The formulation and manufacture of plant medicines
G. Brian Lockwood
Chapter contents
Plant-based products in medicinal use
Quality control of crude plant drugs
Production methods used to obtain plant-derived active constituents
Extraction of active constituents
Concentration, purification and drying of extracts
Formulation and manufacture of plant-based medicines
Active-constituent considerations
Key points
• Quality control of crude plant drugs is more complex than for single chemical entities.
• A wide range of formulations of plant medicines are increasingly available.
Introduction
Drugs obtained directly from plant sources are notably alkaloids, glycosides and phenolic compounds. Plant material is also a favoured source for volatile (essential) oils. They are used in a number of different dosage forms; conventional plant derived pharmaceuticals, OTC preparations for minor ailments, herbal remedies, homoeopathic mother tinctures and medicines, volatile (essential) oils used medicinally and in aromatherapy, nutraceuticals (single and complex entities), antioxidants plus a vast array of traditional usages worldwide.
The European Pharmacopoeia alone contains more than 166 monographs for herbal drugs and 77 for herbal drug preparations, with an increasing number under study for future inclusion (Vlietinck et al, 2009).
Plant-based products in medicinal use
There is a wide range of plant derivatives in use for the manufacture of medicinal products. These include fresh and dried plant material, acellular products, a wide range of types of extracts including standardized extracts, and pure and in-vitro biotechnology-derived individual compounds. A range of conventional single-component pharmaceuticals derived from plants is listed in Table 44.1.
Table 44.1
Single chemical entities available after extraction from plant sources
| Chemical entity | Current prescription medicine application | Plant source |
| Atropine | Antispasmodic, ophthalmic | Atropa belladonna |
| Codeine, morphine | Analgesic | Papaver somniferum |
| Colchicine | Gout treatment | Colchicum autumnale |
| Digoxin | Cardiac glycoside | Digitalis lanata |
| Ephedrine | Bronchospasm, nasal congestion treatment | Ephedra spp. |
| Galanthamine | Alleviation of Alzheimer’s disease | Narcissus spp |
| Pilocarpine | Treatment of xerostomia, myotic | Pilocarpus jaborandi |
| Quinidine | Treatment of ventricular fibrillation | Cinchona succiruba |
| Sennosides | Laxative | Cassia senna |
| Vinblastine, vincristine | Anti-cancer agents | Catharanthus roseus |
The problems involved with the use of collected wild plant material include dramatic variability in quality as a result of the genetic variability of the wild stock, poor knowledge of the plants’ life cycle and the effects of differing habitats on levels of active constituents. Uncontrolled collection from the wild has led to devastation of certain supplies.
In order to control the influences of agro-ecological factors on levels of active constituents with the plant, cultivation is employed for production of the best quality raw materials. Medicinal plants should ideally be grown from homogeneous, genetically selected strains chosen for high yield of the relevant constituent(s) or other useful traits such as insect/fungal resistance.
Transport delays between collection and processing is a particular problem with the use of fresh plant material, which further compromises quality, leading to the possibility of degradation of the active constituent(s) as a result of microbial infestation, oxidation, reduction, hydrolysis and numerous other reactions. In spite of these disadvantages herbalists are still convinced of their benefits.
Quality control of crude plant drugs
Quality control (QC) techniques are described in a range of monographs in national and international pharmacopoeias, as well as in herbal and homoeopathic pharmacopoeias. QC procedures should be applied to the herbal starting materials, their extracts and the finished products. Quality control techniques used for plants and their extracts are outlined in Table 44.2. Modern chromatographic techniques are also used for separation and quantification of specific individual constituents. This chapter will not detail the specific analytical techniques described and the reader is referred to other texts for this information.
Table 44.2
| Standard | Technique | Purpose |
| Sampling | Selecting representative samples for analysis. Pharmacopoeias may suggest the number of samples from large consignments. | To ensure all analytical data obtained truly represent the characteristics of the batch |
| Preliminary investigation | Organoleptic testing; observation of colour, odour, taste | Observation for evidence of poor quality or adulteration, to ensure high quality of final product |
| Foreign matter | Observation for excreta, mould, etc. | To ensure high quality of final product |
| Moisture content | Loss on drying at 100–105 °C, Dean & Stark measurement, GC, Karl Fischer method, IR, UV, NMR spectroscopy | Inhibit or minimize enzymic or microbial degradation |
| Extractive values | Water soluble extractive, ethanol (45–90%) extractives, range of non-polar solvent extractives | To determine whether low levels of compounds of specific polarity are present or even absent |
| Ash values | Incineration at 450 °C for total ash | Indication of level of inorganic matter or silica |
| Insoluble ash values | Water- and acid-insoluble ash contents | Indication of level of contamination with earth or silica |
| Crude fibre | Defatting followed by boiling | Confirmation of normal level or detection of excess material, stalk for example |
| Macroscopical analysis | Comparison with botanical description | Initial identity of material |
| Microscopical analysis | Description of cells, inclusions and structures | Identification of material |
| Tannin content, bitterness value, swelling index | Quantitative measurements | Used for specific plants, containing either tannins, bitter substances or those used for swelling ability, e.g. laxatives |
| Microbiological contamination | Limits for levels of specific organisms | Check for levels of organisms above 103–104 microorganisms per gram |
(Courtesy of Evans 2009, with permission.)
Plant preparations are often considered to be active due to their combination of constituents, and these often complex mixtures can be identified by a semi-quantitative proof of content, such as a chromatographic fingerprint in combination with an appropriate assay of major constituents (Vlietinck et al, 2009). A combination of data from three types of chromatography are able to provide much qualitative and quantitative information.
Thin layer chromatography (TLC) is a semi-qualitative technique using specified standards. By determination of Rf (retardation factor) values, this technique allows comparison between extracts of different origins and composition with known standards. This will give evidence for the presence of the component(s) of the standards, plus indicative quantitative data as to their levels in the materials being tested.
To obtain true quantitative data either high-performance liquid chromatography (HPLC) or gas chromatography (GC) should be used. These techniques are predominantly used for assays of either polar or volatile constituents, respectively. GC is increasingly widely available coupled with mass spectroscopy (MS). This GC-MS combination allows identification and quantification of a wide range of components without the need for standards.
In addition to these chromatographic techniques, a number of spectroscopic techniques are widely used such as visible, infra-red (IR) and ultraviolet (UV) spectroscopy for determining semi-quantitative levels of constituents. In addition to these latter techniques, assays for specific constituents have been devised using NMR spectroscopy, immunoassay, radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA) and fluorescence analysis. Near infra-red spectroscopy (NIR) has recently been used for routine analysis of dry plant material and formulated products (both liquid and solid) and has the added advantage that it is non-invasive and can therefore be used for quality control in production and in packaging lines.
Herbal remedies often contain numerous herbal extracts, in many examples numbering more than 10. This creates analytical difficulties and this challenge, associated with the increasing usage of all herbal remedies particularly traditional and complementary medicines (TCM), has inspired analysts to produce more inclusive techniques, such as chemical pattern recognition, spectral correlation, etc. (Liang et al, 2004). DNA fingerprinting has recently been used to establish the identity of highly expensive raw materials, particularly prone to substitution.
Production methods used to obtain plant-derived active constituents
The wide variety of medicinal plants, types of plant parts and varying textures of material, makes it impossible to standardize mechanical procedures, from harvesting through to drying, size reduction, or even essential oil extraction. The fibrous texture of in vivo or field-grown plant material and also unorganized crude drugs often requires severe mechanical disruption prior to extraction. Table 44.3 outlines the basic processes involved in production of plant extracts. These are applied to both conventional plant-based pharmaceuticals and complementary herbal medicines.
Table 44.3
The major stages in the conversion of plant material into a concentrated extract
| Production process | Purpose | Constraints |
| Harvesting | Stop metabolism at optimum time | Weather |
| Drying | Inactivate enzymes, inhibit microbial infestation | Plant part and temperature determine speed. Essential in tropical conditions |
| Size reduction (comminution) | Increase surface area for effective solvent extraction | Solvent flow impeded if particles too small, possible release of excessive mucilage which hinders later filtration |
| Extraction of active constituents | Production of most active base for formulation | Financial constraints to complete (100%) extraction |
| Extract concentration | Minimize volume/weight of extract, for ease of transport, storage, and ease of distribution in the final formulation | As above, but extra investment |
Harvesting
The first stage, harvesting, is strictly an agricultural and not a pharmaceutical process, but it can have a great influence on the quality of the final product. Each procedure requires specialized equipment, often using modified versions of commercial agricultural machinery.
Further mechanical processing techniques are often required, which may include cleaning or washing. Procedures are needed to eliminate unwanted foreign matter, such as other plant material, minerals, any other organisms and agrochemical residues. In some instances, manual techniques are still superior to mechanization.
Drying
Drying is usually an essential process, as medicinal plant material contains water. This water must be removed to maintain the quality of the raw plant material. Often this degradation is simply monitored by macroscopical investigation of colour, form and absence of microbiological and fungal growth. The moisture content of the raw material is affected by the prevailing humidity; hence there is a greater risk of degradation in crops grown in tropical climates that are subject to higher humidity and high temperature.
Drying is necessary in almost all cases in order to protect the active constituent content. The three main techniques used for drying of plant material are:
• Natural drying – direct sunlight, which may have adverse effects
• Hot air drying – the operational temperature depends on the nature of the active constituents and may range from 40 °C for essential oils to 100 °C for glycoside-containing material. Similar equipment to that used in pharmaceutical operations is employed (Chapter 29)
• Microwave drying – this is often used in combination with hot air drying. It may cause browning of the material, but it is useful in reduction of microbial contamination (Oztekin and Martinov, 2007).
Size reduction
The main aim of size reduction of the dried material is to create particles of similar size which permits uniform and maximum extraction of the required plant material. The rate of extraction is dependent upon the rate of diffusion of solvent into the plant material and of solutes from the material into the surrounding solvent. Hence reasonably fine powders are preferred, with diameters approaching 0.5 mm.
Care must be taken during size reduction of fresh (undried) material as in some cases it can lead to degradation of constituents via a number of chemical reactions and also endogenous enzymatic action. Low temperature can be used to reduce these possibilities and deep freezing may be required during storage of fresh materials prior to comminution (Bombardelli, 1991).
Size reduction can be carried out using a variety of crushers and mills, usually fitted to a magnetic separator to collect extraneous metal particles. Dust collection devices are imperative for this process. Typical types of size reduction apparatus (see Chapter 10) used for plant material include:
• cutting and shredding mills for leaves and herbs
• hammer and pin mills for herbs with high fat content
Size reduction of plant material is a very inefficient operation, with only about 1% of the energy input directly responsible for the size reduction (Chaudhri, 1996).
Extraction of active constituents
Types of extracts
The next step in the process is to remove the active constituents from the dried and powdered plant. This is achieved mainly by the process of extraction. In its most common form, extraction consists of soaking the powdered material in a liquid (usually aqueous or ethanolic) solvent. The solvent diffuses into the powdered material, dissolves the ingredients which then diffuse out into the liquid. Decreasing particle size of the plant matrix, within limits, will therefore decrease extraction time.
The major types of liquid product obtained by this process and then used in the manufacture of medicines include (Bonati 1980, Vlietinck et al, 2009):
Each of these products has particular advantages and disadvantages.
Liquid extracts are preferred over decoctions (plant boiled with water) and infusions (plant stood in hot or cold water) because of the higher concentration of active constituents in the extract. Liquid extracts are produced by extracting 1 part of plant material with 1–2 parts of solvent, whilst tinctures require 1 part of plant material with 5–10 parts of solvent. These liquid extracts can be incorporated directly into semi-solid formulations such as ointments or into liquid formulations such as drops or solutions (Vlietinck et al, 2009).
The choice of extract type depends on the intended application; dry extracts (liquid extracts that have been subsequently dried) are suitable for tablet/capsule formulations, while solvent extracts are more widely used in liquid formulations.
Purified (refined) and standardized extracts are intermediate between crude extracts and single chemical entities and as such have widespread application. They avoid the need to separate complex mixtures, but provide knowledge of the levels of constituents.
It is important to appreciate that with many plant materials a single chemical entity usually has the greatest activity, although synergistic interactions may increase the activity of complex mixtures.
All types of extracts need to be made with knowledge of the polarity of targeted constituents, which may impact on cost of the most appropriate solvent for the process, waste solvent disposal, extract stability, etc. The composition of most end materials is highly dependent on the procedures used for extraction, and often the most valuable constituents are produced using the most sophisticated processes.
Extraction procedures
Table 44.4 lists the types of extraction procedures that are widely used.
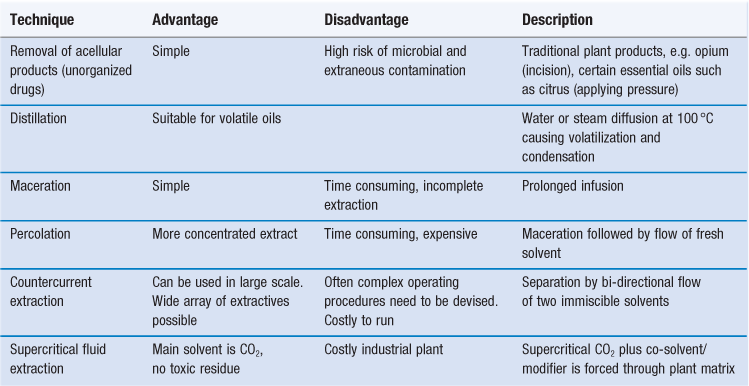
Removal of acellular products.
This techniques is really a collection method specific to a few plant products, but is also classed by some as a method of ‘extraction’. As shown in Table 44.4, this is only used for a few specific examples of materials obtained by simple traditional techniques. Although crude in design, the products are the commercially available material, and often need highly specialized treatment before incorporation into formulated products.
Distillation.
If the active constituent is a volatile oil it is most often removed by the process of distillation. Whist this ‘extracts’ the active constituents it is not, in a chemical engineering sense (as described above), a true extraction process. Three different types of distillation processes are employed for the removal of volatile oils from plant material:
Maceration.
Maceration involves the steeping of raw material in a solvent, which is later strained out. It is widely used for the preparation of tinctures, but is very inefficient for the collection of solutes.
Percolation.
Percolation involves the subjection of raw material to continuous flow of fresh solvent. This produces stronger extracts, but at increased solvent cost. Repeated percolation using a number of extractors with solution percolates (solvent already containing extracted components), partially decreases the problem.
Countercurrent extraction.
Countercurrent extraction is effected by continuous distribution of solutes from raw material between moving tubes of immiscible upper and lower phases (basically a series of interconnected separating funnels). This gives improved extraction yields, but the equipment is expensive and needs lengthy extraction times for best results.
Newer extraction techniques.
A number of alternative techniques can be employed in laboratory conditions. These include:
• Supercritical fluid extraction
• Subcritical water extraction can be used from 100–374 °C under pressure
• Sonication-assisted extraction
• Phytol/florasol extraction using hydrofluoroalkane (HFA) 134a is carried out at −26 °C, and is useful for thermolabile materials (Oztekin and Martinov, 2007).
The supercritical fluid extraction technique involves elevating the temperature and pressure of the solvent above its critical value. It is now widely used in industrial scale procedures. Supercritical CO2 is a good solvent for non-polar compounds, but not for most plant compounds with biological activity (notably alkaloids, glycosides and phenolics) which are polar in nature. For these, a co-solvent or modifier must be added to the CO2. Industrial processes have been reported for naringin, colchicine and oleoresins (Wang and Weller, 2006).
Choice of extraction technique
Usually the method of choice is determined by the size of the batch of plant material to be extracted. Solvent extraction may include risks of toxicity when using particular solvents.
The solubility of plant entities determines the choice of the best medium, but this is usually fixed by formulation constraints resulting in either partial or complete lack of solubility. This problem is confounded by extracts containing ranges of chemicals of differing solubilities (Bonati, 1980). Alternative solvents may result in lower solute recoveries; therefore co-solvents may be added to polar solvents to increase recovery.
Concentration, purification and drying of extracts
Concentration of extracts
Liquid extracts typically contain 2–5% of the plant constituents, and further concentration by evaporation is required. The Roberts concentrator was once commonly used but it is slow and inefficient. It is being replaced by either the descending film concentrator or the plate concentrator, both of which are fast acting and thus reduce the risk of degradation. If water is the solvent, no solvent recovery is required, although some degree of clean-up may be necessary before disposal. With other solvents, evaporated solvent must be collected by condensation under cooled conditions and the collection vessel enclosed to avoid evaporative losses.
Purification of extracts
Following concentration of the original extracts, further purification is often required. A number of procedures are available to remove extraneous plant material or undesirable material formed during extraction. These include decantation, pressure filtration, vacuum filtration, centrifugation and drying.
Many extracts are sticky due to their hygroscopic nature, and this causes processing problems. A number of crude plant drugs may need to undergo preliminary treatment such as defatting to avoid high fat levels in the extract, or enzyme inactivation to avoid degradation of active constituents (Bonati, 1991).
Drying of extracts
After purification, extracts may be dried, and several types of equipment are available to carry out this procedure. The range of dryer types available is outlined in Table 44.5 (see Chapter 29 for further details).
Table 44.5
| Type | Comments |
| Heated tray dryers | Deterioration due to long time in contact with heat, possible risk of oxidation of active constituents, labour intensive |
| Cabinet vacuum dryers | Oven temperatures 60–80 °C, heterogeneous drying, possible risk of oxidation of active constituents, labour intensive tray layout, uncertain endpoint |
| Drum/belt dryers | Evaporation provided by hot air stream, uncertain endpoint, possible risk of oxidation of active constituents, large scale possibilities, requires regranulation stage |
| Atomizers/spray dryers | Lower temperatures used, less time in contact with heat, cheaper than freeze drying, product in powder form, widely used |
| Freeze dryers | Low temperature, expensive to buy and operate, high quality product in layer form, requiring regranulation |
Dry extracts have a superior stability profile over time and are likely to have lower levels of microbiological contamination. In addition to this, gamma irradiation can easily be used, if necessary, to eradicate any remaining microbiological contamination.
Formulation and manufacture of plant-based medicines
Active-constituent considerations
Purity of active constituent(s)
Production and formulation of plant-based medicines involves technology and stability problems far greater than those for single natural isolated chemicals or synthetic compounds. One major cause of the problem is inclusion of compounds which are either pharmacologically inactive, are active but possess additive, synergistic or even opposite or dissimilar activities. Ideally, these constituents should have been removed during primary production, but this is often unrealistic due to constraints of cost, lack of knowledge of their identities, and technical inability to remove them. Formulation with plant extracts requires a complete knowledge of the composition of the extract, so that the formulator can choose the most suitable excipients and formulation. Ideally, only one plant extract should be included in any formulation, but there are many examples where there are more than one. This may cause formulation problems due to interaction of components of one with the other, causing instability.
The problems associated with converting fresh or dried plant material into medicinal products are highlighted in the third column of Table 44.3. This lists the constraints to producing the best quality products. In addition to these general problems, there are additional problems relating to specific plants and their constituents.
Standardized extracts are essential in order to get as complete a list of constituents actually present in the extract (and hence) into the formulation. A number of these constituents are exceedingly unstable, e.g. valepotriates from Valerian, when in acidic or basic medium in combination with water. Turbidity of reconstituted solutions is a widespread problem, sometimes dealt with by incorporation of polyvinylpyrrolidone (PVP). Emulsions of particular extracts containing saponins (widespread in plant extracts) are often found to exhibit phase separation (Crippa, 1980)
Drying plant material inactivates endogenous enzymes, but extracts which contain glycosides are then subject to degradation when formulated into aqueous media, as the enzymes are re-activated.
The greatest risk to the quality of constituents is heating during manufacture, but the quality of water is also important. pH has a major effect on the stability of a number of types or formulations and their active constituents. Both acidic and basic conditions have been shown to have detrimental effects.
Variability of crude drug material
Supply of raw material is a major problem, due to wide-scale variations in composition. Published surveys show that there can be a distinct difference in constituent levels even between batches from the same supplier.
Adulteration (deliberate or accidental substitution with inferior material) has long been a problem with herbal remedies. Traditional QC procedures have been adopted to detect this situation, with a number of parameters specifically designed to identify the problem of adulteration with inferior material.
With the increased use of herbal remedies the causes may be more sophisticated, for example attempting to improve the characteristics of the extracts with material not detected using standard assays. Bilberry extracts have been found adulterated with the food dye, amaranth, added specifically to make it appear more intense in colour.
Finished-product considerations
There exists a wide range of typical formulations widely used for plant extracts, which include most of the types used for conventional pharmaceuticals. Most conventional dosage forms can be produced from plant liquid or dried extracts using conventional techniques.
As with conventional pharmaceuticals, solid dosage forms are the preferred type of formulation, while liquids such as syrups are losing favour due to lack of patient acceptability and poor stability during storage. Controlled-release solid dosage forms of plant-based material can also be manufactured.
Preparation of solid dosage forms
Approximately 75% of European herbal medicines are constituted from dry extracts. These dry extracts are invariably hygroscopic. A high porosity excipient, such as microcrystalline cellulose, is usually added to the formulation, together with a cellulose derivative binder. As is the case with conventional tablets (Chapter 30), these extra excipients are responsible for improved physical characteristics of the tablets, notably hardness, friability, and disintegration, when used in the correct proportions for a particular plant extract (Crippa, 1978).
Freeze-dried extracts have been shown to have far greater solubility than powdered plant material, with a direct effect on bioavailability.
Pharmaceutical parameters, such as flowability and hygroscopicity can vary widely depending on the source of the raw plant material. Likewise compactability varies considerably and all these factors may affect tablet/capsule weights and levels of active constituents (Jin et al, 2008).
Preparation of liquid dosage forms
Nearly all types of extracts can be used in formulating liquid dosage forms. If dry extracts are used, the material must be re-dissolved, which may result in precipitation of components or at least the presence of turbidity. To avoid these issues, it is advisable to re-dissolve at precisely the same concentration in the solvent as that which was used to prepare the extract. On some occasions, the downstream processing of the initial extract may modify the composition of the extract so that dissolution does not occur, in which case co-solvents or surfactants may need to be added. The solubility and stability of some extracts can be improved by pH manipulation, particularly when reduction favours salt formation for the active, such as in the case of an alkaloid. The stability of these dosage forms is adversely affected by fermentation, which is prone to occur in extracts containing nutritive plant constituents, but this can be reduced by regulating the alcohol content or by using traditional preservatives, such as p-hydroxybenzoic acid esters (Bonati, 1991).
One claimed advantage of plant-based liquid formulations is that they have improved bioavailability, and that unique formulations can be produced for individual patients (Bone, 2003).
Newer delivery systems
Methods used to manufacture liposomes, nanoparticles, phytosomes, emulsions, and microspheres from numerous plant extracts are regularly reported in the scientific literature. Liposomes have been produced for markedly different products including paclitaxel, curcumin, garlic and quercetin.
Herbal medicines are now being formulated and administered via the most up-to-date delivery technologies available. Transferosomes and ethosomes are being used for a range of topical applications. A range of herbal entities have also been formulated into microspheres, as small as 6 µm, which can be ingested or injected and targeted to specific organs of the body (Saraf, 2010).
Excipients
Preservatives.
The p-hydroxybenzoic acid esters, such as the methyl and propyl esters (parabens), are widely used. However, in a number of formulations such as herbal cosmetics and external medicaments, bronopol is also widely used. The possibility of manufacturing ‘organic products’ without addition of preservatives has been commercially exploited in a number of herbal products. This strategy normally results in products having a reduced shelf-life.
Antioxidants.
The use of antioxidants to limit oxidation in pharmaceuticals and foods are widespread, ascorbate is widely employed for this purpose.
Colouring materials.
As concerns about the dangers of artificial colours continues worldwide, plant derived colours are increasingly being used, because their use obviates the use of synthetic dyes. An example is Turmeric from the roots of Curcuma longa, which contains curcuminoids that have a yellow or orange colour. Beta-carotene is another example and has an orange-yellow colour. Commercial beta-carotene is derived from algae or is synthesized and is oil-soluble, but it can also be made into a water-dispersible emulsion. These natural yellow to orange colours are an alternative to synthetic yellow dyes. Other plant-based colours include anthocyanins or tomato extract, which can produce a range of red colours in place of synthetic red dyes.
The possible use of plant extracts for colouring formulations has been extensively researched over a number of years, but the major problem is that they usually tend to be unstable in varying pH conditions, and are particularly prone to degradation.
Flavours.
Plant extracts are often bitter or astringent and masking this with sweeteners or flavours is widely employed. Flavours are invariably included in liquid oral formulations to mask these bitter or unpleasant tastes, to improve patient compliance. Apart from volatile oils, which are selectively used for flavouring formulations designed for different patient groups, a wide range of soft fruit flavours such as banana or strawberry, either natural or synthetic, are also used for flavouring.
Biotechnological production of plant products
A number of major prescription medicines are currently produced using in vitro techniques. Currently, digoxin, taxol and vincristine amongst a small number of medicines, are derived from plant cell culture techniques, and commercially manufactured and extracted in a complete ‘in-house’ procedure. These procedures are excessively expensive, consequently only products with the highest value can be commercially exploited using plant cell culture.
Due to commercial sensitivity, biotechnology procedures are not published in detail, however they are similar to those used for whole plant material, without the need for drying and size reduction, but are produced by simply leaching out the active constituents followed by purification and later formulation as for single chemical entities.
Quality of finished products
Quality of formulated herbal products
Unlike prescription products, there are few agreed standards for formulated herbal remedies. A survey of a large number of herbal products from a range of manufacturers found incorrect/inadequate labelling, products with wide ranging content claims, wide ranging recommended daily dosage, a range of different plant parts used and from a number of claimed sources of botanical origins. Wide ranging values for active constituent content have been reported for parthenolide in Feverfew and for the active constituent(s) in Ginseng, Gingko, Echinacea, Hypericum and others (Ruparel and Lockwood, 2011). This demonstrates the possible risk to patients and the urgent need for standards (Heptinstall et al, 1992).
Shelf-life of formulated products
Labelling of shelf-life or expiry date is presently not mandatory for all formulated plant products. Scientific knowledge about degradation and acceptable shelf-life is obviously necessary for these complex products. Improved packaging designs are now being used to limit degradation, but control of storage conditions from warehouse to point-of-sale are of major importance. There are a number of difficulties in conducting shelf-life determinations with complex products, and often simplistic parameters are used, such as colour and consistency of the formulation, in addition to chemical evaluation. Further, detailed real-time and accelerated testing may be carried out at specific temperature and humidities, as for conventional pharmaceuticals. These techniques are particularly useful to speed up data collection and for determining suitable formulations (Houghton and Mukherjee, 2009).
Bioequivalence of different formulations
The issue of bioequivalence of different formulations of conventional pharmaceuticals is well researched. However, such information is less detailed when comparing different formulations of plant medicines. Data from a limited number of single component herbal medicines, showing variable plasma concentrations for each, are available. However, major complication occur when a herbal medicine’s activity is derived from a range of components. In this instance, plasma concentrations are often insufficient to determine levels of activity, and therefore assays for effects on biomarkers are required in order to show comparative activities of different formulations. Further problems are clearly evident when the active constituent(s) of a plant-based product are unknown, which is often the case (Loew and Kaszkin, 2002).
Adverse effects and drug interactions
The public are slowly appreciating the fact that complementary medicines are not necessarily safe plant products simply because they are ‘natural’. An increasing number of potential and actual adverse effects have been reported.
Synergy.
Synergy is enhancement of the activity of one constituent by more than simply adding the two individual activities. The chances of synergy occurring is increasingly likely with these complex products. The degree of synergy will depend on the concentration of the entities involved, therefore product variability has increased consequences.
Drug interactions.
Drug interactions with herbal medicines are poorly researched, however there is always the probability that one medicine will react with the activities of another, especially when they have marked pharmacological activity. The most likely plants to be involved are those which are metabolized by the P450 CYP enzyme system. Echinacea, Garlic, Cloves, Evening Primrose oil and Soy constituents are examples of the major herbs responsible for these interactions. St John’s Wort is responsible for the largest amount of data on interactions, and it has proven hepatic enzyme inducing properties (Williamson et al, 2009).
Summary
This chapter covers the use of plant products, featuring conventional and complementary medicines, herbal, homoeopathic and aromatherapy products, and their levels of usage and the numerous forms of plants in use.
Production methods used for plant derived conventional pharmaceuticals and complementary medicines are outlined. Formulation techniques and problems specific to plant products have been discussed.
References
1. Bombardelli E. Technologies for the processing of medicinal plants. In: Wijesekera ROB, ed. The Medicinal Plant Industry. Boca Raton: CRC Press; 1991;85–98.
2. Bonati A. Formulation of plant extracts into dosage forms. In: Wijesekera ROB, ed. The Medicinal Plant Industry. Boca Raton: CRC Press; 1991;107–113.
3. Bonati A. Medicinal plants and industry. Journal of Ethnopharmacology. 1980;2:167–171.
4. Bone K. A Clinical Guide to Blending Liquid Herbs: Herbal Formulations for the Individual Patient. Edinburgh: Churchill Livingstone; 2003.
5. Chaudhri RD, ed. Herbal Drugs Industry. New Delhi: Eastern Publishers; 1996.
6. Crippa F. Problems of pharmaceutical techniques with plant extracts. Fitoterapia. 1978;49:257–263.
7. Crippa F. Problems involved in pharmaceutical and cosmetic formulations containing extracts. Fitoterapia. 1980;51:59–66.
8. Evans WC. Trease and Evans’ Pharmacognosy. 16th edn Edinburgh: Saunders; 2009.
9. Heptinstall S, Awang DVC, Dawson BA, et al. Parthenolide content and bioactivity of feverfew (Tanacetum parthenium (L.) Schultz-Bip.) Estimation of commercial and authenticated feverfew products. Journal of Pharmacy and Pharmacology. 1992;44:391–395.
10. Houghton P, Mukherjee PK. Evaluation of Herbal Medicines. London: Pharmaceutical Press; 2009.
11. Jin P, Madieh S, Augsburger L. Selected physical and chemical properties of Feverfew (Tanacetum parthenium) extracts important for formulated product quality and performance. AAPS PharmSciTech. 2008;9:22–30.
12. Liang Y-Z, Xie P, Chan K. Quality control of herbal medicines. Journal of Chromatography B. 2004;812:53–70.
13. Loew D, Kaszkin M. Approaching the problem of bioequivalence of herbal medicinal products. Phytotherapy Research. 2002;16:705–711.
14. Oztekin S, Martinov M. Medicinal and Aromatic Crops: Harvesting, Drying and Processing. U.S: Food Products Press; 2007.
15. Ruparel P, Lockwood B. The quality of commercially available herbal products. Natural Product Communications. 2011;6:1–12.
16. Saraf AS. Applications of novel drug delivery system for herbal formulations. Fitoterapia. 2010;81:680–689.
17. Vlietinck A, Pieters L, Apers S. Legal requirements for the quality of herbal substances and herbal preparations for the manufacturing of herbal medicinal products in the European Union. Planta Medica. 2009;75:683–688.
18. Williamson EM, Driver S, Baxter K. Stockley’s Herbal Medicines Interactions. London: Pharmaceutical Press; 2009.
Bibliography
1. Lockwood GB. Complementary and alternative medicine. In: Rees J, Smith I, Wingfield AJ, eds. Pharmaceutical Practice. 4th edn. Edinburgh: Churchill Livingstone; 2009;199–210.
2. Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends in Food Science and Technology. 2006;17:300–312.