The dural concept
Hypothesis
The dural concept was first defined by James Cyriax in 1945.1 His hypothesis was that lumbago and backache originate when a subluxated fragment of disc tissue impinges on the sensitive dura mater. This concept – lumbar pain may be of dural origin – is based on two premises:
• Disc degeneration and disc displacements are of themselves painless events.
• The dura mater is sensitive and translates deformations of the posterior border of the disc into pain.
Clinical evidence for insensibility of the disc
• Data obtained from postmortem studies show the existence of large, symptomless disc protrusions in almost 40% of the cadavers.2
• Several controlled studies have failed to show a relationship between radiological changes seen in disc degeneration and the existence of clinical syndromes.3–5
• Myelograms in asymptomatic patients show defects in 37% of cases,6 and the incidence of asymptomatic disc herniations demonstrated by computed tomography (CT) in subjects over 40 years of age is more than 50%.7
• Recent magnetic resonance imaging (MRI) studies8–11 have demonstrated anew the high incidence of disc degeneration and displacement in an asymptomatic group of patients.
Clinical evidence for sensibility of the dura mater
One of the most striking clinical features to support the pain-mediating role of the dura is the chronological evolution from backache to sciatica. Almost every instance of sciatica starts with a period of central or unilateral backache, but once leg pain supervenes, the backache usually disappears. Since the work by Mixter and Barr,12 it has been widely accepted that most radicular pain is caused by a disc protrusion compressing the dural investment around the nerve root and, if this is so, it is logical to argue that the earlier backache was brought about by the same disc lesion. If sciatica is referred pain from the dural sleeve, by analogy the prior backache must have originated from the dura mater. Sciatic pain is thus only the final stage of a progression. A small posterior protrusion, bulging out of the intervertebral joint, lifts the posterior longitudinal ligament, touches the dura mater and causes backache. Kept under control by the posterior ligament, the bulge can recede, resulting in spontaneous recovery, or stay unaltered, causing chronic backache. If it increases, however, the counterpressure exerted by the stretched posterior longitudinal ligament pushes it laterally. No longer subject to any resistance, it immediately swells and compresses the nerve root. At the same time, pressure against the dural tube is released and backache ceases (see p. 459).
Another proof of the role of the dura in lumbar pain syndromes is the effect of diagnostic local anaesthesia. A weak solution of procaine, induced via the sacral hiatus into the epidural space, and thus forced between the dural tube and the boundaries of the neural canal, causes contact anaesthesia of the dura mater (see p. 556). Because procaine 0.5% is too weak to penetrate the ligaments or the dural membrane, it acts as a surface anaesthetic, thus only desensitizing the structures with which it comes into contact. If the patient had backache before the injection, and anaesthesia affords temporary relief of symptoms and signs, the dura is most likely to be the source of pain. In all cases of acute lumbago and in most cases of acute or recurrent backache, epidural local anaesthesia immediately abates the pain, thus strongly suggesting a dural origin.
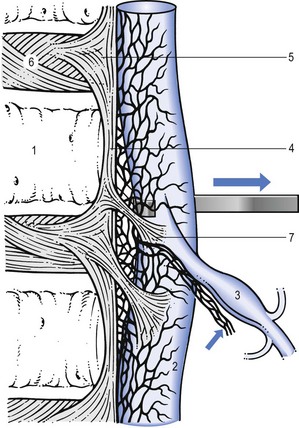
During the last few decades, numerous neuroanatomical studies have shown that the ventral half of the dura mater is supplied by small branches of the sinuvertebral nerve.13,14 Immunohistochemical studies further demonstrate a significant number of free nerve endings in the dura that contain substance P, calcitonin gene-related peptides and other neurotransmitters contributing to nociception.15,16
The mechanism of dural pain is dual
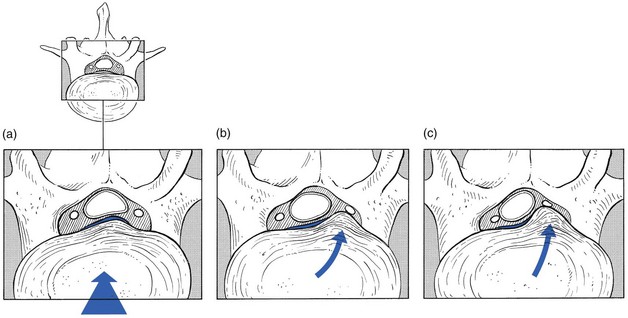
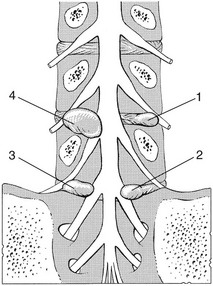
The original concept was quite simple: a subluxated (but of itself painless) component of the disc impinges on the dura or the dural sleeves of the nerve roots. These pain-sensitive structures translate the anatomical changes into back pain or root pain, respectively (Fig. 33.1).
However, recent anatomical and biochemical studies have slightly changed this original concept:
• The outer border of the disc is innervated. Although earlier anatomical studies demonstrated the disc to be totally deprived of innervation,17 more recent research could detect sparse nerve fibres and free nerve endings in the three outer lamellae of the annulus fibrosus,18–20 penetrating to a maximum depth of 0.9 mm into the annulus. This means that, except at the surface, a normal intervertebral disc remains almost without innervation.
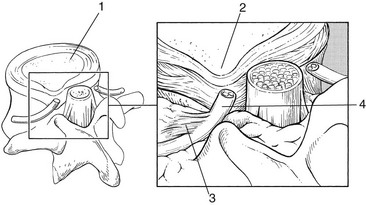
• Dura mater attachments exist between the anterior part of the dura and the posterior longitudinal ligament (Fig. 33.2). Recent anatomical and MRI studies have demonstrated that the dura mater is not totally disconnected from the vertebral column but also attached to the posterior longitudinal ligament by connective tissue, consisting of ventral and lateral fibrous bands.21–24 Although these ligaments are sufficient to allow for displacement of the dural sac during movement, they could act to place traction on the dural sac in the event of nuclear bulge or herniation.25
• Pain is not only mechanical: inflammatory mechanisms are also involved. Apart from being stimulated mechanically, nociceptors in dura mater may also be activated chemically. An increasing number of experimental studies suggest that disc lesions and/or displacements may induce sufficient chemical changes to irritate the dura mater and to elicit dural pain.26–31
• Articular signs and symptoms are those that are related to the mechanical behaviour of the disc: certain postures and movements create biomechanical changes, which force the protrusion against the dura mater.
• Dural signs and symptoms are those that are related to the increase of dural irritation: traction exerted from a distance (straight leg raising and neck flexion) pulls on the inflamed dura or, via the dural ligaments, on the posterior longitudinal ligament or outer annular rim. Also, a sudden increase in spinal fluid pressure pushes the dura against the protrusion (painful coughing and sneezing).
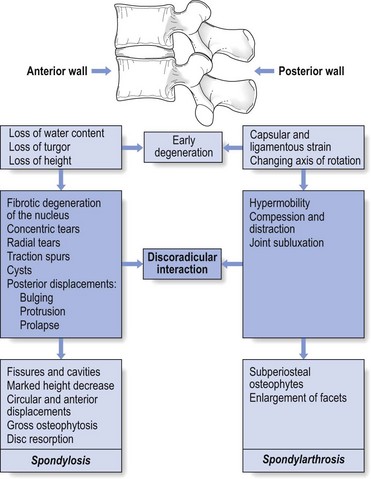
The dural concept in the natural history of the ageing disc
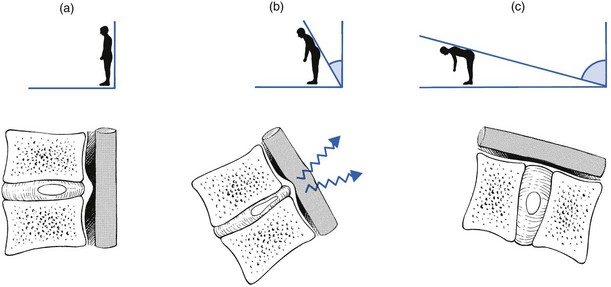
One of the factors involved in the dural concept is a subluxated portion of the disc and so the biomechanical conditions to allow such a displacement must be present. First, there must be some degeneration of the disc, leading to weakness of the annular fibres and to radiating fissures. These changes are present very early in the degeneration cycle and, for a number of biomechanical and biochemical reasons, occur most frequently at the rear side of the disc. Second, repeated wear and tear, together with shearing forces and slight decrease of disc height, creates some ligamentous laxity which results in an instability of the whole ‘motion segment’. Third, through enzymatic depolymerization of macromolecules in the disc, the oncotic pressure temporarily rises.32 This means that during a particular period of life (between the ages of 20 and 50 years), the osmotic pressure within the nucleus pulposus increases.
Raised intradiscal pressure together with increased segmental laxity is the perfect foundation for disc displacement. A kyphosis imposed on such a predisposed intervertebral joint not only increases the intradiscal pressure but also tends to shift disc material backwards in the direction of the convexity.33 The intensity of the contact between disc and dura determines whether lumbago or backache will result. When the protrusion is more posterolateral, the dural investment and the content of the nerve root, rather than the dural tube, are compressed, with the symptomatic outcome of root pain.
Further degeneration of the disc results in its ‘deflation’ and a decline of intradiscal pressure. Decrease in disc height leads to reactive changes at the intervertebral joint and at the posterior structures, which stiffen and stabilize the segment and so diminish the tendency for disc displacements during the later stages of ageing of the spine (Fig. 33.3).
Clinical syndromes
Lumbago
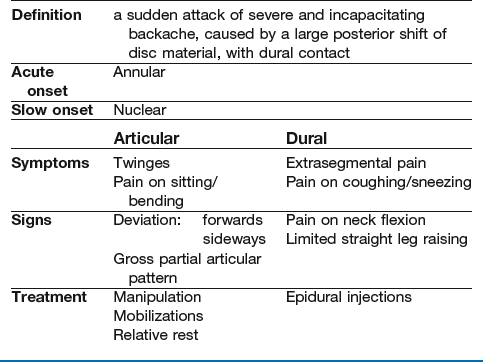
The most striking example of a discodural interaction is acute lumbago – a sudden attack of severe and incapacitating backache, with obvious limitation of movement, together with gross dural signs and symptoms, summarized in Box 33.1.
History
Annular lumbago
The patient states that, during some trivial activity, a sudden ‘snap’ was felt and agonizing pain in the back immediately followed. Very often, this acute event has occurred during a simple movement: coming up after bending, rising from a chair or picking up a light object. Initially, the pain is central and spreads bilaterally over the lower lumbar area and the buttocks. Later, it often tends to radiate more and more unilaterally. Although centralized in the lumbar and/or gluteal area, it spreads to the groin and abdomen, downwards to one or both legs as far as the ankles, or upwards in the trunk as far as the inferior aspect of the scapulae (Fig. 33.4).
An attack of acute annular lumbago is caused by posterior subluxation of part of the annular rim, pressing the posterior longitudinal ligament against the dura mater (Fig. 33.5a). It is obvious that a history of sudden pain, immediately followed by a ‘locking’ in flexion, indicates some internal derangement, just as a sudden pain in the knee, followed by inability to straighten it, indicates subluxation of a meniscus. The dural extrasegmental reference of the pain, together with pain on coughing and sneezing, implicates the dura mater and therefore excludes locking of the posterior facet joints. In displacement at the back of the intervertebral joint, the lumbar spine is held in flexion because extension squeezes the protrusion, which in turn increases the painful pressure on the dura. In order to keep the protrusion away from the dura and as immobile as possible, the patient adopts a flexed position. Muscle spasm prevents any further movement at the lumbar spine.
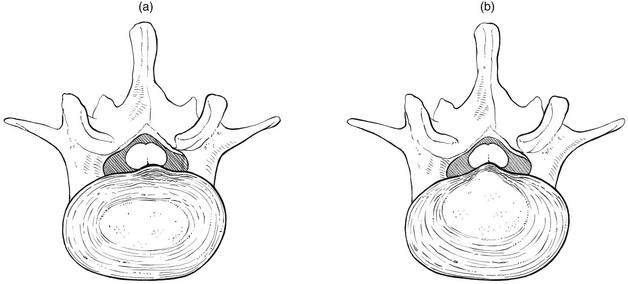
Fig 33.5 (a) Annular and (b) nuclear lumbago.
Nuclear lumbago
The pain, although equally incapacitating, does not appear suddenly but gradually increases over the course of a number of hours or days. Alternatively, after heavy work involving much stooping and lifting or sitting for an unusual length of time in an uncomfortable position, slight backache is felt but is initially regarded as trivial. However, by the next morning the backache is sufficiently severe to make getting out of bed impossible. The pain radiates in a way that is typical of dural involvement (see Fig. 33.4). The patient is immobilized in flexion or side flexion and every attempt to straighten the back is followed by an agonizing twinge in the lumbar area and the buttocks. Sometimes even simple neck flexion is impossible or coughing or sneezing creates a twinge. As in an attack of annular lumbago, the patient has to go to bed to cope with the pain. As a rule the pain eases after a few days or weeks.
In gradually increasing lumbago, the protrusion presumably consists of soft and pulpy nuclear material, oozing slowly backwards. This typically happens during the maintenance of a kyphotic posture (sitting, bending or lifting). The displaced nuclear material gradually presses more and more against the outermost layers of the annulus and the posterior longitudinal ligament and makes them protrude (Fig. 33.5b). This provokes dural irritation, resulting in the typical dural pain in lumbar area, buttocks and limbs.
Clinical examination
During clinical examination the following are important.
Inspection
Deviation towards flexion is noted and the sacrospinalis muscles are seen and felt to be in contraction to maintain the adaptive posture. Because the flexed position places the upper trunk in front of the centre of gravity, the muscles contract to prevent further forward toppling. Lumbago is not caused by muscle spasm – as was maintained by some authorities for many years36 – but is the result of a disorder at the posterior aspect of the intervertebral joint.
A lateral shift associated with acute lumbago is a common clinical event, undoubtedly associated with a disc protrusion.37 The lateral shift can be either towards the dominant side of pain (ipsilateral) or away from the side of the pain (contralateral). The majority of affected patients have a contralateral shift.38 Occasionally, the shift may change from side to side, which has been termed an alternating scoliosis. The lateral shift is explained as avoidance of compression or irritation of the dura mater, either actively or reflexively through muscle spasm.39,40
Spinal movements
Extension
As a rule, extension is considerably limited (Fig. 33.6) on account of the posterior displacement of the disc causing a block at the back of the joint.
Dural tests
Neck flexion
In lumbago, neck flexion often hurts in the lower back, which proves the involvement of the dura mater in the origin of the pain41 (see p. 502).
Straight leg raising (SLR)
We have discussed the evidence that SLR is a dural sign (see p. 427) and, just as neck flexion stretches the dura from above, so SLR stretches it from below. A lesion resulting in such a gross discodural interaction as acute lumbago, therefore, must influence SLR. Acute lumbago with full and painless SLR should make the clinician reluctant to accept the diagnosis of a displaced disc. If the acute pain in the back is so severe that the patient cannot move out of bed, but dural symptoms and signs – including a positive SLR – are absent, gross bony lesions such as osteomyelitis or metastases should be considered (see Ch. 39).
Lumbago usually causes bilateral limitation of SLR: because the bulge and the dura mater both lie centrally, raising both legs pulls on the dura equally. In unilateral lumbago there will often be more limitation of SLR on the painful side. Occasionally this may be reversed, when the crossed SLR phenomenon is present (see p. 498).
The degree of limitation of SLR is an indication of the intensity of the discodural interaction.42 In hyperacute lumbago, any attempt to move the straight leg upwards results in considerable pain, whereas in more moderate lumbago the SLR is limited at 45–60°. During recovery, when the reduction is almost complete, SLR will probably only be painful at the end of range or show a painful arc at mid-range. The progress of SLR is therefore a very sensitive clinical index in following the position of the protrusion during manipulation.
Tests of conduction
Neither muscle weakness nor cutaneous analgesia is present in cases of acute lumbago. Because protrusion is more or less central, nerve roots are not involved. Care should be taken, however, not to miss a compression of the fourth sacral root. Because it lies centrally, partly protected by the posterior longitudinal ligament, a central protrusion can endanger it, especially if the protrusion overstretches the ligament. Physical findings are non-existent and the diagnosis is made entirely on the history. If pain deep in the sacral area, pain and paraesthesia in the penis, vagina or rectum, numbness in the saddle area or problems with continence are mentioned, damage to the fourth sacral root should be considered and the patient immediately referred for further assessment.43 A fourth sacral lesion occurs at a level proximal to the posterior ganglion and permanent interference with bladder function can result if decompression is not carried out.44 Therefore its onset, however slight, is an indication for laminectomy. Cyriax45 (his p. 284) recommends operation even when bladder function is returning after the attack of lumbago, because there is no guarantee that lasting incontinence may not follow the next attack.
Natural history
With, without or despite treatment, most cases of acute lumbago recover spontaneously and completely within 2–6 weeks (Dixon46; Chöler, cited by Nachemson47; Spitzer48). The tension in the posterior longitudinal ligament exerts counterpressure on the bulge, which moves gradually anteriorly, until compression of the dura mater ceases and symptoms disappear. However, as cartilage has little tendency to reunite, a fragment that has moved backwards once will sooner or later move again, which implies that, although complete recovery after an attack of acute lumbago is the rule, recurrences are to be anticipated.49
Alternatively, the lumbago disappears but there is simultaneous onset of root pain. As has already been discussed (see p. 442), the protrusion has moved from the centre to one side.
Treatment
Most cases of acute lumbago recover without treatment. MacNab put it well when he remarked that ‘The physician must constantly remind himself that even if he elected to treat the patient by rubbing peanut butter on each buttock, in the balance of probabilities, the patient would get well fairly quickly.’50 However, keeping the intradiscal pressure as low as possible will, of course, ease symptoms and hasten the reduction of the bulge. It is therefore wise to adopt the supine lying position from time to time, with the knees and the hips flexed to a right angle51; this decreases the load on the disc to about 30 kPa52 (the ‘psoas position’; Fig. 33.7). It is also sensible to avoid movements and positions that cause high intradiscal pressures, such as sitting or bending (Nachemson53: p. 708).
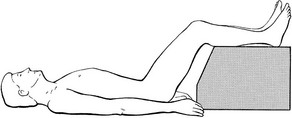
Fig 33.7 The ‘psoas position’.
Standard textbooks almost unanimously recommend bed rest as the first line of treatment for acute lumbago.54,55 However, it has never been proven that complete and continuous bed rest decreases the time of recovery. In a comparative trial, statistically significant differences between bed rest, early mobilization and no treatment have not been found, although results tended to favour early mobilization.56 Others have reported little difference between mobilization and rest,57 and 2 days in bed was found to be even better than 7 days; put the other way around, 7 days in bed was more harmful than 2 days.58,59 Also, a recent Cochrane review concluded that there is no difference in effect between advice to stay in bed and advice to stay active.60 Therefore bed rest is only necessary if bed is the only place where the patient is comfortable. If, after a couple of days, walking around is possible without a considerable increase in pain, such a regime should be followed. Furthermore, a patient should never be forced to stay in bed against his or her will.
Nuclear lumbago
In nuclear types of acute lumbago, classic manipulative reduction usually fails. A slow onset of symptoms usually indicates that the protrusion is too soft to be pushed back. Manipulation is also apt to fail in lateral deviation away from the painful side. A good alternative, then, is a sustained manual stretching technique. Positioning the patient in increasing but still comfortable lordosis (McKenzie technique) is another alternative in treating acute nuclear lumbago.61,62
Although effective in chronic nuclear backache, where the dural symptoms are much milder, traction should never be used in acute nuclear lumbago. Experience shows that, if traction is applied on a patient who mentions the presence of ‘twinges’, considerable worsening of the condition for several days may be expected. The reason for this is not completely understood. Presumably the size of the bulge increases when the hydrostatic and osmotic conditions within the disc change during traction (see p. 420).
Hyperacute lumbago
If the lumbago is really hyperacute, which means that the dural symptoms are so intense that repeated and agonizing twinges force the patient to lie motionless, any attempt at manipulative reduction is unthinkable. It is obvious that manipulation cannot be done when the patient can hardly move or when it takes some minutes to roll from a prone to supine-lying position on the examination couch. In these cases, the only alternative to several weeks of bed rest is epidural local anaesthesia, which affords immediate and complete relief of symptoms over 1 or 2 hours. Curiously enough, and although the anaesthesia only works for 2 hours, there is lasting relief from the next day on. The injection probably has some long-term effect on the inflamed dura, rendering it less sensitive. Once the immobilizing twinges have been abolished, the patient is capable of getting up and travelling for manipulative reduction of the residual displacement. This combination of epidural local anaesthesia and manipulation is rapidly successful in nearly all cases of hyperacute lumbago.63
Backache
About 80% of all cases of low back pain relate directly to the intervertebral discs.64 Discodural backache presents a typical complex of symptoms and signs, both articular and dural. The mechanism of pain and dysfunction is exactly the same as described in lumbago, but the signs and symptoms are less acute because the discodural interaction is more moderate.
History
Depending on which part of the dura is irritated, the pain is central or unilateral or shifts in location.65 Shifting pain is a common history and indicates that the lesion has moved from one side of the intervertebral joint to the other. Shifting pain in the back is one of the most characteristic phenomena in discodural backache. An alternating ache in the buttock, however, suggests sacroiliac arthritis rather than a disc problem (see Ch. 41).
The localization of pain not only varies according to the site of compression of the dural tube but is also determined by the intensity of the stimulation. One of the rules of referred pain is that the stronger the stimulus, the further the pain will be referred. This has some practical bearings when it comes to evaluation of therapy: when the pain has originally been located in a buttock but, during a manipulative session, tends towards the centre and becomes paravertebral, this implies that the pain stimulus has been reduced and discodural contact is now less pronounced than it was. ‘Centralization’ of the pain is thus a good predictor of a successful outcome.66,67 The reverse change – pain moving more and more distally – indicates that the situation has worsened.
Relation between posture/movement and pain is also important. In minor disc lesions, the ache probably depends entirely on the level of exertion. Any work involving stooping, lifting or sitting for too long is followed by pain, which may, however, be almost or completely absent at rest. In more advanced instances, particular positions are very painful or even impossible. It is obvious that contact will increase in positions and activities that increase intradiscal pressure and thus discodural contact. Bending forwards and lifting result in higher pressure than standing erect. To most patients with backache, walking around is more comfortable than sitting, because the latter imposes more load on the disc.68 Sitting without support causes yet more load and consequently more pain than does sitting with a reclined back rest.53
A paradoxical symptom complex is sometimes encountered. The dynamics of the disc, described above (see p. 420), suggest that intradiscal pressure should decrease and any bulge become less prominent when the patient lies down. Yet some patients have more pain during and after bed rest, wake during the night and have to get out of bed before dawn. The explanation is probably an increase in swelling when the external load diminishes. Diurnal changes in disc hydration and pressure have been demonstrated both in vitro69 and in vivo,70,71 and it is estimated that around 25% of the disc fluid is expressed and re-absorbed during each diurnal cycle.72 A small posterior bulge that becomes more hydrated swells to increase dural contact. This phenomenon is also mirrored in the diurnal changes in the range of the SLR; comparison of the range of SLR after recumbency and after 2 hours erect shows an increase in range of 10% or more.73
The characteristics of discodural pain are summarized in Box 33.2.
Clinical examination
Spinal movements
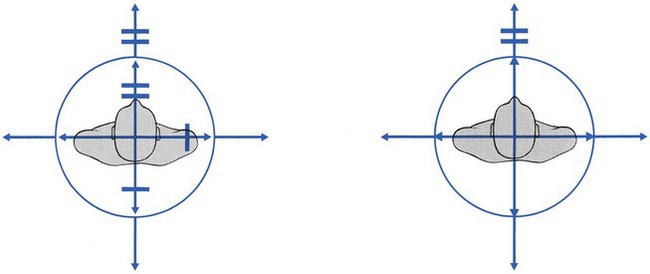
A partial articular pattern is present on the four active movements. The degree of limitation is unequal in different directions (Fig. 33.8); if there is no limitation, some movements are painful at their extremes and some not. All these findings are typical of internal derangement – some movements increase the annular or nuclear bulge, so increasing dural contact, while others reduce it.
Side flexion
Usually this is unequally limited (Fig. 33.8a). Alternatively there is pain at the end of one side flexion only, the other being full-range and painless. If side flexion away from the pain is the more difficult to achieve, manipulative reduction will almost certainly succeed, but in the reverse situation quick and lasting relief by manipulation is more uncertain. Sometimes there is a painful arc (Fig. 33.8d): momentary pain is experienced on moving the trunk from one side to the other. The arc may be quite extensive and is only overcome with considerable effort. Therefore the patient should be encouraged to continue movement and not stop the moment pain is felt; otherwise, the presence of an arc could be missed. Sometimes both side flexions are full and painless. This does not eliminate a disc protrusion but probably indicates that it is too small and too centrally localized to come in contact with the dura during side flexions. Only extension or flexion will then influence the pain and asymmetry is probably only shown by some momentary deviation, a painful arc during flexion or unilateral localization.
Painful arc
Frequently, a painful arc is encountered, with a transient pain somewhere at mid-range (Fig. 33.8c, d). Alternatively, slight deviation may be seen at the midpoint of flexion. Careful observation is needed to detect this visible arc, of which the patient is usually unaware. A painful arc during flexion can be associated with a partial articular pattern but it can also be an isolated finding. It always means that a small fragment of disc tissue impinges momentarily against the dura mater. At the beginning of flexion, an increase in both intradiscal pressure and convexity of the posterior aspect of the intervertebral joint provokes discodural contact (Fig. 33.9). Flexion beyond the horizontal imposes more distraction than compression on the intervertebral joint. The backward pressure on the disc then decreases and is replaced by a more centripetal force on the disc, which is supported by the tightening of the posterior longitudinal ligament. The small posterior displacement is then removed from contact with the dura and pain ceases.
Natural history of discodural backache
It is extremely difficult to predict the natural history and therefore unwise to tell the patient that backache will very soon abate. Although it is true that most episodes are self-limiting,74 the disability often becomes chronic.75 Though an acute bout shows some tendency to spontaneous cure,76 recent research shows that the course of back pain is merely episodic, with repeated recurrences following an acute episode.77–79 A substantial minority of patients may not even experience resolution of their pain and disability, and suffer for years from chronic lower lumbar pain.80–83
Particular types of backache
Bruised dura
One possible explanation for this type of backache is ‘bruising’ of the dura mater.84 The acute lumbago has induced inflammation of the dura mater. Although the disc displacement has receded after some time, the dura has remained inflamed, which results in continuous pain. Obviously this type of backache – chronic pain unaltered by posture or exertion and with a negative clinical examination – can also exist as the result of pain referred to the back from other (visceral) structures.
Nocturnal or morning backache
This nocturnal backache often occurs in middle-aged people. It is best explained by an increase in intradiscal oncotic pressure at an early stage of degeneration. When the external load is diminished in the horizontal position, there is a considerable increase in water content. Expansion forces the disc against the pain-sensitive dura. Resumption of the upright position raises the hydrostatic pressure, water is extruded and the disc deflates, which alleviates the tension on the dura mater so that pain disappears.72
Because the pain is of dural origin, epidural injection is the treatment of choice and succeeds in about 70% of cases.63 Should the injection fail, ligamentous sclerosis is used, in order to stabilize the lower lumbar segments.
Treatment
Before specific treatment is given, a few questions must be answered:
• Is the backache caused by an activity-related spinal disorder?
• If this is the case, is the disorder a discodural interaction or not?
When there is a clear combination of both articular and dural symptoms and signs, the answer is obvious. In moderate discodural backache, however, when the patient presents with articular signs only, it may be more difficult to make a certain diagnosis of internal derangement. However, a partial articular pattern always indicates a disc lesion, except in a few cases (see Ch. 39). A deviation, whether in the upright position or in flexion, signifies a protrusion. Also, the presence of a painful arc, whether during side or forward flexion, is the sign of a small posterior bulge.
• What is the level of the lesion and what is the size and composition of the bulge?
• Is the subluxated fragment an acute and occasional event, or does the patient have recurrent attacks of backache? How long does the disability last? Does the pain disappear completely between bouts or is there a continuous ache?
• What is the degree of pain and how much is the dura inflamed?
• What attitude does the patient have towards the problem?
• Does the patient want to get better? Is there any compensation claim or does the patient show clear evidence of psychological disorder? (see online chapter Psychogenic pain).
• Reduction of the displacement
• Desensitization of the dura (in acute or gross inflammation of the dura, it is sometimes better to desensitize it in order to abate the pain instead of trying to move the disc back into place).
Reduction
If the displacement is annular, the treatment of choice is manipulative reduction. A nuclear displacement is an indication for sustained traction. Although the two techniques are, to some extent, interchangeable, some protrusions prove irreducible by traction, yet reducible by manipulation and vice versa. It is obvious that a protrusion composed of hard annular material will respond better to manipulation but that a soft nuclear bulge requires traction. Cyriax said45: ‘You can hit a nail with a hammer, but treacle must be sucked.’ If sufficient data on the onset of the complaints cannot be obtained and the choice of treatment is in doubt, manipulation should be tried first. If it fails, the patient should attend for traction from the next day on. If considerable improvement is achieved by manipulation but, despite further attempts, a residual displacement cannot be reduced, traction should be substituted so as to complete the process.
Manipulation
The simple and easy-to-learn manipulation measures are usually speedily effective. Overall, acute backache is relieved by one session of manipulation in 46–57% of patients.85,86 As a rule, small annular displacements are cured by a single manipulative session. In larger protrusions, 2–4 sessions may be required. When the patient presents with a marked lateral deviation, up to four manipulation sessions are sometimes needed. Manipulation is successful after a small number of sessions or not at all. Hence, if the patient does not improve almost immediately and lastingly, it is unwise to continue treatment and daily traction should be used instead.
Maintenance of reduction
Strengthening the muscles of the trunk does not increase the stability of the disc. Back muscles do not directly control the intervertebral content and in consequence stability will not depend on their strength but on the position in which they keep the body. Exercise and strengthening of the abdominal and sacrospinalis muscles are therefore futile and may make backache worse because intradiscal pressure increases significantly with prone-lying extension exercises89 and sit-up and curl-up exercises.90,91
Sciatica
Since Mixter and Bar published their classic paper in 1934,12 it has been generally acknowledged that lateral disc displacements are the main source of radicular pain. Pressure of the protruded disc against the nerve root causes mechanical nerve fibre deformation and changes in the nerve root circulation, which result in pain and functional changes.
Mechanism
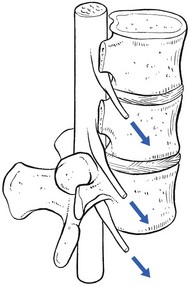
A posterior disc displacement (Fig. 33.10a) usually remains more or less under the physical influence of the posterior longitudinal ligament. The resistance of the ligament keeps the bulge in place or tends to push the protrusion forwards again, as happens during the spontaneous or manipulative reduction of a disc in acute lumbago: the pressure exerted by the ligament is higher than the intradiscal pressure and moves the bulge gradually forwards.
Sometimes, however, when intradiscal pressure remains high, the displaced tissue is pushed more and more laterally towards the posterolateral edge of the disc – a zone of lesser resistance (Fig. 33.10b).92,93 Moved laterally, and freed from the counterpressure of the strong central part of the posterior longitudinal ligament, the bulge enlarges, lifts or ruptures the lateral ligamentous expansion and herniates into the lateral compartment of the epidural space where it compresses the nerve root. This is the typical development of a secondary posterolateral protrusion leading to a classic attack of sciatica.
For a good understanding of the clinical picture, it is important to remember that the severity of the symptoms depends not only on the mass of protruded disc material94 but also on other factors (Fig. 33.11). Among these, the distension within the mass – in other words, the softness or hardness of the bulge – plays an important role.95,96 Furthermore, the relative fixation of the root to the bony elements of the intervertebral foramen can determine the degree of traction on it.97–99 Finally the degree of inflammation of the nerve is a significant element in producing symptoms.100 There can be a direct chemical injury to the nerve root,101–105 or extra- and intraneural swelling,106,107 with further compression.108 Many experts have emphasized that pain is provoked mainly when the nerve root is the site of a chronic irritation,109–111 and experimental confirmation of this has been obtained by inflating Fogarthy balloon catheters placed around the roots, which produce sciatica only if the pressure is maintained long enough to set up an inflammatory reaction.112 During operations using progressive local anaesthesia, sciatica can be produced only by direct pressure or stretch on an inflamed nerve root, whereas pressure on a normal root is painless.65
It should thus be clear that the severity of sciatic symptoms and signs is a function of the magnitude of the mass, the intensity of the discoradicular contact and the inflammatory responses around the nerve root.113 For all these reasons, sciatica is not simply the existence of a bulge, demonstrable on CT. Judging the severity of sciatica therefore depends only on data obtained from the history and clinical examination. Technical investigations usually add little information.
The dural sleeve
Symptom: segmental pain
The dural investment is first to be compressed and inflamed, and causes the appearance of radicular pain. Unlike extrasegmental pain from pressure on the dura, pain stemming from the dural root sleeve follows exactly the rules of segmentally referred pain114 (see Ch. 1). However, an inexperienced examiner may sometimes find it difficult to differentiate segmental radicular pain from extrasegmental dural pain. The following features may then be of value. First, dural pain is never felt beyond the ankle, whereas radicular pain of L4, L5, S1 and S2 origin usually spreads to the foot and the toes. Second, dural pain is not restricted to precise dermatomes of which the patient can give an accurate description but is felt vaguely over a large area. The patient will therefore be more imprecise in describing the area.
Sign: alterations in mobility
The dural sheath of the nerve root moves in relation to the neighbouring structures. As the course of the nerve root is downwards and slightly anterior and the nerve root is loosely bound to the pedicle below by the lateral root ligament, it will be caught against the posterolateral aspect of the disc when downward traction is exerted115 (Fig. 33.12).
During SLR, the L4, L5, S1 and S2 roots undergo a downward and anterior excursion of 2–4 mm at the level of the intervertebral foramen116 (see p. 430). It is obvious that, in large posterolateral disc protrusions, the mobility of these roots is impaired and SLR painfully limited. Sometimes a painful arc rather than limitation is observed: pain appears during the movement but disappears when the leg is raised higher – an indication of a small projection, which the nerve catches against and then slips over.117 Such a momentary pain is an encouraging sign for conservative treatment.
The third lumbar root continues into the femoral nerve, which remains relaxed during SLR. Therefore lack of pain with this manœuvre is not an indication that the L3 root is intact. A better test is knee flexion in the prone position.118,119
There are no clinical tests for the mobility of the S3 and S4 roots.
The parenchyma
Mechanical factors are mainly responsible for intraneural blood flow and formation of intraneural oedema, which in turn causes structural damage to the nerve fibres. It has also been suggested that breakdown products from the degenerating nucleus pulposus may leak through the root and induce a ‘chemical radiculitis’100,120 and that autoimmune mechanisms play a role in the inflammatory tissue reactions seen around degenerating discs.121,122 The details of disturbances in nerve tissue during discoradicular interaction are not yet fully understood; however, their clinical consequences and functional changes are clear.123 On the one hand, hyperexcitability of the fibres results in paraesthesia124 and muscle fasciculations.125 On the other hand, there is loss of nerve function – muscle weakness, sensory deficit and reflex changes.
Symptom: paraesthesia
Pins and needles only appear as the result of hyperexcitability of nervous tissue. They are therefore pathognomonic for peripheral nerve lesions. In nerve root compression they are strictly limited to the respective dermatome and occupy an area at its distal extremity. As a rule, paraesthesiae tend to disappear when numbness begins – hyperexcitability ceases when pressure has induced a sensory deficit (see Ch. 2).
Signs: sensory deficit, motor deficit and reflex changes
On account of the obliquity of the nerve roots and the fact that the sensory and motor rootlets have separate courses within the dural sleeve, it is possible for one protrusion to compress one root, half of a root, two roots or the halves of two consecutive roots (see Fig. 33.16 below). This is particularly so in a lesion at the fifth lumbar level, where the same protrusion compresses the motor rootlet of L5 and the S1 sensory fibres.
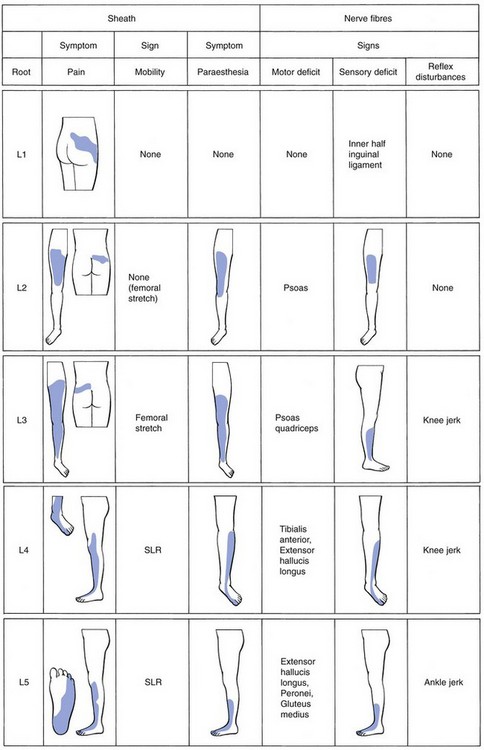
The root signs in discoradicular interactions (see Fig. 33.17 below) are as follows (see reference 126 and Cyriax45: pp. 283–286):
• L1: cutaneous analgesia at, and just below the inner half of the inguinal ligament.
• L2: cutaneous analgesia from the groin to the patella, and weakness of the psoas muscle.
• L3: cutaneous analgesia over the anterior aspect of the leg, from the patella to the ankles, weakness of the psoas and quadriceps muscles and a sluggish or absent knee jerk.
• L4: cutaneous analgesia over the outer ankle, dorsum and inner aspect of the foot and big toe, and weak tibialis anterior and extensor hallucis muscles.
• L5: cutaneous analgesia over the outer leg, the dorsum of the foot and the inner three toes, weak extensor hallucis and peroneal muscles; the ankle jerk may be absent or sluggish.
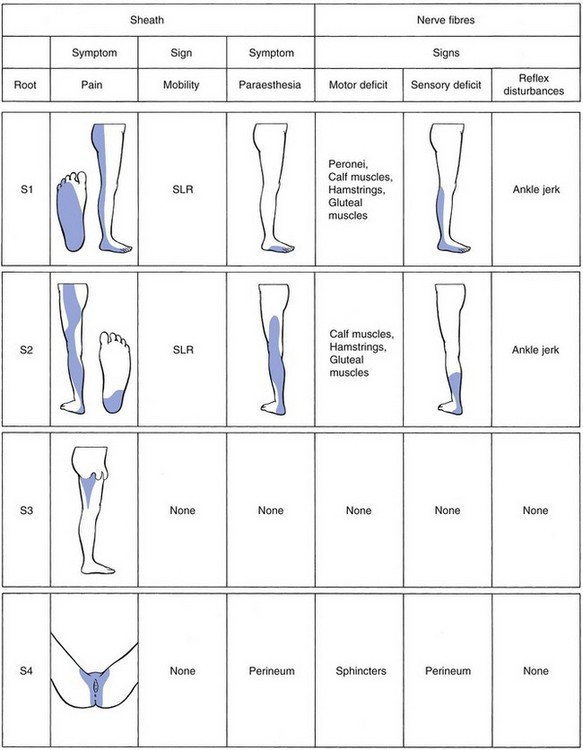
• S1: cutaneous analgesia of the posterolateral aspect of the leg, behind the lateral ankle, the lateral aspect of the foot and the two outer toes; the peronei, calf hamstrings and gluteus medius muscles may be weak; the ankle jerk is sluggish.
• S2: cutaneous analgesia of the dorsum of the leg and the heel, and weakness of the calf, hamstrings and gluteus medius muscles.
• S3: cutaneous analgesia at the inner aspect of the thigh; no muscle weakness.
• S4: numbness of the saddle area and dysfunction of bladder and rectum.
History
Secondary posterolateral protrusion
The symptoms caused by a posterolateral disc displacement have a striking similarity.127 The history is vital in the diagnosis of sciatica and is probably the most important diagnostic technique. The onset and development of symptoms, their relation to posture and exertion, the exact localization of the pain and the presence of paraesthesia and numbness are extremely important features in diagnosis and decision making for both treatment and prophylaxis. The pain often increases on sitting, and eases in recumbency, especially when the patient adopts the ‘psoas position’ – supine, with the hips and knees flexed (see Fig. 33.7). In severe instances, however, when the continuous pressure has induced a considerable inflammation of the dural sheath, the pain may be continuous, sometimes increasing at night. As a rule, standing is better than sitting, but sometimes walking can be difficult, especially if nerve root mobility is impaired in such a way that moving the affected leg forwards during the swing phase drags on the sciatic nerve. The patient then walks with an adaptive gait. In lesions of the third lumbar root, pain increases on standing or reclining and eases only in sitting, because the latter is the only position which relaxes the tension on the femoral nerve and the third lumbar root. These patients often prefer to sleep sitting up. In discoradicular interactions the symptoms are usually worse in the morning, probably as a result of the increased swelling pressure in the disc (Krämer32: pp. 17–21). In an active patient, the pain decreases somewhat around midday and increases again by the evening. Coughing and sneezing may cause pain in the gluteal area or in the limb.
Primary posterolateral protrusion
The history is typical. A young patient states that a calf aches when sitting is prolonged. Alternatively, the pain may be at the lateral side of the knee and the leg, but seldom spreads to the foot. Very occasionally, the onset is with numbness in the heel, later spreading to an ache in the calf and thigh. The moment the patient stands up, the pain disappears. Previous backache has not occurred and the patient usually does not associate the pain in the calf or knee with a disorder in the back; however, a cough or sneeze hurts in the leg. The ache gets slowly worse over a period of months, during which it spreads upwards to the posterior aspect of the thigh. By the time the pain has reached the buttock, it may be constant except in bed. Lumbar flexion and SLR gradually decrease. In the end, even extending the knee becomes painful, which forces the patient to adopt a waddling gait with flexed knee.128
‘Bruised’ dural sleeve
A possible explanation for this unusual pain syndrome is probably a persisting inflammation of the root sleeve, resulting from a past disc lesion that has undergone spontaneous reduction or has been surgically removed. Although there is no more discal contact, the sleeve remains irritated.94
Differential diagnosis
• Sciatica in the elderly is more often caused by a lateral recess stenosis and, especially if the pain appears during standing or walking, the existence of a narrow radicular canal should be suspected (see Ch. 35).129
• Bilateral sciatica is seldom caused by one disc lesion, unless there is a massive protrusion of the disc with rupture of the posterior longitudinal ligament. Evidence of an S4 lesion will also be present (see above). Another, although uncommon, possibility is the presence of two posterolateral protrusions, one at L5 and another at L4 on the other side. Alternatively, one disc has developed two posterolateral disc protrusions, one at each side of the posterior longitudinal ligament. In bilateral sciatica in younger patients, spondylolisthesis should be considered; in elderly patients, suspect spinal or lateral recess stenosis.
• Alternating sciatica is rarely caused by a disc lesion but suggests the sacroiliac arthritis of an early ankylosing spondylitis.
• Increasing backache together with worsening sciatica indicates a serious disorder, especially if the pain does not vary with exertion but steadily gets worse, irrespective of posture or exertion (see Ch. 39).
Clinical examination
Inspection
A lateral pelvic tilt or a deviation may be present. As in lumbago, the deviation can be towards or away from the painful side, depending on the position of the protrusion. If the latter occurs lateral to the nerve root, there is a lateral shift towards the painless side, which reduces contact with the root (Fig. 33.13). If the protrusion is located at the ‘axilla’ between the dura and dural sleeve of the root, the spine is deviated towards the painful side in an attempt to decrease pressure on the root.
Spinal movements
If side flexion or extension hurts in the leg instead of at the lumbar or gluteal area, manipulation nearly always fails, especially if the patient is less than 60 years old (Fig. 33.14).
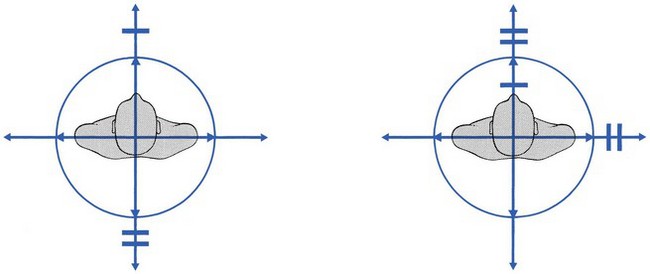
Fig 33.14 Unfavourable patterns in sciatica.
Root tests
Testing the mobility of the root
Straight leg raising examines the mobility of the nerve root sleeves of L4 and S2, whereas prone-lying knee flexion tests that of the L3 root. It is important to remember that each nerve root is incompletely fixed by a ligamentous band, running from the sheath of the nerve root to the inferior pedicle of the respective foramen.99,130,131 During SLR, the sciatic nerve is pulled downwards and the root dragged forwards. Because of its fixation, the nerve root cannot slip away and it is caught against any space-occupying lesion at the front of the canal.98 In contrast, compression of the nerve root from above or from behind does not result in a decrease in root mobility. The anterior and relatively fixed position of the root protects it from a posterior compression when SLR is performed. This observation is extremely important in the differential diagnosis of radicular pain. Lateral recess stenosis or hypertrophy of the facets causes compression from behind (posterior wall lesions) and does not influence the mobility of the root. Thus, SLR (or femoral stretch) specifically tests the mobility between the nerve root and the posterior aspect of the intervertebral joint (anterior wall).
However, limitation of nerve root mobility is not pathognomonic of a disc lesion,132,133 as the specificity of the SLR test is about 90%.134
• Any space-occupying lesion at the anterior aspect of the nerve root canal which interferes with the anterior aspect of the nerve root will cause the same clinical feature. Such is the case in neuromas and tumours, for instance, which cause as much limitation of SLR as do disc lesions.
• Lesions in the buttock can also produce significant limitation of SLR. The combination of a limitation of SLR with serious limitation of flexion of the hip immediately draws attention to such lesions (see Section 12).
• Lesions of the hamstrings and sacroiliac joints also cause pain at the extreme of SLR, as the result of direct tension being exerted on tender structures.
On the other hand, full and painless SLR does not exclude a disc lesion135,136:
• Lesions at L1, L2 and L3 are not detected by SLR because the sciatic nerve does not directly pull on the roots of these levels. However, the L3 root can be subject to some traction at the extreme of SLR because of the downward pull on the dura mater, exerted from the nerve roots below.
• Small posterolateral protrusions are sometimes not large enough to impinge on the dural sleeve during mobilization of the roots in the supine position. In contrast, trunk flexion with the patient erect can provoke pain in the leg because the joint is now compressed by the body weight and the bulge is squeezed in the direction of the root. There is therefore no inconsistency in a patient being unable to bend fully forwards and yet having a full and painless SLR.
• In root atrophy, SLR is also of full range and painless.
• When sciatica causes gross limitation of extension of the trunk, SLR is also often of full range and painless, although at laminectomy a large disc protrusion may be seen. These cases of sciatica are resistant to conservative treatment. It has been suggested that the nerve root emerges here a little higher up in the foramen and therefore is not affected during SLR or bending.
Sometimes SLR on the painless side causes pain in the other limb and sometimes may even be limited. This phenomenon – crossed SLR – is encountered more frequently at the L4–L5 level,137 and indicates axillary protrusion: the downward movement of the dura mater drags the medial aspect of the root against the protrusion (Fig. 33.15).
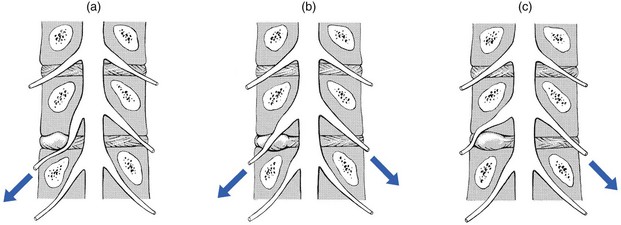
Fig 33.15 Straight leg raising: (a) unilateral limitation; (b) bilateral limitation; (c) crossed limitation.
When neurological deficit is not present, the degree of restriction of SLR is proportional to the pressure exerted on the nerve root. The course of SLR over time is then the best objective criterion by which the development of sciatica can be followed. However, this situation changes when conduction becomes impaired, and then the degree of interference with this affords the best measure of the size of protrusion.45
Testing root conduction
Because of the oblique course of the nerve roots, a disc lesion can compress one single root or two consecutive roots. It is also possible for compression to affect just the upper part of the root and cause sensory deficit, whereas pressure from below will result in motor palsy. A large protrusion can compress two consecutive roots, or the motor fibres of one root and the sensory part of the root below (Fig. 33.16). A fourth–fifth root compression, resulting in a permanent drop foot, can stem from a large protrusion at the fourth level. A fifth–first sacral compression can occur at the fifth level.
Combined third–fourth palsies are extremely rare, and seem to occur only in congenital anomalies of the nerve roots.138,139 Also triple palsies are not possible in single disc lesions. Because L2 disc lesions are extremely rare, an L2 palsy (psoas) always suggests a non-discogenic lesion. Also, bilateral palsies are scarcely ever caused by disc lesions; hence neoplasm should be suspected when there is bilateral weakness. Serious lesions should also be suspected if total loss of power is present, as it is unusual for a disc lesion to cause a complete palsy.
Sometimes, in severe sciatica, the affected leg is found to be colder than the other. Attention may be drawn to this by the patients and confirmation obtained during the clinical examination or by thermography.140–142 In our experience, a cold limb only occurs in combination with neurological deficit.
Ankle and knee jerks sometimes disappear earlier than the muscle power or skin sensitivity (Fig. 33.17). Loss of ankle jerk is permanent in about half of the cases, whereas the knee jerk recovers more often. It is a curious phenomenon that both ankle jerks occasionally disappear during a unilateral sciatica. Bilateral loss of ankle jerk should therefore not be a cause for concern.
Natural history
The majority of patients suffering from a discoradicular interaction heal spontaneously without surgery. Although low back pain can continue for years, sciatica usually has a natural history of spontaneous improvement, even if there is clinical evidence of weakness or radiological evidence of disc extrusion. Despite an abundant literature that proves the contrary, there is still a belief among doctors and patients alike that a herniated disc should be treated operatively. Especially if the signs and symptoms have not improved after a few weeks of bed rest, and if the diagnosis is confirmed by a positive CT or MRI, surgical intervention is recommended.135 This opinion is not supported by studies, however, which show equally good or better results after conservative treatment.143 As soon as the early 1970s, studies found no difference between the final results of surgical and non-surgical therapy after 7–10 years of observation.144–146 These conclusions have been confirmed by more recent work which found that conservative treatment has as good a result as the operative approach after 1 and 2 years of follow-up.147–150,1,32 Obviously, a better knowledge of the natural history of discal herniations and of the mechanisms leading to changes in the extruded discal tissue would be of great help in planning the therapeutic procedure.151
The different mechanisms that result in spontaneous recovery in sciatica, enumerated by Cyriax (his pp. 233–234),45 are spontaneous reduction, erosion of the posteroinferior aspect of the vertebral body, bulge shrinkage and root atrophy. The most important mechanisms are bulge shrinkage and root atrophy.152
Erosion of the posteroinferior aspect of the vertebral body
As described by Young, this is probably not a very important mechanism in recovery from sciatica.153 However, there have also been recent reports of disc herniation eroding bone and thus effectively creating more space and less pressure.154–157 It is considered likely that the defect is caused by a purely mechanical effect.156,157
Bulge shrinkage
The protrusion slowly shrivels away over the course of a few weeks or months, and this probably accounts for the slow and progressive spontaneous recovery from uncomplicated sciatica without neurological deficit.158,159 CT and MRI studies have demonstrated that a high proportion of intervertebral disc herniations have the potential to resolve spontaneously.160,161 The largest herniations appear to be the most likely to undergo a significant decrease in size. The presence of large herniations and/or disc extrusions should therefore not be considered as indications for surgery.162–165 On the contrary, MRI reports confirmed that the more degenerate the disc and the larger the initial herniation, the more the size of the herniated fragment decreased.166–169 There also seems to be a higher incidence of diminution of lateral hernias, compared to central hernias,170,171 and the further the herniated nucleus pulposus migrated, the more rapid decrease in size could be observed,172,173 with full regression of an extruded fragment in all cases.174
The precise mechanism is not totally understood, but one plausible explanation could be that the dissolution of disc material is accelerated when the latter enlarges and becomes deprived of the nutrient influence of the endplates and the posterior longitudinal ligament.175,176 Loss of water content then deflates the protrusion, which decreases the pressure on the nerve root. Additionally, cellular infiltration of the epidural space promotes phagocytosis177–179 of the offending nuclear material, which is transformed into scar tissue.180 Later on, inflammation108 and the resultant venous congestion181 decrease, which in turn further reduces pressure on the root. The spontaneous shrinkage of the protruded material is probably comparable with the disc shrinkage induced by chemonucleolysis (see p. 590).182,183
Root atrophy
The clinical picture is as follows. A patient with sciatica suddenly experiences an increase in pain. After a certain length of time (hours to days), the pain ceases and the skin in the respective dermatome becomes numb. From this time, there may be some weakness in the foot or the leg. Examination shows full range of SLR but complete root dysfunction, both motor and sensory. In root atrophy, there seems to be a relationship between the degree of pain relief and the neurological deficit: the more marked the neurological weakness, the quicker the pain disappears. Neurophysiological recovery is usually slow and not always complete – the atrophy may lead to some slight permanent weakness if two consecutive roots are paretic. A large posterolateral protrusion at the fourth level, for instance, compressing both L4 and L5, may occasionally result in permanent foot drop. In general, however, and when only one nerve root is involved, complete return of strength within 1 year is the rule. The spontaneous recovery of neurological deficit has been studied in monoradicular weakness: in all cases, full recovery was complete in an average of 7 months; when there was multiradicular weakness, only 13% recovered fully.184 The present author re-examined 42 patients with a monoradicular deficit due to a discoradicular interaction, 1–4 years after they had recovered from their sciatic pain; all were completely rehabilitated and muscular weakness could not be detected. Some cutaneous analgesia may be permanent: for instance, the outer side of the foot stays numb after an S1 palsy, or the dorsum of the foot after an L4 root palsy. Some permanent sensory dysfunction remains in about 35% of patients after 10 years.145 In about half, the ankle jerk will not recover but the knee jerk usually does.
The speed of recovery from neurological deficits is very variable and difficult to predict. Usually, a nerve root recovers slowly over 6–12 months, but it can recover with inexplicable rapidity, sometimes within 2–4 weeks and before the pain has ceased completely. This cannot be explained by a simple regrowth of the axons – as regrowth of nerve takes place at a rate of about 1.5 mm a day – and it has been suggested that there might be a peripheral reinnervation of the muscle from intact nerve endings.185,186
It is important to remember that myelograms130 and CT can remain positive for up to 15 months after the pain has disappeared. The same phenomenon has been reported after successful treatment with chymopapain.187 It is therefore unwise to rely on CT for evaluation of the course of sciatica, and again the clinical facts are more important than the radiological appearances.
Once there has been a spontaneous recovery from sciatica, whether by erosion, shrinkage or atrophy, there is no likelihood of recurrence of sciatica at the same level. All the mechanisms of spontaneous cure seem to encourage some stabilization at the joint and therefore recurrence is not the rule. This does not imply that there might not be some chronic or recurrent backache because of a fresh lesion at another level or other mechanisms (ligamentous laxity, and posterior wall problems – see Ch. 34). However, and as a rule, patients who have recovered without surgical treatment do not need to take more care than others. They can therefore continue their normal lifestyle and perform any sports they used to do before the episode. This contrasts strongly with the attitude to be taken to those who have had a laminectomy. The tendency to recurrence then prohibits heavy work, and even with care a constant or intermittent ache may make them aware of their back. Seventy percent of patients who have undergone surgery still complain of backache and 45% of sciatica 4–17 years after the intervention and 37% continue to receive some form of treatment.188,189 The incidence of re-operation ranges from 17 to 23%.190–193 The decision to intervene surgically should therefore not be taken lightly and not until all possible non-operative management, including epidural local anaesthesia, has been tried. Even in an ‘unrelenting’ case, with tolerable root pain, the patient should be made aware of the chance of spontaneous recovery and encouraged to wait at least 8–12 months before opting for operation. With such a conservative approach, very few patients will need an operation. Our personal experience is that such an attitude is appropriate, provided pain remains reasonably controlled.
Treatment
The possibility of spontaneous resolution must influence any evaluation of treatment. Placebo treatment can be effective; for instance, in randomized trials, a placebo for chymopapain injection gave relief in 42–60%.194–197
As for the treatment of discodural interactions, there is no clear-cut overall treatment for sciatica (Fig. 33.18). As the anatomical basis of sciatica differs completely from one patient to another, treatment will always be chosen in relation to the symptoms and signs: ‘Sciatica has many faces, and treatment should always be selective’ (James Cyriax).
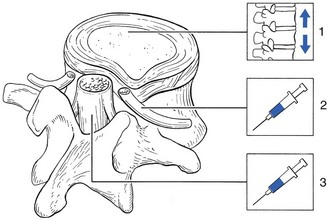
Epidural local anaesthesia
The mechanism of caudal epidural injection is still a matter of debate. Probably the fluid has some hydrostatic effects – epidural injections with 50% of 0.5% procaine produce a hydrostatic pressure that removes the dural tube and the nerve roots from the bulge.198 The effect is not just temporary but persists for the next few weeks. Another explanation for the results obtained after procaine injections in sciatica is that they might influence the chemical mediators of inflammation. Procaine seems to produce better results than lidocaine, perhaps because of the higher pH of procaine (6.5), which may have an influence on the chemical radiculitis.100,199,200
References
1. Cyriax, JH. Lumbago: the mechanism of dural pain. Lancet. 1945; ii:427.
2. MacRae, DL, Asymptomatic intervertebral disc protrusion. Actal Radiol 1956; 46:9. ![]()
3. Torgerson, W, Dotter, WE, Comparative röntgenographic study of the asymptomatic and symptomatic lumbar spine. J Bone Joint Surg 1976; 58A:850. ![]()
4. Wiltse, LL, The effect of the common anomalies of the lumbar spine upon disc degeneration and low back pain. Orthop Clin North Am 1971; 2:569–582. ![]()
5. Waddell, G, A new clinical model for the treatment of low-back pain. Spine 1987; 12:632–644. ![]()
6. Hitselberger, WE, Whitten, RM, Abnormal myelograms in asymptomatic patients. J Neurosurg 1968; 28:204. ![]()
7. Wiesel, SW, Tsourmas, N, Feffer, HL, et al, A study of computer-assisted tomography: 1. The incidence of positive CAT scans in an asymptomatic group of patients. Spine 1984; 9:549–551. ![]()
8. Powell, MC, Wilson, M, Szypryt, P, Symonds, EM, Prevalence of lumbar disc degeneration observed by magnetic resonance in symptomless women. Lancet 1986; 13:1366–1367. ![]()
9. Boden, SD, Davis, DO, Dina, TS, et al, Abnormal magnetic resonance scans of the lumbar spine in asymptomatic subjects. J Bone Joint Surg 1990; 72:403–408. ![]()
10. Jensen, M, Brandt-Zawadzki, M, Obuchowski, N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994; 331:67–73.
11. Boos, N, Rieder, R, Schade, V, The diagnostic accuracy of magnetic resonance imaging, work place perception, and psychosocial factors in identifying symptomatic disc herniations. Spine. 1995;20(24):2613–2625. ![]()
12. Mixter, WJ, Barr, JS. Ruptures of the intervertebral disc, with involvement of the spinal canal. N Engl J Med. 1934; 211:210–215.
13. Cuatico, W, Parker, JC, Further investigations on spinal meningeal nerves and their role in pain production. Acta Neurochir 1989; 101:126–128. ![]()
14. Groen, GJ, Baljet, B, Drukker, J, Nerves and nerve plexuses of the human vertebral column. Am J Anat 1990; 188:289–296. ![]()
15. Ahmed, M, Bjurholm, A, Kreicbergs, A, Schultzberg, M, Neuropeptide Y, tyrosine hydroxylase and vasoactive intestinal polypeptide – immunoreactive nerve fibers in the vertebral bodies, discs, dura mater and spinal ligaments of the rat lumbar spine. Spine 1993; 18:268–273. ![]()
16. Kallakari, S, Cavanaugh, JM, Blagoev, DC, An immunohistochemical study of innervation of lumbar spinal dura and longitudinal ligaments. Spine 1998; 23:403–411. ![]()
17. Wyke, B. The neurology of low back pain. In Jayson MIV, ed.: The Lumbar Spine and Back Pain, 2nd ed, Tunbridge Wells: Pitman Medical, 1980.
18. Roberts, S, Eisenstein, SM, Menage, J, et al, Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides. Spine 1995; 20:2645–2651. ![]()
19. Yamashita, T, Minaki, Y, Oota, I, et al, Mechanosensitive afferent units in the lumbar intervertebral disc and adjacent muscle. Spine 1993; 18:2252–2256. ![]()
20. Palmgren, T, Gronblad, M, Virri, J, et al, An immunohistochemical study of nerve structures in the annulus fibrosus of human normal lumbar intervertebral discs. Spine 1999; 24:2075–2079. ![]()
21. Wiltse, LL, Fonseca, AS, Amster, J, et al, Relationship of the dura, Hofmann’s ligaments, Batson’s plexus, and a fibrovascular membrane lying on the posterior surface of the vertebral bodies and attaching to the deep layer of the posterior longitudinal ligament. An anatomical, radiologic, and clinical study. Spine 1993; 18:1030–1043. ![]()
22. Scapinelli, R, Anatomical and radiologic studies on the lumbosacral meningo-vertebral ligaments of humans. J Spinal Disord 1990; 3:6–15. ![]()
23. Plaisant, O, Sarrazin, JL, Cosnard, G, et al, The lumbar anterior epidural cavity: the posterior longitudinal ligament, the anterior ligaments of the dura mater and the anterior internal vertebral venous plexus. Acta Anal (Basel) 1996; 155:274–281. ![]()
24. Ohshima, H, Hirano, N, Osada, R, et al, Morphologic variation of lumbar posterior longitudinal ligament and the modality of disc herniation. Spine 1996; 18:2408–2411. ![]()
25. Bashline, SD, Bilott, JR, Ellis, JP, Meningovertebral ligaments and their putative significance in low back pain. J Manipul Physiol Ther 1996; 19:592–596. ![]()
26. Grönblad, M, Virri, J, Tolonen, J, et al, A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine 1994; 24:2744–2751. ![]()
27. Kang, JD, Georgescu, HI, McIntyre-Larkin, L, et al, Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine 1996; 21:271–277. ![]()
28. Kayama, S, Konno, S, Olmarker, K, et al, Incision of the annulus fibrosus induces nerve root morphologic, vascular and functional changes. Spine 1996; 22:2539–2543. ![]()
29. Takahashi, H, Suguro, T, Okazima, Y, et al, Inflammatory cytokines in the herniated disc of the lumbar spine. Spine 1996; 21:218–224. ![]()
30. Chen, CC, Cavanaugh, JM, Ozaktay, AC, et al, Effects of phospholipase A2 on lumbar nerve root structure and function. Spine 1997; 22:1057–1064. ![]()
31. Nygaard, OP, Mellgren, SI, Osterud, B, The inflammatory properties of contained and noncontained lumbar disc herniation. Spine 1997; 22:2484–2488. ![]()
32. Krämer, J. Intervertebral Disk Diseases: Causes, Diagnosis, Treatment and Prophylaxis. Stuttgart: Thieme; 1981.
33. Krag, MH, Seroussi, RE, Wilder, DG, Thoracic and lumbar internal disc displacement distribution from in vitro loading of human spinal motion segments: experimental results and theoretical predictions. Spine 1987; 12:1001–1007. ![]()
34. Kelsey, J, White, AA, Epidemiology and impact of low back pain. Spine 1980; 6:133–142. ![]()
35. Cochrane Report. Working Group on Back Pain. London: HMSO; 1979.
36. Gowers, W, Lumbago. BMJ 1904; 1:117. ![]()
37. Suk, KS, Lee, HM, Moon, SH, Kim, NH, Lumbosacral scoliotic list by lumbar disc herniation. Spine (Phila Pa 1976). 2001;26(6):667–671. ![]()
38. Tenhula, JA, Rose, SJ, Delitto, A, Association between direction of lateral lumbar shift, movement tests, and side of symptoms in patients with low back pain. Phys Ther 1990; 70:480–486. ![]()
39. Matsui, H, Ohmori, K, Kanamori, M, et al, Significance of sciatic scoliotic list in operated patients with lumbar disc herniation. Spine 1998; 23:338–342. ![]()
40. Weitz, EM, The lateral bending sign. Spine 1981; 6:388–397. ![]()
41. Troup, JD. Biomechanics of the lumbar spinal canal. Clin Biomech. 1986; 1:31–43.
42. Edgar, MA, Park, WM, Induced pain patterns on passive straight-leg raising in lower lumbar disc protrusions. J Bone Joint Surg 1974; 56B:658–667. ![]()
43. Mooney, V. Differential diagnosis of low back disorders. Principles of classification. In: Frymoyer J, ed. The Adult Spine. New York: Raven Press; 1991:1559.
44. Kostuik, JP, Harrington, I, Alexander, D, et al, Cauda equina syndrome and lumbar disc herniations. J Bone Joint Surg 1986; 68A:386–391. ![]()
45. Cyriax, JH. Textbook of Orthopaedic Medicine, vol. 1, Diagnosis of Soft Tissue Lesions, 8th ed. London: Baillière Tindalll; 1982.
46. Dixon, ASTJ. Diagnosis of low back pain – sorting the complainers. In Jayson MIV, ed.: The Lumbar Spine and Back Pain, 2nd ed, Tunbridge Wells: Pitman Medical, 1980.
47. Nachemson, A, Advances in low back pain. Clin Orthop 1985; 200:266. ![]()
48. Spitzer, WO. Scientific approach to the assessment and management of activity-related spinal disorders, a monograph for clinicians, report of the Quebec Task Force on Spinal Disorders. Spine. 1987; 12.
49. Valkenburg, HA, Haanen, HCM. The epidemiology of low back pain. In: White AA, III., Gordon SL, eds. American Academy of Orthopaedic Surgeons Symposium on Idiopathic Low Back Pain. St Louis: Mosby; 1982:9–22.
50. MacNab, I. Backache. Baltimore: Williams & Wilkins; 1983.
51. Hirschberg, G, Treating lumbar disc lesions. Tex St J Med 1974; 70:58. ![]()
52. Nachemson, AL, Elfström, G, Intravital dynamic pressure measurements in lumbar discs: a study of common movements, maneuvers and exercises. Scand J Rehab Med. 1970;(suppl 1):1–40. ![]()
53. Nachemson, AL. Lumbar intradiscal pressure. In: Jayson M, ed. The Lumbar Spine and Back Pain. London: Pitman Medical, 1987.
54. Waddell, G, 1987 Volvo Award in clinical sciences: a new clinical model for the treatment of low back pain. Spine 1987; 12:632–644. ![]()
55. Deyo, RA, Tsui-Wu, YJ, Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine 1987; 12:264–268. ![]()
56. Gilbert, JR, Taylor, DW, Hildebrand, A, Clinical trial of common treatments for low back pain in family practice. BMJ 1985; 291:791–794. ![]()
57. Lidstrom, A, Zachrisson, M, Physical therapy on low back pain and sciatica: an attempt at evaluation. Scand J Rehabil Med 1970; 2:37–42. ![]()
58. Deyo, RA, Diehl, AK, Rosenthal, M, How many days of bed rest for acute low back pain? N Engl J Med 1986; 315:1064–1070. ![]()
59. Atlas, SJ, Volinn, E, Classics from the spine literature revisited: a randomized trial of 2 versus 7 days of recommended bed rest for acute low back pain. Spine (Phila Pa 1976). 1997;22(20):2331–2337. ![]()
60. Hagen, KB, Hilde, G, Jamtvedt, G, Winnem, M, WITHDRAWN: Bed rest for acute low-back pain and sciatica. Cochrane Database Syst Rev. 2010;16(6). ![]()
61. McKenzie, RA, May, S. Mechanical Diagnosis and Therapy: The Lumbar Spine, 2nd ed. Waikanae: Spinal Publications; 2003.
62. Stankovic, R, Johnell, O, Conservative treatment of acute low back pain; a prospective randomised trial: McKenzie method of treatment versus patient education in ‘mini back school’. Spine 1990; 15:120–123. ![]()
63. Ombregt L, Cyriax JH, eds. Illustrated Manual of Orthopaedic Medicine. Butterworths: London, 1983:212.
64. Frymoyer, JW, Gordon, SL, Research perspectives in low-back pain: report of a 1988 workshop. Spine 1989; 14:1384–1388. ![]()
65. Kuslich, SD, Ulstrom, CL, Michael, CJ, The tissue origin of low back pain and sciatica. Orthop Clin North Am 1991; 22:181–187. ![]()
66. Donelson, R, Silva, G, Murphy, K, Centralization phenomenon; its usefulness in evaluating and treating referred pain. Spine 1990; 15:211–213. ![]()
67. Aina, A, May, S, Clare, H, The centralization phenomenon of spinal symptoms – a systematic review. Man Ther. 2004;9(3):134–143. ![]()
68. Nachemson, AL, Disc pressure measurements. Spine 1981; 6:93–97. ![]()
69. Urban, JPG, McMullin, JF, Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition and degeneration. Spine 1988; 13:179–187. ![]()
70. Adams, MA, Dolan, P, Hutton, WC, Diurnal variations in the stresses on the lumbar spine. Spine 1987; 2:130–137. ![]()
71. Tyrrell, AR, Reilly, T, Troup, JDG, Circadian variations in stature and the effects of spinal loading. Spine 1985; 10:161–164. ![]()
72. Sivan, S, Neidlinger-Wilke, C, Würtz, K, et al, Diurnal fluid expression and activity of intervertebral disc cells. Biorheology. 2006;43(3–4):283–291. ![]()
73. Porter, RW, Trailescu, IF, Diurnal changes in straight leg raising. Spine 1990; 15:103–106. ![]()
74. Andersson, GBJ, Svensson, H-O, Oden, A, The intensity of work recovery in low back pain. Spine 1983; 8:880–884. ![]()
75. Cassidy, JD, Côté, P, Carroll, L, Kristman, V, The incidence and course of low back pain in the general population: a population-based cohort study. Spine 2005; 30:2817–2823. ![]()
76. Bergquist-Ullman, M, Larsson, U, Acute low back pain in industry. Acta Orthop Scand. 1977;170(suppl):1–117. ![]()
77. Troup, JDG, Causes, prediction and prevention of back pain at work. Scand J Work Environ Health 1984; 10:419–428. ![]()
78. Hestbaek, L, Leboeuf, YdeC, Manniche, C, Low back pain what is the long-term course? A review of studies of general patient populations. Eur Spine J 2003; 12:149–165. ![]()
79. Côté, P, Baldwin, ML, Johnson, WG, et al, Patterns of sick-leave and health outcomes in injured workers with back pain. Eur Spine J. 2008;17(4):484–493. ![]()
80. Troup, JDG, Martin, JW, Lloyd, DCEF, Back pain in industry: a prospective survey. Spine 1981; 6:61–69. ![]()
81. Van Korff, M, Saunders, K, The course of back pain in primary care. Spine 1996; 24:2833–2839. ![]()
82. Van Korff, M, Studying the natural history of back pain. Spine 1994; 19:2041S–2046S. ![]()
83. Klenerman, L, Slade, PD, Stanley, IM, et al, The prediction of chronicity in patients with an acute attack of low back pain in a general practice setting. Spine 1995; 20:478–484. ![]()
84. Otani, K, Arai, I, Mao, G-P, et al, Experimental disc herniation; evaluation of the natural course. Spine 1997; 22:2894–2899. ![]()
85. Barbor, R. Low backache. BMJ. 1955; 1:55.
86. Fisk, B, Manipulation in general practice. NZ Med J 1971; 74:172. ![]()
87. Armstrong, J. Lumbar Disc Lesions. Baltimore: Williams & Wilkins; 1965.
88. Lancourt, JE. Traction techniques for low back pain. J Musculoskel Med. 1986.
89. Nachemson, A. Review of mechanics of the lumbar disc. Rheumat Rehabil. 1975; 14:129.
90. Andersson, GBJ, Örtengren, R, Nachemson, A, Intradiscal pressure, intra-abdominal pressure and myo-electric back muscle activity related to posture and loading. Clin Orthop 1977; 129:156. ![]()
91. Nachemson, AL, Disc pressure measurements. Spine 1981; 6:93–97. ![]()
92. Farfan, HF. Mechanical disorders of the low back. Philadelphia: Lea & Febiger; 1973.
93. Nachemson, A. The lumbar spine – an orthopaedic challenge. Spine. 1976; 1:59.
94. Fagerlund, MKJ, Thelander, U, Friberg, S, Size of lumbar disc hernias measured using computed tomography and related to sciatic symptoms. Acta Radiologica 1990; 31:555–558. ![]()
95. McCullogh, JA. Computed tomography before and after chemonucleolysis. In: Post MFD, ed. Computed Tomography of the Spine. Baltimore: Williams & Wilkins, 1983.
96. Boumphrey, FRS, Bell, GR, Modic, M, et al, Computed tomography scanning after chymopapain injection for herniated nucleus pulposus: a prospective study. Clin Orthop 1987; 219:120–123. ![]()
97. Yu, QY, Yang, CR, Yu, LT, Imaging study of lumbar intervertebral disc herniation and asymptomatic lumbar intervertebral disc herniation. Zhongguo Gu Shang. 2009;22(4):279–282. ![]()
98. Bertolini J, Miller J, Spencer D. The effect of intervertebral disc space narrowing on the contact force between the nerve root and a simulated disc protrusion. Presented at the annual meeting of the International Society for the Study of the Lumbar Spine, Toronto, Canada, 10 June 1982.
99. Spencer, DL, Irwin, GS, Miller, JAA, Anatomy and significance of the lumbosacral nerve roots in sciatica. Spine 1983; 8:672–679. ![]()
100. Marshall, LL, Trethewie, ER, Curtain, CC, Chemical radiculitis: a clinical, physiological and immunological study. Clin Orthop 1977; 129:61–67. ![]()
101. Gertzbein, S, Degenerative disc disease of the lumbar spine. Clin Orthop 1977; 129:68–71. ![]()
102. Marshall, L, Trethewie, E, Curtain, C, Chemical irritation of nerve-root in disc-prolapse. Lancet 1973; 11:320. ![]()
103. Saal, JS, Franson, RC, Dobrow, R, et al, High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine 1990; 15:674–678. ![]()
104. Franson, RC, Saal, JS, Saal, JA, Human disc phospholipase A2 is inflammatory. Spine. 1992;17(suppl):129–132. ![]()
105. Olmarker, K, Rydevik, B, Norborg, C, Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine 1993; 18:1425–1432. ![]()
106. Rydevik, B, Brown, MD, Ehira, T, et al. Effects of graded compression and nucleus pulposus on nerve tissue – an experimental study in rabbits. Proceedings of the Swedish Orthopaedic Association, Götenborg, Sweden, 27 Aug 1982. Acta Orthop Scand. 1983; 54:670–671.
107. Thelander, U, Fagerlund, M, Friberg, S, Larsson, S, Straight leg raising test versus radiologic size, shape, and position of lumbar disc hernias. Spine 1992; 17:395–399. ![]()
108. Takata, K, Inoue, S, Takahashi, K, Ohtsuka, Y, Swelling of the cauda equina in patients who have herniation of a lumbar disc. J Bone Joint Surg 1988; 70A:361–368. ![]()
109. Smyth, MJ, Wright, V, Sciatica and the intervertebral disc. An experimental study. J Bone Joint Surg 1958; 40A:1401–1418. ![]()
110. MacNab, I. The mechanism of spondylogenic pain. In: Hirsch C, Zotterman Y, eds. Cervical Pain. Oxford: Pergamon Press, 1972.
111. Rydevik, B, Brown, MD, Lundborg, G, Pathoanatomy and pathophysiology of nerve root compression. Spine 1984; 9:7–15. ![]()
112. Howe, JF, Loeser, JD, Calvin, WH, Mechanosensitivity of dorsal root ganglia and chronologically injured axons: a physiological basis for the radicular pain of nerve root compression. Pain 1977; 3:25–41. ![]()
113. Garfin, SR, Rydevik, BL, Brown, RA, Compressive neuropathy of spinal nerve roots. A mechanical or biological problem? Spine 1991; 16:162–166. ![]()
114. Mumenthaler, M, Schliack, H. Läsionen peripherer Nerven. Stuttgart: Thieme; 1973.
115. Hofmann, M. Die Befestigung der Dura mater im Wirbelkanal. Arch Anat Physio (Anat Abt). 1899; 403.
116. Goddard, MD, Reid, JD, Movements induced by straight leg raising in the lumbo-sacral roots, nerves and plexus and in the intrapelvic section of the sciatic nerve. J Neurol Neurosurg Psychiatry 1965; 28:12–18. ![]()
117. Smith, SA, Massie, JB, Chesnut, R, Garfin, SR, Straight leg raising. Anatomical effects on the spinal nerve root without and with fusion. Spine 1993; 18:992–999. ![]()
118. Estridge, MN, Rouhe, SA, Johnson, NG, The femoral stretching test. J Neurosurg 1982; 57:813–817. ![]()
119. Lee, SH, Choi, SM. L1-2 disc herniations: clinical characteristics and surgical results. J Korean Neurosurg Soc. 2005; 38:196–201.
120. Nachemson, A, Intradiscal measurements of pH in patients with lumbar rhizopathies. Acta Orthop Scand 1969; 40:23–42. ![]()
121. Gertzbein, SD, Tile, M, Gross, A, Falk, R, Auto-immunity in degenerative disc disease of the lumbar spine. Orthop Clin North Am 1975; 6:67–73. ![]()
122. Kliliuk, PS, Pountain, GD, Keegan, AL, Jayson, MIV, Serial measurements of fibrinolytic activity in acute low back pain and sciatica. Spine 1987; 12:925–928. ![]()
123. Rydevik, B, Nordborg, C, Changes in nerve function and nerve fibre structure induced by acute, graded compression. J Neurol Neurosurg Psychiatry 1980; 43:1070–1082. ![]()
124. Brodal, A. Neurological Anatomy in Relation to Clinical Medicine, 2nd ed. New York: Oxford University Press; 1969.
125. Rasminsky, M. Ectopic generation of impulses in pathological nerve fibres. In: Jewett DL, McCarroll HR, Jr., eds. Nerve Repair and Regeneration – its Clinical and Experimental Basis. St Louis: Mosby; 1980:178–185.
126. The Guarantors of Brain. Aids to the Examination of the Peripheral Nervous System. London: Baillière Tindall; 1986.
127. Leavitt, S, Garron, DC, Whisler, WW, Sheinkop, MB, Affective and sensory dimensions of back pain. Pain 1978; 4:273–281. ![]()
128. Atalay, A, Akbay, A, Atalay, B, Akalan, N, Lumbar disc herniation and tight hamstrings syndrome in adolescence. Childs Nerv Syst. 2003;19(2):82–85. ![]()
129. Hall, S, Bartleson, JD, Onofrio, BM, et al, Lumbar spinal stenosis. Clinical features, diagnostic procedures and results of surgical treatment in 68 patients. Ann Intern Med. 1985;103(2):271–275. ![]()
130. Falconer, MA, McGeorge, M, Begg, AC, Observations on the cause and mechanism of symptom production in sciatica and low back pain. J Neurol Neurosurg Psychiatry 1948; 11:13. ![]()
131. O’Connell, JEA, Protrusions of the lumbar intervertebral discs: a clinical review based on five hundred cases treated by excision of the production. J Bone Joint Surg 1951; 33B:8. ![]()
132. Spangfort, E, Lasègue’s sign in patients with lumbar disc herniation. Acta Orthopaed Scand 1971; 42:459–460. ![]()
133. Hakelius, A, Hindmarsh, J, The comparative reliability of preoperative diagnostic methods in lumbar disc surgery. Acta Orthop Scand 1972; 43:234–238. ![]()
134. Majlesi, J, Togay, H, Unalan, H, Toprak, S, The sensitivity and specificity of the Slump and the Straight Leg Raising tests in patients with lumbar disc herniation. J Clin Rheumatol. 2008;14(2):87–91. ![]()
135. Spengler, DM, Freeman, CW, Patient selection for lumbar discectomy: an objective approach. Spine 1979; 4:129–134. ![]()
136. Blower, PW, Neurologic patterns in unilateral sciatica: a prospective study of 100 new cases. Spine 1981; 6:175–179. ![]()
137. Spangfort, EV, The lumbar disc herniation: a computer-aided analysis of 2504 operations. Acta Orthop Scand. 1978;142(suppl):1–95. ![]()
138. Kikuchi, S, Hasue, M, Nishiyama, K, Ito, T, Anatomic and clinical studies of radicular symptoms. Spine 1984; 9:23–30. ![]()
139. Kadish, LJ, Simmons, EH, Anomalies of the lumbosacral nerve roots. J Bone Joint Surg 1984; 66B:411–416. ![]()
140. Stary, O, Pathogenesis of discogenic disease. Rev Czech Med 1956; 2:1. ![]()
141. Ash, CJ, Shealy, CN, Young, PA, et al, Thermography and the sensory dermatome. Skel Radiol 1986; 15:40–46. ![]()
142. So, YT, Aminoff, MJ, Olney, RK, The role of thermography in the evaluation of lumbosacral radiculopathy. Neurology 1989; 39:1154–1158. ![]()
143. Weinstein, JN, Tosteson, TD, Lurie, JD, et al, Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296(20):2441–2450. ![]()
144. Hakelius, A, Prognosis in sciatica. A clinical follow-up of surgical and nonsurgical treatment. Acta Orthop Scand. 1970;129(suppl). ![]()
145. Nashold, BS, Hrubec, Z. Lumbar Disc Disease. A Twenty-year Clinical Follow-up Study. St Louis: Mosby; 1971.
146. Weber, H, Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine 1983; 8:131–140. ![]()
147. Atlas, SJ, Keller, RB, Chang, Y, et al, Surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: five-year outcomes from the Maine Lumbar Spine Study. Spine. 2001;26(10):1179–1187. ![]()
148. Peul, WC, van Houwelingen, HC, van den Hout, WB, et al, Surgery versus prolonged conservative treatment for sciatica. N Engl J Med. 2007;356(22):2245–2456. ![]()
149. Van Tulder, MW, Koes, B, Seitsalo, S, Malmivaara, A, Outcome of invasive treatment modalities on back pain and sciatica: an evidence-based review. Eur Spine J. 2006;15(1):S82–S92. ![]()
150. Gibson, JN, Waddell, G, Surgical interventions for lumbar disc prolapse. Cochrane Database Syst Rev 2007; 2. ![]()
151. Awad, JN, Moskovich, R, Lumbar disc herniations: surgical versus nonsurgical treatment. Clin Orthop Relat Res 2006; 443:183–197. ![]()
152. Benoist, M, The natural history of lumbar disc herniation and radiculopathy. Joint Bone Spine. 2002;69(2):155–160. ![]()
153. Young, RH, Results of surgery in sciatica and low back pain. Lancet 1952; i:245. ![]()
154. Briceno, C, Fazl, M, Willinsky, RA, Gertzbein, S, Sequestered intervertebral disc associated with vertebral erosion. Spine 1989; 14:898–899. ![]()
155. Nofray, JF, Gadom, J, Becker, RC, et al, Extruded nucleus pulposus causing osseous erosion of the lumbar vertebral body. Spine 1988; 13:941–944. ![]()
156. Vadala, G, Dore, R, Garbagna, P, Unusual osseous changes in lumbar herniated discs: CT features. J Comput Assist Tomogr 1985; 9:1045–1049. ![]()
157. Vincent, JM, Baldwin, JE, Sims, C, Dixon, AK, Vertebral ‘corner’ defect associated with lumbar disc herniation shown by magnetic resonance imaging. Spine 1993; 18:109–113. ![]()
158. Saal, JA, Saal, JS, Herzog, RI, The natural history of lumbar intervertebral disc extrusions treated nonoperatively. Spine 1990; 15:683–686. ![]()
159. Teplick, JG, Haskin, ME, Spontaneous regression of herniated nucleus pulposus. AJNR 1985; 6:331–335. ![]()
160. Masui, T, Yukawa, Y, Nakamura, S, et al, Natural history of patients with lumbar disc herniation observed by magnetic resonance imaging for minimum 7 years. J Spinal Disord Tech. 2005;18(2):121–126. ![]()
161. Cribb, GL, Jaffray, DC, Cassar-Pullicino, VN, Observations on the natural history of massive lumbar disc herniation. J Bone Joint Surg Br. 2007;89(6):782–784. ![]()
162. Maigne, JY, Rime, B, Deligne, B, Computed tomographic follow-up study of forty-eight cases of nonoperatively treated lumbar intervertebral disc herniation. Spine 1992; 17:1071–1074. ![]()
163. Bush, K, Cowan, N, Katz, D, Gishen, P, The natural history of sciatica associated with disc pathology. Spine 1992; 17:1205–1222. ![]()
164. Delauche-Cavalier, MC, Budet, C, Laredo, JD, et al, Lumbar disc herniation; computed tomography scan changes after conservative treatment of nerve root compression. Spine 1992; 17:927–933. ![]()
165. Ellenberg, MR, Ross, ML, Honet, JC, et al, Prospective emulation of the course of disc herniations in patients with proven radiculopathy. Arch Phys Med Rehabil 1993; 74:3–8. ![]()
166. Matsubara, Y, Kato, F, Mimatsu, K, Serial changes on MRI in lumbar disc herniations treated conservatively. Neuroradiology 1995; 37:378–383. ![]()
167. Modic, MT, Ross, JS, Obuchowski, NA, et al, Contrast-enhanced MR imaging in acute lumbar radiculopathy: a pilot study of the natural history. Radiology 1995; 195:429–435. ![]()
168. Fraser, RD, Sandhu, A, Gogan, WJ, Magnetic resonance imaging findings 10 years after treatment for lumbar disc herniation. Spine 1995; 20:710–714. ![]()
169. Erly, WK, Munoz, D, Beaton, R, Can MRI signal characteristics of lumbar disk herniations predict disk regression? J Comput Assist Tomogr. 2006;30(3):486–489. ![]()
170. Ahn, SH, Park, HW, Byun, WM, et al, Comparison of clinical outcomes and natural morphologic changes between sequestered and large central extruded disc herniations. Yonsei Med J. 2002;43(3):283–290. ![]()
171. Dullerud, R, Nakstad, PH, CT changes after conservative treatment for lumbar disk herniation. Acta Radiologica 1994; 35:415–419. ![]()
172. Komori, H, Shinomiya, MD, Nakai, O, et al, The natural history of herniated nucleus pulposus with radiculopathy. Spine 1996; 21:225–229. ![]()
173. Benson, RT, Tavares, SP, Robertson, SC, et al, Conservatively treated massive prolapsed discs: a 7-year follow-up. Ann R Coll Surg Engl. 2010;92(2):147–153. ![]()
174. Splendiani, A, Puglielli, E, De Amicis, R, et al, Spontaneous resolution of lumbar disk herniation: predictive signs for prognostic evaluation. Neuroradiology. 2004;46(11):916–922. ![]()
175. Hendry, NGC, The hydration of the nucleus pulposus and its relation to intervertebral disc derangement. J Bone Joint Surg 1958; 40B:132–144. ![]()
176. Naylor, A, The biomechanical changes in the human intervertebral disc in degeneration and nuclear prolapse. Orthop Clin North Am. 1971;2(2):343. ![]()
177. Ito TakuiYamada, M, Ikuta, F, et al, Histologic evidence of absorption of sequestration-type herniated disc. Spine 1996; 21:230–234. ![]()
178. Ikeda, T, Nakamura, T, Kikuchi, T, et al, Pathomechanism of spontaneous regression of the herniated lumbar disc: histologic and immunohistochemical study. J Spinal Disord 1996; 9:136–140. ![]()
179. Slavin, KV, Raja, A, Thornton, J, Wagner, FC, Jr., Spontaneous regression of a large lumbar disc herniation: report of an illustrative case. Surg Neurol. 2001;56(5):333–336. ![]()
180. Hirabayashi, S, Kumano, K, Tsuiki, T, et al, A dorsally free fragment of lumbar disc herniation and its interesting histologic findings. A case report. Spine 1990; 15:1221–1223. ![]()
181. Parke, WW, Watanabe, R, The intrinsic vasculature of the lumbosacral spine nerve roots. Spine 1985; 10:508–515. ![]()
182. Suguro, T, Oegema, T, Bradford, D, The effects of chymopapain on prolapsed human intervertebral disc. Clin Orthop 1986; 213:223–231. ![]()
183. Spencer, DL, Miller, JAA. The mechanism of sciatic pain relief by chemonucleolysis. Orthopedics. 1983; 6:1600–1603.
184. Yates, DAH. Indications for spinal manipulation. Ann Phys Med. 1964; 10:146.
185. Woolf, AL, Till, K, Pathology of the lower motor neurone in the light of new muscle biopsy techniques. Proc R Soc Med 1955; 48:189. ![]()
186. Yates, DAH, Unilateral lumbosacral root compression. Ann Physiol Med. 1964;7(5):169–179. ![]()
187. Macnab, I, McCulloch, JA, Weiner, DS, et al, Chemonucleolysis. Can J Surg 1971; 14:280. ![]()
188. Loupasis, GA, Stamos, K, Katonis, PG, et al, Seven- to 20-year outcome of lumbar discectomy. Spine (Phila Pa 1976). 1999;24(22):2313–2317. ![]()
189. Yorimitsu, E, Chiba, K, Toyama, Y, Hirabayashi, K, Long-term outcomes of standard discectomy for lumbar disc herniation: a follow-up study of more than 10 years. Spine (Phila Pa 1976). 2001;26(6):652–657. ![]()
190. Dvorak, J, Gauchat, M-H, Valach, L, The outcome of surgery for lumbar disc herniation I. A 4–17 years’ follow-up with emphasis on somatic aspects. Spine 1988; 13:1418–1422. ![]()
191. Rish, BL, A critique of the surgical management of lumbar disc disease in private neurosurgical practice. Spine 1984; 9:500–504. ![]()
192. Berney, J. Sciatiques chirurgicales et chirurgie des sciatiques. Med Hyg. 1980; 38:2006–2013.
193. Vik, A, Zwart, JA, Hulleberg, G, Nygaard, OP, Eight year outcome after surgery for lumbar disc herniation: a comparison of reoperated and not reoperated patients. Acta Neurochir (Wien). 2001;143(6):607–610. ![]()
194. Martins, AN, Ramirez, A, Johnston, J, Schwetzenau, PR. Double blind evaluation of chemonucleolysis for herniated lumbar discs. A prospective study with random assignment. Clin Orthop. 1983; 174:236–242.
195. Javid, MJ, Nordby, EJ, et al, Safety and efficacy of chymopapain (chymodiactin) in herniated nucleus pulposus with sciatica – results of a randomized, double blind study. JAMA 1983; 249:2489–2494. ![]()
196. Feldman, J, Menkes, CJ, Pallardy, G, et al, Etude en double-aveugle du traitement de la lombosciatique discale par chimionucléolyse. Rev Rhum Mal Osteoartic 1986; 53:147–152. ![]()
197. Fraser, RD, Chymopapain for the treatment of intervertebral disc herniation – the final report of a double blind study. Spine 1984; 9:815–818. ![]()
198. Troisier, O. Sémilogie et traitement des algies discales et ligamentaires du rachis. Paris: Masson; 1973.
199. Troisier, O. Communication on the symposium on low back pain. Antwerp, De Haan, Belgium: BSSOM; 1982.
200. Manchikanti, L, Cash, KA, McManus, CD, et al, One-year results of a randomized, double-blind, active controlled trial of fluoroscopic caudal epidural injections with or without steroids in managing chronic discogenic low back pain without disc herniation or radiculitis. Pain Physician. 2011;14(1):25–36. ![]()
201. Ombregt L. Epidural local anaesthesia – results after two years of use in a general practice. Symposium on low back pain. Antwerp, De Haan, Belgium: BSSOM; 1982.