CHAPTER 53 The overactive bladder syndrome
Introduction
The normal adult human bladder is under voluntary control and does not contract, except during micturition. Conversely, an overactive bladder is one which contracts involuntarily or can be provoked to do so. Raised bladder pressure was first reported in certain neurological conditions over 70 years ago (Rose 1931, Langworthy et al 1936), but its clinical significance was not appreciated until 1963 when Hodgkinson et al demonstrated urinary incontinence as a result of detrusor contractions in 64 neurologically normal women. They called this condition ‘dyssynergic detrusor dysfunction’. Various other names have been used, including ‘detrusor instability’, ‘detrusor reflex instability’, ‘overactive bladder’ and ‘detrusor hyperreflexia’. The terminology of lower urinary tract dysfunction has been revised and standardized by the International Continence Society in order to clarify understanding of patient symptoms and diagnosis (Abrams et al 2002).
Overactive Bladder
The term ‘overactive bladder’ (then ‘unstable bladder’) was first used by Bates et al (1970) to describe ‘the objectively measured loss of ability to inhibit detrusor contraction even when it is provoked to contract by filling, change of posture, coughing, etc.’ More recently, overactive bladder has been redefined as the symptom-based diagnosis of ‘urgency, with or without urge incontinence usually with frequency and nocturia’ (Abrams et al 2002).
Detrusor Overactivity
The symptoms of overactive bladder are due to involuntary contractions of the detrusor muscle during the filling phase of the micturition cycle. These involuntary contractions are termed ‘detrusor overactivity’ (Abrams et al 2002) and are mediated by acetylcholine-induced stimulation of bladder muscarinic receptors (Andersson 1997). However, overactive bladder is not synonymous with detrusor overactivity, as the former is a symptom-based diagnosis whilst the latter is a urodynamic diagnosis. It has been estimated that 64% of patients with overactive bladder have urodynamically proven detrusor overactivity, and 83% of patients with detrusor overactivity have symptoms suggestive of overactive bladder (Hashim and Abrams 2006).
Incidence
Epidemiological studies have reported the overall prevalence of overactive bladder in women to be 16.9%, suggesting that there could be 17.5 million women in the USA who suffer from the condition. The prevalence increases with age, being 4.8% in women under 25 years of age and 30.9% in those over 65 years of age (Stewart et al 2001). This is supported by recent prevalence data from Europe in which 16,776 interviews were conducted in a population-based survey (Milsom et al 2001). The overall prevalence of overactive bladder in individuals aged 40 years and above was 16.6%, and this increased with age. Frequency was the most commonly reported symptom (85%), whilst 54% complained of urgency and 36% complained of urge incontinence. When considering management, 60% had consulted a physician, although only 27% were currently receiving treatment.
More recently, a further population-based survey of lower urinary tract symptoms in Canada, Germany, Italy, Sweden and the UK has reported on 19,165 men and women over the age of 18 years (Irwin et al 2006). Overall, 11.8% were found to complain of symptoms suggestive of overactive bladder and 64.3% reported at least one urinary symptom. Nocturia was the most prevalent lower urinary tract symptom, being reported by 48.6% of men and 54.5% of women.
Aetiology
During infancy, prior to toilet training, it is normal for the bladder to contract uninhibitedly at a critical volume, and overactive bladder may be the result of poorly learnt bladder control. Zoubek et al (1990) studied 46 toilet-trained children, all of whom developed isolated urinary frequency. In 40% of cases, a ‘trigger’ was identified prior to the onset of symptoms. This often involved problems at school. All cases were self-limiting or resolved following counselling or removal of the ‘trigger’. In addition, there is a strong association between childhood nocturnal enuresis and overactive bladder presenting in adult life (Whiteside and Arnold 1975).
The psychoneurotic status of women with overactive bladder has been assessed by several authors, with conflicting results. Walters et al (1990) evaluated 63 women with incontinence and 27 continent controls using formal psychometric testing. They reported no difference in the test results between women with urodynamic stress incontinence and those with overactive bladder. Women with overactive bladder scored significantly higher than controls on the hypochondriasis, depression and hysteria scales. They concluded that these abnormalities may be related to incontinence in general and not to the specific diagnosis. Norton et al (1990) psychiatrically assessed 117 women prior to urodynamic investigation. There was no increased psychiatric morbidity in women with detrusor overactivity compared with women with urodynamic stress incontinence. Interestingly, women in whom no urodynamic abnormality could be detected had the highest scores for anxiety and neuroticism. These levels were comparable with those of psychiatric outpatients.
In addition, following incontinence surgery, there is an increased incidence of detrusor overactivity (Cardozo et al 1979, Steel et al 1985, Brown and Hilton 1999) for which no specific cause has been found, but it may be due to extensive dissection around the bladder neck as it is more commonly seen after multiple previous operations. Alternatively, it may be failure to diagnose the abnormality prior to surgery or relative outflow obstruction caused by the operation itself. Outflow obstruction is rare in women and does not seem to cause detrusor overactivity in the same way that prostatic hypertrophy does in men. The increased incidence of detrusor overactivity in the elderly may be due to the onset of occult neuropathy (e.g. senile atherosclerosis or dementia).
Muscarinic Receptors
Molecular cloning studies have revealed five distinct genes for muscarinic acetylcholine receptors in rats and humans, and it has been shown that five receptor subtypes (M1–M5) correspond to these gene products (Caulfield and Birdsall 1998). In the human bladder, the occurrence of mRNA encoding M2 and M3 subtypes has been demonstrated, although not for M1 (Yamaguchi et al 1996). The M3 receptor is thought to cause a direct smooth muscle contraction (Harris et al 1995). Whilst the role of the M2 receptor has not yet been clarified, it may oppose sympathetically mediated smooth muscle relaxation (Hegde et al 1997) or result in the activation of a non-specific cationic channel and inactivation of potassium channels (Hegde and Eglen 1999). In general, it is thought that the M3 receptor is responsible for the normal micturition contraction, although in certain disease states, such as neurogenic bladder dysfunction, the M2 receptors may become more important in mediating detrusor contractions (Braverman and Ruggieri 1998).
Pathophysiology
Acetylcholine is released by the postganglionic nerves at the neuromuscular junction, and results in a coordinated detrusor contraction mediated through muscarinic receptors. However, adenosine triphosphate (ATP) also has a role (Burnstock 2001) mediated through non-adrenergic, non-cholinergic receptors (O’Reilly et al 2002).
The pathophysiology of detrusor overactivity remains unclear. In-vitro studies have shown that in cases of idiopathic detrusor overactivity, the detrusor muscle contracts more than normal. These detrusor contractions are not nerve mediated and can be inhibited by the neuropeptide vasoactive intestinal polypeptide (Kinder and Mundy 1987). Other studies have shown that increased α-adrenergic activity causes increased detrusor contractility (Eaton and Bates 1982). There is evidence to suggest that the pathophysiology of idiopathic and obstructive overactive bladder is different. From animal and human studies on obstructive overactivity, it seems that the detrusor develops postjunctional supersensitivity, possibly due to partial denervation (Sibley 1997), with reduced sensitivity to electrical stimulation of its nerve supply but greater sensitivity to stimulation with acetylcholine (Sibley 1985). If outflow obstruction is relieved, the detrusor can return to normal behaviour and reinnervation may occur (Speakman et al 1987).
Relaxation of the urethra is known to precede contraction of the detrusor in a proportion of women with detrusor overactivity (Wise et al 1993a). This may represent primary pathology in the urethra which triggers a detrusor contraction, or may merely be part of a complex sequence of events which originate elsewhere. It has been postulated that incompetence of the bladder neck, allowing passage of urine into the proximal urethra, may result in an uninhibited contraction of the detrusor. However, Sutherst and Brown (1978) were unable to provoke a detrusor contraction in 50 women by rapidly infusing saline into the posterior urethra using modified urodynamic equipment.
Brading and Turner (1994) suggest that the common feature in all cases of detrusor overactivity is partial denervation of the detrusor, which may be responsible for altering the properties of the smooth muscle, leading to increased excitability and increased ability of activity to spread between cells, resulting in coordinated myogenic contractions of the whole detrusor (Brading 1997). They dispute the concept of neurogenic detrusor overactivity (i.e. increased motor activity to the detrusor) as the underlying mechanism in detrusor overactivity, proposing that there is a fundamental abnormality at the level of the bladder wall, with evidence of altered spontaneous contractile activity consistent with increased electrical coupling of cells, a patchy denervation of the detrusor and a supersensitivity to potassium (Mills et al 2000). Charlton et al (1999) suggest that the primary defect in the idiopathic and neuropathic bladders is a loss of nerves accompanied by hypertrophy of the cells, and an increased production of elastin and collagen within the muscle fascicles.
Clinical Presentation
Symptoms and signs
Overactive bladder usually presents with a multiplicity of symptoms. Those most commonly seen are urgency, daytime frequency, nocturia, urge incontinence, stress incontinence, nocturnal enuresis and often coital incontinence. However, it is important to remember that there are numerous other causes of urgency and frequency (Box 53.1).
Iatrogenic frequency and polyuria due to numerous cups of tea or coffee and fizzy drinks should be detected by means of a frequency/volume chart. Most women who are incontinent develop voluntary frequency, initially in order to try to reduce leakage. Nocturia is also a common symptom in overactive bladder, occurring in almost 70% of cases (Cardozo and Stanton 1980). However, being woken from sleep for some other reason and voiding because one is awake does not constitute nocturia. There is an increasing incidence of nocturia with increasing age, and it is normal for women over 70 years of age to void twice during the night, and women over 80 years of age to void three times during the night.
Urge incontinence is usually preceded by urgency (a sudden compelling desire to pass urine which is difficult to defer) and is due to an involuntary detrusor contraction. However, some women are unaware of any sensation associated with their detrusor contractions, and just notice that they are wet. There seems to be a strong correlation between nocturnal enuresis, either childhood or current, and idiopathic detrusor overactivity (Whiteside and Arnold 1975). Some women complain of incontinence during sexual intercourse, and they can be broadly divided into two groups: those who leak during penetration and tend to have urodynamic stress incontinence, and those who leak at orgasm who tend to have detrusor overactivity (Hilton 1988).
Quality of Life
Quality of life (QoL) has been defined as including ‘those attributes valued by patients including their resultant comfort or sense of well being; the extent to which they were able to maintain reasonable physical, emotional, and intellectual function; the degree to which they retain their ability to participate in valued activities within the family and the community’ (Naughton and Shumaker 1996). This helps to emphasize the multidimensional nature of QoL, and the importance of considering the patient’s perception of their own situation with regard to non-health-related aspects of their life (Gill and Feinstein 1974).
QoL is assessed by the use of questionnaires completed by the patient alone or as part of the consultation, and its measurement allows the quantification of morbidity and the evaluation of treatment efficacy. It also acts as a measure of how lives are affected and coping strategies adopted. It is estimated that 20% of adult women suffer some degree of life disruption secondary to lower urinary tract dysfunction (Burgio et al 1991).
Generic questionnaires, such as the Short Form 36 (Jenkinson et al 1993), are general measures of QoL and are therefore applicable to a wide range of populations and clinical conditions, whilst disease-specific questionnaires have also been designed to focus on lower urinary tract symptoms. Generic questionnaires are not specific to a particular disease, treatment or age group and hence allow broad comparisons to be made. Consequently, they lack sensitivity when applied to women with lower urinary tract symptoms, and may be unable to detect clinically important improvement.
The King’s Health Questionnaire is a reliable, validated, disease-specific tool used to assess women with lower urinary tract dysfunction (Kelleher et al 1997a). Experience using this questionnaire has shown that incontinence impact scores were significantly worse for women with detrusor overactivity than for those with urodynamic stress incontinence, and significantly better in women with normal urodynamics.
Investigations
Frequency/volume chart
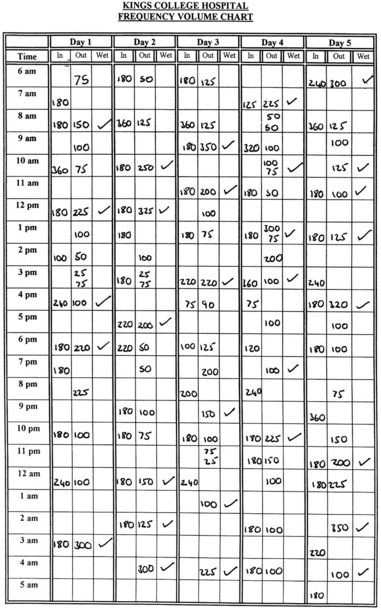
It is the authors’ practice to send all patients a frequency/volume chart with their appointment for urodynamic investigations, so that their fluid intake and voiding pattern can be evaluated. As well as the number of voids and incontinence episodes, the mean volume voided over a 24-h period can also be calculated as well as the diurnal and nocturnal volumes. They are asked to complete the chart (Figure 53.1) for 5 days, but are told that they need not measure their voided volumes when at work if this proves difficult. In addition, there is now evidence that 3- and 4-day frequency/volume charts are as accurate as 7-day diaries (Brown et al 2003). Some women find that this is a useful exercise, similar to home bladder drill.
Cystometry
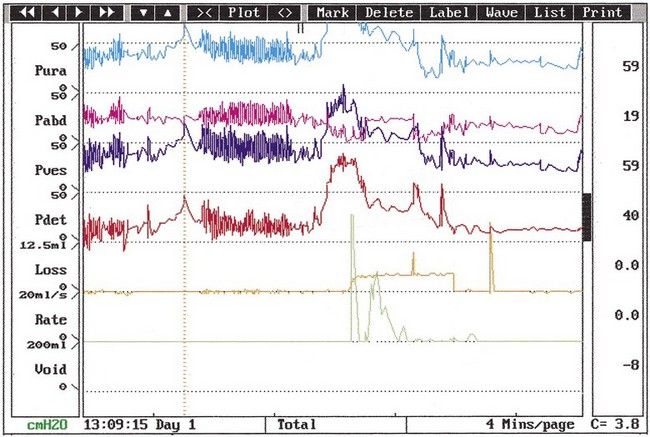
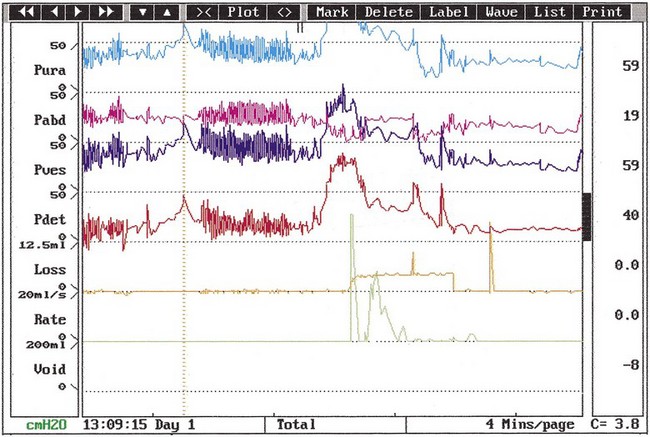
The urodynamic diagnosis of detrusor overactivity is made when detrusor contractions are seen on a cystometrogram. Detrusor overactivity is defined as ‘a urodynamic observation characterised by involuntary detrusor contractions during filling which may be spontaneous or provoked’ (Abrams et al 2002). The recorded detrusor pressure rise may take different forms on the cystometrogram trace. Most commonly, uninhibited systolic contractions occur during bladder filling (Figures 53.2 and 53.3). Not all cases of detrusor overactivity will be diagnosed on supine filling alone (Turner-Warwick 1975). Some show an abnormal detrusor pressure rise on a change of posture and may void precipitately on standing (Figure 53.4), or there may be detrusor contractions provoked by coughing which manifest as stress incontinence. Sometimes, a steep detrusor pressure rise occurs during bladder filling. This usually represents low compliance of the detrusor, but may be due to involuntary detrusor activity in some cases. It can be difficult to differentiate between systolic (phasic) detrusor overactivity and low compliance, which may coexist. Both conditions usually produce similar symptoms.
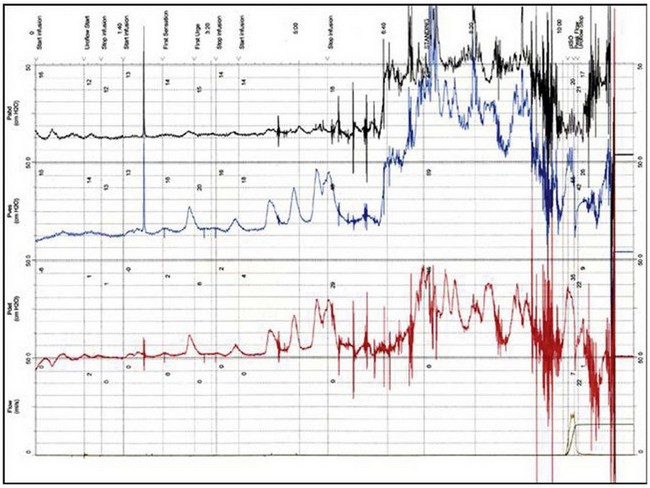
Figure 53.2 Cystometrogram showing severe systolic and provoked detrusor contractions during filling.
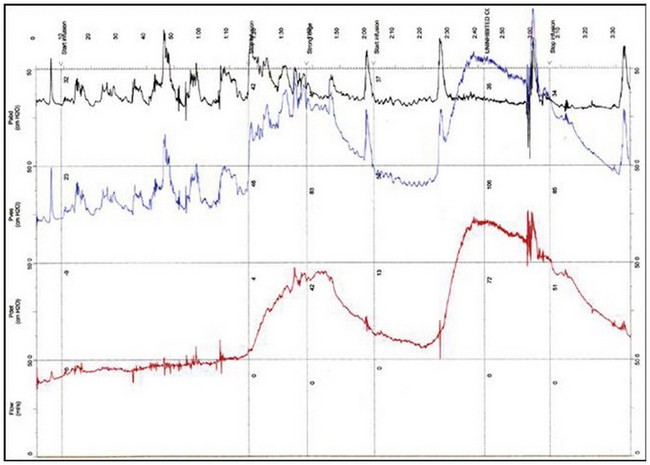
Figure 53.3 Cystometrogram showing neurogenic detrusor overactivity during filling in a patient with multiple sclerosis.
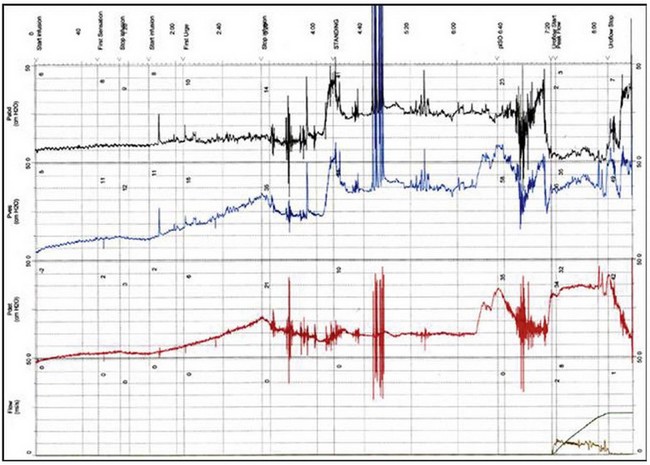
Figure 53.4 Cystometrogram showing low compliance with a high isometric contraction (Piso) during bladder filling.
During the cystometrogram, it is important to ask the patient about her symptoms and relate them to the recorded changes. Most patients will complain of urgency when a detrusor contraction occurs, or urge incontinence if the detrusor pressure exceeds the urethral pressure. Thus, in order to diagnose or exclude detrusor overactivity, subtracted provocative cystometry must be employed. Other common, although not universal, features of the cystometrogram in women with detrusor overactivity are early first sensation, small bladder capacity and inability/or difficulty in interrupting the urinary stream. The latter may be associated with a high isometric detrusor contraction (Figure 53.4) or, if videocystourethrography is performed, slow or absent ‘milk-back’ of contrast medium from the proximal urethra into the bladder.
Ambulatory urodynamics
There are three main components to an ambulatory urodynamic system: the transducers, the recording unit and the analysing system. The transducers are solid state and are mounted on 5 French and 7 French bladder and rectal catheters. It is the authors’ practice to use two bladder transducers in order to reduce artifact. The recording system should be portable in order to allow freedom of movement, with a digital memory aiding compression and expansion of the traces which are obtained. An event marker is attached to the recording unit allowing the patient to mark episodes of urgency and also to document voids. In addition, the recording unit is attached to an electronic (Urilos) pad to document episodes of leakage during the study, and should have the facility to attach to a flow meter in order to record pressure flow voiding studies. The ambulatory protocol at Kings College Hospital consists of a 4-h period during which time the patient is asked to drink 200 ml of fluid every 30 min and also to keep a diary of events and symptoms. On completion of the test, the trace is analysed with the patient using a personal computer and the urinary diary. Detrusor overactivity should only be diagnosed if there is a detrusor contraction noted on both bladder lines in the presence of symptoms (Figure 53.5).
The clinical usefulness of ambulatory urodynamics is limited by the high prevalence of abnormal detrusor (38–69%) contractions in asymptomatic volunteers (van Waalwijk van Doorn et al 1992, Robertson et al 1994, Heslington and Hilton 1996). However, the diagnosis of detrusor overactivity is highly dependent on interpretation of the results; in a prospective study of 26 asymptomatic women, the incidence of detrusor overactivity varied from 11.5% to 76.9% depending on the criteria used (Salvatore et al 1998, 2001). If the criteria for defining abnormal detrusor contractions are a simultaneous pressure rise on both bladder lines in addition to patient-reported symptoms of urgency or urge incontinence, the findings are normal in 90% of women; this is similar to that reported in laboratory urodynamics. In order to improve the diagnostic discrimination of ambulatory urodynamic studies, a standardization document has been published (van Waalwijk van Doorn et al 2000).
Videocystourethrography
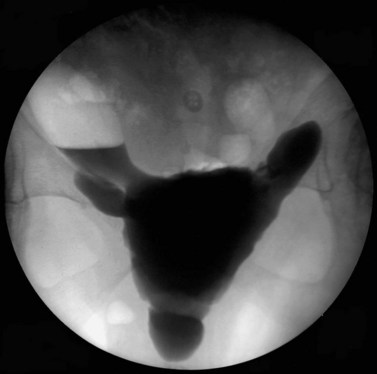
Additional information may be acquired by undertaking videocystourethrography with pressure and flow studies, rather than subtracted cystometry, and this may also increase the diagnostic accuracy. Bladder diverticulae (Figure 53.6) and trabeculation (Figure 53.7) may be observed during videocystourethrography, and vesicoureteric reflux may be observed when severe detrusor overactivity has caused upper urinary tract damage.
Treatment
Historically, other types of treatment which have been tried include vaginal denervation (Hodgkinson and Drukker 1977, Warrell 1977, Ingleman-Sundberg 1978), caecocystoplasty, selective sacral neurectomy (Torrens and Griffiths 1974), cystodistension (Ramsden et al 1976, Higson et al 1978, Pengelly et al 1978) and bladder transection (Mundy 1983). All give some short-term benefit in carefully selected cases, but may produce significant morbidity. None have stood the test of time.
General management
All incontinent women benefit from advice regarding simple measures which they can take to help alleviate their symptoms. Many patients drink too much and they should be told to reduce their fluid intake to between 1 and 1.5 l/day (Swithinbank et al 2005), and to avoid tea, coffee and alcohol if these exacerbate their problem. In addition, there is also increasing evidence to suggest that weight loss may improve symptoms of urinary incontinence (Subak et al 2009). The use of drugs which affect bladder function, such as diuretics and α-adrenreceptor antagonists, should be reviewed and stopped if possible. If there is coexistent urodynamic stress incontinence, pelvic floor exercises may also be helpful.
It is usually preferable, in cases of mixed incontinence, to treat the overactive bladder prior to resorting to surgery for urethral sphincter incompetence. Such treatment may obviate the need for surgery (Karram and Bhatia 1989). In addition, there is always the risk that the incontinence operation may exacerbate the symptoms of overactive bladder.
Pharmacology
Drug therapy has an important role in the management of women with urinary symptoms caused by overactive bladder, although there are no drugs which act specifically on the bladder and urethra and do not have systemic effects. The large number of drugs available is indicative of the fact that none are ideal, and it is often their systemic adverse effects which limit their use in terms of efficacy and compliance (Kelleher et al 1997b). The pharmacology of drugs and recommendations for usage have been reviewed recently by the 4th International Consultation on Incontinence (Andersson et al 2009) (Table 53.1).
Table 53.1 Drugs used in the treatment of overactive bladder
| Level of evidence* | Grade of recommendation# | |
|---|---|---|
| Antimuscarinic drugs | ||
| Tolterodine | 1 | A |
| Trospium | 1 | A |
| Solifenacin | 1 | A |
| Darifenacin | 1 | A |
| Fesoterodine | 1 | A |
| Propantheline | 2 | B |
| Atropine, hyoscamine | 3 | C |
| Drugs acting on membrane channels | ||
| Calcium-channel antagonists | 2 | D |
| Potassium-channel openers | 2 | D |
| Drugs with mixed actions | ||
| Oxybutynin | 1 | A |
| Propiverine | 1 | A |
| Flavoxate | 2 | D |
| α Antagonists | ||
| Alfuzosin | 3 | C |
| Doxazosin | 3 | C |
| Prazosin | 3 | C |
| Terazosin | 3 | C |
| Tamsulosin | 3 | C |
| β Agonists | ||
| Terbutaline | 3 | C |
| Salbutamol | 3 | C |
| Antidepressants | ||
| Imipramine | 3 | C |
| Duloxetine | 2 | C |
| Prostaglandin synthesis inhibitors | ||
| Indomethacin | 2 | C |
| Flurbiprofen | 2 | C |
| Vasopressin analogues | ||
| Desmopressin | 1 | A |
| Other drugs | ||
| Baclofen | 3 | C (intrathecal) |
| Capsaicin | 2 | C (intravesical) |
| Resiniferatoxin | 2 | C (intravesical) |
| Botulinum toxin (idiopathic) | 3 | B (intravesical) |
| Botulinum toxin (neurogenic) | 2 | A (intravesical) |
Source: Andersson KE, Chapple CR, Cardozo L et al 2009 Pharmacological treatment of urinary incontinence. In: Abrams P, Cardozo L, Khoury S, Wein A (eds) Incontinence, 4th edn. Health Publication Ltd, Paris, pp 631–700.
Antimuscarinic drugs
Tolterodine
Tolterodine is a competitive muscarinic receptor antagonist with relative functional selectivity for bladder muscarinic receptors (Ruscin and Morgenstern 1999). Whilst it shows no specificity for receptor subtypes, it does target the muscarinic receptors in the bladder rather than those in the salivary glands (Nilvebrant et al 1997). Several randomized, double-blind, placebo-controlled trials have demonstrated a significant reduction in incontinent episodes and micturition frequency (Jonas et al 1997, Hills et al 1998, Millard et al 1999), whilst the incidence of adverse effects has been shown to be no different to placebo (Rentzhog et al 1998). When compared with oxybutynin in a randomized, double-blind, placebo-controlled, parallel-group study, it was found to be equally efficacious and to have a lower incidence of side-effects, notably dry mouth (Abrams et al 1998). A pooled analysis of the safety, efficacy and acceptability of tolterodine in 1120 patients in four randomized, double-blind, parallel, multicentre trials found that both tolterodine and oxybutynin decreased incontinent episodes significantly, although tolterodine was associated with fewer adverse events, dose reductions and patient withdrawals than oxybutynin (Appell 1997).
Tolterodine has also been developed as an extended-release, once-daily preparation. A double-blind, multicentre trial of 1235 women compared extended-release tolterodine with immediate-release tolterodine and placebo. Whilst both formulations were found to reduce the mean number of urge incontinence episodes per week, the extended-release preparation was found to be significantly more effective (Swift et al 2003). In addition to increased efficacy, extended-release tolterodine has been shown to have better tolerability. In a double-blind, multicentre, randomized placebo-controlled trial of 1529 patients, extended-release tolterodine was found to be 18% more effective in the reduction of episodes of urge incontinence, whilst having a 23% lower incidence of dry mouth (van Kerrebroeck et al 2001).
Extended-release oxybutynin and extended-release tolterodine were compared in the OPERA (Overactive bladder: Performance of Extended Release Agents) study, which involved 71 centres in the USA. Improvements in episodes of urge incontinence were similar for the two drugs, although extended-release oxybutynin was significantly more effective than extended-release tolterodine in reducing the frequency of micturition. Significantly more women taking oxybutynin were also completely dry (23% vs 16.8%; P = 0.03), although dry mouth was significantly more common in the oxybutynin group (Diokno et al 2003).
Trospium chloride
Trospium chloride is a quaternary ammonium compound which is non-selective for muscarinic receptor subtypes and shows low biological availability (Schladitz-Keil et al 1986). It crosses the blood–brain barrier to a limited extent and hence appears to have few cognitive effects (Fusgen and Hauri 2000). A placebo-controlled, randomized, double-blind, multicentre trial has shown trospium to increase cystometric capacity and bladder volume at the first unstable contraction, leading to significant clinical improvement without an increase in adverse effects over placebo (Cardozo et al 2000). When compared with oxybutynin, it has been found to have comparable efficacy but was associated with a lower incidence of dry mouth (4% vs 23%) and patient withdrawal (6% vs 16%) (Madersbacher et al 1995). At present, trospium chloride appears to be as effective as oxybutynin and may be associated with fewer adverse effects. More recently, an extended-release preparation (Dmochowski et al 2008) has been introduced in the USA and is soon to be licensed in the UK.
Solifenacin
The clinical efficacy of solifenacin has been assessed in a multicentre, randomized, double-blind, parallel group, placebo-controlled study of solifenacin 5 mg and 10 mg once daily in patients with overactive bladder (Cardozo et al 2004a). The primary efficacy analysis showed a statistically significant reduction of micturition frequency following treatment with both 5-mg and 10-mg doses compared with placebo, although the largest effect was with the higher dose. In addition, solifenacin was found to be superior to placebo with respect to the secondary efficacy variables of mean volume voided per micturition, episodes of urgency per 24 h, number of incontinence episodes and episodes of urge incontinence. The most frequently reported adverse events leading to discontinuation were dry mouth and constipation. These were also found to be dose related. In order to assess the long-term safety and efficacy of solifenacin (5 mg and 10 mg once daily), a multicentre, open-label, long-term follow-up study has been reported. This was essentially an extension of two previous double-blind, placebo-controlled studies in 1637 patients (Haab et al 2005). Overall, the efficacy of solifenacin was maintained in the extension study with a sustained improvement in symptoms of urgency, urge incontinence, frequency and nocturia over the 12-month study period. The most commonly reported adverse events were dry mouth (20.5%), constipation (9.2%) and blurred vision (6.6%), and these were the primary reason for discontinuation in 4.7% of patients.
More recently, solifenacin 5 mg and 10 mg od have been compared with extended-release tolterodine 4 mg od (Chapple et al 2005). This was a prospective, double-blind, double-dummy, two-arm, parallel-group, 12-week study of 1200 patients with the primary aim of demonstrating non-inferiority of solifenacin to extended-release tolterodine. Solifenacin was not inferior to extended-release tolterodine with respect to change from baseline in the mean number of micturitions per 24 h (reduction of 2.45 micturitions/24 h vs 2.24 micturitions/24 h; P = 0.004). In addition, solifenacin resulted in a statistically significant improvement in urgency (P = 0.035), urge incontinence (P = 0.001) and overall incontinence compared with extended-release tolterodine. In addition, 59% of solifenacin-treated patients who were incontinent at baseline became continent by the study endpoint compared with 49% of patients on extended-release tolterodine (P = 0.006).
Darifenacin
Darifenacin is a tertiary amine with moderate lipophilicity, and is a highly selective M3 receptor antagonist which has been found to have a 5-fold higher affinity for the human M3 receptor relative to the M1 receptor (Alabaster 1997).
A review of the pooled darifenacin data from the three Phase III, multicentre, double-blind clinical trials in patients with overactive bladder has been reported in 1059 patients (Chapple et al 2004a). Darifenacin resulted in a dose-related significant reduction in median number of incontinence episodes per week. Significant decreases in the frequency and severity of urgency, micturition frequency and number of incontinence episodes resulting in a change of clothing or pads were also apparent, along with an increase in bladder capacity. Darifenacin was well tolerated. The most common treatment-related adverse events were dry mouth and constipation, although these resulted in few discontinuations. The incidence of central nervous system and cardiovascular adverse events were comparable with placebo.
Fesoterodine
Fesoterodine is a new and novel derivative of 3,3-diphenylpropyl-amine, which is a potent antimuscarinic agent that has recently been developed for the management of overactive bladder. A Phase II dose-finding study was conducted in 728 patients (Chapple 2004b). Fesoterodine 4 mg, 8 mg and 12 mg were all found to show significantly greater decreases in micturition frequency than placebo. The most commonly reported side-effect was dry mouth, with an incidence of 25% in the 4-mg group rising to 34% in the 12-mg group. Subsequently, a Phase III randomized placebo-controlled trial has been reported comparing fesoterodine 4 mg and 8 mg with extended-release tolterodine 4 mg in 1135 patients complaining of overactive bladder (Chapple et al 2006a, 2008). Both doses of fesoterodine demonstrated significant improvements over placebo in reduction of daytime frequency and number of urge incontinence episodes per day, and were found to be superior to tolterodine. The current evidence suggests that fesoterodine may offer some advantages over tolterodine in terms of efficacy and flexible dosing regimens.
Drugs that have a mixed action
Oxybutynin
Oxybutynin is a tertiary amine that undergoes extensive first-pass metabolism to an active metabolite, N-desmethyl oxybutynin (Waldeck et al 1997), which occurs in high concentrations (Hughes et al 1992) and is thought to be responsible for a significant part of the action of the parent drug. It has a mixed action consisting of both an antimuscarinic and a direct muscle relaxant effect in addition to local anaesthetic properties. The latter is important when given intravesically but probably has no effect when given systemically. Oxybutynin has been shown to have a high affinity for muscarinic receptors in the bladder (Nilvebrant et al 1985), and has a higher affinity for M1 and M3 receptors than M2 receptors (Nilvebrant and Sparf 1986).
The effectiveness of oxybutynin in the management of patients with detrusor overactivity is well documented. A double-blind, placebo-controlled trial found oxybutynin to be significantly better than placebo in improving lower urinary tract symptoms, although 80% of patients complained of significant adverse effects, principally dry mouth or dry skin (Cardozo et al 1987). The antimuscarinic adverse effects of oxybutynin are well documented and are often dose limiting (Baigrie et al 1988), with 10–23% of women discontinuing medication (Kelleher et al 1994a). Using an intravesical route of administration, higher local levels of oxybutynin can be achieved whilst limiting the systemic adverse effects. Using this method, oxybutynin has been shown to increase bladder capacity and lead to a significant clinical improvement (Weese et al 1993). Intravesical administration of oxybutynin is an effective and useful alternative for patients with neurogenic detrusor overactivity who need to self-catheterize or who suffer from ‘bypassing’ an indwelling catheter (Madersbacher and Jilg 1991). Rectal administration has also been shown to be associated with fewer adverse effects compared with oral administration (Collas and Malone-Lee 1997).
A controlled-release oxybutynin preparation using an osmotic system has been developed which has been shown to have comparable efficacy when compared with immediate-release oxybutynin, and which is associated with fewer adverse effects (Anderson et al 1999). In order to maximize efficacy and minimize adverse effects, alternative delivery systems are currently under evaluation. An oxybutynin transdermal delivery system has been developed and compared with extended-release tolterodine in 361 patients with mixed urinary incontinence. Both agents significantly reduced incontinence episodes, increased volume voided and led to an improvement in QoL compared with placebo. The most common adverse event in the oxybutynin arm was application site pruritus (14%), although the incidence of dry mouth was reduced to 4.1% compared with 7.3% in the tolterodine arm (Dmochowski et al 2003).
More recently, a large prospective, multicentre, randomized, double-blind, placebo-controlled study has been reported investigating the use of oxybutynin gel in the management of overactive bladder in 704 patients (Staskin et al 2009). Overall, there was a significant reduction in urge incontinence episodes in the gel arm compared with the placebo arm (−3.0 vs −2.5/day; P<0.0001), a significant reduction in daytime frequency (−2.7, P = 0.0017) and an increase in volume voided. When considering adverse events, dry mouth was more common in the treatment arm compared with the placebo arm (6.9% vs 2.8%), and skin site reactions were infrequent in both arms (5.4% and 1.0%, respectively). Consequently, oxybutynin gel may represent an important development over the oxybutynin patch in terms of patient acceptability.
Propiverine
Propiverine has both antimuscarinic and calcium-channel-blocking actions (Haruno et al 1989). Open studies have demonstrated a beneficial effect in patients with overactive bladder (Mazur et al 1995) and neurogenic detrusor overactivity (Stoher et al 1999). Dry mouth was experienced by 37% in the treatment group as opposed to 8% in those taking placebo, with dropout rates of 7% and 4.5%, respectively. Propiverine has also been compared with oxybutynin in the management of neurogenic detrusor overactivity in children and adolescents (Madersbacher et al 2009). Overall, propiverine was found to have comparable efficacy to oxybutynin but was better tolerated in terms of adverse effects.
The efficacy and cardiac safety of propiverine has been assessed in a double-blind, multicentre, placebo-controlled, randomized study in 98 elderly patients suffering from urgency, urge incontinence or mixed urge/stress incontinence. After a 2-week placebo run-in period, the patients received propiverine (15 mg tds) or placebo (tds) for a 4-week period. Propiverine caused a significant reduction in micturition frequency and a significant decrease in episodes of incontinence. Only 2% of patients complained of dry mouth, and resting and ambulatory electrocardiograms showed no significant changes (Dorschner et al 2000).
More recently, extended-release propiverine has been introduced and been shown to be as effective as the immediate-release preparation in the management of overactive bladder (Jünemann et al 2006).
Tricyclic antidepressants
These drugs have a complex pharmacological action. Imipramine has antimuscarinic, antihistaminic and local anaesthetic properties. It may increase outlet resistance by peripheral blockage of noradrenaline uptake and it also acts as a sedative. The side-effects are antimuscarinic, together with tremor and fatigue. Imipramine is particularly useful for the treatment of nocturia and nocturnal enuresis (Castleden et al 1981), although other studies have reported little effect (Diokno et al 1972). Other tricyclic antidepressants such as amitriptyline may be substituted for imipramine if specific side-effects are a problem, whilst doxepin has been found to be more potent in its musculotropic relaxant and antimuscarinic activity (Lose et al 1989). A tricyclic antidepressant given prophylactically before sexual intercourse may be of benefit to patients with coital incontinence at orgasm (Cardozo 1988).
Calcium-channel antagonists
Contractile activity in the bladder smooth muscle is activated by the movement of extracellular calcium into the cell. Spontaneous and evoked contractile activity are mediated by membrane depolarization and the movement of calcium into the smooth muscle cell through L-type Ca2+ channels (Brading 1997). Inhibition of the entrance of Ca2+ can prevent spontaneous and evoked contractile activity (Levin et al 1991) with L-type Ca2+ blocking agents, such as nifedipine, inhibiting the entry of extracellular calcium.
Potassium-channel-opening drugs
The opening of K+ ion channels in the membrane of the detrusor muscle cell leads to an increase in K+ movement out of the cell, resulting in membrane hyperpolarization (Andersson 1997). This reduces the opening probability of ion channels involved in membrane depolarization and hence excitability is reduced (Andersson 1992). These agents act during the filling phase and, whilst abolishing spontaneous detrusor contractions, are not thought to affect normal bladder contractions. Their clinical usefulness is limited by significant cardiovascular effects, with cromakalin and pinacidil being found to be up to 200 times more potent as inhibitors of vascular preparations than of detrusor muscle (Edwards et al 1991). At present, none are commercially available, although the development of subtype-selective drugs may lead to a role in the management of overactive bladder (Lawson 1996).
More recently, newer drugs with KATP-channel-opening properties have been described (Andersson and Arner 2004). These may be useful for the treatment of bladder overactivity, although there is no evidence at present to suggest that potassium channel openers represent a viable treatment alternative. This is supported by a recently reported randomized, double-blind, placebo-controlled Phase II study evaluating the efficacy and safety of a novel ATP-sensitive potassium channel opener in 299 patients with detrusor overactivity. Whilst treatment was safe and well tolerated, there was no difference over placebo in mean volume voided per micturition and micturition frequency (Chapple et al 2006b).
Antidiuretic agents
Desmopressin
Synthetic vasopressin (desmopressin) has been shown to reduce nocturnal urine production by up to 50%. It can be used for children or adults with nocturia or nocturnal enuresis (Norgaard et al 1989), but must be avoided in patients with hypertension, ischaemic heart disease or congestive cardiac failure (Hilton and Stanton 1982). There is good evidence to show that it is safe to use in the long term (Knudsen et al 1989, Rew and Rundle 1989), and it may be given orally or as a buccal preparation.
Desmopressin has also been used as a ‘designer drug’ for daytime incontinence (Robinson et al 2004), and also in the treatment of overactive bladder (Hashim et al 2009).
Desmopressin is safe for long-term use; however, the drug should be used with care in the elderly due to the risk of hyponatraemia. The current recommendations are that serum sodium should be checked in the first week following the start of treatment. To identify the safety of desmopressin, a recent systematic review and meta-analysis has been performed of cohort studies and randomized controlled trials using the oral and intranasal preparations in the treatment of nocturia. In total, 75 papers were identified of which seven reported the incidence of hyponatraemia, giving an overall pooled estimate of 7.6% (95% confidence interval 3.7–15.1). The authors concluded that hyponatraemia is a relatively common adverse event associated with the use of desmopressin, and regular monitoring should be used, particularly in the elderly (Weatherall 2004)
Prostaglandin synthetase inhibitors
Bladder mucosa has been shown to have the ability to synthesize eicosanoids (Jeremy et al 1987), although it is uncertain whether they contribute to the pathogenesis of overactive detrusor contractions. However, they may have a role in sensitizing sensory afferent nerves, increasing the afferent input produced by a given bladder volume. A double-blind controlled study of flurbiprofen in women with detrusor overactivity was shown to have an effect, although it was associated with a high incidence of adverse effects (43%) including nausea, vomiting, headache and gastrointestinal symptoms (Cardozo et al 1980). Indomethacin has also been reported to give symptomatic relief, although the incidence of adverse effects was also high (59%) (Cardozo et al 1980). At present, this evidence does not support the use of prostaglandin synthetase inhibitors in detrusor overactivity.
α-Adrenoreceptor antagonists
The adrenergic receptors found at the bladder neck are α1 adrenergic, and three subtypes have been identified: α1A, α1B and α1D (Malloy et al 1998). Those receptors present in the bladder are predominantly α1A and α1D, whilst α1B receptors are found in the vasculature and are involved in blood pressure control. Consequently, α1-adrenergic blocking agents with subselectivity for α1A and α1D may be most useful in the management of lower urinary tract dysfunction, and it has been speculated that the α1D receptor may mediate the overactive symptoms of overactive bladder while the α1A receptor mediates the obstructive symptoms (Schwinn 2000). Anecdotal evidence has demonstrated that tamsulosin, a selective α1-adrenoceptor antagonist, may improve urinary symptoms secondary to detrusor overactivity in men, and it has been used ‘off label’ in women with symptoms of overactive bladder for some time.
A randomized, double-blind, placebo-controlled study evaluating the efficacy of tamsulosin in the management of women complaining of overactive bladder has been reported recently (Robinson et al 2007). Overall, 364 women (53.3% treatment naïve) were randomized to receive one of four doses of tamsulosin (0.25 mg, 0.5 mg, 1.0 mg or 1.5 mg), extended-release tolterodine 4 mg or placebo for 6 weeks. The primary efficacy analysis showed that the difference from placebo in the mean number of micturitions/24 h was not statistically significant for tamsulosin 1.5 mg (P = 0.189). Interestingly, no statistically significant difference was observed for extended-release tolterodine 4 mg (P = 0.353). Similarly, when considering the secondary outcome parameters, there was no statistically significant difference between tamsulosin and placebo. Although women taking extended-release tolterodine 4 mg od demonstrated a consistently greater increase in mean volume voided and consistent decreases in incontinence episodes/24 h, urgency episodes/24 h and episodes of nocturia/24 h, this was not statistically significant compared with placebo. In addition, there was no significant improvement in QoL scores across the treatment groups. The results from this study suggest that α-adrenoreceptor antagonists alone are not clinically useful in the management of overactive bladder in women.
Oestrogens in the Management of Overactive Bladder
Oestrogens have been used in the treatment of urinary urgency and urge incontinence for many years, although there have been few controlled trials to confirm their efficacy. A double-blind, placebo-controlled, crossover study using oral oestriol in 34 postmenopausal women produced subjective improvement in eight women with mixed incontinence and 12 women with urge incontinence (Samsicoe et al 1985). However, a double-blind, multicentre study of the use of oestriol (3 mg/day) in postmenopausal women complaining of urgency has failed to confirm these findings (Cardozo et al 1993), showing both subjective and objective improvement but not significantly better than placebo. The use of sustained-release 17β-oestradiol vaginal tablets has also been examined in postmenopausal women with urgency and urge incontinence or a urodynamic diagnosis of sensory urgency or detrusor overactivity. These vaginal tablets have been shown to be well absorbed from the vagina and to induce maturation of the vaginal epithelium within 14 days (Nilsson and Heimer 1992). However, following a 6-month course of treatment, the only significant difference between active and placebo groups was an improvement in the symptom of urgency in those women with a urodynamic diagnosis of sensory urgency (Benness et al 1992). A further double-blind, randomized, placebo-controlled trial of vaginal 17β-oestradiol vaginal tablets has shown lower urinary tract symptoms of frequency, urgency, urge and stress incontinence to be significantly improved, although no objective urodynamic assessment was performed (Eriksen and Rasmussen 1992). In both of these studies, the subjective improvement in symptoms may simply represent local oestrogenic effects reversing urogenital atrophy rather than a direct effect on bladder function.
A randomized, parallel group, controlled trial has been reported, comparing the oestradiol-releasing vaginal ring with oestriol vaginal pessaries in the treatment of postmenopausal women with bothersome lower urinary tract symptoms (Lose and Englev 2000). Low-dose vaginally administered oestradiol and oestriol were found to be equally efficacious in alleviating lower urinary tract symptoms of urge incontinence (58% vs 58%), stress incontinence (53% vs 59%) and nocturia (51% vs 54%), although the vaginal ring was found to have greater patient acceptability.
A meta-analysis of the use of oestrogen in women with symptoms of overactive bladder has been reported by the Hormones and Urogenital Therapy Committee to try and clarify the role of oestrogen therapy in the management of women with urge incontinence (Cardozo et al 2004b). In a review of 10 randomized placebo-controlled trials, oestrogen was found to be superior to placebo when considering symptoms of urge incontinence, frequency and nocturia, although vaginal oestrogen administration was found to be superior for symptoms of urgency. In those taking oestrogens, there was also a significant increase in first sensation and bladder capacity compared with placebo.
Intravesical Therapy
Capsaicin
Capsaicin is the pungent ingredient found in red chillies and is a neurotoxin of substance-P-containing (C) nerve fibres. Patients with neurogenic detrusor overactivity secondary to multiple sclerosis appear to have abnormal C fibre sensory innervation of the detrusor, which leads to premature activation of the holding reflex arc during bladder filling (Fowler et al 1992). Intravesical application of capsaicin dissolved in 30% alcohol solution can be effective for up to 6 months. The effects are variable (Chandiramani et al 1994) and the long-term safety of this treatment has not yet been evaluated.
Resiniferatoxin
Resiniferatoxin is a phorbol-related diterpene isolated from the cactus, and is a potent analogue of capsaicin that appears to have similar efficacy but fewer side-effects of pain and burning during intravesical instillation (Kim and Chancellor 2000). It is 1000 times more potent than capsaicin at stimulating bladder activity (Ishizuka et al 1995). As with capsaicin, the currently available evidence does not support the routine clinical use of the agents, although they may prove to have a role as an intravesical preparation in neurological patients with neurogenic detrusor overactivity.
Botulinum toxin
Clostridium botulinum produces its effect by production of a neurotoxin; different strains produce seven distinct serotypes designated A–G. All seven have a similar structure and molecular weight, consisting of a heavy and a light chain joined by a disulphide bond (Dolly 1997). They interfere with neural transmission by blocking the calcium-dependent release of neurotransmitter (acetylcholine), causing the affected muscle to become weak and atrophic. The affected nerves do not degenerate, but as the blockage is irreversible, only the development of new nerve terminals and synaptic contacts allows recovery of function.
The use of intravesical botulinum toxin was first described in the treatment of intractable neurogenic detrusor overactivity in 31 patients with traumatic spinal cord injury (Schurch et al 2000). Subsequently, a larger European study has reported on 231 patients with neurogenic detrusor overactivity (Reitz et al 2004). All were treated with 300 units of botulinum-A toxin which was injected cystoscopically into the detrusor muscle at 30 different sites, sparing the trigone. At 12- and 36-week follow-up, there was a significant increase in cystometric capacity and bladder compliance. Patient satisfaction was high, the majority stopped taking antimuscarinic medication and there were no significant complications. More recently, the first randomized placebo-controlled trial has been reported in 59 patients with neurogenic detrusor overactivity (Schurch et al 2005). At 6 months, there was a significant reduction in incontinence episodes in the botox group compared with the placebo group, and a corresponding improvement in QoL evaluation.
Whilst the role of botulinum toxin has been established in the treatment of neurogenic detrusor overactivity, the data regarding its use in intractable idiopathic detrusor overactivity is less robust. A prospective open-label study has recently been reported assessing the use of botulinum-A toxin in both neurogenic (300 units) and idiopathic (200 units) detrusor overactivity in 75 patients (Popat et al 2005). When considering urodynamic outcome parameters in both groups, there was a significant increase in cystometric capacity and a decrease in maximum detrusor pressure during filling in both groups. Clinically, there was also a significant reduction in frequency and episodes of urge incontinence. Interestingly, however, 69% of patients with neurogenic detrusor overactivity required self-catheterization following treatment compared with 19.3% of those with idiopathic detrusor overactivity.
At present, the evidence suggests that intravesical administration of botulinum toxin may offer an alternative to surgery in those women with intractable detrusor overactivity, although the effect is only temporary and there is little long-term data regarding the efficacy and complications associated with repeat injections (Grosse et al 2005).
Behavioural Therapy
As continence is normally learned during infancy, it is logical to suppose that it can be relearned during adult life. Bladder re-education includes bladder drill, biofeedback and hypnotherapy. Bladder discipline was first described as a method of treating urgency incontinence by Jeffcoate and Francis (1966) in the belief that it was exacerbated or even caused by underlying psychological factors. Since then, Frewen (1970) has shown that many women with overactive bladder are able to correlate the onset of their symptoms with some untoward event, which can be identified by taking a careful history. He showed that both inpatient and outpatient bladder drill can be effective forms of treatment for many such women.
The technique for performing inpatient bladder drill has been established by Jarvis (1989) and is shown below. It may be performed either in the inpatient or outpatient setting.
Technique for bladder drill
Jarvis and Millar (1980) performed a controlled trial of bladder drill in 60 consecutive incontinent women with idiopathic overactive bladder. They showed that following inpatient treatment, 90% of the bladder drill group were continent and 83.3% remained symptom-free after 6 months. In the control group, 23.2% were continent and symptom-free due to the placebo effect. Despite the excellent early results, it has been shown that up to 40% of patients relapse within 3 years (Holmes et al 1983).
Biofeedback
Biofeedback is a form of learning or re-education in which the patient is given information about a normally unconscious physiological process in the form of an auditory, visual or tactile signal. The objective effects of biofeedback in the treatment of detrusor overactivity can be recorded on a polygraph trace, but the subjective changes may be difficult to separate from the placebo effect. This technique was originally described by Cardozo et al (1978a). Thirty women aged between 16 and 65 years suffering from idiopathic overactive bladder which was resistant to conventional therapy were treated in two centres (Cardozo et al 1978b). Some 80% of the women were cured or significantly improved subjectively, and 60% were cured or improved objectively. Long-term follow-up revealed a relatively high relapse rate (Cardozo and Stanton 1984) consistent with the long-term effects of bladder drill. Other reports suggest that bladder training with or without biofeedback can be effectively used to treat incontinence due to overactive bladder (Millard and Oldenberg 1983, Burgio et al 1986). However, this type of treatment is time-consuming and requires trained personnel. At present, biofeedback is used almost exclusively to treat children with maladaptive voiding problems.
Hypnotherapy
Hypnotherapy can be used in one of two ways: either symptom removal by suggestion alone, or by attempting to help the patient to disclose hidden emotions or memories which may be pathogenic. Freeman and Baxby (1982) treated 61 women with idiopathic overactive bladder using 12 sessions of hypnosis over a period of 1 month. They achieved an overall improvement rate of over 80%, but unfortunately only nine of the women remained symptom-free at 2-year follow-up (Freeman 1989). Consequently, this type of treatment is not currently employed in the management of overactive bladder.
Acupuncture
Acupuncture is thought to act by increasing levels of endorphins and encephalins in the cerebrospinal fluid. Encephalins are known to inhibit detrusor contractility in vitro (Klarskov 1987). Naloxone, an opiate antagonist, conversely causes decreased bladder capacity and increased detrusor pressure (Murray and Feneley 1982). Several studies have shown symptomatic improvement (Philp et al 1988) or decreased frequency and urinary leakage (Pigne et al 1985) in patients with overactive bladder treated with acupuncture. Gibson et al (1990) utilized infra-red low-power laser on acupuncture points with initial success, but unfortunately there was a high relapse rate at 6 months. Acupuncture is as effective as oxybutynin in the treatment of symptoms associated with idiopathic low compliance, and it is acceptable to patients but time-consuming for the operator (Kelleher et al 1994b). Acupuncture has also been compared with placebo treatment in a randomized controlled trial of 85 women (Emmons and Otto 2005). Overall, there was a greater reduction in incontinence episode frequency in the acupuncture arm compared with the placebo arm (59% vs 40%; P > 0.05), with a significant reduction in frequency (14%; P = 0.013) and improvement in QoL (P < 0.001).
All forms of bladder retraining in the treatment of overactive bladder are advantageous because there are few unpleasant side-effects and no patient is ever made worse. Mild-to-moderate overactive bladder can be cured or improved significantly by re-educating the bladder. However, the relapse rate is very high, and although this type of treatment avoids the morbidity associated with surgery and the side-effects of drug therapy, it requires skilled personnel and is time-consuming for both the patient and the operator. A meta-analysis has concluded that bladder retraining is more effective than placebo and medical therapy, although there is insufficient evidence to support the effectiveness of electrical stimulation and too few studies to evaluate the effect of pelvic floor exercises and biofeedback in women with urinary urge incontinence (Berghmans et al 2000).
Neuromodulation
Sacral neuromodulation
Stimulation of the dorsal sacral nerve root using a permanent implantable device in the S3 sacral foramen has been developed for use in patients with overactive bladder and neurogenic detrusor overactivity. Prior to implantation, temporary cutaneous sacral nerve stimulation is performed to check for a response; if successful, a permanent implant is inserted under general anaesthesia. Initial studies in patients with overactive bladder refractory to medical and behavioural therapy have demonstrated that after 3 years, 59% of 41 urinary urge incontinent patients showed greater than 50% reduction in incontinence episodes, with 46% of patients being completely dry (Seigel et al 2000).
Peripheral neuromodulation
Stimulation of the posterior tibial nerve in patients with urge incontinence was first reported in 1983 (McGuire et al 1983), and has also been proposed for pelvic floor dysfunction (Stoller 1999). The tibial nerve is a mixed nerve containing L4–S3 fibres and originates from the same spinal cord segments as the innervation to the bladder and pelvic floor. Consequently, peripheral neural modulation may have a role in the management of urinary symptoms.
In a prospective multicentre study, 35 patients with urge incontinence underwent 12 weekly sessions of posterior tibial nerve stimulation (PTNS), with 70% of patients reporting a greater than 50% reduction in urinary symptoms and 46% being completely cured (Vandoninick et al 2003). More recently, a prospective, randomized, multicentre North American study has been reported comparing PTNS with extended-release tolterodine 4 mg in 100 patients. Overall, there was an improvement in 75% of patients with PTNS compared with 55.8% with extended-release tolterodine, and there was a significant improvement in QoL in both groups (Peters et al 2008).
Surgery
Clam cystoplasty
In clam cystoplasty (Bramble 1990, Mast et al 1995), the bladder is bisected almost completely and a patch of gut (usually ileum) equal in length to the circumference of the bisected bladder (approximately 25 cm) is sewn in place. This often cures the symptoms of overactive bladder (McRae et al 1987) by converting a high-pressure system into a low-pressure system, although inefficient voiding may result. Patients have to learn to strain to void or may have to resort to clean intermittent self-catheterization, sometimes permanently. In addition, mucus retention in the bladder may be a problem, but this can be partially overcome by ingestion of 200 ml of cranberry juice each day (Rosenbaum et al 1989) in addition to intravesical mucolytics such as acetylcysteine. The chronic exposure of the ileal mucosa to urine may lead to malignant change (Harzmann and Weckerman 1992). There is a 5% risk of adenocarcinoma arising in ureterosigmoidostomies, where colonic mucosa is exposed to N-nitrosamines found in both urine and faeces, and a similar risk may apply to enterocystoplasty. Biopsies of the ileal segment taken from patients with clam cystoplasties show evidence of chronic inflammation of villous atrophy (Nurse and Mundy 1987), and diarrhoea due to disruption of the bile acid cycle is common (Barrington et al 1995). This may be treated using cholestyramine. In addition, metabolic disturbances such as hyperchloraemic acidosis, B12 deficiency and, occasionally, osteoporosis secondary to decreased bone mineralization may occur.
Detrusor myectomy
Detrusor myectomy offers an alternative to clam cystoplasty by increasing functional bladder capacity without the complications of bowel interposition. In this procedure, the whole thickness of the detrusor muscle is excised from the dome of the bladder, thereby creating a large bladder diverticulum with no intrinsic contractility (Cartwright and Snow 1989). Whilst there is a reduction in episodes of incontinence, there is little improvement in functional capacity and thus frequency remains problematic (Kennelly et al 1994, Snow and Cartwright 1996).
National Institute for Health and Clinical Excellence Guidelines
The medical management of overactive bladder has been reviewed recently by the National Institute for Health and Clinical Excellence (2006). In the first instance, bladder retraining lasting for a minimum of 6 weeks should be offered to all women with mixed or urge incontinence. In those women who do not achieve satisfactory benefit from bladder retraining alone, an antimuscarinic agent in addition to bladder retraining should be considered.
Conclusion
KEY POINTS
Appendix
Levels of evidence
Grades of recommendations
Sources: Hadom DC, Baker D, Hodges JS, Hicks N 1996 Journal of Clinical Epidemiology 49: 749–754. Harbour R, Miller J 2001 BMJ (Clinical Research Ed) 323: 334–336.
Abrams P, Freeman R, Anderstrom C, Mattiasson A. Tolterodine, a new antimuscarinic agent: as effective but better tolerated than oxybutynin in patients with an overactive bladder. British Journal of Urology. 1998;81:801-810.
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function. Report from the Standardisation Committee of the International Continence Society. Neurourology and Urodynamics. 2002;21:167-178.
Alabaster VA. Discovery and development of selective M3 antagonists for clinical use. Life Science. 1997;60:1053-1060.
Anderson RU, Mobley D, Blank B, Saltzstein D, Susset J, Brown JS. Once daily controlled versus immediate release oxybutynin chloride for urge urinary incontinence. OROS Oxybutynin Study Group. Journal of Urology. 1999;161:1809-1812.
Andersson KE. Clinical pharmacology of potassium channel openers. Pharmacology and Toxicology. 1992;70:244-245.
Andersson KE. The overactive bladder: pharmacologic basis of drug treatment. Urology. 1997;50:74-89.
Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiological Reviews. 2004;84:935-986.
Andersson KE, Chapple CR, Cardozo L, et al. Pharmacological treatment of urinary incontinence. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. 4th edn. Paris: Health Publication Ltd; 2009:631-700.
Appell RA. Clinical efficacy and safety of tolterodine in the treatment of overactive bladder: a pooled analysis. Urology. 1997;50:90-96.
Baigrie RJ, Kelleher JP, Fawcett DP, Pengelly AW. Oxybutynin: is it safe? British Journal of Urology. 1988;62:319-322.
Barrington JW, Fern Davies H, Adams RJ, Evans WD, Woodcock JP, Stephenson TP. Bile acid dysfunction after clam enterocystoplasty. British Journal of Urology. 1995;76:169-171.
Bates CP, Whiteside CG, Turner-Warwick RT. Synchronous cine-pressure-flow cystourethrography with special reference to stress and urge incontinence. British Journal of Urology. 1970;50:714-723.
Benness C, Wise BG, Cutner A, Cardozo LD. Does low dose vaginal oestradiol improve frequency and urgency in postmenopausal women. International Urogynecology Journal. 1992;3:281.
Berghmans LC, Hendricks HJ, de Bie RA, van Waalwijk van Doorn ES, Bo K, van Kerrebroeck PE. Conservative treatment of urge urinary incontinence in women: a systematic review of randomized clinical trials. BJU International. 2000;85:254-263.
Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50:57-67.
Brading AF, Turner WH. The unstable bladder: towards a common mechanism. British Journal of Urology. 1994;73:3-8.
Bramble FJ. The clam cystoplasty. British Journal of Urology. 1990;66:337-341.
Braverman AS, Ruggieri MR. The M2 receptor contributes to contraction of the denervated rat urinary bladder. American Journal of Physiology. 1998;275:1654.
Brown JS, McNaughton KS, Wyman JF, et al. Measurement characteristics of a voiding diary for use by men and women with overactive bladder. Urology. 2003;61:802-809.
Brown K, Hilton P. The incidence of overactive bladder before and after colposuspension: a study using conventional and ambulatory urodynamic monitoring. BJU International. 1999;84:961-965.
Burgio KL, Robinson JC, Engel BT. The role of biofeedback in Kegel exercise training for stress incontinence. American Journal of Obstetrics and Gynecology. 1986;154:64-88.
Burgio KL, Matthews KA, Engel BT. Prevalence, incidence and correlates of urinary incontinence in healthy, middle-aged women. Journal of Urology. 1991;146:1255-1259.
Burnstock G. Purinergic signaling in lower urinary tract. In: Abbracchio MP, Williams M, editors. Purinergic and Pyrimidinergic Signalling I: Molecular, Nervous and Urogenitary System Function. Berlin: Springer; 2001:423-515. 151
Cardozo LD. Sex and the bladder. BMJ (Clinical Research Ed.). 1988;296:587-588.
Cardozo LD, Stanton SL, Allan V. Biofeedback in the treatment of overactive bladder. British Journal of Urology. 1978;50:250-254.
Cardozo LD, Abrams PH, Stanton SL, Feneley RCL. Idiopathic overactive bladder treated by biofeedback. British Journal of Urology. 1978;50:521-523.
Cardozo LD, Stanton SL, Williams JE. Detrusor instability following surgery for urodynamic instability. British Journal of Urology. 1979;51:204-207.
Cardozo LD, Stanton SL. Genuine stress incontinence and overactive bladder: a review of 200 cases. BJOG: an International Journal of Obstetrics and Gynaecology. 1980;87:184-190.
Cardozo LD, Stanton SL, Robinson H, Hole D. Evaluation on flurbiprofen in detrusor instability. BMJ (Clinical Research Ed.). 1980;280:281-282.
Cardozo LD, Stanton SL. Biofeedback: a five year review. British Journal of Urology. 1984;56:220.
Cardozo LD, Cooper D, Versi E. Oxybutynin chloride in the management of idiopathic overactive bladder. Neurourology and Urodynamics. 1987;6:256-257.
Cardozo LD, Rekers H, Tapp A, et al. Oestriol in the treatment of postmenopausal urgency: a multicentre study. Maturitas. 1993;18:47-53.
Cardozo LD, Chapple CR, Toozs-Hobson P, et al. Efficacy of trospium chloride in patients with overactive bladder: a placebo-controlled, randomized, double-blind, multicentre clinical trial. BJU International. 2000;85:659-664.
Cardozo L, Lisec M, Millard R, et al. Randomised, double blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. Journal of Urology. 2004;172:1919-1924.
Cardozo L, Lose G, McClish D, Versi E. A systematic review of the effects of oestrogens for symptoms suggestive of overactive bladder. Acta Obstetricia et Gynecologica Scandinavica. 2004;83:892-897.
Cartwright PC, Snow BW. Bladder autoaugmentation: partial detrusor excision to augment the bladder without use of bowel. Journal of Urology. 1989;142:1050-1053.
Castleden CM, George CF, Renwick AG, Asher MJ. Imipramine — a possible alternative to current therapy for urinary incontinence in the elderly. Journal of Urology. 1981;125:318-320.
Caulfield MP, Birdsall NJ. International Union of Pharmacology XVII. Classification of muscarinic acetylcholine receptors. Pharmacological Reviews. 1998;50:279.
Chandiramani VA, Peterson T, Beck RO, Fowler CJ. Lessons learnt from 44 intravesical instillations of capsaicin. Neurourology and Urodynamics. 1994;13:348-349.
Chapple CR. Darifenacin is well tolerated and provides significant improvement in the symptoms of overactive bladder: a pooled analysis of phase III studies. European Urology. 2004;171(Suppl):130.
Chapple C. Fesoterodine: a new effective and well tolerated antimuscarinic for the treatment of urgency–frequency syndrome: results of a phase II controlled study. Neurourology and Urodynamics. 2004;23:598-599.
Chapple CR, Martinez-Garcia R, Selvaggi L, et al. A comparison of the efficacy and tolerability of solifenacin succinate and extended release tolterodine at treating overactive bladder syndrome: results of the STAR trial. STAR study group. European Urology. 2005;48:464-470.
Chapple C, van Kerrebroeck P, Tubaro A, Millard R. Fesoterodine in non-neurogenic voiding dysfunction — results on efficacy and safety in a phase III trial. European Urology. 2006;5(Suppl):117.
Chapple C, Patroneva A, Raines S. Effect of an ATP-sensitive potassium channel opener in subjects with overactive bladder: a randomized double-blind placebo controlled study (ZD0947IL/0004). European Urology. 2006;49:879-886.
Chapple CR, van Kerrebroeck PE, Junemann KP, Wang JT, Brodsky M. Comparison of fesoterodine and tolterodine in patients with overactive bladder. BJU International. 2008;102:1128-1132.
Charlton RG, Morley AR, Chambers P, Gillespie JI. Focal changes in nerve, muscle and connective tissue in normal and unstable human bladder. BJU International. 1999;84:953-960.
Collas D, Malone-Lee J. The pharmacokinetic properties of rectal oxybutynin — a possible alternative to intravesical administration. Neurourology and Urodynamics. 1997;16:346-347.
Diokno AC, Hyndman CW, Hardy DA, Lapides J. Comparison of action of imipramine (Tofranil) and propantheline (Probanthine) on detrusor contraction. Journal of Urology. 1972;107:42-43.
Diokno AC, Appell RA, Sand PK, et al. Prospective, randomised, double blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. OPERA Study Group. Mayo Clinic Proceedings. 2003;78:687-695.
Dmochowski RR, Sand PK, Zinner NR, Gittelman MC, Davila GW, Sanders SW, Transdermal Oxybutynin Study Group. Comparative efficacy and safety of transdermal oxybutynin and oral tolterodine versus placebo in previously treated patients with urge and mixed urinary incontinence. Urology. 2003;62:237-242.
Dmochowski RR, Sand PK, Zinner NR, Staskin DR. Trospium 60 mg once daily for overactive bladder syndrome: results from a placebo controlled interventional study. Urology. 2008;71:449-454.
Dolly JO. Therapeutic and research exploitation of botulinum neurotoxins. European Journal of Neurology. 1997;4(Suppl 2):S5-S10.
Dorschner W, Stolzenburg JU, Griebenow R, et al. Efficacy and safety of propiverine in elderly patients — a double blind, placebo controlled clinical study. European Urology. 2000;37:702.
Eaton AC, Bates CP. An in vitro physiological, study of normal and unstable human detrusor muscle. British Journal of Urology. 1982;54:653-657.
Edwards G, Henshaw M, Miller M, Weston WH. Comparison of the effects of several potassium-channel openers on rat bladder and rat portal vein in vitro. British Journal of Pharmacology. 1991;102:679-686.
Emmons SL, Otto L. Acupuncture for overactive bladder: a randomised controlled trial. Obstetrics and Gynecology. 2005;106:138-143.
Eriksen PS, Rasmussen H. Low dose 17β-oestradiol vaginal tablets in the treatment of atrophic vaginitis: a double-blind placebo controlled study. European Journal of Obstetrics, Gynaecology and Reproductive Biology. 1992;44:137-144.
Fowler CJ, Jewkes D, McDonald WI, Lynn B, DeGroat WC. Intravesical capsaicin for neurogenic bladder dysfunction. The Lancet. 1992;339:1239.
Freeman RM. Hypnosis and psychomedical treatment. In: Freeman RM, Malvern J, editors. The Unstable Bladder. Bristol: Wright; 1989:73-80.
Freeman RM, Baxby K. Hypnotherapy for incontinence caused by the unstable detrusor. BMJ (Clinical Research Ed.). 1982;284:1831-1834.
Frewen WK. Urge and stress incontinence: fact and fiction. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1970;77:932-934.
Fusgen I, Hauri D. Trospium chloride: an effective option for medical treatment of bladder overactivity. International Journal of Clinical Pharmacology and Therapeutics. 2000;38:223-234.
Gibson JS, Pardley J, Neville J 1990 Infra-red low power laser therapy on acupuncture points for treatment of the unstable bladder. Proceedings of the 20th Meeting of the International Continence Society, Aarhus, Denmark, pp 146–147.
Gill TM, Feinstein AR. A critical appraisal of the quality of life measurements. Journal of the American Medical Association. 1974;272:619-626.
Grosse J, Kramer G, Stoher M. Success of repeat detrusor injections of botulinum-A toxin in patients with severe neurogenic detrusor overactivity and incontinence. European Urology. 2005;47:653-659.
Haab F, Cardozo L, Chapple C, Ridder AM, Solifenacin Study Group. Long-term open label solifenacin treatment associated with persistence with therapy in patients with overactive bladder syndrome. European Urology. 2005;47:376-384.
Hadom DC, Baker D, Hodges JS, Hicks N. Rating the quality of evidence for clinical practice guidelines. Journal of Clinical Epidemiology. 1996;49:749-754.
Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ (Clinical Research Ed.). 2001;323:334-336.
Harzmann R, Weckerman D. Problem of secondary malignancy after urinary diversion and enterocystoplasty. Scandinavian Journal of Urology and Nephrology. 1992;142(Suppl):56.
Haruno A, Yamasaki Y, Miyoshi K, et al. Effects of propiverine hydrochloride and its metabolites on isolated guinea pig urinary bladder. Folia Pharmacologica Japonica. 1989;94:145-150.
Harris DR, Marsh KA, Birmingham AT, et al. Expression of muscarinic M3 receptors coupled to inositol phospholipid hydrolysis in human detrusor cultured smooth muscle cells. Journal of Urology. 1995;154:1241.
Hashim H, Abrams P. Is the bladder a reliable witness for predicting detrusor overactivity? Journal of Urology. 2006;175:191-195.
Hashim H, Malmberg L, Graugaard-Jensen C, Abrams P. Desmopressin as a ‘designer-drug’ in the treatment of overactive bladder syndrome. Neurourology and Urodynamics. 2009;28:40-46.
Hegde SS, Chopin A, Bonhaus D, et al. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. British Journal of Pharmacology. 1997;120:1409.
Hegde SS, Eglen RM. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sciences. 1999;64:419.
Heslington K, Hilton P. Ambulatory urodynamic monitoring. BJOG: an International Journal of Obstetrics and Gynaecology. 1996;103:393-399.
Higson RH, Smith JC, Whelan P. Bladder rupture: an acceptable complication of distension therapy. British Journal of Urology. 1978;50:529-534.
Hills CJ, Winter SA, Balfour JA. Tolterodine. Drugs. 1998;55:813-820.
Hilton P. Urinary incontinence during sexual intercourse: a common but rarely volunteered symptom. BJOG: an International Journal of Obstetrics and Gynaecology. 1988;95:377-381.
Hilton P, Stanton SL. Use of desmopressin (DDAVP) in nocturnal urinary frequency in the female. British Journal of Urology. 1982;54:252-255.
Hodgkinson CP, Ayers MA, Drukker BH. Dyssynergic detrusor dysfunction in the apparently normal female. American Journal of Obstetrics and Gynecology. 1963;87:717-730.
Hodgkinson CP, Drukker BH. Infravesical nerve resection for detrusor dyssynergia (the Ingleman-Sundberg operation). Acta Obstetricia et Gynecologica Scandinavica. 1977;56:401-408.
Holmes DM, Stone AR, Barry PR, Richards CJ, Stephenson TP. Bladder training — years on. British Journal of Urology. 1983;55:660-664.
Hughes KM, Lang JCT, Lazare R, et al. Measurement of oxybutynin and its N-desethyl metabolite in plasma, and its application to pharmacokinetic studies in young, elderly and frail elderly volunteers. Xenobiotica. 1992;22:859-869.
Ingleman-Sundberg A. Partial bladder denervation for detrusor dyssynergia. Clinical Obstetrics and Gynaecology. 1978;21:797-805.
Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder and other lower urinary tract symptoms in five countries; results of the EPIC study. European Urology. 2006;50:1306-1315.
Ishizuka O, Mattiasson A, Andersson K-E. Urodynamic effects of intravesical resiniferatoxin and capsaicin in conscious rats with and without outflow obstruction. Journal of Urology. 1995;154:611-616.
Jarvis GT. Bladder drill. In: Freeman R, Malvern J, editors. The Unstable Bladder. Bristol: Wright; 1989:55-60.
Jarvis GT, Millar DR. Controlled trial of bladder drill for overactive bladder. BMJ (Clinical Research Ed.). 1980;281:1322-1323.
Jeffcoate TNA, Francis WJA. Urgency incontinence in the female. American Journal of Obstetrics and Gynecology. 1966;94:604-618.
Jenkinson C, Coulter A, Wright L. Short Form 36 (SF-36) health survey questionnaire. Normative data for adults of working age. BMJ (Clinical Research Ed.). 1993;306:1437-1440.
Jeremy JY, Tsang V, Mikhailidis DP, Rogers H, Morgan RJ, Dandona P. Eicosanoid synthesis by human urinary bladder mucosa: pathological implications. British Journal of Urology. 1987;59:36-39.
Jonas U, Hofner K, Madesbacher H, Holmdahl TH. Efficacy and safety of two doses of tolterodine versus placebo in patients with detrusor overactivity and symptoms of frequency, urge incontinence, and urgency: urodynamic evaluation. World Journal of Urology. 1997;15:144-151.
Jünemann KP, Hessdörfer E, Unamba-Oparah I, et al. Propiverine hydrochloride immediate and extended release: comparison of efficacy and tolerability in patients with overactive bladder. Urology International. 2006;77:334-349.
Karram MM, Bhatia NW. Management of coexistent stress and urge urinary incontinence. Obstetrics and Gynecology. 1989;73:4-7.
Kelleher CJ, Cardozo LD, Khullar V, Salvatore S, Hill S. Anticholinergic therapy: the need for continued surveillance. Neurourology and Urodynamics. 1994;13:432-433.
Kelleher CJ, Filsche J, Burton G, Cardozo LD. Acupuncture and the treatment of irritative bladder symptoms. Acupuncture in Medicine. 1994;12:9-12.
Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. BJOG: an International Journal of Obstetrics and Gynaecology. 1997;104:1374-1379.
Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A medium-term analysis of the subjective efficency of treatment for women with detrusor instability and low bladder compliance. BJOG: an International Journal of Obstetrics and Gynaecology. 1997;104:988-993.
Kennelly MJ, Gormley EA, McGuire EJ. Early clinical experience with adult bladder autoaugmentation. Journal of Urology. 1994;152:303-306.
Kim DY, Chancellor MB. Intravesical neuromodulatory drugs: capsaicin and resiniferatoxin to treat the overactive bladder. Journal of Endourology. 2000;14:97-103.
Kinder RB, Mundy AR. Pathophysiology of idiopathic overactive bladder and detrusor hyperreflexia — an in vitro study of human detrusor muscle. British Journal of Urology. 1987;60:509-515.
Klarskov P. Gukephaline inhibits presynaptically the contractility of urinary tract smooth muscle. British Journal of Urology. 1987;59:31-35.
Knudsen UB, Rittig S, Pedersen JP, Norgaard JP, Djurhuus JC. Long-term treatment of nocturnal enuresis with desmopressin — influence on urinary output and haematological parameters. Neurourology and Urodynamics. 1989;8:348-349.
Langworthy DR, Kolb LG, Dees JE. Behaviour of the human bladder freed from cerebral control. Journal of Urology. 1936;36:577-597.
Lawson K. Is there a therapeutic future for ‘potassium channel openers’? Clinical Science. 1996;91:651-663.
Levin RM, Kitada S, Hayes L, et al. Experimental hyperreflexia: effect of intravesical administration of various agents. Pharmacology. 1991;42:54.
Lose G, Jorgensen L, Thunedborg P. Doxepin in the treatment of female detrusor overactivity: a randomized double-blind crossover study. Journal of Urology. 1989;142:1024-1027.
Lose G, Englev E. Oestradiol-releasing vaginal ring versus oestriol vaginal pessaries in the treatment of bothersome lower urinary tract symptoms. BJOG: an International Journal of Obstetrics and Gynaecology. 2000;107:1029-1034.
Madersbacher H, Jilg S. Control of detrusor hyperreflexia by the intravesical instillation of oxybutynin hydrochloride. Paraplegia. 1991;29:84-90.
Madersbacher H, Stoher M, Richter R, et al. Trospium chloride versus oxybutynin: a randomised, double-blind multicentre trial in the treatment of detrusor hyperrflexia. British Journal of Urology. 1995;75:452-456.
Madersbacher H, Murtz G, Alloussi S, et al. Propiverine vs oxybutynin for treating neurogenic detrusor overactivity in children and adolescents: results of a multicentre observational cohort study. BJU International. 2009;103:776-781.
Malloy BJ, Price DT, Price RR, et al. α1 Adrenergic receptor subtypes in human detrusor. Journal of Urology. 1998;160:937-943.
Mast P, Hoebeke P, Wyndale JJ, Oosterlinck W, Everaert K. Experience with clam cystoplasty. A review. Paraplegia. 1995;33:560-564.
Mazur D, Wehnert J, Dorschner W, Schubert G, Herfurth G, Alken RG. Clinical and urodynamic effects of propiverine in patients suffering from urgency and urge incontinence. Scandinavian Journal of Urology and Nephrology. 1995;29:289-294.
McGuire EJ, Shi-Chun Z, Horwinski ER, et al. Treatment of motor and sensory detrusor instability by electrical stimulation. Journal of Urology. 1983;129:78.
McRae P, Murray KH, Nurse DE, Stephenson JP, Mundy AR. Clam entero-cystoplasty in the neuropathic bladder. British Journal of Urology. 1987;60:523-525.
Millard RJ, Oldenberg BF. The symptomatic, urodynamic and psycho-dynamic results of bladder re-education programmes. Journal of Urology. 1983;130:717-719.
Millard R, Tuttle J, Moore K, et al. Clinical efficacy and safety of tolterodine compared to placebo in detrusor overactivity. Journal of Urology. 1999;161:1551-1555.
Mills IW, Greenland JE, McMurray G, et al. Studies of the pathophysiology of idiopathic overactive bladder: the physiological properties of the detrusor smooth muscle and its pattern of innervation. Journal of Urology. 2000;163:646-651.
Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of overactive bladder and how are they managed? A population-based prevalence study. BJU International. 2001;87:760-766.
Mundy AR. The long-term results of bladder transection for urge incontinence. British Journal of Urology. 1983;55:642-644.
Murray KH, Feneley RCL. Endorphins — a role in lower urinary tract function The effect of opioid blockade on the detrusor and urethral sphincter mechanism. British Journal of Urology. 1982;54:638-640.
Naughton MJ, Shumaker SA. Assessment of health related quality of life. In: Furberg CD, DeMets DL, editors. Fundamentals of Clinical Trials. 3rd edn. St Louis: Mosby Press; 1996:185.
National Institute for Health and Clinical Excellence. The Management of Urinary Incontinence in Women. NICE Guideline 40. London: Department of Health; 2006. Available at www.nice.org.uk
Nilsson K, Heimer G. Low dose oestradiol in the treatment of urogenital oestrogen defiiciency — a pharmacokinetic and pharmacodynamic study. Maturitas. 1992;15:121-127.
Nilvebrant L, Andersson KE, Mattiasson A. Characterization of the muscarinic cholinoreceptors in the human detrusor. Journal of Urology. 1985;134:418-423.
Nilvebrant L, Sparf B. Dicyclomine, benzhexol and oxybutynin distingush between subclasses of muscarinic binding sites. European Journal of Pharmacology. 1986;123:133-143.
Nilvebrant L, Andersson K-E, Gillberg P-G, Stahl M, Sparf B. Tolterodine — a new bladder selective anti-muscarinic agent. European Journal of Pharmacology. 1997;327:195-207.
Norgaard JP, Rillig S, Djurhuus JC. Nocturnal enuresis: an approach to treatment based on pathogenesis. Journal of Pediatrics. 1989;114:705-709.
Norton KRW, Bhat AV, Stanton SL. Psychiatric aspects of urinary incontinence in women attending an outpatient clinic. BMJ (Clinical Research Ed.). 1990;31:271-272.
Nurse DE, Mundy AR. Cystoplasty infection and cancer. Neurourology and Urodynamics. 1987;6:343-344.
O’Reilly BA, Kosaka AH, Knight GF, et al. P2X receptors and their role in female idiopathic detrusor instability. Journal of Urology. 2002;167:157-164.
Pengelly AW, Stephenson TP, Milroy EJG, Whiteside CG, Turner-Warwick R. Results of prolonged bladder distension as treatment for overactive bladder. British Journal of Urology. 1978;50:243-245.
Peters KM, Leong FC, Shoberi SA, et al. A randomised multicentre study comparing percutaneous tibial nerve stimulation with pharmaceutical therapy for the treatment of overactive bladder. Poster Presentation. Orlando, FL: American Urological Association; 2008.
Philp T, Shah PJR, Worth PHL. Acupuncture in the treatment of bladder instability. British Journal of Urology. 1988;61:490-493.
Pigne A, Degansac C, Nyssen C, Barratt J 1985 Acupuncture and the unstable bladder. In: Proceedings of the 15th International Continence Society Meeting, pp 186–187.
Popat R, Apostolidis A, Kalsi V, Gonzales G, Fowler CJ, Dasgupta P. A comparison between the response of patients with idiopathic detrusor overactivity and neurogenic detrusor overactivity to the first intradetrusor injection of botulinum-A toxin. Journal of Urology. 2005;174:984-989.
Ramsden PD, Smith JC, Dunn M, Ardran GM. Distension therapy for the unstable bladder: late results including an assessment of repeat distensions. Journal of Urology. 1976;48:623-629.
Reitz A, Stroher M, Kramer G, et al. European experience of 200 cases treated with botulinum-A toxin injections into the detrusor muscle for urinary incontinence due to neurogenic detrusor overactivity. European Urology. 2004;45:510-515.
Rentzhog L, Stanton SL, Cardozo LD, Nelson E, Fall M, Abrams P. Efficacy and safety of tolterodine in patients with overactive bladder: a dose ranging study. British Journal of Urology. 1998;81:42-48.
Rew DA, Rundle JSH. Assessment of the safety of regular DDAVP therapy in primary nocturnal enuresis. British Journal of Urology. 1989;63:352-353.
Robertson AS, Griffiths CJ, Ramsden PD, Neal DE. Bladder function in healthy volunteers: ambulatory monitoring and conventional urodynamic studies. British Journal of Urology. 1994;73:242-249.
Robinson D, Cardozo L, Akeson M, Hvistendahl G, Riis A, Norgaard J. Anti-diuresis — a new concept in the management of daytime urinary incontinence. BJU International. 2004;93:996-1000.
Robinson D, Cardozo L, Terpstra G, Bolodeoku J. A randomised double blind placebo controlled study to evaluate the efficacy of tamsulosin OCAS in the management of women with overactive bladder. BJU International. 2007;100:840-845.
Rose DK. Clinical application of bladder physiology. Journal of Urology. 1931;26:91-105.
Rosenbaum TP, Shah PJR, Rose GA, Lloyd-Davies RW. Cranberry juice helps the problem of mucus production in enterouroplasties. Neurourology and Urodynamics. 1989;8:344-345.
Ruscin JM, Morgenstern NE. Tolterodine use for symptoms of overactive bladder. Annals of Pharmacotherapy. 1999;33:1073-1082.
Salvatore S, Khullar V, Anders K, Cardozo LD. Reducing artifacts in ambulatory urodynamics. British Journal of Urology. 1998;81:211-214.
Salvatore S, Khullar V, Cardozo L, Anders K, Zocchi G, Soligo M. Evaluating ambulatory urodynamics: a prospective study in asymptomatic women. BJOG: an International Journal of Obstetrics and Gynaecology. 2001;108:107-111.
Samsicoe G, Jansson I, Mellstrom D, Svanberg A. 1985 Urinary incontinence in 75 year old women. Effects of oestriol. Acta Obstetricia et Gynecologica Scandinavica. 1985;93:57.
Schladitz-Keil G, Spahn H, Mutschler E. Determination of bioavailability of the quaternary ammonium compound trospium chloride in man from urinary excretion data. Arzneimittel Forschung/Drug Research. 1986;36:984-987.
Schurch B, Stohrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured pateints: a new alternative to anticholinergic drugs? Preliminary results. Journal of Urology. 2000;164:692-697.
Schurch B, de Seze M, Denys P, et al. Botulinum toxin type a is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. Botox Detrusor Hyperreflexia Study Team. Journal of Urology. 2005;174:196-200.
Schwinn DA. Novel role for alpha 1-adrenergic receptor sub-types in lower urinary tract symptoms. BJU International. 2000;86(Suppl 2):11-20.
Seigel SW, Cantanzaro F, Dijkema HE, et al. Long term results of a multicentre study on sacral nerve stimulation for treatment of urinary urge incontinence, urgency–frequency and retention. Urology. 2000;56:87-91.
Sibley GN. An experimental model of overactive bladder in the obstructed pig. British Journal of Urology. 1985;57:292-298.
Sibley GN. Developments in our understanding of overactive bladder. British Journal of Urology. 1997;80:54-61.
Snow BW, Cartwright PC. Bladder autoaugmentation. Urologic Clinics of North America. 1996;23:323-331.
Speakman MJ, Brading AF, Gilpin CJ, Dixon JS, Gilpin SA, Gosling JA. Bladder outflow obstruction — cause of denervation supersensitivity. Journal of Urology. 1987;183:1461-1466.
Staskin DR, Dmochowski RR, Sand PK, et al. Efficacy and safety of oxybutynin chloride topical gel for overactive bladder: a randomised double-blind, placebo controlled, multicentre study. Journal of Urology. 2009;181:1764-1772.
Steel SA, Cox C, Stanton SL. Long-term follow-up of overactive bladder following the colposuspension operation. British Journal of Urology. 1985;58:138-142.
Stewart WF, Corey R, Herzog AR, et al. Prevalence of overactive bladder in women: results from the NOBLE program. International Urogynecology Journal. 2001;12:S66.
Stoher M, Madersbacher H, Richter R, Wehnert J, Dreikorn K. Efficacy and safety of propiverine in SCI-patients suffering from detrusor hyperreflexia: a double-blind, placebo-controlled clinical trial. Spinal Cord. 1999;37:196-200.
Stoller ML. Afferent nerve stimulation for pelvic floor dysfunction. European Urology. 1999;135:32.
Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. PRIDE Investigators. New England Journal of Medicine. 2009;360:481-490.
Sutherst JR, Brown M. The effect on the bladder pressure of sudden entry of fluid into the posterior urethra. British Journal of Urology. 1978;50:406-409.
Swift S, Garely A, Dimpfl T, Payne C, Tolterodine Study Group. A new once-daily formulation of tolterodine provides superior efficacy and is well tolerated in women with overactive bladder. International Urogynecology Journal and Pelvic Floor Dysfunction. 2003;14:50-54.
Swithinbank L, Hashim H, Abrams P. The effect of fluid intake on urinary symptoms in women. Journal of Urology. 2005;174:187-189.
Torrens MJ, Griffiths HB. The control of the uninhibited bladder by selective sacral neurectomy. British Journal of Urology. 1974;46:639-644.
Turner-Warwick RT. Some clinical aspects of detrusor dysfunction. Journal of Urology. 1975;113:539-544.
Vandoninick V, van Balken MR, Finazzi Agro E, et al. Posterior tibial nerve stimulation in the treatment of urge incontinence. Neurourology and Urodynamics. 2003;22:17-23.
van Ermenegen E. Uber einen neuen anaeroben Bacillus und seine Beziehungen zum Botulismus. Zeitschrift fuer Hygiene und Infektionskrankheiten. 1897;26:1-56.
van Kerrebroeck P, Kreder K, Jonas U, Zinner N, Wein A, Tolterodine Study Group. Tolterodine once-daily: superior efficacy and tolerability in the treatment of overactive bladder. Urology. 2001;57:414-421.
van Waalwijk van Doorn ESC, Remmers A, Janknegt RA. Conventional and extramural ambulatory urodynamic testing of the lower urinary tract in female volunteers. Journal of Urology. 1992;47:1319-1326.
van Waalwijk van Doorn ESC, Anders K, Khullar V, et al. Standardisation of ambulatory urodynamic monitoring: report of the Standardisation Sub-committee of the ICS for Ambulatory Urodynamic Studies. Neurourology and Urodynamics. 2000;19:113-125.
Waldeck K, Larsson B, Andersson KE. Comparison of oxybutynin and its active metabolite, N-desmethyl-oxybutynin, in the human detrusor and parotid gland. Journal of Urology. 1997;157:1093-1097.
Walters MD, Taylor S, Schoenfeld LS. Psychosexual study of women with overactive bladder. Obstetrics and Gynecology. 1990;75:22-26.
Warrell DW. Vaginal denervation of the bladder nerve supply. Urology International. 1977;32:114-116.
Weatherall M. The risk of hyponatraemia in older adults using desmopressin for nocturia: a systematic review and meta-analysis. Neurourology and Urodynamics. 2004;23:302-305.
Weese DL, Roskamp DA, Leach GE, Zimmern PE. Intravesical oxybutynin chloride: experience with 42 patients. Urology. 1993;41:527-530.
Whiteside CG, Arnold GP. Persistent primary enuresis: a urodynamic assessment. BMJ (Clinical Research Ed.). 1975;1:364-369.
Wise BG, Cardozo LD, Cutner A, Benness CJ, Burton G. The prevalence and significance of urethral instability in women with overactive bladder. British Journal of Urology. 1993;72:26-29.
Wise BG, Benness CJ, Cardozo LD, Cutner A 1993b Vaginal oestradiol for lower urinary tract symptoms in post-menopausal women. A double blind placebo-controlled study. In: Proceedings of the 7th International Congress on the Menopause, Stockholm, 1993, p 15.
Yamaguchi O, Shisida K, Tamura K, et al. Evaluation of mRNAs encoding muscarinic receptor subtypes in human detrusor muscle. Journal of Urology. 1996;156:1208.
Zoubek J, Bloom D, Sedman AB. Extraordinary urinary frequency. Pediatrics. 1990;85:1112-1114.