Chapter 4 The Eye
A. Generalities
2 What is needed to measure visual acuity?
Snellen chart, pinhole occluder, and pocket-size near-vision test card. All are readily available.
10 How is vision measured in illiterate patients or children?
By using the single “E” chart and asking patients to designate the strokes’ direction.
11 How is vision measured in bedridden patients?
By using the Rosenbaum card, which can be found at any medical supply store.
12 What is the significance of reduced visual acuity?
It may indicate any of the following ocular processes:
 Refractive and correctable errors (myopia, astigmatism, and presbyopia)
Refractive and correctable errors (myopia, astigmatism, and presbyopia)
 Treatable and reversible blinding disease (cataracts or uveitis)
Treatable and reversible blinding disease (cataracts or uveitis)
 Manifestations of systemic disorders, progressive if untreated (diabetes or hypertension)
Manifestations of systemic disorders, progressive if untreated (diabetes or hypertension)
 Vision-impairing and possibly life-threatening CNS disease (multiple sclerosis, gliomas)
Vision-impairing and possibly life-threatening CNS disease (multiple sclerosis, gliomas)
 Congenital disorders (rubella or toxoplasmosis)
Congenital disorders (rubella or toxoplasmosis)
 Infectious diseases (cytomegalovirus, retinitis, or toxoplasmosis)
Infectious diseases (cytomegalovirus, retinitis, or toxoplasmosis)
13 How can one confirm the cause of reduced visual acuity?
 Uncorrected refractive errors of the eye are usually suspected whenever vision improves with a pinhole occluder.
Uncorrected refractive errors of the eye are usually suspected whenever vision improves with a pinhole occluder.
 Opacities of the light-transmitting media are diagnosed by either ophthalmoscopy or by simply examining the red reflex.
Opacities of the light-transmitting media are diagnosed by either ophthalmoscopy or by simply examining the red reflex.
 Neurologic or retinal disorders are revealed by ophthalmoscopy, the swinging flashlight test, or visual field testing.
Neurologic or retinal disorders are revealed by ophthalmoscopy, the swinging flashlight test, or visual field testing.
 Amblyopia is suggested by a childhood history of reduced visual acuity often accompanied by strabismus.
Amblyopia is suggested by a childhood history of reduced visual acuity often accompanied by strabismus.
15 When should you refer a patient with subnormal visual acuity?
 No symptoms, but visual acuity of 20/40 (or worse) in one or both eyes
No symptoms, but visual acuity of 20/40 (or worse) in one or both eyes
 A visual acuity difference between eyes of two lines or more
A visual acuity difference between eyes of two lines or more
 Middle-aged or elderly patients with presbyopia, even if distance visual acuity is preserved (for prescription of reading glasses)
Middle-aged or elderly patients with presbyopia, even if distance visual acuity is preserved (for prescription of reading glasses)
B. Color Vision
C. Visual Fields
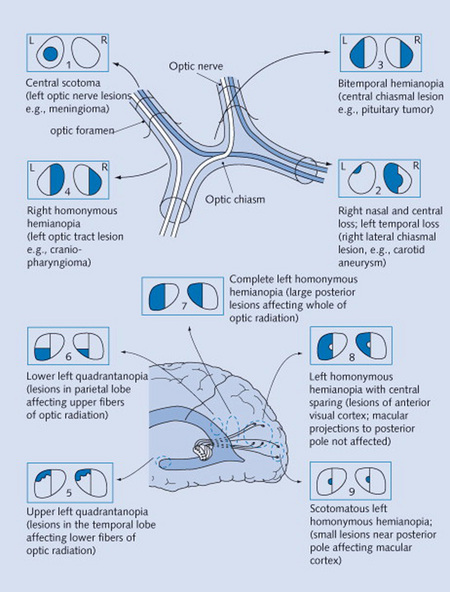
21 Describe the normal anatomy of the visual pathways.
This is quite complex and yet fundamental for the understanding of visual field defects (Fig. 4-1). Our visual world (i.e., visual field) is divided into right and left hemifields. Each eye gets information from both.
22 How do you test visual fields?
 Static testing: The examiner sits 3 feet away from the patient, who covers one eye with the palm of one hand while fixating on the examiner’s opposite eye. While the patient is doing so, the examiner outstretches an arm and briefly holds up one or two fingers. These are then displayed in each quadrant, and the patient is asked to report how many fingers are seen. In fact, the maneuver is often carried out by testing two quadrants simultaneously (by presenting both examiner’s hands to the two quadrants at the same time). This may unmask a parietal lesion that would otherwise allow a patient to see a single object (but not a double) by relying on the contralateral field. The test is then repeated for the opposite eye.
Static testing: The examiner sits 3 feet away from the patient, who covers one eye with the palm of one hand while fixating on the examiner’s opposite eye. While the patient is doing so, the examiner outstretches an arm and briefly holds up one or two fingers. These are then displayed in each quadrant, and the patient is asked to report how many fingers are seen. In fact, the maneuver is often carried out by testing two quadrants simultaneously (by presenting both examiner’s hands to the two quadrants at the same time). This may unmask a parietal lesion that would otherwise allow a patient to see a single object (but not a double) by relying on the contralateral field. The test is then repeated for the opposite eye.
 Dynamic testing: This is carried out very much like its static counterpart, except that the examiner continuously wiggles his or her fingers while gradually (and very slowly) bringing them into the patient’s visual field from a peripheral angle. The patient is then asked to report when (s)he sees the fingers.
Dynamic testing: This is carried out very much like its static counterpart, except that the examiner continuously wiggles his or her fingers while gradually (and very slowly) bringing them into the patient’s visual field from a peripheral angle. The patient is then asked to report when (s)he sees the fingers.
25 What is the difference between anterior and posterior visual field defects?
 Anterior defects are unilateral (monocular)—typically crossing the vertical meridian.
Anterior defects are unilateral (monocular)—typically crossing the vertical meridian.
 Posterior defects are instead bilateral, with borders aligned to (and not crossing) the vertical meridian of the visual field. They are referred to as chiasmal if the defect is bitemporal and retrochiasmal if homonymous (see later).
Posterior defects are instead bilateral, with borders aligned to (and not crossing) the vertical meridian of the visual field. They are referred to as chiasmal if the defect is bitemporal and retrochiasmal if homonymous (see later).
26 What are the causes of visual field defects?
 Prechiasmal defects are due to ocular lesions (glaucoma, retinal emboli, optic neuritis).
Prechiasmal defects are due to ocular lesions (glaucoma, retinal emboli, optic neuritis).
 Chiasmal defects are due to pituitary lesions (mostly neoplastic).
Chiasmal defects are due to pituitary lesions (mostly neoplastic).
 Postchiasmal defects are due to cortical lesions (temporal, parietal, or occipital).
Postchiasmal defects are due to cortical lesions (temporal, parietal, or occipital).
 Optic tracts lesions are instead rare, representing only 5% of all postchiasmal defects.
Optic tracts lesions are instead rare, representing only 5% of all postchiasmal defects.
D. Pupils
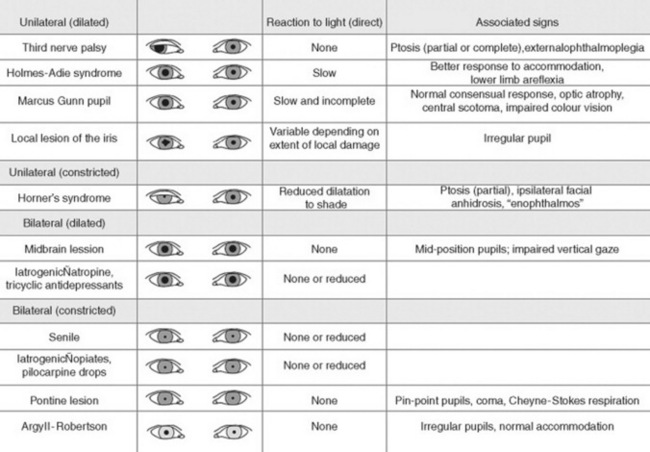
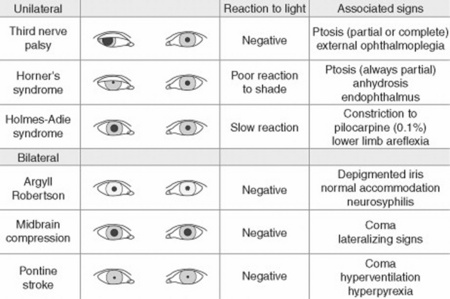
37 Why is examination of the pupils important?
Because attention to pupillary shape, size, and response to external stimuli provides extremely valuable clinical information. Figures 4-2 and 4-3 summarize the most common pupil abnormalities.
44 What are the most common abnormalities in pupillary shape?
 The most common is probably the irregular, pear-shaped contour of pupils that have undergone an intraocular surgical procedure, such as cataract excision. There also are other causes of oval pupils, such as an incipient transtentorial uncal herniation (before the pupil becomes dilated, fixed, and fully round) and Adie’s pupil.
The most common is probably the irregular, pear-shaped contour of pupils that have undergone an intraocular surgical procedure, such as cataract excision. There also are other causes of oval pupils, such as an incipient transtentorial uncal herniation (before the pupil becomes dilated, fixed, and fully round) and Adie’s pupil.
 Blunt trauma to the eye. This may tear the iris sphincter, making the affected pupil larger and slightly irregular.
Blunt trauma to the eye. This may tear the iris sphincter, making the affected pupil larger and slightly irregular.
 Inflammation of the iris (iritis). This may cause adhesions (synechiae) of the iris to the anterior capsule of the lens, thus making the pupil irregular.
Inflammation of the iris (iritis). This may cause adhesions (synechiae) of the iris to the anterior capsule of the lens, thus making the pupil irregular.
 A coloboma of the iris. This is a congenital defect characterized by incomplete closure of the embryonic fissure of the optic cup. The involved iris usually has a keyhole shape, with the defect being most commonly in an inferonasal position.
A coloboma of the iris. This is a congenital defect characterized by incomplete closure of the embryonic fissure of the optic cup. The involved iris usually has a keyhole shape, with the defect being most commonly in an inferonasal position.
47 What are the most common causes of anisocoria?
 Simple (physiologic) anisocoria: A normal variant characterized by a physiologic difference of at least 0.4 mm between the two pupils, due, in turn, to an imbalance in muscular tone of the right and left sphincters. It is the most common anisocoria, present at all times in 3% of the population and at some times in up to 20%, with presence or absence depending on day of observation. In contrast to pathologic anisocoria, the physiologic form is characterized by a pupillary difference that does not change with various levels of illumination. Moreover, physiologic anisocoria is chronic, rarely >1 mm and always isolated (i.e., never associated with ptosis double vision or light-near dissociation see later). Hence, presence of concomitant findings makes anisocoria a much more ominous condition.
Simple (physiologic) anisocoria: A normal variant characterized by a physiologic difference of at least 0.4 mm between the two pupils, due, in turn, to an imbalance in muscular tone of the right and left sphincters. It is the most common anisocoria, present at all times in 3% of the population and at some times in up to 20%, with presence or absence depending on day of observation. In contrast to pathologic anisocoria, the physiologic form is characterized by a pupillary difference that does not change with various levels of illumination. Moreover, physiologic anisocoria is chronic, rarely >1 mm and always isolated (i.e., never associated with ptosis double vision or light-near dissociation see later). Hence, presence of concomitant findings makes anisocoria a much more ominous condition.
 Pharmacologic dilation: Another common and benign cause. This can be due to conscious or inadvertent instillation of mydriatic drops (like in Italian Renaissance women) or even to improper nebulization of anticholinergic agents. The latter may create a diagnostic dilemma in intensive care unit patients, whose mental status often waxes and wanes. A response to cholinergic eye drops (such as pilocarpine) may help separating pharmacologic paralysis (which will remain unresponsive) from either Adie’s pupil or a third nerve palsy (which, instead, will constrict in response to topical cholinergics—previously discussed).
Pharmacologic dilation: Another common and benign cause. This can be due to conscious or inadvertent instillation of mydriatic drops (like in Italian Renaissance women) or even to improper nebulization of anticholinergic agents. The latter may create a diagnostic dilemma in intensive care unit patients, whose mental status often waxes and wanes. A response to cholinergic eye drops (such as pilocarpine) may help separating pharmacologic paralysis (which will remain unresponsive) from either Adie’s pupil or a third nerve palsy (which, instead, will constrict in response to topical cholinergics—previously discussed).
 Third cranial nerve palsy (i.e., parasympathetic denervation): This is characterized by (1) ipsilateral mydriasis plus; (2) ptosis (from paralysis of the levator palpebrae); and (3) weakness of all extraocular muscles, with the exception of the lateral rectus and superior oblique (which are the only ones not controlled by the third cranial nerve). Hence, the affected eye will be deviated outward and downward, thus resulting in diplopia. Note that the dilated pupil will still constrict if a cholinergic drop is instilled in the eye. Moreover, due to defective constriction, the anisocoria of third-nerve palsy is greater in bright illumination.
Third cranial nerve palsy (i.e., parasympathetic denervation): This is characterized by (1) ipsilateral mydriasis plus; (2) ptosis (from paralysis of the levator palpebrae); and (3) weakness of all extraocular muscles, with the exception of the lateral rectus and superior oblique (which are the only ones not controlled by the third cranial nerve). Hence, the affected eye will be deviated outward and downward, thus resulting in diplopia. Note that the dilated pupil will still constrict if a cholinergic drop is instilled in the eye. Moreover, due to defective constriction, the anisocoria of third-nerve palsy is greater in bright illumination.
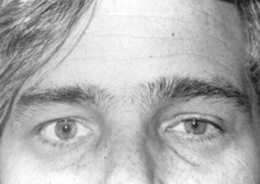
 Horner’s syndrome (Fig. 4-4): First described in 1860 by the Swiss ophthalmologist Johann Friedrich Horner, this is characterized by miosis of the affected pupil (from paralysis of the pupillodilator muscle) plus dysautonomic findings, including (1) ipsilateral ptosis (from paralysis of the superior tarsal muscle), and (2) anhidrosis (from damage to sudomotor fibers). In contrast to physiologic anisocoria, the difference between pupils in Horner’s varies with illumination: greater in the dark (revealing defective dilation) and smaller in bright light (demonstrating intact constriction). Response to cocaine drops is also different: Horner’s worsens, whereas simple anisocoria improves. This has strong predictive value, with sensitivity and specificity > 95%, a positive likelihood ratio (LR) of 96.8, and a negative LR of 0.1. Horner’s should always prompt a search for lesions of (1) the first-order neuron (such as a brainstem stroke, the most common cause of Horner’s on a neurology service; hence, the need for a thorough neurologic exam, with special attention to a lateral medullary syndrome); (2) the second-order neuron (usually a tumor of lung or thyroid, the most common cause of Horner’s on a medical service; hence, the need for a thorough neck/supraclavicular/respiratory exam); or, finally (3) the third-order neurons. These are less common causes of Horner’s, usually due to vascular headache (migraine), trauma or inflammation of the orbit, and cavernous sinus syndrome. Lesions of third-order neurons may preserve facial sweating.
Horner’s syndrome (Fig. 4-4): First described in 1860 by the Swiss ophthalmologist Johann Friedrich Horner, this is characterized by miosis of the affected pupil (from paralysis of the pupillodilator muscle) plus dysautonomic findings, including (1) ipsilateral ptosis (from paralysis of the superior tarsal muscle), and (2) anhidrosis (from damage to sudomotor fibers). In contrast to physiologic anisocoria, the difference between pupils in Horner’s varies with illumination: greater in the dark (revealing defective dilation) and smaller in bright light (demonstrating intact constriction). Response to cocaine drops is also different: Horner’s worsens, whereas simple anisocoria improves. This has strong predictive value, with sensitivity and specificity > 95%, a positive likelihood ratio (LR) of 96.8, and a negative LR of 0.1. Horner’s should always prompt a search for lesions of (1) the first-order neuron (such as a brainstem stroke, the most common cause of Horner’s on a neurology service; hence, the need for a thorough neurologic exam, with special attention to a lateral medullary syndrome); (2) the second-order neuron (usually a tumor of lung or thyroid, the most common cause of Horner’s on a medical service; hence, the need for a thorough neck/supraclavicular/respiratory exam); or, finally (3) the third-order neurons. These are less common causes of Horner’s, usually due to vascular headache (migraine), trauma or inflammation of the orbit, and cavernous sinus syndrome. Lesions of third-order neurons may preserve facial sweating.
 Miscellaneous: These include (1) inflammatory processes (e.g., unilateral iritis), (2) old trauma, (3) acute angle closure glaucoma, (4) various neurologic disorders, and (5) previous intraocular surgery (i.e., cataract extraction). In case of the red eye (see later), presence of anisocoria argues in favor of a more serious disease.
Miscellaneous: These include (1) inflammatory processes (e.g., unilateral iritis), (2) old trauma, (3) acute angle closure glaucoma, (4) various neurologic disorders, and (5) previous intraocular surgery (i.e., cataract extraction). In case of the red eye (see later), presence of anisocoria argues in favor of a more serious disease.
E. External Eye
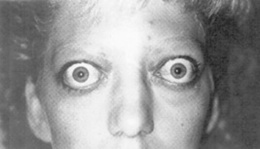
79 What is the most common cause of exophthalmos in adults?
Graves’ ophthalmopathy (Fig. 4-5). The next most common reason is a space-occupying lesion, such as a metastatic or primary tumor (benign or malignant). Unilateral exophthalmos is instead almost always due to a tumor. For other ocular manifestations of Graves’ ophthalmopathy, also see Chapter 8, The Thyroid, questions 70 and 71.
81 What is the difference between preseptal and orbital cellulitis?
 Preseptal cellulitis involves only the eyelids, which are erythematous and swollen. Hence, the process violates neither the orbital septum nor its contents. Patients with preseptal cellulitis do not appear systemically ill. Vision, pupils, conjunctiva, and ocular motility are entirely normal.
Preseptal cellulitis involves only the eyelids, which are erythematous and swollen. Hence, the process violates neither the orbital septum nor its contents. Patients with preseptal cellulitis do not appear systemically ill. Vision, pupils, conjunctiva, and ocular motility are entirely normal.
 Orbital cellulitis is instead a medical emergency, since it involves not only the eyelids but also the orbital contents. Patients look systemically ill and have a host of symptoms and signs, including (1) pain on eye movement, (2) erythema and edema of the eyelids, (3) conjunctival injection and chemosis (swelling), (4) limitation of ocular motility on the affected side, (5) proptosis, (6) decreased vision, and possibly, (7) an afferent pupillary defect.
Orbital cellulitis is instead a medical emergency, since it involves not only the eyelids but also the orbital contents. Patients look systemically ill and have a host of symptoms and signs, including (1) pain on eye movement, (2) erythema and edema of the eyelids, (3) conjunctival injection and chemosis (swelling), (4) limitation of ocular motility on the affected side, (5) proptosis, (6) decreased vision, and possibly, (7) an afferent pupillary defect.
(4) Extraocular Movements
84 How valuable is testing of extraocular movements?
Valuable primarily when the patient reports new double vision. However, a subtle ocular misalignment of neurologic importance may be present even if the eye movements appear normal. These patients still merit prompt referral to the appropriate consultant. For strabismus and pseudostrabismus, see the Corneal Light Reflex (questions 119–123).
85 What is diplopia?
It is double vision. Due to ocular misalignment, typically from paralysis of one or more of the external ocular muscles (usually because of lesions of cranial nerves III, IV, or VI, but also myasthenia gravis or extraocular myopathy; see question 89). Note, however, that more than one half of all diplopias actually have no cranial nerve lesions.
87 What is heterotropia?
It is strabismus (i.e., a condition characterized by visual axes that are not parallel) (see later).
88 What are the causes of monocular diplopia?
They are either ocular or extraocular.
 Ocular causes may involve the eyelids (sty), cornea (astigmatism, keratitis), or lens (early cataract). They typically resolve by asking the patient to look through a pinhole card (which eliminates stray rays caused by abnormalities in the media).
Ocular causes may involve the eyelids (sty), cornea (astigmatism, keratitis), or lens (early cataract). They typically resolve by asking the patient to look through a pinhole card (which eliminates stray rays caused by abnormalities in the media).
 Extraocular causes instead include problems with glasses or contact lenses and typically resolve by removing the lenses or adjusting the glasses.
Extraocular causes instead include problems with glasses or contact lenses and typically resolve by removing the lenses or adjusting the glasses.
89 What is the approach to the patient with binocular diplopia?
The first step is to recognize diplopias that are not due to cranial nerve lesions, but instead to eye muscle disease (15% of all binocular diplopias). These include (1) the diplopia of myasthenia gravis (which typically worsens with progression of the day and may be associated with ptosis); (2) the diplopia of thyroid myopathy (which typically occurs with other signs of Graves’ disease, such as proptosis and lid edema–retraction lag); (3) the diplopia of orbital fracture (14% of all binocular diplopias) should be easily recognizable by the history of trauma; and (4) finally, presence of ptosis should suggest third-nerve palsy or myasthenia gravis, whereas presence of other neurologic findings would argue in favor of supranuclear causes (such as internuclear ophthalmoplegia and skew deviation, responsible for 7% of all binocular diplopias). Once these conditions have been eliminated, the physician should identify the weak ocular muscle(s) caused by cranial neuropathy (i.e., third-, fourth-, or sixth-nerve palsy), which is responsible for 40% of all binocular diplopias. Note that a third-nerve palsy can be the presenting manifestation of a posterior communicating artery aneurysm, which has suddenly expanded and is about to rupture (see questions 48 and 49).
102 What is the significance of a palpable preauricular lymph node?
It indicates viral conjunctivitis. It is just anterior to the tragus and usually tender.
104 How can one differentiate the injected vessels of uveitis from those of conjunctivitis?
By looking for prominent pericorneal vessels at the limbus (i.e., the interface between cornea and sclera). This phenomenon is known as “ciliary flush,” and it is typical of uveitis (see questions 111–114). By contrast, conjunctivitis causes diffuse vascular prominence, plus a typical discharge.
107 Can heterochromia occur in Horner’s syndrome?
Yes, and it usually indicates congenital Horner’s, with the affected iris being lighter than the other; in acquired Horner’s syndrome, on the other hand, heterochromia is rare to absent. Other less frequent causes include a ferrous intraocular foreign body, which causes the affected iris to appear darker, and Waardenburg syndrome (see Chapter 3, The Skin, question 123).
114 What are the symptoms of uveitis?
It depends. In anterior uveitis the affected eye may have a smaller pupil, yet the predominant symptom is aching ocular pain and photophobia, often requiring the use of dark glasses. Uveitis is different from keratitis since its pain is unrelieved by topical anesthetics; it also is different from conjunctivitis since it has no discharge, and it is different from glaucoma since it has lower intraocular pressure. Note that intermediate or posterior uveitis causes no pain but rather blurred vision and floaters (see also questions 139 and 226).
116 What is a hyphema?
It is blood in the anterior chamber, typically from trauma (from the Greek hyphaimos, suffused with blood). When bleeding is significant, blood can even be seen with a penlight, as a dark red layer in the inferior portion of the anterior chamber (see also question 132).
F. Ophthalmoscopy
124 Is ophthalmoscopy an important skill?
It is a fundamental skill. Practice, as always, is paramount.
G. Optic Disc
141 What is the optic disc?
It is the point where axons of retinal ganglion cells form the optic nerve (nerve head).
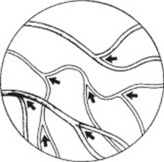
143 In following vessels toward the disc, how do you know the direction is right?
By noticing that the caliber of the vessels increases. Another way is to follow the “arrowheads” formed by the bifurcations of the retinal vessels (Fig. 4-6).
144 What does a normal optic disc look like?
Like an oval, with sharp margins and a yellowish-pinkish color.
150 Does pallor of the disc always suggest a disorder of the optic nerve?
No. It often represents a normal variant, particularly in myopic eyes.
156 What is chronic (or open-angle) glaucoma? What are its symptoms?
It is a different condition from acute (closed-angle) glaucoma, since the angle here is not closed, but simply not working well. This results in a slow drainage that is typically chronic and with no symptoms in its early stages. In this (most common) form of glaucoma, visual loss progresses slowly, compromising initially only the peripheral field, so that patients do not usually notice the deficit. Hence, the need to screen for glaucoma. The diagnosis is made by finding pathologic optic disc cupping (see question 159) and characteristic visual field loss. Elevated intraocular pressure also may be present.
157 What are the symptoms of acute (or angle-closure) glaucoma?
167 Is there any loss of visual activity in papilledema?
No. Unless the papilledema is long standing, visual acuity is usually well preserved.
172 What is papillitis?
A form of optic neuritis characterized by visible swelling of the disc. As in other optic neuritides, the chief complaint is acute and unilateral loss of vision, accompanied by eye pain, an afferent pupillary defect (due to the fact that one eye is more involved than the other; see questions 60–63), and an acquired impairment in color vision (i.e., dyschromatopsia, see questions 18 and 19).
175 What are the manifestations of AION?
Acute, painless, and monocular loss of visual acuity, visual field cut, and an afferent pupillary defect (i.e., Marcus Gunn pupil) on the affected side. Loss of vision can often be complete and irreversible. Ophthalmoscopy reveals a pale, swollen optic nerve head. Dyschromatopsia also may be present (see questions 18 and 19).
H. Retinal Circulation
I. Retinal Background
192 What retinal lesions can be identified by ophthalmoscopy?
Three groups: (1) yellow-white spots, (2) red spots, and (3) brown-black spots.
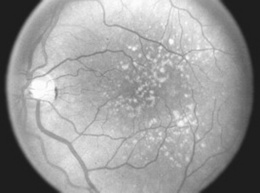
198 What are drusen?
From the German term for geode (due to their glittering appearance), drusen are discrete, round, and yellowish lipoproteinaceous deposits in the retinal pigment epithelium (RPE, Fig. 4-7). First described by Donders in 1854, they are a normal byproduct of aging, often located in the macular region, but rarely causing visual disturbances. Yet, when in great number and large size, they are the earliest finding of age-related macular degeneration. They are totally unrelated to Optic Disc Drusen, which are instead globular deposits located on the optic nerve head (optic disk), found in 1–3% of the population, made of mucoproteins and mucopolysaccharides, often calcifying, and possibly representing a clue to the presence of retinitis pigmentosa.
203 What other lesions may be associated with red spots?
Yellow-white spots, especially hard exudates in the perimacular region. These are due to damaged retinal vessels, leaking not only blood (responsible for red spots) but also plasma (causing hard exudates—see questions 196 and 197).
204 Why do red lesions have different shapes and sizes?
Shape and size depend on the location of the hemorrhage:
 When the hemorrhage occurs in the superficial (nerve fiber) layer of the retina, it follows the orientation of nerve fibers and assumes a linear shape (flame or splinter hemorrhage). Red lesions of this type are most common in hypertension.
When the hemorrhage occurs in the superficial (nerve fiber) layer of the retina, it follows the orientation of nerve fibers and assumes a linear shape (flame or splinter hemorrhage). Red lesions of this type are most common in hypertension.
 When the hemorrhage occurs in the middle retinal layer, it obeys the vertical orientation of that part of the retina, assuming a circular shape (dot hemorrhage if the border is sharp, blot hemorrhage if indistinct). Red lesions of this type are most common in diabetes mellitus.
When the hemorrhage occurs in the middle retinal layer, it obeys the vertical orientation of that part of the retina, assuming a circular shape (dot hemorrhage if the border is sharp, blot hemorrhage if indistinct). Red lesions of this type are most common in diabetes mellitus.
(3) Brown-Black Spots
210 What are brown-black retinal lesions?
Abnormal melanin proliferation. The most common pigmented retinal lesions are (1) retinitis pigmentosa, (2) retinal pigment epithelium hypertrophy, and (3) melanomas/benign choroidal nevi. Black borders often occur around the yellow-white lesions of chorioretinal scars (see question 199).
215 What are choroidal melanomas and benign nevi?
 Melanomas are the most common malignancies of the eye. They appear as raised and highly pigmented and are often asymptomatic.
Melanomas are the most common malignancies of the eye. They appear as raised and highly pigmented and are often asymptomatic.
 Benign nevi consist instead of clumps of choroidal melanocytes, presenting ophthalmoscopically as flat, grayish/greenish pigmented lesions with indistinct borders. They do not compromise vision.
Benign nevi consist instead of clumps of choroidal melanocytes, presenting ophthalmoscopically as flat, grayish/greenish pigmented lesions with indistinct borders. They do not compromise vision.
J. Diabetic Retinopathy
216 What is the relevance of diabetic retinopathy?
It is the most severe of all ocular complications of diabetes. A major cause of blindness.
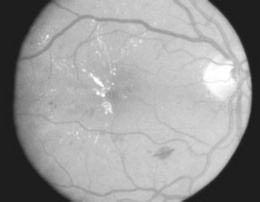
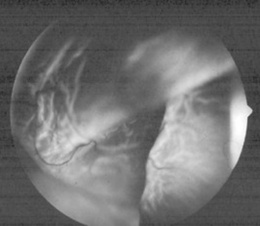
219 What is proliferative diabetic retinopathy (PDR)?
The most advanced stage of diabetic retinopathy. It consists of chronic retinal ischemia resulting in new vessel formation (neovascularization) on the inner surface of retina, optic disc, or vitreous (Fig. 4-8). If untreated, this may lead not only to preretinal or vitreous hemorrhage, but also to tractional retinal detachments. The new vessels appear tiny, irregular, and often with a fibrous component. They may develop quite rapidly, even within 1 year after the initial appearance of an isolated cotton-wool spot. They often resemble the spokes of a wheel, insofar as they radiate outward toward a circumferential vessel. Neovascularization of the disc carries a worst prognosis than new vessel formation in other areas. Yet, all patients with PDR are at risk of losing vision, and thus should be candidates for photocoagulation. Patients with diabetes should be referred at least once a year for ophthalmologic evaluation.
K. Retinal Detachment
224 What are the causes of retinal detachment?
 The most common is a degenerative tear in the sensory retina, which, in turn, allows the entry of fluid from the vitreous cavity and subsequent separation of the sensory layer from its underlying pigmented epithelial layer.
The most common is a degenerative tear in the sensory retina, which, in turn, allows the entry of fluid from the vitreous cavity and subsequent separation of the sensory layer from its underlying pigmented epithelial layer.
 The second most common cause is mechanical traction on the retina, as in diabetics with PDR, whose contracting neovascular membranes pull the retina away from the retinal pigment epithelium.
The second most common cause is mechanical traction on the retina, as in diabetics with PDR, whose contracting neovascular membranes pull the retina away from the retinal pigment epithelium.
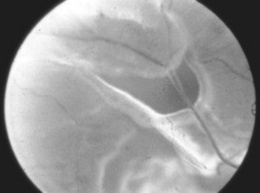
226 How does retinal detachment present?
With three “F’s”: floaters, flashes, and field loss. The most common initial complaint is the sudden appearance of floaters (i.e., opacities that float in the vitreous of the affected eye). These represent either clusters of red cells released at time of retinal tear or a fibrous condensation in the vitreous. Subsequently, there will be flashes of light (i.e., phospheni, from the Greek phos, light, and phaino, to show). These “shows of light” represent mechanical activation of the sensory retinal layer, stretched by traction from the vitreous. Eventually, with progression of the detachment, a “curtain” will gradually obscure the vision (field loss). This may occur as early as few hours or as late as few weeks since the initial event (see Fig. 4-9).
227 What are the ophthalmoscopic findings in retinal detachment?
It depends. If the entire retina is detached, there will be loss of the red reflex. Conversely, if only part of the retina is detached, the affected section will appear whiter, with fine folds on its surface. Most often, however, retinal detachment is difficult to visualize under direct ophthalmoscopy, except when performed with special instruments that allow better visualization of the peripheral retina (see Fig. 4-10).
L. Macula
229 What are the most common macular abnormalities?
In addition to edema (see question 222), they are drusen, hemorrhages, exudates, and scars.
232 How does macular degeneration appear ophthalmoscopically?
 The dry type has drusen as precursors, followed by loss of pigment in the retinal pigment epithelium. This may be total, causing choroidal vessels to show through.
The dry type has drusen as precursors, followed by loss of pigment in the retinal pigment epithelium. This may be total, causing choroidal vessels to show through.
 The wet type is associated with thickening of the retinal pigment epithelium and a choroidal neovascular membrane, which appears as a gray-green area deep to the retina. Hemorrhage and exudates may emanate from the choroidal membrane, leading to a disciform scar in the macula.
The wet type is associated with thickening of the retinal pigment epithelium and a choroidal neovascular membrane, which appears as a gray-green area deep to the retina. Hemorrhage and exudates may emanate from the choroidal membrane, leading to a disciform scar in the macula.
M. Red Eye
234 What causes a red eye?
All the major eye layers, if involved, may produce a red eye. The most important are:
 Conjunctiva: Conjunctivitis, allergic, bacterial, and especially viral, is the most common (and usually benign) cause of a red eye. The same is true for subconjunctival hemorrhage. Note that allergic conjunctivitis is always binocular, while viral and bacterial conjunctivitis are often monocular.
Conjunctiva: Conjunctivitis, allergic, bacterial, and especially viral, is the most common (and usually benign) cause of a red eye. The same is true for subconjunctival hemorrhage. Note that allergic conjunctivitis is always binocular, while viral and bacterial conjunctivitis are often monocular.
 Cornea: Inflammation of the cornea or keratitis (from the Greek keras, horn or cornea) is also common but potentially a much more serious disorder. This is because keratopathies violate the corneal epithelium, enter the corneal stroma, and result in scarring that may cause blindness. This is common in herpes simplex keratitis, but also may occur in Graft-versus-host disease, and also in conditions producing an excessive drying of the cornea (Sjögren’s syndrome, rheumatoid arthritis). In herpetic keratitis a fluorescein stain of the basement membrane of the cornea (made possible by the injury of its superficial layer) will demonstrate the typical dendritic ulcer. Finally, a surface keratopathy may also result from corneal abrasion, its classic symptom being a foreign body sensation.
Cornea: Inflammation of the cornea or keratitis (from the Greek keras, horn or cornea) is also common but potentially a much more serious disorder. This is because keratopathies violate the corneal epithelium, enter the corneal stroma, and result in scarring that may cause blindness. This is common in herpes simplex keratitis, but also may occur in Graft-versus-host disease, and also in conditions producing an excessive drying of the cornea (Sjögren’s syndrome, rheumatoid arthritis). In herpetic keratitis a fluorescein stain of the basement membrane of the cornea (made possible by the injury of its superficial layer) will demonstrate the typical dendritic ulcer. Finally, a surface keratopathy may also result from corneal abrasion, its classic symptom being a foreign body sensation.
 Episclera: Episcleritis is inflammation of the connective tissue between the sclera and conjunctiva. It is a less-common and usually benign condition.
Episclera: Episcleritis is inflammation of the connective tissue between the sclera and conjunctiva. It is a less-common and usually benign condition.
 Sclera: Inflammation of the sclera (scleritis) is also less common but more serious. It usually indicates an underlying systemic process, such as connective tissue disease.
Sclera: Inflammation of the sclera (scleritis) is also less common but more serious. It usually indicates an underlying systemic process, such as connective tissue disease.
 Iris and ciliary body: These structures belong to the uvea, the synovium of the eye. The iris represents its anterior region, the ciliary body its intermediate, and the choroid its posterior. Acute iridocyclitis (from the Greek irid, iris, and kyklos, circle or ciliary body) is inflammation of both the iris and ciliary body. Photophobia is the hallmark of uveitis (like in keratopathy, but without the foreign body sensation). The iris is typically irregular, because adherent through synechiae to the lens (see question 235), and there may even be residual blindness, especially when the choroid is involved (posterior uveitis). Anterior uveitis may instead result in glaucoma. Overall, uveitis can be part of a systemic disease (like sarcoidosis), but it is much more commonly idiopathic and recurrent.
Iris and ciliary body: These structures belong to the uvea, the synovium of the eye. The iris represents its anterior region, the ciliary body its intermediate, and the choroid its posterior. Acute iridocyclitis (from the Greek irid, iris, and kyklos, circle or ciliary body) is inflammation of both the iris and ciliary body. Photophobia is the hallmark of uveitis (like in keratopathy, but without the foreign body sensation). The iris is typically irregular, because adherent through synechiae to the lens (see question 235), and there may even be residual blindness, especially when the choroid is involved (posterior uveitis). Anterior uveitis may instead result in glaucoma. Overall, uveitis can be part of a systemic disease (like sarcoidosis), but it is much more commonly idiopathic and recurrent.
 Adnexal structures: This may include a tear or sebaceous glands. Dacryocystitis and sties are common disorders.
Adnexal structures: This may include a tear or sebaceous glands. Dacryocystitis and sties are common disorders.
235 What other ocular signs may accompany a red eye?
The most important include the following:
 Ciliary flush. This is dilation or hyperemia (which ophthalmologists refer to as “injection”) of the deep conjunctival and episcleral and pericorneal vessels, which appear as a deep red ring encircling the cornea. Ciliary flush is a serious sign, usually indicating one of three disorders: (1) iridocyclitis, (2) acute glaucoma, or (3) keratitis. It is typically absent in more benign conditions, such as conjunctivitis. Hence, always refer it.
Ciliary flush. This is dilation or hyperemia (which ophthalmologists refer to as “injection”) of the deep conjunctival and episcleral and pericorneal vessels, which appear as a deep red ring encircling the cornea. Ciliary flush is a serious sign, usually indicating one of three disorders: (1) iridocyclitis, (2) acute glaucoma, or (3) keratitis. It is typically absent in more benign conditions, such as conjunctivitis. Hence, always refer it.
 Corneal opacities. These are always serious findings in patients with a red eye. Corneal opacities may be either localized (as in keratitis or corneal ulcers) or diffuse (as in acute glaucoma, in which the edema of the cornea creates a haze that obscures the iris). They also may result from cellular deposits on the cornea (as in iridocyclitis).
Corneal opacities. These are always serious findings in patients with a red eye. Corneal opacities may be either localized (as in keratitis or corneal ulcers) or diffuse (as in acute glaucoma, in which the edema of the cornea creates a haze that obscures the iris). They also may result from cellular deposits on the cornea (as in iridocyclitis).
 Disruption of the corneal epithelium. This is usually the result of trauma. Corneal abrasions are easily visualized under cobalt blue light and after application of fluorescein. They can even be detected by noticing a distortion of the corneal light reflex.
Disruption of the corneal epithelium. This is usually the result of trauma. Corneal abrasions are easily visualized under cobalt blue light and after application of fluorescein. They can even be detected by noticing a distortion of the corneal light reflex.
 Anisocoria. This is usually a sign of iridocyclitis, and overall argues in favor of more serious disease (such as corneal abrasion or foreign body, keratitis, and uveitis), rather than benign processes (such as conjunctivitis—19% sensitivity, 97% specificity, +LR 6.5, and −LR 0.8. The pupil of the involved eye is smaller (because of a reflex constriction of the iris sphincter muscle) and may be distorted (because of inflammatory adhesions between lens and iris).
Anisocoria. This is usually a sign of iridocyclitis, and overall argues in favor of more serious disease (such as corneal abrasion or foreign body, keratitis, and uveitis), rather than benign processes (such as conjunctivitis—19% sensitivity, 97% specificity, +LR 6.5, and −LR 0.8. The pupil of the involved eye is smaller (because of a reflex constriction of the iris sphincter muscle) and may be distorted (because of inflammatory adhesions between lens and iris).
 Proptosis. This is usually a serious sign, indicating swelling or a mass in the orbit or cavernous sinus.
Proptosis. This is usually a serious sign, indicating swelling or a mass in the orbit or cavernous sinus.
 Eye discharge. This is instead a benign finding, most commonly associated with a process such as conjunctivitis. A watery, clear discharge usually indicates viral conjunctivitis, whereas a purulent discharge is the hallmark of a bacterial etiology.
Eye discharge. This is instead a benign finding, most commonly associated with a process such as conjunctivitis. A watery, clear discharge usually indicates viral conjunctivitis, whereas a purulent discharge is the hallmark of a bacterial etiology.
 Preauricular lymph node enlargement. This is much more common in viral than bacterial conjunctivitis. Hence, it may provide a clue to the etiology of the process.
Preauricular lymph node enlargement. This is much more common in viral than bacterial conjunctivitis. Hence, it may provide a clue to the etiology of the process.
236 What other ocular symptoms may accompany a red eye?
 Blurred or reduced vision. This is always absent in conjunctivitis but frequently present in disorders such as keratitis, iridocyclitis, or acute glaucoma. The key maneuver is to have the patient blink. An improvement in vision indicates the cleaning of inflammatory debris from the cornea (as in the case of conjunctivitis), whereas persistent blurring indicates a much more serious disorder.
Blurred or reduced vision. This is always absent in conjunctivitis but frequently present in disorders such as keratitis, iridocyclitis, or acute glaucoma. The key maneuver is to have the patient blink. An improvement in vision indicates the cleaning of inflammatory debris from the cornea (as in the case of conjunctivitis), whereas persistent blurring indicates a much more serious disorder.
 Pain. This is always absent in conjunctivitis (in which patients usually complain of a scratchy, itchy feeling but never of pain) but common in three serious conditions associated with ciliary flush: keratitis, iridocyclitis, or acute glaucoma. Conversely, itchiness is so typical of allergic conjunctivitis to become almost a sine qua non.
Pain. This is always absent in conjunctivitis (in which patients usually complain of a scratchy, itchy feeling but never of pain) but common in three serious conditions associated with ciliary flush: keratitis, iridocyclitis, or acute glaucoma. Conversely, itchiness is so typical of allergic conjunctivitis to become almost a sine qua non.
 Halos. These are indicative of corneal edema, usually resulting from a sudden increase in intraocular pressure (as in acute glaucoma). Patients with this complaint describe a rainbow-like ring surrounding a point of light (i.e., the halo).
Halos. These are indicative of corneal edema, usually resulting from a sudden increase in intraocular pressure (as in acute glaucoma). Patients with this complaint describe a rainbow-like ring surrounding a point of light (i.e., the halo).
 Photophobia. This is also absent in conjunctivitis but common in serious conditions like iritis.
Photophobia. This is also absent in conjunctivitis but common in serious conditions like iritis.
 Mid-dilated and unreactive pupil. This is typically associated with acute angle closure glaucoma.
Mid-dilated and unreactive pupil. This is typically associated with acute angle closure glaucoma.
1 Aiello LM, Cavallerano J. Diabetic retinopathy. Curr Ther Endocrinol Metab. 1997;6:475-485.
2 Alio J, Hernandez I, Millan A, et al. Pupil responsiveness in diabetes mellitus. Ann Ophthalmol. 1989;21:132-137.
3 American Diabetes Association. Diabetic retinopathy. Diabetes Care. 1998;21:547-549.
4 Buxton MJ, Sculpher MJ, Ferguson BA, et al. Screening for treatable diabetic retinopathy: A comparison of different methods. Diab Med. 1991;8:371-377.
5 Capo H, Warren F, Kupersmith MJ. Evolution of oculomotor nerve palsies. J Clin Neuroophthalmol. 1992;12:12-15.
6 Cogan DG, Mount HTJ. Intracranial aneurysms causing ophthalmoplegia. Arch Ophthalmol. 1963;70:757-771.
7 Dacso CC. The Argyll Robertson pupil in clinical medicine. Am J Med. 1989;86:199-202.
8 David NJ. Optokinetic nystagmus: A clinical review. J Clin Neuroophthalmol. 1989;9:258-266.
9 Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. Ophthalmology. 1991;98:823-833.
10 Enyedi LB, Dev S, Cox TA. A comparison of the Marcus Gunn and alternating light tests for afferent pupillary defects. Ophthalmology. 1998;105:871-873.
11 Federman J, Gouras P, Schubert H, et al: Retina and vitreous Podos S, Yanoff M, editors:Textbook of Ophthalmology. St. Louis;Mosby: 1991;Vol. 9