18 The immune system
Acquired Immunity: The ability of the human body to develop an extremely powerful specific immunity against most invading agents.
Active Acquired Immunity: Immunity that develops when a person comes into direct contact with a pathogen either by contracting the disease produced by the pathogen or by vaccination against the disease.
Antibody: A globulin molecule with the potential to attack agents that are foreign to the host.
Antigen: A protein, large polysaccharide, or large lipoprotein complex that stimulates the process of acquired immunity.
B Lymphocytes or Bursa-Dependent Cells: Immunocompetent lymphocytes that are named for the preprocessing that occurs in the bursa of Fabricius of birds and is responsible for humoral immunity.
Cellular or Cell-Mediated Immunity: A type of acquired immunity that uses sensitized lymphocytes as the primary defense.
Clone: A group of cells that originate from a single parent cell.
Humoral Immunity: A type of acquired immunity that uses antibodies as the primary defense.
Immunity: The ability of the human body to resist almost all types of organisms or toxins that can damage tissues and organs.
Immunodeficiency Disease: Immunosuppression that results from a deficiency of a single humoral antibody group or from a combined deficiency of both T cell and B cell systems.
Immunosuppression: A state of nonresponsiveness of the immune system to antigenic challenge.
Innate Immunity: General processes in the human body, other than those of acquired immunity, that are responsible for protection against organisms and toxins.
Lymphopenia: Decreased function of the lymphoid organs.
Passive Acquired Immunity: Immunity that results when a person receives immune cells or immune serum produced by someone else.
Phagocytosis: The envelopment and digestion of bacteria or other foreign substances.
Sensitized Lymphocytes: Lymphocytes that are made competent by processing to facilitate immunologic activity, such as attachment to and destruction of a foreign agent.
Stem Cells: Unspecialized cells that give rise to specific specialized cells such as T and B lymphocytes.
T Lymphocytes: Sensitized lymphocytes that are responsible for cellular immunity.
Physical and chemical barriers
The body’s first line of immunologic defense is the mechanical barrier provided by the epithelial surface. Some parts of the epithelium have extensions from their surface, such as the cilia and the mucus in the respiratory system. These extensions provide an additional physical barrier to the entrance of foreign substances and an efficient removal system. Hydrochloric acid, which is thought to have bactericidal action, is secreted in the stomach. As an additional defense, the skin produces chemicals that inactivate bacteria. The surfaces of the boundary tissues also have specific defenses in the form of secretory antibodies. Consequently, surgical incisions, intravenous cannulation, and many other invasive procedures can cause major breaks in the first line of defense. Therefore the perianesthesia nurse should use good aseptic or sterile technique to prevent an overwhelming bacterial invasion through the boundary tissues.
Acquired immunity
Humoral immunity
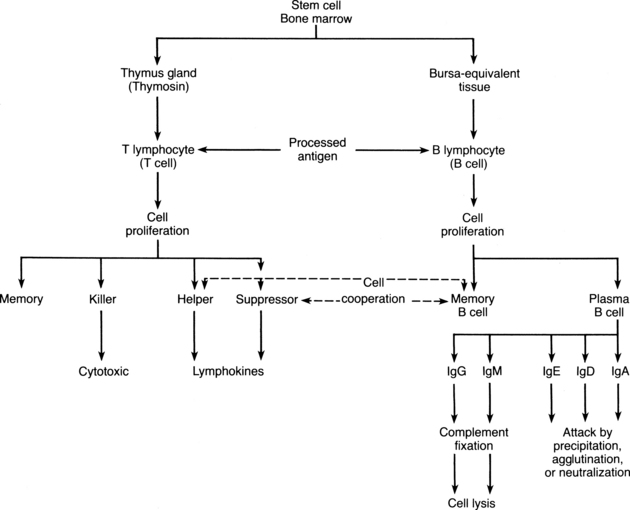
On the second stimulation by the same antigen, a second response occurs. This secondary response, in which a massive amount of antibody specific to the antigen is produced within 1 or 2 days, lasts for months. The secondary response is more rapid, stronger, and more persistent than the primary response because of the memory cells and clones that are produced by the initial exposure to the antigen. If the T lymphocytes are activated by the same antigen, the T lymphocyte helper cells enhance the response of the B lymphocytes; therefore, because of this cooperative effort, the total number of lymphocytes in the lymphoid tissue increases markedly. On second exposure to an antigen, the same plasma cell can produce the particular antibody needed and can convert from one type of antibody secretion to another as needed. When the specific antibodies from the plasma cells are no longer needed, further production of the antibodies is suppressed by the antibodies themselves or by T lymphocyte suppressor cells (Fig. 18-1).
Immunoglobulins, or antibodies, once secreted by the plasma cells, protect the body against invading agents with the following three mechanisms of action: (1) attacking the antigen; (2) activating the complement system, which results in cell lysis; and (3) activating the immediate hypersensitivity reaction, which localizes the invader and may negate its virulence. More specifically, antibodies can inactivate the invading antigen with precipitation, agglutination, neutralization, or complement fixation. Precipitation occurs when an insoluble antibody forms a complex with a soluble antigen, such as tetanus toxin, and the resulting antigen-antibody complex becomes insoluble and precipitates. When antigens are bound together and react with an antibody, agglutinated aggregates occur. Neutralization is achieved when antibodies cover the toxic sites of an antigenic agent or when antibodies counteract toxins released by bacteria. Rarely are the potent antibodies able to attack a cell membrane directly and cause lysis. However, one of the powerful effects of the binding of the antigen-antibody complex is the activation of complement, which serves to amplify this interaction. More specifically, when IgG or IgM binds to an antigen, the complement system is activated and a cascade system of nine different enzyme precursors (C1 through C9) reacts sequentially. The final result of the activation of the complement system is puncture of the antigen’s cell membrane (cell lysis) and rupture of its cellular agents.
IgA is a small molecule that constitutes approximately 15% of the total immunoglobulins and is present in most body secretions. Secretory IgA is effective against viruses and some bacteria that invade the mucous membranes. Secretory immunity is also mediated by IgA. The secretory antibodies are found on the mucosal surfaces of the oral cavity (saliva), the lungs (sputum), and the intestinal and urogenital tracts and in mammary secretions. This secretory IgA differs from other antibodies in that it has a protein molecule, called a secretory piece, attached to it. IgA activates the complement system through a particular sequence of events called the properdin pathway. The complement system is complex cascade of activations of more than 20 proteins that result in the improved ability of phagocytes’ cell killing.
IgD constitutes about 1% of the total immunoglobulins. The exact function of IgD is unknown. Similar to IgA, IgD is situated in the upper respiratory mucosa and works to activate B lymphocytes. IgD has been described as “an ancestral surveillance system at the interface between immunity and inflammation.”1 IgD has also been suggested for relationships in antibody activity directed toward insulin, penicillin, milk proteins, diphtheria toxoid, thyroid antigens, and the products of abnormal tissue growth.
Cellular immunity
The T lymphocytes, like the B lymphocytes, originate from primitive stem cells and go through stages of maturation (see Fig. 18-1). When the immature lymphocyte leaves the bone marrow, it migrates to the thymus gland, where it is acted on by the hormone thymosin. The T lymphocyte then becomes mature and immunocompetent. The origin of the name T lymphocyte is derived from this thymus-dependent maturation. These mature T lymphocytes can circulate in the blood and lymph, or they may come to rest in the inner cortex of the lymph nodes, where they may form subgroups of T lymphocytes.
The direct cellular cytotoxicity that is mediated by cellular immunity involves cytotoxic lymphocytes, or killer cells, and macrophages. The role of these cytotoxic T lymphocytes is not well established; however, they are believed to be involved in nonspecific killing of viruses, rejection of allografts, and immune surveillance of malignant diseases.
Hypersensitivity reactions
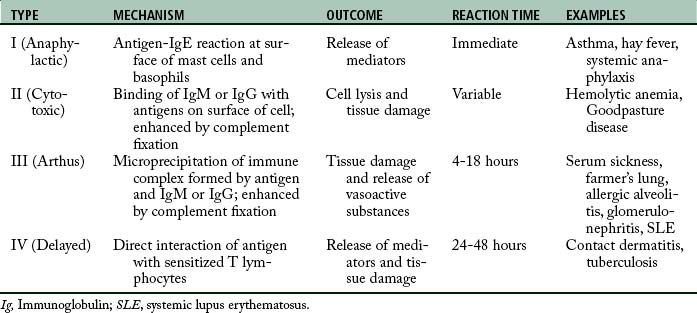
The immune system serves mainly as protection from harmful substances; however, in some instances, the activation of the immune system can cause deleterious effects, which is termed allergic response or hypersensitivity reaction. This response represents a magnified or inappropriate reaction by the host to an antigenic substance; it can result in immunologic disease. Hypersensitivity reactions are divided into four major categories: type I, type II, type III, and type IV hypersensitivity reactions (Table 18-1).
Type III hypersensitivity reaction (arthus)
The type III hypersensitivity reaction involves the formation of immune complexes of antigen and antibody (IgG or IgM). These immune complexes precipitate in and around small vessels and damage the target tissue by activating complement over several hours. Also involved in this process is an inflammatory reaction that is initiated by the gathering of inflammatory cells and the release of vasoactive amines from platelets. As this process continues, polymorphonuclear leukocytes phagocytize the immune complexes and cause inflammation and necrosis of the blood vessels and surrounding tissue because of the release of lysosomal enzymes. Serum sickness and systemic lupus erythematosus are clinical examples of type III hypersensitivity reactions.
Latex allergy
Of major concern is the type I immediate hypersensitivity, which is the IgE-mediated response. This response usually occurs within minutes of exposure to the latex; however, it can occur within a few hours in some cases. A mild reaction is characterized by skin redness, urticaria, and itching. Severe reactions can produce acute rhinorrhea, sneezing, angioedema, bronchospasm, and anaphylactic shock.2
Many organizations are investigating latex allergies, and the American Society of Anesthesiologists has a document that can be found on the Internet (http://ecommerce.asahq.org/publicationsAndServices/latexallergy.pdf). Also, the American Association of Nurse Anesthetists has an excellent web site on latex allergies (http://www.anesthesiapatientsafety.com/patients/latex/fact_sheet.asp). Another expert guideline for management of latex allergies and safe latex use is from the American College of Allergy, Asthma, and Immunology (http://www.acaai.org/allergist/allergies/Types/latex-allergy/Pages/latex-allergies-safe-use.aspx).
The best treatment for a latex allergic response is avoidance. Patients who are at risk for an allergic reaction caused by latex are listed in Box 18-1. Health care personnel with known sensitivity should carry their own nonlatex gloves, usually made of vinyl or neoprene. They also should use nonlatex tourniquets and latex-free or glass syringes and should use stopcocks to inject drugs. Intravenous line tubing should have no latex ports, or if the latex ports exist, they should be taped. Box 18-2 summarizes the various recommendations for patients with known latex allergic reactions or a risk of reactions.
BOX 18-1 Patients Who Should Be Strongly Considered at Risk for an Allergic Reaction to Latex
• History of atopic immunologic reactions
• History of contact dermatitis
• History of documented immunologic reactions of unknown etiology during a medical or surgical procedure
• History of allergies to food products, including fruits, nuts, bananas, avocados, celery, figs, chestnuts, and papayas
• Neural tube defects, including spina bifida, myelomeningocele/meningocele, and lipomyelomeningocele
Preoperative and intraoperative care
• Remove all latex products from the operating room.
• Use a latex-free reservoir bag on the anesthesia machine, oral airways, endotracheal tubes, and laryngeal mask airways.
• Use a latex-free breathing circuit with plastic mask and bag.
• Anesthesia ventilator must have a latex-free bellows.
• Place all monitoring devices, cords, and tubes (oximeter, blood pressure cuffs, electrocardiograph wires) in stockinet and secure with tape to prevent direct skin contact.
• Cover all rubber injection ports on intravenous bags with tape and label them “Do not inject fluid through ports.”
• Use intravenous tubing without latex ports or cover with tape.
• Draw medication directly from opened multidose vials.
• Draw up medications immediately before the beginning of the case.
Postanesthesia phase of care
• Place a sign on the door and flag the chart of the patient to alert personnel to the hypersensitivity.
• Ensure that the patient recovers in a private area.
• Use good handwashing techniques.
• Use only latex-free syringes and gloves.
• Use ampules or remove stoppers from vials before use.
• Cover injection ports on minibags and use latex-free tubings.
• Remain alert for signs and symptoms (hypotension, tachycardia, and bronchospasm) of a latex allergy response and have appropriate emergency drugs and equipment available.
Treatment for the type I reaction should consist of first carefully looking for the latex allergen, such as rubber drains in the wound, inadvertent use of latex gloves, latex that contains urinary draining tubes, or intravenous tubing. In addition, remove all latex from the patient bedside; change gloves, discontinue antibiotics and blood administration, maintain the airway, administer 100% oxygen, intubate the trachea if necessary, and maintain intravascular volume support. Once the diagnosis of type I IgE-mediated anaphylaxis has been confirmed, epinephrine should be administered intravenously in doses of 0.1 mg/kg and titrated to effect. These small doses stabilize the patient’s condition and avoid ventricular tachycardia and malignant arrhythmias.
The increasing recognition of latex allergy and accurate testing that is available has led to a decrease in the number of latex allergic persons. Advances in the understanding of these processes should allow this trend to continue.2 In the meantime, many health care facilities have become latex free environments to protect both employees and patients.
Immunosuppression
Forms of immunosuppression
The unresponsive state of the immune system may be caused by a natural tolerance to self-antigens, to a pathologic state, or to induced immunosuppression. Researchers are attempting to understand immunosuppression by artificially manipulating the immune system to produce a natural tolerance to self-antigens. Pathologic states such as lymphoma and leukemia are examples of the second form of immunosuppression, in which the immune system becomes unresponsive because of the pathologic changes in the immunocompetent cells. Induced immunosuppression can be accomplished with the administration of an antigen, antisera, or antibody; hormones and cytotoxic drugs; radiation; or surgery. For the most part, induced immunosuppression is used for tissue and organ transplants.
Anesthetics and immune function
Anesthetic agents themselves have long been associated with immune dysfunction. Over two decades ago, Nakagawara and colleagues demonstrated that inflammatory precursors were inhibited when exposed to inhalation anesthetics.3 Macrophage phagocytotic ability is also reduced by the common inhalation anesthetic isoflurane.4 Similarly, monocyte, neutrophil, and macrophages are inhibited by intravenous propofol in a dose dependent fashion.5 Proper universal precautionsand careful caution against contamination of the described vectors, are crucial for positive patient outcomes.
Summary
The physiology of the immune system has been presented. This area of medical physiology has been enhanced in its degree of research and treatment with the onset of AIDS and the organ transplant surgeries that depend on drugs and such for suppression of the immune system. Many new pathophysiologic processes will be discovered in the near future that are related directly to the immune system.
1. Chen K, Cerutti A. The function and regulation of immunoglobulin D. Current Opinion in Immunology. 2011;23(3):345–352.
2. Shah D, Chowdhury MMU. Rubber allergy. Clin in Dermatology. 2011;29:278–286.
3. Nakagawara M, et al. Inhibition of superoxide production and Ca21 mobilization in human neutrophils by halothane, enflurane, and isoflurane. Anesthesiology. 1986;64:4–12.
4. Kotani N, et al. Intraoperative modulation of alveolar macrophage function during isoflurane and propofol anesthesia. Anesthesiology. 1998;89:1125–1132.
5. Mikawa K, et al. Propofol inhibits human neutrophil functions. Anesthesia & Analgesia. 1998;87:695–700.
American Association of Nurse Anesthetists: Talking points about latex allergies. available at: http://www.anesthesiapatientsafety.com/patients/latex/talking_points.asp, June 2, 2011. Accessed
American Society of Anesthesiologists: Natural rubber latex allergy considerations for anesthesiologists. available at: http://ecommerce.asahq.org/publicationsAndServices/latexallergy.pdf, June 2, 2011. Accessed
Atlee J. Complications in anesthesia. ed 2. St. Louis: Saunders; 2007.
Brunton L, et al. Goodman and Gilman’s the pharmacological basis of therapeutics, ed 12. New York: McGraw-Hill; 2011.
Fleisher L. Anesthesia and uncommon diseases, ed 5. St. Louis: Saunders; 2007.
Gorringe-Moore R. Immunology and the lung. Traver G, ed. Respiratory nursing: the science and the art. New York: John Wiley & Sons; 1982.
Groenwald S. Physiology of the immune system. Heart Lung. 1980;9(4):645–650.
Hall J. Guyton and Hall textbook of medical physiology, ed 12. Philadelphia: Saunders; 2011.
Jocius M. Immunohematology and transfusion reaction. AANA J. 1982;50(1):42–48.
Murray J. The normal lung, ed 2. Philadelphia: Saunders; 1986.
Paskawicz J, Chatwani A. Latex allergy: a concern for anesthesia personnel. Am J Anesth.2001;28:435–441.
Rana A, Luskin A. Immunosuppression, autoimmunity, and hypersensitivity. Heart Lung. 1980;9(4):651–657.
Spindler J, et al. Intramuscular ketorolac and morphine in the treatment of moderate to severe pain after major surgery. Pharmacotherapy. 1990;10:51S–58S.
Sussman G, Gold M. Guidelines for the management of latex allergies and safe latex use in health care facilities. Arlington Heights, IL: American College of Allergy; 2010. available at: http://www.acaai.org/allergist/allergies/Types/latex-allergy/Pages/latex-allergies-safe-use.aspx, June 2, 2011. Accessed
Trevor AJ, et al. Basic and clinical pharmacology, ed 11. New York: McGraw Hill; 2009.