12 The limbic system
Anatomical components of the limbic system
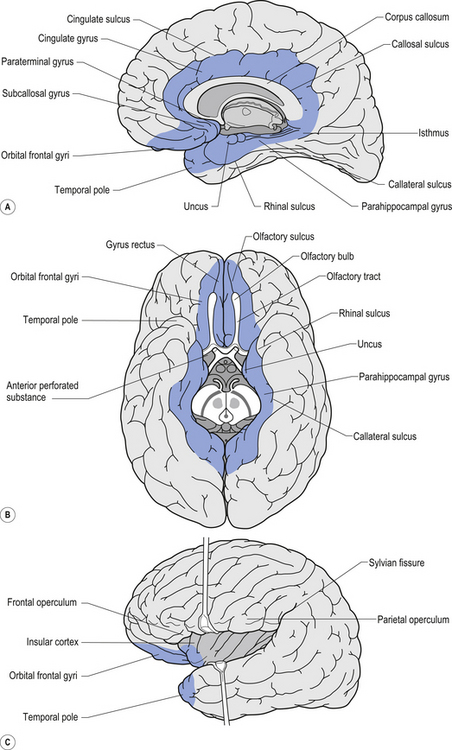
The exact components of the limbic system remain open for debate. For our purposes in this text the following structures will be considered as components of the limbic system (Figs 12.1 and 12.2):
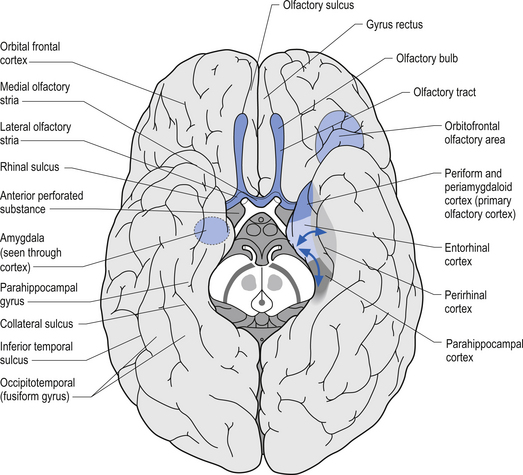
The olfactory bulb, tract, and cortex
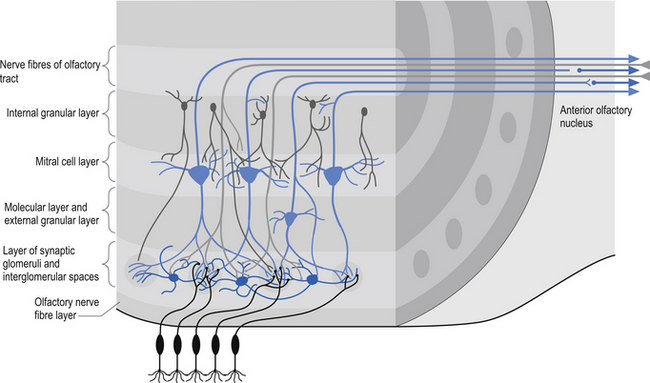
The olfactory bulb and tract develop from ectodermal extensions of the anteromedial part of the primitive cerebral hemisphere. The bulb is radial in structure with six defined layers, which include, from the surface to the central core (Fig. 12.3):
1. The olfactory nerve fibre layer;
2. The area of synaptic glomeruli;
3. The molecular and external granule layers;
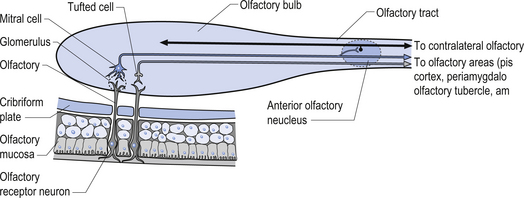
The olfactory nerves (CN I) emerge as the axons of the olfactory cells of the nasal mucosa. These axons are then collected into complex intercrossing bundles, which gather into about 20 nerve axon bundles that traverse the cribriform plate of the ethmoid bone to synapse in the glomeruli of the olfactory bulb (Fig. 12.4). The olfactory epithelium is bathed in lipid substance produced by Bowman’s glands that dissolves particles in the air and makes them accessible to the receptors of the olfactory nerves.
The glomeruli of the olfactory bulb are composed of a complex of axons, dendrites, and neurons including the dendrites of external granule, mitral and tuft cells, and axons from the contralateral olfactory bulb and corticofugal fibres supplying descending modulation. The axons of the mitral and tuft cells course centrally through the olfactory tract to the anterior olfactory nucleus where some axons synapse and others project collaterals but continue with the postganglionic axons to form the olfactory stria. The axons continue posteriorly and divide into the medial and lateral olfactory stria at the junction of the anterior perforated substance, circumventing the olfactory tubercle. The lateral olfactory stria project to the anterior perforated substance, the piriform lobe of the cerebrum, the lateral olfactory gyrus, and the corticomedial group of amygdaloid nuclei. This group of structures is referred to as the primary olfactory cortex (Fig. 12.5). The medial olfactory stria give collaterals to the anterior perforated substance. Some fibres cross the midline via the anterior commissure and synapse in the contralateral anterior olfactory nucleus. These fibres are the only sensory fibres known to reach the cortex without synapsing in one of the thalamic nuclei. The entorhinal area of the parahippocampal gyrus, which forms the caudal area of the piriform lobe, is considered the secondary olfactory cortex. The secondary olfactory cortex mediates emotional and autonomic reflexes associated with smell, along with the hypothalamus. The primary and secondary olfactory cortical areas are responsible for the human perception of smell.
The amygdala
The amygdala, so named because it resembles an almond in shape, is also referred to as the amygdaloid body or the amygdaloid nuclear complex. The amygdala is composed of groups of neurons and their associated nerve fibres in the dorsal medial part of the temporal lobe. The amygdaloid complex is composed of two main groups of nuclei: the corticomedial or basomedial circuit and the basolateral circuit. The corticomedial circuit is composed of the central, medial, and cortical amygdaloid nuclei, the nucleus of the lateral olfactory stria, and the anterior amygdaloid area. The corticomedial division contains irregular groups of pyramidal and granule neurons that resemble a rudimentary cortical structure. The basolateral circuit includes the lateral, basal, and accessory basal amygdaloid nuclei and through its transitional zone is continuous with the parahippocampal gyrus. The amygdala receives projections from the anterior olfactory nucleus, the olfactory bulb, the lateral olfactory stria, various hypothalamic nuclei, various thalamic nuclei, the reticular formation of the brainstem, and a number of areas of cortex. Some neurons of the amygdala project via the stria terminalis to the septal areas and preoptic areas of the hypothalamus, while others project their axons via the amygdalofugal fibres to many hypothalamic nuclei, the medial dorsal nucleus of the thalamus, and the mesencephalic reticular formation (Williams & Warwick 1982).
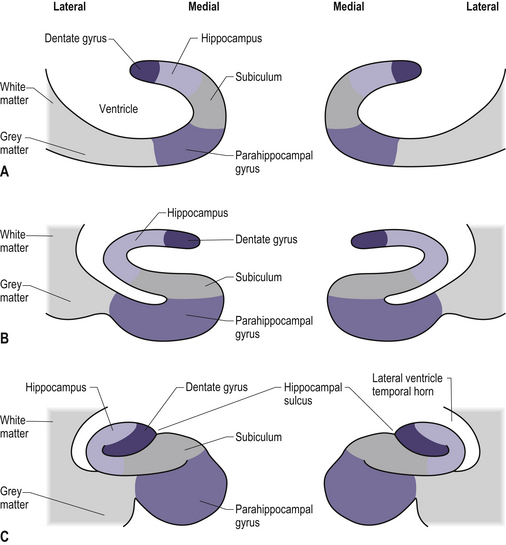
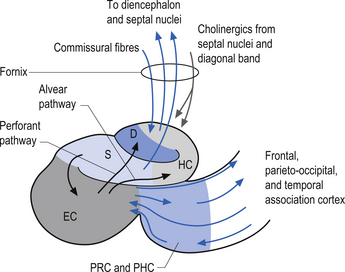
The hippocampal formation
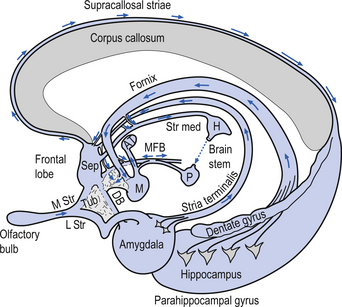
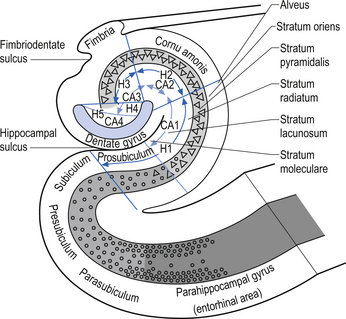
The hippocampal formation is composed of a curved column of phylogenically ancient brain called the archipallium (Fig. 12.6). The hippocampal formation includes the hippocampus proper, the subiculum, and the dentate gyrus. Following the structure of the archipallium from a central point in the dentate gyrus in a radial clockwise fashion, the archipallium can be divided into three zones: the dentate gyrus, the cornu ammonis, and the subiculum (Fig. 12.7). The dentate gyrus and cornu ammonis display the three-layered cortical structure of the ancient cortices; the subiculum demonstrates a gradual shift from four layers to six layers through its length (Fig. 12.8). The hippocampal formation includes the following structures:
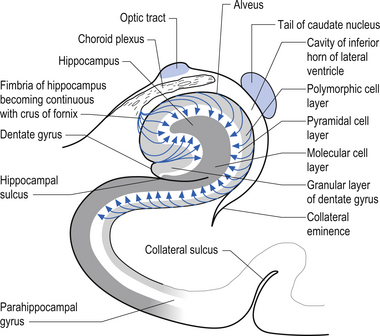
Afferent projections received by the hippocampus include (Fig. 12.9):
The hippocampus has several important functions including a primary role in the acquisition of associative behaviour. It is also involved in the identification of contiguity between spatial and temporal events through memory recall mechanisms (McIntosh & Gonzalez-Lima 1998).
Disorders of temporolimbic function
The hypothalamus, amygdala, and prefrontal cortex are the critical neural structures implicated in the expression of aggression (Weiger & Bear 1988). The ventromedial hypothalamic area, in particular, mediated primarily via cholinergic neurotransmission, has been associated with predatory-type aggression. Bilateral lesions in this area may lead to aggressive outbursts referred to as hypothalamic rage that are provoked by frustrated attempts to satisfy basic drives such as hunger, thirst, and sexual need. Hypothalamic rage typically involves outbursts of simple behaviours such as kicking, biting, scratching, or throwing objects and is usually a wild, lashing out response not directed against specific targets (Reeves & Plum 1969). After the event, patients may express remorse and exhibit some insight about their uncontrolled impulses. Aggressive behaviour is also the hallmark of intermittent explosive disorder, which likely involves frontotemporolimbic circuitry. Patients with this disorder present with impulsive loss of behavioural control episodes, in response to minimal provocation which often leads to serious violence. The prefrontal cortex is intimately connected with the hypothalamus and the amygdala and is essential in the hierarchy of aggression control. Orbitofrontal lesions frequently lead to affective disinhibition, most commonly irritability with angry outbursts but also including silliness, euphoria, loud behaviour, and interpersonal and social disinhibition (Lichter & Cummings 2001). The actions of individuals that exhibit aggressive behaviour and violent criminal activity resulting from disinhibited frontal lobe syndromes are usually impulsive, and lack elaborate planning and consideration of consequences. The actions are usually simple in nature and committed without remorse. Abnormalities in prefrontal and subcortical circuitry may also underlie the aggressive and violent acts seen in patients with antisocial personality disorder. Aggressive behaviour has also been linked to disturbed neurotransmission involving specific neural circuitry in the limbic system. Serotonergic, noradrenergic, dopaminergic, and GABA-ergic circuits have all been identified as playing a role in modulating behaviours including aggressive behaviour. The most consistent findings relate to the serotonergic system. Both high and low concentrations of serotonin levels have been implicated in abnormal behaviours. For example, low serotonin levels have been found in the cerebrospinal fluid of people who have attempted suicide and of those who have successfully completed suicide (Virkkunen et al. 1995).
As outlined above, the amygdala receives sensory input from various cortical areas and projects to the hypothalamus and temporolimbic cortex via the ventral amygdalofugal pathway and stria terminalis (Othmer et al. 1998). This connectivity provides a neural mechanism whereby external stimuli receive emotional colouring. Hence, a limbic hyperconnection syndrome may account for the heightened emotional responsivity that is part of the rare and controversial behavioural syndrome, the Gastaut-Geschwind syndrome, which is characterised by dysfunction in three distinct areas of psychosocial interactions including heightened emotional responses, exaggerated behaviours, and lability in physiological drives. The altered emotional responses include periods of intense metaphysical preoccupation with hyperreligiosity, and exaggerated philosophic or moral concerns. They may also experience changes in affect such as depression, paranoia, or irritability. Their behavioural ’viscosity’ manifests as exaggerated verbal, motor, and writing behaviours, nonrational adherence to ideas, interpersonal adhesiveness with prolonged encounters, obsessive preoccupation with detail, excessive need to collect background information, and copious description of thoughts and feelings with a moral or religious twang (Trimble et al. 1997).
Finally, they experience prolonged and powerful alterations of physiological drives resulting in hyposexuality, aggression, and fear responses (Lichter & Cummings 2001).
The amygdala theory of autism
It has been proposed that in humans a network of neural regions may comprise the ’social brain’; this network includes the amygdala. Since the childhood psychiatric condition of autism involves deficits in ’social intelligence’, it has been proposed that autism may be caused by an amygdala dysfunction that results in deficits in social behaviour in these individuals. Recent studies involving the use of functional magnetic resonance imaging (fMRI) found that patients with autism did not activate the amygdala when making mentalistic inferences from the eyes, whilst people without autism did show amygdala activity. The amygdala is therefore proposed to be one of several neural regions that are abnormal in autism (Baron-Cohen et al. 2000).
Learning and memory
Implicit memory, which is the memory we use to perform a previously learned task and does not require conscious recall, and includes various types of memory such as procedural, priming, associative, and nonassociative. Procedural memory is involved with recall of how to perform previously learned skills or habits. It is thought to rely heavily on areas of the striatum. Priming is a type of memory in which the recall of words or objects is enhanced with prior exposure to the words or objects. This type of memory utilises neocortical circuits. Associative learning or memory involves the association of two or more stimuli and includes classical conditioning and operant conditioning (Kandel et al. 2000).
Classical conditioning involves the presynaptic facilitation of synaptic transmission that is dependent on activity in both pre- and postsynaptic cells. The neuron circuit learns to associate one type of stimulus with another. When stimuli are paired in this manner the result is a greater and longer-lasting enhancement. For this form of activity-dependent facilitation to occur, the conditioned and unconditional stimuli must occur at closely spaced intervals in time. Influx of calcium in response to action potentials in the conditional stimulus pathway leads to potentiation of the stimulus by binding of activated calcium/calmodulin to adenylyl cyclase. This occurs in a fashion similar to sensitisation due to serotonin release described above. Adenylyl cyclase acts as a coincidence detector, recognising molecular response to both a conditioned stimulus and an unconditional stimulus present simultaneously or within a required space of time. The postsynaptic component of classical conditioning is a retrograde signal to the sensory neurons that potentiation of the stimulus has indeed occurred.
1. Activation of adenylyl cyclase by calcium influx (conditioned);
2. Activation of serotonergic receptors coupled to adenylyl cyclase (unconditioned); and
3. Retrograde signal indicating that the postsynaptic cell has been adequately activated by the unconditioned stimulus.
Long-term storage of implicit memory involving both sensitisation and classical conditioning involves the cyclic AMP (cAMP)–protein kinase A (PKA)–mitogen-activated protein kinase (MAPK)–cAMP response element-binding protein (CREB) pathway (see Chapter 3).
Nonassociative learning or memory occurs when a person or neuron is exposed to a novel stimulus either once or repeatedly. This type of learning or memory involves the neurophysiological processes of habituation and sensitisation of synaptic function, which are important processes in nonassociative learning and memory (Kandel et al. 2000).
1. Episodic memory, which involves the recall of events in time and space; and
2. Semantic memory, which involves the recall of facts, words, names, and meanings.
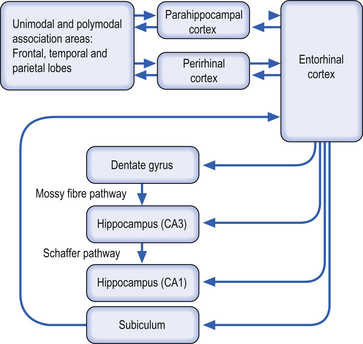
Explicit memory involves the process of long-term potentiation of synapses in the hippocampus. The entorhinal cortex acts as both the primary input and primary output of the hippocampus in this process. The unimodal and polymodal areas of association cortex project to the parahippocampal gyrus and the perirhinal cortex, which both project to the entorhinal cortex. Information then flows from the entorhinal cortex to the hippocampus in three possible pathways including the perforant, the mossy fibre, and the Schaffer collateral pathways (Kandel et al. 2000).
The perforant pathway projects from entorhinal cortex to granular cells of the dentate gyrus. This is the primary conduit for polymodal information from the association cortices to the hippocampus. The mossy fibre pathway, which contains axons of the granule cells and runs to the pyramidal cells in the CA3 region of hippocampus, is dependent on noradrenergic activation of beta-adrenergic receptors, which activate adenylyl cyclase. This pathway is nonassociative in nature and can be modified by serotonin. The Schaffer collateral pathway consists of excitatory collaterals of the pyramidal cells in the CA3 region and ends on the pyramidal cells in the CA1 region. Long-term potentiation in the Schaffer collateral and perforant pathways is associative in nature (Fig. 12.10).
Memory processing can be divided into four processes which include:
1. Encoding—the process in which new material is attended to. The degree to which attention is allotted to the new information is directly related to the strength of the memory.
2. Consolidation—processes that make the memory more stable; includes expression of genes and synthesis of proteins in the neuron. Loss of consolidation of a memory occurs because of inhibition of mRNA or protein synthesis to block long-term memory selectively. Consolidation involves three processes which include:
3. Storage—the process of solidifying the memory into long-term memory which seems to have unlimited storage capacity.
4. Retrieval—the process in which the memory is brought back to conscious awareness and is dependent to some extent on a working short-term memory circuit called the attentional control system.
Case 12.2
12.2.1
The components of the limbic system include:
• reticular formation of the brainstem
• tegmentum of the mesencephalon
The hypothalamus, amygdala, and prefrontal cortex are the critical neural structures implicated in the expression aggression (Weiger & Bear 1988). The ventromedial hypothalamic area, in particular, mediated primarily via cholinergic neurotransmission, has been associated with predatory-type aggression. Bilateral lesions in this area may lead to aggressive outbursts, referred to as hypothalamic rage, that are provoked by frustrated attempts to satisfy basic drives such as hunger, thirst, and sexual need. Hypothalamic rage, typically involves outbursts of simple behaviours such as kicking, biting, scratching or throwing objects and is usually a wild, lashing out, response not directed against specific targets (Reeves & Plum 1969). After the event, patients may express remorse and exhibit some insight about their uncontrolled impulses. Aggressive behaviour is also the hallmark of intermittent explosive disorder, which likely involves frontotemporolimbic circuitry.