Chapter 91 The High-Risk Infant
Neonates at risk should be identified as early as possible to decrease neonatal morbidity and mortality (Chapter 87). The term high-risk infant designates an infant who should be under close observation by experienced physicians and nurses. Factors that define infants as being high-risk are listed in Table 91-1. Approximately 9% of all births require special or neonatal intensive care. Usually needed for only a few days, such observation may last from a few hours to several months. Some institutions find it advantageous to provide a special or transitional care nursery for high-risk infants, often within the labor and delivery suite. This facility should be equipped and staffed like a neonatal intensive care area.
DEMOGRAPHIC SOCIAL FACTORS
PAST MEDICAL HISTORY
PREVIOUS PREGNANCY
PRESENT PREGNANCY
LABOR AND DELIVERY
NEONATE
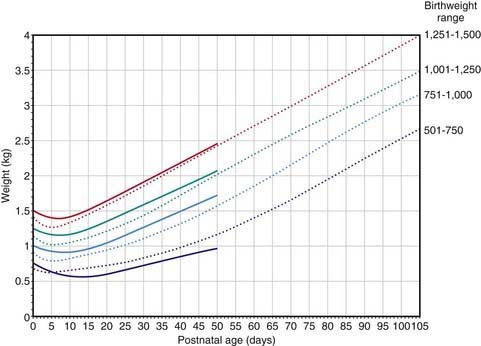
For many infants who are born prematurely, are small for gestational age (SGA), have significant perinatal asphyxia, are breech, or are born with life-threatening congenital anomalies, there are no previously identified risk factors. For any given duration of gestation, the lower the birthweight, the higher the neonatal mortality; for any given birthweight, the shorter the gestational duration, the higher the neonatal mortality (Fig. 91-1). The highest risk of neonatal mortality occurs in infants who weigh <1,000 g at birth and whose gestation was <28 wk. The lowest risk of neonatal mortality occurs in infants with a birthweight of 3,000-4,000 g and a gestational age of 38-42 wk. As birthweight increases from 500 to 3,000 g, a logarithmic decrease in neonatal mortality occurs; for every week of increase in gestational age from the 25th to the 37th wk, the neonatal mortality rate decreases by approximately half. Nevertheless, approximately 40% of all perinatal deaths occur after 37 wk of gestation in infants weighing 2,500 g or more; many of these deaths take place in the period immediately before birth and are more readily preventable than those of smaller and more immature infants. Neonatal mortality rates rise sharply for infants weighing over 4,000 g at birth and for those whose gestational period is 42 wk or longer. Because neonatal mortality largely depends on birthweight and gestational age, Figure 91-1 can be used to help identify high-risk infants quickly. This analysis is based on total live births and therefore describes the mortality risk only at birth. Because most neonatal mortality occurs within the 1st hours and days after birth, the outlook improves dramatically with increasing postnatal survival.
(From Lemons JA, Bauers CR, Oh W, et al: Very low birthweight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996, Pediatrics 107:2001; available at www.pediatrics.org.cgi/content/full/107/1/el.)
91.1 Multiple Gestation Pregnancies
Monozygotic Versus Dizygotic Twins
Examination of the Placenta
In the fetal transfusion syndrome, an artery from one twin acutely or chronically delivers blood that is drained into the vein of the other. The latter becomes plethoric and large, and the former is anemic and small. Generally, with chronicity, 5 g/dL hemoglobin and 20% body weight differences can be noted in this syndrome. Maternal hydramnios in a twin pregnancy suggests fetal transfusion syndrome. Anticipating this possibility by preparing to transfuse the donor twin or bleed the recipient twin may be lifesaving. Death of the donor twin in utero may result in generalized fibrin thrombi in the smaller arterioles of the recipient twin, possibly as the result of transfusion of thromboplastin-rich blood from the macerating donor fetus. Disseminated intravascular coagulation may develop in the surviving twin. Table 91-2 lists the more frequent changes associated with a large uncompensated arteriovenous shunt from the placenta of one twin to that of the other. Treatment of this highly lethal problem includes maternal digoxin, aggressive amnioreduction for polyhydramnios, selective twin termination, and Nd:YAG laser or fetoscopic ablation of the anastomosis.
Table 91-2 CHARACTERISTIC CHANGES IN MONOCHORIONIC TWINS WITH UNCOMPENSATED PLACENTAL ARTERIOVENOUS SHUNTS
| TWIN ON | |
|---|---|
| ARTERIAL SIDE—DONOR | VENOUS SIDE—RECIPIENT |
| Prematurity | Prematurity |
| Oligohydramnios | Polyhydramnios |
| Small premature | Hydrops |
| Malnourished | Large premature |
| Pale | Well nourished |
| Anemic | Plethoric |
| Hypovolemia | Polycythemic |
| Hypoglycemia | Hypervolemic |
| Microcardia | Cardiac hypertrophy |
| Glomeruli small or normal | Myocardial dysfunction |
| Arterioles thin walled | Tricuspid valve regurgitation |
| Right ventricular outflow obstruction | |
| Glomeruli large | |
| Arterioles thick walled | |
Prognosis
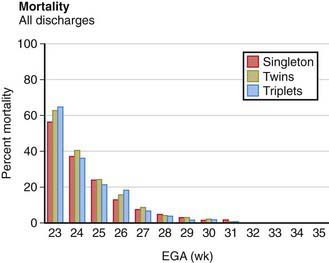
Most twins are born prematurely, and maternal complications of pregnancy are more common than with single pregnancies. The risk for twins is most often associated with twin-twin transfusion, assisted reproductive technology, and early-onset discordant growth. Although monochorionic twins have a significantly higher perinatal mortality, there is no significant difference between the neonatal mortality rates of twin births and single births in comparable weight and gestational age groups (Fig. 91-2). Because most twins are premature, their overall mortality is higher than that of single-birth infants. The perinatal mortality of twins is about four times that of singletons. Monoamnionic twins have an increased likelihood of entangling the cords, which may lead to asphyxia. Theoretically, the 2nd twin is more subject to anoxia than the 1st because the placenta may separate after birth of the 1st twin and before birth of the 2nd. In addition, delivery of the 2nd twin may be difficult because it may be in an abnormal presentation (breech, entangled), uterine tone may be decreased, or the cervix may begin to close after the 1st twin’s birth. The mortality for multiple gestations with four or more fetuses is excessively high for each fetus. Because of this poor prognosis, selective fetal reduction (with transabdominal intrathoracic fetal injection of KCl) to two to three fetuses has been offered as a treatment option. Monozygotic twins have an increased risk of one twin dying in utero. The surviving twin has a greater risk for cerebral palsy and other neurodevelopmental sequelae.
Airas U, Heinonen S. Clinical significance of true umbilical knots: a population-based analysis. Am J Perinatol. 2002;19:127-132.
American Academy of Pediatrics Committee on Fetus and Newborn. Hospital discharge of the high-risk neonate. Pediatrics. 2008;122:1119-1126.
Anderson AN, Pinborg A, Loft A. Neonatal outcome in singletons conceived after ART. Lancet. 2008;372:694-695.
Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. PNAS. 2005;102:10606-10609.
Garite TJ, Clark RH, Elliott JP, et al. Twins and triplets: the effect of plurality and growth on neonatal outcome compared with singleton infants. Am J Obstet Gynecol. 2004;191:700-707.
Hansen M, Colvin L, Petterson B, et al. Twins born following assisted reproductive technology: perinatal outcome and admission to hospital. Hum Reprod. 2009;9:2321-2331.
Mari G, Roberts A, Detti L, et al. Perinatal morbidity and mortality rates in severe twin-twin transfusion syndrome: results of the International Amnioreduction Registry. Am J Obstet Gynecol. 2001;185:708-715.
Ortibus E, Lopriore E, Deprest J, et al. The pregnancy and long-term neurodevelopment outcome of monochorionic diamniotic twin gestations: a multicenter prospective cohort study from the first trimester onward. Am J Obstet Gynecol. 2009;200:e1-494.e8.
Pearn J. Bioethical issues in caring for conjoined twins and their parents. Lancet. 2001;357:1968-1971.
Steer P. Perinatal death in twins. BMJ. 2007;334:545-546.
Strömberg B, Dahiquist G, Ericson A, et al. Neurological sequelae in children born after in-vitro fertilisation: a population-based study. Lancet. 2002;359:461-465.
91.2 Prematurity and Intrauterine Growth Restriction
Definitions
Liveborn infants delivered before 37 wk from the 1st day of the last menstrual period are termed premature by the World Health Organization. LBW (birthweight of 2,500 g or less) is due to prematurity, poor intrauterine growth (IUGR, also referred to as SGA), or both. Prematurity and IUGR are associated with increased neonatal morbidity and mortality. Ideally, definitions of LBW for individual populations should be based on data that are as genetically and environmentally homogeneous as possible. As previously mentioned, Figure 91-1 presents variations in mortality based on birthweight, gestational age, and gender.
Incidence
There is an increasing percentage of deaths in children <5 yr of age that occur in the neonatal period. Approximately 57% of deaths in this age group occur within the 1st mo of life, of which approximately 36% are attributable to premature birth. In 2008, 8.2% of liveborn neonates in the USA weighed <2,500 g; the rate for blacks was almost twice that for whites. Over the past 2 decades, the LBW rate has increased primarily because of an increased number of preterm births. Women whose 1st births are delivered before term are at increased risk for recurrent preterm delivery. Approximately 30% of LBW infants in the USA have IUGR and are born after 37 wk. At LBW rates >10%, the contribution of IUGR increases and that of prematurity decreases. In developing countries, approximately 70% of LBW infants have IUGR. Infants with IUGR have greater morbidity and mortality than do appropriately grown, gestational age–matched infants (see Fig. 91-1). Although U.S. infant mortality rates have fallen since 1971, the ethnic disparity between black infants and white or Hispanic infants remains unchanged. Black infants have higher neonatal mortality rates and comprise a larger percentage of low birthweight births in the USA.
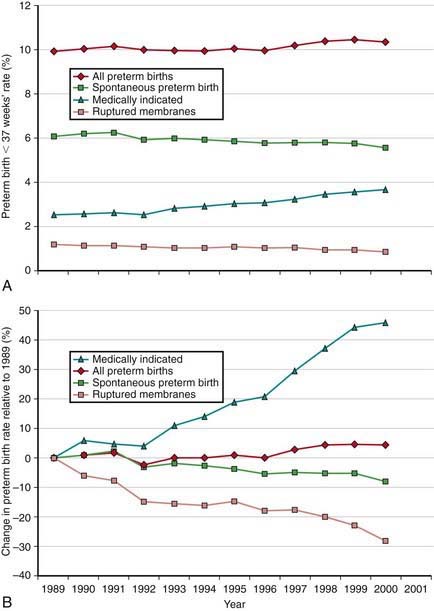
The incidence of preterm births in the USA continues to rise (Fig. 91-3) and is due in part to multiple gestation pregnancies. In single births, the overall incidence has been stable, but premature births due to medically indicated deliveries have increased, whereas premature births due to spontaneous preterm birth or ruptured membranes have declined (Fig. 91-4).
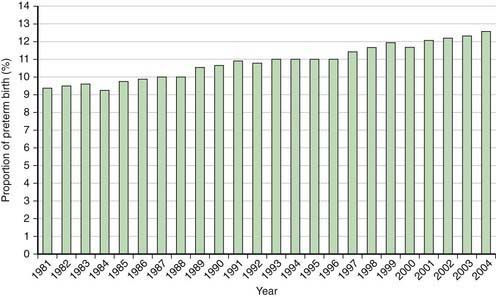
Figure 91-3 Percentage of all births classified as preterm in the USA, 1981-2004.
(From Martin JA, Kochanek KD, Strobino DM, et al: Annual summary of vital statistics—2003, Pediatrics 115:619–634, 2005.)
Factors Related to Premature Birth and Low Birthweight
It is difficult to separate completely the factors associated with prematurity from those associated with IUGR (Chapters 88 and 89). A strong positive correlation exists between both preterm birth and IUGR and low socioeconomic status. Families of low socioeconomic status have higher rates of maternal undernutrition, anemia, and illness; inadequate prenatal care; drug misuse; obstetric complications; and maternal history of reproductive inefficiency (abortions, stillbirths, premature or LBW infants). Other associated factors, such as single-parent families, teenage pregnancies, short interpregnancy interval, and mothers who have borne more than four previous children, are also encountered more frequently in such families. Systematic differences in fetal growth have also been described in association with maternal size, birth order, sibling weight, social class, maternal smoking, and other factors. The degree to which the variance in birthweight among various populations is due to environmental (extrafetal) rather than genetic differences in growth potential is difficult to determine.
The etiology of preterm birth is multifactorial and involves a complex interaction between fetal, placental, uterine, and maternal factors (Table 91-3).
Table 91-3 IDENTIFIABLE CAUSES OF PRETERM BIRTH
FETAL
PLACENTAL
UTERINE
MATERNAL
OTHER
IUGR is associated with medical conditions that interfere with the circulation and efficiency of the placenta, with the development or growth of the fetus, or with the general health and nutrition of the mother (Table 91-4). Many factors are common to both prematurely born and LBW infants with IUGR. IUGR is associated with decreased insulin production or insulin (or insulin-like growth factor [IGF]) action at the receptor level. Infants with IGF-I receptor defects, pancreatic hypoplasia, or transient neonatal diabetes have IUGR. Genetic mutations affecting the glucose-sensing mechanisms of the pancreatic islet cells that result in decreased insulin release (loss of function of the glucose-sensing glucokinase gene) give rise to IUGR.
IUGR may be a normal fetal response to nutritional or oxygen deprivation. Therefore, the issue is not the IUGR but rather the ongoing risk of fetal malnutrition or hypoxia. Similarly, some preterm births signify a need for early delivery from a potentially disadvantageous intrauterine environment. IUGR is often classified as reduced growth that is symmetric (head circumference, length, and weight equally affected) or asymmetric (with relative sparing of head growth) (see Fig. 90-1). Symmetric IUGR often has an earlier onset and is associated with diseases that seriously affect fetal cell number, such as conditions with chromosomal, genetic, malformation, teratogenic, infectious, or severe maternal hypertensive etiologies. It is important to assess gestational age carefully in infants suspected to have symmetric IUGR because incorrect overestimation of gestational age may lead to the diagnosis of symmetric IUGR. Asymmetric IUGR is often of late onset, demonstrates preservation of Doppler waveform velocity to the carotid vessels, and is associated with poor maternal nutrition or with late onset or exacerbation of maternal vascular disease (preeclampsia, chronic hypertension). Problems of infants with IUGR are noted in Table 91-5.
Table 91-5 PROBLEMS OF INFANTS SMALL FOR GESTATIONAL AGE OR WITH INTRAUTERINE GROWTH RETARDATION*
| PROBLEM | PATHOGENESIS |
|---|---|
| Intrauterine fetal demise | Hypoxia, acidosis, infection, lethal anomaly |
| Perinatal asphyxia | ↓ Uteroplacental perfusion during labor ± chronic fetal hypoxia-acidosis; meconium aspiration syndrome |
| Hypoglycemia | ↓ Tissue glycogen stores, ↓ gluconeogenesis, hyperinsulinism, ↑ glucose needs of hypoxia, hypothermia, large brain |
| Polycythemia-hyperviscosity | Fetal hypoxia with ↑ erythropoietin production |
| Reduced oxygen consumption/hypothermia | Hypoxia, hypoglycemia, starvation effect, poor subcutaneous fat stores |
| Dysmorphology | Syndrome anomalads, chromosomal-genetic disorders, oligohydramnios-induced deformation, TORCH (toxoplasmosis, other agents, rubella, cytomegalovirus, herpes simplex) infection |
↓, Decreased; ↑, increased.
* Other problems include pulmonary hemorrhage and those common to the gestational age-related risks of prematurity if born at less than 37 wk.
Assessment of Gestational Age at Birth
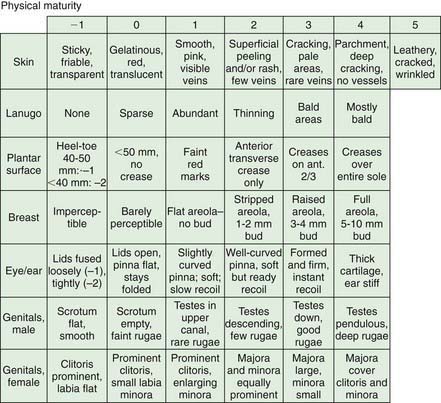
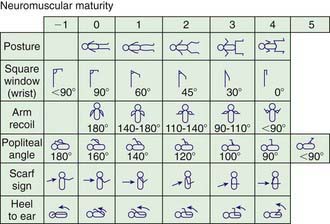
When compared with a premature infant of appropriate weight, an infant with IUGR has a reduced birthweight and may appear to have a disproportionately larger head relative to body size; infants in both groups lack subcutaneous fat. Neurologic maturity (nerve conduction velocity), in the absence of asphyxia, correlates with gestational age despite reduced fetal weight. Physical signs may be useful in estimating gestational age at birth. Commonly used, the Ballard scoring system is accurate to ±2 wk (Figs. 91-5 to 91-7). An infant should be presumed to be at high risk for mortality or morbidity if a discrepancy exists between the estimation of gestational age by physical examination, the mother’s estimated date of her last menstrual period, and fetal ultrasonographic evaluation.
Spectrum of Disease in Low-Birthweight Infants
Immaturity increases the severity but reduces the distinctiveness of the clinical manifestations of most neonatal diseases. Immature organ function, complications of therapy, and the specific disorders that caused the premature onset of labor contribute to the neonatal morbidity and mortality associated with premature, LBW infants (Table 91-6). Among VLBW infants, morbidity is inversely related to birthweight. Respiratory distress syndrome is noted in approximately 80% of infants weighing 501-750 g; in 65% of those 751-1,000 g; in 45% of those 1,001-1,250 g; and in 25% of those 1,251-1,500 g. Severe intraventricular hemorrhage (IVH) is noted in approximately 25% of infants weighing 501-750 g; in 12% of those 751-1,000 g; in 8% of those 1,001-1,250 g; and in 3% of those 1,251-1,500 g. Overall, the risk of late sepsis (24%), bronchopulmonary dysplasia (23%), severe IVH (11%), necrotizing enterocolitis (7%), and prolonged hospitalization (45-125 days) is high in VLBW infants. Problems associated with IUGR LBW infants are noted in Table 91-5; these added problems are often superimposed on those noted in Table 91-6 if an infant with IUGR is also premature. Poor postnatal growth is an important problem for both preterm and IUGR infants.
Table 91-6 NEONATAL PROBLEMS ASSOCIATED WITH PREMATURE INFANTS
RESPIRATORY
CARDIOVASCULAR
HEMATOLOGIC
GASTROINTESTINAL
METABOLIC-ENDOCRINE
CENTRAL NERVOUS SYSTEM
RENAL
OTHER
Nursery Care
At birth, the measures needed to clear the airway, initiate breathing, care for the umbilical cord and eyes, and administer vitamin K are the same for immature infants as for those of normal weight and maturity (Chapter 88). Special care is required to maintain a patent airway. Additional considerations are the need for (1) thermal control and monitoring of the heart rate and respiration, (2) oxygen therapy, and (3) special attention to the details of fluid requirements and nutrition. Safeguards against infection can never be relaxed. Routine procedures that disturb these infants may result in hypoxia. The need for regular and active participation by the parents in the infant’s care in the nursery, the need to instruct the mother in at-home care of her infant, and the question of prognosis for later growth and development require special consideration.
Initiation of Feeding
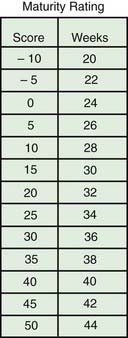
If an infant is well, is making sucking movements, and is in no distress, oral feeding may be attempted, although most infants weighing <1,500 g require tube feeding because they are unable to coordinate breathing, sucking, and swallowing. Intestinal tract readiness for feeding may be determined by active bowel sounds, passage of meconium, and the absence of abdominal distention, bilious gastric aspirates, and emesis. For infants <1,000 g, the initial trophic feedings can be given at 10-20 mL/kg/24 hr as a continuous nasogastric tube drip (or given by intermittent gavage every 2-3 hr) for 5-10 days. If the initial feedings are tolerated, the volume is increased by 20-30 mL/kg/24 hr. Once a volume of 150 mL/kg/24 hr has been achieved, the caloric content may be increased to 24 or 27 kcal/oz. With high caloric density, infants are at risk for dehydration, edema, lactose intolerance, diarrhea, flatus, and delayed gastric emptying with emesis. Intravenous fluids are needed until feedings provide approximately 120 mL/kg/24 hr. The feeding protocol for premature infants weighing >1,500 g is initiated at a volume of 20-30 mL/kg/24 hr with increments in total daily formula volume of 20-30 mL/kg/24 hr. The expected weight increments for premature infants of various birthweights are projected from Figure 91-8. Infants with IUGR may not demonstrate the marked initial weight loss noted in premature infants.
Regurgitation, vomiting, abdominal distention, or gastric residuals from previous feedings should arouse suspicion of sepsis, necrotizing enterocolitis, or intestinal obstruction; these conditions are indications to stop feedings, at least temporarily, and to increase subsequent feedings slowly only as tolerated or to change to intravenous alimentation and evaluate the infant for more serious problems (Chapter 96.2). Weight gain may not be achieved for 10-12 days. Alternatively, in infants whose feeding schedule is advanced successfully in calories or volume, weight gain may appear within a few days.
Vitamins
Although formula in amounts necessary for adequate growth probably contains adequate quantities of all vitamins, the volume of milk sufficient to satisfy these requirements may not be ingested for several weeks. Therefore, LBW and preterm infants should be given supplemental vitamins. Because requirements for these infants have not been precisely established, the recommended daily allowances for term infants should be given (Chapter 41). Furthermore, infants may have a special need for certain vitamins. Intermediary metabolism of phenylalanine and tyrosine depends, in part, on vitamin C. Decreased fat absorption with increased fecal fat loss may be associated with decreased absorption of vitamin D, other fat-soluble vitamins, and calcium in premature infants. VLBW infants are particularly prone to the development of osteopenia, but their total intake of vitamin D should not exceed 1,500 IU/24 hr. Folic acid is essential for the formation of DNA and production of new cells; serum and erythrocyte levels decrease in preterm infants during the 1st few wk of life and remain low for 2-3 mo. Therefore, folic acid supplementation is recommended, although it does not result in improved growth or an increased hemoglobin concentration. Deficiency of vitamin E is uncommon but is associated with increased hemolysis and, if severe, with anemia and edema in premature infants. Vitamin E functions as an antioxidant to prevent the peroxidation of excessive polyunsaturated fatty acids in red blood cell membranes; its need may increase because of the higher membrane content of these fatty acids when formulas with high polyunsaturated fatty acids are used. Vitamin A supplementation reduces bronchopulmonary dysplasia in ELBW infants. Vitamin K deficiency is discussed in Chapter 97.4.
Immaturity of Drug Metabolism
Many drugs apparently safe for adults on the basis of toxicity studies may be harmful to newborn infants, especially premature ones. Oxygen and a number of drugs have proved toxic to premature infants in amounts not harmful to term infants (Table 91-7). Thus, administering any drug, particularly in high doses, that has not undergone pharmacologic testing in premature infants should be undertaken carefully after risks have been weighed against benefits.
Table 91-7 POTENTIAL ADVERSE REACTIONS TO DRUGS ADMINISTERED TO PREMATURE INFANTS
| DRUG | REACTION(S) |
|---|---|
| Oxygen | Retinopathy of prematurity, bronchopulmonary dysplasia |
| Sulfisoxazole | Kernicterus |
| Chloramphenicol | Gray baby syndrome—shock, bone marrow suppression |
| Vitamin K analogs | Jaundice |
| Novobiocin | Jaundice |
| Hexachlorophene | Encephalopathy |
| Benzyl alcohol | Acidosis, collapse, intraventricular bleeding |
| Intravenous vitamin E | Ascites, shock |
| Phenolic detergents | Jaundice |
| NaHCO3 | Intraventricular hemorrhage |
| Amphotericin | Anuric renal failure, hypokalemia, hypomagnesemia |
| Reserpine | Nasal stuffiness |
| Indomethacin | Oliguria, hyponatremia, intestinal perforation |
| Cisapride | Prolonged QTc interval |
| Tetracycline | Enamel hypoplasia |
| Tolazoline | Hypotension, gastrointestinal bleeding |
| Calcium salts | Subcutaneous necrosis |
| Aminoglycosides | Deafness, renal toxicity |
| Enteric gentamicin | Resistant bacteria |
| Prostaglandins | Seizures, diarrhea, apnea, hyperostosis, pyloric stenosis |
| Phenobarbital | Altered state, drowsiness |
| Morphine | Hypotension, urine retention, withdrawal |
| Pancuronium | Edema, hypovolemia, hypotension, tachycardia, vecuronium contractions, prolonged hypotonia |
| Iodine antiseptics | Hypothyroidism, goiter |
| Fentanyl | Seizures, chest wall rigidity, withdrawal |
| Dexamethasone | Gastrointestinal bleeding, hypertension, infection, hyperglycemia, cardiomyopathy, reduced growth |
| Furosemide | Deafness, hyponatremia, hypokalemia, hypochloremia, nephrocalcinosis, biliary stones |
| Heparin (not low-dose prophylactic use) | Bleeding, intraventricular hemorrhage, thrombocytopenia |
| Erythromycin | Pyloric stenosis |
Prognosis
Infants born weighing 1,501-2,500 g have a 95% or greater chance of survival, but those weighing still less have significantly higher mortality (see Fig. 91-1). Intensive care has extended the period during which a VLBW infant is at increased risk of dying of complications of prematurity, such as bronchopulmonary dysplasia, necrotizing enterocolitis, and nosocomial infection (Table 91-8). The postdischarge mortality rate of LBW infants is higher than that of term infants during the 1st 2 yr of life. Because many of the deaths are attributable to infection (e.g., respiratory syncytial virus [RSV]), they are at least theoretically preventable. In addition, premature infants have an increased incidence of failure to thrive, sudden infant death syndrome, child abuse, and inadequate maternal-infant bonding. The biologic risk associated with poor cardiorespiratory regulation due to immaturity or complications of underlying perinatal disease and the social risk associated with poverty also contribute to the high mortality and morbidity of these infants. Congenital anomalies are present in approximately 3-7% of LBW infants.
| IMMEDIATE | LATE |
|---|---|
| Hypoxia, ischemia | Mental retardation, spastic diplegia, microcephaly, seizures, poor school performance |
| Intraventricular hemorrhage | Mental retardation, spasticity, seizures, hydrocephalus |
| Sensorineural injury | Hearing, visual impairment, retinopathy of prematurity, strabismus, myopia |
| Respiratory failure | Bronchopulmonary dysplasia, cor pulmonale, bronchospasm, malnutrition, subglottic stenosis |
| Necrotizing enterocolitis | Short-bowel syndrome, malabsorption, malnutrition, infectious diarrhea |
| Cholestatic liver disease | Cirrhosis, hepatic failure, malnutrition |
| Nutrient deficiency | Osteopenia, fractures, anemia, growth failure |
| Social stress | Child abuse or neglect, failure to thrive, divorce |
| Other | Sudden infant death syndrome, infections, inguinal hernia, cutaneous scars (chest tube, patent ductus arteriosus ligation, intravenous infiltration), gastroesophageal reflux, hypertension, craniosynostosis, cholelithiasis, nephrocalcinosis, cutaneous hemangiomas |
In the absence of congenital abnormalities, central nervous system injury, VLBW, or marked IUGR, the physical growth of LBW infants tends to approximate that of term infants by the 2nd yr; the approximation occurs earlier in premature infants with larger birth size. VLBW infants may not catch up, especially if they have severe chronic sequelae, insufficient nutritional intake, or an inadequate caretaking environment (see Table 91-8). Infrequently, infants with IUGR (SGA) grow poorly and do not demonstrate catch-up growth. These infants may benefit from recombinant human growth hormone therapy beginning at age 4 yr.
Discharge from the Hospital
Before discharge, a premature infant should be taking all nutrition by nipple, either bottle or breast (Table 91-9). Some medically fragile infants may be discharged home while receiving gavage feedings after the parents have received appropriate training and education. Growth should be occurring at steady increments of approximately 30 g/day. Temperature should be stabile in an open crib. Infants should have had no recent episodes of apnea or bradycardia, and parenteral drug administration should have been discontinued or converted to oral dosing. Stable infants recovering from bronchopulmonary dysplasia may be discharged on a regimen of oxygen given by nasal cannula as long as careful follow-up is arranged with frequent pulse oximetry monitoring and outpatient visits. All infants with birthweight <1,500 g and those with birthweights between 1,500 and 2,000 g with an unstable clinical course requiring oxygen should undergo an eye examination to screen for retinopathy of prematurity. All infants should have a hearing test prior to discharge. In those who had indwelling umbilical arterial catheters, blood pressure should be measured to check for renal vascular hypertension. The hemoglobin level or hematocrit should be determined to evaluate for possible anemia. If all major medical problems have resolved and the home setting is adequate, premature infants may then be discharged when their weight approaches 1,800-2,100 g; close follow-up plus easy access to health care providers is essential for early discharge protocols. Alternatively, if the medical or social environment is not ideal, high-risk neonates who have been transported to neonatal intensive care units and whose major illnesses have resolved may be returned to their hospital of birth for an additional period of hospitalization. Standard vaccinations with full doses should commence after discharge or, if infants are still in the hospital, with vaccines that do not contain live viruses. For RSV prophylaxis, see Chapter 252.
Table 91-9 RECOMMENDATIONS FOR THE DISCHARGE OF HIGH-RISK LOW-BIRTHWEIGHT INFANTS
Adapted from American Academy of Pediatrics, American College of Obstetricians: Guidelines for perinatal care, ed 5, EIk Grove Village, IL, 2002, American Academy of Pediatrics.
Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, et al. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717-728.
Adams MM, Barfield WD. The future of very preterm infants. JAMA. 2008;299:1477-1478.
Alberry M, Soothill P. Management of fetal growth restriction. Arch Dis Child Fetal Neonatal Ed. 2007;92:F62-F67.
Ambalavanan N, Carlo WA, Bobashev G, et al. Prediction of death for extremely low birth weight neonates. National Institute of Child Health and Human Development Neonatal Research Network. Pediatrics. 2005;116:1367-1373.
Bell EF, Zumbach DK. The tiniest babies: a registry of survivors with birth weight less than 40 grams. Pediatrics. 2011;127:58-61.
Bonhoeffer J, Siegrist CA, Heath PT. Immunisation of premature infants. Arch Dis Child. 2006;91:929-935.
Bruckner TA, Saxton KB, Anderson E, et al. From paradox to disparity: trends in neonatal death in very low birth weight non-Hispanic black and white infants, 1989–2004. J Pediatr. 2009;155:482-487.
Bukowski R, Malone FD, Porter FT, et al. Preconceptional folate supplementation and the risk of spontaneous preterm birth: a cohort study. PLoS Med. 2009;6:e1000061.
Caple J, Armentrout D, Huseby V, et al. Randomized, controlled trial of slow versus rapid feeding volume advancement in preterm infants. Pediatrics. 2004;114:1597-1600.
Clandinin MT, van Aerde JE, Merkel KL, et al. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. J Pediatr. 2005;146:461-468.
Costantine MM, Weiner SJ. Effects of antenatal exposure to magnesium sulfate on neuroprotection and mortality in preterm infants. Obstet Gynecol. 2009;114:354-364.
Dall’Oglio AM, Rossiello B, Coletti MF, et al. Do healthy preterm children need neuropsychological follow-up? Preschool outcomes compared with term peers. Dev Med Child Neurol. 2010;52:955-961.
DeFelice C, Tassi R, De Capua B, et al. A new phenotypical variant of intrauterine growth restriction? Pediatrics. 2007;119:e983-e990.
De Kieviet JF, Pick JP, Aarnoudse-Moens CS, et al. Motor development in very preterm and very low-birth-weight children from birth to adolescence. JAMA. 2009;302:2235-2242.
Doyle LW. Antenatal progesterone to prevent preterm birth. Lancet. 2009;373:2000-2001.
Doyle LW, Anderson PJ. Adult outcomes of extremely preterm infants. Pediatrics. 2010;126:342-351.
Express GroupFellman V, Hellström-Westas L, Norman M, et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. 2009;301:2225-2232.
Fawzi WW, Msamanga GI, Urassa W, et al. Vitamins and perinatal outcomes among HIV-negative women in Tanzania. N Engl J Med. 2007;356:1423-1431.
Fewtrell MS, Bishop NJ, Edmonds CJ, et al. Aluminum exposure from parenteral nutrition in preterm infants: bone health at 15-year follow-up. Pediatrics. 2009;124:1372-1379.
Gardner F, Johnson A, Yudkin P, et al. Behavioral and emotional adjustment of teenagers in mainstream school who were born before 29 weeks’ gestation. Pediatrics. 2004;114:676-682.
Gargus RA, Vohr BR, Tyson JE, et al. Unimpaired outcomes for extremely low birth weight infants at 18 to 22 months. Pediatrics. 2009;124:112-121.
Goldenberg RL, Culhane JF, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75-84.
Gupta P, Ray M, Dua T, et al. Multimicronutrient supplementation for undernourished pregnant women and the birth size of their offspring. Arch Pediatr Adolesc Med. 2007;161:58-64.
Hack M, Wilson Costello D. Decrease in frequency of cerebral palsy in preterm infants. Lancet. 2007;369:7-8.
Hartley RS, Emanuel I, Hitti J. Perinatal mortality and neonatal morbidity rates among twin pairs at different gestational ages: optimal delivery timing at 37 to 38 weeks’ gestation. Am J Obstet Gynecol. 2001;184:451-458.
Hintz SR, Kendrick DE, Wilson-Costello DE, et al. Early-childhood neurodevelopmental outcomes are not improving for infants born at <25 weeks’ gestational age. Pediatrics. 2011;127:62-70.
Hollo O, Rautava P, Korhonen T, et al. Academic achievement of small for gestational age children at age 10 years. Arch Pediatr Adolesc Med. 2002;156:179-187.
Johnston LB, Savage MO. Should recombinant human growth hormone therapy be used in short small for gestational age children? Arch Dis Child. 2004;89:740-744.
Makrides M, Gibson RA, McPhee AJ, et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid. JAMA. 2009;301:175-182.
Mariorana A, Cianfarani S. Impact of growth hormone therapy on adult height of children born small for gestational age. Pediatrics. 2009;124:e519-e531.
Marlow N, Wolke D, Bracewell MA, et al. Neurologic and developmental disability at six years of age after extremely preterm birth. N Eng J Med. 2005;352:9-19.
McCowan LME, Dekker GA, Chan E, et al. Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: prospective cohort study. SCOPE Consortium. BMJ. 2009;338:b1081.
McGuire W, Henderson G, Fowlie PW. Feeding the preterm infant. Br Med J. 2004;329:1227-1230.
McGuire W, McEwan P, Fowlie PW. Care in the early newborn period. Br Med J. 2004;329:1087-1089.
Modi N. Management of fluid balance in the very immature neonate. Arch Dis Child. 2004;89:F108-F111.
Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359:262-273.
Nongena P, Ederies A, Azzopardi DV, et al. Confidence in the prediction of neurodevelopmental outcome by cranial ultrasound and MRI in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2010;95:F388-F390.
Platt MJ, Cans C, Johnson A, et al. Trends in cerebral palsy among infants of very low birthweight (<1500 g) or born prematurely (<32 weeks) in 16 European centres: a database study. Lancet. 2007;369:43-50.
Pylipow M, Spector LG, Puumala SE, et al. Early postnatal weight gain, intellectual performance, and body mass indexed at 7 years of age in term infants with intrauterine growth restriction. J Pediatr. 2009;154:201-206.
Ruiz-Pelaez JG, Charpak N, Cuervo LG. Kangaroo mother care, an example to follow from developing countries. Br Med J. 2004;329:1179-1181.
Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261-269.
Sedin G. To avoid heat loss in very preterm infants. J Pediatr. 2004;145:720-722.
Spittle AJ, Orton J, Doyle LW, et al: Early developmental intervention programs post hospital discharge to prevent motor and cognitive impairments in preterm infants, Cochrane Database Syst Rev (18):CD005495, 2007.
Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics. 2010;126:443-456.
Tyson JE, Parikh NA, Langer J, et al. Intensive care for extreme prematurity—moving beyond gestational age. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 2008;358:1672-1681.
Walsh MC, Morris BH, Wrage LA, et al. Extremely low birthweight neonates with protracted ventilation mortality and 18-month neurodevelopmental outcomes. J Pediatr. 2005;146:798-804.
Wilson-Costello D, Friedman H, Minich N, et al. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight in infants in the 1990s. Pediatrics. 2005;115:997-1003.
Zayek MM, Trimm RF, Hamm CR, et al. The limit of viability. Arch Pediatr Adolesc Med. 2011;165(2):126-133.
Zeitlin J, El Ayoubi M, Jarreau PH, et al. Impact of fetal growth restriction on mortality and morbidity in a very preterm birth cohort. J Pediatr. 2010;157:733-739.
91.3 Post-Term Infants
Clinical Manifestations
Post-term infants have normal length and head circumference but may have decreased weight if there is placental insufficiency. Infants born post-term in association with presumed placental insufficiency may have various physical signs. Desquamation, long nails, abundant hair, pale skin, alert faces, and loose skin, especially around the thighs and buttocks, give them the appearance of having recently lost weight; meconium-stained nails, skin, vernix, umbilical cord, and placental membranes may also be noted (see Fig. 88-1). Common complications of postmaturity include perinatal depression, meconium aspiration, persistent pulmonary hypertension, hypoglycemia, hypocalcemia, and polycythemia.