The Immunocompromised Patient
Perspective
Compared with individuals with an intact immune system, infections in immunocompromised patients are more common, progressive, and severe, and they are caused by a wider variety of microorganisms. Immunocompromised persons who present with acute infections may appear deceptively benign initially, their symptoms and signs often mimicking noninfectious complications, only to deteriorate rapidly if they are not aggressively treated. Many interrelated factors cause patients to become immunocompromised and predispose them to the development of infections with potentially pathogenic microorganisms. These include disruption of the body’s protective surfaces, such as skin and mucosal barriers (oral and respiratory mucosa and intestinal and genitourinary surfaces); disorders that directly impair the function of the body’s immune system (e.g., lymphoma, asplenism, and myeloma); drugs and irradiation that suppress or alter immune function; alterations in body substances (hyperglycemia) or solid organ function (kidney and liver failure); and malnutrition, aging, and exposure to antimicrobial agents that inhibit the normal protective resident bacterial flora.1
Principles of Disease
The body’s defense mechanisms consist of surface barriers, such as skin, enzymes, and mucus, as well as innate (natural) and acquired (adaptive) responses. Innate responses occur to the same extent regardless of how often the body encounters the infectious agent, whereas acquired responses improve on repeated exposure.2 Innate immunity is activated immediately on exposure to an infecting agent, rapidly controlling replication and allowing the requisite 3 to 5 days for the adaptive component to clone sufficient T and B cells to respond more specifically.3–5
Non–Microbe-Specific Immunity
The first line of defense against microorganisms consists of physical barriers. These include intact skin, gastrointestinal and respiratory mucosa, cilia, biofilm, gastric acid, antibacterial substances in pancreatic and biliary secretions, antimicrobial peptides and proteins on skin and mucous membranes, and resident microflora.6
In the respiratory tract, mucociliary transport and the cough reflex remove particulate matter and microbes, but this mechanism is impaired with smoking and ineffective cough. Mechanical ventilation or tracheostomy introduces large numbers of microbes that often overwhelm natural clearance.7
Initial Inflammatory Response and Innate Immunity
The first response to microbial invasion, the initial inflammatory response (formerly called the acute-phase response), acts to promote phagocytosis and microbial killing and to activate the immune system.8 Sentry cells detect pathogens, immediately triggering inflammation. This innate immune response is not dependent on prior exposure to the pathogen. The initial inflammatory response factors, mainly produced in the liver, activate many cell types to synthesize and to release cytokines, chemokines, and “trigger molecules” that kill the invading organism.3
This response delivers humoral and cellular immune components to sites of inflammation and initiates antibody responses. Cytokines, platelet-activating factor, and hormone-like proteins, including interferons, are secreted from various immune cells and play important roles in mediation of this response.9 These cytokines result in migration and adhesion of polymorphonuclear leukocytes and monocytes to sites of bacterial invasion. These cells release granules of substances that mediate vasodilation and increased vascular permeability, leading to edema, warmth, and redness, but also allow both phagocytic cells and humoral components to be concentrated at the site of infection.
A family of distinct transmembrane proteins, called Toll-like receptors, are found on many cell types, including macrophages, neutrophils, dendritic cells, mucosal epithelial cells, and endothelial cells. They recognize molecular patterns associated with microorganisms even in the absence of prior exposure, alert the host to the presence of the infectious agent and rapidly initiate a cascade of processes to activate innate immune responses, and help bridge innate and adaptive immune responses.3,6
Adaptive (Microbe-Specific) Immunity
Antibodies.: Antibodies are produced by B lymphocytes, and each B cell produces a single microbe-specific antibody type. Stimulation by an antigen (or microbe) causes proliferation of this particular B cell so that large quantities of a specific circulating antibody can be produced. Furthermore, B cells are active in presenting antigens to T lymphocytes, which promotes cell-mediated immunity.
Immunoglobulins.: IgM is the first immunoglobulin to appear in response to a new antigen. Although it has less affinity at binding antigens than IgG does, IgM provides some recognition of antigens and begins B-cell proliferation before the subsequent development of IgG.10 IgM is detectable earlier in serum than IgG and serves as a marker for a patient’s early response to acute infection.
Secretory IgA is the predominant immunoglobulin present in gastrointestinal fluids, nasal and oral secretions, tears, and other mucous fluids. IgA inhibits cell adherence of viral, bacterial, and protozoan pathogens and therefore prevents invasion by organisms through the respiratory or gastrointestinal tract.10
Complement.: The complement cascade, consisting of a complex interaction of 30 proteins, is another crucial component of humoral response. Complement is important in producing inflammation and leukocytosis and in recruiting leukocytes to sites of infection by production of chemoattractants. Complement also neutralizes viruses, enhances opsonization of bacteria, and produces bacterial cell wall and membrane lysis.
Individuals with inherited complement deficiencies are predisposed to frequent and recurrent infections with S. pneumoniae, H. influenzae, and especially Neisseria meningitidis and Neisseria gonorrhoeae.11 The risk of meningococcal infection is increased several thousand-fold and most often develops in people deficient in C3 and in late complement components (C5-C8). Paradoxically, the disease is usually milder with complement deficiency, and mortality is likewise reduced fivefold to tenfold.12 This suggests that the host response may be, in part, responsible for the severity of disease in normal individuals and is attenuated in complement deficiency. People with meningococcemia should be tested for inherited complement deficiencies because they may benefit from immunization.
Acquired deficiencies of complement function may develop in people with rheumatologic diseases, especially systemic lupus erythematosus (SLE). Approximately 40% of patients with SLE have an inhibitor of C5a-derived chemotaxis in their serum that results in enhanced susceptibility to infection.13
Cell-Mediated Immunity
Only 5% of lymphocytes are in circulating blood. Most mature and are active in the marrow, thymus, spleen, and lymph nodes. The last two sites expose T cells to circulating antigen from invading microbes.6 Specialized antigen-presenting cells in the lymphoid system sequester antigen and antigen-antibody complexes and present them to T cells. This process involves internalization and processing of the antigen, followed by formation of peptides that bind to a cell surface molecule called the major histocompatibility complex (MHC). Only with this specific presentation can a T lymphocyte become activated against a particular antigen.
Two major types of T lymphocytes are CD4 (helper cell) and CD8 (suppressor cell), corresponding to type II and type I of MHC, respectively. CD4 lymphocytes provide help for other cells in the immune system, including enhanced B-cell antibody production and production of cytokines. CD8 lymphocytes are generally cytotoxic and mediate the eradication of virally infected target cells and certain tumors. A decline in the number of CD4 cells, with predominance of CD8 cells, is responsible for the increased susceptibility to infection in patients with acquired immunodeficiency syndrome (AIDS).6 Despite the cytotoxicity of CD8 cells, immunity is reduced without adequate numbers of CD4 cells.
Patients with defects in CMI are at increased risk for disseminated infection with intracellular bacteria, such as Mycobacterium tuberculosis, Listeria monocytogenes, and Salmonella species. The DNA viral infections, such as cytomegalovirus, herpes simplex, and varicella-zoster, also affect these patients more severely, as do fungal infections with Candida, Cryptococcus, Mucor, Aspergillus, and Pneumocystis. Finally, some protozoa are pathogenic without intact CMI, as infections with Toxoplasma gondii demonstrate.14,15 Some infections are seen only below a certain CD4 cell count. Pneumocystis pneumonia is seen almost exclusively in patients with counts below 200 cells/mL (2 × 105 cells/L), whereas almost all patients with toxoplasmosis or cryptococcal meningitis have counts below 100 cells/mL (1 × 105 cells/L).
NK cells, closely related to lymphocytes but neither B nor T cells, are important in the innate immune response and are found in high concentrations in blood and spleen.3,6 NK cells recognize infected cells and respond by directly killing these cells, and they secrete cytokines that activate macrophages to destroy phagocytosed microbes. These cells are important in defense against intracellular microbes, particularly viruses and intracellular bacteria such as L. monocytogenes.
Granulocytic Phagocytes
Two other types of granulocytes, eosinophils and basophils, are less involved in the ingestion of organisms.16 Eosinophils mediate the destruction of certain parasitic helminths through release of toxic proteins. Normally only 3% of total granulocytes, this cell type can reach 20% during times of high parasite load. Basophils (rare in circulation) and their tissue counterparts, mast cells, have high affinity for IgE. On exposure to antigens, they release granules with histamine, prostaglandins, and leukotrienes, which affect the allergic-inflammatory response with increased vascular permeability, bronchospasm, and vasodilation.2
Neutrophils constitute 90% of circulating granulocytes and spend only 6 to 8 hours of their average 4-day life in circulation (the remainder in tissues). Effective antibacterial activity depends on the ability of neutrophils to travel to sites of infection, a process known as chemotaxis. The locomotion of neutrophils along vascular endothelium is facilitated by adherence to cell surface proteins whose production is enhanced in the initial inflammatory response.16
In addition to phagocytosis, macrophages (located in the spleen, alveoli, liver, and lymph nodes) modulate the immune response by presenting antigens to lymphocytes and releasing cytokines and complement components. Activation of macrophages to ingest bacteria depends on interaction with interferon-γ, a cytokine manufactured by T cells.6 Thus the once clear demarcation between cellular and humoral immunity is breaking down as more is understood about the interdependent immune system.
Specific Immunocompromised States
Cancer
Patients with cancer frequently have multiple immune defects, such as neutropenia and impaired function of T and B cells, induced by cancer chemotherapy or by the disease process itself, which predisposes them to infection. Other factors leading to infection are defects in physical barriers (skin and mucous membranes), including cytotoxic effects of chemotherapy on cells lining the gastrointestinal tract. In addition, splenic dysfunction or splenectomy, use of long-term intravascular catheters, frequent use of complex invasive diagnostic and therapeutic procedures, toxic effects of radiation therapy, and frequent colonization with antimicrobial-resistant pathogens are predisposing factors. Cancer treatments (e.g., allogeneic bone marrow and autologous stem cell transplantation, platelet transfusion, granulocyte colony-stimulating factor, and implanted central venous catheters) increase survival during episodes of profound immunosuppression, allowing patients to receive more intense cytotoxic cancer chemotherapy regimens. This results in long survival of patients with neoplastic diseases that were formerly rapidly fatal. Despite many advances in supportive care, infections continue to result in serious morbidity and mortality. Furthermore, increasing resistance to antimicrobials is occurring among common pathogens along with the emergence of new opportunistic pathogens. Infection is much more common in patients with acute leukemia and lymphoma (75% of patients) and multiple myeloma (50% of patients) than in those with solid tumors.17 Factors predisposing to infection in immunocompromised patients are listed in Box 183-1.
Neutropenia
Principles of Disease.: Neutropenia is defined as a neutrophil count of less than 500 cells/mL (5 × 105 cells/L), including band forms, or less than 1000 cells/mL (1 × 106 cells/L) and expected to fall to less than 500 cells/mL.18,19 It usually results from cytotoxic chemotherapy or radiation therapy or the disease process, especially in hematologic malignant neoplasms. In addition, cancer chemotherapeutic agents and radiation therapy can cause functional defects in granulocytes. The risk of febrile neutropenia and mortality is higher in the first one or two cycles of multicycle cytotoxic chemotherapy regimens.20
The incidence and severity of infection in cancer patients with neutropenia are inversely proportional to the absolute neutrophil count and directly proportional to the duration of neutropenia. Although the incidence begins to rise as the neutrophil count falls below 500 cells/mL (5 × 105 cells/L), most severe infections and almost all bacteremias occur when the neutrophil count is less than 100 cells/mL.21 Fever in the neutropenic patient is defined as a single temperature of 38.3° C (101° F) or higher or a temperature of 38.0° C (100.4° F) or higher during 1 or 2 hours.19 In neutropenic patients, the temperature should be measured orally or tympanically, not rectally. Although fever can be suppressed or lessened by immunosuppressive agents such as corticosteroids and nonsteroidal anti-inflammatory drugs, most cancer patients with infection manifest fever despite the use of these agents.22 Also, although it is uncommon, immunocompromised patients can have serious local or systemic infections without fever. This is manifested by unexplained tachypnea or tachycardia, mental status changes, metabolic acidosis, increased volume requirements, rapid changes in serum glucose or sodium concentration, or acute abdominal pain. Because the onset of life-threatening infections can be rapid in cancer patients with severe neutropenia or a history of splenectomy, urgent evaluation and initiation of antimicrobial therapy are essential. A prospective multicenter observational study of febrile neutropenic cancer patients in EDs in France found that critically ill patients were poorly recognized and undertreated, low-risk patients were overtreated, and compliance with established guidelines was low.23
The most common sites of infection in neutropenic patients are the lung (25%); mouth and pharynx (25%); gastrointestinal tract (15%); skin, soft tissue, and intravascular catheters (15%); perineum and anorectal area (10%); urinary tract (5%); and nose and sinuses (5%).24 Pneumonia and anorectal infection are more likely to be associated with bacteremia. Bacteremia may occur without an obvious source despite intensive investigation. Historically, the most important bacteria are three gram-negative bacilli—Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa—and four gram-positive cocci—Staphylococcus epidermidis, viridans group streptococci, Enterococcus species, and S. aureus. Many centers that treat large numbers of cancer patients note a decrease in these gram-negative bacilli and an increase in infections caused by others, such as Enterobacter, Citrobacter, and Serratia species, which are capable of rapidly developing resistance to cephalosporins and extended-spectrum penicillins. Anaerobes are uncommon but may be important in certain mixed infections (e.g., mouth, abdominal, and perianal).
During the past 25 years, infection with gram-positive organisms (e.g., coagulase-negative staphylococci, S. aureus, viridans streptococci, and Enterococcus species) has increased, and this is now the leading cause of bacterial infection (50-70% at some centers) in febrile neutropenic cancer patients in the United States, Canada, and western Europe. Gram-negative organisms still predominate in developing countries.25,26 With the exception of viridans streptococci, most of these gram-positive organisms do not produce immediately life-threatening infections compared with the rapid lethality of many gram-negative infections. Life-threatening bloodstream infections caused by viridans streptococci (especially Streptococcus mitis) are common in many cancer centers and often respond poorly to penicillins and cephalosporins. Risk factors for serious viridans streptococcal infections include aggressive cytoreduction therapy for acute leukemia or allogeneic bone marrow transplantation (especially after high-dose cytosine arabinoside treatment), profound neutropenia, and severe oral mucositis. Other factors include prophylactic use of trimethoprim-sulfamethoxazole or fluoroquinolones, use of antacids or H2 receptor antagonists, and childhood.27,28
Aspergillus and Candida species are the most common fungi producing infection in cancer patients with fever and neutropenia.24,29,30 Infection is most likely to develop in neutropenic patients treated with broad-spectrum antimicrobials and in those whose fever persists for more than 7 days. Aspergillus species usually produce necrotizing infections in the lung or sinuses. Pulmonary aspergillosis often is manifested with pleuritic pain, hemoptysis, and localized wheezing. The chest radiograph demonstrates pleural effusion or focal infiltrates. Computed tomography (CT) is more sensitive in detection of pulmonary infiltrates compatible with aspergillosis, and it may demonstrate a distinct halo of low attenuation surrounding a pulmonary infiltrate. This pattern is highly suggestive of invasive aspergillosis, although mucormycosis and other disorders may mimic the halo. Invasive aspergillosis originating in the paranasal sinuses may extend to the surrounding bone and brain. Often, an initial red-purple lesion on the nasal turbinate or palate turns pale and then black as vascular invasion produces infarction of the mucosa and bone. The black eschar on the nose or palate is easily misdiagnosed as dried blood. Patients presenting with head or facial pain or swelling, or proptosis, should be rapidly evaluated for invasive aspergillosis and mucormycosis. Candida species produce infections of the skin, oral cavity, and esophagus as well as fungemia. The sudden onset of generalized rash consisting of pink-purple, nontender subcutaneous nodules is characteristic of candidemia.
Clinical Features.: Certain clinical findings are characteristic of specific pathogens (Table 183-1). Noninfectious causes of fever also need to be considered, such as drug toxicity, drug allergy, transfusion reactions, and pulmonary emboli.17 Fever is frequently the only sign of infection because these patients are unable to mount a full inflammatory response at a site of infection.24 Usual symptoms and signs of infection may not be present, especially when the neutrophil count is less than 100 cells/mL (1 × 105 cells/L). When pneumonia develops, purulent sputum may be absent, and the initial chest radiograph may not show an infiltrate. Pyuria may be absent in the presence of urinary tract infection. Areas of cellulitis may have diminished or absent induration and redness and no purulent drainage. Tenderness may be the only finding in perineal and anal infections. The neutropenic patient with a documented infectious cause of fever may be difficult to distinguish from the patient with fever not caused by infection. The performance of a procedure before the onset of fever, presence of chills, “toxic appearance,” and lack of localized findings do not help determine whether the patient is bacteremic.31 Only 20% of febrile neutropenic patients have a clinical focus of infection identified at presentation, and only 30% of patients have positive blood cultures.
Table 183-1
| CHARACTERISTIC CLINICAL FINDINGS | SUSPECT PATHOGENS |
| Ulcerative lesions in the mouth | Viridans streptococci, herpes simplex, Candida, anaerobes |
| Necrotizing skin lesions | Pseudomonas aeruginosa, Aeromonas hydrophila, Aspergillus, Mucor |
| Nontender subcutaneous nodules | Nocardia, Cryptococcus |
| Nontender pink skin papules | Candida |
| Black eschar of nose or palate | Aspergillus, Mucor |
| Generalized macular red rash | Viridans streptococci |
| Right lower quadrant abdominal pain, tenderness, distention, bloody diarrhea | Typhlitis (neutropenic enterocolitis) caused by Pseudomonas aeruginosa, Escherichia coli, Clostridium septicum |
| Perineal pain and tenderness without inflammation or abscess | Gram-negative bacilli, anaerobes |
| Redness or pain at vascular catheter sites | Coagulase-negative staphylococci, Corynebacterium, Bacillus species |
Diagnostic Strategies.: The evaluation of the cancer patient with fever and neutropenia should include a meticulous search for subtle symptoms and signs of inflammation at common sites: oral cavity and pharynx, lower esophagus, lung, skin, perineum including anus, bone marrow aspiration sites, vascular catheter sites, and tissue around the nails.21 In nearly two thirds of patients, the initial evaluation does not identify a focus of infection.22 Two sets of blood culture specimens should be obtained. If the patient has a central venous catheter, culture specimens of blood should be obtained from each lumen of a multilumen catheter and from at least one peripheral site.19 Specimens for culture should also be obtained from any site of inflammation, including inflamed or draining catheter exit sites. Patients with severe mucositis should have herpes simplex cultures performed if they are not receiving antiherpes prophylaxis, and they should have a smear for Candida pseudohyphae. Complete blood count, electrolyte values, transaminase levels, blood urea nitrogen concentration, and creatinine concentration should be determined to plan management and to monitor the occurrence of drug toxicity.
A chest radiograph should be obtained in patients with respiratory symptoms and signs.19 If the chest radiograph is normal or inconclusive but there is still suspicion for pneumonia, high-resolution CT or thin-section multislice CT scanning of the chest without contrast enhancement should be obtained because pneumonia is often detected by chest CT in febrile neutropenic patients with normal findings on the chest radiograph.32 CT evaluation of the sinuses should be performed if facial pain or swelling is present. In patients with abdominal pain and tenderness, CT scanning of the abdomen is useful for diagnosis of neutropenic enterocolitis (“typhlitis”), a necrotizing infection of the bowel wall that usually affects the cecum. This is more commonly seen in acute leukemia and is not generally treated surgically. Ultrasonography over a subcutaneous tunneled catheter track and its vein of insertion may reveal the presence of an abscess or infected thrombus.33
Management of Febrile Neutropenia:
Antibiotic Therapy.: Broad-spectrum antimicrobial therapy should be initiated promptly in the febrile neutropenic patient if the neutrophil count is less than 500 cells/mL (5 × 105 cells/L) or if the neutrophil count is 500 to 1000 cells/mL (5 to 10 × 105 cells/L) and expected to drop.19,34 Moreover, even afebrile neutropenic patients who have symptoms and signs (e.g., abdominal pain and tenderness) compatible with an infection should be treated empirically (Table 183-2).
Table 183-2
Selected Antimicrobial Agents Useful in the Immunocompromised Patient
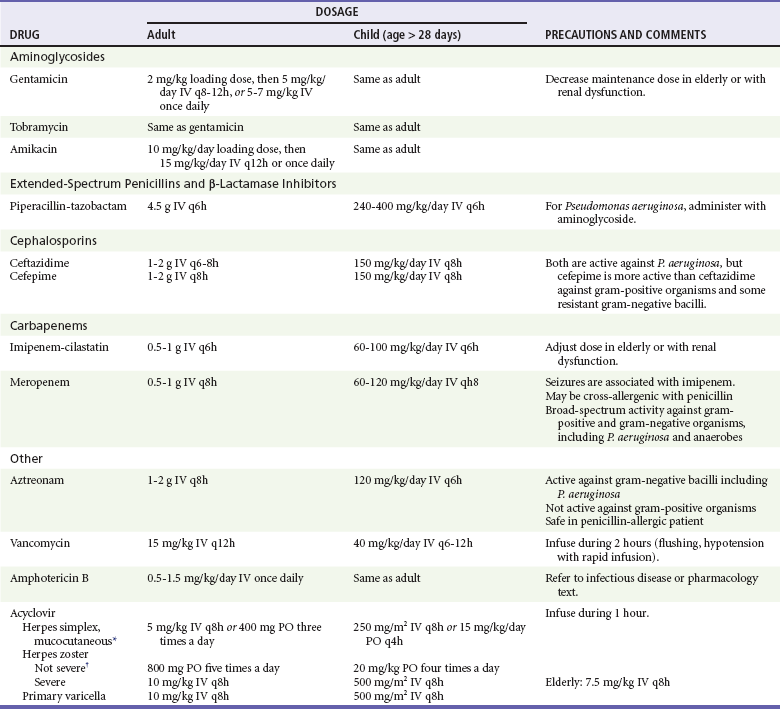
*Alternative for herpes simplex: famciclovir 250 mg three times a day, or valacyclovir 1 g two times a day PO.
†Alternative for herpes zoster: famciclovir 500 mg three times a day, or valacyclovir 1 g three times a day PO q8-12h.
Use of a single antimicrobial agent is preferred in most patients because there is no conclusive evidence of a benefit from multiple drugs.19,35,36 Factors that should be considered in choosing an antimicrobial agent include renal and hepatic function, drug allergies, suspected site of infection or organism, and cost. Antimicrobial resistance varies widely, and in the absence of a written hospital-specific protocol, immediate consultation with an oncologist or an infectious diseases specialist may be of great assistance.
Monotherapy with intravenous cefepime, ceftazidime, imipenem, meropenem, or piperacillin-tazobactam is preferred, with an aminoglycoside (gentamicin, tobramycin, or amikacin) added for the more seriously ill patient.19,24,37,38 Monotherapy, without an aminoglycoside, may be advantageous in the patient with mild to moderate renal dysfunction or for patients receiving nephrotoxic agents, such as cisplatin, cyclosporine, or amphotericin B. Ceftazidime-resistant gram-negative bacilli are common pathogens at some centers. In addition, ceftazidime is the least active against gram-positive organisms, compared with cefepime, cefotaxime, or ceftriaxone. Cefepime, a broad-spectrum cephalosporin with excellent activity against both gram-positive and gram-negative organisms, including P. aeruginosa, is preferred at many centers. The carbapenems imipenem and meropenem provide excellent activity against gram-negative organisms (including P. aeruginosa), gram-positive organisms, and anaerobic bacteria. None of the antimicrobial agents previously listed is active against vancomycin-resistant Enterococcus species or methicillin-resistant staphylococci.
For patients who are allergic to β-lactam antibiotics (e.g., penicillins, cephalosporins, imipenem, and meropenem), coverage of gram-negative bacilli, including P. aeruginosa, can be provided by aztreonam. Because aztreonam is not active against gram-positive or anaerobic bacteria, it should be combined with an antimicrobial such as vancomycin. If anaerobes are suspected (i.e., oral, abdominal, or perianal infection) in the β-lactam–allergic patient or in the patient receiving cephalosporin monotherapy, an antianaerobic drug such as clindamycin or metronidazole should be administered. Empirical treatment with intravenous fluoroquinolones is not recommended in the febrile neutropenic cancer patient because of frequent prophylactic use of these agents in the cancer patient, risk for rapid emergence of resistance in gram-negative bacilli, and predisposition to C. difficile infection.19,39,40
Routine empirical use of vancomycin for the febrile neutropenic cancer patient is not recommended because of concern about the development of vancomycin-resistant organisms.19,27,41,42 Randomized clinical trials show no survival advantage when vancomycin is in the initial therapy for all neutropenic patients, even those with indwelling catheters. Because most infections with gram-positive bacteria are indolent, vancomycin therapy can be safely delayed for 24 to 48 hours in most patients until a vancomycin-requiring gram-positive infection is identified.43
Patients previously colonized or infected with methicillin-resistant S. aureus, vancomycin-resistant enterococci, extended-spectrum β-lactamase–producing gram-negative bacteria, and carbapenemase-producing organisms may require modifications to initial empirical therapy.19
Amphotericin B (and its lipid formulations) is the drug of choice for treatment of invasive fungal infections in patients with neutropenia.19,41,44 Up to one third of febrile neutropenic patients not responding to 1 week of antibiotics have systemic fungal infections, usually Candida or Aspergillus. Antifungal agents, such as caspofungin, voriconazole, or posaconazole, may be indicated in selected cases. Empirical use of fluconazole is not recommended because of lack of activity against Aspergillus and some Candida species.
Cell Stimulation Therapy: For prevention and treatment of neutropenia, some centers routinely use human recombinant hematopoietic or colony-stimulating growth factors (granulocyte colony-stimulating factor: filgrastim, pegfilgrastim; granulocyte-macrophage colony-stimulating factor: sargramostim) to stimulate the proliferation and maturation of bone marrow progenitor cells and to increase the number and function of these committed cell populations. Although they are safe and well tolerated, they are very expensive. Treatment with these agents after chemotherapy may shorten the hospital stay and the duration of fever, but no good evidence exists that they prolong survival or affect the frequency of severe infections. Many authorities recommend that use of these agents should be limited to high-risk neutropenic patients, such as elders and those with severe sepsis, multiorgan failure, or recurrent febrile neutropenia.19,45,46
Risk Assessment for the Patient with Febrile Neutropenia, Including the Concept of Brief Observation and Early Discharge of the Low-Risk Patient: Febrile neutropenic cancer patients can be classified into high-risk and low-risk groups.19,47–49 Factors associated with high-risk patients include the following: status as inpatient when fever and neutropenia develop; presence of comorbid medical conditions; uncontrolled cancer; acute leukemia; hemodynamically unstable; evidence of organ failure; presence of pneumonia, severe soft tissue infection, infection of a central line, abdominal pain, or neurologic or mental status abnormalities; and neutropenia expected to last more than 10 days. These patients should be treated in the hospital with intravenous antibiotics.
Low-risk patients generally have an excellent outcome with therapy and are clinically stable outpatients with solid tumors, lymphomas, or chronic leukemia who lack any of the high-risk factors noted previously and who are not receiving fluoroquinolone prophylaxis. Low-risk patients with fever and neutropenia may be treated in the hospital with oral antibiotics, such as ciprofloxacin plus amoxicillin-clavulanate (or ciprofloxacin plus clindamycin in penicillin-allergic patients).47–49 Furthermore, oral antibiotic therapy with early discharge is safe and effective in carefully selected low-risk patients.19,49–52 Hospitalization exposes low-risk patients to potential iatrogenic complications and antimicrobial-resistant nosocomial pathogens, and early discharge followed by outpatient treatment allows an improved quality of life.
Low-risk patients may be hospitalized initially, stabilized during 12 to 48 hours, and then discharged to continue parenteral or oral antibiotics. Some authorities recommend a period of observation less than 12 hours, which may allow discharge from the ED, an observation unit, or physician’s office.49 The patient should be observed for at least 2 hours after administration of the first dose of antibiotics and discharged only after consultation with the patient’s oncologist. Patients sent home need adequate instructions, family support, and easy access to a hospital in case of emergency.49–57 The 2011 clinical practice guidelines of the Infectious Diseases Society of America and the National Comprehensive Cancer Network support outpatient or short-stay oral antibiotic therapy in carefully selected low-risk patients with neutropenic fever.19,49
Non-Neutropenic Conditions in the Cancer Patient
The Solid Cancer Patient without Neutropenia.
Prompt initiation of antimicrobial therapy in the febrile non-neutropenic solid cancer patient is not always indicated. Rapid surgical intervention may be more important than the urgent initiation of empirical antibiotics. In febrile non-neutropenic cancer patients who are not ill-appearing and have no identified focus of infection, it may be appropriate to obtain culture specimens and to observe the patient. After consultation with an oncologist, some patients can be discharged home with close follow-up. Indications for urgent antibiotics include signs of sepsis, mental status changes, lactic acidosis, shock, abdominal pain, history of splenectomy, and identification of a focal site of infection.58,59
Impaired Cell-Mediated Immunity.
Bacterial Infections.: Listeria monocytogenes is one of the more common bacterial organisms infecting cancer patients with impaired CMI.60–63 Listeria infection is also seen in patients with organ transplants, diabetes, cirrhosis, and AIDS and in those receiving high-dose corticosteroids. No early characteristics distinguish Listeria infection from bacteremias caused by other organisms. Meningitis, which may be accompanied by cerebritis or brain abscess, is the most common focus of infection and may be manifested with personality changes or focal neurologic signs.64 Cerebrospinal fluid examination frequently does not reveal the organism on Gram’s stain, but protein is elevated and pleocytosis is present. Treatment should be with ampicillin and gentamicin. Trimethoprim-sulfamethoxazole is the alternative drug for patients with penicillin allergy. Vancomycin is not effective in treatment of Listeria infections even when in vitro susceptibility is shown. Cephalosporins, such as ceftriaxone and cefotaxime, are not active against Listeria.
Infections caused by Salmonella species are common in patients with impaired CMI and usually are manifested with fever with or without enteritis.65 Bacteremia can result in infection of bones, joints, central nervous system, and endovascular devices. Multidrug-resistant Salmonella species are increasing. Treatment usually includes a third-generation cephalosporin or a fluoroquinolone because many isolates are resistant to ampicillin and trimethoprim-sulfamethoxazole.66
Patients with solid tumors, lymphoma, and leukemia (especially hairy cell leukemia) are at increased risk for pneumonia from Legionella species, with the highest risk in cancer patients receiving high-dose corticosteroids.67,68 Non-pneumophila species of Legionella (e.g., Legionella micdadei and Legionella bozemanii) are particularly common in these patients.69 Clinical and radiographic manifestations of Legionella infection in the immunocompromised patient often differ from those in the immunocompetent host. For example, pleuritic chest pain may be a prominent symptom in the immunocompromised patient and may mimic pulmonary embolism. These patients can have fever without any other symptoms of pneumonia despite the presence of radiographic pulmonary infiltrates. In addition, the chest radiograph may reveal an expanding pulmonary nodule or cavitation of a nodule or infiltrate rather than the usual lower lobe alveolar filling defects. Hyponatremia (serum sodium <130 mEq/L [mmol/L]) is particularly common. Although gastrointestinal and neurologic symptoms and elevated serum transaminase levels are common in patients with Legionella infections, these are not more common in patients with Legionella than in those with other causes of pneumonia. The treatment of choice for immunocompromised patients with Legionella infection is a fluoroquinolone or azithromycin (alternatively, doxycycline or erythromycin, each combined with rifampin).
Nocardiosis is an uncommon but often severe bacterial infection caused by a weakly acid-fast gram-positive branching filamentous rod. It occurs in cancer patients, in those receiving high-dose corticosteroids, and in others with defective CMI.70,71 Subacute pneumonia with nodular infiltrates is the most common manifestation, but Nocardia may also produce cellulitis, subcutaneous abscesses, meningitis, and brain abscess. Diagnosis requires biopsy, tissue stains, and culture. Treatment is with sulfonamides often combined with other agents.
Mycobacterial Infections.: Tuberculosis and other mycobacterial diseases may produce severe disease in those with defective CMI and be manifested as fever of undetermined origin, pneumonia, lymphadenopathy, or skin lesions.72,73 It is easily mistaken for signs caused by the patient’s underlying disease or treatment. Disseminated nontuberculous mycobacterial infections are more common in patients with hairy cell leukemia or chronic myelogenous leukemia.
Fungal Infections.: Infections with Cryptococcus neoformans and Cryptococcus gattii occur in patients with Hodgkin’s and non-Hodgkin’s lymphoma, chronic myelogenous leukemia, and chronic lymphocytic leukemia, especially those taking high-dose corticosteroids.74 Patients with HIV infection, solid organ transplants, diabetes, renal insufficiency, and cirrhosis are also at risk, as are patients receiving prolonged high-dose corticosteroids for connective tissue diseases. Meningitis is the most common manifestation, often with the insidious onset of low-grade fever and subacute (and often intermittent) headache. Many other organ systems can become infected, including the lung, skin, bones, and joints. Diagnosis is made by measurement of cryptococcal antigen (not antibody) in serum and cerebrospinal fluid and by fungal cultures and tissue biopsy.75
Parasitic Infections.: Reactivation of central nervous system infection with the protozoan T. gondii occurs most often in cancer patients with lymphoma and leukemia as well as in HIV infection. Strongyloides stercoralis, an intestinal nematode, is the only helminthic organism producing severe infection in patients with deficient CMI, almost exclusively in those receiving high-dose corticosteroids.76,77 Larvae of the parasite disseminate from intestine to the lung and other organs, including the central nervous system and skin. Wheezing, cough, dyspnea, hemoptysis, and rash are common symptoms. Chest radiographs may show focal or diffuse infiltrates. Dissemination is often accompanied by bacterial infection, usually caused by enteric gram-negative bacilli carried by the parasites from the intestinal tract. Diagnosis includes examination of sputum and stool for parasites. Treatment of choice is ivermectin; thiabendazole is a less effective alternative.
Viral Infections.: The most common viruses producing serious infections in cancer patients with defective CMI are varicella-zoster, herpes simplex, and cytomegalovirus.78 Visceral dissemination is common in primary varicella in nonimmune immunocompromised children and adults. When a nonimmune immunocompromised child or adult is exposed to varicella, varicella-zoster immune globulin (VariZIG, approved by the Food and Drug Administration in the United States for this indication) can be given within 96 hours of exposure to ameliorate the disease.79 Herpes zoster infection is common in cancer patients, particularly those with Hodgkin’s and non-Hodgkin’s lymphoma and leukemia. Disease usually remains localized to the primary dermatome, but dissemination occurs in approximately 11% of patients. Dissemination is usually limited to the skin, but visceral involvement (lung and liver) occasionally occurs. Skin lesions in primary varicella or zoster often become hemorrhagic in these patients.
Cytomegalovirus infection may occur in cancer patients treated with corticosteroids. Measles virus, although uncommon, may produce severe infection with defective CMI. Fever, rash, pneumonia, and encephalitis are common manifestations. Immune serum globulin may be given after exposure to ameliorate disease. Common community respiratory viruses, such as respiratory syncytial virus, influenza, and adenovirus, may produce severe or fatal pneumonia.80
Humoral Immune (B-Cell) Defects.
Hypogammaglobulinemia is common in patients with chronic lymphocytic leukemia and myeloma. Low immunoglobulin levels predispose to infections with encapsulated bacteria, such as S. pneumoniae, H. influenzae, and N. meningitidis.81–84 Pneumonia is the most common manifestation, but sepsis, otitis media, cellulitis, and urinary tract infection may occur. After receiving cytotoxic agents and corticosteroids for treatment, these patients become susceptible to infections associated with impaired CMI as well as bacterial infections caused by S. aureus and gram-negative bacilli. Regular infusions of intravenous immune globulin may decrease the incidence of infection but do not prolong survival. Patients should receive pneumococcal vaccine, but many do not respond.
Disruption of Natural Barriers.
Disruption of natural anatomic barriers (e.g., mucous membranes and skin) by ulcerating tumors, chemotherapy, radiation therapy, diagnostic and therapeutic procedures, and catheters can lead to infection by gram-positive and gram-negative organisms, including anaerobes.85 Oral mucositis, a debilitating and intensely painful condition associated with radiation therapy and high-dose chemotherapy, frequently results in serious local and systemic infections, including life-threatening sepsis with viridans streptococci.86,87 Cancers may cause partial or total obstruction of body lumens and cavities. Stenosis of a lumen may result from radiation therapy. Bronchial obstruction by tumor can lead to pneumonia. Obstruction of the urinary tract may result in infection. Gastrointestinal tract obstruction can lead to perforation and peritonitis.
Pulmonary Infections in the Immunocompromised Patient.
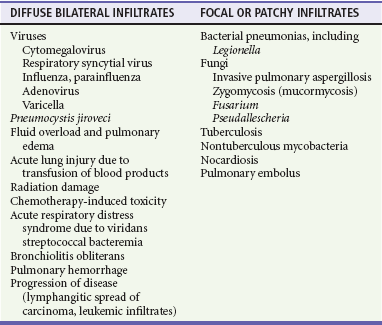
In neutropenic cancer patients, pneumonia is commonly caused by gram-negative bacilli early in neutropenia and by fungal organisms such as Aspergillus late. In those with impaired CMI, cytomegalovirus, Pneumocystis, Legionella, Nocardia, mycobacteria, and fungal organisms predominate. Pneumococcal pneumonia is most common in patients with impaired humoral immunity. Patients with primary lung cancer or with pulmonary metastases from other cancers develop postobstructive pneumonia, lung abscess, and empyema related to S. aureus, gram-negative bacilli, and anaerobes. Mimics of pneumonia in the immunocompromised host include pulmonary emboli and infarction, congestive heart failure, metastatic or primary carcinoma, lymphangitic spread of carcinoma, alveolar hemorrhage, leukoagglutinin reactions, and radiation- and drug-induced pneumonitis.88 An acute presentation of “pneumonia” suggests bacterial pneumonia, pulmonary emboli, congestive heart failure, or pulmonary hemorrhage. Subacute presentation suggests a fungal, nocardial, mycobacterial, or viral etiology (Table 183-3).89,90
Diabetes
Diabetic patients have increased susceptibility to infection because of defects in immune function, excess substrate for fungal and bacterial growth, vascular insufficiency related to microangiopathy and atherosclerosis, and sensory neuropathy that leads to wound neglect.91–93
Neutrophil and monocyte-macrophage functions are impaired in diabetic patients, including adherence to bacteria, chemotaxis, phagocytosis, and intracellular killing. These defects are exacerbated by hyperglycemia and improved by tight glucose control. Although decreased lymphocyte proliferative responses to phytohemagglutinin and certain pathogens are described, cellular immunity appears normal or only minimally affected by diabetes. Humoral immunity is normal in diabetics.94,95
Alcoholism and Cirrhosis
Alcohol consumption predisposes to infection through direct suppression of the immune system, alterations in blood flow, depression of mental status, and delay in seeking medical care.96
With alcoholic cirrhosis, there is deficient hepatic clearance and killing of bacteria by reticuloendothelial cells as well as splenic hypofunction.97 Complement deficiency occurs because the liver is the primary site of C3 synthesis. Neutrophils show impaired recruitment to infective sites and defective chemotaxis and phagocytosis.98,99 Cellular immune deficiency occurs and is exacerbated by malnutrition. Bactericidal activity of IgM antibody against gram-negative pathogens such as E. coli and H. influenzae is decreased.
Common infections include spontaneous bacteremia and sepsis caused by E. coli, K. pneumoniae, Salmonella, streptococci, Vibrio vulnificus, and Aeromonas; spontaneous bacterial peritonitis, usually caused by E. coli, K. pneumoniae, S. pneumoniae, or enterococci; pneumonia related to pneumococci, gram-negative bacilli (E. coli, K. pneumoniae, and H. influenzae), and anaerobes; tuberculosis; meningitis caused by S. pneumoniae and L. monocytogenes; and skin and soft tissue infections with S. aureus, streptococci, and gram-negative bacilli. Nasopharyngeal and cutaneous diphtheria also occurs.100
Renal Failure
Infections cause up to 20% of all deaths among patients with chronic renal failure and are the second most common cause of mortality after coronary artery disease.101,102 Disruption of cutaneous barriers at vascular access sites and peritoneal dialysis catheter sites and numerous immune system defects are responsible for the increased incidence of infection. Uremic pruritus with excoriation, epidermal and sweat gland atrophy, dryness, and vesicular eruptions also compromise the cutaneous barrier. Reduced renal clearance of unknown toxins, nutritional deficiencies, and administration of immunosuppressive medications lead to aberrant immune regulation early in the course of renal failure.
Chronic kidney failure leads to a state of generalized immune hyporesponsiveness. Neutrophils show reduced mobility, chemotaxis, adherence, phagocytosis, and intracellular bactericidal activity, and leukopenia is commonly present. CMI is severely impaired, with decreased activation and proliferation of T lymphocytes and reduced NK cell activity, which cannot be reversed by hemodialysis. Furthermore, humoral immunity is adversely affected, resulting in deficient production of certain IgG subclass antibodies. Poor response to vaccines is common but can be improved by reinforced vaccination schedules, increased vaccine dosage, and adjunct immunomodulators.103
Additional predisposing factors to infection in uremic patients include low serum albumin, iron overload, increased intracellular calcium, circulating low-molecular-weight uremic toxins, metabolic acidosis, circulating inhibitors to chemotactic factors, decreased production of endogenous pyrogens, and invasive vascular procedures for dialysis access. The annual mortality rate from sepsis in dialysis patients is increased 100 to 300 times.104
Splenectomy, Hyposplenia, and Functional Asplenia
The spleen is the most important organ in the reticuloendothelial system and the primary site for IgM synthesis, the first early immune response of the body. Opsonin production in the spleen facilitates phagocytosis of bacteria by intracellular macrophages. Patients without a spleen also have decreased production of neutrophils, NK cells, and immunomodulating cytokines.105,106
The spleen is the principal site of clearance of S. pneumoniae from the blood. Splenectomy or functional asplenia predisposes to overwhelming pneumococcal infection and fulminant infection with other encapsulated organisms (H. influenzae, N. meningitidis, and Capnocytophaga canimorsus after dog bites) and gram-negative bacilli (E. coli and P. aeruginosa). Asplenic people who become infected with Babesia microti, a malaria-like protozoan transmitted by tick bite in the United States, develop severe and often fatal hemolysis. Human granulocytic anaplasmosis (formerly ehrlichiosis), another tick-borne infection, is severe and sometimes fatal in asplenic patients. In addition, the gram-negative coccobacillus Bordetella holmesii produces a non–life-threatening acute febrile illness with bacteremia in patients with asplenia. Pneumococcal sepsis represents 50 to 90% of cases. Most healthy adults who die after fulminating pneumococcal sepsis have had a splenectomy or have a congenitally small or abnormal spleen.107–110
Overwhelming postsplenectomy sepsis is rare, and the true incidence is unknown because of a lack of prospective studies.111 The risk is greater in children than in adults, and children younger than 2 years are at greatest risk. The risk is highest in the first few years after splenectomy but persists throughout life into old age. People undergoing splenectomy for a hematologic disorder or lymphoma are at much higher risk for overwhelming postsplenectomy infection than are those undergoing splenectomy for trauma. This is probably because of the occurrence of splenic implants (splenosis) or accessory spleens in traumatized patients. Patients with functional asplenia from sickle cell anemia or thalassemia major are at high risk for overwhelming bacterial infections as well.
When overwhelming postsplenectomy infection occurs, often no obvious source of infection is found. Prodromal symptoms, such as fever, rigors, malaise, myalgias, headache, vomiting, and diarrhea, may be present for 1 or 2 days.112 Patients seen at this time may be misdiagnosed as having a viral illness, gastroenteritis, or food poisoning. Abrupt deterioration then occurs during hours, with rapid progression to septic shock with disseminated intravascular coagulation, purpura, and multiorgan dysfunction. The mortality rate is high (50-70%), with younger children having the highest mortality rate. In addition, meningitis without overwhelming infection or shock is a common presentation of pneumococcal infection in asplenic patients.113 When fever develops in a person at risk for this disorder, treatment with an antimicrobial agent effective against S. pneumoniae should be initiated without delay. After blood culture is performed, the patient should receive ceftriaxone or cefotaxime, with addition of vancomycin in areas where penicillin resistance is prevalent. Clindamycin, levofloxacin, and moxifloxacin are alternatives for patients with serious penicillin allergy.
Use of pneumococcal vaccine in patients at risk is especially important now that antimicrobial-resistant S. pneumoniae is prevalent.114 Asplenic people should be immunized against pneumococcus, H. influenzae type b, N. meningitidis, and influenza virus. People with functional hyposplenism related to serious underlying diseases often respond poorly to pneumococcal vaccine. Children should receive prophylaxis with oral penicillin or amoxicillin up to the age of 5 years and for at least 1 or 2 years after splenectomy, provided they have not had an invasive pneumococcal infection and have received pneumococcal immunizations. Long-term antimicrobial prophylaxis is generally not recommended in adults. These patients should have standby oral antibiotics at home (amoxicillin-clavulanate, levofloxacin, or moxifloxacin) with instructions to self-administer at the first sign of infection, and they should be provided with information and a medical alert bracelet. Fatal pneumococcal infection has occurred in patients immunized with pneumococcal vaccine who were also taking penicillin. An ED-based pneumococcal vaccine program, if it is implemented, could reduce mortality in high-risk patients while remaining cost-effective.115,116
Immunosuppressive Therapy
High doses of corticosteroids alter the distribution and function of neutrophils, monocytes, and lymphocytes.117 Corticosteroids suppress inflammation and enhance susceptibility to infection by impairing the mobilization and function of neutrophils and mononuclear cells at sites of primary lodgment of microorganisms in tissues. Corticosteroids inhibit neutrophil adherence to endothelium, decrease chemotaxis of neutrophils and monocytes, and inhibit phagocytosis and intracellular killing of microorganisms. Corticosteroids also severely impair CMI, probably a result of inhibition of the migration of lymphocytes to the site of antigen challenge, inhibition of lymphokine production, and consequent inhibition of lymphocyte proliferation. They also inhibit both classical and alternative pathways of complement activation. The hyperglycemia that occurs with corticosteroid use also contributes significantly to infection risk. Moreover, patients receiving high-dose corticosteroids have infection risks related to anatomic abnormalities of the underlying disease, treatment with other immunosuppressive agents, cancer chemotherapeutic agents, radiation therapy, and implantation of foreign bodies.118–120
The most common infections occurring in patients receiving high-dose corticosteroids are those caused by pyogenic bacteria (S. aureus, streptococci, and gram-negative bacilli). Despite the profound depression of CMI that occurs in patients taking corticosteroids, these patients generally have few infections commonly recognized as associated with defective CMI. The most common are tuberculosis and severe or disseminated infections caused by varicella-zoster and herpes simplex viruses. Patients receiving moderate doses of corticosteroids for asthma and other disorders are at increased risk for lethal primary varicella infection.121 Other infections seen with corticosteroid use include those caused by Listeria, Salmonella, Legionella, Nocardia, Candida, Aspergillus, Cryptococcus, Histoplasma, Coccidioides, Pneumocystis, Toxoplasma, Cryptosporidium, and Strongyloides. Patients with neurologic diseases have much higher rates of infectious complications than do patients with intestinal, hepatic, or renal disease. The infectious complications related to corticosteroid use increase with doses of prednisone equivalents of more than 20 mg/day in adults, with total doses of more than 700 mg, and with treatment longer than 30 days. The risk of adrenal suppression can be decreased by use of prednisone doses less than 7.5 mg/day, administration of doses early in the day, avoidance of split doses, and use of alternate-day dosing.
Corticosteroids decrease leukocyte accumulation at inflammatory sites, and the whole cascade of responses leading to local manifestations of infection is slowed. These effects result in late presentation of serious infections. The ability of parietal cavities to localize sepsis is reduced. In addition, prolonged administration of corticosteroids results in delayed wound healing. For example, skin sutures should be left in place 50 to 100% longer than in normal patients. Short-term treatment has little effect on wound healing.122
The diagnosis of peritonitis resulting from perforation of colonic diverticula, appendicitis, peptic ulcer, or another primary intra-abdominal condition is particularly difficult.123 These patients have abdominal discomfort, but they may have few abdominal findings and need rapid and aggressive investigation for life-threatening abdominal disease. CT scan of the abdomen and pelvis and surgical consultation may be needed emergently in these patients. Broad-spectrum antimicrobials to cover for gram-negative enteric bacilli and anaerobes should be administered without delay.
Other Immunosuppressive Medications
Commonly used immunosuppressives include cyclosporine, tacrolimus, sirolimus, mycophenolate, azathioprine, methotrexate, and cyclophosphamide. They treat a wide variety of conditions, including rheumatoid arthritis, psoriasis, nephrotic syndrome, and inflammatory bowel disease, and they are used in the prevention and treatment of organ transplant rejection.124 These drugs depress immune function, especially CMI. In addition, they have a narrow therapeutic window, wide-ranging toxic side effects, and many significant drug-drug and drug-food interactions. Patients may present for evaluation of symptoms caused by an adverse drug reaction or an infection. Before altering current medications, the physician needs to check carefully for drug interactions. For a more detailed discussion of the toxic effects of these agents, refer to Chapter 184.
Immunomodulating agents are available for treatment of a variety of immune-mediated inflammatory diseases, including rheumatoid arthritis, psoriasis and psoriatic arthritis, ankylosing spondylitis, and inflammatory bowel disease. Some of these drugs include inhibitors of tumor necrosis factor alpha (infliximab, adalimumab, certolizumab, golimumab, etanercept), inhibitors of interleukins (tocilizumab, anakinra), inhibitor of pyrimidine synthesis (leflunomide), and inhibitor of T-cell activation (abatacept). These agents, particularly the tumor necrosis factor inhibitors, are associated with increased susceptibility to infection, particularly disseminated infection with various intracellular pathogens. Reactivation of latent infection with Mycobacterium tuberculosis, nontuberculous mycobacterial infection, histoplasmosis, and coccidioidomycosis is frequently disseminated and extrapulmonary at presentation. Additional infections seen at increased frequency include cryptococcosis, listeriosis, legionellosis, salmonellosis, aspergillosis, candidiasis, and pneumocytosis. The clinician should be alert to unusual manifestations of infection in patients taking these agents as misdiagnosis and delayed diagnosis increase mortality.125–127 These drugs may also cause impaired wound healing, so skin sutures should be left in place for a longer time than is usual.128
References
1. Donnelly, JP, et al. Infections in immunocompromised hosts: General principles. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier/Churchill Livingstone; 2010:3781–3791.
2. Delves, PJ, Roitt, IM. The immune system: First of two parts. N Engl J Med. 2000;343:37.
3. Kaufmann S, Rouse B, Sacks D, eds. The Immune Response to Infection. Washington, DC: American Society of Microbiology Press, 2011.
4. Zabriskie, JB. Essential Clinical Immunology. New York: Cambridge University Press; 2009.
5. Medzhitov, R, Janeway, CJr. Innate immunity. N Engl J Med. 2000;343:338.
6. Abbas AK, Lichtman AH, Pillai S, eds. Cellular and Molecular Immunology, 6th ed, Philadelphia: Saunders, 2007.
7. Newhouse, M, Sanchis, J, Biennenstock, J. Lung defense mechanisms. N Engl J Med. 1976;295:990.
8. Dieffenback, CW, et al. Innate (general or nonspecific) host defense mechanisms. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier/Churchill Livingstone; 2010:37–47.
9. Delves, PJ, Roitt, IM. The immune system: Second of two parts. N Engl J Med. 2000;343:108.
10. Birdsall, HH. Antibodies. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier/Churchill Livingstone; 2010:59–75.
11. Figueroa, JE, Densen, P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359.
12. Ross, SC, Densen, P. Complement deficiency states and infection: Epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore). 1984;63:243.
13. Perez, HD, Lipton, M, Goldstein, IM. A specific inhibitor of complement (C5a)–derived chemotactic activity in serum from patients with systemic lupus erythematosus. J Clin Invest. 1978;78:29.
14. Klastersky, J. Infections in cancer patients with suppressed cellular immunity. Recent Results Cancer Res. 1993;132:147.
15. Francis, P, Walsh, TJ. Current approaches to the management of fungal infections in cancer patients. Oncology. 1992;6:81.
16. Nauseef, WM, Clark, RA. Granulocytic phagocytes. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier/Churchill Livingstone; 2010:99–127.
17. Rolston, KVI, Bodey, GP. Infections in patients with cancer. In Hong WI, et al, eds.: Holland-Frei Cancer Medicine, 8th ed, Shelton, Conn: People’s Medical Publishing House, 2010.
18. Alexander, SW, Pizzo, PA. Current considerations in the management of fever and neutropenia. Curr Clin Top Infect Dis. 1999;19:160.
19. Freifeld, AG, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56.
20. Crawford, J, Dale, DC, Lyman, GH. Chemotherapy-induced neutropenia: Risks, consequences, and new directions for its management. Cancer. 2004;100:228.
21. Schimpff, SC, Scott, DA, Wade, JC. Infections in cancer patients: Some controversial issues. Support Care Cancer. 1994;2:94.
22. Pizzo, PA. Fever in immunocompromised patients. N Engl J Med. 1999;341:893.
23. Andre, S, et al. Febrile neutropenia in French emergency departments: Results of a prospective multicentre survey. Crit Care. 2010;14:R68.
24. Giamarellou, H, Antoniadou, A. Infectious complications of febrile leukopenia. Infect Dis Clin North Am. 2001;15:457.
25. Zinner, SH. Changing epidemiology of infections in patients with neutropenia and cancer: Emphasis on gram-positive and resistant bacteria. Clin Infect Dis. 1999;29:490.
26. Wisplinghoff, H, Seifert, H, Wenzel, RP, Edmond, MB. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin Infect Dis. 2003;36:1103.
27. Haslam, DB. Managing the child with fever and neutropenia in an era of increasing microbial resistance. J Pediatr. 2002;140:5.
28. Tunkel, AR, Sepkowitz, KA. Infections caused by viridans streptococci in patients with neutropenia. Clin Infect Dis. 2002;34:1524.
29. Patterson, TF, et al. Invasive aspergillosis: Disease spectrum, treatment practices, and outcomes. Medicine (Baltimore). 2000;79:250.
30. Walsh, TJ, et al. Treatment of aspergillosis: Clinical practice guideline of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327.
31. Pizzo, PA, Robichaud, KJ, Wesley, R, Commers, JR. Fever in the pediatric and young adult patient with cancer: A prospective study of 1001 episodes. Medicine (Baltimore). 1982;61:153.
32. Heussel, CP, Kauczor, HU, Ullmann, AJ. Pneumonia in neutropenic patients. Eur Radiol. 2004;14:256.
33. Lordick, F, et al. Ultrasound screening for internal jugular vein thrombosis aids the detection of central venous catheter–related infections in patients with haemato-oncological diseases: A prospective observational study. Br J Haematol. 2003;120:1073.
34. Lin, MY, et al. Delay of active antimicrobial therapy and mortality among patients with bacteremia: Impact of severe neutropenia. Antimicrob Agents Chemother. 2008;52:3188.
35. Furno, P, Bucaneve, G, Del Favero, A. Monotherapy or aminoglycoside-containing combinations for empirical antibiotic treatment of febrile neutropenic patients: A meta-analysis. Lancet Infect Dis. 2002;2:231.
36. Paul, M, Soares-Weiser, K, Leibovici, L. Beta lactam monotherapy versus beta lactam–aminoglycoside therapy for fever with neutropenia: Systematic review and meta-analysis. BMJ. 2003;326:1111.
37. Raad, II, et al. Treatment of febrile neutropenic patients with cancer who require hospitalization: A prospective randomized study comparing imipenem and cefepime. Cancer. 2003;98:1039.
38. Drugs for bacterial infections. Treat Guidel Med Lett. 2010;8:43.
39. Gafter-Gvili, A, et al. Meta-analysis: Antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med. 2005;142:979.
40. Leibovici, L, et al. Antibiotic prophylaxis in neutropenic patients. Cancer. 2006;107:1743.
41. Viscoli, C, Castagnola, E. Prophylaxis and empirical therapy of infection in cancer patients. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier/Churchill Livingstone; 2010:3793–3807.
42. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 1995;44:1.
43. Paul, M, et al. Additional anti–gram-positive treatment for febrile neutropenia. Cochrane Database Syst Rev. (3):2005.
44. Gotzsche, PC, Johansen, HK. Routine versus selective antifungal administration for control of fungal infections in patients with cancer. Cochrane Database Syst Rev. (2):2002.
45. Kuderer, NM, Dale, DC, Crawford, J, Lyman, GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: A systematic review. J Clin Oncol. 2007;25:3158.
46. Sung, L, et al. Meta-analysis: Effect of prophylactic hematopoietic colony-stimulating factors on mortality and outcomes of infection. Ann Intern Med. 2007;147:400.
47. Koh, A, Pizzo, PA. Empirical oral antibiotic therapy for low risk febrile cancer patients with neutropenia. Cancer Invest. 2002;20:420.
48. Kamana, M, Escalante, C, Mullen, CA. Bacterial infections in low-risk, febrile neutropenic patients: Over a decade of experience at a comprehensive cancer center. Cancer. 2005;104:422.
49. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Prevention and Treatment of Cancer-Related Infections, version 1. NCCN.org, 2011.
50. Freifeld, A, et al. A double-blind comparison of empirical oral and intravenous antibiotic therapy for low-risk febrile patients with neutropenia during cancer chemotherapy. N Engl J Med. 1999;341:305.
51. Kern, WV, et al. Oral versus intravenous empirical antimicrobial therapy for fever in patients with granulocytopenia who are receiving cancer chemotherapy. N Engl J Med. 1999;341:312.
52. Innes, HE, et al. Oral antibiotics with early hospital discharge compared with inpatient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: A prospective randomized controlled single centre study. Br J Cancer. 2003;89:43.
53. Cherif, H, et al. The feasibility of early hospital discharge with oral antimicrobial therapy in low risk patients with febrile neutropenia following chemotherapy for hematologic malignancies. Haematologica. 2006;91:215.
54. Klastersky, J, et al. Outpatient oral antibiotics for febrile neutropenic cancer patients using a score predictive for complications. J Clin Oncol. 2006;24:4129.
55. Vidal, L, et al. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database Syst Rev. (4):2004.
56. Mullen, CA. Ciprofloxacin in treatment of fever and neutropenia in pediatric cancer patients. Pediatr Infect Dis J. 2003;22:1138.
57. Uzun, O, Anaissie, EJ. Outpatient therapy for febrile neutropenia: Who, when, and how? J Antimicrob Chemother. 1999;43:317.
58. Gea-Banacloche, J, Segal, BH. Infections in the cancer patient. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011:2262–2299.
59. Rolston, KVI, Rubenstein, EB. Neutropenia and sepsis in cancer patients. In: Shaw AD, et al, eds. Acute Care of the Cancer Patient. Boca Raton, Fla: Taylor & Francis; 2005:539–566.
60. Gellin, BG, Broome, CV. Listeriosis. JAMA. 1989;261:1313.
61. Khardori, N, et al. Spectrum and outcome of microbiologically documented Listeria monocytogenes infection in cancer patients. Cancer. 1989;64:1968.
62. Lorber, B. Listeriosis. Clin Infect Dis. 1997;24:1.
63. Safdar, A, Armstrong, D. Listeriosis in patients at a comprehensive cancer center. Clin Infect Dis. 2003;37:359.
64. Pruitt, A. Nervous system infections in patients with cancer. Neurol Clin North Am. 2003;21:193.
65. Cohen, JI, Bartlett, JA, Corey, GR. Extraintestinal manifestations of Salmonella infections. Medicine (Baltimore). 1987;66:349.
66. Pegues, DA, Miller, SI. Salmonella species, including Salmonella typhi. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier/Churchill Livingstone; 2010:2887–2903.
67. Edelstein, PH. Legionnaires’ disease. Clin Infect Dis. 1993;16:741.
68. Stout, JE, Yu, VL. Legionellosis. N Engl J Med. 1997;337:682.
69. Fang, GD, Yu, VL, Vickers, RM. Disease due to the Legionellaceae (other than Legionella pneumophila): Historical, microbiological, clinical and epidemiological review. Medicine (Baltimore). 1989;68:116.
70. Berkey, P, Bodey, GP. Nocardial infection in patients with neoplastic disease. Rev Infect Dis. 1989;11:407.
71. Lerner, PI. Nocardiosis. Clin Infect Dis. 1996;22:891.
72. Kaplan, MH, Armstrong, D, Rosen, P. Tuberculosis complicating neoplastic disease: A review of 201 cases. Cancer. 1974;33:850.
73. Akiyama, H, Maruyama, T, Uetake, T. Systemic infection due to atypical mycobacteria in patients with chronic myelogenous leukemia. Rev Infect Dis. 1991;13:815.
74. Kaplan, MH, Rosen, P, Armstrong, D. Cryptococcosis in a cancer hospital: Clinical and pathological correlates in forty-six patients. Cancer. 1977;39:2265.
75. Chayakulkeeree, M, Perfect, JR. Cryptococcosis. Infect Dis Clin North Am. 2006;20:507.
76. Siddiqui, AA, Berk, SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040.
77. Keiser, PB, Nutman, TB. Strongyloides stercoralis infection in the immunocompromised population. Clin Microbiol Rev. 2004;17:208.
78. Wade, JC. Viral infections in patients with hematological malignancies. Hematology Am Soc Hematol Educ Program. 2006:368.
79. VariZIG for prophylaxis after exposure to varicella. Med Lett Drugs Ther. 2006;48:69.
80. Whimbey, E, Englund, JA, Couch, RB. Community respiratory virus infections in immunocompromised patients with cancer. Am J Med. 1997;102:10.
81. Blade, J, Rosinol, L. Complications of multiple myeloma. Hematol Oncol Clin North Am. 2007;21:1231.
82. Wadhwa, PD, Morrison, VA. Infectious complications of chronic lymphocytic leukemia. Semin Oncol. 2006;33:240.
83. Morrison, VA. Infectious complications of chronic lymphocytic leukemia: Pathogenesis, spectrum of infection, preventive approaches. Best Pract Res Clin Haematol. 2010;23:145.
84. Nucci, M, Anaissie, E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. 2009;49:1211.
85. Glauser, M. Alterations of host defenses: The key to the multifaceted spectrum of infections in immunocompromised patients. Recent Results Cancer Res. 1991;121:321.
86. Wilkes, JD. Prevention and treatment of oral mucositis following cancer chemotherapy. Semin Oncol. 1998;25:538.
87. Epstein, JB. Mucositis in the cancer patient and immunocompromised host. Infect Dis Clin North Am. 2007;21:503.
88. Shorr, AF, Susla, GM, O’Grady, NP. Pulmonary infiltrates in the non–HIV-infected immunocompromised patient. Etiologies, diagnostic strategies, and outcomes. Chest. 2004;125:260.
89. Cunha, B. Pneumonias in the compromised host. Infect Dis Clin North Am. 2001;15:591.
90. Rolston, KV. The spectrum of pulmonary infections in cancer patients. Curr Opin Oncol. 2001;13:218.
91. Geerlings, SE, Hoepelman, AI. Immune dysfunction in patients with diabetes mellitus. FEMS Immunol Med Microbiol. 1999;26:259.
92. Calvet, HM, Yoshikawa, TT. Infections in diabetes. Infect Dis Clin North Am. 2001;15:407.
93. Delamaire, M, et al. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14:29.
94. Gupta, S, et al. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am. 2007;21:617.
95. Peleg, AY, et al. Common infections in diabetes: Pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2007;23:3.
96. MacGregor, RR, Louria, DB. Alcohol and infection. Curr Clin Top Infect Dis. 1997;17:291.
97. Szabo, G, Mandrekar, P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 2009;33:220.
98. Engelich, G, Wright, DG, Hartshorn, KL. Acquired disorders of phagocyte function complicating medical and surgical illnesses. Clin Infect Dis. 2001;33:2040.
99. Christou, L, Pappas, G, Falagas, ME. Bacterial infection–related morbidity and mortality in cirrhosis. Am J Gastroenterol. 2007;102:1510.
100. Johnson, DH, Cunha, BA. Infections in cirrhosis. Infect Dis Clin North Am. 2001;15:363.
101. Minnaganti, VR, Cunha, BA. Infections associated with uremia and dialysis. Infect Dis Clin North Am. 2001;15:385.
102. Bloembergen, WE, Port, FK. Epidemiological perspectives on infections in chronic dialysis patients. Adv Ren Replace Ther. 1996;3:201.
103. Johnson, DW, Fleming, SJ. The use of vaccines in renal failure. Clin Pharmacokinet. 1992;22:434.
104. Foley, RN. Infections in patients with chronic kidney disease. Infect Dis Clin North Am. 2007;21:659.
105. Sumaraju, V, Smith, LG, Smith, SM. Infectious complications in asplenic hosts. Infect Dis Clin North Am. 2001;15:551.
106. William, BM, Corazza, GR. Hyposplenism: A comprehensive review. Part I: Basic concepts and causes. Hematology. 2007;12:1.
107. Brigden, ML. Detection, education and management of the asplenic or hyposplenic patient. Am Fam Physician. 2001;63:499.
108. Davidson, RN, Wall, RA. Prevention and management of infections in patients without a spleen. Clin Microbiol Infect. 2001;7:657.
109. Lutwick, LI. Life threatening infections in the asplenic or hyposplenic individual. Curr Clin Top Infect Dis. 2002;22:78.
110. Shepard, CW, et al. Bordetella holmesii bacteremia: A newly recognized clinical entity among asplenic patients. Clin Infect Dis. 2004;38:799.
111. Price, VE, Blanchette, VS, Ford-Jones, EL. The prevention and management of infections in children with asplenia or hyposplenia. Infect Dis Clin North Am. 2007;21:697.
112. Lutwick, LI. Infections in asplenic patients. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Elsevier/Churchill Livingstone; 2010:3865–3873.
113. Schutze, GE, et al. Invasive pneumococcal infections in children with asplenia. Pediatr Infect Dis J. 2002;21:278.
114. William, BM, et al. Hyposplenism: A comprehensive review. Part II: Clinical manifestations, diagnosis, and management. Hematology. 2007;12:89.
115. Stack, SJ, Martin, DR, Plouffe, JF. An emergency department–based pneumococcal vaccination program could save money and lives. Ann Emerg Med. 1999;33:299.
116. Rimple, D, et al. An emergency department–based vaccination program: Overcoming the barriers for adults at high risk for vaccine-preventable diseases. Acad Emerg Med. 2006;13:922.
117. Fareau, GG, Vassilopoulou-Sellin, R. Hypercortisolemia and infection. Infect Dis Clin North Am. 2007;21:639.
118. Stuck, AE, Minder, CE, Frey, FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11:954.
119. Lionakis, MS, Kontoyiannis, D. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828.
120. Klein, NC, Go, H, Cunha, BA. Infections associated with steroid use. Infect Dis Clin North Am. 2001;15:423.
121. Dowell, SF, Bresee, JS. Severe varicella associated with steroid use. Pediatrics. 1993;92:223.
122. Nohr, C. Host defenses. In: Meakins JL, ed. Surgical Infections: Diagnosis and Treatment. New York: Scientific American; 1994:21–28.
123. Nathans, A, et al. Peritonitis and other intra-abdominal infections. In: Howard RJ, Simmons RL, eds. Surgical Infectious Diseases. 3rd ed. Norwalk, Conn: Appleton & Lange; 1995:959–1009.
124. Smith, JM, Nemeth, TL, McDonald, RA. Current immunosuppressive agents: Efficacy, side effects, and utilization. Pediatr Clin North Am. 2003;50:1283.
125. Bongartz, T, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies. JAMA. 2006;295:2275.
126. Raychaudhuri, SP, et al. Incidence and nature of infectious disease in patients treated with anti-TNF agents. Autoimmun Rev. 2009;9:67.
127. Koo, S, Marty, FM, Baden, LR. Infectious complications associated with immunomodulating biologic agents. Infect Dis Clin North Am. 2010;24:285.
128. Busti, AJ, et al. Effects of perioperative anti-inflammatory and immunomodulating therapy on surgical wound healing. Pharmacotherapy. 2005;25:1566.