6 The knee
Introduction
Before magnetic resonance (MR) imaging became readily available in the 1990s, comprehensive imaging of the frequently injured internal structures of the knee joint was primarily accomplished with arthroscopy. In comparison to MR imaging, this procedure is both invasive and expensive and, in modern clinical practice, arthroscopies are no longer routinely performed; they do, however, still have a role in clarifying inconclusive findings and, of course, for therapeutic benefits. Magnetic resonance is now considered the gold standard for intra-articular imaging of the knee1,2; indeed, the knee is the joint most commonly imaged using this modality, partly because the joint is particularly vulnerable to both acute and chronic injury and partly because of the accuracy and detail afforded to both the surgeon and the physician.3,4
Since the first successful nuclear resonance experiment on biological tissue in 1946,5 there have been tremendous improvements in equipment, computer software, protocols and clinical interpretation. These advances have helped to improve image quality and diagnostic accuracy (including the appearance of normal variants) and reduce the effects of imaging artifacts.
History and examination
Perhaps more than any other joint, the knee has an embarrassment of recognized orthopaedic tests, usually eponymous and often of unproven sensitivity and reliability6,7; however, a standard examination routine need contain no more than a handful of such tests if the history and examination are properly structured.
As with any conscious and articulate patient, obtaining a full and competent history should form the backbone of the clinician’s diagnostic work-up. There are several factors that are of particular importance in the knee; these are detailed in Box 6.01 and should be capable of guiding the physician to the most likely involved structures, informing their differential diagnosis and guiding their physical examination.8,9
This should commence not with the knee itself but with general observation of the patient and their height, weight and somatotype, with calculation of the body mass index (BMI) if necessary; more than in any other joint, the incidence of degenerative change in the knee is linked to obesity.10–14 Evaluation of gait can give a good indication of the severity of the injury and the degree of disability, and assessment of stance can direct the clinician to asymmetries of alignment; genu varum or genu valgum; and tibial torsion.9 Pes planus, which can significantly increase valgus knee stress, can also be readily identified at this stage. This observation can be extended to the local area about the knee, wherein any discoloration, swelling, atrophy or displacement should be noted and investigated further.15
This should be augmented by palpation, which should assess any areas of abnormal temperature and the quality of any swelling as well as attempting to elicit specific site tenderness and identify trigger points; in particular, the hypertonic popliteus muscle is a commonly overlooked cause of pain and perpetuation of knee pathomechanics.16 The femoral, popliteal and dorsalis pedis pulses should all be checked and compared. Many conditions, such as vascular disease, bursitis, tendonitis, ligamentous sprain, patellar subluxation, Baker’s cyst, neuroma and some osteochondral defects, can be identified, if not positively confirmed, by history, observation and expert palpation, and local joint line tenderness has been shown to be a sensitive and reliable test for determining a meniscal lesion.17
In the younger patient, where symptoms allow, there is a range of functional tests that can be performed; these are particularly useful in assessment of the high-performance athlete with persistent yet subtle lesions and of knee laxity or instability9 and are detailed in Table 6.01. In the less able patient, active and passive ranges of motion will suffice: flexion, extension plus valgus and varus stress, which can best assess collateral ligament injury; these should be compared to the unaffected side. Movement of the knee may also elicit clicking, popping or grating, suggestive of a foreign body or meniscal tear; this can be further assessed using McMurray’s test, which consists of flexing and extending the knee in neutral, then with internal and external tibial rotation whilst the thumb and fingers are placed over the joint line.
| Test | Ask the patient to… |
|---|---|
| Stationary jog | Jog gently on the spot |
| Fast jog | Increase the tempo and increase their knee lift |
| ‘Housemaid’s knee’ | Kneel upright |
| ‘Parson’s knee’ | Kneel whilst leaning backwards |
| ‘Duck waddle’ | ‘Walk’ whilst fully squatting |
| Hopping | Hop on the affected limb with the toe pointing inwards and then outwards |
Special tests are also needed to assess the cruciate ligaments. The more commonly injured anterior cruciate ligament is evaluated using the modified Lachman’s test whereby the supine patient lies with their hip and knee flexed to approximately 20° and the examiner, thumbs on the proximal tibia, draws the distal extremity anteriorly; instability is noted if the excursion is greater than on the unaffected side. The posterior drawer test is used to assess the posterior cruciate ligament and involves the same position for both patient and examiner but this time the tibia is drawn posteriorly.9
Although there is a plethora of special tests and measurements for assessment of the patella in the patient with patellofemoral pain syndrome, many of these are of dubious or, at best, unproven value.18 A competent assessment of the patella consists mainly of observation of the structure both statically and dynamically. Conditions involving frank abnormalities of the patella such as luxation, patella alta, patella baja or agenesis are usually immediately obvious19; palpation can also readily determine bipartite patellae, the absence of pain or previous trauma will usually be sufficient to differentiate this from fracture or non-union.
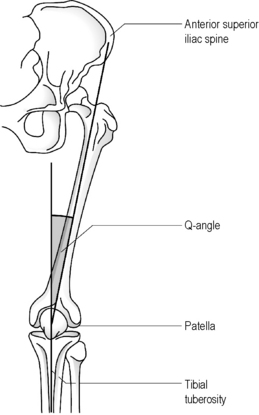
Although radiographs may be required to precisely determine the quadriceps angle or Q-angle (Figure 6.01), the experienced practitioner should be able to determine when this falls outside the normal range of 10–15° for men and 15–23° for women9,20,21; an increased Q-angle is strongly linked to patellofemoral pain.20
Assessment of muscle bulk can also be a key indicator of patellar tracking syndrome. Unless bilateral MR images have been acquired, this assessment will not generally be aided by specialist imaging. Although protocols have been developed for kinematic MR imaging, enabling the patella motion to be captured during early flexion, the expense involved coupled with technical difficulties in reliably acquiring the sequences have severely restricted the application of MR imaging to patellar tracking assessment.18,22
The diagnosis can best be checked by the squat test; in healthy subjects the patella will rotate medially at 90° of knee flexion, whilst in patient with abnormal patellar function the rotation will be lateral.23 During the squat, it is also possible to ascertain and, with practice, source any crepitus or clunking which may be associated with patellar subluxation or degeneration or with intra-articular pathology.9,15
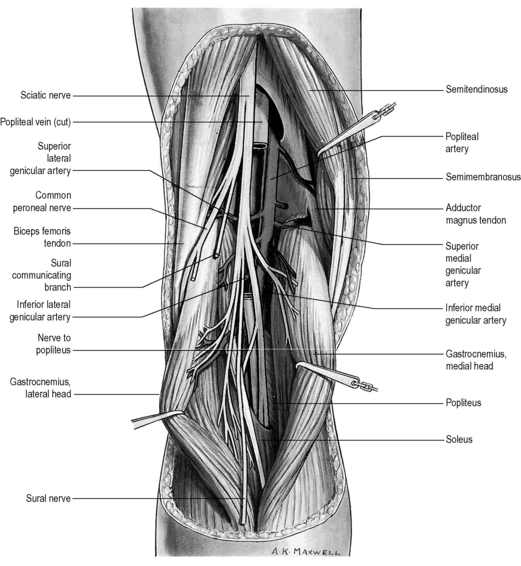
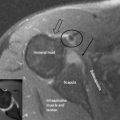
Finally, any assessment of the knee articulation should include a thorough neurological assessment24; not only can lumbar radiculitis refer pain to the knee, it can also disrupt the functionality of the major muscle groups attaching about the joint. Local nerve damage also needs to be eliminated; the peroneal nerve is vulnerable as it passes superficially over the head of the fibula and the popliteal fossa houses the tibial, common peroneal, sural and posterior femoral cutaneous nerves as well as an articular branch from the obturator nerve (Figure 6.02).25–27
Differential diagnosis
Several challenges face the clinician seeking to establish and refine a differential diagnosis for the patient with knee pain. Although the history can give strong clues from the mechanism of injury, the onset of pain can often be insidious or inconclusive: the symptoms can often be due to dysfunction elsewhere in the kinematic chain either from biomechanical consequence or by referred pain. This latter is particularly crucial when considering paediatric cases; both slipped capital femoral epiphysis and Legg–Calvé–Perthes disease can present as knee pain with no hip symptoms – and the cost of a missed diagnosis can be serious for the patient. The age of the patient should help to direct the clinical thinking.28–30
Knee pain can also be a symptom of systemic disease: crystal disease, or inflammatory or septic arthropathy. A list of common conditions causing knee pain is given in Table 6.02.28,31 As well as the history, the location of the knee pain should also help to guide and clarify the clinician’s thinking; a list of conditions that can be suggested by location alone is given in Box 6.02.28
| Children and adolescents | Adults | Older adults |
|---|---|---|
| Patellofemoral pain syndrome | Patellofemoral pain syndrome | Osteoarthritis |
| Osgood–Schlatter disease | Medial plica syndrome | Crystal-induced inflammatory arthropathy: gout, calcium pyrophosphate crystal deposition disease (pseudogout) |
| Jumper’s knee (patellar tendonitis) | Pes anserine bursitis | Popliteal cyst (Baker’s cyst) |
| Referred pain: slipped capital femoral epiphysis, Legg–Calvé–Perthes disease | Trauma | Neoplasm: metastatic cancer |
| Osteochondral defect | Inflammatory arthropathy: rheumatoid arthritis, Reiter’s syndrome (reactive arthritis), pigmented villonodular synovitis | Trauma |
| Juvenile rheumatoid arthritis (Still’s disease) | Tendonitis/bursitis | Synoviochondrometaplasia |
| Trauma | Septic arthritis | |
| Neoplasm: Ewing’s sarcoma, osteosarcoma | Stress fracture/Stress reaction | |
| Referred pain: neurogenic, hip and leg pathology | ||
| Neoplasm: osteochondroma, aneurysmal bone cyst |
Protocols
Most imaging protocols for the knee include sagittal, axial and coronal images with the field of view ranging from 14 cm to 16 cm, depending on patient size. The slice thickness usually varies from 3 mm to 5 mm with an interstitial gap of about 0.5 mm. Gaps, or areas of non-imaged anatomy in between the slices, are used to decrease ‘cross-talk’ in two-dimensional imaging acquisition;32–35 cross-talk is the presence of unwanted signals coming from adjacent anatomy.
Normal anatomy and common variants
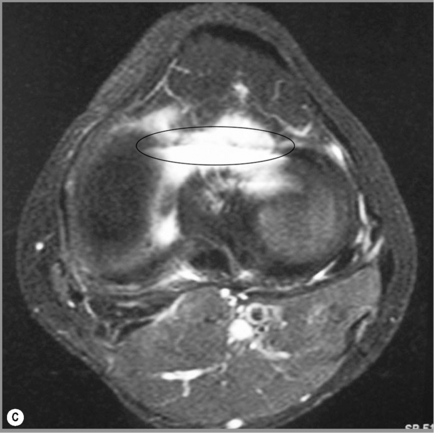
The knee is often described as a synovial hinge joint;27,36,37 however, this is a gross simplification from both an anatomical and biomechanical perspective; the femorotibial joint is the largest in the body and also one of the most complicated (Figure 6.03). The presence of the menisci make the joint complex and, of course, the knee consists of more than just this single joint; the proximal tibiofibular joint, a synovial plane joint, and the patellofemoral articulation make the knee, functionally, a compound joint, most correctly defined as a bicondylar joint.25,38,39 Unlike a true hinge joint, the knee demonstrates limited rotation about two other orthogonal axes, it can increase its valgus and varus angles passively and is reliant on femoral rotation to obtain its highly stable close packed position.38,39
In general, the menisci can be evaluated using single echo proton density images. Spin echo T1-weighted images and gradient echo techniques can also be very useful. When assessing ligaments, tendons or other soft tissues, the preferred techniques will be fluid-sensitive, or T2-weighted, sequences. Hyaline cartilage and loose bodies can be best evaluated using gradient echo techniques, with or without fat suppression, and fat-suppressed spin echo sequences.32–34
Menisci
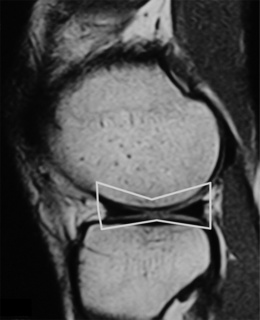
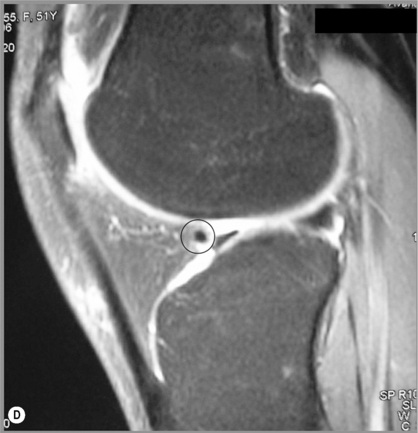
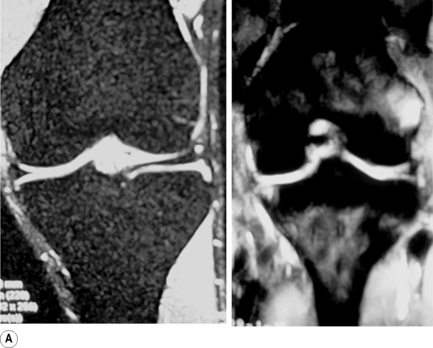
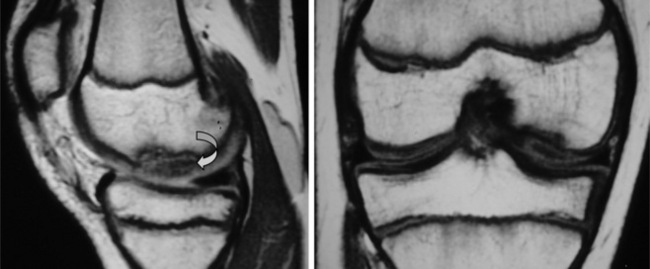
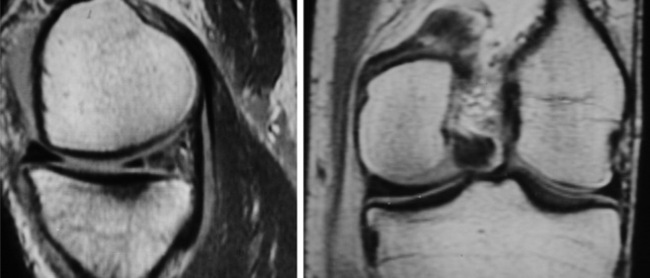
The menisci of the knee are well visualized and commonly evaluated with MR imaging. The normal meniscus is a semicircle of cartilage and collagen fibres, thicker peripherally and thinner centrally; they display low signal intensity on all sequences (Figure 6.04).
The medial meniscus is slightly bigger and has a larger radius than its lateral counterpart. The ‘horns’ of the latter are usually of equal size; in the medial meniscus, the anterior horn is larger than the posterior.3,25,27
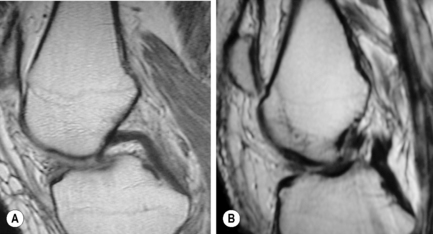
On the sagittal slices, each meniscus has a ‘bow-tie’ appearance (Figures 6.05, 6.06). Towards the midline, the menisci have the appearance of two triangles.32–34,40 In some individuals, the morphology of the meniscus is more representative of a disc. ‘Discoid’ menisci are variable in appearance; many are not true discs but merely have wider-than-normal bodies.41,42 The incidence of discoid meniscus is estimated at being between 3% and 4.5%; they are nearly always found in the lateral meniscus.43–45 On the sagittal views, the meniscus will not demonstrate the typical ‘bow-tie’ appearance; the pathological implication of this morphological variant will be discussed later in the chapter.
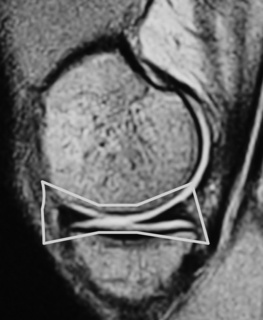
Figure 6.06 • Sagittal T1-weighted MR image of the right knee of a 27-year-old male at the level of the medial compartment demonstrates the ‘bow-tie’ appearance of the medial meniscus. Notice the wider configuration of the medial meniscus compared with that of the lateral meniscus noted in Figure 6.05.
Intrasubstance signal may be present on the peripheral edge, representing vascularity; this finding is more prominent in younger patients and may be mistaken for a tear. The popliteus tendon adjacent to the lateral meniscus and the normal attachments of the meniscofemoral ligaments on both the medial and lateral side are other structures occasionally mimicking tears.32–35
A common normal variant that can mimic pathology is a ‘speckled’ anterior horn lateral meniscus. Present in as many as 60% of normal patients, this appearance is due to fibres of the anterior cruciate ligament inserting into the meniscus; it can present an appearance similar to that of a macerated anterior horn.46
Ligaments
Cruciate ligaments
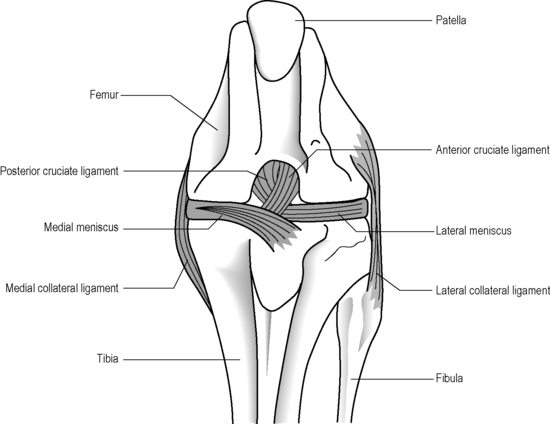
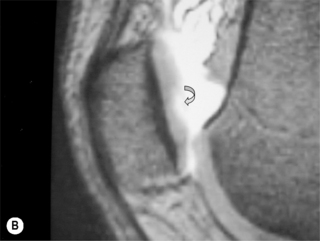
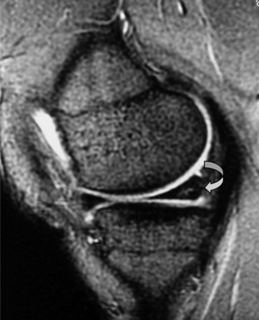
The anterior and posterior cruciate ligaments are two other structures very commonly evaluated with MR imaging. Both ligaments are intra-articular but extrasynovial structures linking the femur and tibia (Figure 6.07).25,36
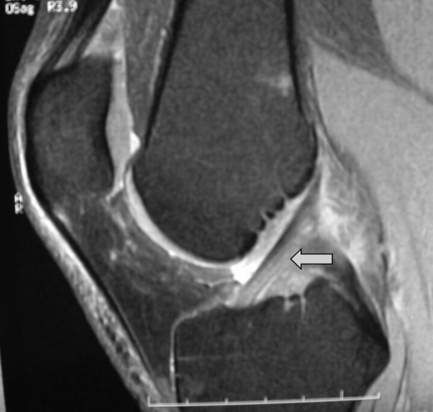
The anterior cruciate ligament (ACL), which is frequently injured during athletic activities, is a very important structure providing stability to the knee and preventing anterior translation of the tibia. It also prevents excessive internal rotation and hyperextension. The ACL attaches at the posterior aspect of the lateral femoral condyles and on the medial anterior aspect of the tibial plateau. It does not, contrary to popular belief, insert on the tibial spines.25,27 The ACL is best visualized on sagittal images (Figures 6.08, 6.09). On some scans, the small anteromedial and larger posterolateral fibre bundles can be differentiated, especially if the patient’s lower extremity is placed with approximately 5° of external rotation in order to align the fibres with the plane of the scan; this can give the structure a striated appearance.3 On the coronal slices, the ACL appears as a flattened structure adjacent to the lateral femoral condyles. There can often be some high signal within the ligament, most particularly near its insertion on the tibia.2
In approximately 1% of cases, the ligament can contain a cyst; whilst normally clinically insignificant – if sufficiently large, it can occasionally cause a feeling of stiffness – this can mimic the appearance of a tumour.47–49
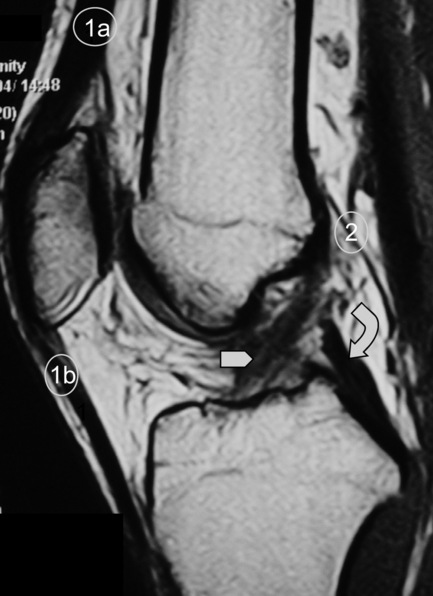
The posterior cruciate ligament (PCL) is seen in all MRI planes and sequences as a band of low signal intensity. It is seen in its entirety on the sagittal slices, appearing as a thick fibrous band originating from the medial aspect of the femoral condyle. From the condyle, it extends inferiorly to the posterior aspect of the tibial plateau, taking the appearance of an ‘inverted hockey stick’ (Figure 6.10A).50 Injury to the PCL, which classically occurs as a result of an anterior-to-posterior force applied to the proximal tibia, such as may occur with contact of the femur against the dashboard in an automobile accident, is relatively rare in comparison to ACL injuries.32–34
Collateral ligaments
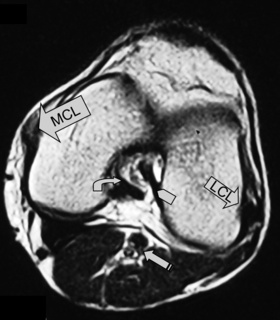
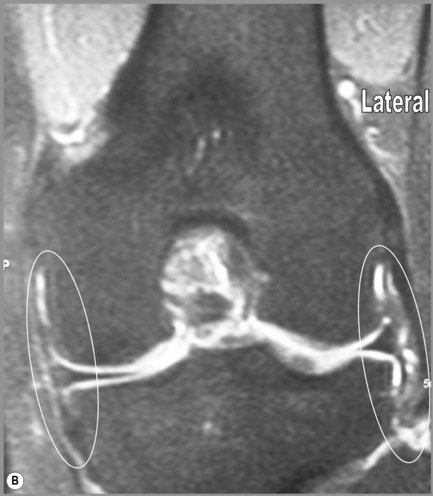
The medial (tibial) and lateral (fibular) collateral ligaments are slightly more difficult to evaluate than the cruciate ligaments. The medial collateral ligament (MCL) is a flat band running from the epicondyles of the femur and attaching into the medial tibia. The MCL fibres are continuous with the joint capsule in its entirety; they also insert into the medial meniscus.25,51 The MCL is, however, extrasynovial; it prevents valgus angulation.9,27 Isolated injuries to this ligament are rare because of the close contact with the other medial structures.25,32–35 This ligament is best visualized on coronal slices, appearing as an area of dark signal typical of most ligaments and tendons.
The lateral or fibular collateral ligament (LCL) is a round, cord-like structure joining the distal femur to the fibular head conjointly with the biceps femoris; it is also intimately related to the iliotibial band. Unlike the medial ligament, the LCL is not in contact with the meniscal surface; these structures are separated by the popliteus tendon. The LCL is only continuous with the articular capsule along the femur.25,51 The LCL resists varus angulation and some internal rotation.9,25 Injuries to the LCL are associated with trauma to other posterolateral structures such as the capsule and cruciate ligaments.32–34
Other ligaments
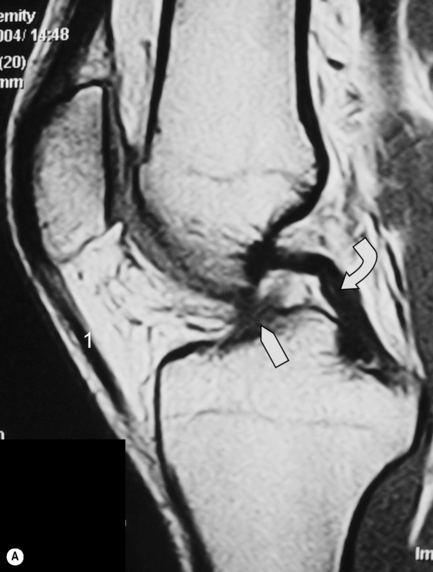
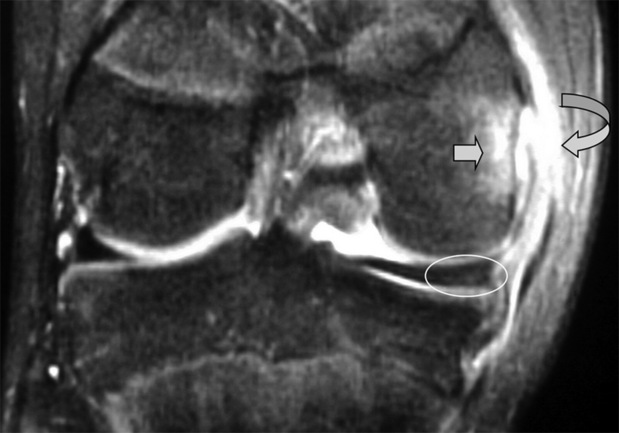
One of the commonest normal variants of the knee is the presence of a transverse (geniculate) ligament, which runs between the anterior horns of medial and lateral menisci, within Hoffa’s fat pad (Figure 6.10C,D). Its purpose remains unknown; however, it is present in 58% of knees and, importantly, can frequently mimic a tear at the point of its insertion on the anterior horn of the lateral meniscus.52–54
There are two other common meniscal ligamentous variants that can mimic tears: the ligament of Humphrey, the anterior branch of the meniscofemoral ligament, is present in 37% of patients; the posterior meniscofemoral ligament (the ligament of Wrisberg) in 84%.55,56 The meniscofemoral ligament runs from the medial femoral condyle via the intracondylar notch to the posterior horn of the lateral meniscus, where is can give rise to the appearance of a (pseudo)tear. The ligament of Humphrey passes anteriorly to the posterior cruciate ligament; the ligament of Wrisberg, posteriorly.3,25
Bursae
Other important soft tissue structures identified on MR imaging are bursae, of which there is a wealth about the knee. These are detailed in Table 6.03;32–34 inflammation of these structures (bursitis) can mimic other intra-articular pathologies. Clinical data as well as a good knowledge of the location and morphology of the bursae are again very useful in establishing an accurate diagnosis and evaluating comorbidities. The popliteal bursa, located between the medial head of the gastrocnemius and the semimembranosus muscle, is usually the most vulnerable to injury and may be seen even when asymptomatic as an area of increased T2-weighted signal in the posterior aspect of the knee.3,25 As fluid accumulation increases, secondary problems such as compartment syndromes may arise. The other bursae are not easily visualized unless inflammation is present, when they become prominent on the fluid-sensitive sequences.
| Location between … | Comment |
|---|---|
| Anterior | The anterior bursae are the most clinically important |
| Lower patella and skin | Prepatellar bursa. Inflammation causes ‘housemaid’s knee’ |
| Tibia and infrapatellar tendon | Deep infrapatellar bursa |
| Distal tibial tuberosity and skin | Superficial infrapatellar bursa. Inflammation causes ‘parson’s knee’ |
| Superior extension of synovium | Suprapatellar bursa |
| Posterior | Most posterior bursae are variable |
| Medial tendon of gastrocnemius and semimembranosus | Popliteal bursa |
| Lateral | |
| Joint capsule and lateral head of the gastrocnemius muscle | This bursa can be continuous with the joint cavity and thus contain synovial fluid |
| The tendon of biceps femoris and the lateral collateral ligament | |
Synovial plicae
Another frequently seen variant is the presence of a thin, fibrous band running between the facet of the patella and medial joint capsule. Most easily noted on the axial sequences, this represents an embryological remnant, the medial patella plica. Although superior and inferior plicae can also be found, it is the medial plica, an inward fold of the synovial lining, that is most commonly noted; one or more plicae can be found in over half of all knees.57,58
The plicae are only clinically significant if they become thickened or stiffen; in medial patella plica syndrome, the structure can become entrapped between the femur and patella, causing pain, clicking and locking. There is no definitive measurement for a thickened plica; the diagnosis is a matter of correlation with clinical presentation and radiological judgment.3,57
Pathology
Bone marrow patterns vary with age. In children of 12 years and under, most of the marrow space is usually filled with haematopoietic cells or red marrow, typically of intermediate signal intensity on both T1- and T2-weighted images. In adults, the bone marrow signal is usually high on T1-weighted images and intermediate on T2-weighted images, consistent with yellow or fatty marrow.32–35
Marrow signal changes, or reconversion, can be indicative of systemic diseases and may be found as an incidental finding in the work-up of an orthopaedic condition (Figure 6.11).59,60 Injuries to bone, such as contusions or stress fractures, are easily detected with MRI as a change in bone marrow signal (Figure 6.11B). When the signal from the marrow is eliminated by fat suppression, bone marrow oedema becomes more evident. It can be demonstrated on fluid-sensitive and fat-suppressed images by focal areas of increased signal.32–34
Osseous structures
Bone contusions
Many injuries to the soft tissue structures of the knee are associated with osseous damage. Blunt fractures are usually well visualized on conventional radiography; however, bone bruising requires MR imaging to be identified. Bone bruises, microfractures or trabecular injuries are characterized principally by bone marrow oedema and will present as decreased signal areas on T1-weighted images and increased signal areas on T2-weighted and on fat-suppressed images (Figure 6.12).32–34
Chondromalacia patellae
Softening of the articular cartilage of the deep surface of the patella can lead to crepitus, foreign body sensation and retropatellar pain, typically aggravated during flexion. A typical complaint will be of pain when descending stairs or walking downhill.30,61
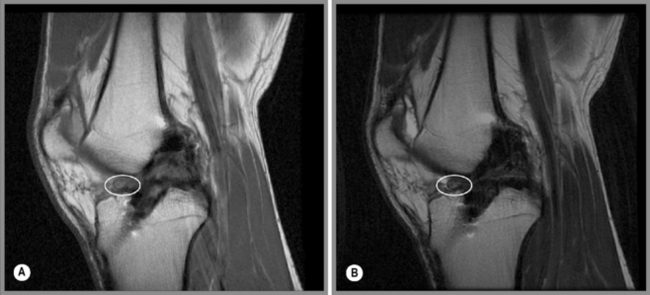
Chondromalacia patellae is common and affects almost every age group but with a variety of aetiologies. Idiopathic chondromalacia patellae is seen in growing children and teenagers; degenerative chondromalacia patellae is more likely to be seen in a middle to older age population.62 For both groups, imaging features will be similar (Figure 6.13A,B). In the beginning stages of the disease, areas of high signal will be present on fluid-sensitive sequences. This finding may be associated with an increase in overall cartilage thickness and represents oedema.61 As the disorder progresses, the articular surface will appear more irregular with focal thinning that can expand to and expose the subchondral bone.
Osteochondral injuries
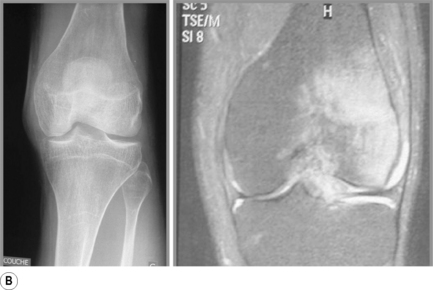
Osteochondral injuries (osteochondritis dissecans) predominantly affect younger individuals. These injuries are considered the result of an accumulation of microtraumatic events at the level of the osteochondral region leading to a series of avascular events. They are characterized by bone necrosis, subsequent re-ossification and healing.63–65 A history of trauma is found in about half of those patients affected.3,61 Depending on the extent of the involvement, patients may present with deep joint pain and locking, clicking or a catching sensation.30,33 In the knee, osteochondral defects are most common at the lateral aspect of the medial femoral condyle at the most convex area of the articular surface (Figure 6.14).61 If the injury is purely cartilaginous, plain films will be normal except for evidence of mild soft tissue swelling.
MR imaging is very sensitive in the early stages of the development of an osteochondral injury; depending on the severity of the disease, the imaging presentation can range from a focal thin rim of abnormal signal intensity in the cartilage (on the fluid-sensitive images) to a well-visualized fragment surrounded by synovial fluid. All these findings are usually best visualized on T2-weighted or fat-suppressed images. Changes can be bilateral in approximately 25% of patients. The treatment options range from conservative pain management to debridement and grafting.30,33,61
Patella
The diagnosis with MR images is usually quite straightforward: there will be a characteristic contusion on the anterior aspect of the lateral femoral condyle from the impaction of the patella; this may or may not be accompanied by a ‘kissing’ contusion on the medial patella itself. There will also be concomitant damage to the medial retinaculum. The sequences can also be used to establish a prognosis: the presence of a piece of loose cartilage usually necessitates arthroscopic intervention; an intact patella cartilage can normally be managed conservatively.66
Meniscal injuries
Injuries to the two ‘cartilages’ are one of the most common causes of knee pain and disability and are well visualized on MR images. Presumably in response to mechanical stress or degeneration, the chemical composition of the meniscus changes, resulting in intrasubstance signal alterations, referred to as myxoid degeneration (Figure 6.15).67 This change is generally asymptomatic and is not linked to a higher incidence of meniscal tearing61; it can, however, be mistaken for a tear.3
In the acute patient, the mechanism of injury usually involves rotation combined with a varus or valgus stress. This mechanism is also likely to compromise collateral and cruciate ligaments, explaining in part the common association and comorbidity between these structures.9 Degenerative changes are usually as the result of repetitive microtrama and are also commonly associated with dysfunction elsewhere in the lower kinematic chain and with obesity.
Patients with meniscal damage usually present with pain, a sensation of their knee ‘giving way’, swelling and locking. The swelling may be sporadic, influenced by the activity level.30,33,61
Meniscal tears have multiple imaging presentations and grading systems related to the imaging features.30,33,61 The definition of a true meniscal tear is an area of increased signal intensity in the meniscus extending to one or more articular surfaces; therefore, high signal regions that do not extend to the articular surface or communicate with the joint should not generally be considered as true tears.68–70
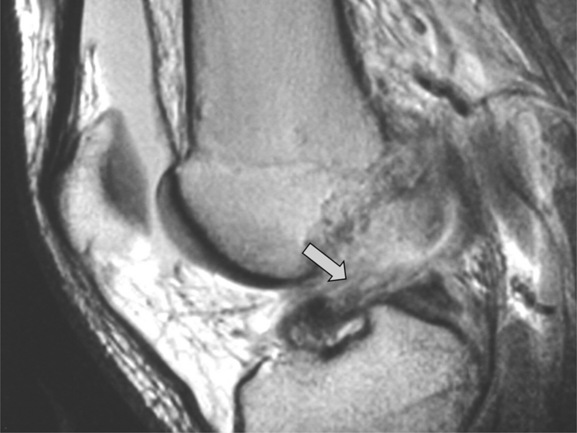
Tears of the menisci are described with reference to their orientation, length, location and presence of any free fragment.71,72 The most common location for an isolated meniscal tear is the posterior horn of the medial meniscus (Figure 6.16); the most common types of tear are oblique (or horizontal), radial (free edge or ‘parrot-beak’) and ‘bucket-handle’.3
Tears are also often seen as an element in more complex knee injuries involving capsular or ligamentous disruption.30,33,61 When associated with injuries to structures such as the anterior cruciate ligament, meniscal tears may often be overlooked because they tend to be located in atypical areas such as the posterior horn of the lateral meniscus or in the periphery of the medial meniscus.61 It is also worth recalling the normal variants discussed above that may simulate the presence of a meniscal injury.43–45
Depending on the type of tear, the location of the defect and the condition of the patient, various treatment options are available. Comprehensive rehabilitation programmes, suturing and meniscectomy (partial or total) are the most common treatment options.30 When evaluating images of a postoperative patient, correlation with preoperative films is usually essential as the signal intensity of the repaired area can remain high for years after.33,61 MR arthrography may be helpful in postoperative knees to differentiate scar tissue from a re-tear of the meniscus.73
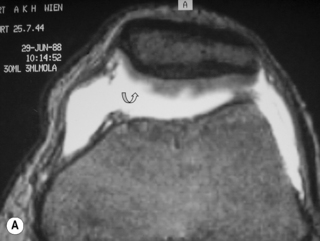
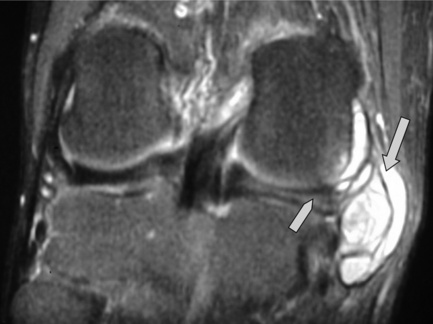
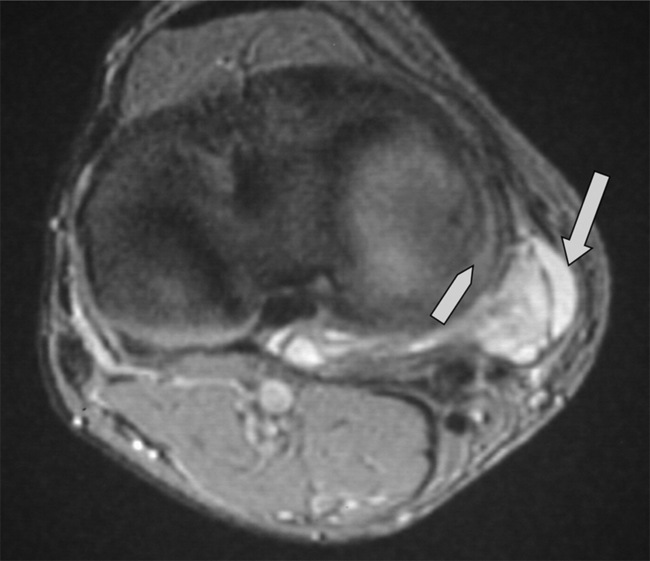
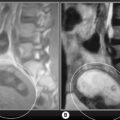
Discoid menisci are often enlarged and can therefore be symptomatic; they are also more prone to tears, degeneration and cyst formation. Meniscal cysts develop from accumulation of fluid in an existing tear. On examination, a palpable, painful mass may be found, especially if the cyst is very large. The mucinous and proteinaceous mass may fluctuate in size according to the degree of knee flexion.30,61 On MR images, the cyst appears as high signal intensity on fluid-sensitive sequences and is situated adjacent to the articular space (Figures 6.17, 6.18).74
Ligamentous injuries
Cruciate ligaments
Cruciate ligament tears are also common, particularly those to the anterior ligament, which are typically caused by anterior translation of the tibia, external rotation of the femur on the tibia with valgus stress on a weight-bearing leg. It is a common injury for athletes practising sports involving constant acceleration and deceleration whilst changing direction, such as football (in all of its forms and variations), basketball, downhill skiing and tennis.61,75–82
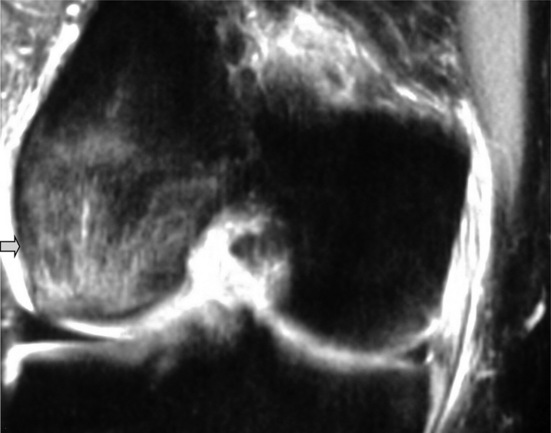
Owing to the mechanism of injury, mid-substance tears are by far the most common type of injury. Discontinuity and an alteration of the orientation of the fibres are two direct signs of a tear (Figure 6.19). Joint effusion and haemarthrosis are non-specific but useful indicators of joint pathology; however, these findings can obscure the ligament fibres, so other indirect signs may be needed to confirm the diagnosis. These include anterior tibial subluxation (the floppy sign), deepening of the lateral femoral condylar notch (to more than the normal limit of 2 mm) and specific patterns of bone bruises (Figure 6.20).3,61
Figure 6.20 • MR imaging of the knee, sagittal STIR (short tau inversion recovery) image in a patient with an acute anterior cruciate ligament tear. This image demonstrates the deep lateral femoral notch, joint effusion and a large bone bruise extending to the supracondylar region of the distal femur and is the same patient as seen in Figure 6.18.
Partial tears of the ACL can be seen as intrasubstance high signal zones. They can be post-traumatic or chronic in nature. Secondary signs such as bone bruises, prominent joint effusion or the presence of associated meniscal injuries can be once again helpful to establish a diagnosis. Chronic tears may be associated with mild effusion but the main differential feature is the prominent fibrosis and associated scar tissue formation.61
Different treatment options for ACL injuries result in different postoperative imaging presentations. Again, observation of the preoperative images is very helpful. Reconstructed ligaments, especially when using autografts, have a tendency to remain avascular (low signal intensity) for years after implantation. The fibrous appearance will not be maintained postoperatively but rather there will be an irregular appearance.61
A common cause of postsurgical pain following ACL reconstruction is the development of scar tissue in Hoffa’s fat pad. Although this can present in different ways, depending on its precise location, it most commonly affects knee extension and/or patellar motion. The MR appearance can also vary; however, the classic presentation is that of a Cyclops’ lesion (Figure 6.21).83,84
Injuries to the posterior cruciate ligament are very rarely seen as isolated incidents. The most common mechanism of injury is by direct blunt force to the anterior knee as in a severe motor vehicle accident where the flexed knee hits the dashboard.9,61 As with ACL injuries, tears of the PCL can be partial or complete. Partial tears can present as focal zones of increased signal intensity and disruption of the normal ‘inverted hockey stick’ appearance of the PCL (Figure 6.22)50; however, the PCL is unusual in that damage to it often involves stretching without overt disruption of its fibres and the fluid-sensitive sequences are therefore normal although the T1-weighted and proton density images will have a subtly increased signal. The width of the ligament will also increase; greater than 7 mm is regarded as being clinically significant.3
Management of isolated PCL injuries commonly includes conservative measures. Aggressive rehabilitation plans including strengthening of the hamstring are preferred since increased tone in the large muscle group in the posterior thigh can act as a substitute for the injured ligament by providing pull on the tibia and removing stress from the compromised PCL.33
Collateral ligament injuries
Excessive valgus and varus stress are the main causes of injury to the medial and lateral collateral ligaments, respectively. Damage to the medial and lateral collateral ligaments can be grouped according to the severity of the sprain.30,33
Low-grade injuries (grade 1) present as oedematous areas of increased T2-weighted signal and haemorrhagic changes (mixed signal) extending into the subcutaneous tissues. Haemarthrosis and joint effusion are not as common in LCL sprains because of the extracapsular location of the structure.32–34
High-grade injuries are associated with discontinuity of the fibres and loss of the normal contours of the ligament (grade 2) or complete disruption of the fibres (grade 3). These are demonstrated in Figure 6.23. Once again, isolated collateral injury is a rare phenomenon and, if found, close attention should be paid to the adjacent bone marrow signal and status of the capsulomeniscal complex, especially about the medial compartment, owing to the high risk of collateral damage in these areas.32–34
Damage to the LCL and, indeed, the lateral meniscus, can be difficult to differentiate clinically from iliotibial band syndrome; the only clue may be weakness of the tensor fascia lata muscle. However, with MR imaging, the diagnosis is relatively straightforward: if there is fluid on both sides of the iliotibial band on the T2-weighted images, the diagnosis is confirmed.85,86
1 Galea A., Giuffre B., Dimmick S., et al. The accuracy of magnetic resonance imaging scanning and its influence on management decisions in knee surgery. Arthroscopy. 2009;25(5):473-480.
2 Crawford R., Walley G., Bridgman S., Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;84:5-23.
3 Helms C., Major N., Anderson M.W., et al. The knee. In Musculoskeletal MRI, 2nd ed, Philadelphia: Elsevier Saunders; 2009:353-383.
4 Rowe L., Yochum T. Principles of radiological interpretation. In: Yochum T., Rowe L., editors. Essentials of Skeletal Radiology. 2nd ed. Baltimore: Williams and Wilkins; 1996:547-585.
5 Bloch F., Hansen W., Packard M. Nuclear induction. Phys Rev. 1946;69:127.
6 Smith T.O., Davies L., Donell S.T. The reliability and validity of assessing medio-lateral patellar position: a systematic review. Man Ther. 2009;14(4):355-362.
7 Smith T.O., Davies L., O’Driscoll M.L., Donell S.T. An evaluation of the clinical tests and outcome measures used to assess patellar instability. Knee. 2008;15(4):255-262.
8 Bates B., Hoekelman R. Interviewing and the health history. In: Bates B., editor. A Guide to Physical Examination. 3rd ed. Philadelphia: JB Lippincott; 1983:1-27.
9 Eustace S., Johnston C., O’Neill P., O’Byrne J. The knee and calf. In: Sports Injuries: Examination, Imaging and Management. Edinburgh: Elsevier Churchill Livingstone; 2007:135-218.
10 Grotle M., Hagen K.B., Natvig B., et al. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132.
11 Lohmander L.S., Gerhardsson de Verdier M., Rollof J., et al. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population-based prospective cohort study. Ann Rheum Dis. 2009;68(4):490-496.
12 Zeng Q.Y., Zang C.H., Li X.F., et al. Associated risk factors of knee osteoarthritis: a population survey in Taiyuan, China. Chin Med J (Engl). 2006;119(18):1522-1527.
13 Holmberg S., Thelin A., Thelin N. Knee osteoarthritis and body mass index: a population-based case-control study. Scand J Rheumatol. 2005;34(1):59-64.
14 Andersen R.E., Crespo C.J., Bartlett S.J., et al. Relationship between body weight gain and significant knee, hip, and back pain in older Americans. Obes Res. 2003;11(10):1159-1162.
15 Bates B. The musculoskeletal system. In: Bates B., editor. A Guide to Physical Examination. 3rd ed. Philadelphia: JB Lippincott; 1983:324-370.
16 Travell J., Simons D. Myofascial Pain and Dysfunction. Baltimore: Williams and Wilkins, 1992.
17 Meserve B.B., Cleland J.A., Boucher T.R. A meta-analysis examining clinical test utilities for assessing meniscal injury. Clin Rehabil. 2008;22(2):143-161.
18 Wilson T. The measurement of patellar alignment in patellofemoral pain syndrome: are we confusing assumptions with evidence? J Orthop Sports Phys Ther. 2007;37(6):330-341.
19 Seron M., Yochum T., Barry M., Rowe L. Skeletal dysplasias. In: Yochum T., Rowe L., editors. Essentials of Skeletal Radiology. 2nd ed. Baltimore: Williams and Wilkins; 1996:858-1652.
20 Emami M.J., Ghahramani M.H., Abdinejad F., Namazi H. Q-angle: an invaluable parameter for evaluation of anterior knee pain. Arch Iran Med. 2007;10(1):24-26.
21 Omololu B.B., Ogunlade O.S., Gopaldasani V.K. Normal Q-angle in an adult Nigerian population. Clin Orthop Relat Res. 2009;467(8):2073-2076.
22 Shellock F.G., Stone K.R., Crues J.V. Development and clinical application of kinematic MRI of the patellofemoral joint using an extremity MR system. Med Sci Sports Exerc. 1999;31(6):788-791.
23 Wilson N.A., Press J.M., Koh J.L., et al. In vivo noninvasive evaluation of abnormal patellar tracking during squatting in patients with patellofemoral pain. J Bone Joint Surg Am. 2009;91(3):558-566.
24 Bannister R. Disorders of the nerve roots and peripheral nerves. In Brain and Bannister’s Clinical Neurology, 7th ed, Oxford: Oxford University Press; 1992:420-458.
25 Standring S., editor. Gray’s Anatomy – Pelvic girdle and lower limb – knee (Section 113). Edinburgh: Elsevier, 2009.
26 Standring S., editor. Gray’s Anatomy – Pelvic girdle and lower limb – thigh (Section 112). Edinburgh: Elsevier, 2009.
27 Moore K. The lower limb. In Clinically Oriented Anatomy, 2nd ed, Baltimore: Williams and Wilkins; 1985:396-564.
28 Calmbach W.L., Hutchens M. Evaluation of patients presenting with knee pain: Part II. Differential diagnosis. Am Fam Physician. 2003;68(5):917-922.
29 Calmbach W.L., Hutchens M. Evaluation of patients presenting with knee pain: Part I. History, physical examination, radiographs, and laboratory tests. Am Fam Physician. 2003;68(5):907-912.
30 Hammer W.I. Functional Soft Tissue Examination and Treatment by Manual Methods. Riverwoods, IL: Aspen, 1999.
31 Rowe L., Yochum T. Arthritic disorders. In: Yochum T., Rowe L., editors. Essentials of Skeletal Radiology. 2nd ed. Baltimore: Williams and Wilkins; 1996:795-974.
32 Kaplan P., Helms C., Dussault R., Anderson M. Musculoskeletal MRI. Philadelphia: WB Saunders, 2001.
33 Stoller D. Magnetic Resonance Imaging in Orthopaedics and Sports Medicine, 2nd ed. Philadelphia: Lippincott Williams and Wilkins, 1996.
34 Berquist T. MRI of the Musculoskeletal System 4th ed. Philadelphia: Lippincott Williams and Wilkins, 2000.
35 Mirowitz S. Pitfalls, Variants And Artifacts in Body MR Imaging. St Louis: Mosby, 1996.
36 Green J., Silver P. The muscles and joints of the lower limb. In: An Introduction to Human Anatomy. Oxford: Oxford University Press; 1981:128-146.
37 Joseph J. Bones of the leg and knee joint. In: Hamilton W., editor. Textbook of Human Anatomy. 2nd ed. London: MacMillan; 1976:123-129.
38 Palastanga N., Field D., Soames R. The lower limb. In Anatomy and Human Movement: Structure and Function, 5th ed, Edinburgh: Elsevier Butterworth Heinmann; 2006:235-470.
39 Young M.F. The physics of anatomy. In: Essential Physics for Musculoskeletal Medicine. Edinburgh: Elsevier; 2009.
40 Grenier J.-M., Green N., Wessely M. Knee MRI part 1: basic overview. Clinical Chiropractic. 2004;7(3):147-150.
41 Youm T., Chen A.L. Discoid lateral meniscus: evaluation and treatment. Am J Orthop. 2004;33(5):234-238.
42 Klingele K.E., Kocher M.S., Hresko M.T., et al. Discoid lateral meniscus: prevalence of peripheral rim instability. J Pediatr Orthop. 2004;24(1):79-82.
43 Kocher M.S., Klingele K., Rassman S.O. Meniscal disorders: normal, discoid, and cysts. Orthop Clin North Am. 2003;34(3):329-340.
44 Tachibana Y., Yamazaki Y., Ninomiya S. Discoid medial meniscus. Arthroscopy. 2003;19(7):E12-E18.
45 Rohren E.M., Kosarek F.J., Helms C.A. Discoid lateral meniscus and the frequency of meniscal tears. Skeletal Radiol. 2001;30(6):316-320.
46 Shankman S., Beltran J., Melamed E., Rosenberg Z.S. Anterior horn of the lateral meniscus: another potential pitfall in MR imaging of the knee. Radiology. 1997;204(1):181-184.
47 Fernandes J.L., Viana S.L., Mendonca J.L., et al. Mucoid degeneration of the anterior cruciate ligament: magnetic resonance imaging findings of an underdiagnosed entity. Acta Radiol. 2008;49(1):75-79.
48 Drosos G.I., Pozo J.L. Large extrasynovial intracapsular ganglia of the knee: a report of 3 cases. Arthroscopy. 2005;21(11):1362-1365.
49 Dinakar B., Khan T., Kumar A.C., Kumar A. Ganglion cyst of the anterior cruciate ligament: a case report. J Orthop Surg (Hong Kong). 2005;13(2):181-185.
50 Regatte R.R., Akella S.V., Borthakur A., et al. In vivo proton MR three-dimensional T1rho mapping of human articular cartilage: initial experience. Radiology. 2003;229(1):269-274.
51 Joseph J. Muscles and fascia of the lower limb. In: Hamilton W., editor. Textbook of Human Anatomy. 2nd ed. London: MacMillan; 1976:179-195.
52 Sintzoff S.A.Jr, Stallenberg B., Gillard I., et al. Transverse geniculate ligament of the knee: appearance and frequency on plain radiographs. Br J Radiol. 1992;65(777):766-768.
53 Watanabe A.T., Carter B.C., Teitelbaum G.P., et al. Normal variations in MR imaging of the knee: appearance and frequency. Am J Roentgenol. 1989;153(2):341-344.
54 Watanabe A.T., Carter B.C., Teitelbaum G.P., Bradley W.G.Jr. Common pitfalls in magnetic resonance imaging of the knee. J Bone Joint Surg Am. 1989;71(6):857-862.
55 Ranalletta M., Rossi W., Paterno M., et al. Incidence of the anterior meniscofemoral ligament: an arthroscopic study in anterior cruciate ligament-deficient knees. Arthroscopy. 2007;23(3):275-277.
56 Nagasaki S., Ohkoshi Y., Yamamoto K., et al. The incidence and cross-sectional area of the meniscofemoral ligament. Am J Sports Med. 2006;34(8):1345-1350.
57 Sznajderman T., Smorgick Y., Lindner D., et al. Medial plica syndrome. Isr Med Assoc J. 2009;11(1):54-57.
58 Griffith C.J., Laprade R.F. Medial plica irritation: diagnosis and treatment. Curr Rev Musculoskelet Med. 2008;1(1):53-60.
59 Hartman R.P., Sundaram M., Okuno S.H., Sim F.H. Effect of granulocyte-stimulating factors on marrow of adult patients with musculoskeletal malignancies: incidence and MRI findings. Am J Roentgenol. 2004;183(3):645-653.
60 Ollivier L., Gerber S., Vanel D., et al. Improving the interpretation of bone marrow imaging in cancer patients. Cancer Imaging. 2006;6:194-198.
61 Stoller D.W., Tirman P.F., Bredella M.A. Diagnostic Imaging Orthopaedics. Salt Lake City: Amirsys Inc, 2003.
62 Zhang H., Kong X.Q., Cheng C., Liang M.H. A correlative study between prevalence of chondromalacia patellae and sports injury in 4068 students. Chin J Traumatol. 2003;6(6):370-374.
63 De Smet A.A., Fisher D.R., Graf B.K., Lange R.H. Osteochondritis dissecans of the knee: value of MR imaging in determining lesion stability and the presence of articular cartilage defects. Am J Roentgenol. 1990;155(3):549-553.
64 Fritz R.C. MR imaging of osteochondral and articular lesions. Magn Reson Imaging Clin N Am. 1997;5(3):579-602.
65 Bhosale A.M., Richardson J.B. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77-95.
66 White B.J., Sherman O.H. Patellofemoral instability. Bull NYU Hosp Jt Dis. 2009;67(1):22-29.
67 Ferrer-Roca O., Vilalta C. Lesions of the meniscus. Part I: Macroscopic and histologic findings. Clin Orthop Relat Res. 1980:146:289-300
68 Kaplan P.A., Nelson N.L., Garvin K.L., Brown D.E. MR of the knee: the significance of high signal in the meniscus that does not clearly extend to the surface. Am J Roentgenol. 1991;156(2):333-336.
69 De Smet A.A., Tuite M.J. Use of the “two-slice-touch” rule for the MRI diagnosis of meniscal tears. Am J Roentgenol. 2006;187(4):911-914.
70 De Smet A.A., Norris M.A., Yandow D.R., et al. MR diagnosis of meniscal tears of the knee: importance of high signal in the meniscus that extends to the surface. Am J Roentgenol. 1993;161(1):101-107.
71 Widuchowski W., Widuchowski J., Trzaska T. Articular cartilage defects: study of 25, 124 knee arthroscopies. Knee. 2007;14(3):177-182.
72 Christoforakis J., Pradhan R., Sanchez-Ballester J., et al. Is there an association between articular cartilage changes and degenerative meniscus tears? Arthroscopy. 2005;21(11):1366-1369.
73 Ciliz D., Ciliz A., Elverici E., et al. Evaluation of postoperative menisci with MR arthrography and routine conventional MRI. Clin Imaging. 2008;32(3):212-219.
74 Tyson L.L., Daughters T.C.Jr, Ryu R.K., Crues J.V.3rd. MRI appearance of meniscal cysts. Skeletal Radiol. 1995;24(6):421-424.
75 Alentorn-Geli E., Myer G.D., Silvers H.J., et al. Prevention of non-contact anterior cruciate ligament injuries in soccer players. Part 1: Mechanisms of injury and underlying risk factors. Knee Surg Sports Traumatol Arthrosc. 2009;17(8):859-879.
76 Meyer E.G., Haut R.C. Anterior cruciate ligament injury induced by internal tibial torsion or tibiofemoral compression. J Biomech. 2008;41(16):3377-3383.
77 Davies H., Tietjens B., Van Sterkenburg M., Mehgan A. Anterior cruciate ligament injuries in snowboarders: a quadriceps-induced injury. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1048-1051.
78 Krosshaug T., Nakamae A., Boden B.P., et al. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007;35(3):359-367.
79 Hewett T.E., Myer G.D., Ford K.R. Anterior cruciate ligament injuries in female athletes: Part 1, mechanisms and risk factors. Am J Sports Med. 2006;34(2):299-311.
80 Brooks J.H., Fuller C.W., Kemp S.P., Reddin D.B. Epidemiology of injuries in English professional rugby union: part 2 training injuries. Br J Sports Med. 2005;39(10):767-775.
81 Brooks J.H., Fuller C.W., Kemp S.P., Reddin D.B. Epidemiology of injuries in English professional rugby union: part 1 match injuries. Br J Sports Med. 2005;39(10):757-766.
82 Brown J.R., Trojian T.H. Anterior and posterior cruciate ligament injuries. Prim Care. 2004;31(4):925-956.
83 Papakonstantinou O., Chung C.B., Chanchairujira K., Resnick D.L. Complications of anterior cruciate ligament reconstruction: MR imaging. Eur Radiol. 2003;13(5):1106-1117.
84 Wang J., Ao Y. Analysis of different kinds of cyclops lesions with or without extension loss. Arthroscopy. 2009;25(6):626-631.
85 Stabler A., Glaser C., Reiser M. Musculoskeletal MR: knee. Eur Radiol. 2000;10(2):230-241.
86 Vasilevska V., Szeimies U., Stabler A. Magnetic resonance imaging signs of iliotibial band friction in patients with isolated medial compartment osteoarthritis of the knee. Skeletal Radiol. 2009;38(9):871-875.