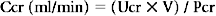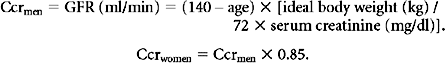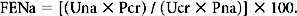CHAPTER 88 THE MANAGEMENT OF RENAL FAILURE: RENAL REPLACEMENT THERAPY AND DIALYSIS
Acute renal failure (ARF) is a common and devastating problem that contributes to morbidity and mortality in critically ill patients. ARF prolongs hospital stays and increases mortality. Although effective renal replacement therapy (RRT) is available, it is not ideal and the best therapy is prevention.
INCIDENCE
Acute renal failure is defined as an abrupt and sustained decline in the glomerular filtration rate (GFR),1 which leads to accumulation of nitrogenous waste products and uremic toxins. In critically ill patients, more than 90% of the episodes of ARF are due to acute tubular necrosis (ATN) and are the result of ischemic or nephrotoxic etiology (or a combination of both). ARF affects nearly 5% of all hospitalized patients and as many as 15% of critically ill patients.2 Like many other medical conditions, there is no gold standard of diagnosis, no specific histopathologic confirmation, and no uniform clinical picture.
The mortality rate of an isolated episode of ARF is approximately 10% to 15%. When it occurs in association with multiple-organ dysfunction, as in the ICU setting, mortality rates are much greater and vary in published series between 40% and 90%.3
In some cases, preexisting conditions may worsen. New major complications, such as sepsis and respiratory failure, may also develop after the onset of renal failure. Although ARF that requires RRT carries a high mortality,4 there is emerging evidence to suggest that milder forms of ARF that do not require supportive therapy with RRT have better patient outcomes.5
MECHANISM OF INJURY/ETIOLOGY
Assessment of Renal Function
Serum concentrations of blood urea nitrogen (BUN) and creatinine are the most commonly used markers of renal function. Urea is the end product of protein and amino acid catabolism. Under normal conditions, 80%–90% of total nitrogen excretion is by the kidneys. Creatinine is formed in muscle by the nonenzymatic degradation of creatine and phosphocreatine, and is excreted primarily by glomerular filtration. A small percentage of creatinine is actively secreted into the glomerular filtrate and tubular reabsorption of creatinine is negligible.5
Creatinine Clearance
where Ucr is urine creatinine, Pcr is serum creatine, and V is volume.
Normal GFR is 125 ± 15 ml/min/1.73 m2 body surface area (BSA).
Here, Una and Ucr are the urinary concentrations of sodium and creatinine, and Pna and Pcr are the serum levels of sodium and creatinine, respectively. If the FENa is very low (<1%), it may indicate inadequate renal arteriolar pressure—suggesting that factors other than intrinsic renal dysfunction are responsible for clinically inadequate renal function.6
Urine Production and Output
The end result of renal function is the production of urine. Quantitative measurements of urine are important for assessing renal function. Urine output is highly sensitive to renal blood flow, making it a key indicator of renal function and total body vascular perfusion7 (Table 1).
| Age | Urine Output (ml/kg/min) |
|---|---|
| Infant (<10 kg) | 2.0 |
| Toddler (10–20 kg) | 1.5 |
| Child (20–50 kg) | 1.0 |
| Adult (>50 kg) | 0.5 |
MANAGEMENT OF PATIENTS
Nonpharmacologic Strategies for Acute Renal Failure Prevention
Fluids
Adequate hydration is the cornerstone of renal failure prevention. One randomized controlled trial (n = 1620) compared hydration using 0.9% saline infusion with 0.45% saline in dextrose for prevention of radiocontrast-induced nephropathy in patients who underwent coronary angiography.8 Hydration with 0.9% saline infusion significantly reduced contrast nephropathy compared with 0.45% saline in dextrose hydration (0.7% vs. 2%, respectively; p = 0.04). This effect was greater in women, diabetics, and patients who received a large volume (>250 ml) of a contrast agent. A recent single-center randomized controlled trial compared the efficacy of sodium bicarbonate with 0.9% saline hydration in preventing contrast nephropathy.9 In this study, 119 patients who had stable serum creatinine of at least 1.1 mg/dl were randomized to 154 mEq/l infusion of sodium chloride (n = 59) or sodium bicarbonate (n = 60) before and after contrast (iopamidol) administration. One of 59 patients (1.7%) in the group that received bicarbonate developed contrast nephropathy (defined as an increase of ≥25% in serum creatinine from baseline within 48 hours) compared with 8 of 60 patients (13.3%) in the group that received saline (p = 0.02).
Nephrotoxin Exposure
Minimizing exposure to potentially nephrotoxic agents is an important strategy to prevent ARF in the ICU setting. Aminoglycosides, other antibiotics, amphotericin, and radiocontrast are the nephrotoxins encountered most commonly in the ICU. A systematic review in patients who had neutropenic fever and received aminoglycosides, however, found no significant differences in efficacy or nephrotoxicity between once daily and three times daily dosing.10
The use of lipid formulations of amphotericin B seems to cause less nephrotoxicity compared with standard formulations, but direct comparisons of long-term safety are lacking. With regard to contrast media, one systematic review (31 randomized controlled trials, 5146 patients) compared low osmolality contrast media with standard contrast media.11 The study showed that low osmolality contrast media did not influence the development of ARF or the need for dialysis.
Pharmacologic Strategies for Acute Renal Failure Prevention
Loop Diuretics
Multiple small clinical trials studied the efficacy of loop diuretics in preventing ARF and have provided conflicting results. They have been underpowered, nonrandomized, or methodologically flawed. One systematic review that compared fluids with diuretics in people who were at risk for ARF from various causes did not show any benefit from diuretics with regard to prevalence of ARF, need for dialysis, or mortality.12
N-Acetylcysteine
Systematic reviews found that NAC plus hydration reduced the incidence of contrast nephropathy more than hydration alone in people who had baseline renal impairment and underwent radiocontrast studies.13 A recent study, however, suggested that NAC could decrease serum creatinine independently without any effect on GFR (as evaluated by other surrogate outcomes, such as serum cystatin C levels).14 Hence, the current implications of reduction in serum creatinine after contrast administration with the use of NAC remain unclear and need to be explored further.
INDICATIONS FOR RENAL REPLACEMENT THERAPY IN ACUTE RENAL FAILURE
As in chronic kidney disease, overt disturbances of ECF volume and body fluid composition remain the objective indications for initiation of RRT in patients with ARF (Table 2). These include volume overload, hyperkalemia, severe metabolic acidosis, uremia, and azotemia.
Volume Overload
Mehta and colleagues15 performed a retrospective analysis of data from 522 critically ill patients who had ARF. Fifty-nine percent of these patients had been treated with diuretics. After adjustment for relevant covariates and the propensity for diuretic use, they observed a significant increase in the risk of death or nonrecovery of renal function (odds ratio 1.77, 95% confidence interval 1.14–2.76). On the basis of this, they concluded that diuretic therapy was potentially deleterious in patients who had ARF. They noted, however, that the increased risk was borne largely by patients who were unresponsive to diuretics. This suggested that this increased risk might reflect selection for a more severe degree of renal injury.
Hyperkalemia
In patients who have severe renal failure, diuretic therapy is generally ineffective in promoting kaliuresis due to lack of diuretic response. Although sodium polystyrene sulfonate can enhance fecal potassium losses, its use is limited in patients with recent intraabdominal or GI surgery, ileus, or bowel ischemia. Dialysis provides the most rapid means of decreasing the serum potassium concentration. However, because of variability in study design and evolution of dialysis techniques it is difficult to determine the expected potassium removal during a single dialysis treatment.16
Even greater clearances of potassium may be achieved by using more permeable synthetic hemodialysis membranes and greater blood flow rates. However, the rate of potassium removal is ultimately limited by the rapid decrease in the concentration gradient between plasma and dialysate.17 As with volume status, a specific threshold level of serum potassium cannot be established as an indication for initiation of RRT. Myocardial toxicity from hyperkalemia is uncommon when the serum potassium concentration is less than 6.5 mmol/l.16 Therefore, decisions regarding the initiation of treatment for control of hyperkalemia must take into consideration the absolute level and rate of increase of serum potassium, the patient’s overall condition, and the likely efficacy of medical therapy.
Metabolic Acidosis
The role of alkali therapy in the treatment of metabolic acidosis, particularly lactic acidosis, is controversial.18 The use of RRT as an alternative to alkali replacement in metabolic acidosis can avoid some of the deleterious effects ascribed to aggressive alkali replacement, specifically volume overload and hypernatremia. Although progressive metabolic acidosis is a generally accepted indication for RRT, clinical trials to establish a threshold blood pH or serum bicarbonate concentration or to demonstrate improved patient outcomes have not been performed.
Other Electrolyte Disturbances
Uremia
The development of overt uremic signs or symptoms represents an obvious indication for initiation of RRT in ARF. Early manifestations of uremia, such as anorexia, nausea and vomiting, and pruritus, are nonspecific and may be difficult to differentiate from other comorbid conditions in patients who have critical illness. Mental status changes, which may represent uremic encephalopathy, also may be difficult to differentiate from other etiologies of delirium in the critically ill patient. Uremic pericarditis is usually a late complication, but requires urgent initiation of renal support given the high risk of intrapericardial hemorrhage and tamponade. As was emphasized more than four decades ago by Teschan et al.,19 optimally RRT should be initiated before the onset of overt uremic manifestations.
TIMING OF INITIATION OF RENAL REPLACEMENT THERAPY
Beginning with the studies by Paul Teschan and colleagues,19 in the years following the Korean War numerous studies have attempted to define the criteria for timing of initiation of RRT in ARF. These studies attempted to determine the balance between three major competing risks: the inherent risk that results from delay in therapy; the potential risk of harm as a result of RRT, including complications of therapy and the potential that dialysis may prolong the course of ARF; and the risk that early initiation of therapy will result in patients undergoing treatment who, if managed conservatively, might recover renal function without requiring RRT.
In their landmark report, Teschan et al.19 described a prospective uncontrolled series of 15 patients who had oliguric ARF who were treated with “prophylactic” hemodialysis defined as the initiation of dialysis before the serum urea nitrogen reached 100 mg/dl.18 Patients received daily dialysis (average duration 6 hours) using twin-coil cellulosic dialyzers at a blood flow of 75–250 ml/min to maintain a predialysis serum urea nitrogen of less than 75 mg/dl. Caloric and protein intake were unrestricted. All-cause mortality was 33%. Mortality due to hemorrhage or sepsis was 20%. Although no control group was studied, the investigators reported that the results contrasted dramatically with their own past experience in patients in whom dialysis was not initiated until “conventional” indications were present.
Acute Renal Failure
ARF is a common complication in critically ill patients and is associated with a mortality rate greater than 50%.20 As many as 70% of these patients require RRT, making it an important component of the management of ARF in the ICU. Ideally, RRT controls volume, corrects acid-base abnormalities, improves uremia through toxin clearance, promotes renal recovery, and improves survival without causing complications (such as bleeding from anticoagulation and hypotension). The available RRT options include intermittent hemodialysis (IHD), continuous RRT (CRRT), and sustained low-efficiency dialysis (SLED). Currently, there is insufficient evidence to establish which modality of RRT is best for ARF in the critically ill patient. There is a general consensus that patients receiving CRRT using lower blood flow rates and lower fluid removal rates have less cardiovascular instability/morbidity. Clearly, there is no significant difference in mortality rates with any of the available modalities. Understanding the advantages and limitations of the various dialysis modalities is essential for appropriate RRT selection in the ICU setting.
Principles of Renal Replacement Therapy
Ultrafiltration achieves volume removal by using a pressure gradient to drive water through a semipermeable membrane. This pressure gradient is known as the transmembrane pressure gradient and is the difference between plasma oncotic pressure and hydrostatic pressure. Determinants of the ultrafiltration rate include the membrane surface area, water permeability of the membrane, and transmembrane pressure gradient.21
Diffusion occurs by movement of solutes from an area of higher solute concentration to an area of lower solute concentration across a semipermeable membrane. The concentration gradient is maximized and maintained throughout the length of the membrane by running the dialysate (an electrolyte solution usually containing sodium, bicarbonate, chloride, magnesium, and calcium) countercurrent to the blood flow. Solutes with a higher concentration in the blood, such as potassium and urea, move down their concentration gradient across the membrane to the dialysate compartment. Conversely, solutes with a higher concentration in the dialysate (such as bicarbonate) diffuse into the blood. Solute concentrations that are nearly equivalent in the blood and dialysate, such as sodium and chloride, move very little across the membrane. Because smaller solutes (such as urea and creatinine) diffuse more rapidly than larger solutes, lower-molecular-weight molecules (<500 daltons) are cleared more efficiently than heavier molecules. The rate of solute diffusion depends on blood flow rate, dialysate flow rate, duration of dialysis, concentration gradient across the membrane, and membrane surface area and pore size.21
Convection occurs when the transmembrane pressure gradient drives water across a semipermeable membrane (as in ultrafiltration) but then “drags” with the water both small-molecular-weight (BUN, creatinine, potassium) and large-molecular-weight (inulin, β2-microglobulin, tumor necrosis factor, vitamin B12) solutes. Membrane pore diameter limits the size of the large solutes that can pass through. Increasing the transmembrane pressure difference allows more fluid and solutes to be “pulled” through the membrane. Because the efficiency of solute removal depends mainly on the ultrafiltration rate, typically at least 1 l of water needs to be pulled through the membrane each hour. The process of increasing the ultrafiltration rate to provide convective clearance of solutes is known as hemofiltration. Ultrafiltration rate is determined by the transmembrane pressure, water permeability of the membrane, and membrane surface area and pore size.21
CLASSIFICATION OF RENAL REPLACEMENT THERAPIES
RRT for ARF can be classified as intermittent or continuous, based on the duration of the treatment. The duration of each intermittent therapy is less than 24 hours, whereas the duration of continuous therapy is at least 24 hours. The intermittent therapies include IHD and SLED. The continuous therapies include peritoneal dialysis and CRRT.22 Peritoneal dialysis is rarely used in the acute setting because it provides inefficient solute clearance in critically ill catabolic patients, increases the risk of peritonitis, compromises respiratory function by impeding diaphragmatic excursion, and is contraindicated in patients with recent abdominal surgery or abdominal sepsis.23
Intermittent Hemodialysis
Traditionally, nephrologists have managed ARF with IHD—empirically delivered three to six times a week, 3–4 hours per session, with a blood flow rate of 200–350 ml/min and a dialysate flow rate of 500–800 ml/min. In IHD, solute clearance occurs mainly by diffusion—whereas volume is removed by ultrafiltration. The degree of solute clearance, also known as the “dialysis dose,” is largely dependent on the rate of blood flow. Increasing the blood flow increases solute clearance. Decisions regarding dialysis duration and frequency are based on patient metabolic control, volume status, and presence of any hemodynamic instability.
Rapid solute removal from the intravascular space can cause cerebral edema and increased intracranial pressure. ARF patients with head trauma or hepatic encephalopathy are at a significant risk of brain edema and even herniation.24 Finally, there is a lack of consensus as to how to assess solute clearance (dialysis dose) and what constitutes an adequate dose in ARF because the kinetics of urea in the end-stage renal disease patient cannot be extrapolated to patients with ARF.
Continuous Renal Replacement Therapy
All types of CRRT use membranes that are highly permeable to water and low-molecular-weight solutes. CRRT modalities are classified by access type and method of solute clearance. Venovenous circuits are now the standard, and the various venovenous modalities of CRRT differ by their mechanism of solute removal. The four main types of CRRT in order of increasing complexity are slow continuous ultrafiltration, continuous venovenous hemofiltration (CVVH), continuous venovenous hemodialysis (CVVHD), and continuous venovenous hemodiafiltration (CVVHDF).25
Replacement fluids can be administered prefilter or postfilter. Postfilter replacement fluid results in hemoconcentration of the filter and increased risk of clotting, especially when the filtration fraction is greater than 30%. The filtration fraction is the ratio of ultrafiltration rate to plasma water flow rate and is dependent on the blood flow rate and hematocrit.25 Prefilter replacement fluid dilutes the blood before the filter, resulting in reduced filter clotting. Dilution of solutes before the filter reduces solute clearance by up to 15% by lowering the diffusion driving force and convective concentration.
Advantages and Disadvantages
The advantages of CRRT include hemodynamic tolerance caused by slower ultrafiltration rates.27 The gradual continuous volume removal makes control of volume status easier and allows administration of medications and nutrition with less concern for volume overload. Because it is a continuous modality, there is less fluctuation of solute concentrations over time and better control of azotemia, electrolytes, and acid-base status. The improved hemodynamic stability may be associated with fewer episodes of reduced renal blood flow, less renal ischemia, and more rapid renal recovery. Mehta et al.28 examined this issue in a prospective study in which 166 ICU patients with ARF were randomized to IHD or to CRRT. CRRT patients who survived were significantly more likely to show renal recovery than those treated with IHD. Because CRRT does not cause rapid solute shifts, it does not raise intracranial pressure like IHD.
The cumulative solute removal with CRRT is greater than that achievable with IHD. Ronco et al.29 provided convincing evidence that increasing solute clearance with CRRT can improve outcome in critically ill patients with ARF. In a prospective randomized controlled trial, 425 critically ill patients with ARF were assigned to CVVH using ultrafiltration rates of 20 ml/kg/hr (group 1), 35 ml/kg/hr (group 2), or 45 ml/kg/hr (group 3). The ultrafiltration rate of 20 ml/kg/hr was based on the average rate used in clinical practice as reported in the literature at the time of the study. The blood flow rates ranged from 120 to 240 ml/min and the replacement fluid was administered postfilter. The primary study outcome was survival at 15 days after discontinuation of CVVH. Secondary outcomes were recovery of renal function and CRRT-related complications. Patient survival after discontinuing CVVH was 41, 57, and 58% in groups 1, 2, and 3, respectively. Survival in group 1 was significantly lower than group 2 (p = 0.0007) and group 3 (p = 0.001), demonstrating a survival advantage for patients treated with CVVH at an ultrafiltration rate of at least 35 ml/kg/hr. It is unclear, however, whether the reduction in mortality was solely caused by small-molecule (urea) clearance or by both small-molecule clearance and increased middle-molecule clearance.
Intermittent Hemodialysis versus Continuous Renal Replacement Therapy: Outcomes
There are few prospective studies comparing IHD with CRRT with respect to outcomes, such as mortality or recovery of renal function. Mehta et al.28 randomized 166 patients to CRRT (CVVH or CVVHDF) or IHD. Univariate intention-to-treat analysis revealed a higher mortality among patients receiving CRRT. Patients randomized to CRRT had higher APACHE III scores and had a higher prevalence of liver failure, confounding the results. Multivariate analysis revealed no impact of RRT modality on all-cause mortality or recovery of renal function. Instead, severity of illness scores (such as APACHE III scores and number of failed organs) were more important prognostic factors. The authors concluded that insufficient data existed to draw strong conclusions, mainly because of the lack of randomized controlled trials and the influence of biases and confounding variables.
Sustained Low-Efficiency Dialysis or Extended Daily Dialysis
Because sustained low-efficiency dialysis and extended daily dialysis can be done intermittently based on the needs of the patient, they also avoid the interruption of therapy for various diagnostic and therapeutic procedures that may be required in such patients. Kumar et al.30 described their prospective experience of 25 patients treated with extended daily dialysis and 17 patients treated with CVVH at University of California Davis Medical Center. No significant differences in mean arterial pressure or inotrope requirements were observed between the two groups. Mortality was higher in the extended daily dialysis group (84% vs. 65%). The APACHE II scores were higher, however, in the extended daily dialysis group at the onset of treatment. The authors argued that extended daily dialysis was more cost effective by removing the need for constant monitoring of dialysis equipment and reducing nursing workload.
1 Nissenson AR. Acute renal failure: definition and pathogenesis. Kidney Int Suppl. 1998;66:7-10.
2 Hou SH, Bushinsky DA, Wish JB. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243-248.
3 Bellomo R, Ronco C. The changing pattern of severe acute renal failure. Nephrology. 1991;2:602-610.
4 Metnitz PG, Krenn CG, Steltzer H. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051-2058.
5 Gehr TWB, Schoolwerth AC. Adult acute and chronic renal failure. In: Ayers SM, et al, editors. Textbook of Critical Care. 3rd ed. Philadelphia: WB Saunders; 1995:1029-1041.
6 Mullins RJ. Acute renal failure. In: Cameron JL, editor. Current Surgical Therapy. 6th ed. St. Louis: Mosby; 1998:1109-1114.
7 Thadhani R, Paucual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448-1460.
8 Mueller C, Buerkle G, Buettner HJ. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329-336.
9 Merten GJ, Burgess WP, Gray LV. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328-2334.
10 Hatala R, Dinh TT, Cook DJ. Single daily dosing of aminoglycosides in immunocompromised adults: a systematic review. Clin Infect Dis. 1997;24:810-815.
11 Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high-and low-osmolality iodinated contrast media. Radiology. 1993;188:171-178.
12 Kellum JA. The use of diuretics and dopamine in acute renal failure: a systematic review of the evidence. Crit Care. 1997;1:53-59.
13 Alonso A, Lau J, Jaber BL. Prevention of radiocontrast nephropathy with N-acetylcysteine in patients with chronic kidney disease: a meta-analysis of randomized, controlled trials. Am J Kidney Dis. 2004;43:1-9.
14 Hoffmann U, Fischereder M, Kruger B. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15:407-410.
15 Mehta RL, Pascual MT, Soroko S. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288:2547-2553.
16 Greenberg A. Hyperkalemia: treatment options. Semin Nephrol. 1998;18:46-57.
17 Hou S, McElroy PA, Nootens J. Safety and efficacy of low-potassium dialysate. Am J Kidney Dis. 1989;13:137-143.
18 Forsythe SM, Schmidt GA. Sodium bicarbonate for the treatment of lactic acidosis. Chest. 2000;117:260-267.
19 Teschan PE, Baxter CR, O’Brien TF. Prophylactic hemodialysis in the treatment of acute renal failure. Ann Intern Med. 1960;53:992-1016.
20 Liano F, Junico E. The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. Kidney IntKidney Int Suppl. 1998;66:S16-S24.
21 Yeun J, Depner T. Principles of dialysis. In: Owen WF, Pereira BJ, Sayegh MH, editors. Dialysis and Transplantation: A Companion to Brenner & Rectors’ The Kidney. Philadelphia: WB Sanders; 2000:1-32.
22 Mehta RL, Chertow GM. Selection of dialysis modality. In: Owen WF, Pereira BJ, Sayegh MH, editors. Dialysis and Transplantation: A Companion to Brenner & Rectors’ The Kidney. Philadelphia: WB Sanders; 2000:403-417.
23 Goel S, Saran R, Nolph KD. Indications, contraindications and complications of peritoneal dialysis in the critically ill. In: Ronco C, Bellomo R, editors. Critical Care Nephrology. Dordrecht: Kluwer Academic Publishers; 1998:1373-1381.
24 Davenport A, Will EJ, Davidson AM. Effect of renal replacement therapy on patients with combined acute renal failure and fulminant hepatic failure. Kidney Int. 1993;43:S245-S251.
25 Bellomo R, Ronco C, Mehta R. Nomenclature for continuous renal replacement therapies. Am J Kidney Dis. 1996;28:S2-S7.
26 Clark WR, Turk JE, Kraus MA. Dose determinants in continuous renal replacement therapy. Artif Organs. 2003;27:815-820.
27 Lameire N, Van Biesen W, Vanholder R. The place of intermittent hemodialysis in the treatment of acute renal failure in the ICU patient. Kidney Int Suppl. 1998;66:S110-S119.
28 Mehta R, McDonald B, Gabbai F. A randomized, clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int. 2001;60:1154-1163.
29 Ronco C, Bellomo R, Homel P. Effects of different doses in continuous veno-venous hemofiltration on outcomes of acute renal failure: a prospective, randomized trial. Lancet. 2000;356:26-30.
30 Kumar VCM, Depner T, Yeun J. Extended daily dialysis: a new approach to renal replacement for acute renal failure in the intensive care unit. Am J Kidney Dis. 2000;36:294-300.