CHAPTER 49 The management of eyelid tumors
Having a comprehensive knowledge of the epidemiology, the clinical features of benign and malignant eyelid tumors, an understanding of the relevant tumor biology, and available diagnostic and therapeutic options is essential for the clinician caring for these patients to be able to deliver an appropriate standard of care1.
Epidemiology
Skin cancer is the most common human malignancy with a corresponding human morbidity, mortality, and health care cost. A variety of benign and malignant primary skin, adnexal and other tumors may involve the eyelids, as well as a range of secondary malignancies2. The four relatively common malignant tumors to involve the eyelids are basal cell carcinoma (BCC), squamous cell carcinoma (SCC), sebaceous carcinoma (SC), and malignant melanoma (MM). Additionally almost any other adnexal process can involve the eyelids but these tumors have a very low incidence.
BCC represents about 80% of eyelid malignancies, SCC about 15%, with SC, MM, and other rarer lesions making up the remainder. The age-standardized incidence of BCC ranges from 53 (Germany) to 1041 (Australia) per 100 000 per year3. The age-standardized incidence of SCC is lower, ranging from 11 (Germany) to 499 (Australia) per 100 000 per year. MM incidence in the United States was 24.6 per 100 000 in 20064–6. The Australian incidence is higher; statistics from an Australian cancer registry show an age-standardized incidence rate of 64.4/100 000/year and a mortality rate of 7.1/100 000/year for MM7. The reported incidence in a United States population of SC in whites is 0.11–0.2/100 000/year8, 0.1 in Asian/Pacific Islanders and 0.05/100 000 in blacks9. The incidence in Asians has been reported as much higher, representing 30–40% of all eyelid malignancies in Chinese, Indian, Thai, and Japanese populations10.
The incidence of Merkel cell carcinoma ranges from 0.17 to 0.44/100 000/year in a number of incidence reports, occurring predominately in elderly white males in the head and neck region11–13.
BCC is usually associated with long-term sun exposure14.
Australian studies, where there is a high baseline incidence of BCC, have not shown a strong occupational association, whereas German studies, with a lower baseline incidence, have shown an association with occupational exposure3,15,16. Higher dietary fat intake is associated with a higher incidence of SCC but not BCC17. A population-based study into the association of antioxidant levels and non-melanoma skin cancer (NMSC) incidence, looking at carotenoids, α-tocopherol, and selenium, showed no association with carotenoids or α-tocopherol, but an inverse association between serum selenium levels and the incidence of BCC and SCC18.
Clinical features, diagnosis, and differential diagnosis
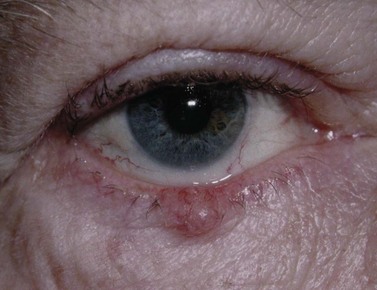
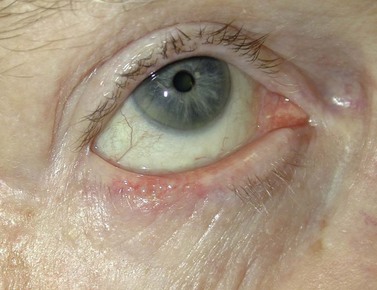
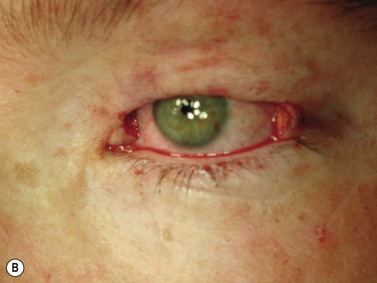
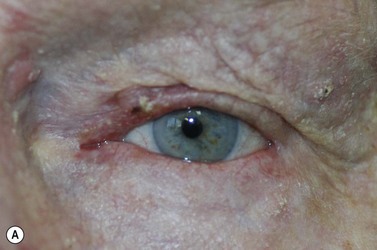
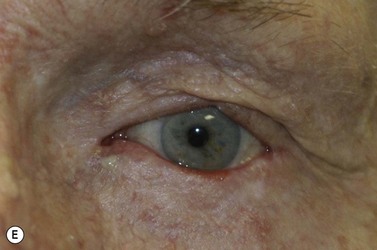
BCC typically presents in the fifth to eighth decade of life, most commonly as a well-circumscribed nodular lesion with pearly edges and some telangiectasia, with or without ulceration (Fig. 49.1). These lesions are relatively easy to diagnose clinically but there are many clinical variants which can confound the clinician. Superficial, multifocal BCC may present as flat erythematous plaques; micronodular lesions as multiple small nodules, while the infiltrating, morpheic lesions may have very subtle features and indistinct margins, which make, diagnosis difficult and margins impossible to assess clinically (Fig. 49.2). Less commonly, BCCs may have cystic or pigmented components19.
SCCs may arise de novo, or from a pre-existing actinic keratosis (AK) or areas of squamous intraepidermal carcinoma (IEC, Bowen’s disease, SCC in situ), with appearances that range from erythematous plaques to raised nodular lesions that may be ulcerated, keratotic, or papillomatous (Fig. 49.3)20–22.
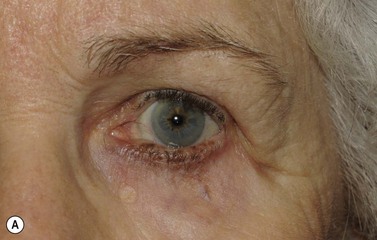
Melanomas are generally variably darkly pigmented, but may be amelanotic. They are usually categorized into lentigo maligna melanoma (Fig. 49.4A), superficial spreading melanoma (Fig. 49.8A), nodular melanoma, and acral melanoma. Most melanomas undergo a radial then a vertical growth phase (to be discussed later), such that they present as fairly large flat pigmented macules. The nodular form has a very short or absent radial growth phase and more commonly presents as a dark or reddish-brown nodule. Signs of ulceration clinically usually indicate progression into the vertical growth phase23.
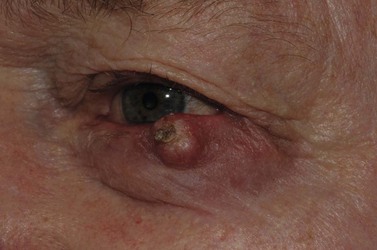
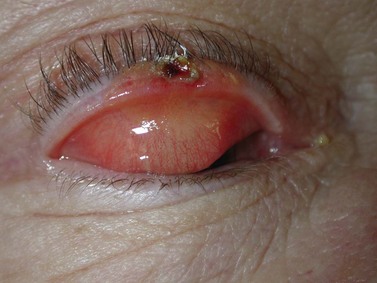
Sebaceous carcinoma is notoriously difficult to diagnose clinically. The lesion may present as a nodule, or as an area of thickening of the tarsal plate associated with a yellow discoloration and ulceration (Fig. 49.5)24–26. Merkel cell carcinoma is also difficult to diagnose clinically, although they usually have a slightly violaceous, purplish hue and grow rapidly.
Probably more important than being able to diagnose each individual lesion correctly at every presentation is the ability to decide whether the lesion is benign or malignant. Further, various clinical features will suggest that a malignant lesion is either low risk or high risk for recurrence27–28.
These include the location and size of the lesion, rate of growth, regular or irregular borders, presence of chronic inflammation, prior irradiation, or neurological symptoms. Clinical diagnosis may need to be confirmed by biopsy. A number of biopsy techniques exist, including shave, punch, incisional, or excisional. Shave biopsies may allow confirmation of diagnosis, but the other techniques give more useful information about the behavior of the tumor in the deeper dermis. Where an attempted excisional biopsy is inadvertently incomplete, identification of the residual disease may be difficult. For these reasons, if biopsy is to be performed prior to definitive treatment, a punch or an incisional biopsy technique is preferred. There are also histological features which are associated with the rate of tumor recurrence. These include degree of differentiation, lymphovascular or perineural invasion, tumor subtype, and depth of invasion27–28. Using these guidelines the National Comprehensive Cancer network has made available guidelines for the management of NMSC. Similarly the Australian Cancer Network, with funding from the Australian Government, and the National Health and Medical Research Council have also published comprehensive multidisciplinary guidelines for the management of NMSC29.
Management
Prevention
Given that sun exposure is one of the main precipitants of NMSC, measures to reduce exposure are very important. Sunscreen has been shown to reduce the incidence of SCC and precursors. Sun exposure should be avoided between 10.00 hours and 14.00 hours, when 60% of the most harmful UV exposure occurs. Application of 30 sun protection factor (SPF) sunscreen should occur prior to sun exposure and be repeated after swimming, or sweating. A broad-brimmed hat and clothing that does not transmit light should be worn outdoors. Additional exposure from solariums increases the incidence of BCCs, SCCs, and MM. Their use should be discouraged. Annually, 281 new melanoma cases, 43 melanoma-related deaths, and 2572 new cases of squamous cell carcinoma were estimated to be attributable to solarium use in Australia30–32.
α-difluoromethylornithine (DFMO) 10% ointment appears to reduce BCC incidence but not SCC incidence, and holds promise as a form of chemoprevention33–35.
Resveratrol
Resveratrol, a polyphenolic phytoalexin, is a chemopreventative agent, present in grapes and red wine, which is currently under investigation in animal studies. SKH-1 hairless mice exposed to UVB and treated with topical application of resveratrol had a statistically significant lower incidence of AK, IEC and SCC development36. Further studies have confirmed this chemoprevention of squamous skin tumor development in SKH-1 hairless mice and suggested that transforming growth factor β2 signaling is targeted37.
Afamelanotide (CUV1647) is an analog of α-MSH which promotes synthesis of melanin in cutaneous keratinocytes and melanocytes and is being evaluated for AK and SCC prevention in organ transplant patients38.
Investigation
The vast majority of periocular tumors need little or no investigation. Diagnosis is usually clinical and treatment can be instituted. There are, however, situations where investigations are necessary. Where a diagnosis is unclear clinically, biopsy can be helpful in order to plan management39. For most NMSC a shave or punch biopsy from the thickest or most representative tumor region can be performed. When radiotherapy is considered as primary treatment for selected cases (too frail for surgical excision, or other specific reasons) biopsy should be performed prior to treatment to confirm diagnosis and plan treatment.
Should a lesion have any clinical sebaceous features, as suggested by location, a diffuse lid margin thickening or yellowish discoloration, generous biopsy to include representative tissue sampling is important and some advocate full-thickness biopsy for this reason. Where a melanoma is suspected, ideally biopsy should be excisional. Shave or punch biopsies may result in incorrect diagnosis or errors in pathological staging, particularly in relation to tumor thickness40–42.
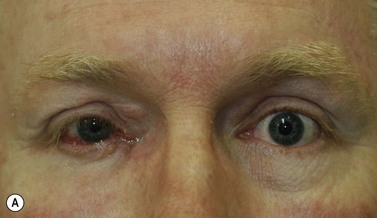
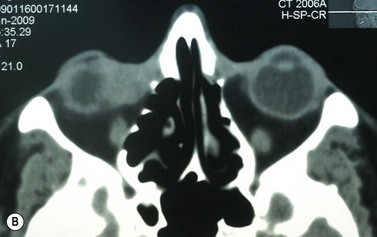
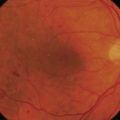
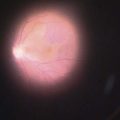
Where a lesion might have invaded the orbit, as suggested by tumor extent, limited eye movements, or enophthalmos, imaging is mandatory to look for the posterior extent of the tumor (Fig. 49.6). Where there might be a possibility of perineural invasion (PNI), imaging is important to obtain preoperatively, if possible. Should PNI be found on histology, imaging should be considered, depending on the size of nerves involved. PNI of small nerves within the excised specimen may be too small to detect with imaging. PNI of larger, named nerves seen on pathology, or patients with clinical evidence of PNI (cranial nerve palsies, altered sensation, pain), may be detected on imaging studies43–47.
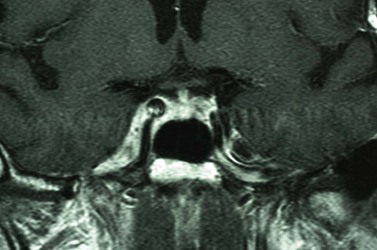
Magnetic resonance imaging (MRI) has better resolution for intraorbital structures and should be used for detection of PNI (Fig. 49.7). Larger tumors are also more likely to have PNI and imaging could be considered. Usually PNI will involve either motor or sensory nerve of the face and within the orbit coursing posteriorly to the brainstem. The possibility of PNI should be included on the imaging request form47.
Management of established disease
Once a tumor has developed and a diagnosis has been established, treatment should be undertaken to eradicate the tumor with minimal damage to surrounding ocular and periocular structures and their function. Currently available treatments for periocular tumors include surgery, radiotherapy, cryotherapy, curettage/diathermy, electro-dissection, photodynamic therapy, and topical agents (imiquimod, 5-fluorouracil, diclofenac gel), as well as emerging medical treatments. Traditionally, for most eyelid malignancies, the treatment has been surgical with excision of the tumor and subsequent repair. Less commonly, some lymphoid, metastatic or other lesions have are managed differently with chemotherapy, radiotherapy, or immunotherapy, reflecting their different tumor biology. The tumor should be staged according to the American Joint Committee on Cancer (AJCC) Eyelid Carcinoma staging protocol, which has been recently revised48. Further, if a melanoma, the AJCC specific melanoma staging system should be used49.
The integument consists of three main layers: the epidermis, the dermis, and the subcutis. The vast majority of cells are keratinocytes, involved in a staged maturation from the basal to superficial skin layers. Other cell types (<5%) include Langerhans cells, which have an important immune function, neural crest derived melanocytes, which are essentially protective, Merkel cells, which perform specialized sensory functions, as well as T-cells50.
Skin homeostasis is mediated by two populations of progenitors: renewable stem cells and their offspring, transient amplifying cells, which can give rise to the various cells of the hair follicle, the interfollicular epidermis (IFE), as well as sebocytes and sebaceous glands. The basal cells undergo an orderly, progressive maturation to suprabasal (or spinous cells), then granular cells. They then undergo a terminal differentiation into the stratum corneum, performing a barrier function before being shed as squames. Under the influence of lower levels of β-catenin they may develop into sebocytes and IFE, rather than hair follicles, suggesting that these pathways are closely linked51–52. Melanocytes migrate from the neural crest to the basal layer of the epidermis, uveal, and meningeal tissues, as well as ectodermal mucosa.
There are a number of important embryonic signaling pathways that influence skin development and homeostasis, influenced by a molecular conversation of their various proteins and genes. The most important of these are the hedgehog pathway (Hh), Wnt (wingless related mouse mammary tumor virus integration site), BMP (bone morphogenic protein), and notch53. Hh signaling influences hair follicle bulge stem cells to produce transit amplifying cells. This process is modulated by Wnt, which increases nuclear β-catenin resulting in upregulation of Hh signaling and Patched homolog 1 (Ptch1) in new follicles. Altered Hh function has been implicated in all BCCs following the finding of a mutant Ptch1 gene in the basal cell nevus syndrome (BCNS or Gorlin’s syndrome). Ptch1 functions as a classic tumor suppressor gene and 90% of sporadic BCCs can be shown to have Ptch1 mutations. Smoothened (SMO) is a downstream activator of the Hh pathway which may show an activating mutation in the remaining 10% of BCCs. Smo activates the Gli family of transcription factors. While genetic abnormalities at these levels are the most common seen in BCC development, other genes including those that influence skin color, repair DNA, or are involved with Wnt, PI3K, and FOXM1 have also been implicated.
SCC conforms to the multi-stage model of cancer, with mutations arising in the p53 tumor suppressor gene. Keratinocytes with multiple p53 mutations do not undergo apoptosis but rather proliferate with clonal expansion leading to SCC in situ and with progression to invasive SCC54.
MM progression from normal melanocytes has been linked to mutations in a number of genes involved: signaling RTK, Pl3K, retinoblastoma, p53, Wnt, and NF-kB pathways55. Five main oncogenes have also been implicated: NRAS, BRAF, MITF, NEDD-9, and KIT, and many others may also be involved.
Sebaceous carcinoma may develop via a number of different pathways. Muir–Torre syndrome is an autosomal dominant genodermatosis caused by mis-sense mutations in genes coding for mismatch repair enzymes, which is the best understood56. Loss of the fragile histidine triad protein has been shown to play a role in some periocular sebaceous carcinomas. Lef-1 mutations, as well as overexpression of β-catenin in the cytoplasm of eyelid sebaceous carcinomas have been reported, suggesting the involvement of multiple developmental pathways56–58.
There has been a great deal of recent activity in trying to inhibit the hedgehog pathway to influence the behavior of BCCs. These have largely come about following the linking of cyclopamine, a naturally occurring teratogenic alkaloid derived from the plant species Veratrum californicum, to its effects on the Hh pathway, and the identification of the importance of the Hh pathway in the development of BCC in the BCNS and sporadically59.
Knowledge of these genetic pathways has provided the opportunity to search for drugs which might alter the behavior of BCCs at a molecular level, without having to resort to surgery or other invasive techniques. Drugs targeting the hedgehog pathway can be thought of as SMO inhibitors of the Hh pathway, or other non-SMO inhibitors60. Unfortunately, cyclopamine, as well as being teratogenic, had poor aqueous solubility and did not lend itself to clinical trials61. Curis-61414 showed promise but had poor skin penetration, which precluded it from ongoing study62. GDC-0449 has been studied in Phase I trials with promising results showing 18 of 33 patients with advanced or metastatic disease showing a clinical or imaging response63. Consequently GDC-0449 is now undergoing Phase II trials for BCC and BCNS64–65. A small number of other SMO antagonists and non-SMO antagonist Hh inhibitors are also currently under investigation66–68.
Surgery
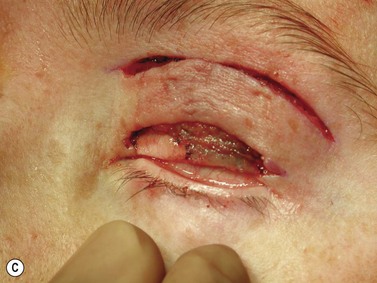
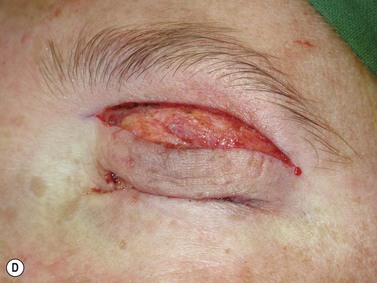
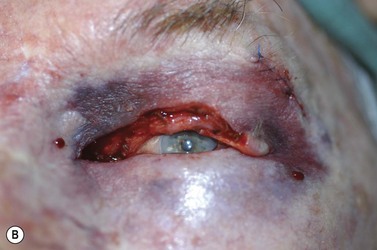
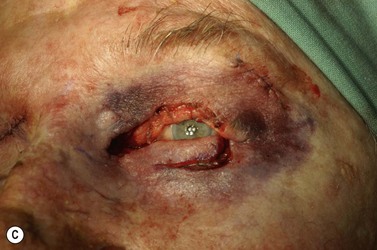
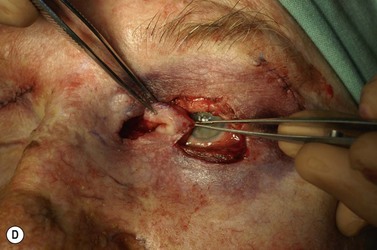
Surgery remains the main therapeutic modality for most malignant eyelid tumors. Management of BCC and SCC can be considered together as NMSC. The aim of surgical excision is to obtain cure, with histologically confirmed complete excision in both width and depth. Ideally normal function is retained and cosmesis is as good as possible. The main advantage of surgical excision is that it provides excellent overall cure rates and is currently superior to all other treatment modalities. Surgical excision also allows pathological examination of the excised specimen. Recommended surgical margins for BCC vary from 2 to 5 mm and for the majority of simple nodular lesions a 2–3 mm margin will be adequate. Factors associated with recurrent disease include the tumor size, site, and type. Morpheic, micronodular, or infiltrative lesions, or those with sclerotic or ulcerated histology, have a higher recurrence rate. Where lesions are reported as incompletely excised on routine histology, careful complete re-excision is the prudent recommendation. SCC is potentially more aggressive with the possibility of spread to draining lymph nodes, via perineural infiltration, or to other distant sites. Recommendations for SCC excision margins vary from 2 to10 mm. Realistically, complete tumor clearance should be the goal, with the initial excision margin varied according to the tumor type, location, and biology (if known from prior biopsy). Histological confirmation of excision may be performed subsequently, or at the time of excision with either frozen section control or Mohs’ micrographic surgery (MMS). Histological confirmation at the time offers the great advantage of being able to deal with any residual disease in the same surgical sitting, until clear margins are obtained, giving the highest cure rates. Simple breadloafing techniques will not examine the entire excision margin and ideally patients having intraoperative margin control should undergo MMS under care of a Mohs’ surgeon, with subsequent repair by an oculoplastic surgeon, or en-face examination of the entire excision margin by a dermatopathologist if frozen section is being performed19,69–71. MMS is primarily used in a tertiary referral setting for difficult to treat tumors arising from anatomically sensitive areas (including the periocular region), tumors with poorly defined borders, or poor tumor biology, recurrence, or extensive disease. Frozen section using en-face examination of the entire excision margin can offer similar cure rates in the periocular area. While intraoperative margin control is the ideal, it may not be practical in all health care systems. Some clinicians perform staged excision with overnight paraffin sections to obtain clear margins prior to surgical repair. Currently, surgical techniques that use comprehensive margin control, such as Mohs’ micrographic surgery, or frozen section with en face excision to examine the entire excision surface, give extremely low recurrence rates while minimizing the extent of the excision to facilitate reconstruction19,69–71. Using these techniques, primary BCC recurrence rates are roughly 1%, while previously recurrent lesions were 5.6%19,69–70.
Using combined series, recurrence rates for primary SCC are roughly 3%, whereas previously recurrent lesions had a higher rate of up to 10%21,71.
Wide local excision is the recommended treatment for cutaneous melanoma, including in the periocular region23,72–74. The surgical margin is determined by the Breslow thickness of the tumor74–75. Generally MMS and frozen section are not performed for cutaneous melanoma because of the difficulty in distinguishing atypical melanocytes, melanoma in situ, and invasive melanoma on frozen specimens. Most authors would perform rapid overnight paraffin section and mapped-serial excision (MSE) in order to obtain clear margins prior to reconstruction of the defect76. Most eyelid melanomas are <1 mm thick and a margin of 1 cm is generally recommended for these thin melanomas (pT1). Even with small lesions this results in large defects best managed by specialist clinics. For thicker lesions (pT2-3) margins of 1–2 cm are recommended. A 2 cm margin is recommended for T4 lesions40–42. Using the updated AJCC classification, sentinel lymph node (SLN) status is an important prognostic factor and is recommended for lesion greater than 1 mm thick and for some thinner melanomas with ulceration or other unfavorable features41–42,74,77–78.
Sebaceous carcinoma also occurs in the periocular region and warrants special mention for a number of reasons. First, it may be sporadic or occur as part of a systemic genodermatosis, the Muir–Torre syndrome. For these patients, the eye lesion may be the first manifestation of a systemic disorder associated with gastrointestinal, genitourinary, or other carcinomas. Diagnosis of an ocular sebaceous carcinoma should prompt systemic screening for the syndrome and other carcinomas. Appropriate genetic referral and counseling should be seen as part of the management. Immunohistochemical staining of the ocular specimen can predict the presence or absence of the systemic genodermatosis79,80. Second, the lesion is notoriously difficult to diagnose and is often regarded as a masquerading lesion, thus a high index of suspicion is required to anticipate the diagnosis81. Chronic blepharitis associated with a thickened lid margin, or the so-called ‘recurrent chalazion’, should prompt consideration of sebaceous carcinoma and appropriate biopsy. Traditionally lipid-based stains, such as Oil Red-O, have been used for confirmation of diagnosis but with advances in immunohistochemical profiles these are now complementary rather than essential. Third, it has a relatively high local recurrence rate due to a high incidence of pagetoid or other intraepithelial spread, requiring special management. Recommendations for excision vary, with some using MMS and others wide excision with 4–5 mm margins82. The overarching principle with all of these techniques is the pursuit of histologically confirmed complete excision. Because of the high incidence of pagetoid spread, multiple map biopsies of the conjunctiva are also advocated25. Should there be positive map biopsies topical treatment with adjunctive mitomycin C has been advocated to avoid exenteration in selected cases83.
Certain aspects of surgical management require further comment. Orbital invasion of malignant eyelid tumors should be managed with the principle of trying to obtain tumor clearance. Traditionally this has called for exenteration, and for extensive orbital involvement; this is still appropriate84. For lesser degrees of orbital involvement Madge et al. have advocated retention of the globe in selected cases85. Where orbital invasion has occurred in the setting of PNI, there appears to be a limited role for exenteration, with limited local resection and adjunctive radiotherapy to the antegrade and retrograde distribution of the involved neural structures, posteriorly to Meckel’s cave43.
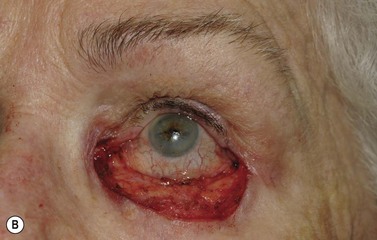
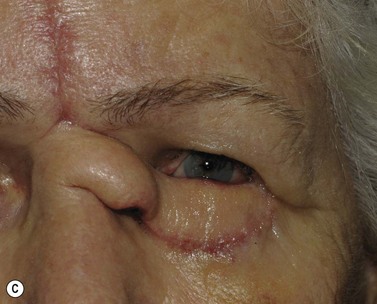
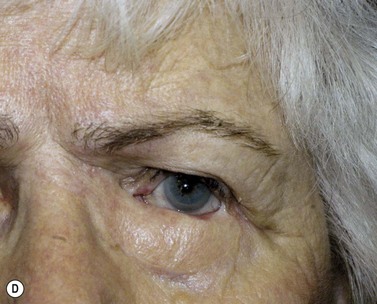
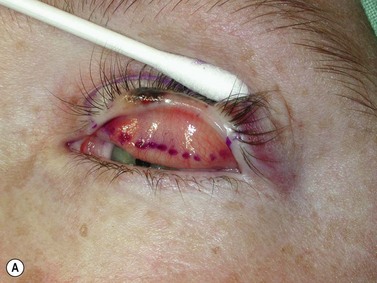
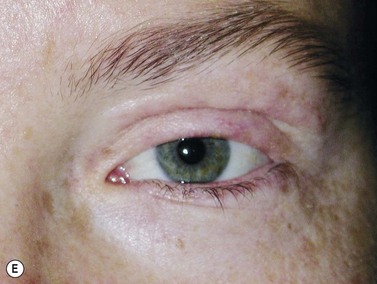
Surgical reconstruction of the defect should ideally be delayed until histologically clear margins have been obtained. Simple principles should be followed to achieve the goal of restoration of function with a cosmetically satisfactory outcome86–88. The periocular region can be broken down into the upper and lower eyelids, the medial and lateral canthi, and specialized structures such as the lacrimal secretory and drainage apparatus. Loss of the anterior lamella (skin and orbicularis) only can be repaired by laissez-faire, skin grafts, or local flaps. Laissez-faire may have a role for small defects, or in patients unable or unwilling to undergo surgical repair. The results using this technique can be surprisingly good; however, eyelid malposition and canthal dystopia are potential complications89. Full-thickness skin grafts can also give pleasing results but tend to have greater shrinkage and potential for infection than vascularized flaps. This is especially true for skin grafts to the lateral aspect of the lower eyelid, particularly when there is concomitant solar damage. Grafts may be fenestrated to allow for drainage of collections and expansion of the graft surface area. Donor sites should be chosen to match like tissue with like tissue and in general the surgeon will look to the upper eyelid, pre- and postauricular, supraclavicular and upper arm skin in descending order of preference. Flaps also have the advantage of generally transposing tissue that is more similar to the excised tissue.
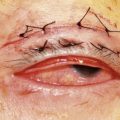
Where the defect involves the full thickness of the eyelid, consideration must be given to reconstructing both the anterior and posterior lamellae. Smaller defects may be closed directly, or with the assistance of a lateral cantholysis for lower and upper eyelid defects. The lower eyelid is more forgiving in terms of reconstruction than the upper eyelid. Loss of the lower eyelid can be better tolerated than loss of, or inadequate reconstruction of, the upper eyelid. Larger lower eyelid defects generally require a two-stage Wendell–Hughes (or Koellner–Hughes) repair, which may be divided as early as 1–2 weeks post primary repair90–93. Posterior lamella tarso-conjunctival grafting with an overlying transposed upper to lower eyelid myocutaneous flap, based at the lateral canthus, can give very pleasing cosmetic and functional results for larger lower eyelid defects. Many other reconstructive techniques have also been described, including Tenzel myocutaneous semicircular flaps, McGregor flaps, and much larger Mustardé cheek rotation flaps when required. The midline forehead flap is versatile for large upper and lower eyelid defects (see Fig. 49.4). All of these techniques have their place and the skilled oculoplastic surgeon will be facile with each of them.
Larger upper eyelid defects present more challenges. Traditionally a Cutler–Beard two-stage repair has been performed. This is a good technique providing the reconstructed upper lid is stabilized with some form of ‘tarsal’ reconstruction, to prevent late entropion. Alternatives are available and might need to be considered when actinic change to the lower eyelid restricts the mobility necessary for a Cutler–Beard flap. Where any of the superior aspects of the posterior lamella have been retained, this can be recessed to contribute to the new posterior lamella. Should this be absent then a tarsoconjunctival (preferably) or hard palate mucosal graft could be utilized. The anterior lamella can be derived from a fully vascularized sub-brow bipedicle myocutaneous flap, transposed to cover the tarsal remnant or tarso-conjunctival graft, with a full-thickness skin graft placed into the donor site of the bipedicle flap (see Fig. 49.8). Another approach for these large upper eyelid defects is to rotate the lower eyelid into the upper eyelid defect with a so-called Mustardé rotation flap (Fig. 49.9). This requires a second stage, to divide the reconstructed upper eyelid and to secondarily repair the lower eyelid donor site.
Radiotherapy
Radiotherapy is a well-established treatment modality that can be effective in the treatment of primary and recurrent periocular NMSC94.
Cryotherapy
Cryotherapy has utility elsewhere in the body, based on its ability to freeze intracellular water components, but has limited applicability in the periocular region. When used elsewhere, histological confirmation prior to treatment may not be obtained, but this can have significant consequences around the eye95. Deeper lesions may not be completely eradicated, extending deeply toward the orbit with little surface abnormality, risking orbital invasion. Lesions close to the lid margin, particularly intraepidermal SCC (IEC) tend to invade down follicular structures and not be affected by superficial applications of cryotherapy. For these reasons cryotherapy is not generally recommended close to the eye. One series of patients with eyelid BCC treated with cryotherapy have a lower cure rate than surgery, with 97% and 95% success for nodular and infiltrative lesions less than 1 cm in diameter but 85% and 82% respectively when the lesion diameter was greater than 1 cm96.
Laser
Lasers have been used to prevent and treat NMSC. CO2 lasers have been used to prevent the progression of AK’s and to treat IE, or Bowen’s disease. NeodymiumYag lasers have been effectively used in the treatment of BCC and SCC, with low recurrence rates of 2–5%97–99.
Topical agents
The two main topical agents include 5-fluorouracil (5FU) and imiquimod. 5FU acts by depleting thymidine in cells resulting in decreased DNA production, reduced cell growth, and subsequent cell death100. Normal cells are relatively resistant to the effects of 5FU, but rapidly proliferating tumor cells are more susceptible. 5FU has been used in the treatment of actinic keratoses, SCC in situ, SCC, keratoacanthoma, and superficial BCC, elsewhere in the body 101,102. Unfortunately, 5FU has poor penetration of the dermis, so has limited applicability to invasive BCC or SCC. There are also reports of its use in the periocular region. There are anatomical reasons that limit its use in this region, as well as poor dermal penetration. First, there is the risk of extension of squamoproliferative lesions downwards along eyelash follicles, or more deeply into the orbit if treatment is unsuccessful. Also, conjunctival and ocular irritation can occur as a complication of topical 5FU applied close to the eye. Having said this, there may well be situations in selected patients too frail to undergo surgery, or those with tumor predispositions such as ongoing immunosuppression (including organ transplant recipients) or the basal cell nevus syndrome (BCNS), who may benefit from the use of this topical agent.
Imiquimod stimulates toll-like receptors (TLRs) on antigen presenting cells to stimulate innate and acquired immunity. It causes the production of a number of inflammatory cytokines, including interferon α, tumor necrosis factor α, as well as interleukins 12 and 6, to prolong the immune reaction and promote Langerhans cell activity103. There is increased cytotoxic T-cell, natural killer cell, and B-cell activity, resulting in powerful anti-viral and anti-tumor activity104. Imiquimod has a proven role against basal cell carcinoma, in-situ and invasive squamous proliferations as well as lentigo maligna. Recently there have been a number of case reports and small series of the benefits of imiquimod in the treatment of periocular BCC, SCC (and related lesions) as well as lentigo maligna, in selected cases105–106. Resiquimod is another TLR7/8 agonist, currently under trial, with greater potency than imiquimod107. There is little doubt that these medical approaches to periocular malignancy will have an increasingly important role.
Photodynamic therapy
Photodynamic therapy (PDT) has a role in the management of squamoproliferative lesions as well as BCC. PDT works by the accumulation of a photosensitizer, light energy, and oxygen within the target cell. The photosensitizer, usually alpha- or methyl aminolevulinic acid, accumulates light energy of a specific wavelength and generates reactive oxygen species (ROS), which causes tissue damage by the induction of necrosis and apoptosis. The technique has a lower success rate than surgical excision or radiotherapy but, like 5FU and imiquimod, may have some application in the periocular region in patients who are unable to undergo surgical excision, or who have multiple lesions, as seen in immunosuppressed patients and those with the BCNS108–109.
Retinoids
Retinoids have been demonstrated to be useful in the management and prevention of NMSC. Systemic toxicity has been a problem with broad-spectrum retinoic receptor agonists. Tazarotene activates a specific group of retinoid receptors and is useful against BCC but with fewer systemic side effects (mainly skin irritation)110. Currently under study, tazarotene may have application in chemoprevention and of BCCs where there is an underlying predisposition to the development of multiple lesions, as in the BCNS.
Diclofenac gel
Cyclooxegenase 2 is expressed in a range of skin lesions, AK, SCC, BCC, and melanoma. UVB induces COX-2 in humans. Studies have shown benefit from the use of selective COX-2 inhibitors in AK, BCC, and SCC. Diclofenac 3.0% gel is effective against Bowen’s disease111–113.
Interferon
Interferon exerts an anti-proliferative effect on lesional cells and enhances surrounding local tissue immune system activity. This has been used as a gel preparation for AKs with no advantage over placebo, although intralesional injections have been successful for BCC and SCC, but limitations include significant cost and the need for multiple injections114–116.
Ingenol mebutate
Ingenol mebutate, initially used as a home treatment for various skin lesions, is derived from the milkweed plant, and is now being subjected to controlled studies for the treatment of actinic keratoses. It acts by causing plasma membrane disruption, and mitochondrial dysfunction in dysplastic keratinocytes. It also induces an antibody dependant cytotoxicity117,
1 Cook BEJr, Bartley GB. Treatment options and future prospects for the management of eyelid malignancies: an evidence-based update. Ophthalmology. 2001;108:2088-2098.
2 Cook BEJr, Bartley GB. Epidemiologic characteristics and clinical course of patients with malignant eyelid tumors in an incidence cohort in Olmsted County, Minnesota. Ophthalmology. 1999;106:746-750.
3 Walter U, Kron M, Sander S, et al. Risk and protective factors for sporadic basal cell carcinoma: results of a two-centre case–control study in southern Germany. Clinical actinic elastosis may be a protective factor. Br J Dermatol. 2004;151:170-178.
4 Data from SEER FastStats. Available at http://seer.cancer.gov/faststats/index.php Accessed January 30, 2009
5 Califono J, Nance M. Malignant melanoma. Facial Plast Surg Clin North Am. 2009;17:337-348.
6 Tucker MA. Melanoma epidemiology. Hematol Oncol Clin N Am. 2009;23:383-395.
7 Queensland Cancer Registry. Cancer in Queensland Incidence and mortality 1982 to 2003. Statistical tables. Available at http://www.health.qld.gov.au/hic/qcr2005/1982_03_text.pdf Accessed November 4, 2010
8 Dores G, Curtis R, Toro J, et al. Incidence of cutaneous sebaceous carcinoma and risk of associated neoplasms insight into Muir–Torre syndrome. Cancer. 2008;113:3372-3381.
9 Dasgupta T, Wilson LD, Yu JB. A retrospective review of 1349 cases of sebaceous carcinoma. Cancer. 2009;115:158-165.
10 Izumi M, Mukai K, Nagai T, et al. Sebaceous carcinoma of the eyelids: Thirty cases from Japan. Path Int. 2008;58:483-488.
11 Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
12 Becker J. Merkel cell carcinoma. Ann Oncol. 2010;21(Suppl. 7):vii81-vii85.
13 Pulitzer MP, Amin BD, Busam KJ. Merkel cell carcinoma. Review Adv Anat Pathol. 2009;16:135-144.
14 Marks R. The epidemiology of non-melanoma skin cancer: who, why and what can we do about it. J Dermatol. 1995;22:853-857.
15 Green A, Battistutta D, Hart V, et al. Skin cancer in a subtropical Australian population: incidence and lack of association with occupation. The Nambour Study Group. Am J Epidemiol. 1996;144:1034-1040.
16 Green A, Beardmore G, Hart V, et al. Skin cancer in a Queensland population. J Am Acad Dermatol. 1988;19:1045-1052.
17 Ibiebele TI, van der Pols JC, Hughes MC, et al. Dietary fat intake and risk of skin cancer: A prospective study in Australian adults. Int J Cancer. 2009;125:1678-1684.
18 Van der Pols JC, Heinen MM, Hughes MC, et al. serum antioxidants and skin cancer risk: an 8-year community-based follow-up study. Cancer Epidemiol Biomarkers Prev. 2009;18:1167-1173.
19 Wong VA, Marshall JA, Whitehead KJ, et al. Management of periocular BCC with modified en face frozen section controlled excision. Ophthal Plast Reconstr Surg. 2002;18:430-435.
20 Sullivan TJ, Boulton JE, Whitehead KJ. Intraepidermal carcinoma of the eyelid. Clin Exp Ophthalmol. 2002;30:23-27.
21 Donaldson MJ, Sullivan TJ, Whitehead KJ, et al. Squamous cell carcinoma of the eyelids. Br J Ophthalmol. 2002;86:1161-1165.
22 Limawararut V, Leibovitch I, Sullivan T, et al. Periocular squamous cell carcinoma. Clin Exp Ophthalmol. 2007;35:174-185.
23 Chan F, O’Donnell BA, Whitehead K, et al. Treatment and outcomes of malignant melanoma of the eyelid. Ophthalmology. 2007;114:187-192.
24 Zürcher M, Hintschich CR, Garner A, et al. Sebaceous carcinoma of the eyelid: a clinicopathological study. Br J Ophthalmol. 1998;82:1049-1055.
25 Shields JA, Demirci H, Marr BP, et al. Sebaceous carcinoma of the eyelids: personal experience with 60 cases. Ophthalmology. 2004;111:2151-2157.
26 Shields JA, Demirci H, Marr BP, et al. Sebaceous carcinoma of the ocular region: a review. Surv Ophthalmol. 2005;50:103-122.
27 Lee D, Miller SJ. Non melanoma skin cancer. Facial Plast Surg Clin North Am. 2009;17:309-324.
28 . NCCN 1.2008 Basal and Squamous Cell Skin Cancers Clinical Practice Guidelines in Oncology Available at http://www.nccn.org. Accessed November 2010
29 Basal cell carcinoma, squamous cell carcinoma (and related lesions). A guide to clinical management in Australia. Cancer Council Australia and Australian Cancer Network, Sydney. November 2008 Available at http://www.cancer.org.au Accessed November 2010
30 International Agency for Cancer Research. The association of the use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116-1122.
31 Gordon L, Hirst N. The health effects of using solaria and potential cost effectiveness of enforcing solaria regulations in Australia. Brisbane: Queensland Institute of Medical Research; 2007 http://www.arpansa.gov.au/Publications/RHC/rhc_reports.cfm Accessed Mar 2008
32 Gordon L, Hirst N, Gies PHF, et al. What impact would effective solarium regulation have in Australia? MJA. 2008;189:375-378.
33 Alberts DS, Dorr RT, Einspahr JG, et al. Chemoprevention of human actinic keratoses by topical 2-(Difluoromethyl)-dlornithine. Cancer Epidemiol Biomarkers Prev. 2000;9(12):1281-1286.
34 Alberts DS. Phase IIB randomized, double-blinded, placebo controlled study to evaluate the safety and efficacy of topical difluoromethylornithine (DFMO) with and without a topical corticosteroid cream (triamcinolone 0.1%) in the therapy of actinic keratoses (AK) on the forearms ClinicalTrials.gov Identifier: NCT00021294 http://clinicaltrials.gov/ct2/show/NCT00021294
35 Carbone PP. Chemoprevention of skin cancers with DFMO: a controlled, randomized clinical trial ClinicalTrials.gov Identifier: NCT00005884 http://clinicaltrials.gov/ct2/show/NCT00005884
36 Aziz MH, Reagan-Shaw S, Wu J, et al. Chemoprevention of skin cancer by grape constituent resveratrol; relevance to human disease. J FASEB. 2005;19:1193-1195.
37 Kim KH, Back JH, Zhu Y, et al. Resveratrol targets transforming growth factor β2 signaling to block UV0induced tumor progression. J Invest Dermatol. 2011;131:195-202.
38 Barnetson RS, Ooi TK, Zhuang L, et al. [Nle4-D-Phe7]-alphamelanocyte-stimulating hormone significantly increased pigmentation and decreased UV damage in fair-skinned Caucasian volunteers. J Invest Dermatol. 2006;126(8):1869-1878.
39 Kersten RC, Ewing-Chow D, Kulwin DR, et al. Accuracy of clinical diagnosis of cutaneous eyelid lesions. Ophthalmology. 1997;104:479-484.
40 Marsden JR, Newton-Bishop JA, Burrows L, et al. Revised UK guidelines for the management of cutaneous melanoma 2010. J Plast Reconstr Aesth Surg. 2010;63:1401-1419.
41 http://www.cancer.org.au/File/HealthProfessionals/ClinicalPracticeGuidelines-ManagementofMelanoma.pdf.
42 http://www.cancer.org.au/File/HealthProfessionals/ManagementofOcularmelanomasupplementarydocument2008.pdf.
43 Bowyer JD, Sullivan TJ, Whitehead KJ, et al. The management of perineural spread of squamous cell carcinoma to the ocular adnexae. Ophthal Plast Reconstr Surg. 2003;19:275-281.
44 Valenzuela AA, Whitehead KJ, Sullivan TJ. Ocular adnexal pseudo-cyst formation as a characteristic feature of perineural spread in squamous cell carcinoma. Ophthalmol Plast Reconstr Surg. 2006;22:201-205.
45 McNab AA, Francis IC, Benger R, et al. Perineural spread of cutaneous squamous cell carcinoma via the orbit. Clinical features and outcome in 21 cases. Ophthalmology. 1997;104:1457-1462.
46 Lin C, Tripcony L, Keller J, et al. Perineural infiltration of cutaneous squamous cell carcinoma and basal cell carcinoma without clinical features. Int J Radiation Oncol Biol Phys 2010 Nov 17. [Epub ahead of print]
47 Gandhi MR, Pannizza B, Kennedy D. Detecting and defining the anatomic extent of large nerve perineural spread of malignancy: comparing targeted MRI with the histologic findings following surgery. Head Neck. 2011;33(4):469-475.
48 Ainbinder DJ, Esmaeli B, Groo C, et al. Introduction of the 7th Edition Eyelid Carcinoma Classification System From the American Joint Committee on Cancer – International Union Against Cancer Staging. Manual Arch Path Lab Med. 2009;133:1256-1261.
49 Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199.
50 Edmondson SR, Thumiger SP, Werther GA. Epidermal homeostasis: The role of the growth hormone and insulin-like growth factor systems. Endocrine Rev. 2003;24(6):737-764.
51 Jayaraj P, Sen S, Kashyap S, et al. Does β-catenin have a role in pathogenesis of sebaceous cell carcinoma of the eyelid? Br J Ophthalmol. 2011 Feb;95:284-287.
52 Niemann C, Unden AB, Lyle S, et al. Indian hedgehog and β-catenin signaling: Role in the sebaceous lineage of normal and neoplastic mammalian epidermis. PNAS. 2003;100(Suppl. 1):11873-11880.
53 Epstein EH. Basal cell carcinoma: attack of the hedgehog. Nat Rev Cancer. 2008;8:743-754.
54 Alam M, Ratner D. Cutaneous squamous cell carcinoma. N Engl J Med. 2001;344(13):975-983.
55 Berger MF, Garraway LA. Applications of genomics in melanoma oncogene discovery. Hematol Oncol Clin N Am. 2009;23:397-414.
56 Lazar AJF, Lyle S, Calonje E. Sebaceous neoplasia and Torre–Muir syndrome. Curr Diagnost Pathol. 2007;13:301-319.
57 Goldberg M, Rummett C, Foja S. Different genetic pathways in the development of periocular sebaceous gland carcinomas in presumptive Muir–Torre syndrome patients. Hum Mutation. 2006;27:155-162.
58 Takeda Lyle S, Lazar AJ. Human sebaceous tumors harbor inactivating mutations in LEF1. Nat Med. 2006;12:395-397.
59 Chen JK. Inhibition of Hedgehog signaling by direct binding of cyclopamine to smoothened. Genes Develop. 2002;16:2743-2748.
60 So P-L, Tang JY, Epstein EH. Novel investigational drugs for basal cell carcinoma. Expert Opin Invest Drugs. 2010;19(9):1099-1112.
61 Tremblay MR, Nevalainen M, Nair SJ, et al. Semisynthetic cyclopamine analogues as potent and orally bioavailable hedgehog pathway antagonists. J Med Chem. 2008;51:6646-6649.
62 Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303-312.
63 Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164-1172.
64 . A study of GDC-0449 (Hedgehog pathway inhibitor) in patients treated with GDC-0449 in a previous Genentech-sponsored Phase I or II cancer study (Phase II for metastatic colorectal cancer, ovarian cancer and BCC Available from http://clinicaltrials.gov/ct2/show/NCT00833417
65 Study to determine the efficacy and safety of a systemic Hedgehog pathway antagonist (GDC-0449) in patients with basal cell nevus syndrome (BCNS) (Phase II). Available from: http://clinicaltrials.gov/ct2/show/NCT00957229..
66 Tremblay MR, Nesler M, Weatherhead R, et al. Recent patents for Hedgehog pathway inhibitors for the treatment of malignancy. Expert Opin Ther Pat. 2009;19:1039-1056.
67 Chen JK, Taipale J, Young KE, et al. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002;99:14071-14076.
68 Bijlsma MF, Peppelenbosch MP, Spek CA. (Pro-)vitamin D as treatment option for hedgehog-related malignancies. Med Hypotheses. 2008;70:202-203.
69 Malhotra R, Huilgol SC, Huynh NT, et al. The Australian Mohs database, Part 1. Periocular basal cell carcinoma experience over 7 years. Ophthalmology. 2004;111:624-630.
70 Malhotra R, Huilgol SC, Huynh NT, et al. The Australian Mohs Database, Part II. Periocular basal cell carcinoma outcome at 5-year follow-up. Ophthalmology. 2004;111:631-636.
71 Malhotra R, Huilgol SC, Huynh NT, et al. The Australian Mohs database: periocular squamous cell carcinoma. Ophthalmology. 2004;111:617-623.
72 Vaziri M, Buffam F, Martinka M, et al. Clinicopathologic features and behavior of cutaneous eyelid melanoma. Ophthalmology. 2002;109:901-908.
73 Esmaeli B, Youssef A, Naderi A, et al. Margins of excision for cutaneous melanoma of the eyelid skin. Ophthal Plast Reconstr Surg. 19, 2003. 96-10
74 Ott PA, Berma RS. Surgical approach to primary cutaneous melanoma. Surg Oncol Clin North Am. 2011;20:39-56.
75 Esmaeli B, Wang B, Deavers M, et al. Prognostic factors for survival in malignant melanoma of the eyelid skin. Ophthal Plast Reconstr Surg. 2000;16:250-257.
76 Malhotra R, Chen C, Huilgol S, et al. Mapped serial excision for periocular lentigo maligna and lentigo maligna melanoma. Ophthalmology. 2003;110:2011-2018.
77 Rhodes AR. Prognostic usefulness of sentinel lymph node biopsy for patients who have clinically node negative, localized, primary invasive cutaneous melanoma. Published online December 2010. Available from: doi:10.1001/archdermatol.2010.371.
78 Savar A, Ross MI, Prieto VG, et al. Sentinel lymph node biopsy for ocular adnexal melanoma: experience in 30 patients. Ophthalmology. 2009;116:2217-2223.
79 Gaskin B, Fernando B, Sullivan CA, et al. The significance of DNA-mismatch repair genes in the diagnosis and management of Muir–Torre syndrome and peri-ocular sebaceous cell carcinoma. BJO June 23 2011 as 11.36/bjo.2011. 203075.
80 Mathiak M, Rütten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir–Torre syndrome and MSH2 or MLH1 germline mutations establishment of immunohistochemical analysis as a screening test. Am J Surg Path. 2002;26:338-343.
81 Yeatts RP, Waller RR. Sebaceous carcinoma of the eyelids: pitfalls in diagnosis. Ophthalmic Plast Reconstr Surg. 1985;1:35-42.
82 Song A, Carter K, Syed N. Nerad J. Sebaceous cell carcinoma of the ocular adnexa: clinical presentations, histopathology, and outcomes. Ophthalmic Plast Reconstr Surg. 2008;24:194-200.
83 Shields CL, Naseripour M, Shields JA, et al. Topical mitomycin-C for pagetoid invasion of the conjunctiva by eyelid sebaceous gland carcinoma. Ophthalmology. 2002;109:2129-2133.
84 Leibovitch I, McNab AA, Sullivant TJ, et al. Orbital invasion by periocular basal cell carcinoma. Ophthalmology. 2005;112:717-723.
85 Madge SN, Khine AA, Thaller VT, et al. Globe-sparing surgery for medial canthal basal cell carcinoma with anterior orbital invasion. Ophthalmology. 2010;117:2222-2228.
86 Collin JRO. Manual of Systematic Eyelid Surgery, 3rd edn. Oxford: Butterworth-Heinemann; 2006.
87 Morley AMS, de Sousa J-L, Selva D, et al. Techniques of upper eyelid reconstruction. Surv Ophthalmol. 2010;55:256-271.
88 deSousa J-L, Leibovitch I, Malhotra R, et al. Techniques and outcomes of total upper and lower eyelid reconstruction. Arch Ophthalmol. 2007;125:1601-1609.
89 Lowry JC, Bartley GB, Garrity JA. The role of second-intention healing in periocular reconstruction. Ophthal Plast Reconstr Surg. 1997;13:174-188.
90 Koellner H. Verfahren fur den plastischen Ersatz des Unterlides. Munch Med Wochenschr. 1911;58:2166-2168.
91 Hughes WL. Total lower eyelid reconstruction: technical details. Trans Am Ophthalmol Soc. 1976;74:321-329.
92 McNab AA, Martin P, Benger R, et al. A prospective randomized study comparing division of the pedicle of modified Hughes flaps at two or four weeks. Ophthalmol Plast Reconstr Surg. 2001;17:317-319.
93 Leibivitch I, Selva D. Modified Hughes flap: division at 7 days. Ophthalmol. 2004;111:2164-2167.
94 Venness MJ. The important role of radiotherapy in patients with non-melanoma skin cancer and other cutaneous entities. J Med Imaging Rad Oncol. 2008;52:278-286.
95 Anderson R. A warning on cryosurgery for eyelid malignancies. Arch Ophthalmol. 1978;96:1289-1290.
96 Fraunfelder FT, Zacarian SA, Wingfield DL, et al. Results of cryotherapy for eyelid malignancies. J Ophthalmol. 1984;97:184-188.
97 Iyer S, Friedli A, Bowes L, et al. Full face laser resurfacing: therapy and prophylaxis for actinic keratosis and non melanoma skin cancer. Lasers Surg Med. 2004;34:114-119.
98 Halachmi S, Lapidoth M. Lasers in skin cancer prophylaxis. Exp Rev Anticancer Therapy. 2008;8:1713-1717.
99 Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high energy pulsed Neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
100 Moore AY. Clinical applications for topical 5-fluorouracil in the treatment of dermatological disorders. J Dermatol Treatment. 2009;20:328-335.
101 Gross K, Kircik L, Kricorian G. 5% 5-Fluorouracil cream for the treatment of small superficial basal cell carcinoma: efficacy, tolerability, cosmetic outcome, and patient satisfaction. Dermatol Surg. 2007;33:433-440.
102 Gupta A, Davey V, Ephail H. Evaluation of the effectiveness of imiquimod and 5-fluorouracil for the treatment of actinic keratosis: critical review and meta-analysis of efficacy studies. J Cutan Med Surg. 2005;9(5):209-214.
103 Testerman TL, Gerster JF, Imbertson LM, et al. Cytokine induction by the immunomodulators imiquimod and S-27609. J Leukoc Biol. 1995;58:365-372.
104 Tomai MA, Gibson SJ, Imbertson LM, et al. Immunomodulating and antiviral activities of the imidazoquinoline S-28463. Antiviral Res. 1995;28:253-264.
105 Ross AH, Kennedy CT, Collins C, et al. The use of imiquimod in the treatment of periocular tumors. Orbit. 2010;29:83-87.
106 Demirci H, Shields CL, Bianciotto C, et al. Topical imiquimod for periocular lentigo maligna. Ophthalmology. 2010;117:2424-2429.
107 Jones T. Resiquimod 3M. Curr Opin Invest Drugs. 2003;4:214-218.
108 Braathen LR, Szeimies RM, Basset-Seguin N, et al. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: an international consensus. International Society for Photodynamic Therapy in Dermatology, 2005. J Am Acad Dermatol. 2007;56:125-143.
109 Apalla Z, Sotiriou E, Chovarda E, et al. Skin cancer: preventive photodynamic therapy in patients with face and scalp cancerization. A randomized placebo controlled study. Br J Dermatol. 2010;162:171-175.
110 Bianchi L, Orlandi A, Campione E, et al. Topical treatment of basal cell carcinoma with tazarotene: a clinicopathological study on a large series of cases. Br J Dermatol. 2004;151:148-156.
111 McEwan LE, Smith JG. Topical diclofenac/hyaluronic acid gel in the treatment of solar keratoses. Australas J Dermatol. 1997;38:187-189.
112 Rivers JK, McLean DI. An open study to study the efficacy and safety 3% diclofenac in a 2.5% hyaluroinic acid gel for the treatment of actinic keratosis. Arch Dermatol. 1997;133:1239-1242.
113 Dawe SA, Salisbury JR, Higgins E. Two cases of Bowen’s disease successfully treated topically with 3% diclofenac in 2.5% hyaluronan gel. Clin Exp Dermatol. 2005;30:712-713.
114 Edwards L, Levine N, Weidner M, et al. Effect of intralesional interferon in actinic keratoses. Arch Derm. 1986;122:779-782.
115 Bostanci S, Kocyigit P, Alp A, et al. Treatment of basal cell carcinoma located in the head and neck region with intralesional interferon alpha-2a: evaluation of long-term follow-up results. Clin Drug Invest. 2005;25:661-667.
116 Edwards L, Berman B, Rapini RP, et al. Treatment of cutaneous squamous cell carcinoma by intralesional interferon alpha 2b therapy. Arch Derm. 1992;128:1486-1489.
117 Siller G, Gebauer K, Welburn P, et al. PEP005 (ingenol mebutate) gel, a novel agent for the treatment of actinic keratosis: results of a randomized, double-blind, vehicle-controlled, multicentre, phase IIa study. Australas J Dermatol. 2009;50:16-22.