Chapter 73 The Genetic Approach in Pediatric Medicine
With the completion of the human genome sequence and the haplotype map, investigative and diagnostic tools are available to determine the genetic contributions to uncommon and common disorders. Information about the genetic aspects of all pediatric diseases is readily available on numerous websites and in other locations (Table 73-1).
Table 73-1 USEFUL INTERNET GENETIC REFERENCE SITES
| WEB ADDRESS | DATABASE |
|---|---|
| www.ncbi.nlm.nih.gov | General reference maintained by National Library of Medicine |
| www.ncbi.nlm.nih.gov/sites/entrez?db=omim | Online Mendelian Inheritance in Man, an extremely useful for clinicians ~20,000 entries of genetic traits indexed by gene name, symptoms, etc |
| www.ncbi.nlm.nih.gov/genemap | General reference to current efforts to map the human genome |
| www.ncbi.nlm.nih.gov/Genbank/GenbankOverview.html | Searchable repository of all DNA sequence data |
| www.ncbi.nlm.nih.gov/ncicgap | Cancer Genome Anatomy Project (National Cancer Institute) |
| www.genome.gov/ | National Human Genome Research Institute; useful information about human genetics and ethics issues |
| www.hgmd.cf.ac.uk/ac/index.php | Human Gene Mutation Database, a searchable index of all described mutations in human genes with phenotypes and references |
| www.genetests.org | |
| http://projects.tcag.ca/variation/ | A database of chromosomal alterations seen in normal controls |
| www.geneletter.com | Health, clinical, legal, social, and ethics issues |
| www.ashg.org | American Society of Human Genetics |
| www.acmg.net | American College of Medical Genetics |
| www.aap.org/VISIT/cmte18.htm | Committee on Genetics of the American Academy of Pediatrics: health supervision guidelines for common genetic disorders |
The Burden of Genetic Disorders in Childhood
Multifactorial disorders are caused by the action of multiple genes and/or gene-environmental effects. Spina bifida and isolated cleft lip or palate are common pediatric disorders that display multifactorial inheritance patterns. These traits can cluster in families but do not have a mendelian pattern of inheritance (Chapter 75). In most cases the causative genes are unknown, and genetic counseling is based on empirical data. The concept of multifactorial inheritance extends to common pediatric disorders, such as asthma and diabetes mellitus.
The Changing Paradigm of Genetics in Medicine
Although specific treatments are not available for most genetic disorders, there are some important exceptions. Inborn errors of metabolism were the first genetic disorders to be recognized, and many are amenable to treatment by dietary manipulation (Chapter 78). These conditions result from genetically determined deficiency of specific enzymes, leading to the buildup of toxic substrates and/or deficiency of critical end products.
Individual metabolic disorders tend to be very rare, but their combined impact on the pediatric population is significant. Tandem mass spectrometry has made it relatively inexpensive to screen for a large number of these disorders in the newborn period. Use of this technology not only dramatically increases the number of metabolic disorders identified within a population but also allows treatment to be initiated at a much earlier stage in development (Chapters 72 and 78).
Genetic testing is increasingly available for a wide variety of both rare and relatively common genetic disorders. Genetic testing is commonly used in pediatric medicine to resolve uncertainty regarding diagnosis and provide a basis for genetic counseling; in some instances, it can serve as a prelude for specific treatment. In the future, predictive testing for predisposition to disease could become more common. It is likely that the expansion of such testing will depend, at least in part, on the extent to which such testing can be linked to strategies to prevent disease or improve outcome (Chapter 72).
Genetics and Pediatric Practice
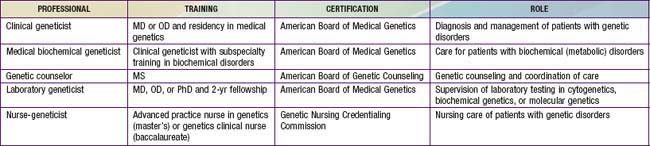
Pediatricians play a critical role in providing and coordinating medical services for families affected by genetic disorders. In this role, pediatricians are likely to come in contact with, or be served by, a variety of genetics professionals. Each of these professionals has undergone a program of training and certification consistent with their clinical role (Table 73-2). In most cases, pediatricians refer children with presumed genetic disorders to a clinical geneticist. Clinical geneticists are physicians who have completed a residency in genetics and are certified by the American Board of Medical Genetics. They can provide expertise in achieving a correct diagnosis, counseling the family regarding natural history and management of the disorder as well as recurrence risk, and implementing a management and treatment plan.
Ethics Issues
Baird PA, Anderson TW, Newcombe HB, et al. Genetic disorders in children and young adults: a population study. Am J Hum Genet. 1988;42:677-693.
Boright AP, Kere J, Scherer SW. The genetics of childhood disease and development: a series of review articles. Pediatr Res. 2003;53:4-9.
Braude P. Preimplantation diagnosis for genetic susceptibility. N Engl J Med. 2006;355:541-543.
Burton PR, Tobin MD, Hopper JL. Key concepts in genetic epidemiology. Lancet. 2005;366:941-950.
Fan HC, Blumenfeld YJ, Chitkara U, et al. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. PNAS. 2008;105:16266-16271.
Fischer A, Cavazzana-Calvo M. Gene therapy of inherited diseases. Lancet. 2008;371:2044-2047.
Goldstein DB. Common genetic variation and human traits. N Engl J Med. 2009;360:1696-1698.
Greely HT. Banning genetic discrimination. N Engl J Med. 2005;353:865-867.
Hall JG, Powers EK, McLlvaine RT, et al. The frequency and financial burden of genetic disease in a pediatric hospital. Am J Med Genet. 1978;1:417.
Kraft P, Hunter DJ. Genetic risk prediction—are we there yet? N Engl J Med. 2009;360:1701-1703.
McCandless SE, Brunger JW, Cassidy SB. The burden of genetic disease on inpatient care in a children’s hospital. Am J Hum Genet. 2004;74:121-127.
Miller JW. Preliminary results of gene therapy for retinal degeneration. N Engl J Med. 2008;358:2282-2284.
Qasim W, Gaspar HB, Thrasher AJ. Update on clinical gene therapy in childhood. Arch Dis Child. 2007;92:1028-1031.
Shaw CJ, Lupski JR. Implications of human genome architecture for rearrangement-based disorders: the genomic basis of disease. Hum Mol Genet. 2004;13:R57-R64.
Stevenson DA, Carey JC. Contribution of malformations and genetic disorders to mortality in a children’s hospital. Am J Med Genet A. 126, 2004. 393–337