Chapter 101 The Endocrine System
The endocrinopathies are discussed in detail in Part XXVI.
Primary hypothyroidism occurs in approximately 1/4,000 births (Chapter 559). Because most infants with serious and treatable disease are asymptomatic at birth, all states screen for it. Thyroid deficiency may also be apparent at birth in genetically determined cretinism or in infants of mothers treated with antithyroid medications or during a pregnancy complicated by maternal hyperthyroidism. Constipation, prolonged jaundice, goiter, lethargy, or poor peripheral circulation as shown by persistently mottled skin or cold extremities should suggest cretinism. Early diagnosis and treatment of congenital thyroid hormone deficiency improve intellectual outcome and are facilitated by screening of all newborn infants for this deficiency.
Transient hypoparathyroidism may manifest as tetany of the newborn (Chapter 565).
Transient diabetes mellitus (Chapter 583) is rare and is encountered only in newborns. It usually manifests as dehydration, loss of weight, or acidosis in infants who are small for gestational age.
101.1 Infants of Diabetic Mothers
Pathophysiology
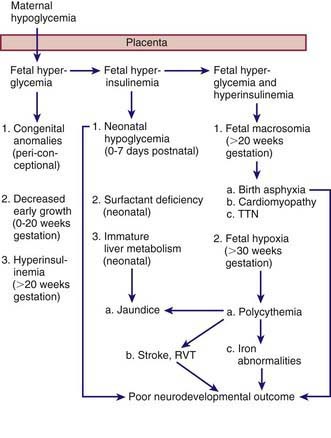
The probable pathogenic sequence is that maternal hyperglycemia causes fetal hyperglycemia, and the fetal pancreatic response leads to fetal hyperinsulinemia; fetal hyperinsulinemia and hyperglycemia then cause increased hepatic glucose uptake and glycogen synthesis, accelerated lipogenesis, and augmented protein synthesis (Fig. 101-1). Related pathologic findings are hypertrophy and hyperplasia of the pancreatic islet β cells, increased weight of the placenta and infant organs except for the brain, myocardial hypertrophy, increased amount of cytoplasm in liver cells, and extramedullary hematopoiesis. Hyperinsulinism and hyperglycemia produce fetal acidosis, which may result in an increased rate of stillbirth. Separation of the placenta at birth suddenly interrupts glucose infusion into the neonate without a proportional effect on the hyperinsulinism, and hypoglycemia and attenuated lipolysis develop during the first hours after birth.
Clinical Manifestations
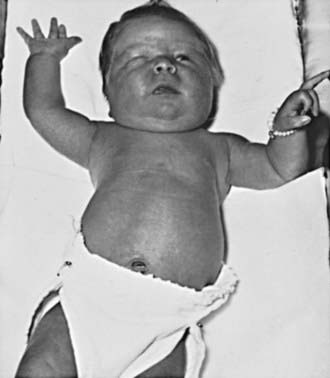
Infants of mothers with diabetic and those of mothers with gestational diabetes often bear a surprising resemblance to each other (Fig. 101-2). They tend to be large and plump as a result of increased body fat and enlarged viscera, with puffy, plethoric facies resembling that of patients who have been receiving corticosteroids. These infants may also, however, be of normal or low birthweight, particularly if they are delivered before term or if their mothers have associated vascular disease.
Treatment
Managing hypoglycemia in sick or symptomatic infants is discussed in the following section. For treatment of hypocalcemia and hypomagnesemia, see Chapter 100; for respiratory distress syndrome treatment, see Chapter 95.3; for treatment of polycythemia, see Chapter 97.3.
Crowther CA, Hiller JE, Moss JR, et al. Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group: Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477-2486.
Esakoff TF, Cheng YW, Sparks TN, et al. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200:672e1-672e4.
Langer O, Yogev Y, Xenakis E, et al. Insulin and glyburide therapy: dosage, severity level of gestational diabetes, and pregnancy outcome. Am J Obstet Gynecol. 2005;192:134-139.
Nold JL, Georgieff MK. Infants of diabetic mothers. Pediatr Clin North Am. 2004;51:619-637.
Reece EA, Leguizamon G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet. 2009;373:1789-1796.
Rowan JA, Hague WM, Gai W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003-2015.
Stothard KJ, Tennant PWG, Bell R, et al. Maternal overweight and obesity and the risk of congenital anomalies. JAMA. 2009;301:636-650.
Teramo K, Kari MA, Eronen M, et al. High amniotic fluid erythropoietin levels are associated with an increased frequency of fetal and neonatal morbidity in type 1 diabetic pregnancies. Diabetologia. 2004;47:1695-1703.
Vela-Huerta MM, Vargas-Origel A, Olvera-López A. Asymmetrical septal hypertrophy in newborn infants of diabetic mothers. Am J Perinatol. 2000;17:89-94.
Wren C, Birrell G, Hawthorne G. Cardiovascular malformations in infants of diabetic mothers. Heart. 2003;89:1217-1220.
Bongers-Schokking JJ, Koot HM, Wiersma D, et al. Influence of timing and dose of thyroid hormone replacement on development in infants with congenital hypothyroidism. J Pediatr. 2000;136:292-297.
Briët JM, van Wassenaer AG, Dekker FW, et al. Neonatal thyroxine supplementation in very preterm children: developmental outcome evaluated at early school age. Pediatrics. 2001;107:712-718.
Fisher DA. The importance of early management in optimizing IQ in infants with congenital hypothyroidism. J Pediatr. 2000;136:273-274.
Osborn DA: Thyroid hormones for preventing neurodevelopmental impairment in preterm infants, Cochrane Database Syst Rev (4):CD001070, 2001.
Rapaport R, Rose SR, Freemark M. Hypothyroxinemia in the preterm infant: the benefits and risks of thyroxine treatment. J Pediatr. 2001;139:182-188.
Rovet JF. In search of the optimal therapy for congenital hypothyroidism. J Pediatr. 2004;144:698-700.