signal centrally, surrounded by decreased signal in the bulk of the mass. D, Coronal, contrast-enhanced, T1-weighted image with fat saturation shows diffuse, intense peripheral enhancement of the bulk of the mass around the central area in addition to surrounding edema.
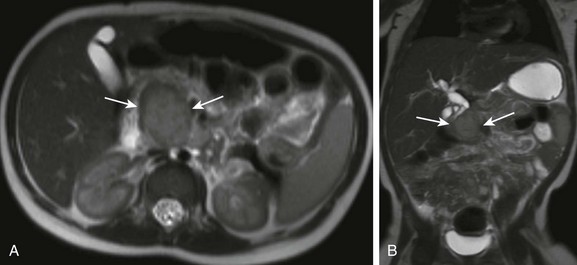
e-Figure 96-23 Primary pancreatic neuroblastoma in a 15-month-old boy with intermittent jaundice.
Axial (A) and coronal (B) T2-weighted magnetic resonance image of the abdomen demonstrates a gently lobulated, T2-hyperintense mass in the head of the pancreas. Pathology following a pylorus-sparing Whipple pancreaticoduodenectomy revealed a poorly differentiated primary pancreatic neuroblastoma.
e-Figure 96-24 Primary lymphoma of bone metastatic to the pancreas.
Axial computed tomographic image of the abdomen in an 8-year-old boy with known lymphoma of bone shows a focal, well-defined, hypoattenuating mass within the body of the pancreas (arrow). This lesion showed mild gallium uptake on a staging gallium scan (not shown).
e-Figure 96-26 Recurrent osteosarcoma metastatic to the pancreas in a 19-year-old man.
Five years earlier, the patient had been treated with multi-drug chemotherapy and limb-sparing surgery for nonmetastatic primary femoral osteosarcoma. He had been well until coming to medical attention with abdominal pain and fatigue. A, Noncontrast axial computed tomography (CT) through the upper abdomen as part of a fluorodeoxyglucose positron emission tomography (FDG-PET) scan shows a large, low-density midabdominal mass arising from the midbody of the pancreas. B, Anterior planar attenuation-corrected PET image (left) shows a subtle blush of uptake in the distribution of the mass (arrows). Fused FDG-PET CT image (right) shows minimal peripheral uptake in the mass and central photopenia consistent with central necrosis; this was confirmed by aspiration and biopsy.
Pancreatic Infections
Overview: Pancreatic hydatid disease, or echinococcosis, is extremely rare and can result from parasitic infestation with Echinococcus granulosus, a parasite that affects humans and other mammals, most notably dogs and sheep. The disease manifests as cysts in the affected organ; only rarely is the pancreas the only organ involved.125
Clinical Presentation: Jaundice and abdominal pain may be the first symptoms of hydatid disease of the pancreas, and hydatid disease may also be a rare cause of recurrent pancreatitis.126
Tuberculosis
Overview and Clinical Presentation: Patients with abdominal tuberculosis are either human immunodeficiency virus positive or otherwise immune compromised, or they are from areas endemic for tuberculosis.
Imaging: Abdominal tuberculosis (Fig. 96-27) can result in focal pancreatic parenchymal lesions or tuberculous abscesses, usually with one or more calcified components and imaging evidence of abdominal tuberculosis elsewhere (retroperitoneal adenopathy that is usually matted and may or may not be centrally necrotic or calcified; tuberculous ascites; matting of bowel loops or omentum, so-called omental cake; and intraperitoneal adhesions).128
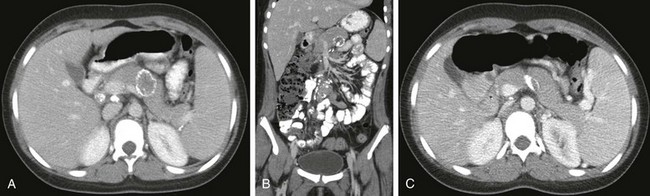
Figure 96-27 Pancreatic tuberculosis in an HIV-positive teen.
A, Axial computed tomography (CT) through the upper abdomen shows a large, peripherally calcified, relatively hypodense lesion in the pancreatic body. B, Coronal CT image through the abdomen demonstrates this lesion again; both axial and coronal images demonstrate calcified retroperitoneal lymph nodes. This patient was on multidrug therapy for abdominal tuberculosis. C, Axial CT through the upper abdomen 2 years later demonstrates interval shrinkage of the peripherally calcified pancreatic body lesion; calcific foci appear more coarse and chunky as a result.
Chung, EM, Travis, MD, Conran, RM. Pancreatic tumors in children: radiologic-pathologic correlation. Radiographics. 2006;26(4):1211–1238.
Nijs, E, Callahan, MJ, Taylor, GA. Disorders of the pediatric pancreas: imaging features. Pediatr Radiol. 2005;35:358.
Nijs, EL, Callahan, MJ. Congenital and developmental pancreatic anomalies: ultrasound, computed tomography, and magnetic resonance imaging features. Semin Ultrasound CT MR. 2007;28(5):395–401.
Shimizu, T, Suzuki, R, Yamashiro, Y, et al. Magnetic resonance cholangiopancreatography in assessing the cause of acute pancreatitis in children. Pancreas. 2001;2:196.
To’o, KF, Raman, SS, Yu, NC, et al. Pancreatic and peripancreatic diseases mimicking primary pancreatic neoplasia. Radiographics. 2005;25:949.
References
1. Shera, AH, Shah, OJ. Congenital common bile duct web in association with hepatobiliary pancreatic ductal anomalies. Eur J Pediatr Surg. 2008;18(5):350–351.
2. Nijs, E, Callahan, MJ, Taylor, GA. Disorders of the pediatric pancreas: imaging features. Pediatr Radiol. 2005;35:358.
3. Siegel, MJ, Martin, KW, Worthington, JL. Normal and abnormal pancreas in children: US studies. Radiology. 1987;165:15.
4. Teele, RL, Share, JC. Ultrasonography of infants and children. Philadelphia: Saunders; 1990. [389].
5. Walsh, E, Cramer, B, Pushpanthan, C. Pancreatic echogenicity in premature and newborn infants. Pediatr Radiol. 1990;20:323.
6. Fuss, M, Salter, BJ, Cavanaugh, SX, et al. Daily ultrasound-based image-guided targeting for radiotherapy of upper abdominal malignancies. Int J Radiat Oncol Biol Phys. 2004;59:1245.
7. Herman, TE, Siegel, MJ. CT of the pancreas in children. AJR Am J Roentgenol. 1991;1547:375.
8. Gong, J-S, Xu, J-M. Role of curved planar reformations using multidetector spiral CT in diagnosis of pancreatic and peripancreatic diseases. World J Gastroenterol. 2004;10:1943.
9. Keppke, AL, Miller, FH. Magnetic resonance imaging of the pancreas: the future is now. Semin Ultrasound CT MR. 2005;26(3):132–152.
10. Matos, C, Cappeliez, O, Winant, C, et al. MR imaging of the pancreas: a pictorial tour. Radiographics. 2002;22:e2.
11. Hakimé, A, Giraud, M, Vullierme, MP, et al. MR imaging of the pancreas. J Radiol. 2007;88(1 Pt 1):11–25.
12. Arcement, CM, Meza, MP, Arumania, S, et al. MRCP in the evaluation of pancreaticobiliary disease in children. Pediatr Radiol. 2001;31:92.
13. Allendorph, M, Werlin, SL, Geenen, JE, et al. Endoscopic retrograde cholangiopancreatography in children. J Pediatr. 1987;110:206.
14. Itoh, Y, Hada, T, Terano, A, et al. Pancreatitis in the annulus of annular pancreas demonstrated by the combined use of computed tomography and endoscopic retrograde cholangiopancreatography. Am J Gastroenterol. 1989;84:961.
15. Bolog, N, Constantinescu, G, Oancea, I, et al. Magnetic resonance imaging of bile and pancreatic ducts: a retrospective study. Rom J Gastroenterol. 2004;13:91.
16. Suarez, F, Bernard, O, Gauthier, F, et al. Bilio-pancreatic common channel in children: clinical, biological and radiological findings in 12 children. Pediatr Radiol. 1987;17:206.
17. Hamada, Y, Tanano, A, Takada, K, et al. Magnetic resonance cholangiopancreatography on postoperative work-up in children with choledochal cysts. Pediatr Surg Int. 2004;20:43.
18. Fulcher, AS, Turner, MA. Orthotopic liver transplantation: evaluation with MR cholangiography. Radiology. 1999;211:715.
19. Van Hoe, L, Mermuys, K, Vanhoenacker, P. MRCP pitfalls. Abdom Imaging. 2004;29(3):360–387.
20. Akisik, MF, Sandrasegaran, K, Aisen, AA, et al. Dynamic secretin-enhanced MR cholangiopancreatography. Radiographics. 2006;26(3):665–677.
21. Calculli, L, Pezzilli, R, Fiscaletti, M, et al. Exocrine pancreatic function assessed by secretin cholangio-Wirsung magnetic resonance imaging. Hepatobiliary Pancreat Dis Int. 2008;7(2):192–195.
22. Fukukura, Y, Fujiyoshi, F, Sasaki, M, et al. Pancreatic duct: morphologic evaluation with MR cholangiopancreatography after secretin stimulation. Radiology. 2002;222:674.
23. Tirkes, T, Akisik, F, Tann, M, Balci, NC. Imaging of the pancreas with secretin enhancement. Top Magn Reson Imaging. 2009;20(1):19–24.
24. Hanninen, EL, Amthauer, H, Hosten, N, et al. Prospective evaluation of pancreatic tumors: accuracy of MR imaging with MR cholangiopancreatography and MR angiography. Radiology. 2002;224:34.
25. Nijs, EL, Callahan, MJ. Congenital and developmental pancreatic anomalies: ultrasound, computed tomography, and magnetic resonance imaging features. Semin Ultrasound CT MR. 2007;28(5):395–401.
26. Benya, EC. Pancreas and biliary system: imaging of developmental anomalies and diseases unique to children. Radiol Clin North Am. 2002;40(6):1355–1362.
27. Lindstrom, E, Ihse, I. Dynamic CT scanning of pancreatic duct after secretin provocation in pancreas divisum. Dig Dis Sci. 1990;35:1371.
28. Manfredi, R, Costamagna, G, Brizi, MG, et al. Pancreas divisum and “Santorinicele”: diagnosis with dynamic MR cholangiopancreatography with secretin stimulation. Radiology. 2000;217:403.
29. Soulen, MC, Zerhouni, EA, Fishman, EK, et al. Enlargement of the pancreatic head in patients with pancreas divisum. Clin Imaging. 1989;13:51.
30. Zeman, RK, McVay, LV, Silverman, PM, et al. Pancreas divisum: thin-section CT. Radiology. 1988;169:395.
31. Kanamori, A, Kumada, T, Kiriyama, S, et al. Endoscopic papillectomy of minor papillar adenoma associated with pancreas divisum. World J Gastroenterol. 2009;15(9):1138–1140.
32. Schnedl, WJ, Piswanger-Soelkner, C, Wallner, SJ, et al. Agenesis of the dorsal pancreas. World J Gastroenterol. 2009;15(3):376–377.
33. Gilinsky, NH, Del Favero, G, Cotton, PB, Lees, WR. Congenital short pancreas: a report of two cases. Gut. 1985;26:304–310.
34. Schnedl, WJ, Piswanger-Soelkner, C, Wallner, SJ, et al. Agenesis of the dorsal pancreas and associated diseases. Dig Dis Sci. 2009;54(3):481–487.
35. Sakpal, SV, Sexcius, L, Babel, N, et al. Agenesis of the dorsal pancreas and its association with pancreatic tumors. Pancreas. 2009;38(4):367–373.
36. Camoglio, FS, Forestler, C, Zanatta, C, et al. Complete pancreatic ectopia in a gastric duplication cyst: a case report and review of the literature. Eur J Pediatr Surg. 2004;14:60.
37. Kilman, WJ, Berk, RN. The spectrum of radiographic features of aberrant pancreatic rests involving the stomach. Radiology. 1977;123:291.
38. Tillig, B, Gerein, V, Coerdt, W, et al. Large supraumbilical pseudocystic tumour due to ectopic pancreatic tissue located in a rest of the omphaloenteric duct. Eur J Pediatr Surg. 2004;14:126.
39. Rahmah, R, Yong, JF, Sharifa, NA, et al. Bilateral adrenal cysts and ectopic pancreatic tissue in Beckwith-Wiedemann syndrome: is a conservative approach acceptable? J Pediatr Endocrinol Metab. 2004;17:909.
40. Ueda, D, Taketazu, M, Itoh, S, et al. A case of gastric duplication cyst with aberrant pancreas. Pediatr Radiol. 1991;21:379.
41. Baggott, BB, Long, WB. Annular pancreas as a cause of extrahepatic biliary obstruction. Am J Gastroenterol. 1991;86:224.
42. Vijayaraghavan, SB. Sonography of pancreatic ductal anatomic characteristics in annular pancreas. J Ultrasound Med. 2002;21(11):1315–1318.
43. Demir, MK, Kilicoglu, G. Rare coincidence of congenital short and annular pancreas with gallbladder agenesis and splenic malrotation. Br J Radiol. 2008;81(968):e204–e206.
44. De Ugarte, DA, Dutson, EP, Hiyama, DT. Annular pancreas in the adult: management with laparoscopic gastrojejunostomy. The American surgeon. 2006;72(1):71–73.
45. To’o, KF, Raman, SS, Yu, NC, et al. Pancreatic and peripancreatic diseases mimicking primary pancreatic neoplasia. Radiographics. 2005;25:949.
46. Handrich, SJ, Hough, DM, Fletcher, JG, et al. The natural history of the incidentally discovered small simple pancreatic cyst: long-term follow-up and clinical implications. AJR Am J Roentgenol. 2005;184:20.
47. Baker, LL, Hartman, GE, Northway, WH, Jr. Sonographic detection of congenital pancreatic cysts in the newborn: report of a case and review of the literature. Pediatr Radiol. 1990;20:488.
48. Choyke, PL, Filling-Katz, MR, Shawker, TH, et al. Von Hippel–Lindau disease: radiologic screening for visceral manifestations. Radiology. 1990;174:815.
49. Clavon, M, Verain, AL, Bigard, MA. Cyst formation in gastroheterotopic pancreas: report of two cases. Radiology. 1988;169:659.
50. Daneman, A, Gaskin, K, Martin, DJ, et al. Pancreatic changes in cystic fibrosis: CT and sonographic appearances. AJR Am J Roentgenol. 1983;141:653.
51. Liu, P, Daneman, A, Stringer, DA, et al. Pancreatic cysts and calcification in cystic fibrosis. J Can Assoc Radiol. 1986;37:279.
52. Tham, RTOT, Heyerman, HGM, Falke, THM, et al. Cystic fibrosis: MR imaging of the pancreas. Radiology. 1991;179:183.
53. Murayama, S, Robinson, AE, Mulvihill, DM, et al. MR imaging of pancreas in cystic fibrosis. Pediatr Radiol. 1990;20:536.
54. Ferrozzi, F, Bova, D, Campodonico, F, et al. Cystic fibrosis: MR assessment of pancreatic damage. Radiology. 1996;198:875.
55. Hernanz-Schulman, M, Teele, RL, Perez-Atayde, A, et al. Pancreatic cystosis in cystic fibrosis. Radiology. 1986;158:629.
56. Berrocal, T, Pajares, MP, Zubillaga, AF. Pancreatic cystosis in children and young adults with cystic fibrosis: sonographic, CT, and MRI findings. AJR Am J Roentgenol. 2005;184(4):1305–1309.
57. Sodhi, KS, Thapa, BR, Khadelwal, S, et al. Pancreatic lipomatosis in an infant with cystic fibrosis. Pediatr Radiol. 2005;35:1157.
58. Mustafa, E, Mustafa, Y, Cengiz, A, et al. Pancreatic involvement in von Hippel–Lindau disease. Indian J Cancer. 2004;41:159.
59. Shen, HC, Adem, A, Ylaya, K, et al. Deciphering von Hippel-Lindau (VHL/Vhl)-associated pancreatic manifestations by inactivating Vhl in specific pancreatic cell populations. PLoS One. 2009;4(4):e4897.
60. Eras, M, Yenigun, M, Acar, C, et al. Pancreatic involvement in von Hippel–Lindau disease. Indian J Cancer. 2004;41:159.
61. Hammel, PR, Vilgrain, V, Terris, B, et al. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d’Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119(4):1087–1095.
62. Weizman, Z, Durie, PR. Acute pancreatitis in childhood. J Pediatr. 1988;113:24.
63. Manfredi, R, Lucidi, V, Gui, B, et al. Idiopathic chronic pancreatitis in children: MR cholangiopancreatography after secretin administration. Radiology. 2002;224:675.
64. Gananadha, S, Smith, RC. Hepatobiliary and pancreatic: anomalous pancreatobiliary junction with choledochal cyst. J Gastroenterol Hepatol. 2006;21(4):776.
65. Roberts-Thomson, IC. Hepatobiliary and pancreatic: anomalous pancreatobiliary ductal junction. J Gastroenterol Hepatol. 2006;21(4):775.
66. Jeong, JB, Whang, JH, Ryu, JK. Risk factors for pancreatitis in patients with anomalous union of pancreatobiliary duct. Hepatogastroenterolgy. 2004;51:1187.
67. Kamisawa, T, Kurata, M, Honda, G, et al. Biliopancreatic reflux-pathophysiology and clinical implications. J Hepatobiliary Pancreat Surg. 2009;16(1):19–24.
68. Lee, SP, Nicholls, JF, Park, HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med. 1992;326:589.
69. Jeffrey, RB, Jr. Sonography in acute pancreatitis. Radiol Clin North Am. 1989;27:5.
70. Chao, HC, Lin, SJ, Kong, MS, et al. Sonographic evaluation of the pancreatic duct in normal children and children with pancreatitis. J Ultrasound Med. 2000;19:757.
71. Swischuk, LE, Hayden, CK, Jr. Pararenal space hyperechogenicity in childhood pancreatitis. AJR Am J Roentgenol. 1985;145:1085.
72. Balthazar, EJ, Robinson, DL, Megabow, AJ, et al. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331.
73. Delhaye, M, Matos, C, Arvanitakis, M, et al. Pancreatic ductal system obstruction and acute recurrent pancreatitis. World J Gastroenterol. 2008;14(7):1027–1033.
74. Shimizu, T, Suzuki, R, Yamashiro, Y, et al. Magnetic resonance cholangiopancreatography in assessing the cause of acute pancreatitis in children. Pancreas. 2001;2:196.
75. Crombleholme, TM, deLorimier, AA, Adzick, NS, et al. Mediastinal pancreatic pseudocysts in children. J Pediatr Surg. 1990;25:843.
76. Ford, EG, Hardin, WD, Jr., Mahour, GH, et al. Pseudocysts of the pancreas in children. Am Surg. 1990;56:384.
77. Mehta, R, Survarna, D, Sadasivan, S, et al. Natural course of asymptomatic pancreatic pseudocyst: a prospective study. Indian J Gastroenterol. 2004;23:140.
78. Garel, L, Burnelle, F, Lallemand, D, et al. Pseudocysts of the pancreas in children: which cases require surgery? Pediatr Radiol. 1983;13:96.
79. Amundson, GM, Towbin, RB, Mueller, DL, et al. Percutaneous transgastric drainage of the lesser sac in children. Pediatr Radiol. 1990;20:590.
80. Haller, J, Greenway, G, Resnick, D, et al. Intraosseous fat necrosis associated with acute pancreatitis: MR imaging. Radiology. 1989;173:193.
81. Yin, WY. The role of surgery in pancreatic pseudocyst. Hepatogastroenterology. 2005;52:1266.
82. Mortelé, KJ, Mergo, PJ, Taylor, HM, et al. Peripancreatic vascular abnormalities complicating acute pancreatitis: contrast-enhanced helical CT findings. Eur J Radiol. 2004;52(1):67–72.
83. Petrov, MS, van Santvoort, HC, Besselink, MG, et al. “Oral refeeding after onset of acute pancreatitis: a review of literature.”. The American Journal of Gastroenterology. 2007;102(9):2079–2084.
84. Villatoro, E, Bassi, C, Larvin, M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. (4):2006. [CD002941].
85. Whitcomb, D. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354(20):2142–2150.
86. Wheatley, MJ, Coran, AG. Obstructive jaundice secondary to chronic pancreatitis in children: report of two cases and review of the literature. Surgery. 1988;104:863.
87. Spencer, JA, Lindsell, DRM, Isaacs, D. Hereditary pancreatitis: early ultrasound appearances. Pediatr Radiol. 1990;20:293.
88. Atkinson, GO, Jr., Wyly, JB, Gay, BB, Jr., et al. Idiopathic fibrosing pancreatitis: a cause of obstructive jaundice in childhood. Pediatr Radiol. 1988;18:28.
89. Rollins, MD, Meyers, RL. Frey procedure for surgical management of chronic pancreatitis in children. J Pediatr Surg. 2004;39:817.
90. American Gastroenterological Association Medical Position Statement: treatment of pain in chronic pancreatitis. Gastroenterology. 1998;115(3):763–764.
91. Stringer, MD. Pancreatic trauma in children. Br J Surg. 2005;92:467.
92. Stringer, MD, Davison, SM, McClean, P, et al. Multidisciplinary management of surgical disorders of the pancreas in childhood. J Pediatr Gastroenterol Nutr. 2005;40:363.
93. Gupta, A, Stuhlfaut, JW, Fleming, KW, et al. Blunt trauma of the pancreas and biliary tract: a multimodality imaging approach to diagnosis. RadioGraphics. 2004;24:1381–1395.
94. Fraser, CJ, Chan, YF, Heath, JA. Anaplastic large cell lymphoma of the pancreas: a pediatric case and literature review. J Pediatr Hematol Oncol. 2004;26:840.
95. Turkish, A, Levy, J, Kato, M, et al. Pancreatitis and probable paraneoplastic cholestasis as presenting manifestations of pancreatic lymphoma in a child. J Pediatr Gastroenterol Nutr. 2004;39:552.
96. Kosmahl, M, Pauser, U, Peters, K, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168.
97. Tien, Y-W, Ser, K-H, Hu, R-H, et al. Solid pseudopapillary neoplasms of the pancreas: is there a pathologic basis for the observed gender differences in incidence? Surgery. 2005;137:591.
98. Moholkar, S, Sebire, NJ, Roebuck, DJ. Solid-pseudopapillary neoplasm of the pancreas: radiological-pathological correlation. Pediatr Radiol. 2005;35:819.
99. Choi, BI, Kim, KW, Han, MC, et al. Solid and papillary epithelial neoplasms of the pancreas: CT findings. Radiology. 1988;166:413.
100. Friedman, AC, Lichtenstein, JE, Fishman, EK, et al. Solid and papillary epithelial neoplasm of the pancreas. Radiology. 1985;154:333.
101. Hassan, I, Celik, I, Nies, C, et al. Successful treatment of solid-pseudopapillary tumor of the pancreas with multiple liver metastases. Pancreatology. 2005;5:289.
102. Huang, H-L, Shih, S-C, Chang, W-H, et al. Solid-pseudopapillary tumor of the pancreas: clinical experience and literature review. World J Gastroenterol. 2005;11:1403.
103. Tang, LH, Aydin, H, Brennan, MF, Kimstra, DS. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol. 2005;29:512.
104. Montemarano, H, Lonergan, GJ, Bulas, DI, et al. Pancreatoblastoma: imaging findings in 10 patients and review of the literature. Radiology. 2000;214:476.
105. Roebuck, DJ, Yuen, MK, Wong, YC, et al. Imaging features of pancreatoblastoma. Pediatr Radiol. 2001;31:501.
106. Dhebri, AR, Connor, S, Campbell, F, et al. Diagnosis, treatment and outcome of pancreatoblastoma. Pancreatology. 2004;4:441. [discussion 452].
107. Stephenson, CA, Kletzel, M, Seibert, JJ, et al. Pancreatoblastoma: MR appearance. J Comput Assist Tomogr. 1990;14:492.
108. Chung, EM, Travis, MD, Conran, RM. Pancreatic tumors in children: radiologic-pathologic correlation. Radiographics. 2006;26(4):1211–1238.
109. Nikou, GC, Toubanakis, C, Nikolaou, P, et al. VIPomas: an updated diagnosis and management in a series of 11 patients. Hepatogastroenterology. 2005;52:1259.
110. Galiber, AK, Reading, CC, Charboneau, JW. Localization of pancreatic insulinoma: comparison of pre- and intraoperative ultrasound with CT and angiography. Radiology. 1988;166:405.
111. Grant, CS, Heerden, J, Charboneau, W. Insulinoma: the value of intraoperative ultrasonography. Arch Surg. 1988;123:843.
112. Brunelle, F, Negre, V, Barth, MO, et al. Pancreatic venous samplings in infants and children with primary hyperinsulinism. Pediatr Radiol. 1989;19:100.
113. Grosfeld, JL, Vane, DW, Rescorla, FJ, et al. Pancreatic tumors in childhood: analysis of 13 cases. J Pediatr Surg. 1990;25:1057.
114. Liang, H, Wang, P, Wang, X-N, et al. Management of nonfunctioning islet cell tumors. World J Gastroenterol. 2004;10:1806.
115. Lau, ST, Kim, SS, Lee, SL, Schaller, RT. Mucinous cystadenoma of the pancreas in a one-year-old child. J Pediatr Surg. 2004;39:1574.
116. Bowlby, LS. Pancreatic adenocarcinoma in an adolescent male with Peutz-Jeghers syndrome. Hum Pathol. 1986;17:97.
117. Giardiello, FM, Welsh, SB, Hamilton, SR, et al. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987;316:1151.
118. Luttges, J, Stigge, C, Pacena, M, Kloppel, G. Rare ductal adenocarcinoma of the pancreas in patients younger than age 40 years: an analysis of its features and a literature review. Cancer. 2004;100:173.
119. Pho, LN, Coffin, CM, Burt, RW. Abdominal desmoid in familial adenomatous polyposis presenting as a pancreatic cystic lesion. Fam Cancer. 2005;4:135.
120. Paal, E, Thompson, LD, Heffess, CS. A clinicopathologic and immunohistochemical study of ten pancreatic lymphangiomas and a review of the literature. Cancer. 1998;82:2150.
121. Rohrer, K, Murphy, R, Thresher, C, et al. Infantile myofibromatosis: a most unusual cause of gastric outlet obstruction. Pediatr Radiol. 2005;35:808.
122. Salimi, J, Karbakhsh, M, Dolatshahi, S, et al. Cystic teratoma of the pancreas: a case report. Ann Saudi Med. 2004;24:206.
123. Kim, SJ, Choi, J-A, Lee, SH, et al. Imaging findings of extrapulmonary metastases of osteosarcoma. J Clin Imaging. 2004;28:291.
124. Carricaburu, E, Enezian, G, Bonnard, A, et al. Laparoscopic distal pancreatectomy for Frantz’s tumor in a child. Surg Endosc. 2003;17:2028.
125. Krige, JE, Mirza, K, Bornman, PC, et al. Primary hydatid cysts of the pancreas. S Afr J Surg. 2005;43:37.
126. Ozmen, MM, Moran, M, Karakahya, M, et al. Recurrent acute pancreatitis due to a hydatid cyst of the pancreatic head: a case report and review of the literature. JOP. 2005;6:354.
127. Borisa, AD, Bakhshi, GD, Tayade, MB, et al. Hydatid cyst of pancreas. Bombay Hospital Journal. 2009;51(1):88–90.
128. Khanna, PC, Merchant, SA, Joshi, AR. Radiological-case-of-the-month: Tuberculous ‘omental cake’. Applied Radiology. 2007;36(5):36–39.