14 The liver and biliary tract
The liver
Anatomy
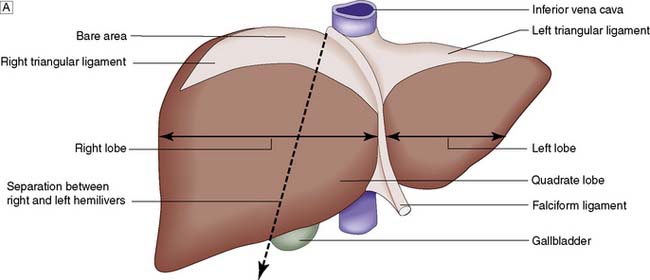
Topographically, the liver is divided by the attachment of the falciform ligament into right and left lobes; fissures on its visceral surface demarcate two further lobes, the quadrate and caudate (Fig. 14.1A). However, it is the liver segmental anatomy, as defined by the distribution of its blood supply, that is important to the surgeon.
Segmental anatomy
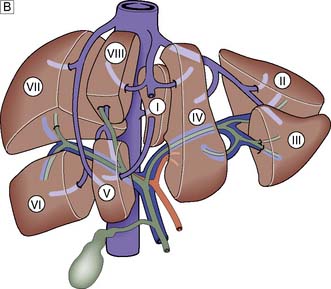
The portal vein and hepatic artery divide into right and left branches in the porta hepatis. Occluding either branch at surgery produces an easily visible line of demarcation that runs from the gallbladder bed behind and to the left of the inferior vena cava, thus separating the two hemilivers. Each hemiliver is further divided into four segments corresponding to the main branches of the hepatic artery and portal vein. In the left hemiliver, segment I corresponds to the caudate lobe, segments II and III to the left lobe (or left lateral section), and segment IV to the quadrate lobe. The remaining segments (V–VIII) comprise the right hemiliver (Fig. 14.1B).
Blood supply and function
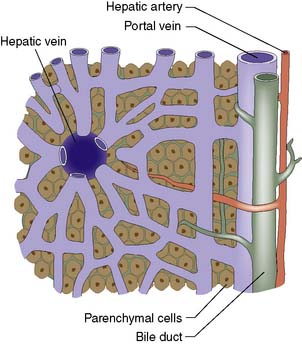
The liver normally receives 1500 ml of blood per minute and has a dual blood supply, 75% coming from the portal vein and 25% from the hepatic artery, which supplies 50% of the oxygen requirements. The principal venous drainage of the liver is by the right, middle and left hepatic veins, which enter the vena cava (Fig. 14.1B). In 25% of individuals, there is an inferior right hepatic vein, and numerous small veins drain direct into the vena cava from the caudate lobe (segment I). The functional unit of the liver is the hepatic acinus. Sheets of liver cells (hepatocytes) one cell thick are separated by interlacing sinusoids through which blood flows from the peripheral portal tract into the hepatic acinus to the central branch of the hepatic venous system. Bile is secreted by the liver cells and passes in the opposite direction along the small canaliculi into interlobular bile ducts located in the portal tracts (Fig. 14.2).
Summary Box 14.1 Surgical anatomy
• The liver is divisible into right and left hemilivers (each having four segments) using a line running from the gallbladder fossa to the inferior vena cava
• Each hemiliver receives a branch of the hepatic artery and portal vein; 75% of liver blood flow and 50% of its oxygen supply are provided by the portal vein
• The hepatocytes are arranged in lobules, each of which has a central branch of the hepatic vein and peripheral portal tracts (containing a branch of the hepatic artery, portal vein and bile duct)
• Liver anatomy allows the surgeon to perform right hepatectomy, left hepatectomy and extended right hepatectomy (i.e. resecting all of the liver to the right of the falciform ligament). Resection of individual segments is also possible.
Jaundice
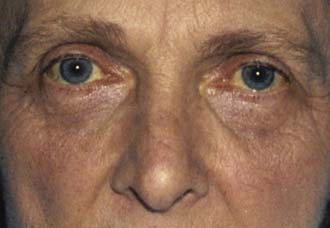
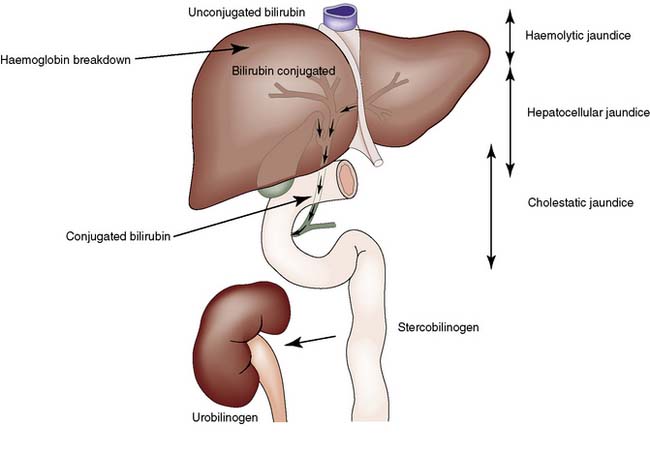
Jaundice is caused by an increase in the level of circulating bilirubin and becomes obvious in the skin and sclera when levels exceed 50 μmol/l (Fig. 14.3). It may result from excessive destruction of red cells (haemolytic jaundice), from failure to remove bilirubin from the blood stream (hepatocellular jaundice), or from obstruction to the flow of bile from the liver (cholestatic jaundice) (Fig. 14.4). Congenital non-haemolytic hyperbilirubinaemia (Gilbert’s syndrome) is a relatively rare cause of jaundice due to defective bilirubin transport; the jaundice is usually mild and transient, and the prognosis is excellent.
To the surgeon, the most important type of haemolytic jaundice is that caused by hereditary spherocytosis, in which splenectomy may be necessary (Ch. 15). Haemolytic jaundice may also occur after blood transfusion and after operative or accidental trauma, when haematoma formation produces a pigment load that exceeds hepatic excretory capacity.
Diagnosis
History and clinical examination
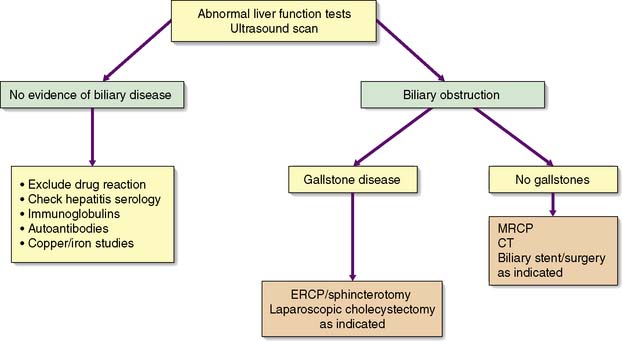
An accurate, rapid diagnosis of the cause of jaundice allows prompt institution of appropriate treatment (Fig. 14.5). The age, sex, occupation, social habits, drug and alcohol intake, history of injections or infusions, and general demeanour of the patient must be considered. A history of intermittent pain, fluctuant jaundice and dyspepsia suggests calculous obstruction of the common bile duct, whereas a history of weight loss and relentless progressive jaundice favours a diagnosis of neoplasia. Obstructive jaundice is likely if there is a history of passage of dark urine and pale stools, and if the patient complains of pruritus (owing to an inability to secrete bile salts into the obstructed biliary system). Hepatocellular jaundice is likely if there are stigmata of chronic liver disease, such as liver palms, spider naevi, testicular atrophy and gynaecomastia. The abdomen must be examined for evidence of hepatomegaly or gallbladder distension, and for signs of portal hypertension such as splenomegaly, ascites and large collateral veins (caput medusae) in the abdominal wall.
Radiological investigations
Congenital abnormalities
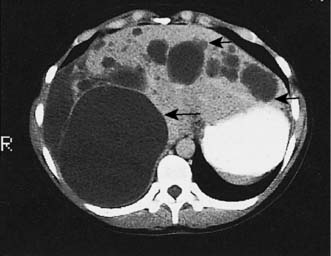
Up to 5% of the population has simple liver cysts. They are lined by biliary epithelium and contain serous fluid, but never communicate with the biliary tree. They rarely produce symptoms, are associated with normal liver function, and on ultrasound or CT have no discernible wall (Fig. 14.6). In the few patients who develop symptoms, cysts tend to recur following aspiration, and sclerosis by alcohol injection is of little value for large symptomatic cysts. Surgical management consists of deroofing and may be undertaken by laparoscopic means. Polycystic disease is a rare cause of liver enlargement and may be associated with polycystic kidneys as an autosomal dominant trait. In symptomatic patients, it may be necessary to combine a deroofing procedure with hepatic resection or to consider liver transplantation.
Summary Box 14.2 Jaundice
• Jaundice is a yellowish discoloration of the tissues that becomes clinically apparent when serum bilirubin levels exceed 50 μmol/l (normal < 20 μmol/l)
• It may be due to excessive haemolysis, hepatic insufficiency or cholestasis; cholestatic (obstructive) jaundice is the type encountered in surgical practice
• The two most common causes of surgical obstructive jaundice are cancer of the head of the pancreas and stones in the common bile duct (choledocholithiasis)
• In cholestatic jaundice, the bilirubin has been conjugated by the hepatocytes and is therefore soluble in water and can be excreted in the urine; patients with obstructive jaundice typically have dark urine and pale stools and may have pruritus (thought to be due to the accumulation of bile salts)
• Obstructive jaundice is characterized by elevated serum alkaline phosphatase levels in addition to hyperbilirubinaemia, and may be accompanied by modest elevations in transaminase (aminotransferase) levels, reflecting liver damage.
Liver trauma
After the spleen, the liver is the solid organ most commonly damaged in abdominal trauma, particularly following road traffic accidents. Stab injuries and gunshot wounds of the liver are also increasing in incidence. These are considered in Chapter 7.
Hepatic infections and infestations
Hydatid disease
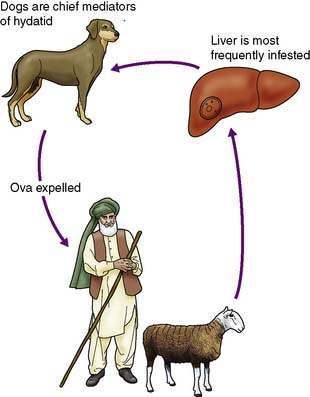
This less common infestation is caused in humans by one of two forms of tapeworm, Echinococcus granulosus and E. multilocularis. The adult tapeworm lives in the intestine of the dog, from which ova are passed in the stool; sheep or goats serve as the intermediate host by ingesting the ova whereas humans are accidental hosts (Fig. 14.7). The condition is most common in sheep- and goat-rearing areas. Ingested ova hatch in the duodenum and the embryos pass to the liver through the portal venous system. The wall of the resulting hydatid cyst is surrounded by an adventitial layer of fibrous tissue and consists of a laminated membrane lined by germinal epithelium, on which brood capsules containing scolices develop.
Portal hypertension
Portal hypertension is caused by increased resistance to portal venous blood flow, the obstruction being prehepatic, hepatic or posthepatic (Table 14.1). Rarely, it results primarily from an increase in portal blood flow. The normal pressure of 5–15 cmH2O in the portal vein is consistently exceeded (above 25 cmH2O). Portal vein thrombosis is a rare cause and is most commonly due to neonatal umbilical sepsis. The most common cause of portal hypertension is cirrhosis resulting from chronic liver disease and is characterized by liver cell damage, fibrosis and nodular regeneration. The fibrosis obstructs portal venous return and portal hypertension develops. Arteriovenous shunts within the liver also contribute to the hypertension.
| Obstruction to portal flow: |
| Prehepatic |
Effects of portal hypertension
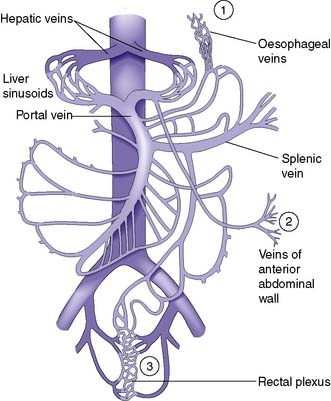
As a result of gradual chronic occlusion of the portal venous system, collateral pathways develop between the portal and systemic venous circulations. Portosystemic shunting occurs at three principal sites (Fig. 14.8). The most important is the development of varices in the submucosal plexus of veins in the lower oesophagus and gastric fundus. Oesophageal varices may rupture, to cause acute massive gastrointestinal bleeding in about 40% of patients with cirrhosis. The initial episode of variceal haemorrhage is fatal in about one-third of patients, and recurrent haemorrhage is common. Bleeding from retroperitoneal and periumbilical collaterals (‘caput medusae’) is troublesome during abdominal surgery, and collaterals may develop and cause bleeding at the site of stomas. Anorectal varices are not uncommonly found at proctoscopy but rarely cause bleeding.
Clinical features
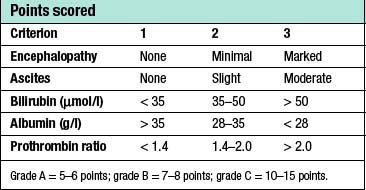
Patients with cirrhosis frequently develop anorexia, generalized malaise and weight loss. Clinical manifestations include hepatosplenomegaly, ascites, jaundice and spider naevi. Slurring of speech, a flapping tremor or dysarthria may point to encephalopathy, and this may be precipitated or intensified by the accumulation of blood in the gastrointestinal tract. The serum bilirubin may be elevated and the serum albumin depressed. Anaemia may be present and the leucocyte count raised (or depressed if there is hypersplenism). The prothrombin time and other indices of clotting may be abnormal. Clinical and biochemical parameters are used as the basis of the modified Child’s classification (Table 14.2). Patients allocated to grade A have a good prognosis, whereas those in grade C have the worst prognosis.
Acute variceal bleeding
Patients presenting with acute upper gastrointestinal bleeding are examined for evidence of chronic liver disease (EBM 14.1). The key investigation during an episode of active bleeding is endoscopy. This allows the detection of varices and defines whether they are or have been the site of bleeding. It is important to remember that peptic ulcer and gastritis are common complaints that occur in 20% of patients with varices.
14.1 Variceal bleeding in cirrhosis: assessment and prophylaxis
Jalan R, Hayes PC, British Society of Gastroenterology. Gut 2000; 46 suppl 3–4:III 1–III 15.
Endoscopy and control of bleeding
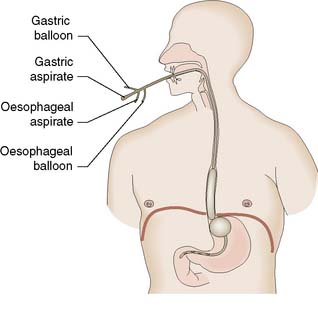
Endoscopy will reveal tortuous varices in three columns most prominent in the lower third of the oesophagus. Haemorrhage usually occurs from varices at the lowest few centimetres of the oesophagus. Rarely, bleeding occurs from varices in the gastric fundus. Although the synthetic form of somatostatin, octreotide, can be used to lower portal venous pressure and arrest bleeding, the injection of a sclerosant such as ethanolamine, or the application of bands is now used to arrest the bleeding at endoscopy (EBM 14.2). If haemorrhage is torrential and prevents direct injection, balloon tamponade may be used to stop the bleeding. The four-lumen Minnesota tube (Fig. 14.9) has largely replaced the three-lumen Sengstaken–Blakemore tube. The four lumina allow:
• aspiration of gastric contents
• compression of the oesophagogastric varices by the inflated gastric balloon
• compression of the oesophageal varices by the inflated oesophageal balloon
• aspiration of the oesophagus and pharynx to reduce pneumonic aspiration.
Prevention of further bleeding
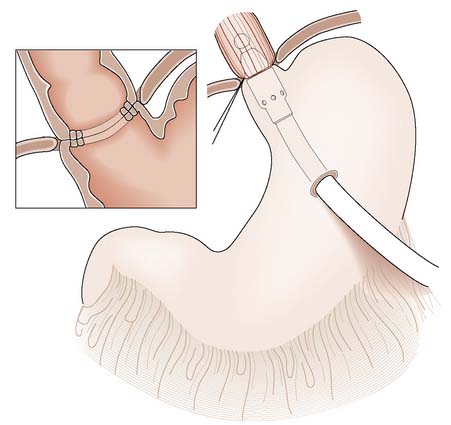
A number of methods are now available to reduce the risk of further variceal bleeding (EBM 14.3). Injection sclerotherapy is repeated at weekly or fortnightly intervals until the varices are completely sclerosed but excessive intervention may cause ulceration and necrosis. Endoscopic banding is favoured since there is a reduced risk of complication. Surgical disconnection (stapled oesophageal transection) is used rarely. The gastric vein and short gastric veins are ligated, and the distal oesophagus is transected and reanastomosed just above the cardia using a stapling gun (Fig. 14.10) to occlude flow into the varices. It carries considerable morbidity and mortality when employed as a last resort in the emergency situation.
14.3 Prevention of further variceal bleeding and infection
Jalan R, Hayes PC, British Society of Gastroenterology. Gut 2000; 46 suppl 3–4:III 1–III 15.
Types of shunt procedure
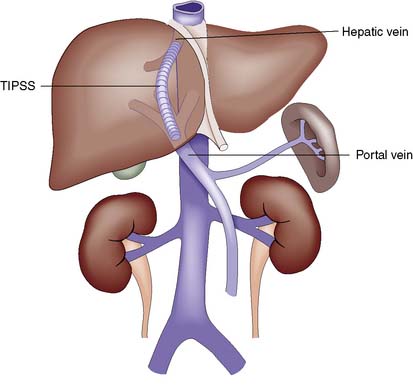
Most portosystemic shunts have been replaced by non-surgical approaches to treatment. In transjugular intrahepatic portosystemic stent shunting (TIPSS, Fig. 14.11), a metal stent is inserted via the transjugular route using a guidewire passed through the hepatic vein to the intrahepatic branches of the portal vein. The technique is a relatively safe means of decompressing the portal system as general anaesthesia and laparotomy are avoided. The risk of encephalopathy is similar to that of a surgical portosystemic shunt, but the procedure is now considered routinely before surgical intervention in both the acute and elective setting.
Summary Box 14.3 Portal hypertension
• Portal hypertension is almost always due to obstruction to portal flow (rather than increased inflow), and may be prehepatic, hepatic or posthepatic
• Cirrhosis of the liver is the most common cause of portal hypertension in developed countries, and alcoholic cirrhosis is most often responsible
• Portosystemic shunts develop between gastric and oesophageal veins, in the retroperitoneum and periumbilical area, and occasionally in the anorectum. Varices in the submucosa of the lower oesophagus are a common source of major bleeding, but gastritis (portal gastropathy) can be responsible
• Child’s grading (A, B or C) is based on encephalopathy, ascites, prothrombin time, bilirubin and albumin levels, and is a valuable prognostic index
• Variceal bleeding may be controlled by endoscopic banding or injection sclerotherapy, although balloon tamponade (four-lumen Minnesota tube) may sometimes be needed
• Surgical portosystemic shunts effectively decompress oesophageal varices and reduce rebleeding, but can cause encephalopathy and have been largely replaced by transjugular intrahepatic portosystemic stent shunting (TIPSS)
• Although banding (or injection sclerotherapy) reduces the risk of rebleeding and may improve survival rates, long-term outcome is determined by the nature and severity of the underlying liver disease.
Ascites
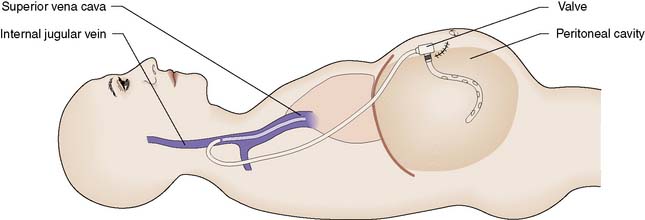
Ascites can be controlled by bed rest, salt and water restriction, and the aldosterone inhibitor spironolactone. If refractory, ascites can be treated by inserting a peritoneojugular (LeVeen) shunt, which allows one-way flow between the peritoneum and the jugular vein (Fig. 14.12). It is unusual for the shunt to remain patent for more than 12 months, but this may suffice for patients with refractory ascites and advanced liver disease who are not candidates for liver transplantation.
Tumours of the liver
Benign hepatic tumours
Focal nodular hyperplasia (FNH)
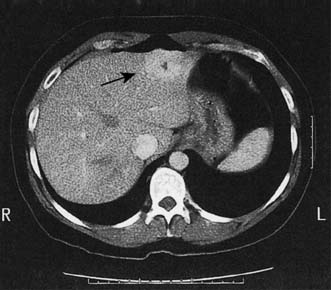
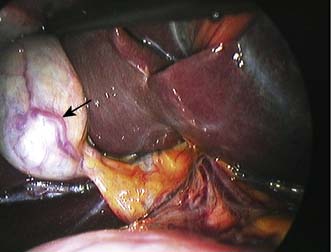
This is more common in females. The lesion is generally asymptomatic and may regress with time or on withdrawal of the contraceptive pill. Hyperplasia can be differentiated from adenoma by the central fibrous scar, which is often visible on ultrasound or CT (Fig. 14.13). Such lesions do not undergo malignant transformation and do not require excision unless symptomatic.
Primary malignant tumours of the liver
Hepatocellular carcinoma (hepatoma)
Investigations
The lesion may be detected and characterized by abnormal ultrasound scanning. Percutaneous needle aspiration cytology and needle biopsy for histological confirmation should be reserved for patients who are not being considered for hepatic resection, as these investigations carry a small but significant risk of tumour dissemination and haemorrhage. There are accepted criteria based on radiology and tumor marker levels that accept a diagnosis of HCC without the need for biopsy (EBM 14.4).
Other primary malignant tumours
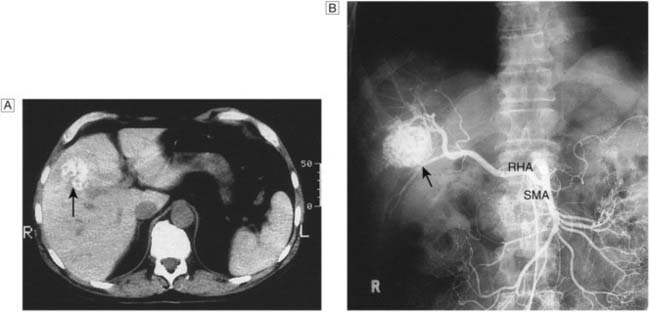
• Angiosarcoma (Fig. 14.14). This rare tumour of the liver may arise after industrial exposure to vinyl chloride or exposure to the previously used radiological contrast medium, Thorotrast
• Haemangioendothelioma. This presents as a diffuse multifocal tumour and is rarely resectable at presentation
• Biliary cystadenoma. This rare condition of the liver, with a marked female predominance, has a 1:4 risk of malignant transformation and should be resected.
The gallbladder and bile ducts
Anatomy of the biliary system
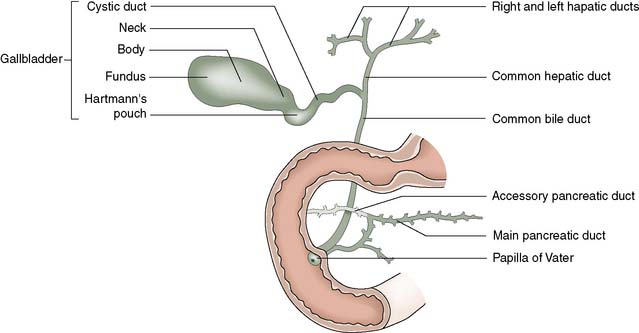
The biliary tree consists of fine intrahepatic biliary radicles that drain individual liver segments before forming the right and left hepatic ducts. The left hepatic duct runs a mainly extrahepatic course and joins the right hepatic duct to form the common hepatic duct. This is joined at a variable position by the cystic duct to form the common bile duct, which ends at the ampulla of Vater, usually in the second part of the duodenum (Fig. 14.15). The common bile duct is approximately 8 cm long and up to 10 mm in diameter. It lies in the free edge of the lesser omentum before passing behind the first part of the duodenum and through the head of the pancreas. It is usually joined by the pancreatic duct just before entering the duodenum.
Physiology
Bile salts and the enterohepatic circulation
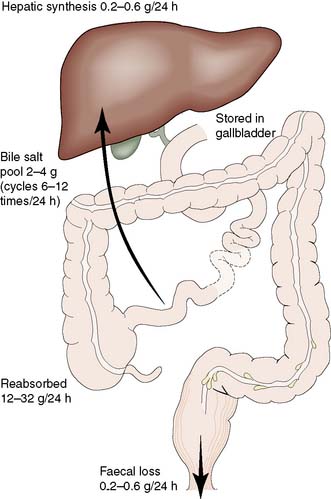
Bile salts can combine with lipids to form water-soluble complexes called micelles, within which lecithin and cholesterol can be transported from the liver. Bile salts are also detergents and a reduction in surface tension allows fat to be emulsified in the intestine, thus facilitating its digestion and absorption. On reaching the distal ileum, 95% of the bile salts are reabsorbed, transported back to the liver and passed once again into the biliary system. This enterohepatic circulation (Fig. 14.16) allows a relatively small bile salt pool (2–4 g) to circulate through the intestine some 6–12 times a day. The daily faecal loss equals that of hepatic synthesis (0.2–0.6 g/24 hrs). When bile is excluded from the intestine, 25% of ingested fat may appear in the faeces and there is marked malabsorption of fat-soluble vitamins, including vitamin K.
Summary Box 14.4 Bile salts
• The primary bile acids, chenodeoxycholic and cholic acid, are conjugated with glycine or taurine and form sodium or potassium bile salts (e.g. sodium taurocholate)
• Bile salts are vital for the excretion of cholesterol in bile; cholesterol is insoluble in water and must be transported in water-soluble complexes (micelles) with bile salts and lecithin
• Bile salts are detergents, and on reaching the intestine they emulsify fat and facilitate the digestion and absorption of fat and fat-soluble vitamins
• Bile salts must not be confused with bile pigments (e.g. bilirubin), which are waste products and excreted in bile. The small bile salt pool (2–4 g) is conserved by reabsorption of bile salts from the terminal ileum
• Disease or resection of the terminal ileum prevents the enterohepatic circulation of bile and is associated with a high incidence of cholesterol gallstones and diarrhoea (owing to the cathartic action of bile salts on the colon).
Congenital abnormalities
Choledochal cysts
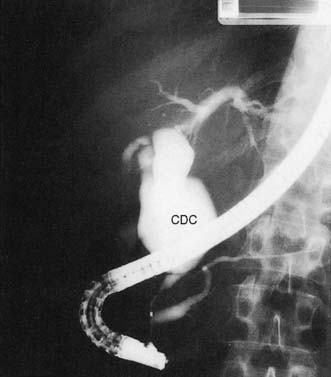
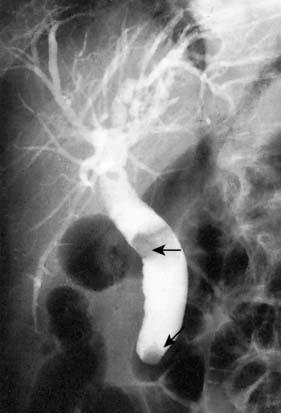
In the neonate, the cyst may present with jaundice or spontaneous perforation. The adult patient usually presents with intermittent pain and jaundice, and may have attacks of pancreatitis. LFTs show a cholestatic pattern, and ultrasonography and cholangiography (MRCP or ERCP) establish the diagnosis (Fig. 14.17). In view of the significant risk of malignant transformation, excision of the cyst is indicated.
Gallstones
Pathological effects of gallstones
Gallstone ileus
Summary Box 14.5 Gallstones
• Most gallstones form because of failure to keep cholesterol in solution. This can result in pure cholesterol stones, but more commonly the stones also acquire a content of bile pigment as they enlarge, forming ‘mixed’ stones
• Pigment stones are the most common type of stone in some Far Eastern countries, but are less common in Western society, where they are associated with chronic haemolysis, biliary stasis and cirrhosis
• Only 15% of stones contain enough calcium to be seen on a plain film
• The majority of individuals with gallstones are asymptomatic and remain so; the presence of gallstones is not in itself an indication for cholecystectomy
• Gallbladder stones may cause flatulent dyspepsia, biliary colic, acute cholecystitis and gallbladder cancer (although the latter is so rare that this consideration does not affect the decision not to treat asymptomatic stones)
• Gallstones that migrate into the bile duct can cause obstructive jaundice, cholangitis and acute pancreatitis, although they often remain asymptomatic
• Gallstone ileus is a rare form of intestinal obstruction; stones large enough to obstruct the gut are usually too large to pass through the ampulla of Vater and have gained access to the gut by an internal fistula involving the gallbladder.
Common clinical syndromes associated with gallstones
Acute cholecystitis
Summary Box 14.6 Acute cholecystitis
• In the great majority of cases acute cholecystitis is associated with gallstones and results from obstruction of gallbladder outflow
• In contrast to biliary colic, which results from obstruction alone, acute cholecystitis is associated with infection and is a systemic illness
• The patient appears unwell, has pyrexia and tachycardia, and is tender in the right hypochondrium; Murphy’s sign is almost always positive
• In 90% of cases, acute cholecystitis will settle with conservative treatment (nil by mouth, intravenous fluids, antibiotics, nasogastric suction if appropriate)
• In 10% of cases, disease progression leads to life-threatening complications: notably, empyema, gangrene and perforation
• Given that the gallbladder is permanently diseased and that complications may supervene, most surgeons now advocate early cholecystectomy (i.e. within 5 days) for acute cholecystitis.
Other benign conditions of the gallbladder
Investigation of patients with suspected gallstones
Ultrasonography
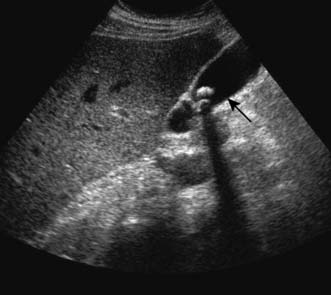
Ultrasonography permits inspection of the gallbladder, its wall and its contents, and demonstrates dilatation of the intrahepatic and extrahepatic biliary tree. Stones reflect the ultrasonic wave and are thrown into prominence by the acoustic shadow they produce (Fig. 14.18). The technique is extremely accurate in skilled hands. As it does not depend on hepatic excretion of contrast, it can be used in both jaundiced and non-jaundiced patients, and therefore has supplanted oral cholecystography.
Surgical treatment of gallstones
Patients with symptomatic gallstones are usually advised to undergo cholecystectomy to relieve symptoms and avoid complications (EBM 14.5). Patients with asymptomatic gallstones are treated expectantly, particularly if they are elderly or suffering from medical conditions likely to increase the risk of surgery. In younger patients, there may be a stronger case for surgery despite the absence of symptoms, particularly if the stones are multiple and likely to cause complications, such as acute pancreatitis.
14.5 Surgical treatment of gallstones
NIH Consensus Statement 1992 Sep 14–16; 10(3):1–20. Kiviluoto T, et al. Lancet 1998; 351:321–325.
Conversion from a laparoscopic procedure to open cholecystectomy should be seen as a limitation of the minimally invasive technique and not as a failure of the surgeon. Laparotomy is mandatory when the anatomy in the area of the cystic duct and artery cannot be defined readily, if uncontrolled bleeding occurs, or if the bile duct is injured (EBM 14.6).
Open cholecystectomy
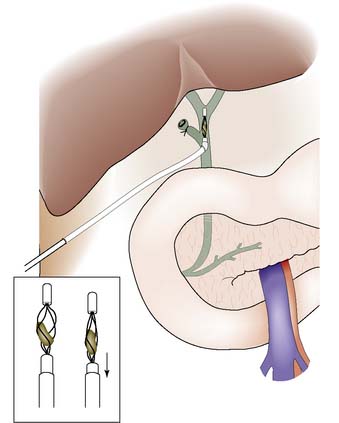
The gallbladder is usually approached through a right subcostal incision. Following careful inspection and palpation of the abdominal contents to exclude other pathology, the cystic duct and artery are identified. Intraoperative cholangiography is performed under image intensification by cannulating the cystic duct and following the injection of contrast. The cholangiogram displays the anatomy of the duct system, identifies ductal stones, and confirms that dye passes freely into the duodenum (Fig. 14.19). The cystic duct and artery are ligated and divided and the gallbladder is removed. A retrograde approach, in which the gallbladder is mobilized ‘fundus first’, can be used when inflammation makes visualization of the biliary anatomy difficult, and in difficult cases a subtotal cholecystectomy may avoid damage of vital structures. Some surgeons pursue a policy of selective cholangiography, obtaining a cholangiogram only in patients at high risk of having ductal stones. The presence of such stones may be suspected if there is a history of jaundice or pancreatitis, if preoperative LFTs are abnormal, or if dilatation of the common bile duct or the presence of multiple gallbladder stones has been detected on ultrasound.
Laparoscopic cholecystectomy
Access to the peritoneal cavity is obtained through three or four cannulae inserted through the anterior abdominal wall and following insufflation of the peritoneal cavity with CO2. The gallbladder is retracted by grasping forceps inserted to display the structures at the porta hepatis. An excellent view of the operating field is obtained with the laparoscope (Fig. 14.20), and the cystic duct and artery are isolated by dissection with instruments passed through the remaining cannulae. Some surgeons have found it difficult to undertake operative cholangiography routinely with this approach, and have either abandoned its use or relied upon MRCP or selective ERCP in the pre- or postoperative period to exclude the presence of common bile duct stones.
Summary Box 14.7 Cholecystectomy
• Cholecystectomy is the standard treatment for symptomatic gallbladder stones; alternatives (stone dissolution, extracorporeal lithotripsy) are now seldom used
• Open cholecystectomy has been largely superseded by laparoscopic cholecystectomy, but conversion to open operation is still sometimes needed
• Cholecystectomy now has a low operative mortality (0.2%); inadvertent injury to the bile duct (0.2% incidence) remains the main source of major morbidity
• Some 5–10% of patients undergoing cholecystectomy have ductal stones, many of which are unsuspected. Opinions vary as to whether intraoperative cholangiography should be undertaken routinely to detect such stones
• In the era of laparoscopic cholecystectomy, there is a growing tendency not to perform routine operative cholangiography, and to extract symptomatic duct stones by non-operative means (i.e. at endoscopic papillotomy)
• If ductal stones cause symptoms, they frequently give rise to cholangitis and Charcot’s triad of pain, jaundice and fever (often with rigors).
Exploration of the common bile duct
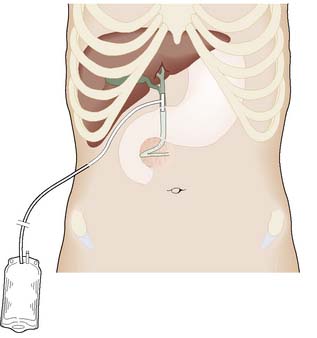
This is undertaken much less often with the free availability of ERCP and sphincterotomy (EBM 14.7). At open surgery, if stones are present in the duct system, the common bile duct is opened between stay sutures (choledochotomy) and the stones are extracted with forceps or a balloon catheter. Following exploration, further check cholangiogram films are obtained, or the interior of the duct can be inspected with a fibreoptic choledochoscope. The opening in the common bile duct is closed around a T-tube, the long limb of which is brought out through a stab incision in the abdominal wall (Fig. 14.21). This serves as a safety valve to allow the escape of bile if there is a temporary obstruction to flow into the duodenum following duct exploration. It also facilitates a T-tube cholangiogram some 7–10 days following surgery. If this shows free flow of dye into the duodenum and no residual duct stones, the T-tube can be clamped before removal.
Complications of cholecystectomy
Infective complications
Wound infection from organisms present in the bile (notably E. coli, Klebsiella aerogenes and Strep. faecalis) can be reduced following cholecystectomy by the intravenous administration of a cephalosporin at the time of induction of anaesthesia, although the routine use of this group of drugs at laparoscopic cholecystectomy has recently been questioned (EBM 14.8). A longer course of antibiotics may be prescribed when significant bile contamination of the peritoneal cavity has occurred at surgery. Collections of bile and/or blood readily become infected after cholecystectomy. Formal drainage may be needed if this progresses to the formation of a subhepatic or subphrenic abscess.
Retained stones
Following bile duct exploration, any retained small stones evident on the postoperative T-tube cholangiogram (see above) may be flushed into the duodenum by irrigating the T-tube with saline. Their passage may be facilitated if glucagon is given to relax the sphincter of Oddi. If the duct cannot be cleared by irrigation, delayed extraction of the stones may be undertaken under radiological control. The patient is discharged with the T-tube in place. This is removed 4–6 weeks later and a steerable catheter passed along its track into the bile duct. A wire (Dormia) basket can be passed along the catheter to catch and withdraw the retained calculus (Fig. 14.22).
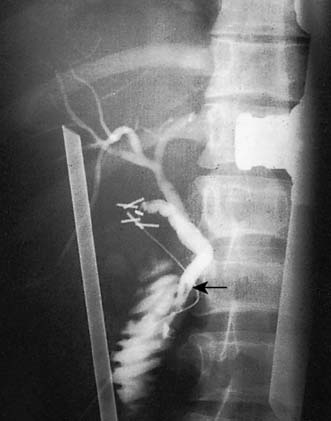
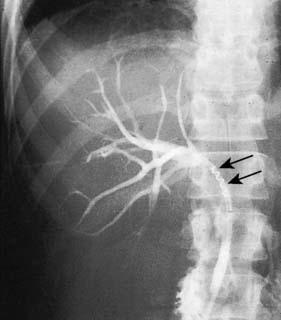
In some patients, unsuspected stones may be left in the bile duct at cholecystectomy. Such stones usually give rise to complications such as jaundice, cholangitis and pancreatitis in the months and years following cholecystectomy. Ultrasonography and MRCP can be used to confirm the presence of such retained stones (Fig. 14.23) and endoscopic retrograde cholangiography and sphincterotomy are performed to recover them (Fig. 14.24). In this technique, a diathermy wire attached to a cannula is passed through the duodenoscope and used to divide the sphincter of Oddi. The stones can then be extracted with a Dormia basket or balloon catheter. This same method can be used to extract stones detected in the immediate postoperative period. If the stones are too large to be withdrawn or the patient is unwell, a stent or a catheter can be left in the biliary system (nasobiliary catheter). The stones can be crushed (lithotripsy) and removed at a later date by means of a repeat endoscopic examination. Surgery may be required to retrieve retained bile duct stones that cannot be dealt in this way.
Bile duct stricture
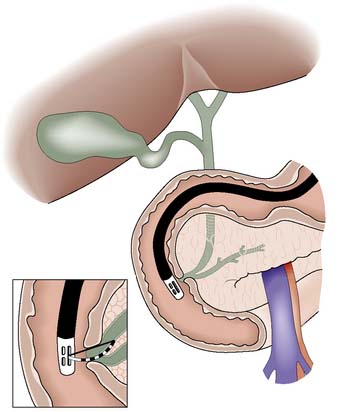
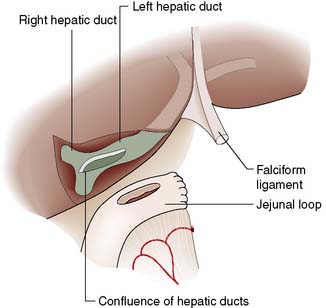
The site and extent of the stricture must be defined radiologically. After ultrasonography has been performed, MRCP, ERCP and/or PTC are undertaken. Reconstructive surgery is carried out in a specialist centre and usually necessitates bringing up a Roux loop of jejunum and anastomosing this to the distended biliary system above the stricture (Fig. 14.25).
Management of acute cholecystitis
Some surgeons delay operation for 2–3 months after the attack in the expectation that the acute inflammatory reaction will have resolved by then, but most now prefer to perform cholecystectomy during the same admission and within 72 hours of the onset of the attack (EBM 14.9). Provided the operation is carried out by an experienced surgeon and under antibiotic cover, ‘early’ cholecystectomy is not associated with an increased incidence of complications. The duration of the illness and hospitalization is reduced, and further attacks of acute cholecystitis during the waiting period for elective surgery are averted. It should be noted that this is a planned procedure carried out after appropriate investigation (ultrasonography) and with all facilities, on a scheduled list. Laparoscopic cholecystectomy is more difficult to perform in the acute setting, but is the method preferred by most surgeons.
Atypical ‘biliary’ pain
More difficulty arises with patients who have attacks of pain consistent with biliary colic but in whom investigations such as ultrasonography, oral cholecystography and ERCP reveal no abnormality. Some of these patients with ‘acalculous biliary pain’ may eventually prove to have non-biliary disease, such as peptic ulceration, chronic pancreatitis or ‘irritable’ colon. In the majority, no explanation for the symptoms can be found, although recent evidence suggests that some may be suffering from a functional disorder of the sphincter of Oddi (Fig. 14.26). Endoscopic manometry may be useful in identifying patients who may benefit from endoscopic sphincterotomy.
Tumours of the biliary tract
Carcinoma of the bile ducts
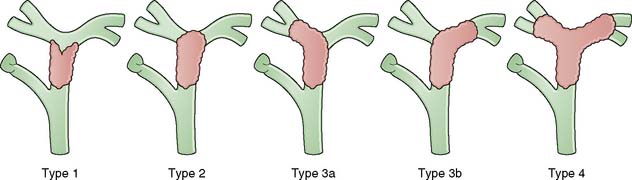
Cholangiocarcinoma is a relatively uncommon cancer that affects the elderly and which is increasing in frequency. Such lesions may arise at any site within the biliary tree and can be multifocal. Tumours can be classified based on the level of involvement of the biliary tree (Fig. 14.27) and are most common at the hilus. Polypoidal tumours are uncommon but carry a more favourable outlook. Sclerotic lesions involving the confluence of the hepatic ducts (Klatskin tumour) pose considerable problems in management. The lesions are said to be slow-growing, but this has been over-emphasized. Cholangiocarcinoma may develop in patients with underlying primary sclerosing cholangitis or choledochal cyst.
Management
Carcinoma of the upper biliary tract is resectable in only 10% of patients, some of whom may require hepatic resection to achieve satisfactory clearance of the tumour. Following resection, the divided intrahepatic ducts are anastomosed to a Roux limb of jejunum. Operative mortality is reported in as many as 10% of patients. In the majority of patients not submitted to resection, palliation can be achieved by insertion of a stent by endoscopic or percutaneous transhepatic techniques (Fig. 14.28). Most stents are liable to occlusion, exposing the patient to repeated attacks of cholangitis and/or jaundice. Quality of life is poor and few patients with cholangiocarcinoma survive for more than 18 months. The role of systemic chemotherapy and/or radiotherapy has yet to be established.