CHAPTER 384 The Natural History of Intracranial Vascular Malformations
Treatment options for intracranial vascular malformations continue to change as microsurgical, radiosurgical, and endovascular procedures evolve. However, before one can define the best management, the natural history of each malformation must be known. Natural history data then allow proper counseling of patients on long-term outcome, anticipation of complications, and management decision making. The natural history of vascular malformations is complicated by a number of factors. First, there are many subtypes of vascular malformations, each with unique characteristics, and the distinction between subtypes is not always clear because of transitional or mixed types of malformations. Second, because of a high frequency of asymptomatic lesions, the study population may not be representative of the general population. Third, patients found to have vascular malformations are often treated; therefore, selection bias is inherent in these studies. Finally, the length of follow-up is frequently variable and inconsistent and thus not always representative of the general population.1
Classic pathoanatomic schemes divide vascular malformations into four categories: arteriovenous malformations (AVMs), venous malformations (VMs), cavernous malformations (CM), and capillary malformations. This classification scheme, although widely used, may be outdated by new information on the pathogenesis and evolution of these lesions. Reports of de novo lesions and the evolution of lesions have challenged the conventional concept of congenital, static malformations. Second, mixed vascular malformations have been poorly reconciled with this conventional scheme. Finally, there are several important arteriovenous shunting malformations (dural arteriovenous fistula [DAVF], carotid-cavernous fistula, galenic malformation) that clearly have distinctive pathoanatomic, radiologic, and natural history characteristics. An integrated classification scheme has been proposed (Table 384-1) to address the inadequacies of conventional pathoanatomic classifications.2 This chapter addresses the natural history of several selected vascular malformation subtypes.
TABLE 384-1 Classification Scheme for Central Nervous System Vascular Anomalies
| Proliferating Vascular Tumor |
| Nonproliferating Vascular Malformations (or Anomalies) |
AVF, arteriovenous fistula; AVM, arteriovenous malformation.
Arteriovenous Malformation
Synonyms: arteriovenous fistulous malformation, pial arteriovenous malformation, parenchymal arteriovenous malformation, arteriovenous anomaly, cryptic arteriovenous malformation, angiographically occult vascular malformation.*
Definition and Pathogenesis
AVMs are vascular abnormalities consisting of fistulous connections of arteries and veins without normal intervening capillary beds. Typically, they are triangular with the base toward the meninges and the apex toward the ventricular system.3 There is an abrupt transition between the arteries that contains variable amounts of smooth muscle and elastic laminae and the dilated veins. The veins have a thickened wall that appears arterialized because of the proliferation of fibroblasts. Within the arteries and veins, evidence of previous thrombosis and recanalization may be evident. Residua of previous hemorrhages, such as dystrophic calcification and blood breakdown products, may surround the AVM along with histologic evidence of hemosiderin-laden macrophages.3 Marked surrounding gliosis may be present as a result of hypoperfusion related to the high-flow, low-resistance AVM shunt “stealing” blood away from surrounding tissue. The overlying leptomeninges are often thickened and sometimes stained with hemosiderin.3,4
AVMs appear as serpiginous isointense or slightly hyperintense vessels that strongly enhance on computed tomography (CT) after the administration of contrast material.5–7 Calcification is identified in 25% to 30% of cases.7,8 In addition to identifying the AVM, CT is useful for demonstrating acute hemorrhage and showing a mass effect or displacement of normal anatomic structures. Magnetic resonance imaging (MRI), however, is superior in sensitivity and specificity. It is useful to determine the size, location, and evidence of previous symptomatic or subclinical hemorrhage, as well as secondary changes such as mass effect, edema, and ischemic changes in adjacent brain tissue.9 The typical AVM appears as a tightly packed “honeycomb” of flow voids on T1- and T2-weighted images as a result of high–flow velocity signal loss. Increased signal may be seen in thrombosed or low-flow vessels.7 Phase-contrast magnetic resonance angiography can be useful in the depiction of flow, although complete definition of complex lesions and their internal angioarchitecuture requires a cerebral angiogram.10,11
On cerebral angiography, parenchymal AVMs appear as tightly packed masses of enlarged feeding arteries and dilated tortuous veins with little or no intervening parenchyma within the nidus. There is little or no mass effect unless an associated hemorrhage or venous varix is present. Arteriovenous shunting with abnormal early filling of veins that drain the lesion is characteristic of AVMs.7 Angiography allows the identification of feeding and draining vessels, as well as associated vascular abnormalities such as aneurysms. Superselective angiography is preferred to delineate the internal angioarchitecture in detail.12 Although angiography is highly sensitive, it may be negative after acute hemorrhage13–16 or after spontaneous AVM thrombosis.17
AVMs are thought to be primarily congenital in origin because of the lack of development of intervening capillary beds.18–20 Although this may be part of the story, other influences may predispose to the development of AVMs. De novo formation may occur rarely,21 and recurrent AVMs have been documented after negative angiography, particularly in young adults.22 In addition, AVMs may be associated with other vascular malformations.23 These examples raise questions about whether abnormalities in venous outflow,24–27 angiogenic humoral factors, and hormonal influences play a role in pathogenesis.22
AVMs may also have a genetic basis in some populations. In addition to the association with hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease), a few rare pedigrees of familial AVM have been described both in the Asian population28 and in non-Asian populations.29 Some have shown an autosomal dominant pattern of inheritance, although this is variable and no gene has yet been identified. Low statistical power in the available genome-wide association studies may be contributing to the negative analyses.30 No clinically distinguishing features have been found between familial and congenital AVMs, although the familial type may occur at an earlier age.28,29,31–34 In families with brain AVMs in successive generations, the age at diagnosis in later generations may be younger than the age of earlier generations.29
Epidemiology
The exact incidence of AVMs in unknown.35 Large autopsy series estimate the frequency of AVM detection to be 1.4% to 4.3%.36 In one population-based study in Olmsted County, Minnesota, the sex- and age-adjusted incidence rate was 1.11 per 100,000 persons.37 In the New York Islands Study, the detection rate of AVMs was 1.34 per 100,000 person-years, and for first-ever AVM-related hemorrhage, it was 0.51 per 100,000 person-years.38,39 In the Scottish Intracranial Vascular Malformation Study,40 the detection rate for an AVM was 0.56 per 100,000 adults per year.41,42 The Australian population-based vascular malformation study revealed an AVM detection rate of 0.89 per 100,000 person-years.43 AVMs are the most frequently detected symptomatic vascular formation44 and account for 2% of all strokes45,46 and 38% of all intracerebral hemorrhages in patients between 15 and 45 years of age.47 AVMs are a seventh as common as aneurysms, the prevalence of which has been estimated to be 0.2% to 0.8% in the general population.2,37,48,49 The prevalence may be slightly higher in Asian populations.50
Patients are typically seen initially between 20 and 40 years of age.51–60 Older patients may be more likely to have hemorrhage and focal neurological deficits and less likely to have seizures.61 Most studies report an equal46,58 or slight male preponderance in patients with AVMs.51–54,62,63
The majority of AVMs are located supratentorially.46,54,57,64,65 Less common sites include the cerebellum and brainstem and within the ventricles. In the posterior fossa, the cerebellum is the most common site.66 Extracranially, AVMs may be present in the spinal cord, as well as in various organs and soft tissues.
Although AVMs are typically solitary, multiple AVMs have rarely been described.67–69 The Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage reported a less than 1% incidence of multiple AVMs.46 Willinsky and colleagues, however, reported a higher incidence of 9% in 203 consecutive patients.68 Occasionally, embolization or definitive treatment of a larger dominant AVM will unmask others as a result of changes in hemodynamics.68
Multiple AVMs may be present without apparent cause or in association with hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease),46,67,68,70,71 Wyburn-Mason syndrome,72 or soft tissue vascular malformations.73 Cases of AVM in the brain, as well as in the spinal cord, have also been reported.58,74
AVMs may be associated with other vascular malformations.23 Mixed vascular malformations are those that contain angiographic or imaging characteristics of more than one type of vascular malformation. Of 280 consecutive patients with vascular malformations, 14 had mixed lesions.23 Although rare, their presence may generate hypotheses regarding a common pathogenesis or evolution of causation among different types of lesions. AVMs have been reported in association with CMs,23 venous angiomas,23,27,46 and aneurysms.55,75–85 Nussbaum and coauthors reported the case of a patient with an AVM that drained into a developmental venous anomaly.27 After regression of the initial AVM, new malformations that drained into the same venous anomaly subsequently appeared, thus suggesting the possible importance of venous outflow in the pathogenesis of AVMs. The association with arterial aneurysms is discussed later.
Clinical Findings
Asymptomatic
In one large autopsy series, only 12% of patients harboring an AVM had symptoms related to it.36 Although the exact number of asymptomatic patients is unknown, clinical studies report that 2% to 4% of AVMs detected are incidental findings.56,86 In a population-based study of patients with intracranial vascular malformations, 40% were asymptomatic.37 Because cross-sectional imaging is being performed more frequently, an increasing number of patients are initially being seen either in an asymptomatic state or with symptoms other than hemorrhage.87
Hemorrhage
AVMs may be manifested as hemorrhage. In two population-based studies, 38%38 and 65%37 of patients with AVMs had hemorrhage, with a peak incidence in the fifth decade. In the cooperative study mentioned earlier,46 72% of patients with hemorrhage were initially seen before the age of 40. Before CT scanning, most studies reported a high incidence of subarachnoid hemorrhage.46,58,62 Since the advent of CT, distinction of hemorrhage type has been made clearer. Intraparenchymal hemorrhage is the most common, followed by intraventricular hemorrhage and subarachnoid hemorrhage.5,88,89 Of 50 consecutive cerebral angiograms in patients with AVM and hemorrhage, 60% had intracerebral hemorrhage, 26% had intracerebral hemorrhage with intraventricular extension, 8% had intraventricular hemorrhage, 4% had subarachnoid hemorrhage, and 2% had evidence of subdural hemorrhage.89 Subarachnoid hemorrhage is more common when an AVM is located cortically and can rarely be associated with vasospasm, depending on the location and thickness of the blood.58,90,91 Of patients with primary subarachnoid hemorrhage, 0.6% were due to AVMs.75
Risk for Hemorrhage
The risk for hemorrhage in patients with AVMs has been estimated to be 2% to 4% per year.51,54,55,63,65,92,93 In a study of 168 patients with no previous history of bleeding and a follow-up greater than 8.2 years, 18% experienced a symptomatic hemorrhage during the follow-up period, for a crude risk of 2.2% per year.65 Using life-table analysis, the annual risk was 1.3% at 1 year, 1.7% at 5 years, 1.5% at 10 years, and 2.2% at 15 years. Graf and associates also evaluated the prospective rate of hemorrhage.55 The risk for a first hemorrhage in patients with seizures was 2% at 1 year, 13% at 5 years, and 30% at 10 years, for an overall rate of 2% to 3% per year. Similarly, other studies have also reported prospective hemorrhage risks of 1% to 3% per year.53,54,94
Ondra and coworkers studied 160 patients with AVMs, which represented 90% of such lesions in the country of Finland.63 Patients were initially evaluated because of hemorrhage, seizures, or other symptoms and were monitored over a 24-year period. This study found 147 new hemorrhagic events in 64 patients during the follow-up period, for an overall hemorrhage risk of 4% per year that was constant over time. The mean interval between diagnosis and hemorrhage was 7.7 years. This study started during the pre-CT era and included multiple hemorrhages in some patients. It is important to note that if one considers the risk for only the first hemorrhage, the hemorrhagic risk is 1.7% per year, similar to other studies. Similarly, Graf and Pollock found the initial risk for hemorrhage to be approximately 2% per year. Pollock and colleagues studied predominantly small AVMs (<3 cm).92 Of 315 patients, 196 were initially seen with hemorrhage, for a retrospective risk of 1.89% per year. A crude overall rate of 2.4% per year was determined when multiple hemorrhages were included.
A prospectively collected database consisting of 678 consecutive brain AVMs was monitored over 1932 person-years. The overall hemorrhage rate was 4.61% per year, but this included the 258 patients who initially had hemorrhage. Among these 258 patients, the annual risk for hemorrhage was 7.48% per year, as opposed to 4.16% for those initially evaluated because of seizures (n = 260). In terms of angiographic features, the annual risk for hemorrhage in those with deep venous drainage was 5.42% per year (n = 365), for those with an associated brain aneurysm it was 6.93% per year (n = 122), and for those without a brain aneurysm the risk was 3.99% per year (n = 556). In univariate analysis, a hemorrhagic manifestation (hazard ratio [HR], 2.21; P < .001), associated aneurysm (HR, 1.83; P = .01), and deep venous drainage (HR, 1.81; P = .01) were associated with an increased risk for hemorrhage. In multivariate analysis, a hemorrhagic manifestation (HR, 2.15; P < .01) was a significant predictor of future hemorrhage. There was a nonstatistically significant trend toward an increased risk for hemorrhage in patients with AVMs associated with the presence of an associated aneurysm (P = .07) and deep venous drainage (P = .07).93
In a study of 305 consecutive patients observed prospectively, hemorrhage occurred in 26 of those initially seen with hemorrhage during 380 person-years, for a risk of 6.84% per year, and in 16 patients without initial hemorrhage during 512 person-years, for a risk of 3.12% per year.95 In those with hemorrhage initially, the rate was higher in the first year (15.4%), 5.3% in the next 4 years, and 1.7% in the next 5 years. Patients initially evaluated because of headache had a 6.5% per year risk for hemorrhage versus an annual risk of 6.44% in asymptomatic patients, 2.2% in those with seizures, and 1.7% in those with neurological deficits. There was a higher risk for rebleeding in children (HR, 2.69), females (HR, 2.93), and those with deep-seated AVMs (HR, 3.07).
In 622 consecutive patients with brain AVMs in the prospective Columbia AVM database, patients were monitored for a mean of 829 days. Intracranial hemorrhage was the initial finding in 282 (45%) patients. During follow-up, hemorrhage occurred in 39 (6%) patients, for an overall risk of 2.8% per year. Independent predictors of hemorrhage during follow-up in multivariate analysis included initial hemorrhagic AVM manifestation (HR, 5.38; 95% confidence interval [CI], 2.64 to 10.96), older age (HR, 1.05; 95% CI, 1.03 to 1.08), deep brain location (HR, 3.25; 95% CI, 1.30 to 8.16), and exclusive deep venous drainage (HR, 3.25; 95% CI, 1.01 to 5.67). Annual hemorrhage rates were 0.9% in patients without hemorrhage initially and in those without deep brain location or exclusive deep venous drainage and 34.4% in those with each of these factors.96
Using the multiplicative law of probability, the lifetime risk for AVM rupture can be assessed by using the formula [1 − (risk for no hemorrhage)n], where n is the number of expected years of life remaining.97 This formula assumes that the lesion is congenital and that the risk is constant over time. Assuming a 3% per year risk for hemorrhage, the formula becomes (1 − 0.97). The n value can be obtained from life tables. For example, a 35-year-old patient with 43 years of life remaining yields (1 − 0.97)43 = 73%. An estimation that correlates well with this formula is simply 105 − age. In addition to the assumptions just mentioned, this formula also assumes that the risk is similar among different patients and that AVM morphology and angioarchitecture do not influence the rate of rupture.
Risk Factors Predicting Hemorrhage
Many studies have attempted to elucidate clinical and angiographic predictors of hemorrhagic AVM to further delineate which patients may be at higher risk. Clinical risk factors for AVM diagnosed after the occurrence of hemorrhage found by some include age,46,61 sex,94 pregnancy,98 and hypertension.99 Hemorrhage in infants and children is distinctly uncommon.46 Before the age of 2 years, patients with AVMs are typically initially evaluated for congestive heart failure, hydrocephalus, or seizures.46 Some data suggest that older patients are more likely to have hemorrhage or a focal neurological deficit and less likely to have seizures initially. Older patients were more likely to have concurrent arterial aneurysms, smaller AVMs, and AVMs in an infratentorial location.61 Regarding gender issues, in a multivariate analysis Mast and coauthors reported that male sex is a risk factor for a hemorrhagic manifestation of AVM.94 Conversely, another study showed a slight preponderance of females initially seen with hemorrhage (62 males, 72 females),55 but this was not statistically significant. Another study suggested that female sex may be a risk factor for hemorrhage in those with previous hemorrhage.95 Pregnancy may increase the likelihood of rupture, but this is controversial and discussed in subsequent sections. One study found a history of hypertension to be independently associated with a hemorrhagic manifestation of AVM.99 The association with recurrent hemorrhages, however, was unclear. Furthermore, no other studies have confirmed this observation. Race/ethnicity may also be of importance in predicting the risk for hemorrhage. In a prospective and retrospective study, Hispanic race/ethnicity (HR, 1.9; P = .02) was an independent risk factor for intracranial hemorrhage. After adjustment for selected angiographic factors, the HR was 3.1. There were trends observed for blacks (HR, 2.1; P = .09) and for Asians (HR, 2.4; P = .11).100,101
Angiographic risk factors for AVMs manifested as hemorrhage have also been studied but are subject to certain biases. Angiography performed after acute hemorrhage must be interpreted carefully because hemorrhage may change the size and morphologic features of AVMs.15 Furthermore, patients undergoing angiography after hemorrhage had survived to that point. That is, patients with early death were excluded from the populations studied after hemorrhage. Early mortality may also be excluded in some studies because the patients represent a selected subgroup of patients referred to a center with angiographic capabilities.
One large study of 168 patients with AVMs detected before rupture evaluated angiographic characteristics predictive of future rupture. Despite meticulous analysis of AVM size, site, and flow characteristics, no angioarchitectural features were found to be predictive of rupture in this multivariate analysis.65
Several studies have indicated that location influences the risk for hemorrhage. The presence of an AVM in a deep location,59,77,95,96,102 such as the basal ganglia,12,56,103 posterior fossa,56,104 intraventricular and periventricular areas,105,106 and arterial border zones,107 may predispose to a hemorrhagic manifestation. Some studies report an increased risk for hemorrhage with cerebellar lesions.84,105,106,108,109 The higher risk in one study was attributed to the relatively high incidence of associated aneurysms.108 In contrast to these studies, others found location to be inconsequential in predicting hemorrhagic risk.60,63,65 Some hypothesize that deeply located AVMs may simply not cause symptoms other than hemorrhage until they reach a size where cortical irritation is possible.
Size has been controversial. Several large studies, including one of unruptured AVMs at the start of follow-up,65 found no difference in risk for hemorrhage based on the size of the AVM.12,53,60,63,65,102,105 Others have found that small AVMs (<3 cm) pose a higher risk for a hemorrhagic manifestation.55–58,62,64,99,110–112 One study found that 90% of patients with small AVMs initially came to medical attention because of hemorrhage.56 Hemodynamic assessment of small AVMs revealed distinct differences in flow patterns and pressure. Spetzler and associates found higher intra-arterial pressure in smaller AVMs, thus suggesting a potential role in hemorrhage.110 In addition, the transnidal pressure gradient is higher in smaller AVMs.113 Others question whether the AVM has just not achieved a size at which it can cause other symptoms, thereby leading to a higher proportion of patients initially being seen with hemorrhage. In contrast to these studies, however, retrospective114 and prospective data115 suggest that larger AVMs have an increased risk for hemorrhage. Furthermore, a study of predominantly small AVMs found the hemorrhage risk rate to be similar to that in other studies, which suggests that there may not be a difference in terms of size.92
Feeding arteries and venous drainage have also been assessed. Norris and colleagues evaluated a number of angiographic features in 31 patients, including size and several arterial and venous parameters.116 The studies were performed several months after hemorrhage to minimize changes caused by acute hemorrhage. The only difference in those with hemorrhage was slower arterial filling with contrast material, thus suggesting high feeding arterial pressure. Mean feeding arterial pressure was confirmed to be an important factor in the pathophysiology of AVM hemorrhage by the Columbia AVM study group.56 This factor was independent of size and location; however, mean arterial pressure was not assessed in patients with small AVMs. Patients may also be predisposed to a hemorrhagic manifestation depending on which artery is feeding the nidus. Various arteries have been implicated, including perforating arteries12,56,77 and the vertebrobasilar trunk.12
Many AVMs that rupture do so from the venous drainage system. Recent studies have focused on the venous drainage pattern of AVMs and its importance in the pathophysiology of hemorrhage. Contributing features include deep venous drainage, often with accompanying stenosis and occlusion, the number of draining veins, and turbulent venous flow, perhaps leading to enhanced platelet aggregation and thrombosis.56
Impaired venous drainage may lead to a higher risk for hemorrhage as a result of increased pressure transmitted through the shunt. This was evaluated mathematically117 and clinically suggested by several studies.58,77,118,119 Vinuela and colleagues found that 21 of 41 patients with intracranial hemorrhage secondary to deep AVMs had vessel wall irregularity, stenosis, or occlusion in the deep venous system.119
Deep venous drainage has frequently been shown to increase the risk for a hemorrhagic AVM manifestation.12,56,77,94,96,99,102,105,118 Because many lesions with deep venous drainage are noncortical and unlikely to cause seizures, some believe that hemorrhage is the only initial manifestation possible. The Columbia AVM study group examined a large number of physiologic indices in 449 patients to determine the relationship of AVM hemorrhage and venous drainage, as well as other parameters.120 Multivariate analysis revealed that size and deep venous drainage were independent risk factors for bleeding, thus arguing against beliefs that deep venous drainage increases risk because of deep location or size. In fact, even large cortical AVMs with deep venous drainage were more likely to hemorrhage. Furthermore, equal draining pressure was found in both the deep and superficial AVMs that bled. From this study, four groups of patients emerged on the model’s prediction of the probability of intracerebral hemorrhage in terms of size and venous drainage: (1) small AVM size and the presence of deep venous drainage only had a probability of 96%, (2) medium or large AVMs and deep venous drainage only had a probability of 80%, (3) small AVM size and superficial venous drainage had a probability of 69%, and (4) medium or large AVMs with superficial venous drainage had a probability of 29%. In a later study of 622 patients in the Columbia AVM database, independent predictors of a hemorrhagic manifestation included AVM size, deep brain location, border zone location, exclusive deep venous drainage, and the presence of associated arterial aneurysms.96 As noted earlier, in a prospective analysis of risk for hemorrhage, there was a trend toward deep venous drainage being an independent risk factor in several studies.93,96
A single draining vein was predictive of hemorrhagic risk in some studies102,109,118 but was not confirmed by others.12,65,92 This may reflect the fact that smaller AVMs are more likely to have single draining veins.56 The presence of, but not the size of, fragile venous aneurysms was significantly associated with risk for hemorrhage in two studies,77,121 but this finding, too, is controversial.12,65,118
Although used primarily as a surgical risk scale, the Spetzler-Martin scale has also been used in considering the risk for hemorrhage.122 In patients with grade IV or V AVMs, the annual pretreatment hemorrhage rate was 10.4% per year, 13.9% in those with hemorrhage and 7.3% in those without hemorrhage at initial evaluation. After treatment, the overall risk was 6.1% per year for all patients, 5.6% for those initially seen with hemorrhage and 6.4% for those without hemorrhage, thus suggesting that the risk for hemorrhage after treatment was either less than or equal to the pretreatment rate.123
Seizures
Approximately 15% to 35% of patients with AVMs will have a seizure as the first symptom.46,48,49,54,55,57,65,93 Seizures may be the result of a mass effect with cortical irritation or flow characteristics leading to steal, ischemia, and neuronal damage or be due to associated hemorrhage and gliosis.49,124 In a series by Morello and Borghi, 35% of patients were initially evaluated because of seizures.57 Of these, 57% had seizures alone and 43% had seizures associated with hemorrhage. The majority of these patients had less than six seizures per year. The seizures are most commonly focal (simple or partial complex) but may also be generalized.
Some risk factors have been delineated for predicting the occurrence of seizures in patients with AVMs. Ninety percent of the seizures were supratentorial. Superficial, large (>6 cm) AVMs57,86 and those in a frontal or temporal location46,57 were more likely to be manifested as seizures. Turjman and colleagues identified six angioarchitectural features predictive of the development of seizures in a multivariate analysis of 100 patients with AVMs.124 The predictive features included cortical location, feeding by the middle cerebral artery, a cortical feeding artery, absence of aneurysms, presence of varices in the venous drainage, and the association of a varix in the absence of an intranidal aneurysm. Interestingly, size and high-flow shunting failed to reach statistical significance and were not predictive. The risk for future hemorrhage in those initially seen with seizures is probably less than in those with hemorrhage.93
Headache
Headaches are a common complaint in patients with AVMs, even in the absence of hemorrhage. Approximately 15% of unruptured AVMs are initially manifested as headache.65 The headache is typically located hemicranially (ipsilateral or contralateral to the lesion) or in the occipital region, and the quality is similar to migraine. Interestingly, the incidence of migraine headache in patients with AVMs does not exceed that of the general population125 and therefore makes it difficult to know which headaches are related to the AVM unless treatment is performed. In fact, successful embolization of AVMs in patients with headache has been reported. The pathologic etiology of the headache is hypothesized to relate to long-standing meningeal artery involvement and recruitment of blood supply by the AVM. Occipital AVM location may be a risk factor for headache.49
Neurological Deficit
Less than 10% of patients will initially have transient, permanent, or progressive focal neurological deficits not ascribed to hemorrhage or seizure.51,54,65,126 Progression of neurological dysfunction may be a result of the long-term effects of recurrent small hemorrhages, mass effect of the AVM, hydrocephalus, or ischemic complications and “steal.” Steal is the term used to describe blood flow away from a region of the brain toward the shunt of the AVM. This flow may cause hypoperfusion, ischemia, and symptoms in the region from where the blood was “stolen.” Consequently, steal may lead to focal or more global neurological deficits. Learning disorders have been documented in 66% of adults with AVMs, thus suggesting that functional brain deficits may be present before other clinical signs.49,127
Risk factors for progressive neurological deficits include size110 and shunt characteristics.128 Large AVMs are more likely to result in neurological symptoms attributed to steal. Spetzler and coworkers hypothesized that the low feeding artery pressure associated with large AVMs provides low perfusion pressure to the surrounding cortex, thereby producing a relative ischemia.110 Patients with progressive deficits are also more likely to have extremely fast shunts with higher flow volumes noted on transcranial Doppler ultrasound.128 In contrast to these studies, Mast and colleagues believe that steal is a rare phenomenon.126 They found no relationship to size or flow velocities in patients with focal deficits.126 Furthermore, although positron emission tomography has shown reduced cerebral blood flow around AVMs, oxygen extraction fractions remain normal, which suggests that surrounding parenchyma compensates for the reduced flow.129
Outcome
Morbidity and Mortality
The mortality associated with the initial symptomatic hemorrhage is 4% to 29%.37,48,55,60,63,65,93,130–132 More recent data, however, suggest that mortality and morbidity may be somewhat lower than previously reported.96,133,134 In a study published in 1988, the 30-day mortality with the initial hemorrhage was 29%, and long-term disability was noted in 23% of survivors.65 In a recent series of 678 patients, 89 hemorrhages occurred during follow-up; 5 patients (6%) died of the hemorrhage and 35% had significant functional impairment.93 There were no specific demographic or angioarchitectural predictors of a poor outcome. In a study of 622 patients monitored prospectively, 39 hemorrhages occurred and caused just one death and a median Rankin score of 2 in survivors.96 In a separate report of the morbidity and mortality in 241 patients initially seen with hemorrhage in the Columbia AVM database, the median Rankin scale score was 2 and the neurological deficit was mild. There was no increase in morbidity if hemorrhage recurred during follow-up.135 Parenchymatous AVM hemorrhage was associated with higher stroke morbidity (odds ratio [OR], 2.9) than nonparenchymatous hemorrhage was but had a better outcome than did non–AVM-related parenchymatous hemorrhage (P < .0001). A population-based study from Australia noted that the initial hemorrhage carried a 4.6% case fatality rate.
Many other studies have reported complete recovery or just mild disability in more than 50% of patients after an initial hemorrhage.131,55,130,136 Outcome may be dependent on the location and type of hemorrhage, but not on the size of the AVM.54,55 Patients were more likely to have neurological deficits if the hemorrhage was parenchymal rather than subarachnoid or intraventricular.55,131,136 In addition, hemorrhage in the posterior fossa was associated with a higher mortality rate. Mortality was reported to occur in 66.7% of patients with posterior fossa hemorrhage in one study.54
In a long-term follow-up study from the Australian population-based study, patients were monitored for a mean of 10.1 years. Assessment of 209 survivors with the Oxford neurological disability scale showed that 74.1% were grade 0 to 2, 17.2% were grade 3, and 9.5% were grade 4 or 5.43
Recurrent Hemorrhage
Recurrent hemorrhage is noted in 23% to 44% of patients, and the risk may be higher in the first year after the initial hemorrhage.54,55,93–95,137 Because the average time from initial evaluation to hemorrhage is 7 to 12 years,55,63,65 few studies have assessed the risk for recurrent hemorrhage. Graf and colleagues observed 134 patients with ruptured AVMs for a median time of 2 years.55 Twenty-four percent of the patients had recurrent hemorrhage during the follow-up period, for an annual rebleeding risk of 6% at 1 year and 2% thereafter. Although this study found size to be predictive of initial hemorrhage, size was not a predictor of subsequent hemorrhage.
Of 315 patients in one study, 196 were seen because of an initial hemorrhage.92 Recurrent hemorrhage was found in 44%, for a recurrence risk of 7.45% per year. Four AVM groups were constructed to predict risk for hemorrhage on the basis of three significant variables in the multivariate analysis. The low-risk group had no history of bleeding, more than one draining vein, and a compact nidus. The intermediate- to low-risk group had no history of bleeding and one draining vein or a diffuse nidus (or both). The intermediate- to high-risk group had a history of bleeding, more than one draining vein, and a compact nidus. The high-risk AVM group had a history of bleeding and one draining vein or a diffuse nidus (or both). Annual rates of hemorrhage were 1.31% in the low-risk group, 2.4% in both intermediate groups, and 8.99% in the high-risk group.
Other studies have confirmed that previous hemorrhage is a risk factor for subsequent hemorrhage.* The Columbia AVM study group found an 18% per year risk for hemorrhage in patients with previous hemorrhage versus 2% per year for all others.120 In a prospectively collected database from Toronto, the annual risk for hemorrhage was 4.61% for the entire cohort and 7.48% for those with hemorrhage.93 Hemorrhage was an independent risk factor for future hemorrhage in a multivariate analysis (HR, 2.15). In a study of 305 patients, the annual risk for hemorrhage was 6.84% per year in those with hemorrhage as opposed to 3.12% per year in those without hemorrhage.95
Mortality did not necessarily increase with subsequent hemorrhagic episodes.54 The mortality associated with recurrent hemorrhage has been estimated to be 12% to 15%.46,51,60,130 In a population-based study with small numbers, 4 of 17 patients had recurrent hemorrhage, with mortality reaching 50%.37
Seizures
Information on risk for the de novo development of seizures over time and the outcome of patients with epilepsy treated conservatively is scant. Crawford and coworkers monitored 245 patients with symptoms other than seizures for a median period of 7 years.86 Ninety-six patients were treated surgically, and these patients had a higher risk for the development of epilepsy. The 20-year risk was 57% in the surgical group and 19% (<1% per year) in the conservatively treated group. In three fourths of the patients in whom seizures developed, they did so within 2 years of treatment. The surgically treated group was more likely to experience seizures if they were younger at diagnosis and if the AVM was in the frontal or parietal lobes. Of the conservatively treated group, those with hemorrhage at a younger age and those with temporally located AVMs were most likely to have seizures. The size of the AVM did not play a significant role. In none of the patients who had nonhemorrhagic focal neurological deficits or were initially asymptomatic did seizures develop. The reason that the surgically treated patients have a higher risk for epilepsy is unclear. It may be a result of selection bias inasmuch as most patients who were selected for surgery had hemorrhage on initial evaluation and the lesions were located superficially.
Piepgras and colleagues studied 280 patients over a period of 7.5 years.112 Fifty-six percent of the patients were initially seen with hemorrhage, 25% had seizures, and 19% had other symptoms. Sixty-nine patients had both seizures and hemorrhage. Patients were treated surgically mainly to reduce the risk for bleeding, not specifically because of seizures. Of the surviving 136 patients who had no history of preoperative seizures, 94% were seizure free and new seizures developed in just 6% after surgery. Of patients with preoperative seizures, 83% were seizure free after surgery (50% were taking antiepileptic medications) and 17% had intermittent seizures, although the majority were improved. The difference between this and older studies probably relates to improved diagnostic and surgical techniques.
In conservatively treated patients, the majority are well controlled with antiepileptic medications.46,139 Murphy found that only 16% of conservatively treated patients were incapacitated by their seizures.139 Similarly, Perret and Nishioka found that just 4 of 39 patients treated with medications alone were unable to work.46
Radiographic Features/Lesion Behavior
AVMs may increase in size, remain stable, decrease in size, or completely regress or thrombose over time.16,58,60,111,140–145 Minikawa and associates found that the patients with enlarging AVMs had their first angiogram at a young age (0 to 11 years).141 Subsequently, three of the four patients in that study rebled. The AVMs that regressed were relatively small and fed by few feeding arteries.
Spontaneous regression can be acute or gradual and occurs in approximately 2% to 3% of patients with AVMs.17 Causes include low flow; occlusion of feeding vessels by atherosclerosis, emboli, or dissection; or regression in association with hemorrhage because of mass effect or vasospasm.16,140,142,146 Thrombosis is thought to be protective. Thrombosed AVMs rarely cause hemorrhage, although the risk for seizures remains.48,144 Caution is suggested in concluding that an AVM is thrombosed after hemorrhage with negative angiographic findings. Transient regression after acute hemorrhage may be secondary to vasospasm and not thrombosis.15 Others have reported recanalization after thrombosis.147
Arteriovenous Malformations And Aneurysms
The association of aneurysms with AVMs has been established.46,55,75–83,85,93,148–152 Numerous studies have attempted to classify the types of aneurysms associated with AVMs, and in general, each classification system takes into account the distance and flow relationship to the AVM. Aneurysms may be flow related, intranidal, or unrelated.77 Flow-related aneurysms refer to saccular aneurysms arising along the course of arteries that eventually supply the AVM. A proximal flow-related aneurysm is one located on the supraclinoid internal carotid artery, the circle of Willis, the middle cerebral artery up to the bifurcation, the anterior communicating artery up to the anterior cerebral artery, or the vertebrobasilar trunk. All flow-related aneuryms beyond these locations are distal flow-related aneurysms.77 The distal type generally consists of those on the main feeding artery of the AVM, and they are also known as pedicle artery aneurysms.76 Some further break the classification down to differentiate whether the feeding artery is deep or superficial.82 Intranidal aneurysms are aneurysms that lie within the AVM nidus. Unrelated or dysplastic aneurysms are those remote to the AVM.
The pathogenesis of aneurysms associated with AVMs is unknown. Three theories have evolved. The first proposes that the association of aneurysms and AVMs is coincidental without a causal relationship. This is probably not the case because the prevalence of aneurysms in patients with AVMs exceeds that of the general population.83,85 The second theory hypothesizes that both AVMs and aneurysms are congenital vascular abnormalities. The third and more favored theory is that aneurysms result from hemodynamic factors caused by the increased flow through the AVM.81 In favor of this hypothesis is the increased occurrence of aneurysms on the feeding arteries,85 evidence of endothelial changes and internal elastic lamina rupture proximal to the AVM shunt, and the observation that distal flow-related aneurysms often regress with definitive treatment of the AVM. Brown and associates, however, found that even patients with low-shunt AVMs harbored pedicle aneurysms.85 They further hypothesized that the pathogenesis of aneurysms may be related to the size of the AVM inasmuch as all aneurysms associated with small AVMs were found at atypical locations. Thus, the pathogenesis may relate to a combination of factors, such as an underlying vascular defect, hemodynamic stimuli, vasoactive substances, and locally generated growth factors.
Depending on the series, aneurysms are present in 2.7%78 to 34%152 of patients with AVMs.46,55,75,76,78–85,93,150–152 The average figure of 8% to 10% exceeds the 2% prevalence of aneurysms in the general population.83 The mean age at initial evaluation may be slightly older than that of the general AVM population. Miyasaka and coworkers found the mean age at diagnosis to be 41 years in those with aneurysms versus 31 in those without.79 The male-to-female ratio is similar to that of the entire AVM population.80 Males may be more likely to harbor flow-related and intranidal aneurysms, whereas females may be more likely to have associated dysplastic or remote aneurysms.80 As with AVMs in isolation, AVMs with associated aneurysms are typically manifested as hemorrhage.82,84 Of 39 patients with 64 aneurysms, Cunha and coauthors reported intracerebral hemorrhage as the initial finding in 63%.82 Forty-six percent of these hemorrhages were secondary to the aneurysm, 33% were related to the AVM, and in 21%, the site of hemorrhage was unclear. Redekop and colleagues reported that 36% of a total of 632 patients with AVMs were initially seen with hemorrhage.77 In those with intranidal aneurysms, hemorrhage occurred in 72%. Similar to the overall group, 40% of patients with flow-related aneurysms experienced hemorrhage. Of patients with an AVM and flow-related aneurysm, 12 of 29 (41.4%) bled from the aneurysm (5 distal, 7 proximal), 15 of 29 (51.7%) bled from the AVM, and 2 of 29 were undetermined.
Aneurysms may be multiple in 30% to 50% of patients with AVMs and aneurysms.79,82,84,151 The majority of studies reveal no difference in the size of the AVM and the type, number, and size of the associated aneurysm.77,82,85 Only one study found that larger AVMs were more likely to be associated with aneurysms.79 The size of the associated aneurysm ranged from 3 mm to 2.5 cm and averaged approximately 7.2 to 8 mm.77,85 The frequency of aneurysm type varied according to the study, with the majority being flow related.46,75,77,79,80,82,151 The frequency of distal feeding artery aneurysms ranged from 37% to 69%.46,75,77,79,149,151 Remote aneurysms are less common and found in 1.6% to 43% of patients.46,77,79,80,82,151 Intranidal aneurysms represent approximately 20% of aneurysms.77 This figure, however, is variable and depends on the type of study performed. Superselective angiography is more likely to detect intranidal aneurysms but was not uniformly used in these studies. Typical locations (at artery bifurcations) were found in association with high-shunt malformations.85 Atypical locations were noted in association with high- or low-flow and high- or low-shunt AVMs.
Several studies reported an increased risk for hemorrhage when an AVM is associated with an aneurysm.77,85,93,148,151,152 Brown and collaborators studied 16 patients with 26 aneurysms associated with unruptured AVMs.85 The risk for hemorrhage in a patient with a coexisting aneurysm was 7% per year at 5 years. In those without a coexisting aneurysm, the rate was 3% per year at 1 year and then declined to 1.7% per year at 5 years. In a multivariate analysis of 678 people with AVMs, the risk for hemorrhage in those with an aneurysm was 6.93% per year versus 3.99% per year in those without an aneurysm. In a multivariate analysis, there was a trend toward significance of the presence of an aneurysm as a predictor of hemorrhage (HR, 1.59; P = .07).93 The association of hemorrhage at initial evaluation and coexisting aneurysms has not been consistently noted.152
Risk factors for hemorrhage in patients harboring both lesions have been evaluated. Neither the size of the AVM nor the size of the aneurysm was important, although the number of patients was small. Several studies have reported that patients with associated intranidal aneurysms are at increased risk for an initial manifestation of hemorrhage and subsequent rebleeding.77,148,151 All the patients in a study of Marks and colleagues with intranidal aneurysms were initially seen because of hemorrhage, as opposed to 58% of those without such aneurysms.148 As mentioned previously, 72% of the patients with intranidal aneurysms in the study by Redekop and associates had hemorrhage initially.77 Thirteen of the patients with intranidal aneurysms in this study could not be treated surgically. Fourteen subsequently hemorrhaged over a period of 143 patient-years, for an annual rate of hemorrhage of 9.8%. This was compared with a risk of 5.3% per year for flow-related aneurysms that could not be treated by surgery. They concluded that patients with intranidal aneurysms had a high risk for rupture and a hemorrhagic manifestation, as well as a high risk for rebleeding. In addition, for flow-related aneurysms, patients had an equal chance of bleeding from the aneurysm as from the AVM. In the Columbia AVM databank, 117 of 463 (25%) prospectively identified patients had a concurrent aneurysm, including 54 with aneurysms on the feeding arteries, 21 with aneurysms in an intranidal location, 18 with aneurysms unrelated to the AVM, and 24 with more than one aneurysm type.151 In multivariate analysis, the presence of a feeding artery aneurysm was an independent predictor of a hemorrhagic manifestation (OR, 2.11), but such was not the case with intranidal or unrelated aneurysms. Pedicle artery aneurysms were considered a risk factor for hemorrhage in other small studies, but no multivariate modeling was available.76,84 Perata and associates demonstrated the significance of pedicle artery aneurysms in 4 patients.76 They hypothesized that short perforators (thalamoperforate or lenticulostriate) are exposed to higher pressure and flow rates and are more likely to undergo aneurysm formation and rupture during the development of an aneurysm. Batjer and coworkers confirmed the danger of pedicle or feeding artery aneurysms. Of 9 patients with intracranial hemorrhage, 7 bled from the aneurysm.84 All 7 aneurysms were atypical aneurysms located on the major feeding vessel.
Aneurysms may increase in size, remain the same, or regress over time. With definitive AVM treatment, distal flow-related aneurysms are the most likely to regress.77 Proximal aneurysms on the circle of Willis or remote aneurysms are unlikely to change.81,83 Furthermore, in patients with untreated AVMs, new aneurysms may arise over time.
Arteriovenous Malformations in Pregnancy
Cerebral hemorrhage during pregnancy is the third leading cause of maternal death from nonobstetric causes. Maternal mortality as a result of intracranial hemorrhage has been reported to be as high as 40% to 50%.153 Intracerebral hemorrhage is most commonly caused by eclampsia, but it may also be due to AVM or aneurysm rupture, venous thrombosis, hypertension, or choriocarcinoma.154 Although referral sources may bias the frequency, estimates of cerebral hemorrhage secondary to AVM or aneurysm rupture during pregnancy range from 0.01% to 0.05%.155
Controversy exists whether pregnancy increases the risk for bleeding from an AVM.53,98,153–158 Robinson and coauthors reported a 10% risk for subarachnoid hemorrhage from AVMs in nonpregnant females of childbearing age and an 87% risk in association with pregnancy.98 Hemorrhage “associated with pregnancy” was defined as pregnancy within 2 years after diagnosis, hemorrhage during pregnancy or delivery, or hemorrhage less than 2 years after pregnancy. Although Robinson and colleagues noted that there was an 87% risk for hemorrhage during pregnancy, the data actually indicate that among AVMs that are diagnosed during pregnancy, 87% will hemorrhage. With conservative treatment, the fetal death rate was 26% and 29% of mothers died or were disabled.
In contrast to these findings, Horton and coworkers studied 451 women (540 pregnancies) with AVMs, 17 of whom had pregnancies complicated by hemorrhage.156 There was no difference in the hemorrhage rate between the months of pregnancy and all other months of the women’s lives. However, the pregnancy term used was somewhat arbitrary, and a definition of pregnancy that was slightly shorter would have led to a “statistically significant” hemorrhage difference. Patients with no history of hemorrhage had a 3.5% risk of bleeding during pregnancy, which is similar to the risk of bleeding during the nongravid months in the same patients with unruptured AVMs. The risk increased to 5.8% in patients with a history of hemorrhage. Of 17 pregnancies complicated by hemorrhage, 4 resulted in maternal disability and 3 in fetal deaths. This study selected patients chosen for proton beam therapy, thereby potentially eliminating early deaths, severely disabled women, or vascular malformations that would not have been proton beam candidates.
Several studies evaluated risk factors for hemorrhage from AVMs during pregnancy. When compared with patients with hemorrhage from aneurysms, patients with AVMs were more likely to be younger,153,154,159 to have borne fewer children,159 and to initially have been seen commonly between 15 and 20 weeks.159 Dias and Sekhar reviewed 154 cases of hemorrhage during pregnancy as a result of ruptured AVMs or aneurysms.153 They found that 77% of the hemorrhages were due to aneurysm rupture and 23% to AVM rupture. Patients with AVM rupture were more likely to be younger than those with aneurysms, but in this study no difference in parity or gestational age was found. It was also noted in this159 and other studies that there is an increased frequency of hemorrhage with advancing gestational age, possibly caused by a combination of hemodynamic, coagulation, and hormonal factors.
Recurrent hemorrhage is not uncommon and increases the rate of mortality.155 Few patients suffer initial AVM rupture during labor and delivery53,155; however, rebleeding may commonly occur during this time.159 Subsequent pregnancies also carry an increased risk for rebleeding.98,154
Arteriovenous Malformations in Children
In large clinical series, children (<20 years of age) accounted for 15% to 33% of all patients initially seen with AVMs.46,55,130 Symptomatic AVMs during childhood are rare, although the most common cause of intracerebral hemorrhage in children is AVM rupture.34,160 In the only population-based study, 3 of 20 patients with hemorrhage because of a ruptured vascular malformation were younger than 20 years.37
Hemorrhage is by far the most common initial manifestation of the lesion (50% to 79%),161–166 followed by seizures (8% to 25%)161,163–165 and congestive heart failure (18%).162,167,168 Children with initial hemorrhage may be at heightened risk for recurrent hemorrhage.95 Symptoms of congestive heart failure from high left-to-right shunting through the AVM and hydrocephalus predominate in newborns.169 Hemorrhage and seizures occur more frequently in children older than 2 years.46,164,165 In addition to these symptoms, patients may rarely exhibit mental deterioration or progressive neurological symptoms secondary to steal phenomena.170
D’Aliberti and colleagues compared the clinical and angiographic features of 19 children (mean age, 11) and 120 adults with AVMs.171 The main differences between the two groups were sex distribution and the size, depth, location, and complexity of the AVM. AVMs were more common in male pediatric patients. AVMs in pediatric patients tended to be smaller and located superficially. Even though 68% of pediatric patients with AVMs suffered hemorrhage, only 6% had deep venous drainage.
AVMs can grow in size, and this is usually most prominent in the pediatric age group. In addition, AVM recurrence after resection and negative postoperative angiographic findings has been reported.161,165,172 Hdlaky and associates hypothesized that persistent microshunts not evident on early postoperative angiograms may increase over time and predispose patients to recurrent hemorrhage.161
Morbidity and Mortality
Even with newer diagnostic techniques and therapeutic interventions, the mortality associated with AVM hemorrhage in children remains high. Clinical studies of pediatric patients with hemorrhage report a 6.5% to 35% mortality rate.162,164,165,173–175 Hemorrhage location and volume predict mortality.165,174 The mortality associated with posterior fossa hemorrhage was far greater than that with supratentorial lesions. In a study of cerebellar AVMs in children, 6 of 17 (35%) patients died.174 In another series, mortality was 67% in 9 patients with brainstem AVMs.172 The higher mortality in pediatric series may relate to the distribution of posterior fossa AVMs in some studies. In addition, the cumulative morbidity and mortality caused by AVMs in children is high because of the prolonged risk period for potential hemorrhage. The risk for rebleeding has been reported to be between 22% and 29%,165,175 and it can increase mortality.175
Despite high mortality rates, children are more likely than adults to improve after intracerebral hemorrhage caused by AVM rupture.171 D’Aliberti and colleagues found that 89% of children versus 79% of adults had good recovery based on the Glasgow outcome score. Similarly, another study found satisfactory outcomes in 81% of children with AVMs observed over a mean period of 8.5 years.161 In this study, 72% of the patients with hemiparesis recovered fully or were left with only mild residual effects. Neonates with congestive heart failure, however, have a very poor prognosis. In the series of seven neonates described by Melville and coworkers, six died in the first 17 days or at the time of intervention. Although some deaths were due to neurosurgical complications, the poor prognosis may also relate to residual shunting after treatment.169
Cavernous Malformation
Synonyms: cavernous angioma, cavernous hemangioma, cryptic vascular formation,* occult vascular formation,* angiographically occult vascular malformation.*
Definition and Pathogenesis
CMs are well-circumscribed, multilobulated, angiographically occult vascular malformations. Pathologically, they are composed of sinusoidal vascular channels (caverns) lined by a single layer of endothelium. The caverns are separated by collagenous stroma devoid of elastin, smooth muscle, or other mature vascular wall elements. The lack of intervening brain parenchyma is a characteristic pathologic marker. Within the lesion, hyalinization, thrombosis with varying degrees of organization, calcification, cysts, and cholesterol crystals are common and lead CMs to be likened to mulberries. The surrounding parenchyma consistently exhibits evidence of previous microhemorrhage, hemosiderin discoloration, and hemosiderin-filled macrophages. A gliomatous reaction of surrounding parenchyma is characteristic and may form a capsule around the lesion.3,4,17,176–180 Electron microscopy reveals a defect in endothelial tight junctions, which has been hypothesized to be the reason that CMs tend to leak.181 There is no difference in pathology between sporadic and familial forms.182
CT is 70% to 100% sensitive but less than 50% specific in detecting CMs.178,183–185 The typical appearance is a well-circumscribed nodular lesion with calcification. Hemorrhage and cystic components may also be present, and faint enhancement may be seen with the administration of contrast material. MRI is the most sensitive in detecting CMs,186 specifically, gradient echo187–189 and susceptibility-weighted imaging.190 CMs appear as well-defined, lobulated lesions on MRI with a central core of mixed signal intensity surrounded by a rim of signal hypointensity.178,191–197 The mixed signal reflects the lesion’s behavior. The decreased rim of T2 intensity is related to repeated subclinical intralesional and perilesional hemorrhage. Low T2 signal within the lesion reflects intralesional calcification. The hyperintensity reflects acute and subacute hemorrhage in different stages. Residua of previously expanded cisterns that have involuted with thrombus organization and resolution are reflected by the presence of cysts. Some studies have further characterized the MRI appearance of CMs for clinical study (Table 384-2).187,189
TABLE 384-2 Categories of Cavernous Malformation Based on Magnetic Resonance Imaging Characteristics
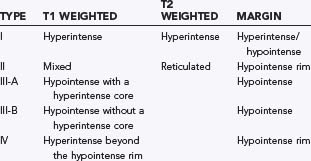
Although MRI is more specific than CT, the MRI appearance is not always pathognomonic. The differential diagnosis should also include thrombosed AVM, calcified tumor (e.g., oligodendroglioma), granuloma, or infectious and inflammatory nodules.178,184,192,194,196,198–200 The absence of systemic neoplasm and surrounding edema and the presence of multiple lesions, calcification, and ossification offer clues distinguishing neoplasms from CMs. A more typical MRI appearance, family history, and multiple lesions may favor CM. Distinguishing CM from other cryptic vascular malformations on imaging studies and even by pathologic evaluation may be more difficult. The designation cryptic or occult vascular malformation refers to angiographically occult vascular malformations. They include CMs but may also include thrombosed AVMs, mixed lesions, or rarely, venous angiomas. The majority of cryptic malformations are CMs.201,202 In a study of 34 consecutive angiographically occult malformations undergoing surgical excision and pathologic examination, 21 were CMs, 3 were AVMs, 3 were VMs, 2 were capillary telangectases, and 5 were mixed lesions.17 One study attempted to find radiographic features that distinguish angiographically occult malformations. Vanefsky and associates found that cryptic CMs were more likely to have hemosiderin rings and absence of edema.203 Rigorous subdivision may not be necessary because angiographically occult vascular malformations of any type may not have a distinctive natural history.17,145,198,204
Because CMs are angiographically occult vascular malformations, angiography is almost always negative.179,180,183,186,195,202,205 Occasional abnormalities seen in association with CMs include an avascular mass, capillary blush, and evidence of neovascularity.180,184,186,195,196,205–210 In addition, CMs are occasionally associated with other vascular malformations that may be detected by angiography, such as AVMs or VMs.
The pathogenesis of CMs is unclear, but ongoing research on familial and acquired forms has helped unravel pieces of the puzzle. Although CMs were originally thought to be congenital, cases of de novo formation have been documented, thus arguing against the congenital theory.189,211–214 De novo formation has been documented in association with radiation therapy,211,212,215,216 along the path of stereotactic biopsy,217 and in association with venous angiomas.218,219 Multiple hypotheses have been presented on why the association of venous angiomas and CMs is common. One group hypothesized that venous hypertension associated with venous angioma may lead to red cell diapedesis, release of angiogenic growth factors, and the subsequent formation of a CM.218,220 Others believe that increased intraluminal pressure within the venous angioma causes reduced tissue perfusion and resultant hypoxia and that the hypoxia stimulates the release of angiogenic factors locally.219 Others are investigating the role of cytomegalovirus infection and CM formation in patients with de novo CM formation after irradiation.221
Several researchers have made considerable progress in understanding the genetics of the familial forms of CM, thereby helping elucidate its pathogenesis. An autosomal dominant pattern of inheritance with incomplete penetrance and variable expression has been described in some families. Investigators have identified three loci in patients with familial CM: CCM1 on chromosome 7q, CCM2 on chromosome 7p, and CCM3 on chromosome 3q.182 Each loci seems to be involved in some way with angiogenesis and integrin signaling pathways (Table 384-3). Familial forms occur more commonly in Hispanic American patients but do appear in the white population as well. Approximately 50% of Hispanic Americans with CMs have a familial form, as compared with 10% to 20% of white individuals with CMs.222
Mutations at the CCM1 loci are responsible for just more than half of the familial forms of CM. The CCM1 locus encodes for the CCM1 or KRIT1 protein and has been mapped to chromosome 7q11.2-q21.182,222–225 The KRIT1 protein has been found in vascular endothelium, astrocytes, and pyramidal cells within the brain.222 KRIT1 knockout mice show marked vascular defects. However, CCM1 gene knockout alone does not result in CM formation; rather, a second gene defect (a tumor suppressor) may be required (two-hit hypothesis).223 The N-terminal of the KRIT1 protein binds to ICAP1α and may be involved in the integrin signaling pathway and cell adhesion to other cells. The KRIT1 protein also colocalizes with β-tubulin in endothelial cells and is thought to interact with the cytoskeleton. It has been hypothesized that this link may result in impaired endothelial cell junctions during a critical phase of angiogenesis and hence result in dilated, leaky capillaries.182
Mutations of the CCM2 locus on chromosome 7p are responsible for 10% to 20% of the familial forms of CM. The malcavernin gene encodes for a protein that interacts with KRIT1 and may influence the p38 mitogen-activated protein kinase pathway, an intracellular kinase that transduces signals necessary for vascular remodeling and maturation.222
The CCM3 locus has been mapped to chromosome 3q25.2-q27 and is responsible for 40% of mutations in the familial forms of CM. The CCM3 or PDCD10 gene encodes for a protein that has been linked to apoptosis, a process important in arterial morphogenesis and in which the β1 integrin cascade is involved.222,223 Integrin molecules are transmembrane receptor proteins that play a critical role in endothelial cell interactions and cell–extracellular matrix interactions, as well as endothelial cell migration in angiogenesis.
Epidemiology
CMs account for approximately 5% to 10% of all vascular malformations66,183,186,226 and are the second most frequent intracranial vascular malformation responsible for hemorrhage in surgical series.227 The exact incidence and prevalence of CMs are unknown because many are asymptomatic. In a population-based study, the age- and gender-adjusted incidence of CM was 0.15 per 100,000 persons37; in another study it was 0.56 per 100,000 persons per year.41 Previous estimates of prevalence were primarily available through autopsy and MRI series. Berry and associates retrospectively found that 0.2% of 6686 consecutive autopsy subjects had CMs; however, they were not specifically looking for vascular malformations and may have underestimated the prevalence.228 Subsequent prospective autopsy series by McCormick4 and Otten and colleagues229 revealed a prevalence of 0.40% (16 of 4069 autopsies) and 0.53% (131 of 24,535 autopsies), respectively. MRI series report similar prevalences ranging from 0.39% to 0.9%.187,230–232
Patients with CMs are typically seen initially between the second and fourth decades.179,180,185,187,230,232–236 In the pediatric age group there is a bimodal distribution, with CMs generally occurring near the ages of 3 and 11.234 The pediatric age group has a higher propensity for overt hemorrhage.179,206,237–239 In contrast, CMs are rarely symptomatic in the elderly.
The overall prevalence in males and females is equal.207,226,230–232,240 Differences arise when patients are stratified by age and the time of initial clinical evaluation. Males typically have symptoms when younger than 30 years, whereas women are usually seen initially between 30 and 60 years of age.231 Above the age of 60, there appears to be an equal prevalence of men and women with CMs. In addition, females are more likely than males to have a CM in the middle fossa, exhibit mixed malformations, and have the CM manifested as hemorrhage.206,231 Males, however, are more likely to initially have seizures.
Familial CMs account for 30% to 50% of patients with CMs186; in other series the figure is slightly lower because of selection bias and aggressiveness in seeking relatives with CMs.236 Most epidemiologic data are similar to sporadic cases of CM.189,241 One difference is the tendency for multiple lesions, up to 84% in one series.189 The prevalence is increased in Hispanic populations. In addition, some studies suggest that successive generations may be affected at earlier ages.
The majority of CMs are supratentorial189,230,231,236 and range between 0.1 and 9 cm with a mean of 1.4 to 1.7 cm.179,180,183,184,187,226,230,231 In the posterior fossa, the pons and cerebellum are the most common sites of involvement. Less common sites that may be involved include the pineal gland, cerebellopontine angle, middle cerebral fossa, cavernous sinus, optic nerve or chiasm, and dura. CMs may also appear extracranially in the orbit or be intraspinal. Most CMs are solitary. Multiple CMs may occur in 10% to 30% of sporadic cases and in up to 84% of familial cases.189,242 The symptoms, however, are usually due to a single dominant lesion.
CMs may be associated with other vascular malformations and central nervous system (CNS) pathology such as CNS tumors, extracerebral soft tissue tumors, and visceral hamartomas.193,207,235,243 Association with VMs, capillary telangiectasia, and AVMs has also been described.17,23,48,184,195,231,244–248 Mixed vascular malformations are thought to be uncommon, but may be underrecognized.
Co-occurrence of CMs and VMs has been reported most commonly, although the exact prevalence is not known. Clinical studies estimate that 2.1% to 36% of patients with CMs have associated venous angiomas.23,248–250 Occasionally, the association is not discovered until the patient undergoes surgery.248 One study suggested a more aggressive clinical course in patients harboring both lesions.250 Patients with associated VMs were more likely to be female, have overt hemorrhage, and suffer repeated hemorrhages. These features were trends but did not reach statistical significance.
Clinical Findings
Asymptomatic
There is a high incidence of asymptomatic lesions, up to 95% in autopsy series.251,252 In MRI series, 11% to 44% of patients referred for unrelated symptoms were found to have CMs.186,231,232,243 This is also evident when evaluating familial patients, in whom few lesions are symptomatic despite the presence of multiple lesions. More males than females are found to have incidental lesions,233 and CMs of all sizes and locations can be clinically silent. Small punctate lesions designated type III (see Table 384-2) by Kim and associates were rarely symptomatic and often found in familial cases.187
Seizures
Because most CMs are supratentorial, it is not surprising that seizures are the most common initial symptom.179,180,185–187,189,208,230,231,240 CMs are more likely than AVMs and VMs to be associated with seizures secondary to recurrent microhemorrhages and local gliomatous reaction.178,186,253,254 Calcification, a thick gliomatous capsule, temporal location, and extensive hemosiderin deposition are frequently associated with the clinical appearance of epilepsy.180,185,207,208,255 Males more commonly have seizures than females.231 All types of seizures have been described in association with CMs—simple partial, complex partial, and generalized tonic-clonic seizures.180,207,226 The epileptogenic mechanism has been proposed to relate to gliosis, blood breakdown products, possible associated venous hypertension, and cellular and humoral inflammatory responses.256
Hemorrhage
Evidence of previous hemorrhage is present in every lesion regardless of clinical history. CMs have persistent intralesional microhemorrhages that occur over time.* Hemorrhage within the area of the hemosiderin ring is rarely associated with symptoms. Overt hemorrhage, however, is less common but more clinically significant. Overt hemorrhage has been defined as (1) MRI signal of acute or subacute blood outside the hemosiderin ring, (2) evidence of previous hemorrhage on lumbar puncture, and (3) fresh clot outside the confines of the lesion at surgery.187,231 Clinical series report that 8% to 37% of patients have hemorrhage as the initial symptom.211 Although patients with CM most commonly have intraparenchymal hemorrhage, subarachnoid and intraventricular hemorrhage has also been described.206,244,259,260 Several clinical series report a higher propensity for overt hemorrhage in women and children.231,239,261,262
Focal Deficit
Patients may be seen with an acute or subacute focal deficit, usually caused by an intralesional or perilesional hemorrhage. The frequency in clinical series is 15.4% to 46.6%.* The deficit may be transient, progressive, recurrent, or fixed. Recurrent symptoms may mimic the course of demyelinating disease. The initial symptoms depend on the location and size of the lesion. Rarely, patients may have cranial neuropathies (trigeminal neuralgia), hypothalamic symptoms, or hydrocephalus.147,179,183,186
Natural History and Outcome
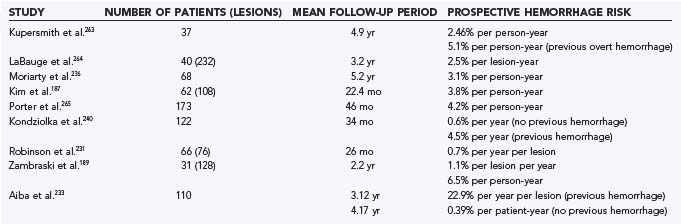
Prospective Hemorrhage Risk
The annual prospective hemorrhage risk in patients with CMs has been estimated to be between 0.7% and 4.2% per patient-year (Table 384-4).187,189,231,233,236,240,263–265 The single most important risk factor for hemorrhage is previous overt hemorrhage.180,183–185,189,197,204,208,231,243,244 This finding has been consistent in most prospective studies. Other risk factors for hemorrhage suggested by some, but not always confirmed in other studies, include female sex,231,236 pregnancy,231,232,257,258 presence of coexistent VM,250 size,263 and deep location.266
Kondziolka and colleagues studied 122 patients over a mean period of 34 months.240 Nine patients had hemorrhage during the prospective follow-up period. Patients with previous hemorrhage had a greater annual risk for hemorrhage (4.5% per year) than did those without previous hemorrhage (0.6% per year). No difference was found with regard to location, sex, or manifestation. Aiba and associates also found previous hemorrhage to be a risk factor for recurrent bleeding, especially in younger females.233 In a retrospective study, 62 of 110 patients with intracranial CMs initially had hemorrhage. The risk for hemorrhage in patients with previous hemorrhage was 22.9% per year per lesion over a 3.1-year follow-up. Recurrent hemorrhage attributable to the initial lesion was found in 23 patients, and 4 had recurrent hemorrhage involving the initial lesion or another lesion (or both). In contrast, in patients initially evaluated for seizure or other symptoms, the hemorrhage rate was just 0.39% per year per patient. The rate of recurrent hemorrhage may be higher in this study than in others because the definition of intracerebral hemorrhage included patients with intralesional or perilesional hemorrhage.
Deep location was found to be a risk factor in only one study. Porter and collaborators studied 110 patients over a mean period of 46 months.266 The overall reported risk for hemorrhage was 4.2% per patient-year (18 events in 427 patient-years of follow-up). Patients with deep CMs (basal ganglia, thalamus, cerebellar nuclei, brainstem) had a significantly higher rate of hemorrhage (10.6% per patient-year) than did those with superficially located lesions (0%).
Female sex was a risk factor for hemorrhage in two prospective studies. Moriarty and associates prospectively studied 68 patients with 228 lesions over a 5.2-year period.236 The overall prospective risk for hemorrhage was 3.1% per patient-year. Female patients had a significantly higher rate (4.2% per patient-year versus 0.9% per patient-year for males), even though a similar number of males and females were initially seen for evaluation of hemorrhage. This study did not find initial findings, size, or location to be a risk factor. Robinson and colleagues also found a significant difference in the rate of hemorrhage between male and female patients.231 Females made up 86% of the hemorrhage group, 2 of whom were in their first trimester of pregnancy.
Case reports and small case series have suggested an aggressive clinical course during pregnancy.204,206,231,232,265,267–273 Sage and coauthors reported a significant number of pregnant women with an acute onset of neurological deficits.232 Others have shown that the size of the CM may increase during pregnancy and decrease afterward.273 Proposed mechanisms for this increased risk include the following: (1) vascular proliferation may occur as a result of hormonal changes,273 (2) estrogen may reduce γ-aminobutyric acid A (GABAA) chloride channels in the temporal lobe and thus increase potential excitability and therefore seizures, (3) increased thrombosis within the CM leads to expansion, and (4) estrogen may degenerate endothelium. Other series, however, have not found an increase in risk for symptoms in women, although the number of pregnancies is not always clear.240 In a series of 292 patients at the Mayo Clinic, 54% of whom were female, none exhibited exacerbation of symptoms during pregnancy (unpublished). In most of these patients, however, the CM was diagnosed many years after pregnancy. It is likely that this risk factor is overestimated in its significance.
Zambraski and colleagues reported the natural history of CMs in familial cases. Fifty-nine members of six families were prospectively evaluated at 6- to 12-month intervals.189 Fifty-three percent (31 patients) had CMs, 19 of which were symptomatic. The patients were observed over a mean period of 2.2 years. The incidence of symptomatic hemorrhage during follow-up was 1.1% per lesion per year. Patients with previous hemorrhage were more likely to experience rebleeding. LaBauge and coworkers found a slightly higher risk of 2.5% per lesion per year in a group of 40 familial CM patients harboring 232 lesions.264
One issue that has been noted by several authors is an apparent temporal clustering of hemorrhages.274,275 Barker and colleagues studied 141 patients, all with clinically overt hemorrhage.274 They noted that the monthly rebleeding rate was 2% initially but fell to less than 1% per month at 2.5 years. Similarly, Duffau and associates noted that 11% of patients in a mixed cohort of patients with CMs had early rebleeding (within 1 month of previous hemorrhage).275
Hemorrhage Morbidity and Mortality
Initial hemorrhages are rarely associated with deterioration or death.231,244 Patients generally achieve fair or good recovery after an initial hemorrhage.231,244,266 In one study, the mortality rate was 5.3% and no deaths were due to the CM.231 All conservatively treated patients had a good or fair outcome, including 2 with overt hemorrhage. Aiba and collaborators similarly found good or excellent recovery in patients with hemorrhage treated surgically or conservatively.233 The only death was due to repeated brainstem hemorrhage. Moderate or severe disability was noted in 10 of the 62 patients, 6 with recurrent bleeding, 2 with initial bleeding, and operative complications in 2. All lesions were in eloquent areas of the brain, and all patients in the nonhemorrhagic group had a good outcome.
Infratentorial location, but not CM size or multiplicity, increases the risk for neurological disability from hemorrhage. Patients with infratentorial bleeding are 6.75 times more likely to be neurologically disabled than those with supratentorial hemorrhage.257 Thirty conservatively treated patients with brainstem CMs were monitored over a mean period of 35.7 months.276 The mortality in this group was 20%. It is notable, however, that 66.6% of the survivors had mild or no deficits.
Recurrent overt hemorrhage may occur from days to years after an initial bleeding episode and is associated with increased morbidity and cumulative disability.183,184,186,230–232,244,257,275 In the series by Tung and colleagues,260 correlation was noted between the number of recurrent hemorrhages, the location of recurrent hemorrhages, and the occurrence of persistent neurological deficits. In most cases (80%) the initial hemorrhage caused only a transient deficit; however, with each successive recurrent bleeding episode, the likelihood of persistent deficits increased.260
Seizure Risk
Few studies have evaluated the prospective risk for seizures. Moriarty and coauthors reported an overall risk of 4.8% per patient-year over a 5.2-year mean follow-up period.236 In the subgroup of patients without previous seizures, the risk was 2.4% per patient-year. Similarly, Kondiziolka and coworkers reported a 4.3% risk for new seizures over a 34-month period.240 The only risk factors determined for seizure occurrence were multiple CMs and supratentorial location. These risk factors have been confirmed by others.230,256 Temporal, frontal, or perilimbic location may have the highest risk.256
Data suggest that the majority of patients with seizures related to CMs are well controlled with medication.254,256 Some suggest that the temporal location may be more refractory to medication and that the disability from seizures was greater in younger patients.258 Patients younger than 40 years were 5.6 times more likely to have disability from seizures than were those initially seen after the age of 40. Although multiplicity may be a risk factor for seizures, it was not found to be a risk factor for disability from seizures.257
Lesion Behavior
Progressive or new focal neurological deficits may occur. They may be due to lesion growth, de novo CM formation, or hemorrhage. CMs are dynamic entities. Robinson and coauthors reported that 40% of initially silent lesions became symptomatic within 6 months to 2 years.231,258 The risk factors for symptomatic transformation are not known, but changes are thought to be due to intralesional hemorrhage, thrombosis, organization, calcification, and involution of caverns.184,189,244,277 Neuroimaging has shown changes in size and lesion characteristics over time in 8% to 38% of patients.48,180,184,189,192,232,244,262,277 Zambraski and colleagues documented 4 patients with increased growth, 1 with regression, and 17 with de novo lesions (0.4 lesions per patient-year).189 Kim and associates found that some type II lesions changed to type IV and vice versa.187
Dural Arteriovenous Fistula
Synonyms: dural arteriovenous malformation,* dural arteriovenous fistulous malformation.
Definition and Pathogenesis
DAVFs are arteriovenous shunts from a dural arterial supply to a dural venous drainage channel. Any dural vessel is a potential source of supply, although most commonly the occipital and meningeal arteries are involved.7 Venous drainage is through the dural sinus or other dural and leptomeningeal venous channels. DAVFs are often described according to the venous sinus to which they are related or the anatomic location of the fistula. On gross pathologic examination, the dural arteries are thickened and the veins dilated in an abnormal vascular network within the wall of a venous sinus.278 Numerous microfistulas are seen on histologic examination. Fistulas may have high or low flow and unilateral or bilateral supply. Demyelination may be seen around leptomeningeal veins as a result of venous hypertension.3
Angiography is the “gold standard” for diagnosis and delineation of the details of the arterial supply and venous drainage. Injection must include the vertebral and internal and external carotid arteries because DAVF may be overlooked with conventional studies alone. Premature appearance during the early arterial phase of a venous structure within or adjacent to the dura mater is characteristic. Visualization of dilated pachymeningeal arteries lends supporting evidence.279 Findings on CT are often normal, although it is the most sensitive in evaluating acute subarachnoid, subdural, or intraparenchymal blood. If the fistulas drain to cortical veins, contrast-enhanced CT may demonstrate serpiginous enhancement. MRI is able to characterize the anatomy and areas of ischemia and chronic hemorrhage. In addition, evidence of dilated cortical veins may suggest a DAVF with venous hypertension. Newer magnetic resonance angiography and venography techniques may indicate the location and extent of the fistula, but these studies do not have sufficient resolution to fully characterize the lesion.280,281 Time-of-flight magnetic resonance angiography demonstrates high-intensity curvilinear or nodular structures adjacent to the dural sinus wall and high-intensity areas in the venous sinues.281 Both MRI and CT can miss DAVF, especially if small281 or if the DAVF drains into the ipsilateral venous sinus.282–284
The vast majority of DAVFs are acquired, idiopathic lesions.1,279,284–293 In a minority of cases a possible cause is found, and the site and characteristics of the fistula may provide a clue to the particular etiology. Possible causes include trauma,294–296 cranial surgery,295,296 sinus infection,289 and association with meningiomas.297–299 Syndromes associated with vascular fragility, such as Ehlers-Danlos syndrome, fibromuscular dysplasia, and neurofibromatosis type 1, may also have associated DAVF. In addition, many studies have shown an association with venous sinus thrombosis.287,288,297–301 In some cases, the thrombosis precedes formation of the DAVF. It has been proposed that thrombosis enlarges the normally present microscopic arteriovenous shunt in the walls of the sinus or stimulates their development.284,302 Other studies have shown sinus thrombosis to follow the formation of a DAVF.303,304 Here, it is believed that turbulence and epithelial damage predispose to clotting. Chaloupka believes that various initiating events lead to a common path of angiogenesis within the dura.303 The resulting budding and proliferation of microarterial or capillary vessels within the dura could create numerous microvascular arteriovenous fistulas. In support of this theory are pathologic specimens of DAVF containing angiogenic growth factor and basic fibroblast growth factor, but it is not clear whether this is a cause or the effect of formation of the DAVF.305,306 Whatever the inciting event, the final common path that may evolve was described by Awad and associates.302 The arteriovenous shunting vessels result in recruitment of arterial feeding vessels (sump effect) with secondary venous hypertension. Venous hypertension can lead to leptomeningeal retrograde drainage and predispose these channels to become varicose and potentially rupture.
Epidemiology
The prevalence of DAVF is unknown because some remain asymptomatic for years. The estimated age- and sex-adjusted incidence from a population-based study was 0.17 per 100,000 persons, but this is likely to underestimate the incidence of DAVF because of the number of asymptomatic lesions not reported.37 Based on imaging studies, DAVFs are found on 1.1% of consecutive angiograms and are a fifth as common as AVMs.302 DAVFs represent approximately 10% to 15% of all intracranial vascular malformations,132,285,286,302,307,308 and patients are typically seen initially between the ages of 40 and 60. Carotid cavernous and transverse/sigmoid DAVFs are more common in women,309 whereas anterior fossa and tentorial DAVFs are more common in men.309
The transverse/sigmoid location is the most common and represents more than 50% of DAVFs. The majority are idiopathic, but they may also occur as a result of a linear fracture across the transverse venous sinus. Approximately 10% to 15% are located in the cavernous sinus. Women may be affected during pregnancy or after menopause, thus suggesting a possible hormonal relationship. In addition, a history of sinusitis has been described in patients with carotid-cavernous fistulas. The anterior fossa, tentorial incisura, sylvian/middle fossa, and sagittal sinus are less common locations.279 DAVFs are usually solitary, but multiplicity has been reported in about 8% of cases.310–313 In a series of 235 patients with dural and cranial arteriovenous fistulas, 19 (8.1%) had multiple fistulas.310
Given the potential association of sinus thrombosis and development of DAVFs, the frequency of potential hypercoagulable states was assessed in a meta-analysis of 121 patients with DAVFs and 178 healthy controls. Thrombophilia mutations were noted in 16 patients with DAVFs and 4 controls. The OR was 4.69 for detection of factor V Leiden positivity and 10.87 for the prothrombin G20210A allele. Widespread screening was not advocated given that a hypercoagulable state is not found in most patients with DAVFs.314
Clinical Findings
The natural history of DAVF is highly variable. Some patients have no symptoms or benign symptomatology for many years and DAVF is only an incidental finding on angiography performed for other reasons.286,287,315–317 These fistulas are generally small or located near the occiput284 and are not associated with cortical venous drainage.318 Others exhibit aggressive and catastrophic behavior. The clinical behavior, including the initial findings, eventual behavior, and prognosis, are a complex function of multiple factors, such as location, mode of venous drainage, and magnitude of flow, as well as a host of angiologic and hormonal factors.
Reversal of flow and venous hypertension in the leptomeningeal draining veins account for the majority of neurological manifestations.319,320 Nonhemorrhagic symptoms most commonly include pulsatile tinnitus and headache. Other manifestations include global or focal (or both) neurological deficits that may be transient or progressive, seizures, and pseudotumor (papilledema with normal MRI findings). Rarely, patients may be initially evaluated for hydrocephalus, dementia,321,322 cranial nerve palsies,323 and cervical myelopathy.324,325 Cervical myelopathy may occur as a result of venous hypertension affecting the spinal cord through medullary veins distant from the cranial DAVF. For cavernous sinus lesions, ocular symptoms are common and include proptosis and chemosis.293
Pulsatile Tinnitus
Pulsatile tinnitus is the most common initial symptom and is frequently associated with high-flow fistulas in the transverse sinus/sigmoid location.279,284,289 Tinnitus occurs because of recruitment of arterial feeders by the DAVF and its close proximity to the middle ear.302 A bruit can often be auscultated over the cranium. Compression of the carotid artery and jugular vein or occipital artery may result in a reduction in bruit intensity and provide a clue to the diagnosis. This is uncommonly seen in patients with DAVFs that have predominately cortical venous drainage, with pulsatile tinnitus occurring in 3% of such patients.326
Hemorrhage
Intraparenchymal hemorrhage, subarachnoid hemorrhage, and subdural hemorrhage may occur, but they are a distinctly uncommon manifestation of DAVF. The risk for hemorrhage has been assessed in terms of both the likelihood of hemorrhage being the initial symptom of a DAVF and the annual risk for hemorrhage when monitoring a cohort of patients with a nonhemorrhagic manifestation. In a group of 402 patients with DAVFs, 73 (18%) were initially seen with intracranial hemorrhage.291 Hemorrhage most commonly occurred in those aged 51 to 60 years, followed by those 61 to 70 years of age. Men were more likely than women to have hemorrhage as the initial symptom (74% of those with hemorrhage were men). With assessment of angiographic characteristics, a higher proportion of patients with posterior fossa and ethmoidal DAVFs and smaller proportion of those with DAVFs in the cavernous sinus exhibited hemorrhage. Venous drainage characteristics were also assessed, and those with hemorrhage as the initial symptom were more likely to have cortical venous drainage (85% of those with hemorrhage versus 22% of those without hemorrhage), retrograde venous drainage (59% versus 36%), and venous sinus occlusion (33% versus 18%). A multivariate logistic regression analysis indicated that independent predictors of DAVF with hemorrhage were male sex (OR, 3.4; P = .001), age older than 50 years (OR, 2.2; P = .046), focal neurological deficits (OR, 4.7; P < .001), posterior fossa location (OR, 3.9; P = .005), and cortical venous drainage (OR, 10.5; P < .001).
A cohort of 54 patients with unruptured DAVFs were observed over a mean of 6.6 years, and the annual risk for hemorrhage was 1.8%.307 Lesions of the petrosal or straight sinus had a higher incidence of hemorrhage, as did those with a venous varix. Sixty percent of DAVFs draining into a venous varix and none without this characteristic experienced hemorrhage associated with the DAVF. The presence of leptomeningeal venous drainage showed a trend toward increased risk for hemorrhage, but this was not found to be significant. Another cohort of 85 patients with cortical venous drainage included 53 without hemorrhage and 32 with hemorrhage. The annual risk for hemorrhage for the 53 without hemorrhage was 1.5% per year (one hemorrhage), and for those with hemorrhage it was 7.4% per year (three hemorrhages).292
The studies available have consistently suggested that patients with retrograde cortical venous drainage have a higher likelihood of hemorrhage initially.290,327 In one series of 91 patients with DAVFs, 29 had cortical venous drainage and 62% of these 29 had hemorrhage as the initial symptom.326 A retrospective study of 93 patients with DAVFs suggested that leptomeningeal venous drainage and dysplastic venous dilation are associated with an increased risk for hemorrhage.328 Persistent cortical venous reflux (Borden grade 2 or 3) was seen in 14 untreated patients observed over a period of 4.3 years in a large single-center series.329 Seven patients had intracranial hemorrhage (8.1% risk per year), nonhemorrhagic neurological deficits occurred in 30% (6.9% risk per year), and 9 patients died. Two had spontaneous closure of the DAVF.330
Other studies have assessed risk factors for the prediction of aggressive DAVFs, specifically, those with a hemorrhagic manifestation or progressive neurological deficit. Most conclude that the risk for hemorrhage is directly related to impedance of outflow. Lesions with anterograde flow through larger sinuses have a lower risk than do those with restricted or retrograde flow into sinuses and cortical veins.284,292,293,308,315,326,331 Awad and colleagues found leptomeningeal venous drainage, variceal or aneurysmal venous structure, and galenic venous drainage to predict an aggressive course.302 In this meta-analysis of 17 personal patients and 360 from the literature, DAVFs associated with the tentorial incisura more often had an aggressive course, but no location was immune. Another study assessing 69 patients with DAVFs evaluated the risk for aggressive manifestations, including hemorrhage, venous infarction, seizures, altered mental status, and intracranial hypertension. Independent risk factors for such manifestations included Cognard types IIb, IIa + b, III, IV, and V,309 as well as DAVFs occurring in “other sinuses” (defined as being outside the cavernous or other “large sinuses”).301 Fistula multiplicity may also increase the risk for hemorrhage as the initial symptom, with hemorrhage being 3 times more likely in those with multiple fistulas in one study.310 Cortical venous drainage was present in 84% of patients with multiple lesions as compared with 46% of patients with single lesions. Another venous pattern associated with an adverse outcome is tortuous, engorged veins in the venous phase of the cerebral circulation, called the pseudophlebitic pattern in one study, in patients with venous congestion related to a DAVF.332 Such findings were present in 42% of the patients overall, including 73% of those with hemorrhage, neurological deficit, or seizure; 21% had a bruit or orbital findings. This pattern was seen in 81% of those with retrograde leptomeningeal venous drainage and 8% with sinus drainage. The aggressive behavior was noted in patients with or without such leptomeningeal venous drainage, thus suggesting that this is another factor predicting an adverse outcome and of importance in making management decisions.332
Numerous classification systems have been proposed and are useful in determining which DAVFs will have a more aggressive course.309,326,329,333–335 Most classification and grading systems take into account the following: the presence of venous stenosis or occlusion, the direction of flow, and the presence of leptomeningeal venous drainage, aneurysms, and single or multiple fistulas. Table 384-5 presents several classification systems and the associated risk for hemorrhage.
Few data are available on the risk for recurrent hemorrhage. Previous case reports suggest that it occurs uncommonly.302,336 A group of 20 patients with DAVFs were observed for a median of 10 months after initial hemorrhage (15 with intracerebral hemorrhage, 3 with subarachnoid hemorrhage, and 2 with subdural hematoma).337 All patients had retrograde cortical venous drainage. Rebleeding occurred in 7 (35%) within 2 weeks after the first hemorrhage. Three patients died and 1 worsened after rebleeding. In a follow-up of 32 patients with cortical venous drainage and hemorrhage, 3 patients had repeated hemorrhage after diagnosis, for an annual risk of 7.4% per year.292
Patients with cortical venous drainage are at higher risk for significant morbidity, whereas those without cortical venous drainage have a more benign course. In a group of 117 patients with such benign DAVFs, observational management occurred in 73 patients. Intolerable bruit or ophthalmologic sequelae led to palliative embolization in 43 and surgery in 1. Overall, a good outcome was achieved in 110 of the 112 patients. Cortical venous drainage developed in 2 untreated patients, thus suggesting that close clinical follow-up in these patients with a “benign” appearance is indicated.318
Specific Symptoms/Location
Carotid-cavernous fistulas may be manifested as proptosis, chemosis, retro-orbital pain, and ophthalmoplegia. These symptoms are related to venous hypertension and reduced venous drainage from the orbits. Over time, this can result in choroid and retinal detachment, central retinal venous thrombosis, and reduction in visual acuity. Treatment is usually necessary. If the patient has already been reduced to light perception only, the prognosis for return of vision is poor.338
DAVFs may change over time. A significant number of benign DAVFs will spontaneously thrombose, occasionally after angiography or compression.339–341 In addition, acute or gradual progression from one grade to the next has been documented. Patients may experience a new neurological deficit or change in the nature of their symptoms. A sustained change in the character or intensity of the bruit may herald a change in the nature of the fistula. A change or reduction in the bruit does not necessarily mean obliteration of the fistula and may in some cases signal development of alternative paths such as the leptomeningeal vein. Therefore, any changes in the bruit or symptoms of a benign DAVF warrant reevaluation of the lesion.
Venous Malformation
Synonyms: developmental venous anomaly, venous angioma.
Definition and Pathogenesis
VMs are congenital venous anomalies.342–346 Saito and Kobayshi hypothesized that an intrauterine ischemic event occurs during formation of the medullary veins and results in collateral venous drainage.342 Pathologically, these malformations are characterized by anomalous veins separated by normal brain tissue. On histologic section, the walls of the veins are thickened and hyalinized and usually lack elastic tissue and smooth muscle.3,347 Despite these histologic abnormalities, venous angiomas drain normal cerebral tissue. Contrast-enhanced imaging studies reveal a stellate mass or linear enhancement of a transcerebral vein without parenchymal abnormalities.348 Angiography typically shows normal findings during the arterial and capillary phase. During the venous phase, however, arcades of veins or caput medusae is visualized converging into a large venous channel.349,350
Epidemiology
The exact prevalence of VMs is unknown. In an autopsy series of 4069 consecutive subjects, 105 (2.6%) were found to have a venous angioma.66 Autopsy studies also reveal that VMs are the most common type of vascular malformation and account for up to 65% of cases.36 Similarly, radiographic series show that VMs are common among vascular malformations. One series found that 50% of patients with vascular malformations diagnosed by MRI were venous angiomas.351 In a population-based study in Olmsted County, Minnesota, the incidence of VMs was second only to that of AVMs, with an age- and sex-adjusted detection incidence rate of 0.41 per 100,000 person-years.37 In the Scottish Intracranial Vascular Malformation Study, the detection rate was 0.43 per 100,000 adults per year.41 The diagnosis is typically made in the third decade351–353; however, VMs have been reported in children, as well as in adults in the eighth decade. There is an equal prevalence in men and women.351,352
VMs are typically located at the junction of the superficial and deep venous systems. They may occur anywhere in the CNS but most commonly within the cerebrum and cerebellum, typically adjacent to a cortical surface or near the ependymal surface of the ventricles. The converging vein of infratentorial VMs is deeply located near or in the choroid plexus and dentate nucleus in the inferior half of the cerebellum. Therefore, infratentorial VMs are commonly found in the cerebellum. Although some report that intracranial VMs are predominantly located in the posterior fossa,308,344,353–356 other series dispute this finding.348,351,352 In one large study the distribution of VMs was as follows: frontal, 42; parietal, 24; occipital, 4; temporal, 2; basal/ventricular, 11; cerebellum, 14; and brainstem, 3.351 They are most commonly solitary, but several cases of multiple VMs have been reported.355
The association of VMs with other vascular malformations is established.218,248,349,357–362 Association has been noted with AVMs,349,359,363,364 as well as CMs.23,184,195,231,244,245,247,248 Mullan and coworkers hypothesized that the association of VMs and AVMs may be related to failure of the development of the cortical venous mantle.364 Komiyama and colleagues reviewed 31 patients with AVMs and VMs and found no difference in the prognosis of those with or without an associated AVM.363 The association of VMs with CMs and occult vascular malformations is also well documented.218,248,249,355,357,361,365,366 Abe and coworkers found that 23% of patients with occult vascular malformations also had a VM.362 Although some may represent a coexistent congenital abnormality, there may be a subgroup of CMs that arise de novo in the vicinity of a VM.218,357,367 It is hypothesized that regional venous hypertension may facilitate CM formation by red cell diapedesis and release of angiogenic growth factors.218,357,367 In addition, a case report described the development of both a CM and capillary telangiectasia after a VM.368
Other pathology, including infarcts and tumors, is found in 10% to 20% of patients with VMs.351,353,369 It is unclear whether the two are related or whether the selection of patients for contrast-enhanced imaging leads to this observation. In addition, a case report of a venous anomaly associated with facial hemangiomas has been described.370
Clinical Findings
VMs rarely cause symptoms and are often incidental findings.344,348,370 They may be considered symptomatic if the location is consistent with the signs and symptoms of the initial illness or if hemorrhage occurs within the VM.
Patients with VMs may rarely be seen initially with hemorrhage,371 venous thrombosis/infarction,372,373 and seizures.342,373,374 Other symptoms have been attributed to VMs in the past but were probably not related to the VM. Other pathology should be sought before attributing signs and symptoms to venous angioma.348
Hemorrhage
Before improved neurodiagnostic techniques, VMs were recognized only at times of catastrophic events, which led to the belief that complications related to VMs were frequent.308,344,347,349,354,358,366,375 Hemorrhage related to venous angiomas has been reported in 8% to 43% of patients in older clinical series. Previous studies have also shown an increased risk for hemorrhage with posterior fossa lesions48,308,344,347,356,375–377 and in association with pregnancy.308 Subsequently, several large studies have evaluated the hemorrhagic risk and prognosis of these vascular malformations and suggested that the risk is small or attributable to coexistent lesions.351,353,378
Garner and colleagues retrospectively reviewed 100 patients with radiographically diagnosed VMs.351 Less than 20% were considered to be symptomatic. Six patients were initially evaluated for hemorrhage, and only one was attributed to the venous angioma. The risk for hemorrhage was calculated to be 0.22% per year (one hemorrhage in 4498 person-years). The lower risk found in this study than in older studies is related to the increased recognition of incidental venous angiomas documented on MRI. In addition, the authors believe this to be a high estimate of risk because of the nature of tertiary care referrals.
McLaughlin and associates retrospectively reviewed the charts of 80 patients with VMs and prospectively observed them over a mean period of 3.6 years.353 There were 16 bleeding episodes at the time of initial registration and 2 additional ones in subsequent follow-up. They calculated a retrospective hemorrhage rate of 0.61% per year (18 bleeding episodes in 2949 patient-years). In the prospective group, the annual rate was 0.34% (2 bleeding events in 298 patient-years). Sex, location, or size did not influence the risk. The authors concluded that the rate of hemorrhage was similar to that for CMs and that perhaps unidentified CMs associated with the venous angioma were the cause of the bleeding.
Others agree that caution should be exercised in attributing hemorrhage to VMs and that other pathologies should be searched for.348,356,379 In one study, two patients sustained hemorrhage initially attributed to a VM. The presence of a CM was suggested on MRI in one patient, but not clearly. Both patients underwent surgery for the hemorrhage and were found to have pathologically confirmed CMs associated with a VM.248
The risk for recurrent bleeding from venous angiomas has not been well defined. A review of 27 cases from the literature reveal that 5 (18.5%) had documented recurrence.354,356 No risk factors for recurrence were identified because of the small numbers. These cases were also reported before 1985, and other pathology may not have been found because of the poorer resolution of imaging at that time.
Seizures
There are several case reports suggesting the role of VMs in seizure disorders. In one study, 11 patients with VMs and no other lesion had seizures. Electroencephalographic (EEG) localization was inconsistent in 8 of the 11 patients.342 In another study by Garner and coworkers, 5 of 23 patients with seizures had EEG localization consistent with the VM.351 Additional case reports suggest a relationship between VMs and seizures, including one in which the VM thrombosed.373,374,380 Several of these studies were published before the availability of gradient echo imaging, and one wonders whether an associated CM was present as the cause of the seizure rather than the VM.
Focal Deficits
Rarely, venous anomalies may thrombose and result in infarction.372,373,381,382 This may be the predisposing event before hemorrhage.371
It is difficult to ascertain the relationship of the venous angioma to a neurological deficit in the absence of hemorrhage or thrombosis. No pathologic evidence of ischemic changes or microhemorrhages has been demonstrated. In addition, MRI findings rarely show a mass effect unless there is associated bleeding. Nonetheless, focal deficits are occasionally attributed to VMs. Eight patients in one series were found to have VMs potentially causing focal deficits in the absence of hemorrhage,351 none of which were disabling. Kondziolka and associates found 2 patients with focal deficits potentially related to a VM.352 One had hemiparesis and a venous angioma in the contralateral posterior internal capsule. The other had a movement disorder and an associated contralateral caudate nucleus VM. Cerebellar lesions may be more likely to result in focal deficits. In one study, 10 of 12 patients with cerebellar VMs had signs or symptoms attributable to the lesion, such as ataxia, diplopia, or dizziness.348
Morbidity/Mortality
VMs can rarely lead to significant morbidity or mortality. In 14 patients with symptomatic VMs monitored over a mean period of 3.7 years, no death or significant morbidity was attributed to the VM.352 Garner and colleagues observed 100 patients over a mean period of 2.46 years.351 Eleven patients had died and 6 were disabled, but none were related to the VM. In another series, just 4 of 30 patients had persistent symptoms (2 with seizure, 1 with ataxia, and 1 with headache). No new hemorrhage or focal deficits developed in the 45-month follow-up in these patients.348
Because VMs drain normal brain parenchyma, treatment may result in devastating venous infarcts.343,354 Therefore, the risk associated with surgery is not outweighed by the benign nature of these lesions. It is not clear whether a subset may exist that will require more aggressive treatment.
Capillary Telangiectasia
Synonym: capillary malformation.
Definition and Pathogenesis
Capillary telangiectases are vascular malformations composed of dilated capillaries with normal intervening neural tissue. Microscopically, they consist of ectatic individual vessels with thin capillary walls that course among normal architectural elements without adjacent gliosis or hemosiderin deposition.3
Imaging studies may often miss capillary telangiectases because of their size and the absence of hemorrhage. These lesions are nearly always angiographically occult.7 CT findings are often normal383 but may display variable enhancement without a mass effect.7 MRI may reveal small enhancing lesions with a brush-like pattern, particularly in the pons, but this is often undetectable on standard T1- and T2-weighted images.384,385 The differential diagnosis of an enhancing pontine lesion includes capillary telangiectasia, neoplasm, subacute infarction, and demyelinating or inflammatory disease. The lack of a mass effect or increased T2 signal argues against other pathology. In addition, capillary telangiectasia is the only one in this group of pathologies that is composed of sacs of stagnant blood with deoxyhemoglobin and therefore exhibits susceptibility dephasing, which is evident only on gradient echo images.384
Controversy exists regarding the pathogenesis of capillary telangiectasia.247,385,386 Some believe that these lesions are congenital, whereas others believe that the association with other vascular malformations and the age at diagnosis suggest an acquired etiology. Rigamonti and colleagues hypothesized that CMs and capillary telangiectases have similar origins but are on different ends of a spectrum.247 The only feature that distinguishes capillary telangiectasia from a CM is the presence or absence of intervening brain parenchyma between the vascular channels. Satellite telangiectases have been found near CMs, and transitional vascular malformations have been described.368 Therefore, Rigamonti and associates suggested a single category of vascular malformation based on pathogenesis. The clear difference in the natural history of these two entities, however, argues against combining CMs and capillary telangiectases.
Epidemiology
Little information exists on the incidence and prevalence of capillary telangiectases. Autopsy studies reveal a prevalence of 0.06% to 0.4%.36,387 McCormick and coworkers found 60 capillary telangiectases, 38 of which were in the posterior fossa and 22 were supratentorial,66 and they were the second most commonly detected vascular malformation in that autopsy series.
Capillary telangiectases are most commonly located in the pons3,4,252 but may occur elsewhere in the CNS, as well as in other organs. Intracranially, they are most often solitary; however, multiple lesions have been described.388
The coexistence of capillary telangiectases with other vascular malformations has been described.23,247,384 Telangiectases may coexist with sporadic or syndromic CNS vascular malformations such as Rendu-Osler-Weber syndrome, ataxia-telangiectasia, or Wyburn-Mason syndrome. They have been described in association with all types of vascular malformations, but the association with CMs has been of particular interest because of the potential common pathogenesis.247
Clinical Findings
Capillary telangiectases are common incidental findings at autopsy and are rarely symptomatic. In the series by Sarwar and McCormick, only 2 of 60 patients had symptoms related to the capillary telangiectases.36 Vague, nonspecific neurological symptoms often lead to the incidental discovery of capillary telangiectasia.
Case reports of all types of neurological symptoms, including hemorrhage, seizures, cranial nerve palsies, extrapyramidal disorders, and focal hemispheric syndromes, have been described. Focal symptoms are thought to be secondary to hemorrhage, ischemic necrosis, or progression of the lesion.252
Hemorrhage
Patients with capillary telangiectases may initially be evaluated because of intraparenchymal or subarachnoid hemorrhage, although this is quite rare.252,388–391 In a population-based study of hemorrhage secondary to vascular malformations, no hemorrhage from capillary telangiectases was found in a 27-year period.37 Of the 60 patients described by Sarwar and McCormick, only 2 had suffered hemorrhage (1 in the cerebellum, 1 in the diencephalon).36 Wijdicks and Schievink reported a patient with a perimesencephalic subarachnoid hemorrhage that was prabably due to capillary telangiectasia, although the capillary telangiectasia could not be proved to be the cause of the hemorrhage.391 Subsequent MRI follow-up in 20 patients with perimesencephalic hemorrhage failed to demonstrate further telangiectases.392 Caution should be taken in attributing hemorrhage to capillary telangiectasia, and other pathology should be sought.393
The risk for hemorrhage is assumed to be low; however, there are no large case series with extensive follow-up. In a study by Lee and colleagues, 18 patients were monitored over a mean of 23 months, with no reported bleeding or neurological deficits.384 Similarly, in another study of 12 patients, no initial symptoms were thought to be definitively related to the capillary telangiectases, and no new symptoms developed in a 3-week to 4-month follow-up.385
Focal Deficit
Several case reports of focal neurological deficits in the absence of hemorrhage have been published.394,395 Farrell and Forno described a 68-year-old patient with progressive gait ataxia and bulbar dysfunction caused by a large pontine capillary telangiectasis.394 They proposed that the deficit was due to local oxygen deficiency caused by the abnormal blood vessels.
Abe M, Kjellberg R, Adams R. Clinical presentations of vascular malformations of the brain stem: comparison of angiographically positive and negative types. J Neurol Neurosurg Psychiatry. 1989;52:167-175.
Aiba T, Tanaka R, Koike T, et al. Natural history of intracranial cavernous malformations. J Neurosurg. 1995;83:56-59.
Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke. 2003;34:1163-1169.
Awad I, Little J, Akrawi W, et al. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg. 1990;72:839-850.
Barker F, Amin-Hanjani S, Butler W, et al. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery. 2001;49:15-25.
Borden J, Wu J, Shucart W. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-179.
Brown RJ, Wiebers D, Forbes G. Unruptured intracranial aneurysms and vascular malformations: frequency of intracranial hemorrhage and relationship of lesions. J Neurosurg. 1990;73:859-863.
Brown RJ, Wiebers D, Forbes G, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68:352-357.
Brown RJ, Wiebers D, Nichols D. Intracranial dural arteriovenous fistulae: angiographic predictors of intracranial hemorrhage and clinical outcome in nonsurgical patients. J Neurosurg. 1994;81:531-538.
da Costa L, Wallace MC, Ter Brugge KG, et al. The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke. 2009;40:100-105.
Garner T, Del Curling OJr, Kelly DLJr, et al. The natural history of intracranial venous angiomas. J Neurosurg. 1991;75:715-722.
Horton J, Chambers W, Lyons S, et al. Pregnancy and the risk of hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1990;27:867-872.
Kondziolka D, Dempsey P, Lunsford L. The case for conservative management of venous angiomas. Can J Neurol Sci. 1991;18:295-299.
Kondziolka D, Lunsford L, Kestle J. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820-824.
McLaughlin M, Kondziolka D, Flickinger J, et al. The prospective natural history of cerebral venous malformations. Neurosurgery. 1998;43:195-201.
Ondra S, Troupp H, George E, et al. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387-391.
Pollock B, Flickinger J, Lunsford L, et al. Factors that predict the bleeding risk of cerebral arteriovenous malformations. Stroke. 1996;27:1-6.
Porter P, Willinsky R, Harper W, et al. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997;87:190-197.
Porter R, Detwiler P, Spetzler R, et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg. 1999;90:50-58.
Robinson JJ, Awad I, Magdinec M, et al. Factors predisposing to clinical disability in patients with cavernous malformations of the brain. Neurosurgery. 1993;32:730-736.
Robinson JJ, Awad I, Masaryk T, et al. Pathological heterogeneity of angiographically occult vascular malformations of the brain. Neurosurgery. 1993;33:547-555.
Singh V, Smith WS, Lawton MT, et al. Risk factors for hemorrhagic presentation in patients with dural arteriovenous fistulae. Neurosurgery. 2008;62:628-635.
Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350-1355.
Stapf C, Mast H, Sciacca RR, et al. The New York Islands AVM Study: design, study progress, and initial results. Stroke. 2003;34:e29-e33.
1 Brown RDJr, Flemming KD, Meyer FB, et al. Natural history, evaluation, and management of intracranial vascular malformations. Mayo Clin Proc. 2005;80:269-281.
2 Chaloupka J, Huddle D. Classification of vascular malformations of the central nervous system. Neuroimaging Clin N Am. 1998;8:295-321.
3 Kalimo H, Kase M, Haltia M. Vascular diseases. In: Graham D, Lantos P, editors. Greenfield’s Neuropathology. 6th ed. New York: Oxford University Press; 1997:345-347.
4 McCormick W. The pathology of vascular malformations. J Neurosurg. 1966;24:807-816.
5 LeBlanc R, Ethier R, Little J. Computerized tomography findings in arteriovenous malformations of the brain. J Neurosurg. 1979;51:765-772.
6 Kumar A, Fox M, Vinuela F, et al. Revisited old and new findings in unruptured larger arteriovenous malformations of the brain. J Comput Assist Tomogr. 1984;8:648-655.
7 Osborn A. Diagnostic Radiology. Chicago: CV Mosby; 1994.
8 Rumbaugh C, Potts D. Skull changes associated with intracranial arteriovenous malformations. Am J Roentgenol Radium Ther Nucl Med. 1966;98:525-534.
9 Prayer L, Wimberger D, Stigbauer R, et al. Haemorrhage in intracerebral arteriovenous malformations: detection with MRI and comparison with clinical history. Neuroradiology. 1993;35:424-427.
10 Kesava P, Turski P. MR angiography of vascular malformations. Neuroimaging Clin N Am. 1998;8:349-370.
11 Huston J, Rufenacht D, Ehman R, et al. Intracranial aneurysms and vascular malformations: comparison of time of flight and phase contrast MR angiography. Radiology. 1991;181:721-730.
12 Turjman F, Massoud T, Vinuela F, et al. Correlation of the angioarchitectural features of cerebral arteriovenous malformations with clinical presentation of hemorrhage. Neurosurgery. 1995;37:856-862.
13 Wakai S, Kumakura N, Nagai M. Lobar intracerebral hemorrhage. A clinical, radiographic, and pathological study of 29 consecutive operated cases with negative angiography. J Neurosurg. 1992;76:231-238.
14 Bavinzski G, Richling B, Killer M, et al. Evolution of different therapeutic strategies in the treatment of cranial dural arteriovenous fistulas—report of 30 cases. Acta Neurochir (Wien). 1996;138:132-138.
15 London D, Enzmann D. The changing angiographic appearance of an arteriovenous malformation after subarachnoid hemorrhage. Neuroradiology. 1981;21:281-284.
16 Wharen RJ, Scheithauer B, Laws EJ. Thrombosed arteriovenous malformations of the brain. J Neurosurg. 1982;57:520-526.
17 Robinson JJ, Awad I, Masaryk T, et al. Pathological heterogeneity of angiographically occult vascular malformations of the brain. Neurosurgery. 1993;33:547-555.
18 Dandy W. Venous abnormalities and angiomas of the brain. Arch Surg. 1928;17:715-793.
19 Padget D. The cranial venous system in man in reference to development, adult configuration and relation to arteries. Am J Anat. 1956;98:307-355.
20 Stein B, Wolpert S. Arteriovenous malformations of the brain. I: Current concepts and treatment. Arch Neurol. 1980;37:1-5.
21 Gonzalez LF, Bristol RE, Porter RW, et al. De novo presentation of an arteriovenous malformation. Case report and review of the literature. J Neurosurg. 2005;102:726-729.
22 Kader A, Goodrich J, Sonstein W, et al. Recurrent cerebral arteriovenous malformations after negative postoperative angiograms. J Neurosurg. 1996;85:14-18.
23 Awad I, Robinson JJ, Mohanty S, et al. Mixed vascular malformations of the brain: clinical and pathogenetic considerations. Neurosurgery. 1993;33:179-188.
24 Deshpande D, Vidyasagar C. Histology of the persistent embryonic veins in arteriovenous malformations of brain. Acta Neurochir (Wien). 1980;53:227-236.
25 Mullan S, Mojtahedi S, Johnson DL, et al. Embryological basis of some aspects of cerebral vascular fistulas and malformations. J Neurosurg. 1996;85:1-8.
26 Mullan S. Reflections upon the nature and management of intracranial and intraspinal vascular malformations and fistulae. J Neurosurg. 1994;80:606-616.
27 Nussbaum E, Heros R, Madison M, et al. The pathogenesis of arteriovenous malformations: insights provided by a case of multiple arteriovenous malformations developing in relation to a developmental venous anomaly. Neurosurgery. 1998;43:347-352.
28 Yokoyama K, Asano Y, Murakawa T, et al. Familial occurrence of arteriovenous malformation of the brain. J Neurosurg. 1991;74:585-589.
29 van Beijnum J, van der Worp HB, Schippers HM, et al. Familial occurrence of brain arteriovenous malformations: a systematic review. J Neurol Neurosurg Psychiatry. 2007;78:1213-1217.
30 Inoue S, Liu W, Inoue K, et al. Combination of linkage and association studies for brain arteriovenous malformation. Stroke. 2007;38:1368-1370.
31 Snead O, Acker J, Morawetz R. Familial arteriovenous malformation. Ann Neurol. 1979;5:585-587.
32 Goto S, Abe M, Tsuji T, et al. Familial arteriovenous malformations of the brain: two case reports. Neurol Med Chir (Tokyo). 1994;34:221-224.
33 Boyd M, Steinbok P, Paty D. Familial arteriovenous malformations. Report of four cases in one family. Neurosurgery. 1985;62:597-599.
34 Brilli R, Sacchetti A, Neff S. Familial arteriovenous malformation in children. Pediatr Emerg Care. 1995;11:376-378.
35 Berman MF, Sciacca RR, Pile-Spellman J, et al. The epidemiology of brain arteriovenous malformations. Neurosurgery. 2000;47:389-396.
36 Sarwar M, McCormick W. Intracerebral venous angioma. Case report and review. Arch Neurol. 1978;35:323-325.
37 Brown RJ, Wiebers DO, Torner JC, et al. Frequency of intracranial hemorrhage as a presenting symptom and subtype analysis: a population based study of intracranial vascular malformation in Olmsted County, Minnesota. J Neurosurg. 1996;85:29-32.
38 Stapf C, Mast H, Sciacca RR, et al. The New York Islands AVM Study: design, study progress, and initial results. Stroke. 2003;34:e29-e33.
39 Stapf C, Labovitz DL, Sciacca RR, et al. Incidence of adult brain arteriovenous malformation hemorrhage in a prospective population-based stroke survey. Cerebrovasc Dis. 2002;13:43-46.
40 Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Scottish Intracranial Vascular Malformation Study (SIVMS): evaluation of methods, ICD-10 coding, and potential sources of bias in a prospective, population-based cohort. Stroke. 2003;34:1156-1162.
41 Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke. 2003;34:1163-1169.
42 Al-Shahi R, Fang JS, Lewis SC, et al. Prevalence of adults with brain arteriovenous malformations: a community based study in Scotland using capture-recapture analysis. J Neurol Neurosurg Psychiatry. 2002;73:547-551.
43 ApSimon HT, Reef H, Phadke RV, et al. A population-based study of brain arteriovenous malformation: long-term treatment outcomes. Stroke. 2002;33:2794-2800.
44 Furlan A, Whisnant J, Elveback L. The decreasing incidence of primary intracerebral hemorrhage: a population study. Ann Neurol. 1979;5:367-373.
45 Gross C, Kase C, Mohr J, et al. Stroke in south Alabama: incidence and diagnostic features—a population based study. Stroke. 1984;15:249-255.
46 Perret G, Nishioka H. Report on the Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage. Section VI. Arteriovenous malformations. J Neurosurg. 1966;25:467-490.
47 Toffol G, Biller J, Adams HJ. Nontraumatic intracerebral hemorrhage in young adults. Arch Neurol. 1987;44:483-485.
48 Wilkins R. The natural history of intracranial vascular malformations: a review. Neurosurgery. 1985;16:421-430.
49 Michelson W. Natural history and pathophysiology of arteriovenous malformations. Clin Neurosurg. 1978;26:307-313.
50 Tay C, Oon C, Lai C, et al. Intracranial arteriovenous malformations in Asians. Brain. 1971;94:61-68.
51 Crawford P, West C, Chadwick D, et al. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986;49:1-10.
52 Albert P. Personal experience in the treatment of 178 cases of arteriovenous malformations of the brain. Acta Neurochir (Wien). 1982;61:207-226.
53 Forster D, Steiner L, Hakanson S. Arteriovenous malformations of the brain. A long-term clinical study. J Neurosurg. 1972;37:562-570.
54 Fults D, Kelly DJ. Natural history of arteriovenous malformations of the brain: a clinical study. Neurosurgery. 1984;15:658-662.
55 Graf C, Perret G, Torner J. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331-337.
56 Kader A, Young W, Pile-Spellman J, et al. The influence of hemodynamic and anatomic features on hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1994;34:801-808.
57 Morello G, Borghi G. Cerebral angiomas. A report of 154 personal cases and a comparison between the results of surgical excision and conservative management. Acta Neurochir (Wien). 1973;28:135-155.
58 Parkinson D, Bachers G. Arteriovenous malformations. Summary of 100 consecutive supratentorial cases. J Neurosurg. 1980;53:285-299.
59 Troupp H, Marttila I, Halonen V. Arteriovenous malformations of the brain. Prognosis without operation. Acta Neurochir (Wien). 1970;22:125-128.
60 Trumpy J, Eldevik P. Intracranial arteriovenous malformations: conservative or surgical treatment? Surg Neurol. 1977;8:171-175.
61 Stapf C, Khaw AV, Sciacca RR, et al. Effect of age on clinical and morphological characteristics in patients with brain arteriovenous malformation. Stroke. 2003;34:2664-2669.
62 Guidetti B, Delitala A. Intracranial arteriovenous malformations. Conservative and surgical treatment. J Neurosurg. 1980;53:149-152.
63 Ondra S, Troupp H, George E, et al. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387-391.
64 Houser O, Baker H, Svien H, et al. Arteriovenous malformations of the parenchyma of the brain. Angiographic aspects. Radiology. 1973;109:83-90.
65 Brown RJ, Wiebers D, Forbes G, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68:352-357.
66 McCormick W, Hardman J, Boulter T. Vascular malformations (angiomas) of the brain with special reference to those occuring in the posterior fossa. J Neurosurg. 1968;28:241-251.
67 Reddy K, West M, McClarty B. Multiple intracerebral arteriovenous malformations: a case report and literature review. Surg Neurol. 1987;27:495-499.
68 Willinsky R, Lasjaunias P, Burrows P. Multiple cerebral arteriovenous malformations (AVMs): review of our experience from 203 patients with cerebral vascular lesions. Neuroradiology. 1990;32:207-210.
69 Utsuki S, Kurata A, Miyasaka Y, et al. Multiple arteriovenous malformations with hemorrhage. Acta Neurochir (Wien). 2002;144:97-101.
70 Yamashita K, Suzuki Y, Yshizumi J, et al. Multiple cerebral arteriovenous malformations. Neurol Med Chir (Tokyo). 1993;33:24-27.
71 Putman C, Chapoupka J, Fulbright R, et al. Exceptional multiplicity of cerebral arterniovenous malformations associated with hereditary hemorrhagic telangiectasia (Osler Weber Rendu). AJNR Am J Neuroradiol. 1996;17:1733-1742.
72 Wyburn-Mason R. Arteriovenous aneurysm of mid-brain and retina, facial nevi, and mental changes. Brain. 1943;66:163-203.
73 Hanieh A, Blumbers P, Carney P. Multiple cerebral arteriovenous malformations associated with soft tissue vascular malformations: case report. J Neurosurg. 1981;54:670-672.
74 Hasegawa S, Hamada J, Morioka M, et al. Multiple cerebral arteriovenous malformations (AVMs) associated with spinal. AVM. Acta Neurochir (Wien). 1999;141:315-319.
75 Suzuki J, Onuma T. Intracranial aneurysms associated with arteriovenous malformations. J Neurosurg. 1979;50:742-746.
76 Perata H, Tomsick T, Tew JJr. Feeding artery pedicle aneurysms: association with parenchymal hemorrhage and arteriovenous malformation in the brain. J Neurosurg. 1994;80:631-634.
77 Redekop G, TerBrugge K, Montanera W, et al. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg. 1998;89:539-546.
78 Paterson J, McKissock W. A clinical survey of intracranial angiomas with special reference to their mode of progression and surgical treatment: a report of 110 cases. Brain. 1956;79:233-266.
79 Miyasaka K, Wolpert S, Prager R. The association of cerebral aneurysms, infundibula, and intracranial arteriovenous malformations. Stroke. 1982;13:196-203.
80 Lasjaunias P, Piske R, Terbrugge K, et al. Cerebral arteriovenous malformations and associated arterial aneurysms. Acta Neurochir (Wien). 1988;91:29-36.
81 Kondziolka D, Nixon B, Lasjaunias P, et al. Cerebral arteriovenous malformations and associated arterial aneuryms: hemodynamic and therapeutic considerations. Can J Neurol Sci. 1988;15:130-134.
82 Cunha E, Sa M, Stein B, et al. The treatment of associated intracranial aneurysms and arteriovenous malformations. J Neurosurg. 1992;77:853-859.
83 Hodgson T, Zaman S, Cooper J, et al. Proximal aneurysms in association with arteriovenous malformations: do they resolve following obliteration of the malformation with stereotactic radiosurgery? Br J Neurosurg. 1998;12:434-437.
84 Batjer H, Suss R, Sampson D. Intracranial arteriovenous malfomations associated with aneurysms. Neurosurgery. 1986;18:29-35.
85 Brown RJ, Wiebers D, Forbes G. Unruptured intracranial aneurysms and vascular malformations: frequency of intracranial hemorrhage and relationship of lesions. J Neurosurg. 1990;73:859-863.
86 Crawford P, West C, Shaw M, et al. Cerebral arteriovenous malformations and epilepsy: factors in the development of epilepsy. Epilepsia. 1986;27:270-275.
87 Stapf C, Mohr JP, Choi JH, et al. Invasive treatment of unruptured brain arteriovenous malformations is experimental therapy. Curr Opin Neurol. 2006;19:63-68.
88 Jomin M, Lesoin F, Lozes G. Prognosis for arteriovenous malformations of the brain in adults based on 150 cases. Surg Neurol. 1985;23:362-366.
89 Aoki N. Do intracranial arteriovenous malformations cause subarachnoid haemorrhage? Review of computed tomography features of ruptured arteriovenous malformations in the acute stage. Acta Neurochir (Wien). 1991;112:92-95.
90 Sasaki T. Cerebral vasospasm with subarachnoid hemorrhage from cerebral arteriovenous malformations. Surg Neurol. 1981;16:183-187.
91 Nishimura K. Cerebral vasospasm with subarachnoid hemorrhage from arteriovenous malformations of the brain. Neuroradiology. 1975;8:201-207.
92 Pollock B, Flickinger J, Lunsford L, et al. Factors that predict the bleeding risk of cerebral arteriovenous malformations. Stroke. 1996;27:1-6.
93 da Costa L, Wallace MC, Ter Brugge KG, et al. The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke. 2009;40:100-105.
94 Mast H, Young W, Koennecke H, et al. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet. 1997;350:1065-1068.
95 Yamada S, Takagi Y, Nozaki K, et al. Risk factors for subsequent hemorrhage in patients with cerebral arteriovenous malformations. J Neurosurg. 2007;107:965-972.
96 Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350-1355.
97 Kondziolka D, McLaughlin M, Kestle J. Simple risk predictions for arteriovenous malformation hemorrhage. Neurosurgery. 1995;37:851-855.
98 Robinson J, Hall C, Sedzimir C. Arteriovenous malformations, aneurysms, and preganancy. J Neurosurg. 1974;41:63-70.
99 Langer D, Lasner T, Hurst R, et al. Hypertension, small size, and deep venous drainage areassociated with risk of hemorrhagic presentation of cerebral arteriovenous malformations. Neurosurgery. 1998;42:481-489.
100 Kim H, Sidney S, McCulloch CE, et al. Racial/Ethnic differences in longitudinal risk of intracranial hemorrhage in brain arteriovenous malformation patients. Stroke. 2007;38:2430-2437.
101 Khaw AV. When race/ethnicity matters more than size. Stroke. 2007;38:2405-2406.
102 Stefani MA, Porter PJ, terBrugge KG, et al. Angioarchitectural factors present in brain arteriovenous malformations associated with hemorrhagic presentation. Stroke. 2002;33:920-924.
103 Fleetwood IG, Marcellus ML, Levy RP, et al. Deep arteriovenous malformations of the basal ganglia and thalamus: natural history. J Neurosurg. 2003;98:747-750.
104 Khaw AV, Mohr JP, Sciacca RR, et al. Association of infratentorial brain arteriovenous malformations with hemorrhage at initial presentation. Stroke. 2004;35:660-663.
105 Marks M, Lane B, Steinberg G, et al. Hemorrhage in intracerebral arteriovenous malformations: angiographic determinants. Radiology. 1990;176:807-813.
106 Nagata S, Matsushima T, Fujii K, et al. Lateral ventricular arteriovenous malformations: natural history and surgical indications. Acta Neurochir (Wien). 1991;112:37-46.
107 Stapf C, Mohr JP, Sciacca RR, et al. Incident hemorrhage risk of brain arteriovenous malformations located in the arterial borderzones. Stroke. 2000;31:2365-2368.
108 Drake C, Friedman A, Peerless S. Posterior fossa arteriovenous malformations. J Neurosurg. 1986;64:1-10.
109 Albert P, Salgado H, Polaina M, et al. A study on the venous drainage of 150 cerebral arteriovenous malformations as related to haemorrhagic risks and size of the lesion. Acta Neurochir (Wien). 1990;103:30-34.
110 Spetzler R, Hargraves R, McCormick P, et al. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76:918-923.
111 Waltimo O. The change in size of intracranial arteriovenous malformations. J Neurol Sci. 1973;19:21-27.
112 Piepgras D, Sundt TJ, Ragoowansi A, et al. Seizure outcome in patients with surgically treated cerebral arteriovenous malformations. J Neurosurg. 1993;78:5-11.
113 Young W, Kader A, Pile-Spellman J, et al. Arteriovenous malformation draining vein physiology and determinants of transnidal pressure gradients. Neurosurgery. 1994;35:389-396.
114 Sadasivan B, Hwang P. Large cerebral arteriovenous malformations: experience with 27 cases. Surg Neurol. 1996;45:245-249.
115 Stefani MA, Porter PJ, terBrugge KG, et al. Large and deep brain arteriovenous malformations are associated with risk of future hemorrhage. Stroke. 2002;33:1220-1224.
116 Norris J, Valiante T, Wallace M, et al. A simple relationship between radiological arteriovenous malformation hemodynamics and clinical presentation: a prospective, blinded analysis of 31cases. J Neurosurg. 1999;90:673-679.
117 Hademenos G, Massoud T. Risk of intracranial arteriovenous malformations rupture due to venous drainage impairment: a theoretical analysis. Stroke. 1996:1072-1083.
118 Miyasaka Y, Yada K, Ohwada T, et al. An analysis of the venous drainage system as a factor in hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76:239-243.
119 Vinuela F, Nombela L, Roach M, et al. Stenotic and occlusive disease of the venous drainage system of deep brain AVM’s. J Neurosurg. 1985;63:180-184.
120 Columbia Arteriovenous Malformation Study Group. Arteriovenous malformations of the brain in adults. N Engl J Med. 1999;340:1812-1818.
121 Pritz M. Ruptured supratentorial arteriovenous malformations associated with venous aneurysms. Acta Neurochir (Wien). 1994;128:150-162.
122 Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
123 Jayaraman MV, Marcellus ML, Do HM, et al. Hemorrhage rate in patients with Spetzler-Martin grades IV and V arteriovenous malformations: is treatment justified? Stroke. 2007;38:325-329.
124 Turjman F, Massoud T, Sayre J, et al. Epilepsy associated with cerebral arteriovenous malformations: a multivariate analysis of angioarchitectural characteristics. AJNR Am J Neuroradiol. 1995;16:345-350.
125 Troost B, Newton T. Occipital lobe arteriovenous malformations. Clinical and radiologic features in 26 cases with comments on differentiation from migraine. Arch Ophthalmol. 1975;93:250-256.
126 Mast H, Mohr J, Osipov A, et al. ‘Steal’ is an unestablished mechanism for the clinical presentation of cerebral arteriovenous malformations. Stroke. 1995;26:1215-1220.
127 Lazar R, Connaire K, Marshall R, et al. Developmental deficits in adult patients with arteriovenous malformations. Arch Neurol. 1999;56:103-106.
128 Manchola I, Salles AD, Foo T, et al. Arteriovenous malformation hemodynamics: a transcranial Doppler study. Neurosurgery. 1993;33:556-562.
129 Fink G. Effects of cerebral angiomas on perifocal and remote tissue: a multivariate positron emission tomography study. Stroke. 1992;23:1099-1105.
130 Svien H, McRae J. Arteriovenous anomalies of the brain. Fate of patients not having definitive surgery. J Neurosurg. 1965;23:23-28.
131 Porter P, terBrugge K, Montanera W. Outcome following hemorrhage from brain arteriovenous malformations at presentation and during follow up: Is it worse than we think? J Neurosurg. 1998;88:184A.
132 Luessenhop A. Dural arteriovenous malformations. In: Wilkins R, Rengachary S, editors. Neurosurgery. New York: McGraw-Hill; 1986:1473-1477.
133 Stapf C, Mohr JP. Unruptured brain arteriovenous malformations should be treated conservatively: yes. Stroke. 2007;38:3308-3309.
134 Hartmann A, Mast H, Choi JH, et al. Treatment of arteriovenous malformations of the brain. Curr Neurol Neurosci Rep. 2007;7:28-34.
135 Choi JH, Mast H, Sciacca RR, et al. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke. 2006;37:1243-1247.
136 Hartmann A, Mast H, Mohr J, et al. Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke. 1998;29:931-934.
137 Itoyama Y, Uemura S, Ushio Y, et al. Natural course of unoperated intracranial arteriovenous malformations: study of 50 cases. J Neurosurg. 1989;71:805-809.
138 Aminoff M. Treatment of unruptured cerebral arteriovenous malformations. Neurology. 1987;37:815-819.
139 Murphy M. Long-term follow-up of seizures associated with cerebral arteriovenous malformations. Results of therapy. Arch Neurol. 1985;42:477-479.
140 Pasqualin A, Vivenza C, Rosta L, et al. Spontaneous regression of intracranial arteriovenous malformation. Surg Neurol. 1993;39:385-391.
141 Minikawa T, Tanaka R, Koike T. Angiographic follow-up study of cerebral arteriovenous malformation with reference to their enlargement and progression. Neurosurgery. 1989;24:68-74.
142 Marconi F, Parenti G, Puglioli M. Spontaneous regression of intracranial arteriovenous malformation. Surg Neurol. 1993;39:385-391.
143 Hook O, Johanson C. Intracranial arteriovenous malformations: a follow-up study with particular attention to their growth. Arch Neurol Psychiatry. 1958;80:39-54.
144 Guazzo E, Zuered J. Spontaneous thrombosis of an arteriovenous malformation. J Neurol Neurosurg Psychiatry. 1994;57:1410-1412.
145 Ebeling J, Tranmer B, Davis K. Thrombosed arteriovenous malformations: a type of occult vascular malformation. Magentic resonance imaging and histopathological considerations. Neurosurgery. 1988;23:605-609.
146 Abdulrauf S, Malik G, Awad I. Spontaneous angiographic obliteration of cerebral arteriovenous malformations. Neurosurgery. 1999;44:280-287.
147 Mizutani T, Tanaka H, Aruga T. Total recanalization of a spontaneously thrombosed arteriovenous malformation. Case report. J Neurosurg. 1995;82:506-508.
148 Marks M, Lane B, Steinberg G, et al. Intranidal aneurysms in cerebral arteriovenous malformations: evaluation and endovascular treatment. Radiology. 1992;183:335-360.
149 Cronqvist S, Troupp H. The intracranial arteriovenous malformation and arterial aneurysm in the same patient. Acta Neurol Scand. 1966;42:307-316.
150 Thompson R, Steinberg G, Levy R, et al. The management of patients with arteriovenous malformations and associated intracranial aneurysms. Neurosurgery. 1998;43:202-212.
151 Stapf C, Mohr JP, Pile-Spellman J, et al. Concurrent arterial aneurysms in brain arteriovenous malformations with haemorrhagic presentation. J Neurol Neurosurg Psychiatry. 2002;73:294-298.
152 Halim AX, Singh V, Johnston SC, et al. Characteristics of brain arteriovenous malformations with coexisting aneurysms: a comparison of two referral centers. Stroke. 2002;33:675-679.
153 Dias M, Sekhar L. Intracranial hemorrhage from aneurysms and arteriovenous malformations during pregnancy and the puerperium. Neurosurgery. 1990;27:855-866.
154 Wiebers D. Subarachnoid hemorrhage in pregnancy. Semin Neurol. 1988;8:226-229.
155 Dias M. Neurovascular emergencies in pregnancy. Clin Obstet Gynecol. 1994;37:337-354.
156 Horton J, Chambers W, Lyons S, et al. Pregnancy and the risk of hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1990;27:867-872.
157 Sadasivan B, Malik G, Lee C, et al. Vascular malformations and pregnancy. Surg Neurol. 1990;33:305-313.
158 Lanzino G, Jensen M, Cappelletto B, et al. Arteriovenous malformations that rupture during pregnancy: a management dilemma. Acta Neurochir (Wien). 1994;126:102-106.
159 Robinson J, Chir B, Hall C, et al. Subarachn oid hemorrhage in pregnancy. J Neurosurg. 1972;36:27-33.
160 Sano K, Ueda Y, Saito I. Subarachnoid hemorrhage in children. Childs Brain. 1978;4:38-46.
161 Hdlaky J, Lejeune J, Blond S, et al. Cerebral arteriovenous malformations in children: report on 62 cases. Childs Nerv Syst. 1994;10:328-333.
162 Mori K, Murata T, Hashimoto N, et al. Clinical analysis of arteriovenous malformations in children: clinical features and outcome of treatment in children and adults. Clin Neurol. 1984;22:43-49.
163 Kelly JJ, Mellinger J, Sundt TJ. Intracranial arteriovenous malformations in childhood. Ann Neurol. 1978;3:338-343.
164 Suarez J, Viano J. Intracranial arteriovenous malformations in infancy and adolescence. Childs Nerv Syst. 1989;5:15-18.
165 Kondziolka D, Humphreys R, Hoffman J, et al. Arteriovenous malformations of the brain in children: a forty-year experience. Can J Neurol Sci. 1992;19:40-45.
166 Lasjaunias P, Hui F, Zerah M. Cerebral arteriovenous malformations in children: management of 179 consecutive cases and review of the literature. Childs Nerv Syst. 1989;5:15-18.
167 Miller C, Bissonnette B, Humphreys R. Cerebral arteriovenous malformations in children. Child Nerv Syst. 1991;7:43-47.
168 Hara H, Burrows P, Flodmark O, et al. Neonatal superficial cerebral arteriovenous malformations. Pediatr Neurosurg. 1994;20:126-136.
169 Melville C, Walsh K, Sreeram N. Cerebral arteriovenous malformations in the neonate: clinical presentation, diagnosis and outcome. Int J Cardiol. 1991;31:175-180.
170 Kurokawa T, Matsuzaki A, Hasuo K, et al. Cerebral arteriovenous malformations in children. Brain Dev. 1985;7:408-413.
171 D’Aliberti G, Talamonti G, Versari P, et al. Comparison of pediatric and adult cerebral arteriovenous malformations. J Neurosurg Sci. 1997;41:331-336.
172 Humphreys R, Hendrick E, Hoffman H. Arteriovenous malformations of the brainstem in childhood. Childs Brain. 1984;11:1-11.
173 Menovsky T, vanOverbeeke J. Cerebral arteriovenous malformations in childhood: state of the art with special reference to treatment. Eur J Pediatr. 1997;156:741-746.
174 Griffiths P, Blaser S, Armstrong D, et al. Cerebellar arteriovenous malformations in children. Neuroradiology. 1998;40:324-331.
175 Gerosa M, Cappellotto P, Licata C, et al. Cerebral arteriovenous malformations in children (56 cases). Childs Brain. 1981;8:356-371.
176 Johnson P, Wascher T, Golfinos J, et al. Definition and pathological features. In: Awad I, Barrow D, editors. Cavernous Malformations. Park Ridge, IL: American Association of Neurological Surgeons; 1993:1-11.
177 Ramina R, Ingunza W, Vonofakos D. Cystic cerebral cavernous angioma with dense calcification. Case report. J Neurosurg. 1980;52:259-262.
178 Rigamonti D, Drayer B, Johnson P, et al. The MRI appearance of cavernous malformations (angiomas). J Neurosurg. 1987;67:518-524.
179 Voigt K, Yasargil M. Cerebral cavernous haemangiomas or cavernomas. Incidence, pathology, localization, diagnosis, clinical features and treatment. Review of the literature and report of an unusual case. Neurochirurgia (Stuttg). 1976;19:59-68.
180 Simard J, Garcia-Bengochea F, Ballinger WJ, et al. Cavernous angioma: a review of 126 collected and 12 new clinical cases. Neurosurgery. 1986;18:162-172.
181 Clatterbuck R, Eberhart C, Crain B, et al. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J Neurol Neurosurg Psychiatry. 2001;71:188-192.
182 Mindea S, Yang B, Shenkar R, et al. Cerebral cavernous malformations: clinical insights from genetic studies. Neurosurg Focus. 2006;21(1):e1.
183 Giombini S, Morello G. Cavernous angiomas of the brain. Account of fourteen personal cases and review of the literature. Acta Neurochir (Wien). 1978;40:61-82.
184 Lobato R, Perez C, Rivas J, et al. Clinical, radiological, and pathological spectrum of angiographically occult intracranial vascular malformations. Analysis of 21 cases and review of the literature. J Neurosurg. 1988;68:518-531.
185 Vaquero J, Leunda G, Martinez R, et al. Cavernomas of the brain. Neurosurgery. 1983;12:208-210.
186 Rigamonti D, Hadley M, Drayer B, et al. Cerebral cavernous malformations. Incidence and familial occurrence. N Engl J Med. 1988;319:343-347.
187 Kim D, Park Y, Choi J, et al. An analysis of the natural history of cavernous malformations. Surg Neurol. 1997;48:9-18.
188 Lehnhardt F, Smekal UV, Ruckriem B, et al. Value of gradient-echo magnetic resonance imaging in the diagnosis of familial cerebral cavernous malformation. Arch Neurol. 2005;62:653-658.
189 Zabramski J, Wascher T, Spetzler R, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg. 1994;80:422-432.
190 Cooper A, Campeau N, Meissner I. Susceptibility-weighted imaging in familial cerebral cavernous malformations. Neurology. 2008;71:382.
191 Bien S, Friedburg H, Harders A, et al. Intracerebral cavernous angiomas in magnetic resonance imaging. Acta Radiol Suppl (Stockh). 1986;369:79-81.
192 Biondi A, Scotti G, Scialfa G, et al. Magnetic resonance imaging of cerebral cavernous angiomas. Acta Radiol Suppl (Stockh). 1986;369:82-85.
193 Bourgouin P, Tampieri D, Johnston W, et al. Multiple occult vascular malformations of the brain and spinal cord: MRI diagnosis. Neuroradiology. 1992;34:110-111.
194 Duong H, Carpio-O’Donovan RD, Pike B, et al. Multiple intracerebral cavernous angiomas. Can Assoc Radiol J. 1991;42:329-334.
195 Rapacki T, Brantley M, Furlow TJ, et al. Heterogeneity of cerebral cavernous hemangiomas diagnosed by MR imaging. J Comput Assist Tomogr. 1990;14:18-25.
196 Perl J, Ross J. Diagnostic imaging of cavernous malformations. In: Awad I, Barrow D, editors. Cavernous Malformations. Park Ridge, IL: American Association of Neurological Surgeons; 1993:37-48.
197 Sigal R, Krief O, Houtteville J, et al. Occult cerebrovascular malformations: follow-up with MR imaging. Radiology. 1990;176:815-819.
198 Ogilvy C, Heros R, Ojemann R, et al. Angiographically occult arteriovenous malformations. J Neurosurg. 1989;70:293.
199 Sze G, Krol G, Olsen W, et al. Hemorrhagic neoplasms: MR mimics of occult vascular malformations. AJR Am J Roentgenol. 1987;149:1223-1230.
200 Steinberg G, Marks M. Lesions mimicking cavernous malformations. In: Awad I, Barrow D, editors. Cavernous Malformations. Park Ridge, IL: American Association of Neurological Surgeons; 1993:151-162.
201 Dillon W. Cryptic vascular malformations: controversies in terminology, diagnosis, pathophysiology, and treatment. AJNR Am J Neuroradiol. 1997;18:1839-1846.
202 Tomlinson F, Houser O, Scheithauer B, et al. Angiographically occult vascular malformations: a correlative study of features on magnetic resonance imaging and histological examination. Neurosurgery. 1994;34:792-800.
203 Vanefsky M, Cheng M, Chang S, et al. Correlation of magnetic resonance characteristics and histopathological type of angiographically occult vascular malformations. Neurosurgery. 1999;44:1174-1181.
204 Abe M, Kjellberg R, Adams R. Clinical presentations of vascular malformations of the brain stem: comparison of angiographically positive and negative types. J Neurol Neurosurg Psychiatry. 1989;52:167-175.
205 Servo A, Porras M, Raininko R. Diagnosis of cavernous haemangiomas by computed tomography and angiography. Acta Neurochir (Wien). 1984;71:273-282.
206 Yamasaki T, Handa H, Yamashita J, et al. Intracranial and orbital cavernous angiomas. A review of 30 cases. J Neurosurg. 1986;64:197-208.
207 Weber M, Vespignani H, Bracard S, et al. Intracerebral cavernous angioma. Rev Neurol (Paris). 1989;145:429-436.
208 Tagle P, Huete I, Mendez J, et al. Intracranial cavernous angioma: presentation and management. J Neurosurg. 1986;64:720-723.
209 Numaguchi Y, Kishikawa T, Fukui M, et al. Prolonged injection angiography for diagnosing intracranial cavernous hemangiomas. Radiology. 1979;131:137-138.
210 Numaguchi Y, Fukui M, Miyake E, et al. Angiographic manifestations of intracerebral cavernous hemangioma. Neuroradiology. 1977;14:113-116.
211 Maraire J, Awad I. Intracranial cavernous malformations: lesion behavior and management strategies. Neurosurgery. 1995;37:591-605.
212 Wilson C. Cryptic vascular malformations. Clin Neurosurg. 1992;38:49-84.
213 Tekkok I, Ventureyra E. De novo familial cavernous malformation presenting with hemorrhage 12.5 years after the initial hemorrhagic ictus: natural hisotory of an infantile form. Pediatr Neurosurg. 1996;25:151-155.
214 Detwiler P, Porter R, Zabramski J, et al. De novo formation of a central nervous system cavernous malformation: implications for predicting risk of hemorrhage. Case report and review of the literature. J Neurosurg. 1997;87:629-632.
215 Larson J, Ball W, Bove K, et al. Formation of intracerebral cavernous malformations after radiation treatment for central nervous system neoplasia in children. J Neurosurg. 1998;88:51-56.
216 Chang S, Vanefsky M, Havton L, et al. Bilateral cavernous malformations resulting from cranial irradiation of a choroid plexus papilloma. Neurol Res. 1998;20:529-532.
217 Ogilvy C, Moayeri N, Golden J. Appearance of a cavernous hemangioma in the cerebral cortex after a biopsy of a deeper lesion. Neurosurgery. 1993;33:307-309.
218 Ciricillo S, Dillon W, Fink M, et al. Progression of multiple cryptic vascular malformations associated with anomalous venous drainage. Case report. J Neurosurg. 1994;81:477-481.
219 Perrini P, Lanzion G. The association of venous developmental anomalies and cavernous malformations: pathophysiological, diagnostic, and surgical considerations. Neurosurg Focus. 2006;21(1):e5.
220 Maeder P, Gudinchet F, Meuli R, et al. Development of a cavernous malformation of the brain. AJNR Am J Neuroradiol. 1998;19:1141-1143.
221 Pozzati E, Musiani M. Cavernous hemangioma. J Neurosurg. 1998;89:498-499.
222 Dashti S, Hoffer A, Hu Y, et al. Molecular genetics of familial cerebral cavernous malformations. Neurosurg Focus. 2006;21(1):e2.
223 Revencu N, Vikkula M. Cerebral cavernous malformation: new molecular and clinical insights. J Med Genet. 2006;43:716-721.
224 Gunel M, Awad I, Anson J, et al. Mapping a gene causing cerebral cavernous malformation to 7q11.2-q21. Proc Natl Acad Sci U S A. 1995;92:6620-6624.
225 Dubovsky J, Zabramski J, Kurth J, et al. A gene responsible for cavernous malformations of the brain maps to chromosome 7q. Hum Mol Genet. 1995;4:453-458.
226 Lonjon M, Roche J, George B, et al. Intracranial cavernoma. 30 cases. Presse Med. 1993;22:990-994.
227 Martin N, Vinters H. Pathology and grading of intracranial vascular malformations. In: Awad I, Barrow D, editors. Intracranial Vascular Malformations. Park Ridge, IL: American Association of Neurological Surgeons; 1990:1-30.
228 Berry R, Alpers B, White J. The site, structure and frequency of intracranial aneurysms, angiomas and arteriovenous abnormalities. In: Milikan CM, editor. Research Publications: Association for Research in Nervous and Mental Disease. Baltimore: Williams & Wilkins; 1966:4-72.
229 Otten P, Pizzolato G, Rilliet B, et al. 131 cases of cavernous angioma (cavernomas) of the CNS, discovered by retrospective analysis of 24,535 autopsies. Neurochirurgie. 1989;35:128-131.
230 Del Curling OJr, Kelly DLJr, Elster AD, et al. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991;75:702-708.
231 Robinson J, Awad I, Little J. Natural history of the cavernous angioma. J Neurosurg. 1991;75:709-714.
232 Sage M, Brophy B, Sweeney C, et al. Cavernous haemangiomas (angiomas) of the brain: clinically significant lesions. Australas Radiol. 1993;37:147-155.
233 Aiba T, Tanaka R, Koike T, et al. Natural history of intracranial cavernous malformations. J Neurosurg. 1995;83:56-59.
234 Edwards M, Baumgartner J, Wilson C. Cavernous and other cryptic vascular malformations in the pediatric age group. In: Awad I, Barrow D, editors. Cavernous Malformations. Park Ridge, IL: American Association of Neurological Surgeons; 1993:163-183.
235 Hsu F, Rigamonti D, Huhn S. Epidemiology of cavernous malformations. In: Awad I, Barrow D, editors. Cavernous Malformations. Park Ridge, IL: American Association of Neurological Surgeons; 1993:13-23.
236 Moriarity J, Clatterbuck R, Rigamonti D. The natural history of cavernous malformations. Neurosurg Clin N Am. 1999;10:411-417.
237 Bergeson P, Rekate H, Tack E. Cerebral cavernous angiomas in the newborn. Clin Pediatr (Phila). 1992;31:435-437.
238 Dobyns W, Michels W, Groover R, et al. Familial cavernous malformations of the central nervous system and retina. Ann Neurol. 1987;21:578-583.
239 Mazza C, Scienza R, Bernardin BD, et al. Cerebral cavernous malformations (cavernomas) in children. Neurochirurgie. 1989;35:106-108.
240 Kondziolka D, Lunsford L, Kestle J. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820-824.
241 Hayman L, Evans R, Ferrell R, et al. Familial cavernous angiomas: natural history and genetic study over a 5-year period. Am J Med Genet. 1982;11:147-160.
242 Gangemi M, Maiuri F, Donati P, et al. Familial cerebral cavernous angiomas. Neurol Res. 1990;12:131-136.
243 Requena I, Arias M, Lopez-Ibor L, et al. Cavernomas of the central nervous system: clinical and neuroimaging manifestations in 47 patients. J Neurol Neurosurg Psychiatry. 1991;54:590-594.
244 Barrow D, Krisht A. Cavernous malformations and hemorrhage. In: Awad I, Barrow D, editors. Cavernous Malformations. Park Ridge, IL: American Association of Neurological Surgeons; 1993:65-80.
245 Diamond C, Torvik A, Amundsen P. Angiographic diagnosis of telangiectasias with cavernous angioma of the posterior fossa: report of two cases. Acta Radiol. 1976;17:281-288.
246 Hirsh L. Combined cavernous-arteriovenous malformation. Surg Neurol. 1981;16:135-139.
247 Rigamonti D, Johnson P, Spetzler R, et al. Cavernous malformations and capillary telangiectasia: a spectrum within a single pathological entity. Neurosurgery. 1991;28:60-64.
248 Rigamonti D, Spetzler R. The association of venous and cavernous malformations. Report of four cases and discussion of the pathophysiological, diagnostic, and therapeutic implications. Acta Neurochir (Wien). 1988;92:100-105.
249 Wilms G, Bleus E, Demaerel P, et al. Simultaneous occurrence of developmental venous anomalies and cavernous angiomas. AJNR Am J Neuroradiol. 1994;15:1247-1254.
250 Abdulrauf S, Kaynar M, Awad I. A comparison of the clinical profile of cavernous malformations with and without associated venous malformations. Neurosurgery. 1999;44:41-47.
251 McCormick W. Pathology of vascular malformations of the brain. In: Wilson C, Stein B, editors. Intracranial Arteriovenous Malformations. Baltimore: Williams & Wilkins; 1984:44-63.
252 Jellinger K. Vascular malformations of the central nervous system: a morphological overview. Neurosurg Rev. 1986;9:177-216.
253 Kraemer D, Awad I. Vascular malformations and epilepsy: clinical considerations and basic mechanisms. Epilepsia. 1994;35(suppl 6):S30-S43.
254 Awad I, Robinson J. Cavernous malformation and epilepsy. In: Awad I, Barrow D, editors. Cavernous Malformations. Park Ridge, IL: American Association of Neurological Surgeons; 1993:49-63.
255 Fargueta J, Iranzo R, Garcia M, et al. Hemangioma calcifications: a benign epileptogenic lesion. Surg Neurol. 1981;15:66-70.
256 Awad I, Jabbour P. Cerebral cavernous malformations and epilepsy. Neurosurg Focus. 2006;21(1):e7.
257 Robinson JJ, Awad I, Magdinec M, et al. Factors predisposing to clinical disability in patients with cavernous malformations of the brain. Neurosurgery. 1993;32:730-736.
258 Robinson J. Clinical spectrum and natural course. In: Awad I, Barrow D, editors. Cavernous Malformations. Park Ridge, IL: American Association of Neurological Surgeons; 1993:25-36.
259 Ueda S, Saito A, Inomori S, et al. Cavernous angioma of the cauda equina producing subarachnoid hemorrhage. Case report. J Neurosurg. 1987;66:134-136.
260 Tung H, Giannotta S, Chandrasoma P, et al. Recurrent intraparenchymal hemorrhages from angiographically occult vascular malformations. J Neurosurg. 1990;73:174-180.
261 Hubert P, Choux M, Houtteville J. Cerebral cavernomas in infants and children. Neurochirurgie. 1989;35:104-105.
262 Scott R, Barnes P, Kupsky W, et al. Cavernous angiomas of the central nervous system in children. J Neurosurg. 1992;76:38-46.
263 Kupersmith M, Kalish H, Epstein F, et al. Natural history of brainstem cavernous malformations. Neurosurgery. 2001;48:47-54.
264 Labauge P, Brunereau L, Lévy C, et al. The natural history of familial cerebral cavernomas: a retrospective MRI study of 40 patients. Neuroradiology. 2000;42:327-332.
265 Porter R, Detwiler P, Spetzler R, et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg. 1999;90:50-58.
266 Porter P, Willinsky R, Harper W, et al. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997;87:190-197.
267 Pozzati E, Acciarri N, Tognetti F, et al. Growth, subsequent bleeding, and de novo appearance of cerebral cavernous angiomas. Neurosurgery. 1996;38:662-669.
268 Hoeldtke N, Floyd D, Werschkul J, et al. Intracranial cavernous angioma initially presenting in pregnancy with new-onset seizures. Am J Obstet Gynecol. 1998;178:612-613.
269 Lopez-Fraile IP, Sanjuan MT, Eiras J, et al. Cerebral cavernous angiomas in pregnancy. Two cases and a review of literature. Neurologia. 1995;10:242-245.
270 Warner J, Rizzo J, Brown E, et al. Recurrent chiasmal apoplexy due to cavernous malformation. J Neuroophthalmol. 1996;16:99-106.
271 Flemming K, Goodman B, Meyer F. Successful brainstem cavernous malformation resection after repeated hemorrhages during pregnancy. Surg Neurol. 2003;60:545-547.
272 Awada A, Watson T, Obeid T. Cavernous angioma presenting as pregnancy-related seizures. Epilepsia. 1997;38:844-846.
273 Zauberman H, Feinsod M. Orbital hemangioma growth during pregnancy. Acta Ophthalmol (Copenh). 1970;48:929-933.
274 Barker F, Amin-Hanjani S, Butler W, et al. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery. 2001;49:15-25.
275 Duffau H, Capelle L, Sichez J, et al. Early radiologically proven rebleeding from intracranial cavernous angiomas: report of 6 cases and review of the literature. Acta Neurochir (Wien). 1997;139:914-922.
276 Fritschi J, Reulen H, Spetzler R, et al. Cavernous malformations of the brain stem. A review of 139 cases. Acta Neurochir (Wien). 1994;130:35-46.
277 Pozzati E, Giuliani G, Nuzzo G, et al. The growth of cerebral cavernous angiomas. Neurosurgery. 1989;25:92-97.
278 Nishijima M, Takaku A, Endo S, et al. Etiological evaluation of dural arteriovenous malformations of the lateral and sigmoid sinuses based on histopathological examinations. J Neurosurg. 1992;76:600-606.
279 Awad I, Little J. Dural arteriovenous malformation. In: Barrow D, editor. Intracranial Vascular Malformation. Park Ridge, IL: American Association of Neurological Surgeons; 1990:219-226.
280 Horie N, Morikawa M, Kitigawa N, et al. 2D Thick-section MR digital subtraction angiography for the assessment of dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2006;27:264-269.
281 Noguchi K, Melhem ER, Kanazawa T, et al. Intracranial dural arteriovenous fistulas: evaluation with combined 3D time-of-flight MR angiography and MR digital subtraction angiography. AJR Am J Roentgenol. 2004;182:183-190.
282 Chen J, Tsurdua J, Halbach V. Suspected dural arteriovenous fistula: results with screening MR angiography in seven patients. Radiology. 1992;183:265-271.
283 DeMarco J, Dillon W, Halbach V, et al. Dural arteriovenous fistulas: evaulation with MR imaging. Radiology. 1990;175:193-199.
284 Malek A, Halbach V, Dowd C, et al. Diagnosis and treatment of dural arteriovenous fistulas. Neuroimaging Clin N Am. 1998;8:445-468.
285 Grady M, Pobereskin L. Arteriovenous malformations of the dura mater. Surg Neurol. 1987;28:135-140.
286 Aminoff M. Vascular anomalies in the intracranial dura mater. Brain. 1973;96:601-612.
287 Chaudhary M, Sachdev V, Cho S, et al. Dural arteriovenous malformation of the major venous sinuses: an acquired lesion. AJNR Am J Neuroradiol. 1982;3:13-19.
288 Houser O, Campbell J, Campbell R, et al. Arteriovenous malformation affecting the transverse dural venous sinus—an acquired lesion. Mayo Clin Proc. 1979;54:651-661.
289 Little N, Piepgras D. Intracranial dural arteriovenous fistula. In: Batjer H, editor. Cerebrovascular Disease. Philadelphia: Lippincott-Raven; 1997:799-809.
290 Sarma D, ter Brugge K. Management of intracranial dural arteriovenous shunts in adults. Eur J Radiol. 2003;46:206-220.
291 Singh V, Smith WS, Lawton MT, et al. Risk factors for hemorrhagic presentation in patients with dural arteriovenous fistulae. Neurosurgery. 2008;62:628-635.
292 Soderman M, Pavic L, Edner G, et al. Natural history of dural arteriovenous shunts. Stroke. 2008;39:1735-1739.
293 Kim MS, Han DH, Kwon OK, et al. Clinical characteristics of dural arteriovenous fistula. J Clin Neurosci. 2002;9:147-155.
294 Dennery J, Ignacio B. Post-traumatic arteriovenous fistula between the external carotid arteries and the superior longitudinal sinus: report of a case. Can J Surg. 1967;10:333-336.
295 Nabors M, Azzam C, Albanna F, et al. Delayed postoperative dural arteriovenous malformations. Report of two cases. J Neurosurg. 1987;66:768-772.
296 Watanabe A, Takahara Y, Ibuchi Y, et al. Two cases of dural arteriovenous malformation occurring after intracranial surgery. Neuroradiology. 1984;26:375-380.
297 Davie J, Hodges F. Arteriovenous fistula after removal of meningioma. Case report. J Neurosurg. 1967;27:364-369.
298 Ugrinovski J, Vrcakovski M, Lozance K. Dural arteriovenous malformation secondary to meningioma removal. Br J Neurosurg. 1989;3:603-607.
299 Yokota M, Tani E, Maeda Y, et al. Meningioma in sigmoid sinus groove associated with dural arteriovenous malformation: case report. Neurosurgery. 1993;33:316-319.
300 Picard L, Bracard S, Mallet J. Spontaneous arteriovenous fistulas. Semin Intervent Radiol. 1987;4:219-240.
301 Tsai LK, Jeng JS, Liu HM, et al. Intracranial dural arteriovenous fistulas with or without cerebral sinus thrombosis: analysis of 69 patients. J Neurol Neurosurg Psychiatry. 2004;75:1639-1641.
302 Awad I, Little J, Akrawi W, et al. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg. 1990;72:839-850.
303 Chaloupka J. Endovascular terapy of dural arteriovenous fistulae. Semin Intervent Radiol. 1994;11:1-13.
304 Chaloupka J, Putman C. Endovascular therapy for surgical diseases of the cranial base. Clin Plast Surg. 1995;22:417-450.
305 Lawton M, Jacobowitz R, Spetzler R. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997;87:267-274.
306 Terada T, Higashida R, Halbach V, et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80:884-889.
307 Brown RJ, Wiebers D, Nichols D. Intracranial dural arteriovenous fistulae: angiographic predictors of intracranial hemorrhage and clinical outcome in nonsurgical patients. J Neurosurg. 1994;81:531-538.
308 Malik G, Morgan J, Boulos R, et al. Venous angiomas: an underestimated cause of intracranial hemorrhage. Surg Neurol. 1988;30:350-358.
309 Cognard C, Gobin Y, Peirot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680.
310 van Dijk JM, TerBrugge KG, Willinsky RA, et al. Multiplicity of dural arteriovenous fistulas. J Neurosurg. 2002;96:76-78.
311 Nakamura M, Tamaki N, Hara Y, et al. Two unusual cases of multiple dural arteriovenous fistulas. Neurosurgery. 1997;41:288-292.
312 Kuwayama N, Takaku A, Nishijima M, et al. Multiple dural arteriovenous malformations. Report of two cases. J Neurosurg. 1989;71:932-934.
313 Ushikoshi S, Kikuchi Y, Miyasaka K. Multiple dural arteriovenous shunts in a 5-year-old boy. AJNR Am J Neuroradiol. 1999;20:728-730.
314 van Dijk JM, TerBrugge KG, Van der Meer FJ, et al. Thrombophilic factors and the formation of dural arteriovenous fistulas. J Neurosurg. 2007;107:56-59.
315 Fermand M, Reizine D, Melki J, et al. Long term follow-up of 43 pure dural arteriovenous fistulae (AVF) of the lateral sinus. Neuroradiology. 1987;29:348-353.
316 Obrador S, Gomez-Bueno J, Silvela J. Spontaneous carotid-cavernous fistula produced by ruptured aneurysm of the meningohypophyseal branch of the internal carotid artery. Case report. J Neurosurg. 1974;40:539-543.
317 Edwards M, Connolly E. Cavernous sinus syndrome produced by communication between the external carotid artery and cavernous sinus. J Neurosurg. 1977;46:92-96.
318 Satomi J, van Dijk JM, Terbrugge KG, et al. Benign cranial dural arteriovenous fistulas: outcome of conservative management based on the natural history of the lesion. J Neurosurg. 2002;97:767-770.
319 Lasjaunias P, Chiu M, Brugge KT, et al. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg. 1986;64:724-730.
320 Vinuela F, Fox AJ, Pelz DM, et al. Unusual clinical manifestations of dural arteriovenous malformations. J Neurosurg. 1986;64:554-558.
321 Zeidman S, Monsein L, Arosarena O, et al. Reversibility of white matter changes and dementia after treatment of dural fistulas. AJNR Am J Neuroradiol. 1995;16:1080-1083.
322 Hurst R, Bagley L, Galetta S, et al. Dementia resulting from dural arteriovenous fistulas: the pathologic findings of venous hypertensive encephalopathy. AJNR Am J Neuroradiol. 1998;19:1267-1273.
323 Ito J, Imamura H, Kobayashi K, et al. Dural arteriovenous malformations of the base of the anterior cranial fossa. Neuroradiology. 1983;24:149-153.
324 Brunereau L, Gobin Y, Meder J, et al. Intracranial dural arteriovenous fistulas with spinal venous drainage: relation between clinical presentation and angiographic findings. AJNR Am J Neuroradiol. 1996;17:1549-1554.
325 Wrobel C, Oldfield E, Chiro GD, et al. Myelopathy due to intracranial dural arteriovenous fistulas draining intrathecally into spinal medullary veins. Report of three cases. J Neurosurg. 1988;69:934-939.
326 van Rooij WJ, Sluzewski M, Beute GN. Dural arteriovenous fistulas with cortical venous drainage: incidence, clinical presentation, and treatment. AJNR Am J Neuroradiol. 2007;28:651-655.
327 Malik GM, Pearce JE, Ausman JI, et al. Dural arteriovenous malformations and intracranial hemorrhage. Neurosurgery. 1984;15:332-339.
328 Lucas Cde P, Caldas JG, Prandini MN. Do leptomeningeal venous drainage and dysplastic venous dilation predict hemorrhage in dural arteriovenous fistula? Surg Neurol. 2006;66(suppl 3):S2-S5.
329 Borden J, Wu J, Shucart W. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-179.
330 van Dijk JM, terBrugge KG, Willinsky RA, et al. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002;33:1233-1236.
331 Lalwani A, Dowd C, Halbach V. Grading venous restrictive disease in patients with dural arteriovenous fistulas of the transverse/sigmoid sinus. J Neurosurg. 1993;79:11-15.
332 Willinsky R, Goyal M, terBrugge K, et al. Tortuous, engorged pial veins in intracranial dural arteriovenous fistulas: correlations with presentation, location, and MR findings in 122 patients. AJNR Am J Neuroradiol. 1999;20:1031-1036.
333 Barrow D, Spector R, Braun I, et al. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62:248-256.
334 Djindjian R, Cophignon J, Theron JR, et al. Superselective arteriographic embolization by the femoral route in neuroradiology. Study of 50 cases. 3. Embolization in craniocerebral pathology. Neuroradiology. 1973;6:143-152.
335 Davies MA, TerBrugge K, Willinsky R, et al. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg. 1996;85:830-837.
336 Grisoli F, Vincentelli F, Fuchs S, et al. Surgical treatment of tentorial arteriovenous malformations draining into the subarachnoid space. Report of four cases. J Neurosurg. 1984;60:1059-1066.
337 Duffau H, Lopes M, Janosevic V, et al. Early rebleeding from intracranial dural arteriovenous fistulas: report of 20 cases and review of the literature. J Neurosurg. 1999;90:78-84.
338 Halbach V, Hieshima G, Higashida R, et al. Carotid cavernous fistulae: indications for urgent treatment. AJR Am J Roentgenol. 1987;149:587-593.
339 Halbach V, Higashida R, Hieshima G, et al. Transvenous embolization of dural fistulas involving the cavernous sinus. AJNR Am J Neuroradiol. 1989;10:377-383.
340 Newton T, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology. 1969;93:1071-1078.
341 Olutola P, Eliam M, Molot M, et al. Spontaneous regression of a dural arteriovenous malformation. Neurosurgery. 1983;12:687-690.
342 Saito Y, Kobayashi N. Cerebral venous angiomas: clinical evaluation and possible etiology. Radiology. 1981;139:87-94.
343 Senegor M, Dohrmann G, Wollmann R. Venous angiomas of the posterior fossa should be considered as anomalous venous drainage. Surg Neurol. 1983;19:26-32.
344 Numaguchi Y, Kitamura K, Fukui M, et al. Intracranial venous angiomas. Surg Neurol. 1982;18:193-202.
345 Lasjaunias P, Burrows P, Planet C. Developmental venous anomalies (DVA): the so-called venous angioma. Neurosurg Rev. 1994;9:232-242.
346 Huang Y, Robbins A, Patel S, et al. Cerebral venous malformations and a new classification of cerebral vascular malformations. In: Kapp J, Schmidek H, editors. The Cerebral Venous System and Its Disorders. New York: Grune & Stratton; 1984:373-474.
347 Wolf A, Brock S. The pathology of cerebral angiomas. A study of nine cases. Bull Neurol Institut N Y. 1935;4:144-176.
348 Rigamonti D, Spetzler R, Medina M, et al. Cerebral venous malformations. J Neurosurg. 1990;73:560-564.
349 Valavanis A, Wellauer J, Yasargil M. The radiological diagnosis of cerebral venous angioma: cerebral angiography and computed tomography. Neuroradiology. 1983;24:193-199.
350 Fierstien S, Pribram H, Hieshima G. Angiography and computed tomography in the evaluation of cerebral venous malformations. Neuroradiology. 1979;17:137-148.
351 Garner T, Del Curling OJr, Kelly DLJr, et al. The natural history of intracranial venous angiomas. J Neurosurg. 1991;75(5):715-722.
352 Kondziolka D, Dempsey P, Lunsford L. The case for conservative management of venous angiomas. Can J Neurol Sci. 1991;18:295-299.
353 McLaughlin M, Kondziolka D, Flickinger J, et al. The prospective natural history of cerebral venous malformations. Neurosurgery. 1998;43:195-201.
354 Biller J, Toffol G, Shea J, et al. Cerebellar venous angiomas. Arch Neurol. 1985;42:367-370.
355 Goulao A, Alvarez H, Monaco RG, et al. Venous anomalies and abnormalities of the posterior fossa. Neuroradiology. 1990;31:476-482.
356 Rothfus W, Albright A, Casey K, et al. Cerebellar venous angioma: Benign entity? AJNR Am J Neuroradiol. 1984;5:61-66.
357 Comey C, Kondziolka D, Yonas H. Regional parenchymal enhancement with mixed cavernous/venous malformations of the brain. Case report. J Neurosurg. 1997;86:155-158.
358 Handa J, Suda K, Sato M. Cerebral venous angioma associated with varix. Surg Neurol. 1984;21:436-440.
359 Hirata Y, Matsukado Y, Nagahiro S, et al. Intracerebral venous angioma with arterial blood supply: a mixed angioma. Surg Neurol. 1986;25:227-232.
360 Meyer B, Stangl A, Schramm J. Assocation of venous and true arteriovenous malformation: a rare entitiy among mixed vascular malformations of the brain. J Neurosurg. 1995;83:141-144.
361 Robinson JJ, Brown A, Spetzler R. Occult malformation with anomalous venous drainage. J Neurol. 1995;82:311-312.
362 Abe T, Singer R, Marks M, et al. Coexistence of occult vascular malformations and developmental venous anomalies in the central nervous system: MR evaluation. AJNR Am J Neuroradiol. 1998;19:51-57.
363 Komiyama M, Yamanaka K, Iwai Y, et al. Venous angiomas with arteriovenous shunts: report of three cases and review of the literature. Neurosurgery. 1999;44:1328-1335.
364 Mullan S, Oojtahedi S, Johnson D, et al. Cerebral venous malformation–arteriovenous malformation transition forms. J Neurosurg. 1996;85:9-13.
365 Scamoni C, Dario A, Basile L. The association of cavernous and venous angioma. Case report and review of the literature. Br J Neurosurg. 1997;11:346-349.
366 Abe M, Asfora W, DeSalles A, et al. Cerebellar venous angioma associated with angiographically occult brain stem vascular malformation: report of two cases. Surg Neurol. 1990;33:400-403.
367 Avman N, Dincer C. Venous malformation of the aqueduct of Sylvius treated by interventriculostomy: 15 years follow-up. Acta Neurochir (Wien). 1980;52:219-224.
368 Abla A, Wait S, Uschold T, et al. Developmental venous anomaly, cavernous malformation, and capillary telangiectasia: spectrum of a single disease. Acta Neurochir (Wien). 2008;150:487-489.
369 Olson E, Gilmor R, Richmond B. Cerebral venous angiomas. Radiology. 1984;151:97-104.
370 Aagaard B, Song J, Eskridge J, et al. Complex right hemisphere developmental venous anomaly associated with multiple facial hemangiomas. Case report. J Neurosurg. 1999;90:766-769.
371 Merten C, Knitelius H, Hedde J, et al. Intracerebral haemorrhage from a venous angioma following thrombosis of a draining vein. Neuroradiology. 1998;40:15-18.
372 Peltier J, Toussaint P, Desenclos C, et al. Cerebral venous angioma of the pons complicated by nonhemorrhagic infarction. Case report. J Neurosurg. 2004;101:690-693.
373 Worrell G, Sencakova D, Jack C, et al. Rapidly progressive hippocampal atrophy: evidence for a seizure-induced mechanism. Neurology. 2002;58:1553-1556.
374 Gümüs A, Yildirim S, Kizilkiliç O, et al. Case report: seizures in a child caused by a large venous angioma. J Child Neurol. 2007;22:787-789.
375 Moritake K, Handa H, Mori K, et al. Venous angiomas of the brain. Surg Neurol. 1980;14:95-105.
376 Sadeh M, Shacked I, Rappaport Z, et al. Surgical extirpation of a venous angioma of the medulla oblongata simulating multiple sclerosis. Surg Neurol. 1982;17:334-337.
377 Wendling L, Moore JJ, Kieffer S, et al. Intracerebral venous angioma. Radiology. 1976;119:141-147.
378 Naff N, Wemmer J, Hoenig-Rigamonti K, et al. A longitudinal study of patients with venous malformations. Neurology. 1998;50:1709-1714.
379 Topper R, Jurgens E, Reul J, et al. Clinical significance of intracranial developmental venous anomalies. J Neurol Neurosurg Psychiatry. 1999;67:234-238.
380 Nemoto H, Nakazora H, Toda T, et al. Venous angioma with epilepsy. Intern Med. 2006;45:345-346.
381 Lai P, Chen P, Pan H, et al. Venous infarction from a venous angioma occurring after thrombosis of a drainage vein. AJR Am J Roentgenol. 1999;172:1698-1699.
382 Konan A, Raymond J, Bourgouin P, et al. Cerebellar infarct caused by spontaneous thrombosis of a developmental venous anomaly of the posterior fossa. AJNR Am J Neuroradiol. 1999;20:256-258.
383 Gomori J, Grossman R, Goldberg H, et al. Occult cerebral vascular malformations: high-field MR imaging. Radiology. 1986;158:707-713.
384 Lee R, Becher M, Benson M, et al. Brain capillary telangiectasia: MR imaging appearance and clinicohistopathologic findings. Radiology. 1997;205:797-805.
385 Barr R, Dillon W, Wilson C. Slow-flow vascular malformations of the pons: capillary telangiectasias? AJNR Am J Neuroradiol. 1996;17:71-78.
386 Guibaud L, Pelizzari M, Guibal A, et al. Slow-flow vascular malformation of the pons: congenital or acquired capillary telangiectasia. AJNR Am J Neuroradiol. 1996;17:1798-1800.
387 Courville C. Pathology of the Central Nervous System, 3rd ed. Moutain View, CA: Pacific Press Publishing Association; 1950.
388 Milandre L, Pellissier J, Boudouresques G, et al. Non-hereditary multiple telangiectasias of the central nervous system. Report of two clinicopathological cases. J Neurol Sci. 1987;82:291-304.
389 McConnell TH, Leonard JS. Microangiomatous malformation with intraventricular hemorrhage. Neurology. 1967;17:618-620.
390 Teilman K. Hemangiomas of the pons. Arch Neurol Psychiatry. 1953;69:208-223.
391 Wijdicks E, Schievink W. Perimesencephalic nonaneurysmal subarachnoid hemorrhage: first hint of a cause? Neurology. 1997;49:634-636.
392 Wijdicks E, Schievink W, Miller G. MR imaging in pretruncal nonaneurysmal subarachnoid hemorrhage: is it worthwhile? Stroke. 1998;29:2514-2516.
393 VanRoost D, Kristof R, Wolf H, et al. Intracerebral capillary telangiectasia and venous malformation: a rare association. Surg Neurol. 1997;48:175-183.
394 Farrell D, Forno L. Symptomatic capillary telangiectasis of the brainstem without hemorrhage. Report of an unusual case. Neurology. 1970;20:341-346.
395 Cushing H, Bailey P. Tumors Arising from the Blood Vessel of the Brain. Springfield, IL: Charles C Thomas; 1928.
* Although the majority of angiographically occult and cryptic vascular malformations are CMs, thrombosed AVMs may appear similar on imaging studies.
* See references 46, 51, 54, 55, 93, 94, 105, 130, 137, 138.
* These terms are all synonyms and encompass CMs as well as thrombosed AVMs and sometimes venous angiomas (see text).
* See references 183, 185, 186, 204, 206, 226, 231, 244, 257, 258.
* See references 180, 183–186, 189, 197, 206, 208, 226, 230, 231, 243, 257.
* Not a preferred term because it implies that these lesions are congenital when the majority are actually idiopathic or acquired.