The Mandible
Embryology
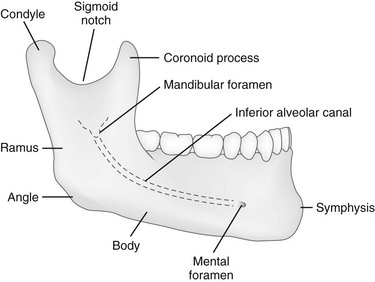
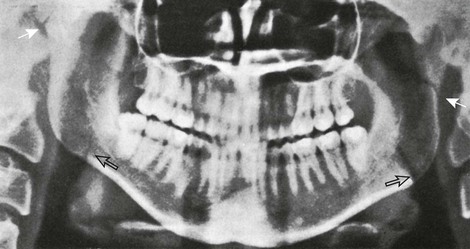
At birth, the mandible consists of two lateral halves united in the midline at the symphysis by a bar of cartilage (Fig. 22-1, e-Fig. 22-2, and Figs. 22-3 and 22-4). Bony fusion of the symphysis usually occurs before the second year, but segments of the fissures may persist beyond puberty. The body of the mandible is large at birth compared with the relatively short rami and poorly differentiated coronoid and condylar processes. The rami form an angle of about 160 degrees with the body at birth.
All or some of the teeth may be missing developmentally, as in persons with anhidrotic ectodermal dysplasia (Fig. 22-4).1 Because infants and children with normal dentition seem to have a plethora of teeth in radiographs (deciduous and permanent dentitions), the condition may be recognized in a newborn by the lack of dental buds. Many diseases cause loss of dentition, and recognition of premature loss of teeth can lead to important diagnoses (Box 22-1).2
The opposite condition—too many teeth with little alveolar bone—can be seen in persons with cleidocranial dysplasia, who have marked delay in shedding of the deciduous teeth. Early tooth extraction has no effect on the subsequent eruption of the permanent dentition and may result in a lengthy edentulous period for the child.3
Anatomy
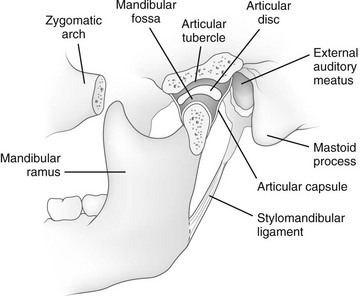
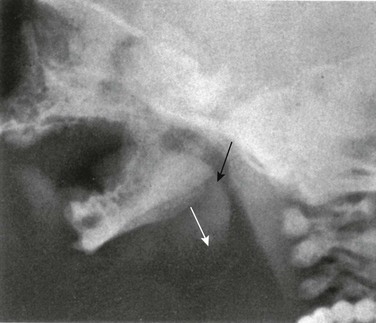
The mandible is the only freely movable bone of the face; it articulates with the temporal bone in the temporomandibular fossa anterior to the external auditory canal (see Fig. 22-3). The range of motion is free in all directions, and the condyle moves downward and forward in the articular fossa upon opening of the jaw.
The temporomandibular joint (see Fig. 22-3) is a complex joint in which a biconcave fibrous disk divides the articular space into upper and lower compartments.4–6 Gliding movements occur in the upper compartment, whereas the lower compartment functions as a true hinge joint. The articulating bony surfaces are not covered by hyaline cartilage as in other joints, but by an avascular, fibrous tissue that is separated from the underlying bone of the condyle by growth cartilage.7
Diseases of the Mandible
Significant congenital malformations of the mandible are rare, with the most important being hypoplasia (micrognathia), which may be a cause of congenital stridor. The short, small mandible apparently causes a retrodisplacement of the tongue and obstruction to airflow (Fig. 22-5).
Micrognathia occurs in a variety of dysmorphic syndromes. The combination of cleft palate and hypoplasia of the mandible defines the Pierre Robin sequence radiographically. The Pierre Robin association is nonspecific and occurs with several genetic and drug-induced syndromes and some loosely associated anomalies, as well as an isolated symptom complex.8 In the cerebrocostomandibular syndrome, it is associated with posterior rib defects, cleft palate, and, occasionally, mental retardation.9
Temporomandibular Joint Disorders
Disorders of the temporomandibular joint are infrequent but not rare.10,11 Adventitious sounds on mandibular movement, muscle tenderness or pain, and deviation of the mandible during movement are the most common signs and symptoms. Magnetic resonance imaging (MRI) is the most precise imaging modality.12,13
Fractures
Direct trauma is the usual cause of fractures, although pathologic fractures occur in association with cysts, destructive inflammations, and neoplasms. More than half of traumatic fractures are found in the body of the mandible near the canine fossa. Fractures high in the ramus frequently are overlooked on standard radiographs but are clearly defined by computed tomography (CT) (Fig. 22-6) and panographic tomography (e-Fig. 22-7). Direct trauma is the usual cause, although pathologic fractures occur in association with cysts, destructive inflammations, and neoplasms. More than half of traumatic fractures are found in the body of the mandible near the canine fossa.
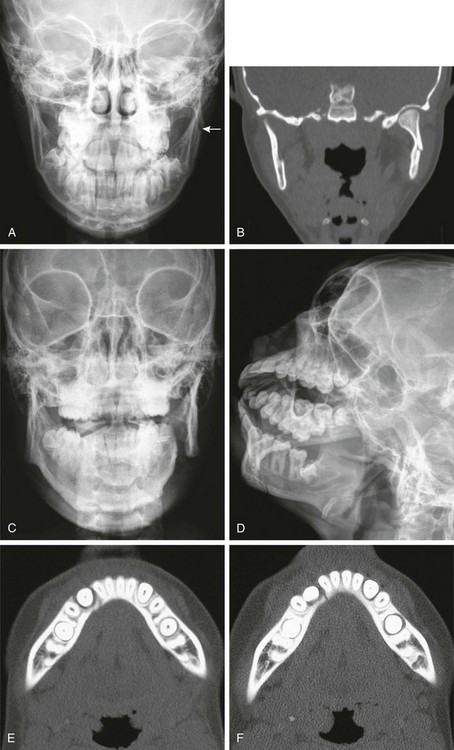
Figure 22-6 A fractured mandible.
A, A 14-year-old patient with a subtle fracture of the left mandibular vertical ramus (arrow). B, Coronal computed tomography reconstruction shows the fracture. C, D, E, and F, A 14-year-old patient with an overt left mandibular fracture of the body and ramus junction and a subtle fracture through the right dentition.
Vehicular trauma is responsible for 35% of mandibular fractures in children, and falls cause 28%. More condylar fractures are found in younger children (<9 years). During childhood, the mandible contains teeth in different stages of eruption, and the pattern of the alveolar bone predisposes it to fractures along the lines of the developing dental crypts. The lack of cortical bone and the relative excess of cancellous bone are responsible for the frequency of incomplete, greenstick fractures with minimal destruction of the bone or cartilage. The condylar neck usually breaks before the condyle itself because of the vascularity and thickness of the latter. During childhood, rapid union of mandibular fractures should be expected because of the rich blood supply, the high osteogenic potential of the periosteum, and the high local metabolic rate.14 The radiographic criteria for healing of fractures in other bones are not applicable to mandibular fractures because strips of rarefaction may persist at the margins of the fracture long after satisfactory union has occurred.
Osteomyelitis
Osteomyelitis rarely occurs in the mandible because it has no metaphyseal-epiphyseal junction structures. Osteomyelitis generally occurs as a result of dental infection or local trauma (e-Fig. 22-8). The immature mandibular structure allows inflammation to extend easily and rapidly. Bone necrosis and sequestra formation are common; destructive changes may not be visible by conventional radiography until weeks after the onset of infection.15 Hyperplastic changes usually predominate and have been confused with the manifestations of infantile cortical hyperostoses (Caffey disease).
Cysts, Neoplasms, and Neoplasm-like Disorders
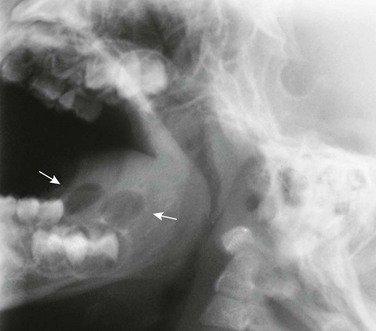
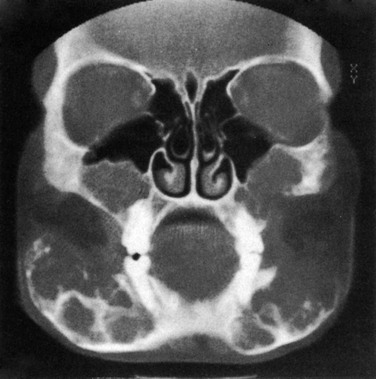
Most primary mandibular cysts and neoplasms are of dental origin (Figs. 22-9 through 22-11, e-Fig. 22-12, and Box 22-2).16 Cystic changes of the jaw may be a manifestation of the basal cell nevus syndrome, in which basal cell carcinomas, skeletal anomalies, ovarian and falx calcification, ovarian fibromas, and pits in the palms and soles also are present (see Fig. 22-9). The jaw cysts may manifest in childhood; the basal cell carcinomas develop only later. Dental root cysts result from proliferation of paradental epithelial cells in the periapical granulation tissue of local infection. Dentigerous cysts are formed by the excessive accumulation of fluid between the enamel and the dental capsule and show a well-formed dental crown without roots with its base adjacent to a large cystic structure that surrounds its upper portion (see Fig. 22-10).
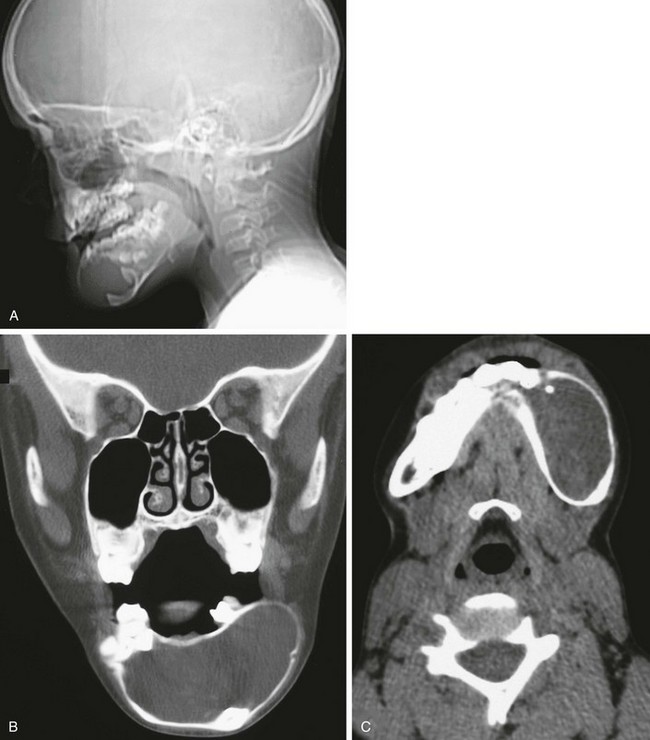
Figure 22-9 An 11-year-old patient with basal cell nevi syndrome.
A, A skull radiograph from the computed tomography (CT) scout image reveals the large round lesion in the central portion (mentum) of the mandible deviating the dentition. B, A coronal nonenhanced CT scan shows that the lesion is homogeneous. C, An axial CT scan with soft tissue windows shows a deviation of dentition and the well-circumscribed lesion.
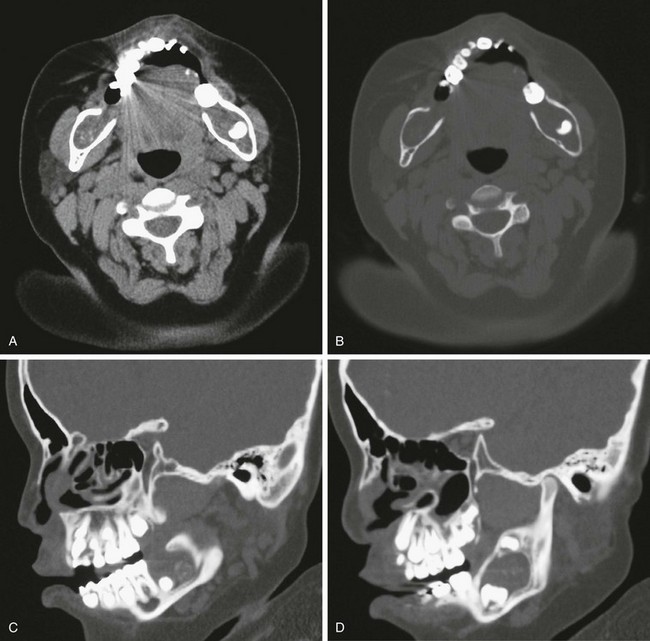
Figure 22-10 A 2-year-old patient with multiple odontogenic keratocysts.
A, An axial scan shows bilateral lesions in the posterior aspect of the body of the mandible that are deviating the dentition. B, Bone windows show further the extent of remodeling on the mandible. C and D, Sagittal reconstruction reveals the isointense lesion and its effect on the teeth and the mandible.
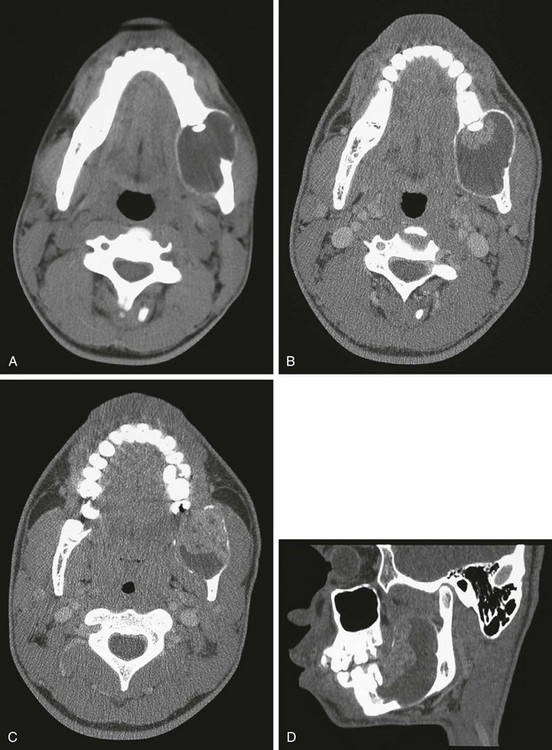
Figure 22-11 A 4-year-old patient with ameloblastoma of the left mandible.
A, An axial computed tomography (CT) scan shows the large destructive lesion of the left mandible. B, Contrast-enhanced axial CT. With further windowing, the two components of the lesion are demonstrated: a more solid component anteriorly and a more cystic component posteriorly. C, A cut slightly inferior to that shown in B reveals the solid component. D, The sagittal reconstruction shows the effect on the mandible; solid and cystic components of the lesion are seen.
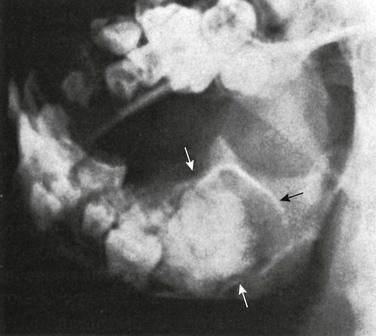
e-Figure 22-12 A composite odontoma (dental embryoma) (arrows) with an intracystic mass in a 9-year-old patient.
Cystic adamantinomas or ameloblastomas result from the proliferation of ectopic ameloblasts derived from the enamel organ (see Fig. 22-11).17 Composite odontomas have increased radiodensity (see e-Fig. 22-12). Osteomas and giant cell tumors resemble similar lesions elsewhere, but tissue diagnosis is necessary in all cases. Osteomas of the mandible and other cranial bones may be a manifestation of Gardner syndrome, which is associated with other connective tissue tumors and particularly polyposis of the large bowel. Benign osteoblastoma has been reported in the mandible. Giant cells, usually osteoclasts, are found in a variety of bone lesions in the jaws.18 Radiographic findings alone do not provide adequate differential characteristics because radiographic, CT, and MRI patterns may be found in association with several different microscopic patterns (e-Fig. 22-13, Fig. 22-14, and e-Fig. 22-15).
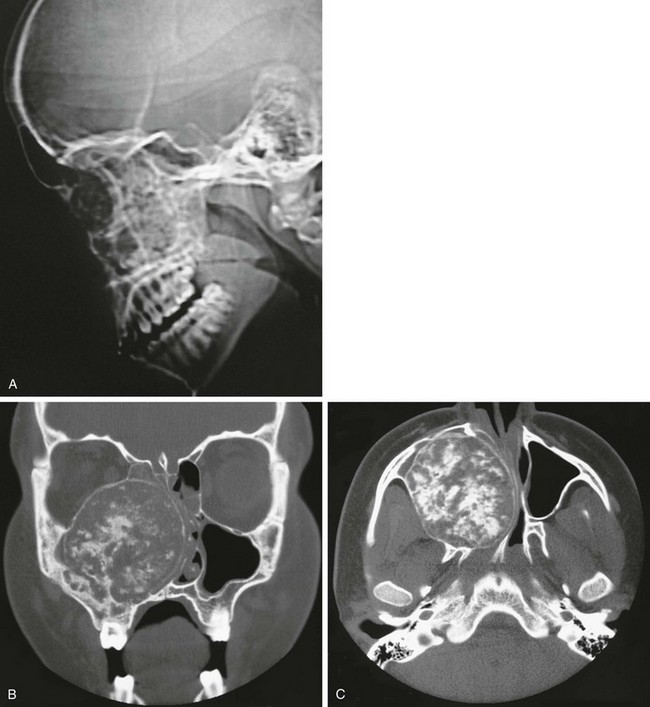
Figure 22-14 Chondrosarcoma of the maxilla.
A, The lateral view of a computed tomography (CT) scout image reveals increased calcification of the midface. B and C, Coronal (B) and axial (C) CT scans show the cartilaginous matrix. This large lesion is destroying the right maxilla, invading the right maxillary sinus, and obstructing the right nasal passage.
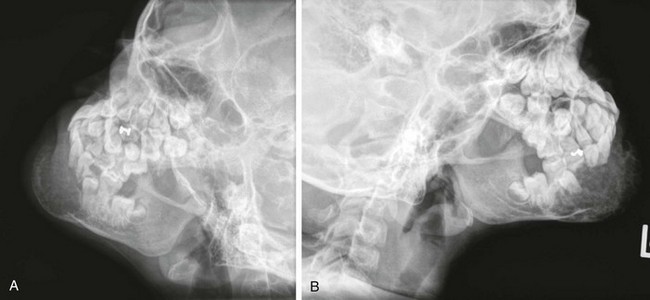
e-Figure 22-13 An 8-year-old girl with osteogenic sarcoma.
A and B, Lateral radiographs of the mandible show the destructive lesion in the mentum, which is producing bone and expanding the mandible.
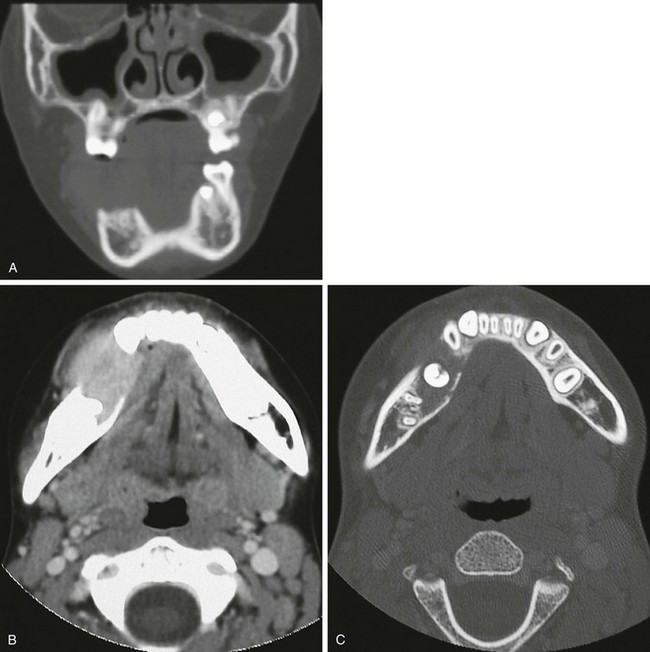
e-Figure 22-15 A benign fibroblastic “pseudotumor”: a 10-year-old girl with a large destructive lesion of the right mandible.
A, A coronal computed tomography (CT) scan shows the soft tissue mass destroying the right mandible. B, An axial-enhanced CT scan again shows the extent of the soft tissue mass and the destruction of bone. C, The mandible at another level with bone windows shows expansion of the body of the mandible and deviation of the dentition.
The radiographic “floating teeth” of Langerhans cell histiocytosis (Fig. 22-16) are not pathognomonic, having been observed in metastatic neuroblastoma, lymphosarcoma, reticulum cell sarcoma, and Ewing sarcoma. Neuroblastoma is the most common metastatic disease to the mandible.19 The common pathologic factor is a destructive process that affects the supporting alveolar structures of the teeth. Melanotic neuroectodermal tumor of infancy occurs most frequently in the maxilla; however, occasionally it may arise in the mandible. In native children of equatorial Africa, Burkitt lymphoma of the jaw accounts for 30% to 50% of all lymphomas, but this frequency is rare in the non-African form of the disease.
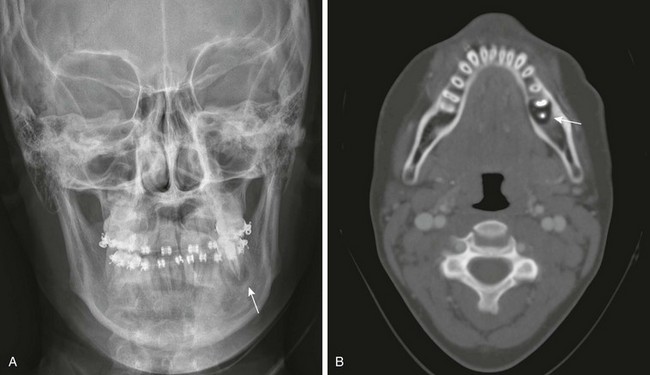
Figure 22-16 Langerhans cell histiocytosis.
A, A radiograph of the skull reveals a lucent area (arrow) around a left molar. This area can represent an abscess, but in this case it is a floating tooth. B, An axial computed tomography scan shows the destructive lesion surrounding the tooth (arrow).
Desmoblastic fibroma, chondrosarcoma, pseudotumors, and chondroid tumors also may occur in the mandible (see Fig. 22-14 and e-Fig. 22-15).
Familial Fibrosis of Jaws (Cherubism)
Cherubism is an autosomal-dominant condition characterized by asymptomatic facial swelling beginning about the third year of life. The mandible and maxilla are generally involved with multilocular cystic changes, but other facial bones are spared. Rarely, cystic lesions in the anterior ends of the ribs have been reported. Stretching of the skin as a result of maxillary involvement tends to pull the lower eyelids down, revealing a band of white sclerae between the eyelid and the iris (Fig. 22-17). This cherubic “eyes raised to heaven” expression has led to the term “cherubism.” Dentition is seriously disturbed; deciduous and permanent teeth are shed prematurely and irregularly, and some of the molar and premolar teeth are congenitally absent.
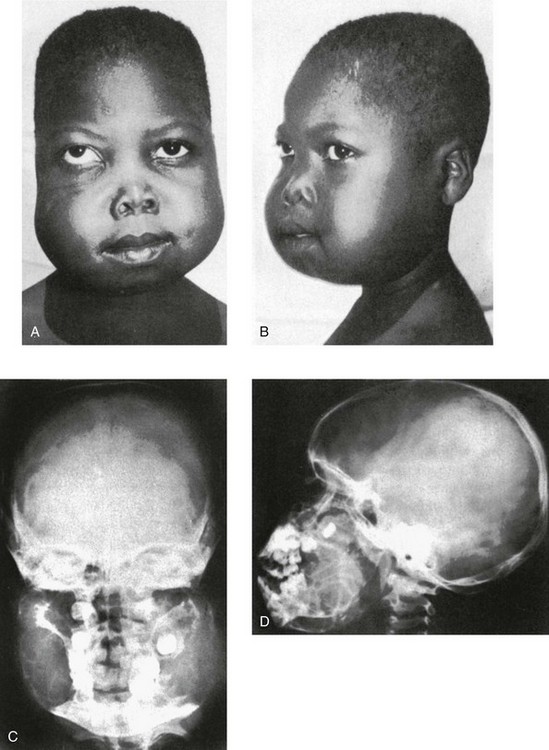
Figure 22-17 Familial fibrosis of the jaws (cherubism) in a 6-year-old boy whose two siblings and father were affected.
A and B, Clinical photographs. Massive painless swelling extends to the preauricular level and obscures the normal external ears in the frontal view. The downward displacement of the lower lids explains the term cherubism (“eyes raised to heaven”). C and D, Frontal (C) and lateral (D) skull projections show cystic swelling of the mandible and, to a lesser degree, the maxilla. The teeth are displaced medially except for the unerupted left maxillary molar. The unerupted right molar and the swollen maxilla impinge on the right maxillary sinus, partially obliterating its cavity. The clouding of the left maxillary sinus extends to the left ethmoid, indicating bony involvement or ostial occlusion. (From Shuler RK, Silverman FN. Familial fibrous dysplasia of the jaws of “cherubism” in a Haitian family. Ann Radiol. 1965;8:45-52.)
Radiographs show diffuse swelling and multiloculated rarefaction of the mandible (see Fig. 22-17 and e-Fig. 22-18). The maxilla is also enlarged, with at least partial obliteration of the sinuses. CT can detect lesions of the upper jaws not observable in conventional radiographs, including involvement of the head of the condyle (e-Fig. 22-18).20,21 Mandibular enlargement and some sclerosis may persist in later life.
Mandibular Involvement in Generalized Diseases of the Skeleton
Fibrocystic changes in the mandible may be a feature of polyostotic fibrous dysplasia.
The disappearance of the lamina dura in persons with hyperparathyroidism was considered a pathognomonic radiographic sign of the disease. More experience has shown that the lamina dura also may be resorbed (in part or in toto) in persons with many other disorders, including rickets, leukemia, Cushing disease, Paget disease, idiopathic osteomalacia, idiopathic chronic familial hyperphosphatemia, and primary and metastatic malignancies, as well as after dental extractions. The bone of the alveolar processes is always affected when the lamina dura has disappeared; the lamina dura does not disappear in the presence of normal alveolar bone.22 Mandibular involvement in infantile cortical hyperostosis is an important diagnostic feature of this condition.
Bodner, L, Woldenberg, V, Bar-Ziv, J. Radiographic features of large cystic lesions of the jaws in children. Pediatr Radiol. 2003;33:3–6.
Dunfee, BL, Sakai, O, Pistey, R, et al. Radiologic and pathologic characteristics of benign and malignant lesions of the mandible. Radiographics. 2006;26:1751–1768.
References
1. Capitanio, MA, Chen, JTT, Arey, JB, et al. Congenital anhydrotic ectodermal dysplasia. AJR Am J Roentgenol. 1968;103:168–172.
2. Hartsfield, JK, Jr. Premature exfoliation of teeth in childhood and adolescence. Adv Pediatr. 1994;41:453–470.
3. Scow, WK, Hertzberg, J. Dental development and molar root length in children with cleidocranial dysplasia. Pediatr Dent. 1995;17:101–105.
4. Sano, T, Yamamoto, M, Okano, T. Temporomandibular joint: MR imaging. Neuroimaging Clin N Am. 2003;13:583–595.
5. Roth, C, Ward, RJ, Tsai, S, et al. MR imaging of the TMJ: a pictorial essay. Appl Radiol. 2005;34(5):9–16.
6. Tomas, X, Pomes, J, Berenguer, J, et al. MR imaging of temporomandibular joint dysfunction: a pictorial review. Radiographics. 2006;26:765–781.
7. Karlo, CA, Stolzmann, P, Habernig, S, et al. Size, shape and age-related changes of the mandibular condyle during childhood. Eur Radiol. 2010;20:2512–2517.
8. Cohen, MM, Jr. Pierre Robin anomalad—its non-specificity and associated syndromes. J Oral Surg. 1976;34:587–593.
9. Silverman, FN, Streffling, AM, Stevenson, DK, et al. Cerebrocostomandibular syndrome. J Pediatr. 1980;97:406–416.
10. Alamondi, N, Farsi, N, Salako, NO, et al. Temporomandibular disorders among school children. J Clin Pediatr Dent. 1998;22:323–328.
11. Cannizzaro, E, Schroeder, S, Müller, LM, et al. Temporomandibular joint involvement in children with juvenile idiopathic arthritis. J Rheumatol. 2011;38:510–515.
12. Abramowicz, S, Cheon, JE, Kim, S, et al. Magnetic resonance imaging of temporomandibular joints in children with arthritis. J Oral Maxillofac Surg. 2011;69(9):2321–2328.
13. Kuseler, A, Pedersen, TK, Herlin, T, et al. Contrast enhanced magnetic resonance imaging as a method to diagnose early inflammatory changes in the temporomandibular joint in children with juvenile chronic arthritis. J Rheumatol. 1998;25:1406–1412.
14. Thoren, H, Iizuka, T, Hallikainen, D, et al. Different patterns of mandibular fractures in children: an analysis of 220 fractures in 157 patients. J Craniomaxillofac Surg. 1992;20:292–296.
15. Gunge, M, Yamamoto, H, Katoh, T, et al. Suppurative osteomyelitis of the mandible in a child. Int J Oral Maxillofac Surg. 1987;16:99–103.
16. Gupta, M, Kaste, SC, Hopkins, KP. Radiographic appearance of primary jaw lesions in children. Pediatr Radiol. 2002;32:153–168.
17. Ord, RA, Blanchaert, RH, Jr., Nikitakis, NG, et al. Ameloblastoma in children. J Oral Maxillofac Surg. 2002;60:762–770.
18. Batsakis, JG, Rice, DH. Diseases affecting the jaws, III: giant cell lesions and fibrous dysplasia of the jaws. Univ Mich Med Cent J. 1969;35:162–169.
19. Deutsch, M, Wollman, MR. Radiotherapy for metastases to the mandible in children. J Oral Maxillofac Surg. 2002;60:269–271.
20. Bianchi, SD, Boccardi, A, Mela, F, et al. The computed tomographic appearance of cherubism. Skeletal Radiol. 1987;16:6–10.
21. Jain, V, Sharma, R. Radiographic, CT and MRI features of cherubism. Pediatr Radiol. 2006;36:1099.
22. Berry, HM, Jr. The lore and the lure of the lamina dura. Radiology. 1973;109:525–528.