Chapter 21 The musculoskeletal system
Drugs used in arthritis, rheumatism and muscle pain
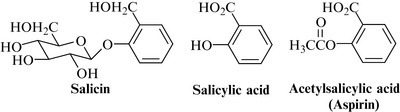
The classic NSAID, aspirin, was originally developed as a result of studies on salicin, obtained from willow bark (see below and Chapter 15 and – for historical aspects – Chapter 3). Although it was thought at first that the effects of salicin were due only to the hydrolysed product salicylic acid, it is now known that plant antiinflammatory agents tend to have fewer gastrointestinal side effects than salicylates in general. There are also several combination herbal products on the market, for which little clinical data are available, but which are very popular and seem to produce few side effects.
Bromelain (Ananase)
Bromelain has been proposed for the treatment of atherosclerosis, dysmenorrhoea, scleroderma, infection and sports injuries. It is antiinflammatory in animal studies and is used clinically to treat bruising, arthritis, joint stiffness and pain, and to improve healing postoperatively, including after dental procedures. It is considered to be an effective alternative to NSAIDs, as shown by a number of clinical trials. A recent study to assess the efficacy of bromelain in controlling the oedema and pain after tooth extraction found it to be effective in treating postoperative oedema after third molar surgery (Inchingolo et al 2010). Bromelain, given once daily in acute tendon injury at a dosage of 7 mg/kg for 14 days, promoted healing by stimulating tenocyte proliferation in rats (Aiyegbusi et al 2011). Bromelain is generally well tolerated, but side effects include minor gastrointestinal upsets.
Devil’s claw, Harpagophytum procumbens DC. ex Meissner (Harpagophyti radix) 
Constituents
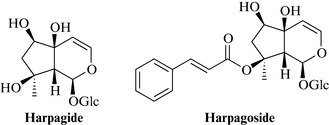
The most important actives are considered to be the bitter iridoids, harpagide and harpagoside (Fig. 21.1), with 8-O–p-coumaroylharpagide, procumbide, 6’-O–p-coumaroylprocumbide, pagide and procumboside; the triterpenoids oleanolic and ursolic acids, β-sitosterol and a glycoside harproside. Other compounds present include phenylethyl glycosides such as verbascoside and isoacteoside, polyphenolic acids (caffeic, cinnamic, and chlorogenic acids), and flavonoids such as luteolin and kaempferol. According to the Eur. Ph. the drug must contain ≥ 1.2% harpagide and harpagoside, expressed as harpagoside.
Therapeutic uses and available evidence
Most pharmacological and clinical research has been conducted using standardized extracts for the treatment of rheumatic conditions and lower back pain. Several clinical studies, including some placebo-controlled double-blind trials, demonstrate the superiority of these extracts to placebo in patients with osteoarthritis, non-radicular back pain and other forms of chronic and acute pain. Other studies show their therapeutic equivalence to conventional forms of treatment. Devil’s claw is generally well tolerated and appears to be a suitable alternative to NSAIDs, which often have gastrointestinal side effects (for review, see Barnes 2009, Cameron et al 2009).
The mechanism of action is not fully known: fractions of the extract containing the highest concentration of harpagoside inhibited COX-1 and COX-2 activity and greatly inhibited NO production, whereas in contrast, the fraction containing mainly the other iridoids increased COX-2 and did not alter NO and COX-1 activities. A fraction containing mainly cinnamic acid was able to reduce only NO production (Anauate et al 2010). An extract of Harpagophytum procumbens showed a significant antiinflammatory effect in the rat adjuvant-induced chronic arthritis model, and harpagoside dose-dependently suppressed the lipopolysaccharide (LPS)-induced production of inflammatory cytokines (IL-1β, IL-6, and TNF-α) in mouse macrophage cells (Inaba et al 2010). These demonstrate that harpagoside is probably the main active constituent responsible for the effect of devil’s claw, but that other components from the crude extract can antagonize or increase the synthesis of inflammatory mediators. In summary, both the pharmacological mechanism and the compounds responsible for this activity have to be investigated further, and by in vivo methods. There are implications for the production methods for preparing devil’s claw extracts, and a recent study of the antiinflammatory activity of various commercial products has demonstrated that there is a great difference in their composition, and concludes that the harpagoside content is not a reliable method of predicting the therapeutic efficacy (Ouitas and Heard 2010). Extracts of devil’s claw are generally well tolerated but should not be used for patients with gastric or duodenal ulceration. The aqueous extract possesses spasmogenic, uterotonic action on rat uterine muscles (Mahomed and Ojewole 2009), leading credence to the folkloric obstetric uses, but suggesting that it should be avoided in pregnant women. Side effects include minor gastrointestinal upsets.
Rosehip, Rosa canina L. (Rosae Pseudofructus; also known as Rosae Fructus or Rosae Pseudofructus cum Fructibus)
Constituents
Antiinflammatory constituents isolated from rosehip extracts include the triterpene acids, oleanolic, betulinic and ursolic acids; oleic, linoleic and alpha-linolenic acids, and a series of galactolipids which are thought to be a major contributor to the effects (Chrubasik et al 2008b).
Therapeutic uses and available evidence
Traditionally, rose hips were used as a source of vitamin C and were made into syrups for that purpose, but modern use is now focused on their antiinflammatory effects (for review, see Chrubasik et al 2008a). In a pilot surveillance study which included 152 patients with acute exacerbations of chronic pain, mainly of the lower back and knee, patients were recommended rose hip and seed powder at a dose providing up to 3 mg of galactolipid/day for up to 54 weeks. Multivariate analysis suggested an appreciable overall improvement, irrespective of type of pain, and this was reflected for most of the individual measures. There were no serious adverse events (Chrubasik et al 2008b). In a recent double-blind placebo-controlled trial of 89 patients with rheumatoid arthritis, treatment with encapsulated rose-hip powder 5 g daily for 6 months suggested that patients with rheumatoid arthritis may benefit from additional treatment with rose hip (Willich et al 2010). A study comparing powdered rose hip with and without the seeds found that extracts derived from rose hip without fruits were more effective in assays carried out for inhibition of COX-1, COX-2 and 5-LOX-mediated leukotriene B(4) formation, as well as for antioxidant capacity (Wenzig et al 2008). Extracts of rosehips have displayed potent antiinflammatory and antinociceptive activities in several in vivo experimental models (Deliorman Orhan et al 2007, but the mechanism of action and the active constituents are still not fully known. Bioassay-guided fractionation of rosehip powder yielded the triterpene acids, oleanolic acid and ursolic acid, as inhibitors of lipopolysaccharide induced interleukin-6 release (Saaby et al 2011), but these are ubiquitous compounds and may only play a part in the overall activity.
Turmeric, Curcuma domestica Val. (Curcumae domesticae rhizoma) 
Constituents
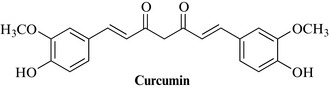
Three classes of compounds are particularly important: the curcuminoids – the mixture known as curcumin (Fig. 21.2) – consisting of several phenolic diarylheptanoids including curcumin, monodemethoxycurcumin and bisdemethoxycurcumin; an essential oil (about 3–5%), containing about 60% sesquiterpene ketones (turmerones), including arturmerone, α-atlantone, zingberene, with borneol, α-phellandrene, eugenol and others; and polysaccharides such as glycans, the ukonans A–D.
Therapeutic uses and available evidence
Turmeric is becoming increasingly popular in the West, as an antiinflammatory and antihepatotoxic agent (see also Chapter 14, Gastrointestinal and biliary system). It is also widely used in Ayurveda and Chinese medicine as an antiinflammatory, digestive, blood purifier, antiseptic and general tonic. It is given internally and also applied externally to wounds and insect bites. Most of the actions are attributable to the curcuminoids, although some of the essential oil components are also antiinflammatory. The efficacy of curcumin and its regulation of multiple targets, as well as its safety for human use, means that turmeric has received considerable interest as a potential therapeutic agent for the prevention and/or treatment of various malignant diseases, arthritis, allergies, Alzheimer’s disease and many other inflammatory illnesses (for review, see Zhou et al 2011). Antiinflammatory properties have been documented in numerous pharmacological models, and the use of turmeric seems promising, despite the limited number of clinical studies and poor bioavailability (for review, see Henrotin et al 2010). Curcumin has been studied as an anticancer drug and inhibits iNOS (inducible nitric oxide synthase) in both in vitro and in vivo mouse models via a mechanism involving the pro-inflammatory transcription factor NF-κB. It has also been shown to inhibit the activation of another transcription factor (AP-1), indicating that curcumin may be a non-specific inhibitor of NF-κB. Reports also indicate cyclo-oxygenase inhibition and free radical scavenging ability as potential targets (for review, see Epstein et al 2010). Immunostimulant activity, due to the polysaccharide fraction, has been shown, and anti-asthmatic effects have been noted, together with antimutagenic and anticarcinogenic effects. It is the subject of much current research but clinical evidence is urgently needed. Turmeric is well tolerated.
Willow bark, Salix spp. (Salicis cortex) 
Constituents
Phenolic glycosides, including salicin (Fig. 21.3), phenolic acids, tannins (mainly dimeric and polymeric procyanidins) and flavonoids are the most prominent groups of compounds. The most commonly used willow bark dry extract has a salicin content of 15–18%.
Very few pharmacological studies of individual compounds from willow bark (and their metabolites) have been conducted. The extract, however, exerts effects on several pro-inflammatory targets, including both isoforms of cyclo-oxygenase and, recently, willow bark water extract STW 33–1 has been shown to produce a significant inhibition of TNFα and NF-κB in activated monocytes (Bonaterra et al 2010).
Therapeutic uses and available evidence
Willow bark has been studied clinically. The effectiveness of an extract of willow bark (which is licensed as a medicine in Germany) has been shown to be superior to placebo for osteoarthritis and lower back pain, and with fewer side effects than for example aspirin (for review, see Vlachlojannis et al 2009). However, further more stringent clinical and mechanistic studies are needed. In very high doses, the side effects of salicylates may be encountered, although these are rarely seen at therapeutic levels of the extract. In general, the effective dose contains lower amounts of salicylate than would be expected by calculation, and a form of synergy is thought to be operating within the extract.
Drugs used in gout
Colchicine 
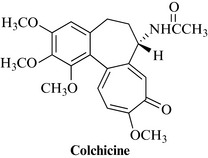
Colchine (Fig. 21.4) is a pure alkaloid extracted from the corms and flowers of Colchicum autumnale L., the autumn crocus or meadow saffron (Colchicaceae, formerly Liliaceae). The plant grows from bulbs in meadows throughout Europe and North Africa, typically appearing during the autumn, with the fruit developing over winter and being dispersed prior to the first mowing of the meadows. The leaves and the fruit appear during spring. The plant extract is not used because colchicine is highly toxic and the dose must be rigorously controlled.
Colchicine is used in the acute phase of gout, particularly when NSAIDs are either ineffective or contraindicated (for review, see Schlesinger et al 2009). Colchicine is occasionally also used as prophylaxis for Mediterranean familial fever. It is an important tool for biochemical research, as an inhibitor of the separation of the chromosomes during mitosis (e.g. used in breeding experiments to produce polyploid organisms). Colchicine causes gastrointestinal upsets such as nausea, vomiting, abdominal pain and diarrhoea. The dose is 1 mg initially, followed by increments of 500 μg every 2–3 hours until relief is obtained, to a maximum of 6 mg. The course should not be repeated within 3 days.
Topical anti-inflammatory agents
Arnica, Arnica montana L. (Arnicae flos) 
Constituents
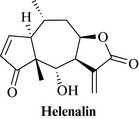
Arnica species are rich in sesquiterpene lactones of the pseudoguianolide type. The most abundant sesquiterpene lactone in A. montana is helenalin (Fig. 21.5), with 11α,13-dihydrohelenalin. Flavonoids, including quercetin and kaempferol derivatives, some coumarins and an essential oil are the other groups of natural products found typically in the flower heads of arnica.
Therapeutic uses and available evidence
Extracts of arnica and the pure sesquiterpene lactones with an exocyclic methylene group (e.g. helenalin) have been shown to exert antiinflammatory effects in vivo in animal models, although few clinical studies have been carried out. A randomised, double-blind study in 204 patients with active osteoarthritis of the hands, carried out to compare ibuprofen gel (5%) with arnica gel (50 g tincture/100 g, drug extract ratio 1:20), found that there were no differences in pain relief and hand function after 21 days’ treatment between the two groups. Adverse events were reported by five patients (4.8%) on arnica, slightly lower than the ibuprofen group (Widrig et al 2007).
However, a recent trial conducted in 53 subjects who were carrying out eccentric calf exercises found that rather than decreasing leg pain, arnica increased leg pain 24 hours after exercise (Adkison et al 2010). However, this effect did not extend to the 48-hour measurement, and it is not clear how this model relates to most of the clinical situations in which arnica is used. There was no difference in muscle tenderness or ankle range of motion.
Helenalin is well known for its in vitro effects on several transcription factors, including NF-κB and NF-AT. Arnica preparations also suppress matrix metalloproteinase-1 (MMP1) and MMP13 mRNA levels in articular chondrocytes at low concentrations, possibly due to inhibition of DNA binding of the transcription factors AP-1 and NF-kappaB (Jäger et al 2009). The cytotoxicity of the sesquiterpene lactones is well documented, and allergic reactions may occur. Arnica is used externally, except in homoeopathic preparations, but the sesquiterpene lactones have been shown to be absorbed through the skin (Tekko et al 2006).
Capsaicin
Therapeutic uses and available evidence
Capsaicin acts on vanilloid receptors, causing inflammation, but it also desensitizes sensory nerve endings to pain stimulation by depleting the neuropeptide Substance P from local C-type nerve fibres. It is used as a local analgesic in the treatment of postherpetic neuralgia, diabetic neuropathy, osteoarthritis and for pruritus (Papoiu and Yosipovitch 2010). In the management of intractable neuropathic pain, it may provide a degree of pain relief to some patients (Derry et al 2009). Capsaicin has long been used in cough and cold remedies, and recent findings that the vanilloid 1 (TRPV1) receptor is a sensor of airway irritation and initiator of the cough reflex (Geppetti et al 2010) may provide a rationale for that usage.
Nocturnal leg cramps
Quinine 
Quinine is isolated from the bark of Cinchona spp. Quinine salts can be effective in reducing their incidence, but should be avoided for routine use in the management of muscle cramps because of their potential for cardiac toxicity. However, in select patients they can be considered once potential side effects are taken into account. It has been recommended that quinine should be used more in patients with cirrhosis, subject to further investigations (Corbani et al 2008). Quinine salts are used in doses of 200–300 mg at bedtime, in ambulatory patients. For further information on quinine, including the structure, see Chapter 18.
Adkison J.D., Bauer D.W., Chang T. The effect of topical arnica on muscle pain. Ann. Pharmacother.. 2010;44:1579-1584.
Aiyegbusi A.I., Duru F.I., Anunobi C.C., Noronha C.C., Okanlawon A.O. Bromelain in the early phase of healing in acute crush Achilles tendon injury. Phytother. Res.. 2011;25:49-52.
Anauate M.C., Torres L.M., de Mello S.B. Effect of isolated fractions of Harpagophytum procumbens D.C. (devil’s claw) on COX-1, COX-2 activity and nitric oxide production on whole-blood assay. Phytother Res.. 2010;24(9):1365-1369.
Barnes J. Devil’s claw (Harpagophytum procumbens). Also known as ‘grapple plant’ or ‘wood spider’. J. Prim. Health Care. 2009;1:238-239.
Bonaterra G.A., Heinrich E.U., Kelber O., Weiser D., Metz J., Kinscherf R. Anti-inflammatory effects of the willow bark extract STW 33-I (Proaktiv(®)) in LPS-activated human monocytes and differentiated macrophages. Phytomedicine. 2010;17:1106-1113.
Cameron M., Gagnier J.J., Little C.V., Parsons T.J., Blümle A., Chrubasik S. Evidence of effectiveness of herbal medicinal products in the treatment of arthritis. Part I: Osteoarthritis. Phytother. Res.. 2009;23:1497-1515.
Chrubasik C., Roufogalis B.D., Müller-Ladner U., Chrubasik S. A systematic review on the Rosa canina effect and efficacy profiles. Phytother. Res.. 2008;22:725-733.
Chrubasik C., Wiesner L., Black A., Müller-Ladner U., Chrubasik S. A one-year survey on the use of a powder from Rosa canina lito in acute exacerbations of chronic pain. Phytother. Res.. 2008;22:1141-1148.
Corbani A., Manousou P., Calvaruso V., Xirouchakis I., Burroughs A.K. Muscle cramps in cirrhosis: the therapeutic value of quinine. Is it underused. Dig. Liver Dis.. 2008;40:794-799.
Deliorman Orhan D., Hartevioğlu A., Küpeli E., Yesilada E. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits. J. Ethnopharmacol.. 2007;112:394-400.
Derry S., Lloyd R., Moore R.A., McQuay H.J. Topical capsaicin for chronic neuropathic pain in adults. Cochrane Database Syst. Rev.. 4, 2009. CD007393
Epstein J., Sanderson I.R., Macdonald T.T. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br. J. Nutr.. 2010;103:1545-1557.
Geppetti P., Patacchini R., Nassini R., Materazzi S. Cough: the emerging role of the TRPA1 channel. Lung. 2010;188:S63-S68.
Henrotin Y., Clutterbuck A.L., Allaway D., et al. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18:141-149.
Inaba K., Murata K., Naruto S., Matsuda H. Inhibitory effects of devil’s claw (secondary root of Harpagophytum procumbens) extract and harpagoside on cytokine production in mouse macrophages. J. Nat. Med.. 2010;64:219-222.
Inchingolo F., Tatullo M., Marrelli M., et al. Clinical trial with bromelain in third molar exodontia. Eur. Rev. Med. Pharmacol. Sci.. 2010;14:771-774.
Jäger C., Hrenn A., Zwingmann J., Suter A., Merfort I. Phytomedicines prepared from Arnica flowers inhibit the transcription factors AP-1 and NF-kappaB and modulate the activity of MMP1 and MMP13 in human and bovine chondrocytes. Planta Med.. 2009;75:1319-1325.
Mahomed I.M., Ojewole J.A. Uterotonic effect of Harpagophytum procumbens DC (Pedaliaceae) secondary root aqueous extract on rat isolated uterine horns. J. Smooth Muscle Res.. 2009;45:231-239.
Ouitas N.A., Heard C. Estimation of the relative antiinflammatory efficacies of six commercial preparations of Harpagophytum procumbens (Devil’s Claw). Phytother. Res.. 2010;24:333-338.
Papoiu A.D., Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin. Pharmacother.. 2010;11:1359-1371.
Saaby L., Jäger A.K., Moesby L., Hansen E.W., Christensen S.B. Isolation of immunomodulatory triterpene acids from a standardized rose hip powder (Rosa canina L.). Phytother. Res.. 2011;25:195-201.
Schlesinger N., Dalbeth N., Perez-Ruiz F. Gout – what are the treatment options? Expert Opin. Pharmacother.. 2009;10:1319-1328.
Tekko I.A., Bonner M.C., Bowen R.D., Williams A.C. Permeation of bioactive constituents from Arnica montana preparations through human skin in-vitro. J. Pharm. Pharmacol.. 2006;58:1167-1176.
Vlachlojannis J.E., Cameron M., Chrubasik S. A systematic review on the effectiveness of willow bark for musculoskeletal pain. Phytother. Res.. 2009;23:897-900.
Wenzig E.M., Widowitz U., Kunert O., et al. Phytochemical composition and in vitro pharmacological activity of two rose hip (Rosa canina L.) preparations. Phytomedicine. 2008;15:826-835.
Widrig R., Suter A., Saller R., Melzer J. Choosing between NSAID and arnica for topical treatment of hand osteoarthritis in a randomised, double-blind study. Rheumatol. Int.. 2007;27:585-591.
Willich S.N., Rossnagel K., Roll S., et al. Rose hip herbal remedy in patients with rheumatoid arthritis – a randomised controlled trial. Phytomedicine. 2010;17:87-93.
Zhou H., Beevers C.S., Huang S. The targets of curcumin. Curr. Drug Targets. 2011;12:332-347.